Abstract
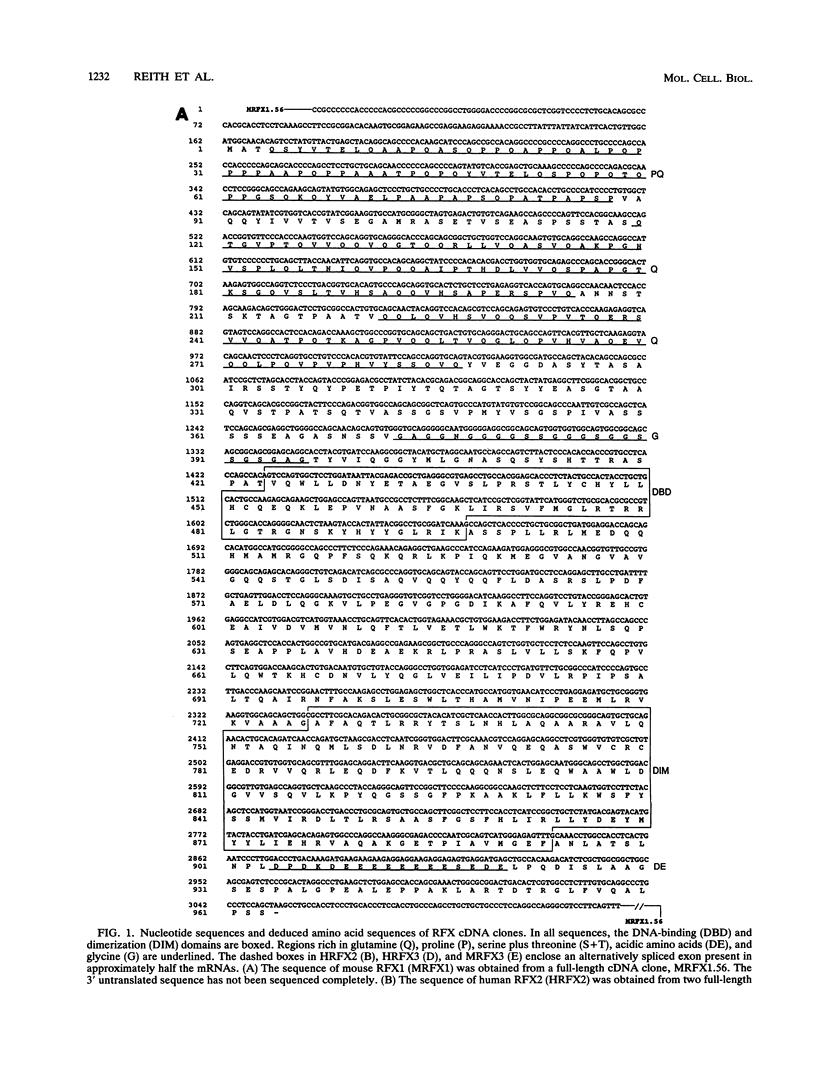
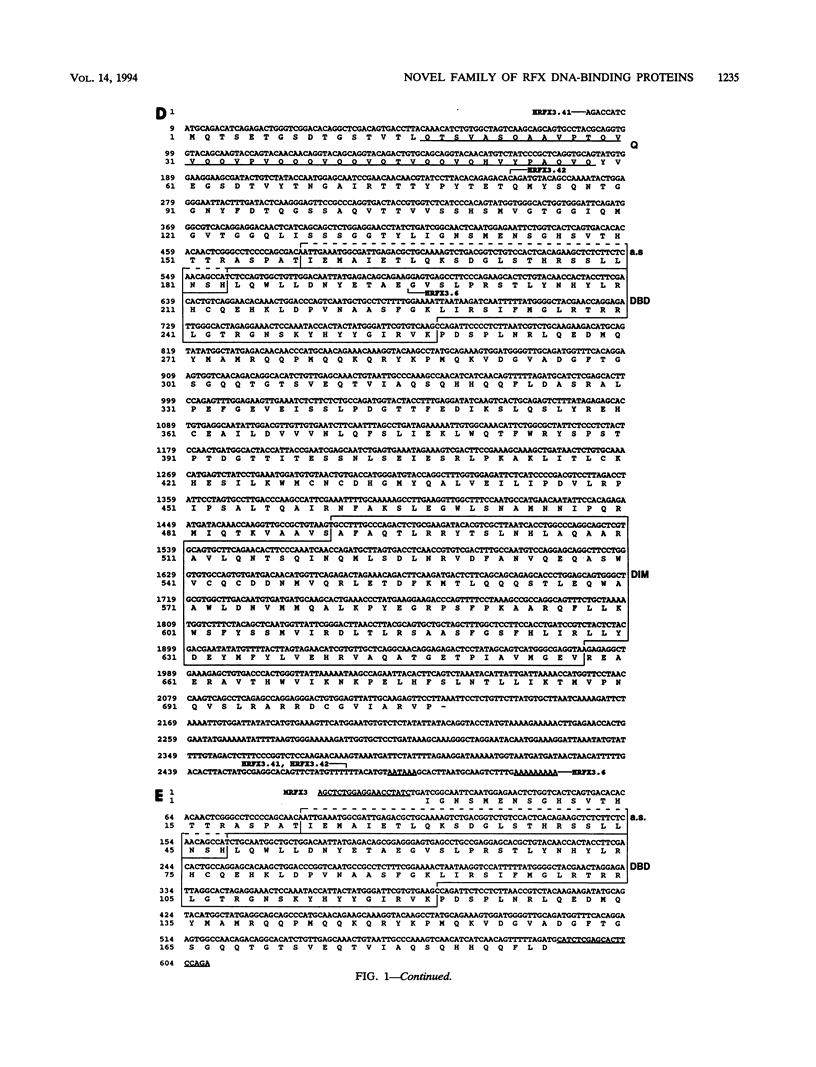
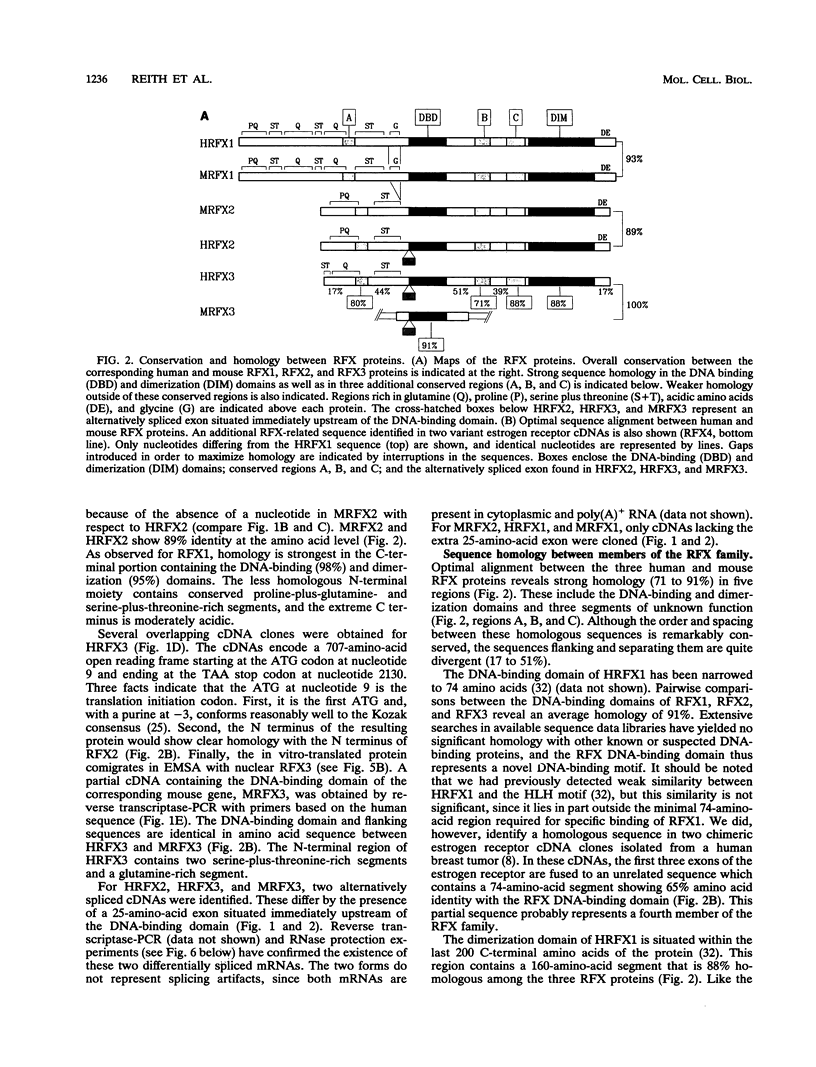
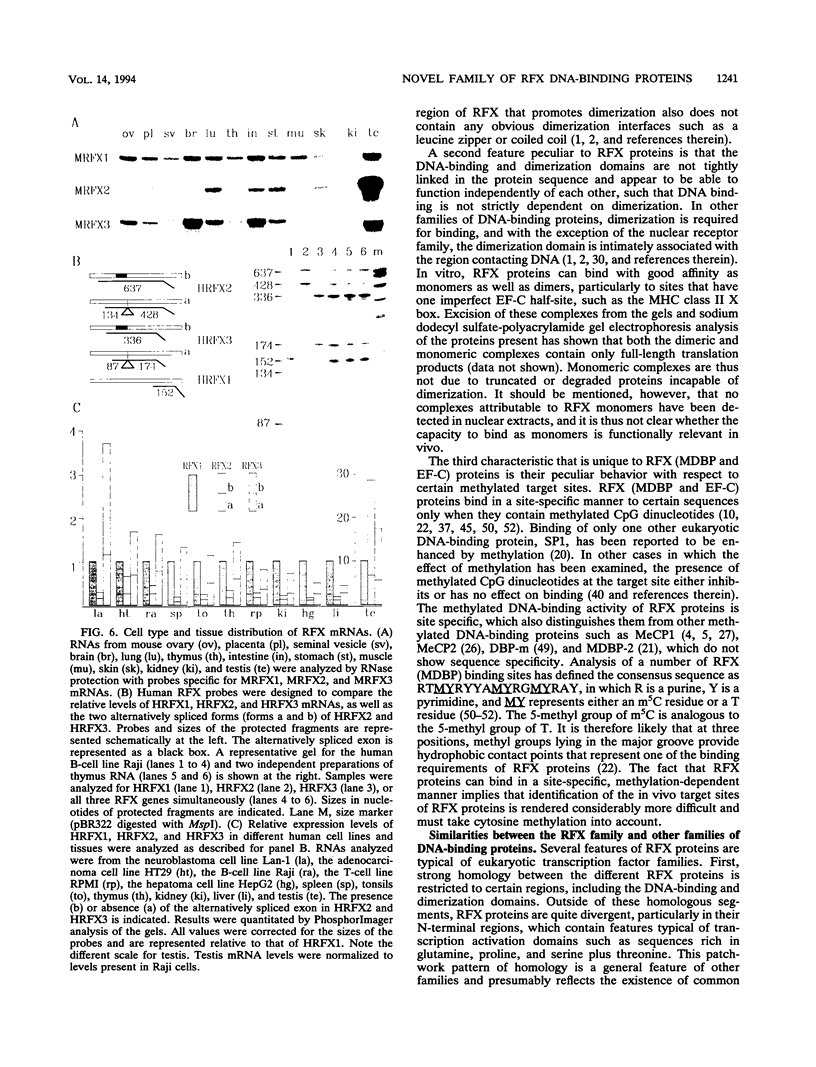
RFX1 is a transactivator of human hepatitis B virus enhancer I. We show here that RFX1 belongs to a previously unidentified family of DNA-binding proteins of which we have cloned three members, RFX1, RFX2, and RFX3, from humans and mice. Members of the RFX family constitute the nuclear complexes that have been referred to previously as enhancer factor C, EP, methylation-dependent DNA-binding protein, or rpL30 alpha. RFX proteins share five strongly conserved regions which include the two domains required for DNA binding and dimerization. They have very similar DNA-binding specificities and heterodimerize both in vitro and in vivo. mRNA levels for all three genes, particularly RFX2, are elevated in testis. In other cell lines and tissues, RFX mRNA levels are variable, particularly for RFX2 and RFX3. RFX proteins share several novel features, including new DNA-binding and dimerization motifs and a peculiar dependence on methylated CpG dinucleotides at certain sites.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alber T. Structure of the leucine zipper. Curr Opin Genet Dev. 1992 Apr;2(2):205–210. doi: 10.1016/s0959-437x(05)80275-8. [DOI] [PubMed] [Google Scholar]
- Baxevanis A. D., Vinson C. R. Interactions of coiled coils in transcription factors: where is the specificity? Curr Opin Genet Dev. 1993 Apr;3(2):278–285. doi: 10.1016/0959-437x(93)90035-n. [DOI] [PubMed] [Google Scholar]
- Benoist C., Mathis D. Regulation of major histocompatibility complex class-II genes: X, Y and other letters of the alphabet. Annu Rev Immunol. 1990;8:681–715. doi: 10.1146/annurev.iy.08.040190.003341. [DOI] [PubMed] [Google Scholar]
- Boyes J., Bird A. DNA methylation inhibits transcription indirectly via a methyl-CpG binding protein. Cell. 1991 Mar 22;64(6):1123–1134. doi: 10.1016/0092-8674(91)90267-3. [DOI] [PubMed] [Google Scholar]
- Boyes J., Bird A. Repression of genes by DNA methylation depends on CpG density and promoter strength: evidence for involvement of a methyl-CpG binding protein. EMBO J. 1992 Jan;11(1):327–333. doi: 10.1002/j.1460-2075.1992.tb05055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dikstein R., Faktor O., Ben-Levy R., Shaul Y. Functional organization of the hepatitis B virus enhancer. Mol Cell Biol. 1990 Jul;10(7):3683–3689. doi: 10.1128/mcb.10.7.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotzlaw H., Alkhalaf M., Murphy L. C. Characterization of estrogen receptor variant mRNAs from human breast cancers. Mol Endocrinol. 1992 May;6(5):773–785. doi: 10.1210/mend.6.5.1603086. [DOI] [PubMed] [Google Scholar]
- Faisst S., Meyer S. Compilation of vertebrate-encoded transcription factors. Nucleic Acids Res. 1992 Jan 11;20(1):3–26. doi: 10.1093/nar/20.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia A. D., Ostapchuk P., Hearing P. Functional interaction of nuclear factors EF-C, HNF-4, and RXR alpha with hepatitis B virus enhancer I. J Virol. 1993 Jul;67(7):3940–3950. doi: 10.1128/jvi.67.7.3940-3950.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia A. D., Ostapchuk P., Hearing P. Methylation-dependent and -independent DNA binding of nuclear factor EF-C. Virology. 1991 Jun;182(2):857–860. doi: 10.1016/0042-6822(91)90629-p. [DOI] [PubMed] [Google Scholar]
- Ghazal P., Lubon H., Hennighausen L. Multiple sequence-specific transcription factors modulate cytomegalovirus enhancer activity in vitro. Mol Cell Biol. 1988 Apr;8(4):1809–1811. doi: 10.1128/mcb.8.4.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher L. H., Kara C. J. Sequences and factors: a guide to MHC class-II transcription. Annu Rev Immunol. 1992;10:13–49. doi: 10.1146/annurev.iy.10.040192.000305. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Guo W. T., Bell K. D., Ou J. H. Characterization of the hepatitis B virus EnhI enhancer and X promoter complex. J Virol. 1991 Dec;65(12):6686–6692. doi: 10.1128/jvi.65.12.6686-6692.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa S. L., Sloan J. H., Reith W., Mach B., Boss J. M. Regulatory factor-X binding to mutant HLA-DRA promoter sequences. Nucleic Acids Res. 1991 Mar 25;19(6):1243–1249. doi: 10.1093/nar/19.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero Sanchez C., Reith W., Silacci P., Mach B. The DNA-binding defect observed in major histocompatibility complex class II regulatory mutants concerns only one member of a family of complexes binding to the X boxes of class II promoters. Mol Cell Biol. 1992 Sep;12(9):4076–4083. doi: 10.1128/mcb.12.9.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L. H., Wang R., Gama-Sosa M. A., Shenoy S., Ehrlich M. A protein from human placental nuclei binds preferentially to 5-methylcytosine-rich DNA. Nature. 1984 Mar 15;308(5956):293–295. doi: 10.1038/308293a0. [DOI] [PubMed] [Google Scholar]
- Jane S. M., Gumucio D. L., Ney P. A., Cunningham J. M., Nienhuis A. W. Methylation-enhanced binding of Sp1 to the stage selector element of the human gamma-globin gene promoter may regulate development specificity of expression. Mol Cell Biol. 1993 Jun;13(6):3272–3281. doi: 10.1128/mcb.13.6.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost J. P., Hofsteenge J. The repressor MDBP-2 is a member of the histone H1 family that binds preferentially in vitro and in vivo to methylated nonspecific DNA sequences. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9499–9503. doi: 10.1073/pnas.89.20.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan R., Zhang X. Y., Supakar P. C., Ehrlich K. C., Ehrlich M. Human methylated DNA-binding protein. Determinants of a pBR322 recognition site. J Biol Chem. 1988 Oct 5;263(28):14374–14383. [PubMed] [Google Scholar]
- Kobr M., Reith W., Herrero-Sanchez C., Mach B. Two DNA-binding proteins discriminate between the promoters of different members of the major histocompatibility complex class II multigene family. Mol Cell Biol. 1990 Mar;10(3):965–971. doi: 10.1128/mcb.10.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouskoff V., Mantovani R. M., Candéias S. M., Dorn A., Staub A., Lisowska-Grospierre B., Griscelli C., Benoist C. O., Mathis D. J. NF-X, a transcription factor implicated in MHC class II gene regulation. J Immunol. 1991 May 1;146(9):3197–3204. [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. D., Meehan R. R., Henzel W. J., Maurer-Fogy I., Jeppesen P., Klein F., Bird A. Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell. 1992 Jun 12;69(6):905–914. doi: 10.1016/0092-8674(92)90610-o. [DOI] [PubMed] [Google Scholar]
- Meehan R. R., Lewis J. D., McKay S., Kleiner E. L., Bird A. P. Identification of a mammalian protein that binds specifically to DNA containing methylated CpGs. Cell. 1989 Aug 11;58(3):499–507. doi: 10.1016/0092-8674(89)90430-3. [DOI] [PubMed] [Google Scholar]
- Ostapchuk P., Diffley J. F., Bruder J. T., Stillman B., Levine A. J., Hearing P. Interaction of a nuclear factor with the polyomavirus enhancer region. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8550–8554. doi: 10.1073/pnas.83.22.8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostapchuk P., Scheirle G., Hearing P. Binding of nuclear factor EF-C to a functional domain of the hepatitis B virus enhancer region. Mol Cell Biol. 1989 Jul;9(7):2787–2797. doi: 10.1128/mcb.9.7.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Transcription factors: structural families and principles of DNA recognition. Annu Rev Biochem. 1992;61:1053–1095. doi: 10.1146/annurev.bi.61.070192.005201. [DOI] [PubMed] [Google Scholar]
- Reith W., Barras E., Satola S., Kobr M., Reinhart D., Sanchez C. H., Mach B. Cloning of the major histocompatibility complex class II promoter binding protein affected in a hereditary defect in class II gene regulation. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4200–4204. doi: 10.1073/pnas.86.11.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith W., Herrero-Sanchez C., Kobr M., Silacci P., Berte C., Barras E., Fey S., Mach B. MHC class II regulatory factor RFX has a novel DNA-binding domain and a functionally independent dimerization domain. Genes Dev. 1990 Sep;4(9):1528–1540. doi: 10.1101/gad.4.9.1528. [DOI] [PubMed] [Google Scholar]
- Reith W., Satola S., Sanchez C. H., Amaldi I., Lisowska-Grospierre B., Griscelli C., Hadam M. R., Mach B. Congenital immunodeficiency with a regulatory defect in MHC class II gene expression lacks a specific HLA-DR promoter binding protein, RF-X. Cell. 1988 Jun 17;53(6):897–906. doi: 10.1016/s0092-8674(88)90389-3. [DOI] [PubMed] [Google Scholar]
- Shapiro D. J., Sharp P. A., Wahli W. W., Keller M. J. A high-efficiency HeLa cell nuclear transcription extract. DNA. 1988 Jan-Feb;7(1):47–55. doi: 10.1089/dna.1988.7.47. [DOI] [PubMed] [Google Scholar]
- Sáfrány G., Perry R. P. Transcription factor RFX1 helps control the promoter of the mouse ribosomal protein-encoding gene rpL30 by binding to its alpha element. Gene. 1993 Oct 15;132(2):279–283. doi: 10.1016/0378-1119(93)90208-k. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate P. H., Bird A. P. Effects of DNA methylation on DNA-binding proteins and gene expression. Curr Opin Genet Dev. 1993 Apr;3(2):226–231. doi: 10.1016/0959-437x(93)90027-m. [DOI] [PubMed] [Google Scholar]
- Ting J. P., Baldwin A. S. Regulation of MHC gene expression. Curr Opin Immunol. 1993 Feb;5(1):8–16. doi: 10.1016/0952-7915(93)90074-3. [DOI] [PubMed] [Google Scholar]
- Trujillo M. A., Letovsky J., Maguire H. F., Lopez-Cabrera M., Siddiqui A. Functional analysis of a liver-specific enhancer of the hepatitis B virus. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3797–3801. doi: 10.1073/pnas.88.9.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng R. W., Fujimura F. K. Multiple domains in the polyomavirus B enhancer are required for productive infection of F9 embryonal carcinoma cells. J Virol. 1988 Aug;62(8):2890–2895. doi: 10.1128/jvi.62.8.2890-2895.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldman G. M., Lupton S., Kamen R. Polyomavirus enhancer contains multiple redundant sequence elements that activate both DNA replication and gene expression. Mol Cell Biol. 1985 Apr;5(4):649–658. doi: 10.1128/mcb.5.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. Y., Zhang X. Y., Khan R., Zhou Y. W., Huang L. H., Ehrlich M. Methylated DNA-binding protein from human placenta recognizes specific methylated sites on several prokaryotic DNAs. Nucleic Acids Res. 1986 Dec 22;14(24):9843–9860. doi: 10.1093/nar/14.24.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann L. M., Perry R. P. Characterization of the expressed gene and several processed pseudogenes for the mouse ribosomal protein L30 gene family. Mol Cell Biol. 1984 Nov;4(11):2518–2528. doi: 10.1128/mcb.4.11.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac-Kaye M., Gelmann E. P., Levens D. A point mutation in the c-myc locus of a Burkitt lymphoma abolishes binding of a nuclear protein. Science. 1988 Jun 24;240(4860):1776–1780. doi: 10.1126/science.2454510. [DOI] [PubMed] [Google Scholar]
- Zajac-Kaye M., Levens D. Phosphorylation-dependent binding of a 138-kDa myc intron factor to a regulatory element in the first intron of the c-myc gene. J Biol Chem. 1990 Mar 15;265(8):4547–4551. [PubMed] [Google Scholar]
- Zhang D. L., Ehrlich K. C., Supakar P. C., Ehrlich M. A plant DNA-binding protein that recognizes 5-methylcytosine residues. Mol Cell Biol. 1989 Mar;9(3):1351–1356. doi: 10.1128/mcb.9.3.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. Y., Asiedu C. K., Supakar P. C., Khan R., Ehrlich K. C., Ehrlich M. Binding sites in mammalian genes and viral gene regulatory regions recognized by methylated DNA-binding protein. Nucleic Acids Res. 1990 Nov 11;18(21):6253–6260. doi: 10.1093/nar/18.21.6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. Y., Inamdar N. M., Supakar P. C., Wu K., Ehrlich K. C., Ehrlich M. Three MDBP sites in the immediate-early enhancer-promoter region of human cytomegalovirus. Virology. 1991 Jun;182(2):865–869. doi: 10.1016/0042-6822(91)90631-k. [DOI] [PubMed] [Google Scholar]
- Zhang X. Y., Supakar P. C., Khan R., Ehrlich K. C., Ehrlich M. Related sites in human and herpesvirus DNA recognized by methylated DNA-binding protein from human placenta. Nucleic Acids Res. 1989 Feb 25;17(4):1459–1474. doi: 10.1093/nar/17.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. Y., Supakar P. C., Wu K. Z., Ehrlich K. C., Ehrlich M. An MDBP site in the first intron of the human c-myc gene. Cancer Res. 1990 Nov 1;50(21):6865–6869. [PubMed] [Google Scholar]