Abstract
Persons with spinal cord injury (SCI) have secondary medical consequences of paralysis and/or the consequences of extreme inactivity. The metabolic changes that result from reduced activity include insulin resistance with carbohydrate disorders and dyslipidemia. A higher prevalence of coronary artery calcification was found in persons with SCI than that in matched able-bodied controls. A depression in anabolic hormones, circulating testosterone and growth hormone, has been described. Adverse soft tissue body composition changes of increased adiposity and reduced skeletal muscle are appreciated. Immobilization is the cause for sublesional disuse osteoporosis with an associated increased risk of fragility fracture. Bowel dysmotility affects all segments of the gastrointestinal tract, with an interest in better defining and addressing gastroesophageal reflux disease and difficulty with evacuation. Developing and testing more effective approaches to cleanse the bowel for elective colonoscopy are being evaluated. The extent of respiratory dysfunction depends on the level and completeness of SCI. Individuals with higher spinal lesions have both restrictive and obstructive airway disease. Pharmacological approaches and expiratory muscle training are being studied as interventions to improve pulmonary function and cough strength with the objective of reducing pulmonary complications. Persons with spinal lesions above the 6th thoracic level lack both cardiac and peripheral vascular mechanisms to maintain blood pressure, and they are frequently hypotensive, with even worse hypotension with upright posture. Persistent and/or orthostatic hypotension may predispose those with SCI to cognitive impairments. The safety and efficacy of anti-hypotensive agents to normalize blood pressure in persons with higher level cord lesions is being investigated.
Keywords: airway disease, anabolic hormones, cardiovascular risk, constipation, hypotension, insulin resistance, osteoporosis
Introduction
Individuals with spinal cord injury (SCI) have an increased prevalence of abnormalities in carbohydrate and lipid metabolism because of immobilization, muscle atrophy, and increased adiposity. In those with SCI, an inverse relationship has been reported between serum high-density lipoprotein (HDL) cholesterol values and abdominal circumference and a direct relationship between serum triglycerides levels and abdominal circumference. Persons with SCI have lower serum HDL cholesterol levels than able-bodied controls. A higher prevalence of insulin resistance and diabetes mellitus, as well as an earlier occurrence of coronary heart disease (CHD), has been reported in persons with SCI than in the general population. Recently, a higher prevalence and greater degree of coronary artery calcification by electron beam computerized tomography has been demonstrated in persons with SCI, even when matched with the able-bodied population for age, gender, ethnicity, and conventional risk factors for CHD. Knowledge of relative risk of CHD in persons with SCI is important for appropriate intervention strategies.
A significant number of individuals with chronic SCI have gastrointestinal (GI) symptoms due to bowel dysfunction, a medical complication that adversely affects health and lifestyle. In persons with chronic SCI, there is delayed transit through the left colon, which may be the result of decreased colonic parasympathetic tone. Adequate bowel care is an important part of their clinical management. An adequate bowel regimen depends on many factors that require an individualized approach for each patient. The goals are to achieve effective evacuation and prevent complications, such as impaction and incontinence. It is important for rehabilitation specialists to understand the pathophysiology of bowel problems after SCI to correctly approach the clinical management of this condition. Preparing an individual with SCI for elective colonoscopy may be a challenging undertaking. It is well appreciated that screening colonoscopy can reduce the incidence of colonic cancer and its consequences. Novel cleansing approaches to achieve high quality colon preparation have been recently employed with associated higher polyp detection rates.
The extent of respiratory dysfunction depends on the level and completeness of SCI. Higher, more complete cord lesions result in loss of function of the major muscles of respiration, leading to ineffective cough and retention of pulmonary secretions. These factors may predispose individuals to atelectasis, pneumonia, and respiratory failure. A high prevalence of persons with SCI report respiratory symptoms, with the frequency directly related to the level of lesion. Our previous work identified the presence of baseline bronchoconstriction, which was unmasked by bronchodilator responsiveness to a beta-2 adrenergic agonist or an anticholinergic agent in persons with cervical SCI. In addition to baseline bronchoconstriction and bronchodilator responsiveness, individuals with tetraplegia have nonspecific airway hyperreactivity (AHR). AHR might result from reductions in baseline airway caliber in persons with tetraplegia due to overriding cholinergic airway tone. An alternative or contributing explanation for reduction in airway caliber in persons with tetraplegia is chronic airway inflammation. In general, scant literature exists in those with chronic SCI which address the relationship between therapeutic interventions with patient outcomes. Electrical stimulation of the diaphragm in high cord lesions and resistive respiratory muscle training to improve expiratory muscle function and cough effectiveness has been demonstrated to be beneficial. In addition, preliminary evidence has suggested that beta-2 adrenergic agonists improve respiratory muscle strength in SCI. A pharmacological approach in conjunction with expiratory muscle training may lead to greater beneficial effects on pulmonary function and cough strength than that of either intervention alone, which would be expected to reduce pulmonary complications.
SCI above the 6th thoracic level interrupts supraspinal control of sympathetic efferent innervation to the heart, the upper and lower extremity and splanchnic bed vasculatures. Thus, individuals with higher cord lesions have restricted chronotropic and inotropic cardiac function, which limits the ability of the heart to respond to metabolic demand, except by vagal withdrawal, and attenuates the heart rate response to orthostasis and exercise. Cord lesions T7 and below affect the lower extremity and splanchnic vasculature but spare the upper extremity vasculature and the heart. Decentralized sympathetic control of the peripheral vasculature results in compromised centrally mediated vasoconstriction, which is directly related to the level and completeness of lesion. Hypotension is associated with cognitive deficits in healthy able-bodied persons, and it would be envisioned that persistent and/or orthostatic hypotension may predispose those with SCI to similar cognitive impairments. The safety and efficacy of anti-hypotensive agents to normalize blood pressure in persons with high level cord lesions is actively being investigated.
Metabolic and Endocrine Disorders
Immobilization and paralysis from SCI result in loss of muscle and a relative gain in adiposity. SCI predisposes the individual to medical complications such as lipid abnormalities, carbohydrate intolerance, and an atherogenic pattern that predisposes to CHD. One likely etiology for these secondary metabolic conditions is that they are the result of adverse body composition changes in conjunction with inactivity, which are progressive with advancing age. Another possible consideration is a global reduction in endogenous anabolic hormones that would be anticipated to worsen body compositional changes in those with SCI.
Soft tissue body composition changes
Longitudinal studies of body composition after acute SCI are limited, but have shown a brisk, marked loss of lean tissue mass.1,2 A recent unpublished observation by our group has shown an increase in total body fat mass on average of 10 kg over the initial 2 years after acute injury. Comparing matched reference populations using cross-sectional designs in individuals with chronic SCI, investigators have demonstrated marked lean tissue loss and/or fat tissue gain.3-5 In a cross-sectional study, Spungen et al reported significant decreases in percent of regional and total body lean tissue in 132 male subjects equally divided with tetraplegia or paraplegia compared with matched controls; compared to able-bodied males, who lost about 1% per decade of total body lean tissue, persons with SCI lost about 3.2% per decade.4 In a monzygotic twin study, discordant for SCI, a loss of total body and extremity muscle mass of about 4 kg of total body lean tissue over each 5-year period of paralysis was observed in the SCI twin.5
In persons with SCI, the usual clinical measures of total body fat (eg, weight or body mass index [BMI]) underestimate the degree of adiposity.4,6 Body fat topography in able-bodied individuals is clearly an important factor associated with metabolic disorders, and it probably has a similar relevance in persons with SCI.7,8 Related to these adverse body compositional changes and reduced levels of activity, individuals with SCI have a pattern of metabolic alteration that would be considered to be atherogenic.8-15
Anabolic hormone deficiency
In persons with SCI, growth hormone (GH) responses to provocative stimulation have been shown to be blunted; younger individuals with SCI have depressed plasma IGF-1 levels.16-18 These deficiencies may reflect a global dysregulation of the hypothalamic-pituitary axis in men with SCI, which is also characterized by an increased prevalence of hypogonadism and elevated levels of gonadotropins and prolactin, possibly suggestive of reduced central dopaminergic tone.19-21 Reduced integrity of pituitary GH secretion would be anticipated to worsen the losses of lean tissue mass and bone mineral density (BMD) below the level of lesion, imparting increased risk of fragility fractures in persons with SCI.22,23 These accelerated adverse body compositional changes in persons with SCI could hamper functional independence and social integration,24 which would adversely affect quality of life and increase health care dependency and costs.
Cross-sectional and longitudinal studies have demonstrated that the levels of total and free testosterone decline with increasing age in able-bodied men.25-30 The etiology of this progressive fall in serum testosterone is multi-factorial and, in part, attributable to various combinations of lifestyle choices, illness, medications, and inappropriate activity of the hypothalamic-pituitary-gonadal axis.25,31,32 Decreased testosterone levels in the general population are associated with lowered libido, fatigue, insomnia, hot flashes, anxiety, depression, poor memory, irritability, impotence, and diminished bone and muscle mass, as well as an increased risk of nonvertebral fractures and cardiovascular disease.33,34 As with able-bodied persons, individuals with SCI have reduced serum concentrations of testosterone from any number of etiologies. In one study, almost half of the total population of men with SCI had low testosterone using the same threshold criterion for the serum total testosterone concentration as that employed by Harman et al in a general population-based study.19,28 Men with SCI had higher percentages of low testosterone for each decade of life by either the total testosterone concentration or the free T index than those reported in the Baltimore Longitudinal Study on Aging19,28; subjects with SCI were excluded from participation if they had a known chronic illness or were prescribed one of several medications known to affect pituitary or testicular secretory function. The prevalence of low testosterone in those with SCI rose strikingly in the third decade of life, a finding that may be associated with deleterious physical and psychological effects of androgen deficiency throughout the remainder of life. Other investigators have also found the prevalence of low testosterone concentrations to be elevated in men with chronic SCI, associated with higher prolactin levels.35,36
One possible etiology of low testosterone is dysfunction of the hypothalamic-pituitary-testicular axis.21,35-38 Early reports have been inconclusive with regard to testicular function, discrepancies that could be attributed to varying factors in population selection, including chronic disease, level of injury, completeness of lesion, duration of SCI, or, in part, differences in study methodology.38-42 Our group has demonstrated that persons with SCI tend to have increased gonadotropin release to standard provocative stimulation to gonadotropin releasing factor compared with able-bodied controls, a finding that confirmed and extended observations of previous investigators.43 In a preliminary report, testicular stimulation with standard doses of human chorionic gonadotropin (hCG) for 2 days was similar in subjects with SCI and able-bodied controls (ie, rise above baseline values with hCG).43
The possibility that an androgenic-deficiency state may contribute to accelerated muscle loss in those with SCI, both above and below the level of lesion should be considered. Muscle wasting reduces energy expenditure and may be associated with increased relative adiposity and, potentially, associated deleterious metabolic consequences.44,45 In a relatively young able-bodied population, producing a hypogonadal state by hormonal manipulation resulted in a loss of lean body tissue.46 Reversal of hypogonadism in younger adults has been shown to improve muscle mass and strength.47 A recent study has demonstrated that testosterone replacement therapy for 12 months significantly improved lean body tissue in persons with SCI.48 A decrement in muscle mass and strength as a consequence of hypogonadism in persons aging with SCI may, eventually, contribute to a loss of physical function and independence. The functional and clinical significance of our laboratory finding of low serum testosterone concentrations in the SCI population has yet to be elucidated. Studies of hormone replacement therapy should assist in determining a possible benefit from reversing low testosterone levels in the individuals with SCI.
Carbohydrate metabolism in SCI
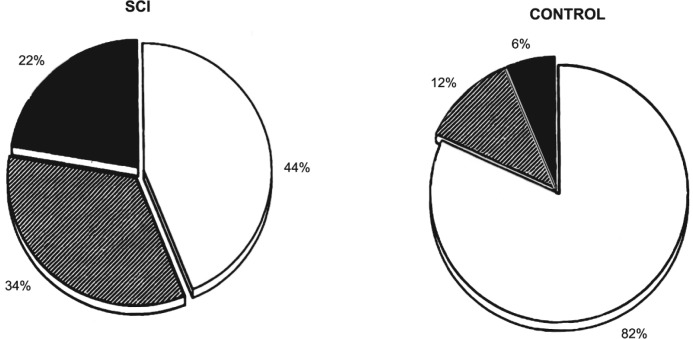
Impaired glucose tolerance and diabetes mellitus occur more frequently in persons with SCI than in the able-bodied population.11,12,49,50 In 100 subjects with SCI, 22% were diabetic by criteria established by the World Health Organization, whereas only 6% of the 50 control subjects were diabetic; 82% of the controls had normal oral glucose tolerance, compared with only 38% of those with quadriplegia and 50% of those with paraplegia.11,51 Subjects with complete tetraplegia had significantly worse carbohydrate tolerance with higher peak and greater sum plasma insulin concentrations, and they were more frequently classified with a disorder in carbohydrate tolerance than the other neurological deficit subgroups (Figure 1).12 Peripheral resistance of insulin to mediate glucose uptake may be demonstrated in most individuals with SCI who have a disorder in oral glucose tolerance, and this metabolic milieu is recognized as being atherogenic.52 Worsening of carbohydrate tolerance will ensue if the compensatory response of the pancreas is insufficient, as may be expected to occur with advancing age.
Figure 1. Pie charts of the distribution of subjects with SCI (left) and able-bodied controls (right) according to the diagnostic classification for oral carbohydrate tolerance as designated by the World Health Organization Committee on Diabetes Mellitus (Second Report, 1980). Normal glucose tolerance is represented by the open sections; impaired glucose tolerance, by the cross-hatched sections; diabetes mellitus, by the solid dark sections. The percentage of each diagnostic group is shown next to each section. The figure was drawn from data presented in Bauman WA, et al. Spinal Cord. 1999;37:765-771.
Lipid metabolism and cardiovascular disease
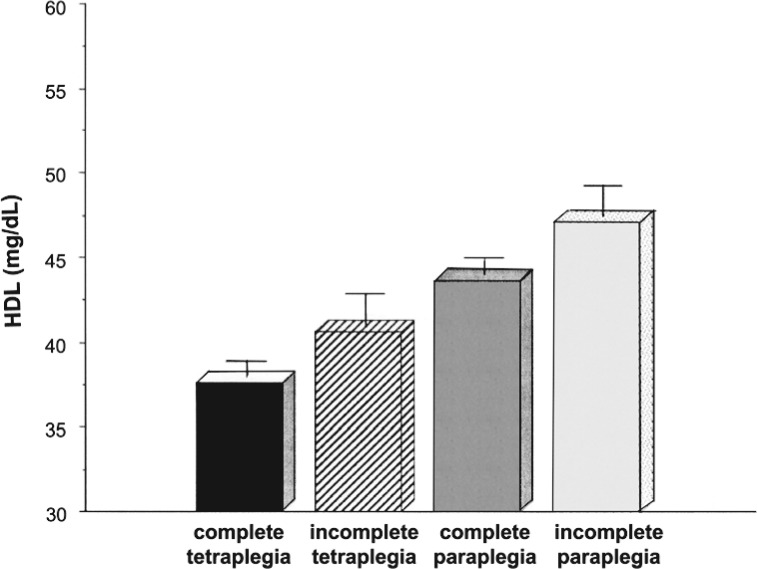
Elevation in low-density lipoprotein (LDL) cholesterol and depression of HDL cholesterol are 2 important risk factors for CHD.53,54 Individuals with SCI are believed to have premature CHD. Approximately 10% of the US population have HDL cholesterol values less than 35 mg/dL, which is an independent risk factor for CHD, whereas about 24% to 40% of those with chronic SCI have levels below this value for HDL cholesterol.13,14,54,55 Although the mechanism by which low levels of HDL cholesterol predispose to CHD is uncertain, it has been postulated that HDL cholesterol is vital for reverse cholesterol transport, which mobilizes cholesterol from the arterial wall, as well as antioxidant, anti-inflammatory, antiplatelet, and anticoagulant effects.56 In subjects with chronic SCI and in controls, an inverse correlation has been demonstrated between serum triglycerides and HDL cholesterol, a relationship that may reflect the effects of elevated plasma insulin levels.57-59 Maki et al found a significant indirect correlation between abdominal circumference and serum HDL cholesterol levels and a direct correlation with serum triglyceride levels, as well as for the ratio of total to HDL cholesterol levels.8 Our group found significant correlations between visceral fat mass and fasting or stimulated insulin levels and fasting or stimulated serum triglyceride levels.60 In persons with SCI, a significant direct relationship was shown between serum HDL cholesterol levels and insulin sensitivity.11 Subjects with chronic tetraplegia had lower levels of serum HDL cholesterol than those with paraplegia (38±0.7 vs 45±0.8 mg/dL, respectively; P < .01).13 Subjects with motor complete injury tended to have lower values of serum HDL cholesterol than those with motor incomplete within the subgroups of tetraplegia and paraplegia (Figure 2); a significant inverse relationship was found for degree of neurological deficit and serum HDL level.13 Men with SCI had lower HDL cholesterol level than able-bodied control men, but there was no significant difference in serum HDL level between predominantly premenopausal females with or without SCI.14
Figure 2. Serum high-density lipoprotein cholesterol levels by neurological deficit. All values are expressed as mean ± SEM. A significant inverse relationship was found for degree of neurological deficit and serum high-density lipoprotein (HDL) cholesterol level (r=0.19; P < .0001). Redrawn, with permission, from Bauman WA, et al. Spinal Cord. 1998;36:13-17.
At the present time, there are several strategies for raising serum HDL cholesterol. Increasing level of activity, smoking cessation, and pharmacological treatment all have been shown to be effective. In persons with or without SCI, increased cardiopulmonary fitness has been demonstrated to raise the serum HDL cholesterol level.11,14,56,61-63 In a prior report by our group, persons with paraplegia had significantly higher serum HDL cholesterol values for relatively modest higher values of maximum oxygen uptake.11 Moderate-intensity exercise interventions have been demonstrated to improve serum HDL cholesterol levels.56,63 In the general population, an increase of 1.0 unit in the ratio of serum total to HDL cholesterol has been found to be associated with a mean increase of 53% in risk of a coronary event.64 Thus, a modest upper extremity exercise regimen that improves cardiovascular fitness may be expected to increase serum HDL cholesterol and reduce CHD risk, although the latter has not been specifically studied in persons with SCI. Inactivity, independent of lipid values or other risk factors for CHD, may be an independent risk factor for CHD.65 Persons with SCI should be strongly encouraged to reach and maintain the highest level of daily activity, compatible with their neurological level of injury. A Model System Collaborative study showed that Niaspan 2 g daily in persons with SCI raised serum HDL cholesterol from 32±3 to 40±7 mg/dL, an average increase of 25%, associated with a reduction in serum LDL cholesterol and a significant improvement in total cholesterol (TC) to HDL ratio from 5.4 to 4.2.66 It should be appreciated that recent studies in the able-bodied population have failed to find a benefit from raising HDL cholesterol levels by pharmacological interventions, albeit the levels were not nearly as profoundly depressed as those frequently found in persons with SCI.67 In addition, a recent report that determined risk of myocardial infarction in the general population by Mendelian randomization failed to find a lower risk of myocardial infarction from genetic mechanisms that raise HDL cholesterol levels.68
CHD in persons with SCI
In the able-bodied population, symptoms of CHD are commonly precipitated by activity, often strenuous. It should be appreciated that the risk of a cardiac event is related to the severity of CHD, not symptoms of CHD.69,70 The ability of a person with SCI, especially those with higher, more complete lesions, to engage in significant physical activity is often difficult and, if at all possible, limited. In addition, if an individual with SCI has an ischemic cardiac event, it may pass unnoticed because of nervous interruption of sensory pathways. Thus, the identification of CHD risk or other macrovascular disease risk equivalents for CHD (such as peripheral vascular disease) may be grossly underestimated in those with SCI, requiring a more aggressive approach to determine the presence of atherosclerotic occlusive vascular disease. Although peripheral vascular disease would be expected to be present, because of multiple metabolic cardiovascular disease risk factors and a cigarette smoking prevalence comparable to that of the able-bodied population, it may be undiagnosed in a nonambulatory, older SCI population.
Whiteneck et al reported that cardiovascular diseases were the most frequent cause of death among persons with SCI more than 30 years after injury (46% of all deaths) and among those more than 60 years of age (35% of all deaths).71 Using nuclear medicine stress testing in a couple of relatively small cohorts, asymptomatic CHD was reported, as determined by upper body exercise stress testing in subjects with paraplegia and by dipyridamole infusion in subjects with tetraplegia.72,73 Coronary artery calcification (CAC) by electron beam computerized tomography was performed in 91 subjects with SCI who were matched 3:1 with non-SCI controls for age, gender, ethnicity, and individual risk factors.74 In the group with SCI, the investigators found that the mean CAC score was significantly greater (75±218 vs 28±104), the prevalence of any CAC was higher (51% vs 39%), the prevalence of those with high scores (>100 HU) was greater (16% vs 7%), and those with tetraplegia had a greater prevalence of severe CAC scores than did those with paraplegia (6.8 vs 2.1%, respectively; P < .05); because of study design, findings suggestive of increased atherogenesis were not explained by traditional CHD risk factors.51,75
The conventional risk factors for CHD, as defined by the Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults [Adult Treatment Panel III (ATP III)], were evaluated in veterans with SCI in order to assign risk and determine target LDL cholesterol levels for therapeutic intervention; as in the general population, those with intermediate CHD risk comprised the predominant proportion of the SCI population.15 Persons with SCI should receive at least comparable care to reduce CHD risk as the able-bodied population. Thus, the conventional risk factors for CHD should be identified and appropriately treated in individuals with SCI, according to current standards of care. However, limitations of ATP III guidelines when applied to individuals with SCI should be appreciated.9,15
Bone loss after acute and chronic SCI
Loss of bone is an important secondary consequence of acute SCI and is due to a state of heightened skeletal resorption, which is restricted to the sublesional skeleton.76,77 Bone loss after neurologically more complete forms of SCI is unique for its rate, distribution, and resistance to currently available treatments. In individuals with neurologically motor complete SCI, bone loss may occur at rates approaching 1% of BMD per week for the first 6 to 12 months after injury.78-80 The rate of bone loss after SCI is substantially greater than that observed with microgravity (0.25% per week),81 bed rest (0.1% per week),82 or in postmenopausal women who are not taking antiresorptive medications (3%-5% per year).83 The increased rate of bone loss appears to continue beyond the first 12 months of injury and continues at least over the next 3 to 7 years, albeit at a slower rate than occurring initially. This dramatic phenomenon may be related to any number of factors including, but not limited to, the loss of endogenous anabolic hormones (ie, circulating testosterone and/or growth hormone); factors in the local bone milieu; the presence of catabolic factors, such as the administration of methylprednisolone at extremely high doses within hours of the acute event; the production of inflammatory mediators/cytokines locally; and/or the loss of positive central and peripheral neural influences on bone.84 There is evidence from a study of monozygotic twins discordant for SCI to indicate that the difference in sublesional BMD between the twins increased with time after SCI for several decades, suggesting that loss of bone may continue at an accelerated rate in those with SCI for an extended period of time.85
The areas of greatest bone loss are the distal femur and proximal tibia, and fractures are most common in these locations. In a cross-sectional study of 8 SCI patients, a change of 35.3% in BMD of the tibial trabecular bone was noted within the first 2 years after SCI, whereas there was only a 12.9% reduction in tibial cortical bone.86 In a cross-sectional study of 31 patients with chronic SCI (≥1 year), Dauty et al demonstrated a demineralization of minus 52% for the distal femur and minus 70% for the proximal tibia,87 in agreement with the findings of several other investigators.88-91 In a cross-sectional report that used peripheral quantitative computed tomography in men with complete SCI to describe trabecular and cortical bone compartments until steady state levels were achieved (3-8 years after acute SCI), bone loss in the epiphysis, which is predominantly trabecular bone, was exponential with time; on average, loss of ephiphyseal bone was 50% in the femur and 60% in the tibia; in the diaphyses, which is predominantly cortical bone, losses were 35% in the femur and 25% in the tibia and involved erosion of the thickness of cortical bone by 0.25 mm/year over the initial 5 to 7 years after SCI.91 When bone loss appeared to plateau, the femoral and tibial epiphyses (trabecular bone) were decreased by 50% and 60%, respectively, and the femoral and tibial shafts (cortical bone) decreased by 35% and 25%, respectively.91 This level of depletion places the bones, particularly at the knee, below clinically accepted thresholds for fracture.
Persons with severe chronic conditions, including disability, have long been recognized to be at high risk for the development of vitamin D deficiency because of reduced sunlight exposure due to lifestyle changes, institutionalization, and/or medications.92,93 Several of the drugs prescribed in persons with SCI, especially anticonvulsants and psychotropic agents, may accelerate hydroxylation of vitamin D and increase renal clearance.93 Persons with SCI often have a lower calcium intake than the general population, including vitamin D-fortified milk, which is the major dietary source of vitamin D, excluding supplements.94 Vitamin D deficiency has been reported to be extremely prevalent in the SCI population, as well as in those with other disabilities.95-97 Our group demonstrated that approximately one-third of a veteran SCI population had an absolute deficiency of vitamin D.95 Other investigators have confirmed this finding in persons with chronic SCI.96 In an effort to restore serum vitamin D levels to a concentration that would serve to optimize GI absorption of calcium,98 cholecalciferol, or vitamin D3, 2000 IU/day was administered to persons with SCI in conjunction with daily oral calcium supplementation of 1.3 g elemental calcium daily. In vitamin D-deficient subjects, vitamin D supplementation for 3 months raised the level of vitamin D into the normal range in 85% of subjects, and the mean serum level of vitamin D for the total group was well above the lower limit of the normal range (25 hydroxyvitamin D >30 ng/mL).99
Bisphosphonates have a strong affinity for bone and inhibit osteoclast bone resorption. This class of agents has been used in an attempt to prevent or reduce bone resorption associated with acute SCI. However, our experience has raised questions concerning the efficacy of this class of medications in persons with complete motor SCI who are non–weight bearing,100 and others have concluded that “data were insufficient to recommend routine use of bisphosphonates for fracture prevention in these patients.”101(p215) The possibility of using a combined mechanical and pharmacological approach to reduce or prevent bone loss after acute SCI may be considered. Because of the obvious difficulty of instituting a mechanical intervention immediately after acute SCI, it may be necessary to begin therapy early after injury with drug intervention(s). It is conceivable that coincident with acute injury a pharmacological approach to target and potently inhibit receptor activator of nuclear factor-κB ligand (RANKL) in an effort to suppress rampant osteoclastosis may prove efficacious, as well as practical.102 Another consideration is that patients who are acutely or subacutely paralyzed frequently have low testosterone levels,103 a hormonal state that may worsen bone loss during acute immobilization. Because high-dose methylprednisolone is frequently prescribed at time of acute paralysis104 and recent animal studies support the role of androgens to antagonize the effects of glucocorticoids on muscle and bone, testosterone replacement therapy may be an appropriate therapeutic option.105-107 When the patient is sufficiently stabilized after acute SCI, a combination approach with mechanical intervention and at least one pharmacological therapy may be envisioned. Albeit purely conjecture at this time, it may be postulated that the sooner a physical intervention can be integrated into the care plan in the form of practical and cost-effective mechanical stimulation to restore forces that had been suddenly removed from the skeleton at time of injury, the greater the likelihood of preserving bone mass. The efficacy of the initiation of a mechanical approach may be reinforced by innovative, and temporally appropriate, pharmacological prescription to stimulate depressed function of the osteoblast.
Gastrointestinal Tract Dysfunction
Gastrointestinal (GI) symptoms due to bowel dysfunction are quite common in persons with SCI and may adversely affect health and lifestyle. Personalizing and providing an appropriate bowel regimen is an important part of rehabilitative management, with the goals being effective evacuation and prevention of bowel complications. Medical professionals who provide care to patients with SCI should have an understanding of the pathophysiology of the GI tract to be able to provide the optimal guidance for initiating and/or maintaining effective and safe bowel management regimens.
Bowel dysfunction in SCI
A significant number of individuals with chronic SCI have GI symptoms due to bowel dysfunction,108 and adequate bowel care is an important part of their clinical management. It is important for rehabilitation specialists to understand the pathophysiology of bowel problems after SCI to correctly approach the management of these individuals. Bowel dysfunction has become a major health and lifestyle issue.108-111 Normal colonic function is important for the process of defecation. It involves muscular activity of the colon and 2 anal sphincters – the internal anal sphincter, which is an involuntary sphincter that is the continuation of the inner circular muscle layer of the colon, and the external anal sphincter, which is made up of striated muscle layers that are under voluntary control and are a major factor in maintaining fecal continence.112
The colon is supplied with both autonomic (parasympathetic and sympathetic) and somatic (ie, sensory and motor) pathways.113 Balance between these distinct neural pathways is controlled by higher centers in the brain and spinal cord. Parasympathetic innervation of the colon is responsible for colonic contractions and motility. The right and proximal transverse colon is innervated through the vagus nerve, whereas the left colon and rectum receives neural input from spinal segments S2-S4 via the pelvic nerve or nervi erigentes.113 Sympathetic supply comes through the lumbar splanchnic nerves and is the major pathway for carrying the sensations to and from the colon. The neuromuscular innervation of the colon is coordinated to produce both nonpropulsive segmental contractions (SC) and high amplitude propagating contractions (HAPC). Various neurotransmitters including acetylcholine, cathecholamines, and serotonin have been shown to regulate colonic motility, although the principal autonomic neurotransmitter is acetylcholine.114
After SCI, there is prolonged mouth to cecum transit time in subjects with tetraplegia and delayed transit through the left colon.115-118 In addition, subjects with SCI show a prominent decrease in HAPC after arousal from sleep compared to able-bodied participants.119 It is clear that prolonged colonic transit time and absence of HAPC contributes to constipation and difficulty with evaluation after SCI. Because colonic motility depends on adequate colonic parasympathetic tone, these findings are consistent with loss of such tone.
Problems with defecation become prominent as time progresses after the acute injury.108 The clinical picture depends on whether the injury is upper motor neuron (above T10) or lower motor neuron (below T10).108-111,120 Problems with defecation occur in both upper and lower cord lesions, which have a significant impact on the quality of life of these individuals, especially in terms of the overall time spent for routine bowel care. In addition, complications such as fecal impaction and autonomic dysreflexia can be anticipated. Fecal impaction is the most common complication, and it may also present with atypical symptoms, such as paradoxical diarrhea, abdominal pain, nausea, vomiting, acute confusion, urinary symptoms, and rectal bleeding due to pressure ulcerations.121 Autonomic dysreflexia is a relatively common complication in patients with an injury above the T6 spinal segment, and it may occur in response to stimuli such as fecal impaction, digital rectal stimulation, and colonoscopy.122-124
Approaches to bowel management
Appropriate and effective bowel management in individuals with SCI is of utmost clinical importance. An adequate bowel regimen depends on many factors and will vary from patient to patient. Regardless of the level of injury, the goals are to achieve effective evacuation and prevent complications, such as impaction and incontinence.125 Whenever possible, bowel care initiated for evacuation should be performed in either the normal position or in the left lateral position.126 This allows use of gravity and facilitates fecal expulsion. Digital rectal stimulation is also useful for promoting bowel evacuation.127,128 Diet plays an important role in these individuals. It is important for these individuals to be on a high-fiber regimen and to consume at least 2 to 3 L of fluids every day.129 Laxatives are often employed as an adjunct to routine bowel care. Laxatives such as docusate, lactulose, and polyethylene glycol are the most commonly employed preparations and are used in conjunction with adequate dietary water.130-133
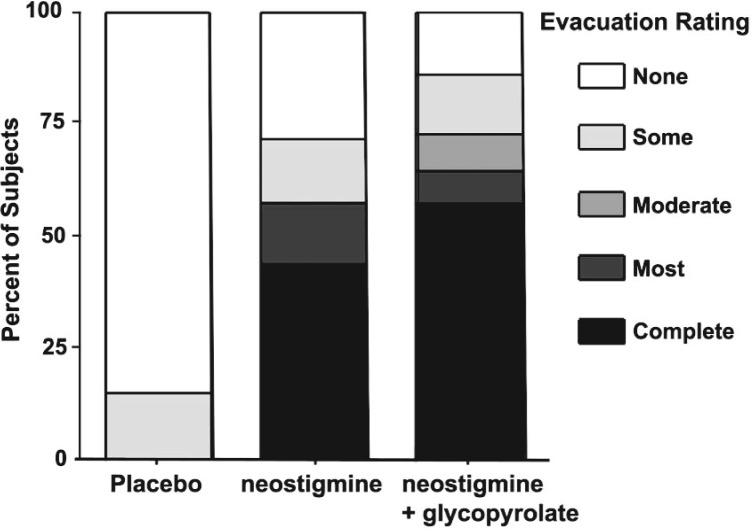
These conventional strategies are time consuming, expensive, and often ineffective. Hence, our research has attempted to develop more effective agents by focusing on the basic pathophysiology after SCI. Such modalities include cisapride (a prokinetic drug that releases acetylcholine from neurons in the gut but has been withdrawn from the market) and neostigmine, an inhibitor of the enzyme acetylchoinesterase that increases levels of acetylcholine at nerve endings and has been employed in patients with acute intestinal pseudo-obstruction.115,134-136 In combination with the anticholingeric agent, glycopyrrolate (to prevent cholinergic-induced bronchoconstriction and bradycardia), we have shown that intravenous neostigmine can be used safely in subjects with SCI to induce bowel evacuation (Figure 3).137 In addition to consideration of intravenous infusion via implantable venous access for routine clinical use, our research is also studying other routes of administration, including intranasal.
Figure 3. Bowel evacuation of barium oatmeal paste after prokinetic agent in subjects with chronic SCI. Prior to intravenous placebo or drug administration, subjects had instillation of barium oatmeal paste in the rectum and descending colon. On separate days, subjects received in randomized-blinded design, 1 of 3 intravenous infusates: placebo (normal saline), neostigmine (2 mg), or neostigmine (2 mg) plus glycopyrrolate (0.4). Evacuation is represented from none (open section of the bar) to complete (darkest section of the bar). Neostigmine alone or neostigmine plus glycopyrrolate were similarly effective in inducing evacuation of the barium oatmeal paste from the bowel compared to negligible bowel evacuation after placebo administration. The figure was drawn from data presented in Korsten MA, et al. Am J Gastroenterol. 2005;100:1560-1565.
Colostomy is an option in those with severe and intractable symptoms, including constipation, fecal incontinence, and perineal pressure ulcers.138,139 It is frequently advocated as an adjunct in the treatment of perineal pressure ulcers. Surgical posterior rhizotomy and sacral anterior root stimulation are other surgical options shown to have therapeutic utility in SCI patients.140 However, this is an expensive approach and its routine use is not a practical alternative.
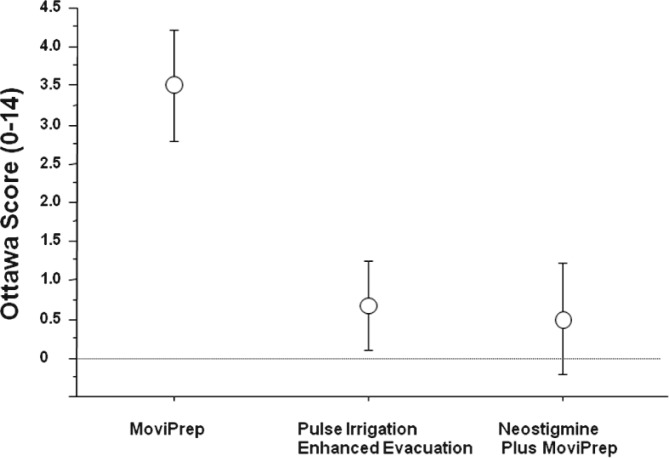
Associated with difficult bowel care, it is often challenging to prepare an individual with SCI for colonoscopy. This has become especially germane with data indicating that screening colonoscopy by polyp detection and removal can reduce the incidence of colonic cancer and the likelihood of death from this neoplasm.141 In ongoing studies, we have reported success in achieving high-quality colon preparation using newer approaches including Moviprep and pulse irrigative enhanced evacuation with and without adjunctive neostigmine (Figure 4).142 With improved bowel cleansing, we have found a trend toward improved polyp detection.
Figure 4. Efficacy of bowel preparation for elective colonic in subjects with SCI. The Ottawa score (0-14) depicts the adequacy of visualization of the bowel wall with lower scores representing increased bowel wall visualization.
Respiratory System Dysfunction
Respiratory complications, excluding pulmonary embolism, are the principal cause of death during the first year following SCI and along with cardiovascular disease a leading cause thereafter.143,144 Injury to the cervical and upper thoracic cord may disrupt innervation to the diaphragm, intercostal muscles, accessory respiratory muscles, and abdominal muscles, resulting in respiratory muscle weakness, ineffective cough, and retention of pulmonary secretions. These factors, in turn, predispose to atelectasis, pneumonia, and potentially respiratory failure. Due to the relative state of physical inactivity, individuals with SCI may lack specific symptoms, such as dyspnea on exertion; however, respiratory symptoms are still reported in a high number of persons with chronic SCI, and they are directly associated with the level of lesion.145,146 The extent of respiratory dysfunction depends on the level and completeness of injury. Respiratory complications during hospitalization after acute injury have been reported to be as high as 84% for C1-C4 levels of injury, 60% for C5-C8 levels of injury, and 65% for thoracic lesions.147 The most common respiratory complications observed are pneumonia, atelectasis, and respiratory failure.144
Pulmonary function after SCI
Paralysis in subjects with cervical and high thoracic cord injury leads to loss of function of the major muscles of respiration. Compared to other neurological disease models such as multiple sclerosis or myasthenia gravis, persons with tetraplegia show greater preservation of inspiratory muscle function relative to the compromise in expiratory muscle function; this is due to relative sparing of diaphragmatic function supplied by the phrenic nerve (C3 to C5), whereas paralysis of intercostal and abdominal musculature results in weak cough and impaired pulmonary toilette. Residual expiratory function has been attributed to the clavicular portion of the pectoralis major muscle originating from C5 to C7.148,149 Immediately following injury, vital capacity (VC), inspiratory capacity (IC), and total lung capacity (TLC) are significantly decreased (VC 30% predicted in C5-C6 injury).150 During the first year of SCI, lung volumes partially recover and inspiratory and expiratory flow rates increase and functional residual capacity (FRC) decreases; after which time, changes in VC and FRC are more gradual.150-153 During the first week after cervical cord injury, a forced vital capacity (FVC) less than 25% predicted is, in particular, associated with the development of respiratory failure requiring ventilator support.153 Initial improvement has beeen attributed to return of functional respiratory muscles coincident with resolution of inflammation and edema above the level of the lesion,152 while later increases have been attributed to improvement in diaphragm function,151,154-156 increased performance of accessory respiratory muscles,151,156 and rib cage/chest wall stability.151,154 Improvement in VC has been attributed to an increase in IC resulting from a decrease in FRC.151
In cross-sectional studies of persons with chronic SCI, the level and completeness of SCI are shown to correlate with the severity of pulmonary dysfunction; higher more complete injuries are associated with significantly reduced parameters of pulmonary function.157,158 In general, however, recovery of pulmonary function in any individual is variable following acute SCI and can only be weakly predicted by knowledge of the initial pulmonary function test values and the level of lesion.159 Significant determinants of longitudinal decline in FVC and forced expiratory volume in 1 second (FEV1) include advancing age, current smoking status, increased BMI, wheezing, and underlying respiratory muscle weakness.160 Spirometric and lung volume studies in persons with chronic tetraplegia and high-level paraplegia demonstrate restrictive dysfunction due to neuromuscular weakness characterized by reduction in VC, FEV1, peak expiratory flow (PEF), TLC, maximum voluntary ventilation (MVV), expiratory reserve volume (ERV), and IC, along with a significant increase in residual volume (RV) and little or no change in FRC.157,161-167
Airway caliber, reactivity, and inflammation after SCI
Our previous work identified the presence of baseline bronchoconstriction unmasked by bronchodilator responsiveness to a beta-2 adrenergic agonist or an anticholinergic agent in persons with cervical SCI.168,169 In 15 subjects with chronic tetraplegia, we found that, compared to individuals with low paraplegia, baseline specific airway conductance (sGaw) was significantly reduced; when administered inhaled ipratropium bromide (anticholinergic agent), a significant bronchodilator response occurred in all subjects with tetraplegia (Figure 5).169 This finding suggests that interruption of sympathetic innervation to the lungs has physiological significance and that a reduction in baseline airway caliber due to heightened cholinergic tone can be reversed following inhalation of an anticholinergic agent.
Figure 5. Specific airway conductance before and after inhalation of ipratropium bromide in subjects with tetraplegia. TETRA = tetraplegia; PARA = paraplegia; BL = baseline; IB = ipratropium bromide. A significant group difference was observed for BL specific airway conductance TETRA vs PARA and controls; *P < .05. BL vs after IB administration; ‡P < .05. The figure was drawn from data presented in Schilero GJ, et al. Chest 2005;127:149-155.
In addition to baseline bronchoconstriction and bronchodilator responsiveness, our group found nonspecific AHR, identified by exaggerated bronchoconstriction evoked by inhalation of nonspecific agents such methacholine or histamine, regardless of smoking history, in individuals with tetraplegia and in individuals with high paraplegia in the presence of decreased lung volumes.170 Another study involving subjects with tetraplegia demonstrated AHR to histamine with significantly lower values for spirometric indices of airway size and airway size relative to lung size.171 AHR might therefore be explained by preexisting airway narrowing, in that further reduction in airway caliber induced by a bronchoconstrictor agent would lead to marked increases in airway resistance.172 Thus, similar to the mechanism postulated to account for bronchodilator responsiveness, AHR might result from reductions in baseline airway caliber in persons with tetraplegia due to overriding cholinergic airway tone.
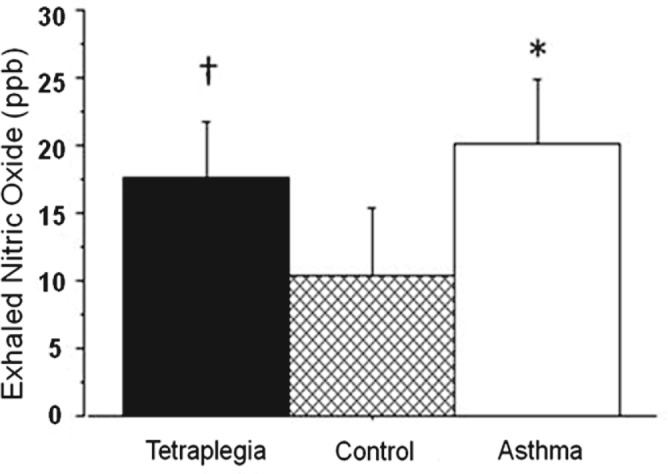
An alternative or contributing explanation for reduction in airway caliber in persons with tetraplegia is chronic airway inflammation. An airway inflammatory response might originate from recurrent respiratory infections as a result of ineffective pulmonary clearance and/or response may be associated with systemic inflammation as a consequence of the acute injury.173 Further airway inflammation might be attributed to chronic microaspiration of stomach contents due to esophageal dysmotility and/or gastroesophageal reflux disorder (GERD). To investigate the potential cause of airway inflammation, a preliminary investigation was performed demonstrating that individuals with tetraplegia had elevated levels of exhaled nitric oxide (NO) comparable to levels associated with mild asthma (Figure 6).174 It is now widely believed that NO generation in the airways of asthmatic subjects represents a physiological mechanism to counteract the bronchoconstriction caused by various stimuli, in which case NO may serve as a marker of the inflammatory response.175 A similar role of NO in airways of individuals with SCI has been postulated. We recently presented evidence that individuals with tetraplegia have significantly elevated levels of 8-isoprostane, an inflammatory biomarker, in exhaled breath condensate (EBC) when compared to mild asthmatics and able-bodied controls; the pH value of their EBC, although not significantly different than the mild asthmatics or controls, trended toward hyperacidity, suggesting the presence of airway acidification associated with airway inflammation.176 Elevation in 8-isoprostane levels and airway acidification have both been demonstrated in asthmatic patients with GERD.177,178 Moreover, decreased 8-isoprostane levels and normalization of EBC pH have been shown following treatment with proton pump inhibitors in persons with GERD-induced asthma.179
Figure 6. Exhaled nitric oxide in subjects with tetraplegia compared to asthmatics or able-bodied controls. Nitric oxide is expressed in parts per billion (ppb). Values are presented as mean ± SD. †P < .01, tetraplegia vs control group; *P < .001, asthma vs control group. Redrawn with permission from Radulovic M, et al. Lung. 2010;188(3):259-262.
Assessment and treatment options
Although work has been done to document the various elements of pulmonary dysfunction and the effectiveness of various pharmacological and mechanical interventions, there is a paucity of systematic research linking therapeutic interventions with patient outcomes following SCI. A therapeutic intervention that has shown promise includes training of the pectoralis major in persons with tetraplegia associated with an increase in ERV and decrease in RV.180 Functional electrical stimulation (FES) to improve expiratory muscle function and cough effectiveness have also been shown to be beneficial in improving pulmonary function.181 Human studies have shown that beta-2 adrenergic agonists increase skeletal muscle strength in young men and in patients with facioscapulohumeral muscular dystrophy.182,183 Findings from 2 small studies in persons with SCI demonstrated an increase in forearm muscle size and strength and total work output FES.184,185
The weight of the evidence suggested by these studies support the notion that beta-2 adrenergic agonists improve respiratory muscle strength in SCI. Guided by this consideration, our group initiated a randomized, prospective, double-blind, placebo-controlled crossover study in subjects with tetraplegia to determine whether administration for 4 weeks of the long-acting beta-2 adrenergic agonist, salmeterol, improved pulmonary function.186 Post treatment with salmeterol, FVC, FEV1, MIP, and MEP all significantly increased. Although increases in MIP and MEP suggest improvement in respiratory muscle strength, it is possible that bronchodilation may also have accounted for some of the observed increases in these parameters because these measurements are also dependent on lung volume.186 Regardless of the cause, significant improvement in pulmonary function parameters following administration of salmeterol suggests that long-term use of beta-2 agonists might improve cough effectiveness and reduce pulmonary complications in persons with cervical or high thoracic SCI. To further investigate the anabolic and bronchodilator effects of beta-2 agonists in persons with tetraplegia and high paraplegia, a randomized, double-blind, placebo-controlled trial to assess the effects of 12 weeks of an oral beta-2 agonist, albuterol repetabs, on spirometric parameters, static mouth pressures, and cough strength is approaching completion. In a second arm of the study, individuals received 6 weeks of expiratory muscle training, subsequent to administration of placebo or study medication, to assess for additional gains in pulmonary function and cough effectiveness. We anticipate that the administration of an oral beta-2 agonist in concert with expiratory muscle training will lead to greater beneficial effects on pulmonary function and cough strength than either intervention alone, which would be expected to translate to a reduction in the risk of pulmonary complications in the SCI population.
Cardiovascular Autonomic Dysregulation
Spinal cord lesions above the 6th thoracic level interrupt supraspinal control of sympathetic efferent innervation to the heart, the upper and lower extremity, and splanchnic bed vasculature, thus affecting heart rate, cardiac output, blood volume distribution, and blood pressure. Lower cord lesions (T7 and below) affect the lower extremity and splanchnic vasculatures but spare the vasculatures of upper extremity and the heart; therefore alterations in cardiovascular function may differ from those with higher cord lesions.
Cardiac effects
Cervical and high thoracic lesions result in partial to complete decentralized cardiac sympathetic control, which restricts chronotropic and inotropic cardiac functions, limits increases in heart rate to levels which can be elicited by vagal withdrawal and diminishing stroke volume.187,188 As a result, many individuals present with resting bradycardia (heart rates below 60 bpm) and diminished heart rate responses to sympathetic provocation such as orthostasis and exercise.189,190 We recently documented resting bradycardia in 25% of individuals with cervical SCI; however, the proportion of bradycardia in those with high thoracic lesions was only 1% (unpublished findings). Paradoxically, individuals with high thoracic lesions were found to have significantly elevated daytime heart rates compared to individuals with tetraplegia and non-SCI controls.191 The disparity in the prevalence of bradycardia between individuals with cervical and those with high thoracic lesions potentially reflects complete versus incomplete decentralized sympathetic cardiac innervation and increased dependence on heart rate to maintain cardiac output in those with high thoracic lesions. Although central cardiac sympathetic control remains intact in individuals with lesions below T7, alterations in heart rate have been documented. Individuals with low thoracic lesions had significantly elevated daytime and nighttime heart rates compared to individuals with tetraplegia and non-SCI controls.191 Evidence is accumulating to support an association between persistently elevated resting heart rate and cardiovascular disease (CVD) in otherwise healthy non-SCI populations.192,193 The association between increased resting heart rate and CVD appears to relate to adverse changes in vasculature morphology that predispose to accelerated arterial stiffening with age.193 Although the association between resting heart rate and arterial stiffness has not been tested in the SCI population, 2 reports do suggest increased arterial stiffness.194,195 However, neither report investigated the association between increased resting heart rate and changes in arterial stiffness; given the evidence in non-SCI cohorts, the possibility of this association being present should be evaluated in the SCI population.
Vascular effects
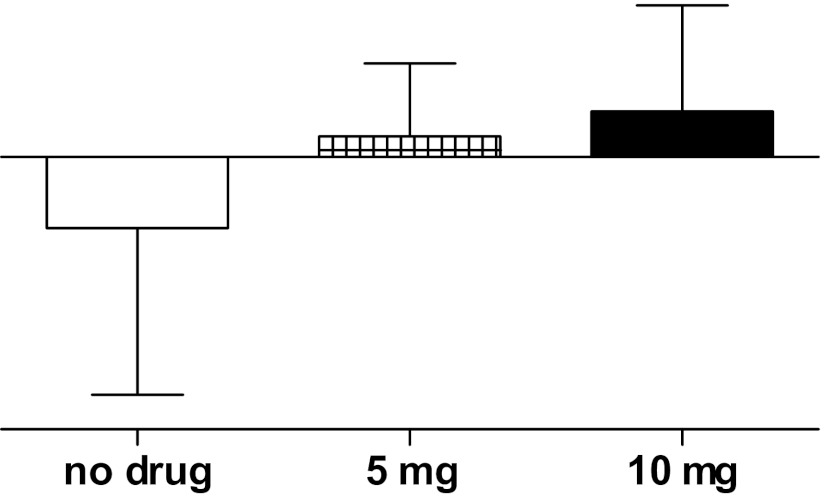
Decentralized sympathetic control of the peripheral vasculature results in compromised centrally mediated vasoconstriction, which is influenced by the level of lesion, with higher, more complete lesions having greater neurovascular impairments.196,197 In general, individuals with tetraplegia are prone to hypotension with periods of even lower blood pressures during orthostasis. Persistent hypotension was documented over the course of a 24-hour day in individuals with tetraplegia, and the nocturnal dip in blood pressure was diminished compared to individuals with thoracic lesions (T1 and below) and non-SCI controls.191 Although many individuals with tetraplegia are persistently hypotensive, most remain asymptomatic and, therefore, do not raise clinical concern. As such, in a recent medical chart review of all SCI patients seen at a large metropolitan VAMC, 29% were diagnosed with hypotension but only 1% were prescribed an antihypotensive agent. Moreover, although there are over 110 US Food and Drug Administration (FDA)–approved medications available to treat hypertension, only the alpha agonist midodrine hydrochloride is approved for treatment of dizziness upon standing. Although this FDA indication may not be not relevant to asymptomatic hypotension in the SCI population, midodrine has been recommended in the SCI literature for treatment of hypotension198; however, data to support safety and efficacy are lacking. As such, our unit has begun testing the safety and efficacy of midodrine (Figure 7), along with several other antihypotensive agents to normalize blood pressure in persons with SCI.199-202Although partially decentralized vascular control is presumed in individuals with high and low thoracic lesions, recent work from our laboratory suggests that individuals with paraplegia remain in the normotensive range throughout a typical 24-hour day.191 In fact, unpublished data from our laboratory suggest that the incidence of systolic hypertension is 19% and 46% in those with high and low thoracic lesions, respectively, although the prevalence of diastolic hypertension is less (7% and 10%, respectively); the increased incidence of systolic hypertension may relate to arterial stiffness, but this association has not been studied.
Figure 7. Mean arterial pressure (MAP) response to head-up tilt. MAP = mean arterial pressure. Open bar = no drug; cross-hatched bar = midodrine 5 mg; solid bar = midodrine 10 mg. *P < .05, midodrine vs no drug. Redrawn, with permission, from Wecht JM, et al. Arch Phys Med Rehabil. 2010;91(9):1429-1435.
Cognitive effects
A well-described association between hypotension and cognitive deficits is reported in healthy non-SCI populations.203-206 Similar to non-SCI individuals, persistent supine and orthostatic hypotension may predispose individuals with SCI to cognitive deficits. Tests of neuropsychological function have been used to describe cognitive function in persons with SCI; the results suggest that between 10% and 60% of the population have measurable deficits encompassing attention,207-210 concentration,208-211 memory,208,209,211 abstract reasoning,211 verbal learning,208,209,211 and information processing.212 Despite these staggering findings, few studies have attempted to clearly define the etiology of the cognitive deficits and most reports attribute the deficits to concomitant traumatic brain injury (TBI) or premorbid conditions in individuals with chronic SCI.207,211,213,214 However, a single report suggests that, although TBI was likely in a sample of individuals with SCI (spinal injury was due to motor vehicle accidents), the pattern of the cognitive deficits suggested multiple etiologies.215 It is conceivable that, analogous to observations in non-SCI population, hypotension and orthostatic hypotension may contribute to the cognitive deficits observed in persons with SCI. We recently reported diminished memory and attention and processing speed in hypotensive individuals with SCI compared to a normotensive SCI cohort, matched for self-reported incidence of TBI (Table 1).216 Although individuals with paraplegia are not chronically hypotensive, previously reported increased resting heart rate217-219 and arterial stiffening194,195 may predispose them to cognitive deficits and accelerated cognitive decline with aging.220,221 Preliminary evidence of significant cognitive impairment in individuals with paraplegia compared to age-matched non-SCI controls was reported by our group.216 Although the cognitive deficits were not related to blood pressure, both groups with paraplegia and tetraplegia displayed a paradoxical decrease in middle cerebral artery blood flow (CBF) velocity during cognitive testing compared to a mean increase in CBF in the non-SCI controls.222 Therefore, the cognitive deficits reported in individuals with SCI may relate to adverse alterations in CBF regulation, secondary to decentralized cardiovascular control, regardless of the level of injury.
Table 1.
Cognitive t-scores in persons with SCI discordant for hypotension
| Hypotensive | Normotensive | P | |
| Total recall | 46.0 ± 10.4 | 59.7 ± 7.0 | .01 |
| Short delay | 44.1 ± 11.8 | 58.9 ± 8.6 | .01 |
| Long delay | 45.0 ± 12.6 | 59.4 ± 9.4 | .02 |
| Recognition | 43.6 ± 13.1 | 56.3 ± 5.2 | .02 |
| Attention & processing | 34.2 ± 19.6 | 48.9 ± 8.9 | .06 |
Recreated with permission from Jegede AB, et al. Clin Auton Res. 2010;20(1):3-9.
Summary
Individuals with SCI have a myriad of medical consequences related to denervation with muscle paralysis and/or autonomic dysfunction, marked adverse soft tissue and skeletal body composition changes, and involuntary and extreme sedentary lifestyles. In this report, some of the endocrine, metabolic, GI, pulmonary, autonomic-cardiovascular, and cognitive secondary manifestations of SCI were discussed. The specific dysfunctions are generally related to the level and completeness of SCI: individuals with higher, more neurologically complete lesions tend to manifest the greatest abnormalities. Advances have been made in improving care, but ongoing investigations will continue to provide additional insight into the pathophysiology of these clinical disorders and test potentially effective interventions to continue to improve function and reduce morbidity. Such an approach would be expected to reduce morbidity, improve quality of life, and extend longevity of persons with SCI.
Acknowledgments
Funding/support: Veteran Affairs Rehabilitation Research and Development Service (#B2468-C, #B4162-C) and the James J. Peters VA Medical Center.
Conflicts of Interest: None to Declare.
References
- 1.Wilmet E, Ismail AA, Heilporn A, Welraeds D, Bergmann P.Longitudinal study of bone mineral content and soft tissue composition after spinal cord section. Paraplegia.1996;33:674–677 [DOI] [PubMed] [Google Scholar]
- 2.Rossier AB, Favre H, Valloton MB.Body composition and endocrine profile in spinal cord injured patients In: Lee BY, Ostrander E, George J, Cochran B, Shaw WW, eds. Comprehensive Management of the Spinal Cord Injured Patient. San Diego: WB Saunders Co; 1991:163–170 [Google Scholar]
- 3.Nuhlicek DN, Spurr GB, Barboriak JJ, Rooney CB, El Ghatt AZ, Bongard RD.Body composition of patients with spinal cord injury. Eur J Clin Nutr. 1988;42:765–773 [PubMed] [Google Scholar]
- 4.Spungen AM, Adkins RH, Stewart CA, Wang J, Pierson RN, Jr, Waters RL, Bauman WA.Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol. 2003;95(6):2398–2407 [DOI] [PubMed] [Google Scholar]
- 5.Spungen AM, Wang J, Pierson RN, Jr, Bauman WA.Soft tissue body composition differences in monozygotic twins discordant for immobilization. J Applied Physiol. 2000;88:1310–1315 [DOI] [PubMed] [Google Scholar]
- 6.Bauman WA, Spungen AM, Zhong YG, Mobbs CV.Plasma leptin is directly related to body adiposity in subjects with spinal cord injury. Horm Metab Res. 1997;28:732–736 [DOI] [PubMed] [Google Scholar]
- 7.Kissebah AH.Vydelingum N, Murray R, et al. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab. 1982;54:254–260 [DOI] [PubMed] [Google Scholar]
- 8.Maki KC, Briones ER, Lanbein WE, et al. Associations between serum lipids and indicators of adiposity in men with spinal cord injury. Paraplegia. 1995;33:102–109 [DOI] [PubMed] [Google Scholar]
- 9.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497 [DOI] [PubMed] [Google Scholar]
- 10.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421 [PubMed] [Google Scholar]
- 11.Bauman WA, Spungen AM.Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism. 1994;43:749–756 [DOI] [PubMed] [Google Scholar]
- 12.Bauman WA, Adkins RH, Spungen AM, et al. The effect of residual neurological deficit on oral glucose tolerance in persons with chronic spinal cord injury. Spinal Cord. 1999;37:765–771 [DOI] [PubMed] [Google Scholar]
- 13.Bauman WA, Adkins RH, Spungen AM, et al. The effect of residual neurological deficit on serum lipoproteins in individuals with chronic spinal cord injury. Spinal Cord. 1998;36:13–17 [DOI] [PubMed] [Google Scholar]
- 14.Bauman WA, Adkins RH, Spungen AM, et al. Is immobilization associated with an abnormal lipoprotein profile? Observations from a diverse cohort. Spinal Cord. 1999;37:485–493 [DOI] [PubMed] [Google Scholar]
- 15.Bauman WA, Spungen AM.Risk assessment for coronary heart disease in a veteran population with spinal cord injury. Top Spinal Cord Inj Rehabil. 2007;12:35–53 [Google Scholar]
- 16.Bauman WA, Spungen AM, Flanagan S, Zhong YG, Alexander LR, Tsitouras PD.Blunted growth hormone response to intravenous arginine in subjects with a spinal cord injury. Horm Metab Res. 1994;26(3):152–156 [DOI] [PubMed] [Google Scholar]
- 17.Tsitouras PD, Zhong YG, Spungen AM, Bauman WA.Serum testosterone and growth hormone/insulin-like growth factor-I in adults with spinal cord injury. Horm Metab Res. 1995;27(6):287–292 [DOI] [PubMed] [Google Scholar]
- 18.Shetty KR, Sutton CH, Mattson DE, Rudman D.Hyposomatomedinemia in quadriplegic men. Am J Med Sci. 1993;305(2):95–100 [DOI] [PubMed] [Google Scholar]
- 19.Bauman WA, La Fountaine MF, Spungen AM. Age-related prevalence of hypogonadism in men with spinal cord injury. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 20.Wang YH, Huang TS, Lien IN.Hormone changes in men with spinal cord injuries. Am J Phys Med Rehabil. 1992;71(6):328–332 [DOI] [PubMed] [Google Scholar]
- 21.Huang TS, Wang YH, Chiang HS, Lien YN.Pituitary-testicular and pituitary-thyroid axes in spinal cord-injured males. Metabolism. 1993;42(4):516–521 [DOI] [PubMed] [Google Scholar]
- 22.Perrini S, Laviola L, Carreira MC, Cignarelli A, Natalicchio A, Giorgino F.The GH/IGF1 axis and signaling pathways in the muscle and bone: mechanisms underlying age-related skeletal muscle wasting and osteoporosis. J Endocrinol. 2010;205(3):201–210 [DOI] [PubMed] [Google Scholar]
- 23.Giustina A, Mazziotti G, Canalis E.Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev. 2008;29(5):535–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janssen TW, van Oers CA, Rozendaal EP, Willemsen EM, Hollander AP, van der Woude LH.Changes in physical strain and physical capacity in men with spinal cord injuries. Med Sci Sports Exerc. 1996;28(5):551–559 [DOI] [PubMed] [Google Scholar]
- 25.Gray A, Feldman HA, McKinlay JB, Longcope C. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 1991;73(5):1016–1025 [DOI] [PubMed] [Google Scholar]
- 26.Longcope C, Goldfield SR, Brambilla DJ, McKinlay J.Androgens, estrogens, and sex hormone-binding globulin in middle-aged men. J Clin Endocrinol Metab. 1990;71(6):1442–1446 [DOI] [PubMed] [Google Scholar]
- 27.Simon D, Preziosi P, Barrett-Connor E, et al. The influence of aging on plasma sex hormones in men: the Telecom Study. Am J Epidemiol. 1992;135(7):783–791 [DOI] [PubMed] [Google Scholar]
- 28.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR.Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86(2):724–731 [DOI] [PubMed] [Google Scholar]
- 29.Morley JE, Kaiser FE, Perry HM, 3rd, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism. 1997;46(4):410–413 [DOI] [PubMed] [Google Scholar]
- 30.Zmuda JM, Cauley JA, Kriska A, Glynn NW, Gutai JP, Kuller LH.Longitudinal relation between endogenous testosterone and cardiovascular disease risk factors in middle-aged men. A 13-year follow-up of former Multiple Risk Factor Intervention Trial participants. Am J Epidemiol. 1997;146(8):609–617 [DOI] [PubMed] [Google Scholar]
- 31.Vermeulen A, Kaufman JM.Ageing of the hypothalamopituitary-testicular axis in men. Horm Res. 1995;43(1-3):25–28 [DOI] [PubMed] [Google Scholar]
- 32.Reddy RG, Aung T, Karavitaki N, Wass JA.Opioid induced hypogonadism. BMJ. 2010;341:c4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tenover JL.Testosterone and the aging male. J Androl. 1997;18(2):103–106 [PubMed] [Google Scholar]
- 34.LeBlanc ES, Nielson CM, Marshall LM, et al. The effects of serum testosterone, estradiol, and sex hormone binding globulin levels on fracture risk in older men. J Clin Endocrinol Metab. 2009;94(9):3337–3346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clark MJ, Schopp LH, Mazurek MO, et al. Testosterone levels among men with spinal cord injury: relationship between time since injury and laboratory values. Am J Phys Med Rehabil. 2008;87(9):758–767 [DOI] [PubMed] [Google Scholar]
- 36.Safarinejad MR.Level of injury and hormone profiles in spinal cord-injured men. Urology. 2001;58(5):671–676 [DOI] [PubMed] [Google Scholar]
- 37.Cortes-Gallegos V, Castaneda G, Alonso R, Arellano H, Cervantes C, Parra A.Diurnal variations of pituitary and testicular hormones in paraplegic men. Arch Androl. 1982;8(3):221–226 [DOI] [PubMed] [Google Scholar]
- 38.Kikuchi TA, Skowsky WR, El-Toraei I, Swerdloff R.The pituitary-gonadal axis in spinal cord injury. Fertil Steril. 1976;27(10):1142–1145 [DOI] [PubMed] [Google Scholar]
- 39.Bors E, Engle ET, Rosenquist RC, Holliger VH.Fertility in paraplegic males: a preliminary report of endocrine studies. J Clin Endocrinol Metab. 1950;10(4):381–398 [DOI] [PubMed] [Google Scholar]
- 40.Nance PW, Shears AH, Givner ML, Nance DM.Gonadal regulation in men with flaccid paraplegia. Arch Phys Med Rehabil. 1985;66(11):757–759 [PubMed] [Google Scholar]
- 41.Ver Voort SM.Infertility in spinal-cord injured male. Urology. 1987;29(2):157–165 [DOI] [PubMed] [Google Scholar]
- 42.Linsenmeyer TA, Perkash I.Infertility in men with spinal cord injury. Arch Phys Med Rehabil. 1991;72(10):747–754 [PubMed] [Google Scholar]
- 43.Bauman WA, La Fountaine MF, Christopher M.Cirnigliaro CM, Emmons RR, Kirshblum SC, Spungen AM. Provocative stimulation of the pituitary-gonadal axis in men with spinal cord injury. Submitted for publication.
- 44.Spungen AM, Bauman WA, Wang J, Pierson RN., Jr.The relationship between total body potassium and resting energy expenditure in individuals with paraplegia. Arch Phys Med Rehabil. 1993;783:965–968 [PubMed] [Google Scholar]
- 45.Mollinger LA, Spurr GB, El Ghatit AZ, et al. Daily energy expenditure and basal metabolic rates of patients with spinal cord injury. Arch Phys Med Rehabil. 1985;66:420–426 [PubMed] [Google Scholar]
- 46.Mauras N, Hayes V, Welch S, et al. Testosterone deficiency in young men: marked alterations in whole body protein kinetics, strength, and adiposity. J Clin Endocrinol Metab. 1998;83(6):1886–1892 [DOI] [PubMed] [Google Scholar]
- 47.Sonmez A, Haymana C, Bolu E, et al. Metabolic syndrome and the effect of testosterone treatment in young men with congenital hypogonadotropic hypogonadism. Eur J Endocrinol. 2011;164(5):759–764 [DOI] [PubMed] [Google Scholar]
- 48.Bauman WA, Cirnigliaro CM, La Fountaine MF, et al. A small-scale clinical trial to determine the safety and efficacy of testosterone replacement therapy in hypogonadal men with spinal cord injury. Horm Metab Res. 2011;43(8):574–579 [DOI] [PubMed] [Google Scholar]
- 49.Duckworth WC, Solomon SS, Jallepalli P, et al. Glucose intolerance due to insulin resistance in patients with spinal cord injuries. Diabetes. 1980;29:906–910 [DOI] [PubMed] [Google Scholar]
- 50.Tharion G, Prasad KR, Gopalan L, Bhattacharji S.Glucose intolerance and dyslipidaemias in persons with paraplegia and tetraplegia in South India. Spinal Cord. 1998;36:228–230 [DOI] [PubMed] [Google Scholar]
- 51.WHO Expert Committee on Diabetes Mellitus: second report. World Health Organ Tech Rep Ser. 1980;646:1–80 [PubMed] [Google Scholar]
- 52.Haffner SM, Stern MP, Hazuda HP, et al. Cardiovascular risk factors in confirmed prediabetic individuals. Does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA. 1990;263:2893–2898 [DOI] [PubMed] [Google Scholar]
- 53.Castelli WP, Leaf A.Identification and assessment of cardiac risk--an overview. Cardiol Clin. 1985;3:171–178 [Google Scholar]
- 54.Grundy SM, DeWitt S, Goodman MD, et al. The place of HDL cholesterol management. A perspective from the National Cholesterol Education Program. Arch Intern Med. 1989;149:505–510 [PubMed] [Google Scholar]
- 55.Brenes G, Dearwater S, Shapera R, et al. High density lipoprotein cholesterol concentrations in physically active and sedentary spinal cord injured patients. Arch Phys Med Rehabil. 1986;67:445–450 [PubMed] [Google Scholar]
- 56.Rader DJ.High-density lipoproteins and atherosclerosis. Am J Cardiol. 2002;90 (suppl):62i–70i [DOI] [PubMed] [Google Scholar]
- 57.Campbell PJ, Mandarino LJ, Gerich JE.Quantification of the relative impairment in actions of insulin on hepatic glucose production and peripheral glucose uptake in NIDDM. Metabolism. 1988;37:15–21 [DOI] [PubMed] [Google Scholar]
- 58.Johansson J, Walldius G, Carlson LA.Close correlation between high-density lipoprotein and triglycerides in normotriglyceridemia. J Int Med. 1992;232:43–51 [DOI] [PubMed] [Google Scholar]
- 59.Golay A, Zech L, Shi MZ, et al. High density lipoprotein (HDL) metabolism in noninsulin-dependent diabetes mellitus: measurement of fHDL turnover using tritiated HDL. J Clin Endocrinol Metab. 1987;65:512–518 [DOI] [PubMed] [Google Scholar]
- 60.Emmons RR, Garber CE, Cirnigliaro CM, et al. The influence of visceral fat on the postprandial lipemic response in men with paraplegia. J Am Coll Nutr. 2010;29(5):476–481 [DOI] [PubMed] [Google Scholar]
- 61.LaPorte RE, Brenes G, Dearwater S, et al. HDL cholesterol across a spectrum of physical activity from quadriplegia to marathon running [letter]. Lancet. 1983;1212–1213 [DOI] [PubMed] [Google Scholar]
- 62.Kraus WE, Houmard JA, Duscha BD, et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347:1483–1492 [DOI] [PubMed] [Google Scholar]
- 63.Nash MS, Jacobs PL, Mendex AJ, Goldberg RB.Circuit resistance training improves the atherogenic lipid profiles of persons with chronic paraplegia. J Spinal Cord Med. 2001; 24:2–9 [DOI] [PubMed] [Google Scholar]
- 64.Stampfer MJ, Sacks FM, Salvini S, Willett WC, Hennekens CH.A prospective study of cholesterol, apolipoproteins, and the risk of myocardial infarction. N Engl J Med. 1992; 325:373–381 [DOI] [PubMed] [Google Scholar]
- 65.Bauman A, Owen N.Habitual physical activity and cardiovascular risk factors. Med J Aust. 1991;154:22–28 [DOI] [PubMed] [Google Scholar]
- 66.Nash MS, Lewis JE, Dyson-Hudson TA, Szlachcic Y, Yee F, Mendez AJ, Spungen AM, Bauman WA. Safety, tolerance, and efficacy of extended-release niacin monotherapy for treating dyslipidemia risks in persons with chronic tetraplegia: a randomized multicenter controlled trial. Arch Phys Med Rehabil. 2011;92(3):399–410 [DOI] [PubMed] [Google Scholar]
- 67.Mureddu GF, Brandimarte F, De Luca L.High density lipoprotein levels and risk of cardiovascular events: a review [published online ahead of print October7, 2011]. J Cardiovasc Med (Hagerstown). [DOI] [PubMed] [Google Scholar]
- 68.Voight BF, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study [published online ahead of print May17, 2012]. Lancet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fleg JL, Gerstenblith G, Zonderman AB, et al. Prevalence of and prognostic significance of exercise induced silent myocardial ischemia detected by thallium scintigraphy. Circulation. 1990;81:428–436 [DOI] [PubMed] [Google Scholar]
- 70.Lee CS, Lu YH, Lee ST, Lin CC, Ding HJ.Evaluating the prevalence of silent coronary artery disease in asymptomatic patients with spinal cord injury. Int Heart J. 2006;47:325–330 [DOI] [PubMed] [Google Scholar]
- 71.Whiteneck GG, Charlifue SW, Frankel HL, et al. Mortality, morbidity, and psychosocial outcomes of persons spinal cord injured more than 20 years ago. Paraplegia. 1992;30:617–630 [DOI] [PubMed] [Google Scholar]
- 72.Bauman WA, Raza M, Spungen AM, et al. Cardiac stress testing with thallium-201 imaging reveals silent ischemia in individuals with paraplegia. Arch Phys Med Rehabil. 1994;75:946–950 [PubMed] [Google Scholar]
- 73.Bauman WA, Raza M, Machac J.Tomograhic thallium201 myocardial perfusion imaging after intravenous dipyridamole in asymptomatic subjects with quadriplegia. Arch Phys Med Rehabil. 1993;I74:740–744 [DOI] [PubMed] [Google Scholar]
- 74.Orakzai SH, Orakzai RH, Ahmadi N, et al. Measurement of coronary artery calcification by electron beam computerized tomography in persons with chronic spinal cord injury: evidence for increased atherosclerotic burden. Spinal Cord. 2007; 45:775–779 [DOI] [PubMed] [Google Scholar]
- 75.Naghavi M, Falk E, Hecht HS, Shah PK; SHAPE Task Force. The first SHAPE (Screening for Heart Attack Prevention and Education) guideline. Crit Pathw Cardiol. 2006;5(4):187–190 [DOI] [PubMed] [Google Scholar]
- 76.Stewart AF, Adler M, Byers CM, Segre GV, Broadus AE.Calcium homeostasis in immobilization: an example of resorptive hypercalciuria. N Engl J Med. 1982;306(19):1136–1140 [DOI] [PubMed] [Google Scholar]
- 77.Naftchi NE, Viau AT, Sell GH, Lowman EW.Mineral metabolism in spinal cord injury. Arch Phys Med Rehabil. 1980;61(3):139–142 [PubMed] [Google Scholar]
- 78.Szollar SM, Martin EM, Sartoris DJ, Parthemore JG, Deftos LJ.Bone mineral density and indexes of bone metabolism in spinal cord injury. Am J Phys Med Rehabil. 1998;77(1):28–35 [DOI] [PubMed] [Google Scholar]
- 79.Garland DE, Adkins RH, Kushwaha V, Stewart C.Risk factors for osteoporosis at the knee in the spinal cord injury population. J Spinal Cord Med. 2004;27(3):202–206 [DOI] [PubMed] [Google Scholar]
- 80.Warden SJ, Bennell KL, Matthews B, Brown DJ, McMeeken JM, Wark JD.Quantitative ultrasound assessment of acute bone loss following spinal cord injury: a longitudinal pilot study. Osteoporos Int. 2002;13(7):586–592 [DOI] [PubMed] [Google Scholar]
- 81.Vico L, Collet P, Guignandon A, et al. Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet. 2000;355(9215):1607–1611 [DOI] [PubMed] [Google Scholar]
- 82.Leblanc AD, Schneider VS, Evans HJ, Engelbretson DA, Krebs JM.Bone mineral loss and recovery after 17 weeks of bed rest. J Bone Miner Res. 1990;5(8):843–850 [DOI] [PubMed] [Google Scholar]
- 83.Recker R, Davies K, Heaney R.Characterization of perimenopausal bone loss: a prospective study. J Bone Miner Res. 2000;15(10):1965–1973 [DOI] [PubMed] [Google Scholar]
- 84.Qin W, Bauman WA, Cardozo CP.Evolving concepts in neurogenic osteoporosis. Curr Osteoporos Rep. 2010;8(4):212–218 [DOI] [PubMed] [Google Scholar]
- 85.Bauman WA, Spungen AM, Wang J, Pierson RN, Jr, Schwartz E.Continuous loss of bone during chronic immobilization: a monozygotic twin study. Osteoporos Int. 1999;10(2):123–127 [DOI] [PubMed] [Google Scholar]
- 86.de Bruin ED, Dietz V, Dambacher MA, Stussi E.Longitudinal changes in bone in men with spinal cord injury. Clin Rehabil. 2000;14(2):145–152 [DOI] [PubMed] [Google Scholar]
- 87.Dauty M, Perrouin Verbe B, Maugars Y, Dubois C, Mathe JF. Supralesional and sublesional bone mineral density in spinal cord-injured patients. Bone. 2000;27(2):305–309 [DOI] [PubMed] [Google Scholar]
- 88.Biering-Sorensen F, Bohr HH, Schaadt OP.Longitudinal study of bone mineral content in the lumbar spine, the forearm and the lower extremities after spinal cord injury. Eur J Clin Invest. 1990;20(3):330–335 [DOI] [PubMed] [Google Scholar]
- 89.Finsen V, Indredavik B, Fougner KJ.Bone mineral and hormone status in paraplegics. Paraplegia. 1992;30(5):343–347 [DOI] [PubMed] [Google Scholar]
- 90.Frey-Rindova P, de Bruin ED, Stussi E, Dambacher MA, Dietz V.Bone mineral density in upper and lower extremities during 12 months after spinal cord injury measured by peripheral quantitative computed tomography. Spinal Cord. 2000;38(1):26–32 [DOI] [PubMed] [Google Scholar]
- 91.Eser P, Frotzler A, Zehnder Y, Wick L, Knecht H, Denoth J, Schiessl H.Relationship between the duration of paralysis and bone structure: a pQCT study of spinal cord injured individuals. Bone. 2004;34(5):869–880 [DOI] [PubMed] [Google Scholar]
- 92.Lifshitz F, Maclaren NK.Vitamin D-dependent rickets in institutionalized, mentally retarded children receiving long-term anticonvulsant therapy. I. A survey of 288 patients. J Pediatr. 1973;83(4):612–620 [DOI] [PubMed] [Google Scholar]
- 93.Hahn TJ, Hendin BA, Scharp CR, Haddad JG., Jr.Effect of chronic anticonvulsant therapy on serum 25-hydroxycalciferol levels in adults. N Engl J Med. 1972;287(18):900–904 [DOI] [PubMed] [Google Scholar]
- 94.Walters JL, Buchholz AC, Martin Ginis KA. Evidence of dietary inadequacy in adults with chronic spinal cord injury. Spinal Cord. 2009;47(4):318–322 [DOI] [PubMed] [Google Scholar]
- 95.Bauman WA, Zhong YG, Schwartz E.Vitamin D deficiency in veterans with chronic spinal cord injury. Metabolism. 1995;44(12):1612–1616 [DOI] [PubMed] [Google Scholar]
- 96.Oleson CV, Patel PH, Wuermser LA.Influence of season, ethnicity, and chronicity on vitamin D deficiency in traumatic spinal cord injury. J Spinal Cord Med. 2010;33(3):202–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nemunaitis GA, Mejia M, Nagy JA, Johnson T, Chae J, Roach MJ.A descriptive study on vitamin D levels in individuals with spinal cord injury in an acute inpatient rehabilitation setting. Phys Med Rehabil. 2010;2(3):202–208; quiz 28. [DOI] [PubMed] [Google Scholar]
- 98.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R.Estimates of optimal vitamin D status. Osteoporos Int. 2005;16(7):713–716 [DOI] [PubMed] [Google Scholar]
- 99.Bauman WA, Emmons RR, Cirnigliaro CM, Kirshblum SC, Spungen AM.An effective oral vitamin D replacement therapy in persons with spinal cord injury. J Spinal Cord. 2011;34(5):455–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bauman WA, Wecht JM, Kirshblum S, Spungen AM, Morrison N, Cirnigliaro C, Schwartz E.Effect of pamidronate administration on bone in patients with acute spinal cord injury. J Rehabil Res Dev. 2005;42(3):305–313 [DOI] [PubMed] [Google Scholar]
- 101.Bryson JE, Gourlay ML.Bisphosphonate use in acute and chronic spinal cord injury: a systematic review. J Spinal Cord Med. 2009;32(3):215–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kearns AE, Khosla S, Kostenuik PJ.Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr Rev. 2008;29(2):155–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Clark MJ, Schopp LH, Mazurek MO, et al. Testosterone levels among men with spinal cord injury: relationship between time since injury and laboratory values. Am J Phys Med Rehabil. 2008;87(9):758–767 [DOI] [PubMed] [Google Scholar]
- 104.Bracken MB, Holford TR.Neurological and functional status 1 year after acute spinal cord injury: estimates of functional recovery in National Acute Spinal Cord Injury Study II from results modeled in National Acute Spinal Cord Injury Study III. J Neurosurg. 2002;96(3 suppl):259–266 [DOI] [PubMed] [Google Scholar]
- 105.Qin W, Pan J, Wu Y, Bauman WA, Cardozo C.Protection against dexamethasone-induced muscle atrophy is related to modulation by testosterone of FOXO1 and PGC-1alpha. Biochem Biophys Res Commun. 2010;403(3-4):473–478 [DOI] [PubMed] [Google Scholar]
- 106.Wu Y, Zhao W, Zhao J, Zhang Y, Qin W, Pan J, Bauman WA, Blitzer RD, Cardozo C.REDD1 is a major target of testosterone action in preventing dexamethasone-induced muscle loss. Endocrinology. 2010;151(3):1050–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Crawford BA, Liu PY, Kean MT, Bleasel JF, Handelsman DJ.Randomized placebo-controlled trial of androgen effects on muscle and bone in men requiring long-term systemic glucocorticoid treatment. J Clin Endocrinol Metab. 2003;88(7):3167–3176 [DOI] [PubMed] [Google Scholar]
- 108.Steins SA, Fajardo NR, Korsten MA. The gastrointestinal system after spinal cord injury In: Lin VW, ed. Spinal Cord Medicine. New York: Demos Medical Publishers; 2003:321–348 [Google Scholar]
- 109.Banwell JG, Graham HC, Aggarwal AC, et al. Management of the neurogenic bowel in patients with spinal cord injury. Urologic Clin North Am. 1993;20:517–526 [PubMed] [Google Scholar]
- 110.Glick ME, Meshkinpour H, Haldeman S, et al. Colonic dysfunction in patients with spinal cord injury. Gastroenterology. 1984;86:287–294 [PubMed] [Google Scholar]
- 111.Steins SA, Bergman SB, Gowtz LL.Neurogenic bowel dysfunction after spinal cord injury: clinical evaluation and rehabilitative management. Arch Phys Med Rehabil. 1997;78:S86–102 [DOI] [PubMed] [Google Scholar]
- 112.Christensen J.The motor function of the colon In: Yamada T, ed. Textbook of Gastroenterology, vol 1. Philadelphia, JB Lippincott; 1991:180–194 [Google Scholar]
- 113.Adams RD, Victor M.Diseases of the spinal cord, peripheral nerve, and muscle In: Adams RD, Victor M, eds. Principles of Neurology. New York: McGraw-Hill Publishers; 1993:1178–1180 [Google Scholar]
- 114.Cook IJ, Brookes SJ. Motility of large intestine In: Feldman M, Friedman LS, Sleisenger MH, eds. Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis and Management. Philadelphia: Elsevier Saunders; 2002:1679–1690 [Google Scholar]
- 115.Rajendran SK, Reiser JR, Bauman W, et al. Gastrointestinal transit after spinal cord injury: effect of cisapride. Am J Gastroenterol 1992;87:1614–1617 [PubMed] [Google Scholar]
- 116.Arhan P, Devroede G, Jehanin B, et al. Segmental colonic transit time. Dis Colon Rectum. 1981;24:625–629 [DOI] [PubMed] [Google Scholar]
- 117.Menardo G, Bausano G, Corazziari E, et al. Large bowel transit in paraplegic patients. Dis Colon Rectum. 1987;30:924–928 [DOI] [PubMed] [Google Scholar]
- 118.Beuret-Blanquart F, Weber J, Gouverneur JP, et al. Colonic transit time and anorectal manometric anomalies in 19 patients with complete transection of the spinal cord. J Auton Nerv Syst. 1990;30:199–208 [DOI] [PubMed] [Google Scholar]
- 119.Ancha HR, Fajardo NR, Bauman WA, Rosman AS, Galea M, Creasey G, Korsten MA.Absence of high amplitude propagating contractions in subjects with chronic spinal cord injury. World J Gastroenterol. 2010;16(43):5435–5439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stone JM, Nino-Murcia M, Wolfe VA, et al. Chronic gastrointestinal problems in spinal cord injury patients: a prospective analysis. Am J Gastroenterol. 1990;85:1114–1119 [PubMed] [Google Scholar]
- 121.Wrenn K.Fecal impaction. N Engl J Med. 1989;321:658–662 [DOI] [PubMed] [Google Scholar]
- 122.Kewalramani LS.Autonomic dysreflexia in traumatic myelopathy. Am J Phys Med. 1979;59:1–21 [PubMed] [Google Scholar]
- 123.Johnson B, Pallares V, Thomason R, et al. Autonomic hyperreflexia: a review. Mil Med. 1975;140:345–349 [PubMed] [Google Scholar]
- 124.Kursh ED, Freehafer A, Persky L.Complications of autonomic dysreflexia. J Urol. 1977;118:70–72 [DOI] [PubMed] [Google Scholar]
- 125.Thomas J.Neurogenic Bowel Management. Spinal Cord Injury Clinical Guidelines. Washington, DC: Paralyzed Veterans of America; 1997 [Google Scholar]
- 126.American Association of Spinal Cord Injury Nurses American Association of Spinal Cord Injury Nurses standards of practice--revised 2003-2004. SCI Nurs. 2004;21(4):228–232 [PubMed] [Google Scholar]
- 127.Shafik A, El-Sibai D, Shafik I.Physiologic basis of digital rectal stimulation for bowel evacuation in patients with spinal cord injury: identification of an anorectal excitatory reflex. J Spinal Cord Med. 2000;23:270–275 [DOI] [PubMed] [Google Scholar]
- 128.Korsten MA, Monga A, Chaparala G, et al. Digital rectal stimulation causes increased left sided colonic motility in patients with SCI. Gastroenterology. 2003;124:A115 [Google Scholar]
- 129.Nelson A, Malassigne P, Amerson T, et al. Descriptive study of bowel care practices and equipment in spinal cord injury. SCI Nursing. 1993;10:65–67 [PubMed] [Google Scholar]
- 130.Chapman R, Sillery J.Fontana D. Effect of oral dioctyl sodium sulfosuccinate on intake-output studies of the human small and large intestine. Gastroenterology. 1985;89:489–493 [DOI] [PubMed] [Google Scholar]
- 131.Pietrusko RG.Use and abuse of laxatives. Am J Hosp Pharm. 1977;34:291–300 [PubMed] [Google Scholar]
- 132.Spiller RC. Pharmacology of dietary fiber. Pharmacol Ther. 1994;62:407–427 [DOI] [PubMed] [Google Scholar]
- 133.Hillemeier C. An overview of the effects of dietary fiber on gastrointestinal transit [review]. Pediatrics. 1995;96:997–999 [PubMed] [Google Scholar]
- 134.Longo WE, Woolsey R, Vernava A, et al. Cisapride for constipation in spinal cord injured patients: a preliminary report. J Spinal Cord Med. 1995;18:240–244 [DOI] [PubMed] [Google Scholar]
- 135.Wysowski DK, Bacsanyi J.Cisapride and fatal arrythmia. N Engl J Med. 1996;335:290–291 [DOI] [PubMed] [Google Scholar]
- 136.Ponec RJ, Saunders MD, Kimmey MB, et al. Neostigmine for the treatment of acute pseudo-obstruction. N Engl J Med. 1999;341:137–141 [DOI] [PubMed] [Google Scholar]
- 137.Korsten MA, Rosman AS, Ng A, et al. Infusion of neostigmine-glycopyrrolate for bowel evacuation in persons with spinal cord injury. Am J Gastroenterol. 2005;100:1560–1565 [DOI] [PubMed] [Google Scholar]
- 138.Saltzstein RJ, Romano J.The efficacy of colostomy as a bowel management alternative in selected spinal cord injury patients. J Am Paraplegia Soc. 1990;13:9–13 [DOI] [PubMed] [Google Scholar]
- 139.Stone JM, Wolfe VA, Nino-Murcia M, et al. Colostomy as treatment for complications of spinal cord injury. Arch Phys Med Rehabil. 1990;71:514–518 [PubMed] [Google Scholar]
- 140.Vignes JR, Bauchet L, Ohanna F.Dorsal rhizotomy combined with anterior sacral root stimulation for neurogenic bladder. Acta Neurochir Suppl. 2007;97(pt 1):323–331 [DOI] [PubMed] [Google Scholar]
- 141.Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366(8):687–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shaheen S, Huq MM, Radulovic M, et al. Adjunctive neostigmine/glycopyrrolate improves colonoscopic bowel preparation and efficacy in subjects with spinal cord injury. Gastroenterology. 2012;142(5 suppl 1):S219 [Google Scholar]
- 143.DeVivo MJ, Black KJ, Stover SL.Causes of death during the first 12 years after spinal cord injury. Arch Phys Med Rehabil. 1993;74(3):248–254 [PubMed] [Google Scholar]
- 144.DeVivo MJ, Krause JS, Lammertse DP, Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil. 1999;80(11):1411–1419 [DOI] [PubMed] [Google Scholar]
- 145.Spungen AM, et al. Self-reported prevalence of pulmonary symptoms in subjects with spinal cord injury. Spinal Cord. 1997;35(10):652–657 [DOI] [PubMed] [Google Scholar]
- 146.Spungen AM, et al. Relationship of respiratory symptoms with smoking status and pulmonary function in chronic spinal cord injury. J Spinal Cord Med. 2002;25(1):23–27 [DOI] [PubMed] [Google Scholar]
- 147.Jackson AB, Groomes TE.Incidence of respiratory complications following spinal cord injury. Arch Phys Med Rehabil. 1994;75(3):270–275 [DOI] [PubMed] [Google Scholar]
- 148.De Troyer A, Estenne M, Heilporn A.Mechanism of active expiration in tetraplegic subjects. N Engl J Med. 1986;314(12):740–744 [DOI] [PubMed] [Google Scholar]
- 149.Fujiwara T, Hara Y, Chino N.Expiratory function in complete tetraplegics: study of spirometry, maximal expiratory pressure, and muscle activity of pectoralis major and latissimus. Am J Phys Med Rehabil. 1999;78(5):464–469 [DOI] [PubMed] [Google Scholar]
- 150.Ledsome JR., Sharp JM.Pulmonary function in acute cervical cord injury. Am Rev Respir Dis. 1981;124(1):41–44 [DOI] [PubMed] [Google Scholar]
- 151.Haas F, Axen K, Pineda H, Gandino D, Haas A.Temporal pulmonary function changes in cervical cord injury. Arch Phys Med Rehabil. 1985;66(3):139–144 [PubMed] [Google Scholar]
- 152.Anke A, Aksnes AK, Stanghelle JK, Hjeltnes N.Lung volumes in tetraplegic patients according to cervical spinal cord injury level. Scand J Rehabil Med. 1993;25(2):73–77 [PubMed] [Google Scholar]
- 153.Mueller G, de Groot S, Van der Woude L, Hopman MT.Time-courses of lung function and respiratory muscle pressure generating capacity after spinal cord injury: a prospective cohort study. J Rehabil Med. 2008;40(4):269–276 [DOI] [PubMed] [Google Scholar]
- 154.McMichan JC, Michel L, Westbrook PR.Pulmonary dysfunction following traumatic quadriplegia. Recognition, prevention, and treatment. JAMA. 1980;243(6):528–531 [PubMed] [Google Scholar]
- 155.Axen K, Pineda H, Shunfenthal I, Haas F.Diaphragmatic function following cervical cord injury: neurally mediated improvement. Arch Phys Med Rehabil. 1985;66(4):219–222 [DOI] [PubMed] [Google Scholar]
- 156.Oo T, Watt JW, Soni BM, Sett PK.Delayed diaphragm recovery in 12 patients after high cervical spinal cord injury. A retrospective review of the diaphragm status of 107 patients ventilated after acute spinal cord injury. Spinal Cord. 1999;37(2):117–122 [DOI] [PubMed] [Google Scholar]
- 157.Roth EJ, Nussbaum SB, Berkowitz M, et al. Pulmonary function testing in spinal cord injury: correlation with vital capacity. Paraplegia. 1995;33(8):454–457 [DOI] [PubMed] [Google Scholar]
- 158.Linn WS, Adkins RH, Gong H, Jr, Waters RL.Pulmonary function in chronic spinal cord injury: a cross-sectional survey of 222 southern California adult outpatients. Arch Phys Med Rehabil. 2000;81(6):757–763 [DOI] [PubMed] [Google Scholar]
- 159.Linn WS, Spungen AM, Gong H, Jr, Adkins RH, Bauman WA, Waters RL.Forced vital capacity in two large outpatient populations with chronic spinal cord injury. Spinal Cord. 2001;39(5):263–268 [DOI] [PubMed] [Google Scholar]
- 160.Stolzmann KL, Gagnon DR, Brown R, Tun CG, Garshick E.Longitudinal change in FEV1 and FVC in chronic spinal cord injury. Am J Respir Crit Care Med. 2008;177(7):781–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hemingway A, Bors E, Hobby RP.An investigation of the pulmonary function of paraplegics. J Clin Invest. 1958;37:773–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fugl-Meyer AR, Grimby G.Ventilatory function in tetraplegic patients. Scand J Rehabil Med. 1971;3:151–160 [PubMed] [Google Scholar]
- 163.Fugl-Meyer AR.A model for treatment of impaired ventilatory function in tetraplegic patients. Scand J Rehabil Med. 1971;3(4):168–177 [PubMed] [Google Scholar]
- 164.Ohry A, Molho M, Rozin R.Alterations of pulmonary function in spinal cord injured patients. Paraplegia. 1975;13(2):101–108 [DOI] [PubMed] [Google Scholar]
- 165.Kokkola K, Möller K, Lehtonen T.Pulmonary function in tetraplegic and paraplegic patients. Ann Clin Res. 1975;7(2):76–79 [PubMed] [Google Scholar]
- 166.Forner JV.Lung volumes and mechanics of breathing in tetraplegics. Paraplegia. 1980;18(4):258–266 [DOI] [PubMed] [Google Scholar]
- 167.Almenoff PL, Alexander LR, Spungen AM, Lesser MD, Bauman WA.Bronchodilatory effects of ipratropium bromide in patients with tetraplegia. Paraplegia. 1995;33(5):274–277 [DOI] [PubMed] [Google Scholar]
- 168.Schilero GJ, Grimm D, Spungen AM, Lenner R, Lesser M.Bronchodilator responses to metaproterenol sulfate among subjects with spinal cord injury. J Rehabil Res Dev. 2004;41(1):59–64 [DOI] [PubMed] [Google Scholar]
- 169.Schilero GJ, Grimm DR, Bauman WA, Lesser M.Assessment of airway caliber and bronchodilator responsiveness in subjects with spinal cord injury. Chest. 2012;127:149–155 [DOI] [PubMed] [Google Scholar]
- 170.Schilero GJ, Spungen AM, Bauman WA, Radulovic M, Lesser M.Pulmonary function and spinal cord injury [review]. Respir Physiol Neurobiol. 2009;166(3):129–141 [DOI] [PubMed] [Google Scholar]
- 171.Grimm DR, et al. Airway hyperreactivity in subjects with tetraplegia is associated with reduced baseline airway caliber. Chest. 2000;118(5):1397–1404 [DOI] [PubMed] [Google Scholar]
- 172.Cockcroft DW, Davis BE.Mechanisms of airway hyperresponsiveness. J Allergy Clin Immunol. 2006;118(3):551–559; quiz 560-561. [DOI] [PubMed] [Google Scholar]
- 173.Garshick E, Stolzmann KL, Gagnon DR, Morse LR, Brown R.Systemic inflammation and reduced pulmonary function in chronic spinal cord injury. Phys Med Rehabil. 2011;3(5):433–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Radulovic M, Schilero GJ, Wecht JM, LaFountaine M, Rosado Rivera D, Bauman W. Exhaled nitric oxide levels are elevated in persons with tetraplegia and comparable to that in mild asthmatics. Lung. 2010;188(3):259–262 [DOI] [PubMed] [Google Scholar]
- 175.Di Maria GU, et al. Role of endogenous nitric oxide in asthma. Allergy. 2000;55(suppl 61):31–35 [DOI] [PubMed] [Google Scholar]
- 176.Radulovic M, Schilero GJ, Wecht JM, LaFountaine M, Rosado Rivera D, Bauman W. Comparison of exhaled breath condensate inflammatory biomarker profiles among persons with tetraplegia, asthma and able-bodied controls. Presented at: Paralyzed Veterans of America’s Summit; 2011; Orlando, Florida.
- 177.Shimizu Y, Dobashi K, Mori M.Exhaled breath marker in asthma patients with gastroesophageal reflux disease. J Clin Biochem Nutr. 2007;41(3):147–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shimizu Y, et al. Assessment of airway inflammation by exhaled breath condensate and impedance due to gastroesophageal reflux disease (GERD). Inflamm Allergy Drug Targets. 2009;8(4):292–296 [DOI] [PubMed] [Google Scholar]
- 179.Shimizu Y, et al. Proton pump inhibitor improves breath marker in moderate asthma with gastroesophageal reflux disease. Respiration. 2007;74(5):558–564 [DOI] [PubMed] [Google Scholar]
- 180.Estenne M.Knoop C, Vanvaerenbergh J, Heilporn A, De Troyer A. The effect of pectoralis mascle training in tetraplegic subjects. Am Rev Respir Dis. 1989;139;1218–1222 [DOI] [PubMed] [Google Scholar]
- 181.DiMarco AF.Restoration of respiratory muscle function following spinal cord injury. Review of electrical and magnetic stimulation techniques. Respir Physiol Neurobiol. 2005;147(2-3):273–287 [DOI] [PubMed] [Google Scholar]
- 182.Caruso JF, Signorile JF, Perry AC, et al. The effects of albuterol and isokinetic exercise on the quadriceps muscle group. Med Sci Sports Exerc. 1995;11:1471–1476 [PubMed] [Google Scholar]
- 183.Kissel JT, McDermott MP, Natarajan R, et al. Pilot trial of albuterurol in facioscapulohumeral muscular dystrophy. Neurology. 1998;50:1402–1406 [DOI] [PubMed] [Google Scholar]
- 184.Signorile JF, Banlovac K, Gomez M, et al. Increased muscle strength in paralyzed patients after spinal cord injury: effect of a beta-2 adrenergic agonist. Arch Phys Med Rehabil. 1995;76:55–58 [DOI] [PubMed] [Google Scholar]
- 185.Murphy RJL, Hartkopp A, Gardiner PF, et al. Salbutamol effect in spinal cord injured individuals undergoing functional electrical stimulation training. Arch Phys Med Rehabil. 1999;80:1264–1267 [DOI] [PubMed] [Google Scholar]
- 186.Grimm DR, et al. Salmeterol improves pulmonary function in persons with tetraplegia. Lung. 2006;184(6):335–339 [DOI] [PubMed] [Google Scholar]
- 187.Faghri PD, Yount JP, Pesce WJ, Seetharama S, Votto JJ.Circulatory hypokinesis and functional electric stimulation during standing in persons with spinal cord injury. Arch Phys Med Rehabil. 2001;82(11):1587–1595 [DOI] [PubMed] [Google Scholar]
- 188.Takahashi M, Sakaguchi A, Matsukawa K, Komine H, Kawaguchi K, Onari K.Cardiovascular control during voluntary static exercise in humans with tetraplegia. J Appl Physiol. 2004;97(6):2077–2082 [DOI] [PubMed] [Google Scholar]
- 189.Wecht JM, Weir JP, Bauman WA.Blunted heart rate response to vagal withdrawal in persons with tetraplegia. Clin Auton Res. 2006;16(6):378–383 [DOI] [PubMed] [Google Scholar]
- 190.Myers JN, Hsu L, Hadley D, Lee MY, Kiratli BJ.Postexercise heart rate recovery in individuals with spinal cord injury. Spinal Cord. 2010;48(8):639–644 [DOI] [PubMed] [Google Scholar]
- 191.Rosado-Rivera D, Radulovic M, Handrakis JP, et al. Comparison of 24-hour cardiovascular and autonomic function in paraplegia, tetraplegia, and control groups: implications for cardiovascular risk. J Spinal Cord Med. 2011;34(4):395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Palatini P.Elevated heart rate in cardiovascular diseases: A target for treatment? Prog Cardiovasc Dis. 2009;52(1):46–60 [DOI] [PubMed] [Google Scholar]
- 193.Tomiyama H, Hashimoto H, Tanaka H, et al. Synergistic relationship between changes in the pulse wave velocity and changes in the heart rate in middle-aged Japanese adults: a prospective study. J Hypertension. 2010;28(4):687–694 [DOI] [PubMed] [Google Scholar]
- 194.Wecht JM, Weir JP, DeMeersman RE, Spungen AM, Bauman WA.Arterial stiffness in persons with paraplegia. J Spinal Cord Med. 2004;27(3):255–259 [DOI] [PubMed] [Google Scholar]
- 195.Miyatani M, Masani K, Oh PI, Miyachi M, Popovic MR, Craven BC.Pulse wave velocity for assessment of arterial stiffness among people with spinal cord injury: a pilot study. J Spinal Cord Med. 2009;32(1):72–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Claydon VE, Krassioukov AV.Orthostatic hypotension and autonomic pathways after spinal cord injury. J Neurotrauma. 2006;23(12):1713–1725 [DOI] [PubMed] [Google Scholar]
- 197.Claydon VE, Steeves JD, Krassioukov A.Orthostatic hypotension following spinal cord injur y: understanding clinical pathophysiology. Spinal Cord. 2006;44(6):341–351 [DOI] [PubMed] [Google Scholar]
- 198.Krassioukov A, Eng JJ, Warburton DE, Teasell R.A systematic review of the management of orthostatic hypotension after spinal cord injury. Arch Phys Med Rehabil. 2009;90(5):876–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wecht JM, Radulovic M, Rosado-Rivera D, Zhang RL, Lafountaine MF, Bauman WA.Orthostatic effects of midodrine versus L-NAME on cerebral blood flow and the renin-angiotensin-aldosterone system in tetraplegia. Arch Phys Med Rehabil. 2011;92(11):1789–1795 [DOI] [PubMed] [Google Scholar]
- 200.Wecht JM, Rosado-Rivera D, Handrakis JP, Radulovic M, Bauman WA.Effects of midodrine hydrochloride on blood pressure and cerebral blood flow during orthostasis in persons with chronic tetraplegia. Arch Phys Med Rehabil. 2010;91(9):1429–1435 [DOI] [PubMed] [Google Scholar]
- 201.Wecht JM, Weir JP, Goldstein DS, et al. Direct and reflexive effects of nitric oxide synthase inhibition on blood pressure. Am J Physiol Heart Circ Physiol. 2008;294(1):H190–197 [DOI] [PubMed] [Google Scholar]
- 202.Wecht JM, Radulovic M, Lafountaine MF, Rosado-Rivera D, Zhang RL, Bauman WA.Orthostatic responses to nitric oxide synthase inhibition in persons with tetraplegia. Arch Phys Med Rehabil. 2009;90(8):1428–1434 [DOI] [PubMed] [Google Scholar]
- 203.Duschek S, Matthias E, Schandry R.Essential hypotension is accompanied by deficits in attention and working memory. Behav Med. 2005;30(4):149–158 [DOI] [PubMed] [Google Scholar]
- 204.Duschek S, Schandry R.Cognitive performance and cerebral blood flow in essential hypotension. Psychophysiology. 2004;41(6):905–913 [DOI] [PubMed] [Google Scholar]
- 205.Duschek S, Schandry R.Reduced brain perfusion and cognitive performance due to constitutional hypotension. Clin Auton Res. 2007;17(2):69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Duschek S, Weisz N, Schandry R.Reduced cognitive performance and prolonged reaction time accompany moderate hypotension. Clin Auton Res. 2003;13(6):427–432 [DOI] [PubMed] [Google Scholar]
- 207.Davidoff G, Morris J, Roth E, Bleiberg J.Cognitive dysfunction and mild closed head injury in traumatic spinal cord injury. Arch Phys Med Rehabil. 1985;66(8):489–491 [PubMed] [Google Scholar]
- 208.Davidoff G, Roth E, Thomas P, et al. Depression and neuropsychological test performance in acute spinal cord injury patients: lack of correlation. Arch Clin Neuropsychol. 1990;5(1):77–88 [PubMed] [Google Scholar]
- 209.Davidoff GN, Roth EJ, Haughton JS, Ardner MS.Cognitive dysfunction in spinal cord injury patients: sensitivity of the Functional Independence Measure subscales vs neuropsychologic assessment. Arch Phys Med Rehabil. 1990;71(5):326–329 [PubMed] [Google Scholar]
- 210.Roth E, Davidoff G, Thomas P, et al. A controlled study of neuropsychological deficits in acute spinal cord injury patients. Paraplegia. 1989;27(6):480–489 [DOI] [PubMed] [Google Scholar]
- 211.Wilmot CB, Cope DN, Hall KM, Acker M.Occult head injury: its incidence in spinal cord injury. Arch Phys Med Rehabil. 1985;66(4):227–231 [DOI] [PubMed] [Google Scholar]
- 212.Dowler RN, O’Brien SA, Haaland KY, Harrington DL, Feel F, Fiedler K.Neuropsychological functioning following a spinal cord injury. Appl Neuropsychol. 1995;2(3-4):124–129 [DOI] [PubMed] [Google Scholar]
- 213.Richards JS, Brown L, Hagglund K, Bua G, Reeder K.Spinal cord injury and concomitant traumatic brain injury. Results of a longitudinal investigation. Am J Phys Med Rehabil. 1988;67(5):211–216 [DOI] [PubMed] [Google Scholar]
- 214.Davidoff G, Thomas P, Johnson M, Berent S, Dijkers M, Doljanac R.Closed head injury in acute traumatic spinal cord injury: incidence and risk factors. Arch Phys Med Rehabil. 1988;69(10):869–872 [PubMed] [Google Scholar]
- 215.Dowler RN, Harrington DL, Haaland KY, Swanda RM, Fee F, Fiedler K.Profiles of cognitive functioning in chronic spinal cord injury and the role of moderating variables. J Int Neuropsychol Soc. 1997;3(5):464–472 [PubMed] [Google Scholar]
- 216.Jegede AB, Rosado-Rivera D, Bauman WA, et al. Cognitive performance in hypotensive persons with spinal cord injury Clin Auton Res. 2010;20(1):3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Houtman S, Oeseburg B, Hughson RL, Hopman MT.Sympathetic nervous system activity and cardiovascular homeostatis during head-up tilt in patients with spinal cord injuries. Clin Auton Res. 2000;10(4):207–212 [DOI] [PubMed] [Google Scholar]
- 218.Jacobs PL, Mahoney ET, Robbins A, Nash M.Hypokinetic circulation in persons with paraplegia. Med Sci Sports Exerc. 2002;34(9):1401–1407 [DOI] [PubMed] [Google Scholar]
- 219.Schmid A, Huonker M, Barturen JM, et al. Catecholamines, heart rate, and oxygen uptake during exercise in persons with spinal cord injury. J Appl Physiol. 1998;85(2):635–641 [DOI] [PubMed] [Google Scholar]
- 220.Triantafyllidi H, Arvaniti C, Lekakis J, et al. Cognitive impairment is related to increased arterial stiffness and microvascular damage in patients with nevertreated essential hypertension. Am J Hypertension. 2009;22(5):525–530 [DOI] [PubMed] [Google Scholar]
- 221.Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB.Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension. 2008;51(1):99–104 [DOI] [PubMed] [Google Scholar]
- 222.Wecht JM, Rosado-Rivera D, Jegede A, et al. Systemic and cerebral hemodynamics during cognitive testing. Clin Auton Res. 2012;22(1):2533. [DOI] [PubMed] [Google Scholar]