Abstract
The tetratricopeptide repeat (TPR) structural motif is known to occur in a wide variety of proteins present in prokaryotic and eukaryotic organisms. The TPR motif represents an elegant module for the assembly of various multiprotein complexes, and thus, TPR-containing proteins often play roles in vital cell processes. As the TPR profile is well defined, the complete TPR protein repertoire of a bacterium with a known genomic sequence can be predicted. This provides a tremendous opportunity for investigators to identify new TPR-containing proteins and study them in detail. In the past decade, TPR-containing proteins of bacterial pathogens have been reported to be directly related to virulence-associated functions. In this minireview, we summarize the current knowledge of the TPR-containing proteins involved in virulence mechanisms of bacterial pathogens while highlighting the importance of TPR motifs for the proper functioning of class II chaperones of a type III secretion system in the pathogenesis of Yersinia, Pseudomonas, and Shigella.
INTRODUCTION
The tetratricopeptide repeat (TPR) structural motif was originally identified in yeasts (1, 2). Now the TPR motif is known to occur ubiquitously, as it has been found in a wide variety of functionally unrelated proteins (more than 20,000 are included in the SMART nrdb database) from all genera.
TPR motifs are minimally conserved (degenerate and variable) 34-residue-long regions. The TPR sequence is centered around the consensus residues W4-L7-G8-Y11-A20-F24-A27-P32 (3). Although no positions are completely invariant, a consensus sequence pattern of small and large hydrophobic residues has been defined; small hydrophobic residues are commonly observed at positions 8, 20, and 27, while large ones are at 4, 7, and 24 (3, 4). Three-dimensional structural data have shown that the TPR motif is a relatively simple solenoid containing two antiparallel α-helical subdomains (termed helix A and helix B) that are equivalent in length. The arrangement in tandem arrays of 3 to 16 TPRs generates a right-handed superhelical structure (Fig. 1) with an amphipathic channel that can accommodate the complementary region of a target protein, as originally described by Das et al. for protein phosphatase 5 (PP5) (5). The interesting issue of possible differences between prokaryotic and eukaryotic TPR domains was discussed in a review by D'Andrea et al. (4); those authors concluded that there are no significant differences in either amino acid preference or sequence consensus (4). Moreover, recent structural studies of the prokaryotic TPR fold have demonstrated that it corresponds to the known structures of eukaryotic TPR folds (6–9).
Fig 1.
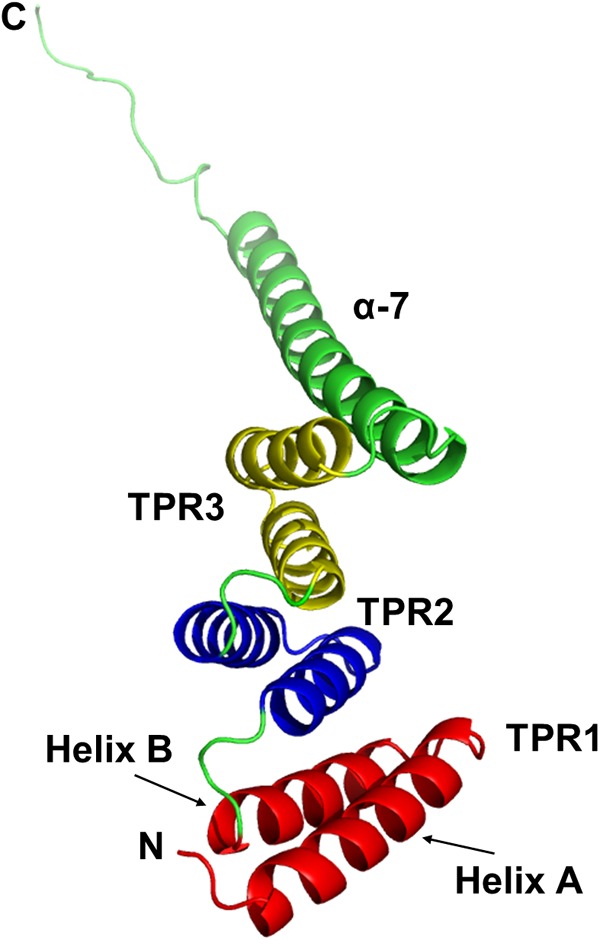
Ribbon diagram of the TPR domain of PP5. PP5 was the first TPR-containing protein whose structure was determined by crystallography (5). We adopted this protein to illustrate the structure of the TPR-containing proteins. PP5 contains three tandem TPR motifs (TPR1 to TPR3), which are depicted in different colors. Each TPR motif is composed of a pair of antiparallel α-helices, termed helices A and B. The structures of all of the TPR motifs are virtually identical. The three TPR motifs are organized in a parallel arrangement, such that sequentially adjacent α-helices are antiparallel. The 35 residues C terminal to the three TPR motifs of PP5 are folded into an extended α-helix (α-7). This helix is packed against helix B of TPR3 in an arrangement similar to that of the helices within the TPR domain. The consequence of the regular repetition of such α-helices is the formation of a right-handed helical conformation that creates an amphipathic channel (5). The image shown was prepared with the PyMOL Molecular Graphics System, version 1.3, Schrödinger, LLC. (Adapted from reference 5 by permission from Nature Publishing Group.)
The basic function of TPR folds is to mediate protein-protein interactions, in which TPR folds reveal a certain level of “promiscuity.” The same fold within different proteins can bind many distinct types of ligands (10). The specificity of the interaction between a TPR domain and its cognate substrate is likely determined by amino acid residues other than the eight TPR consensus residues (11). Thus, in general, the TPR can be seen as a scaffold whose fold is defined by the consensus sequence onto which specific ligand-binding residues are grafted.
The TPR functions in a variety of proteins involved in numerous cell processes, such as gene regulation, mitosis, regulation of steroid receptor function, and protein import (4). The importance of TPR-containing proteins is highlighted by the fact that mutations of the TPR region have possible implications for human diseases, including polycystic ovary syndrome (12), the severe inherited retinopathy known as Leber congenital amaurosis (13), chronic granulomatous disease (14), and Down syndrome (15).
TPR DOMAINS AND PROTEIN-PROTEIN INTERACTIONS
Ligand binding takes place on the concave surface formed by the side chains of amino acids of helix A of the TPR domain (5, 16, 17) as depicted in Fig. 2, although exceptions exist where ligands can make contact with the convex side of the helical array (6, 18). The TPR domain largely recognizes an extended conformation of the target peptide. Upon ligand binding, no substantial structural changes in the TPR folds appear (19).
Fig 2.
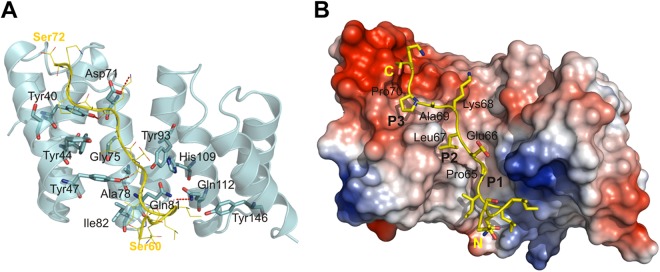
TPR-containing proteins accommodate their cognate substrates in the concave cleft. For illustration, we adopted the interaction between the TTSS class II chaperone of Shigella, IpgC, and the chaperone binding motif of its cognate substrate, IpaB. IpgC binds the chaperone binding domain in an extended conformation that is stabilized by conserved residues present in the cleft. (A) Diagram representing IpgC and a yellow ribbon model of the chaperone binding domain of IpaB. The residues involved in the intermolecular interactions are shown. The H bonds (between the carbonyl of Ile-62, Pro-65, and Lys-68 in the IpaB peptide and the amide of Gln-112 and the hydroxyl of Tyr-47 and Tyr-40 in IpgC) and one salt bridge (Lys-68 in the IpaB peptide and Asp-71 in IpgC) are emphasized as dashed red lines. (B) Same view as in panel A showing a surface representation of IpgC in the cocrystal. The surface is colored according to the electrostatic potential calculated without water and ligand (blue, positive; red, negative). The chaperone binding domain of IpaB (yellow) lining the major groove of IpgC and the three pockets (P1 to P3) interacting with side chains of conserved residues Pro-65, Leu-67, and Pro-70 in the IpaB peptide via hydrophobic and van der Waals interactions, P1, P2, and P3, are indicated. The 65PELKAP70 residues of the IpaB peptide are labeled. (Adapted from reference 9 with the permission of the publisher.)
The TPR domains discriminate between similar ligands in a specific manner, as seen, for example, in the case of the TPR1 and TPR2A domains of Hop (Hsp70-Hsp90 organizing protein), which bind specifically to the C-terminal peptides of Hsp70 and Hsp90, respectively; approximately 10-fold weaker binding was reported in cross-partner binding assays (17, 20). Interactions between Hsp90/Hsp70 and their TPR-containing cochaperones have been widely studied, and thermodynamic dissociation constant (KD) values have been determined; for instance, the TPR2A domain of Hop binds Hsp90 with a KD of 3 ± 2 μM and the TPR1 domain of Hop binds Hsp70 with a KD of 18 ± 8 μM (21). The KD values for the interactions of full-length Hop with Hsp90 and Hsp70 are 1 ± 0.5 and 2 ± 1 μM, respectively (21). Similarly, the KD value for the interaction between Pih1 (Hsp90-interacting cochaperone identified in yeasts) and Hsp90 is 1.3 μM (22) and the KD for the interaction of PP5 and the C-terminal peptide of Hsp90 has been determined to be 660 nM (19).
Most of the studies performed to date have focused on heterocomplexes formed between TPR domains and their ligands. However, it has recently been found that some TPR proteins not only bind heterologous ligands but can also self-assemble into higher-order structures (6, 8, 9, 18, 23–25). This phenomenon seems to be relevant for the fine-tuning of biological processes, since the absence of oligomerization can fatally affect the TPR-containing protein-associated function (6, 9, 23). Although the biological relevance of TPR-mediated oligomerization seems to be apparent, it is still largely unclear whether the interfaces formed between TPR subunits are similar to or distinct from those seen in heterocomplexes.
It has been proposed that TPR-containing proteins dimerize mainly via the convex outer side formed by TPR motifs in the middle of the repeat domain as observed in the crystal structures of human O-linked N-acetylglucosamine transferase (1w3b.pdb) (26), Pseudomonas aeruginosa type 4 pilus protein PilF (2fi7.pdb) (8), and the mitochondrial outer membrane transporter Tom70 (2gw1.pdb) (27). However, whether the interfaces formed in these dimers are biologically relevant has not been confirmed. In addition, a few cases of self-assembling TPR-containing proteins exist where dimer crystal structures do not correspond to biologically relevant arrangements in solution (23).
To address this issue, Krachler et al. introduced Tyr39, Tyr73, and Tyr107 instead of Asp into the TPR three-repeat consensus protein CTPR3 (1na0.pdb) (28, 29), producing CTPR3Y3 (2wqh.pdb) (23). Using this protein construct, the authors suggested a possible mode of assembly for higher TPR-mediated structures, where both the crystal structure and the biologically relevant arrangement of homodimers are in agreement. They observed two distinct classes of interfaces in the case of TPR-mediated homo-oligomerization, none of which involves the TPR domain inner groove. Moreover, they described the displacement of the “capping” helix by competing interactions between TPR subunits as the mechanism of general applicability and relevant for proteins such as Sgt1 (regulator of innate immunity in plants and required for kinetochore assembly in yeast), Fis1 (regulates mitochondrial morphology and fission), and YbgF from Escherichia coli, a protein linked to the Tol system in Gram-negative bacteria (23).
WEB-BASED RESOURCES FOR TPR PREDICTION
The TPR profile is well defined, and thus, the complete repertoire of TPR-containing proteins of organisms with known genomic sequences can be predicted. This provides an opportunity for investigators to identify and study new TPR-containing proteins, including those involved in bacterial pathogenesis. Several web-based resources can be employed to predict TPR-containing proteins, including Pfam (30), SMART (31), and TPRpred (32). Pfam and SMART use hidden Markov model profiles or sequence profiles that are constructed from the repeats believed to belong to the family (30, 31). TPRpred is a profile-sequence comparison method for predicting TPRs and closely related solenoid structural motifs, pentatricopeptide repeats, and Sel1-like repeats (32). TPRpred exploits a P value-dependent score offset to include divergent repeat units, and it exploits the tendency of repeats to occur in tandem (32).
As these web-based tool kits use different algorithms, certain discrepancies in TPR motif prediction in a single protein can appear (Fig. 3). This should be taken into account when utilizing these web-based tool kits.
Fig 3.
Localization of TPR motifs identified by crystallography and/or predicted utilizing the web-based tool kits Pfam (30), SMART (31), and TPRpred (32). Protein names, bacterial origins, and UniProt database accession numbers are shown in columns 1 to 3, respectively. The motifs determined by the particular web-based tool kits are depicted in specific colors. Numbers refer to the first and last residues of the structural units.
TPR-CONTAINING PROTEINS AS BACTERIAL VIRULENCE FACTORS
In the case of bacterial pathogens, TPR-containing proteins have been found to be directly involved in virulence-associated functions, such as the translocation of virulence factors into host cells, adhesion to host cells, and blocking of phagolysosomal maturation (33–36).
Class II chaperones of the type III secretion system (TTSS) are the most-studied TPR-containing proteins related to bacterial pathogenesis (6, 7, 9, 37). Class II chaperones are required for the recognition and presecretory stabilization of two hydrophobic proteins known as translocators. The translocators have been shown to make up a pore in the eukaryotic cell membrane, allowing bacterium-specific effectors to enter the host cell (38). TPR-containing proteins are also essential components for type IV pilus (Tfp) biogenesis. Pili are multifunctional, flexible, filamentous appendages that have been assigned specific virulence traits in several important pathogens. These functions include adhesion, twitching motility, biofilm formation, and competence for DNA transformation (39).
The following section summarizes the current knowledge of the TPR-containing proteins needed for bacterial virulence mechanisms while focusing on the importance of TPR motifs for the proper functioning of class II chaperones of a TTSS in the pathogenesis of Yersinia (LcrH), Pseudomonas (PcrH), and Shigella (IpgC). As TPR motifs are not restricted only to class II chaperones of a TTSS, we also provide salient points on proteins for which limited experimental data on TPR function and/or the TPR structure itself are available. These proteins include P. aeruginosa PilF, Mycobacterium tuberculosis PknG, Porphyromonas gingivalis TprA and PilF, FTL_0205, ComL, and its ortholog FTT1244c from Francisella species, representing potential targets for further studies of TPR motifs in bacterial pathogenesis. All of the TPR-containing virulence factors discussed are listed in Table 1.
Table 1.
Summary of the proteins reviewed
| Protein | Bacterium | Function |
|---|---|---|
| LcrH (SycD) | Genus Yersinia | Class II chaperone of TTSS; negative control of Yop synthesis (34) |
| PcrH | P. aeruginosa | Class II chaperone of TTSS (33, 40) |
| IpgC | Genus Shigella | Class II chaperone of TTSS; coactivator of transcription of virulence genes within MxiE regulon (41, 42) |
| PilF | P. aeruginosa | Tfp biogenesis (43) |
| PilF | F. tularensis | Tfp biogenesis (35) |
| TprA | P. gingivalis | Transduction of stress signals from the environment (affects expression of proteins participating in secretion) (44) |
| PknG | M. tuberculosis | Serine/threonine protein kinase G; inhibits phagosome-lysosome fusion (45) |
| ComL | F. novicida | Competence lipoprotein (needed for growth in macrophages) (46) |
| FTT1244c | F. tularensis subsp. tularensis SchuS4 | Conserved hypothetical protein (needed for growth in hepatic cells) (47) |
| FTL_0205 | F. tularensis subsp. holarctica LVS | Member of genetic locus required for stress resistance and intracellular survival (48) |
LcrH (also termed SycD) from the genus Yersinia.
The genus Yersinia includes three species that are pathogenic to rodents and humans: Yersinia pestis is the causative agent of plague, Y. pseudotuberculosis causes adenitis and septicemia, and Y. enterocolitica, the most prevalent Yersinia species in humans, causes a broad spectrum of gastrointestinal symptoms. In spite of their different routes of infection, these three species share a tropism for host lymphoid tissues and a common capacity to resist nonspecific immune responses (49).
Yersinia relies on a TTSS to deliver toxins directly into the cytosol of target cells. The Yersinia Yop (Yersinia outer protein) effector molecules interfere with signal transduction pathways that regulate the actin cytoskeleton, phagocytosis, apoptosis, and the inflammatory response, thereby favoring survival of the bacteria (50). LcrH, the TTSS class II chaperone from the Ysc-Yop system, binds the Yersinia translocators YopB and YopD (6, 34). Moreover, LcrH is involved in the regulation of Yop synthesis (i) by negative regulation through a complex with YopD and (ii) by the binding of YscY, a regulatory component of Yop synthesis (51).
Analysis of the LcrH monomer crystal structure revealed three TPR-like motifs (6) (Fig. 3). This is in accordance with the previously proposed structural model of the LcrH monomer, which had been created on the basis of the homology between LcrH and tandem TPRs whose structures are known (37). Furthermore, it has been revealed that LcrH can form two structurally different homodimers, i.e., asymmetric back-to-back dimerization via the convex face of the TPR involving TPR2 and the turns between repeats and, additionally, head-to-head dimer with an interface that includes mainly the A and B helices of the first TPR motif. On the basis of mutational analysis, it has been proposed that the biologically relevant LcrH homodimer corresponds to the head-to-head dimer (6). Dimerization seems to be indispensable for appropriate LcrH functioning within the Yersinia TTSS (6). Additional evidence of the importance of TPR motifs located in LcrH has been provided by Edqvist et al., who found that the substitution of canonical amino acids in the TPR significantly reduces chaperone stability and affects the ability of the LcrH to bind one or both translocators (34). Moreover, TPR is required for the interaction between LcrH and YscY. This finding further underlines the importance of TPR within LcrH (52).
PcrH from P. aeruginosa.
A ubiquitous opportunistic pathogen, P. aeruginosa is a versatile Gram-negative bacterium that causes life-threatening infections in patients with compromised immune systems. P. aeruginosa expresses a TTSS for the delivery of effector proteins into the cytoplasm of host cells. Four TTSS translocated effector proteins have been identified in P. aeruginosa. These proteins include the exoenzymes ExoS, ExoT, ExoU, and ExoY (53–56), which subvert host cell signaling pathways and lead to impaired phagocytic functioning in infected macrophages or induce apoptosis in other cell types, such as epithelial and fibroblast cells (57). The TTSS of P. aeruginosa is closely related to that found in Yersinia species at both the structural and functional levels. P. aeruginosa also expresses two translocators, PopB and PopD, that are absolutely essential for toxin translocation. These translocators share high similarity in both sequence and function with Yersinia YopB and YopD (approximately 40% amino acid identity) (58). PopB and PopD are recognized and stabilized within the bacterial cytoplasm of P. aeruginosa by a class II chaperone, PcrH (59).
Structural analysis of PcrH has shown that the overall structure displays a helical arrangement with three TPR units reminiscent of LcrH from Yersinia (7) (Fig. 3). While PcrH has been reported to exist in equilibrium between monomeric and dimeric species in the absence of a substrate, once bound to PopD, PcrH is stabilized as a monomer (7). Two potential biologically relevant dimeric arrangements have been described; the one with the most interactions, observed in the asymmetric unit, is formed by apposition of the convex surfaces of the monomers. A second potential dimeric arrangement was observed between monomers of different asymmetric units that interact through the N terminus. The latter displays more similarity to a biologically relevant head-to-head quaternary arrangement of LcrH (7).
A detailed study by Job et al. showed that four hydrophobic residues in the 49VELNAP54 region of PopD are fully required for an interaction with TPR-folded PcrH leading to the stabilization of PopD within the bacterial cytoplasm (7). Moreover, Bröms et al. reported that substitution of the canonical residues within all three TPR units (Leu-63, Gly-78, Ala-90, Ala-112, and Ala-131) results in a less stable PcrH chaperone, as well as in destabilized and not secreted PopB and PopD translocators (33). Thus, it has been well documented that the TPR domains of PcrH are responsible for the proper functioning of the TTSS of P. aeruginosa and for its virulence. However, PcrH, unlike LcrH, is not involved in the in vitro regulation of the TTSS. This phenomenon can be explained by an unusually high proportion of regulatory factors that may overcome the need for a chaperone-dependent regulatory pathway in P. aeruginosa (40). The functional similarity of LcrH and PcrH has been further demonstrated by employing a Yersinia lcrH deletion mutant in which the introduction of cloned pcrH partly restored wild-type levels of YopB and YopD and thus the virulence phenotype, indicating that PcrH stabilizes YopB and YopD prior to secretion (40).
IpgC from the genus Shigella.
Bacillary dysentery caused by Shigella species represents a global threat to human health. The mechanisms of Shigella colonization of the intestinal epithelium involve the penetration of epithelial cells, intracellular multiplication, and spreading to adjacent cells, altogether eliciting a strong inflammatory response (60). The process of host invasion depends on a TTSS. In Shigella, four genes—ipgC, ipaB, ipaC, and ipaD—from the 31-kb entry region of the virulence plasmid are essential for colonization of the intestinal epithelium (61–63).
IpaB and IpaC are two translocator proteins of the Shigella TTSS that are responsible for membrane lysis of the phagocytic vacuole, contact hemolysis, and macrophage cell death (42, 63–65). Both proteins are present in the bacterial cytoplasm, where they are stabilized by a class II chaperone, IpgC (66). Upon the secretion of IpaB and IpaC, IpgC is available to take on its subsequent role in Shigella virulence mechanisms; IpgC functions as a coactivator of the transcription of virulence genes within the MxiE regulon (41, 67).
IpgC displays a helical arrangement folded into three TPR-like motifs that together create a cleft-like scaffold where IpgC binds its cognate partners (9) (Fig. 2 and 3). Analyses of crystal and biologically relevant structures showed that IpgC likely adopts asymmetric and head-to-head dimer arrangements (9, 68). Dimerization is required for both the stabilization and the secretion of IpaB and, thus, efficient colonization of the intestinal epithelium. Both arrangements may have important physiological roles. Transition between the two modes can both promote effective delivery of translocators to the secretion system and accommodate its broad range of interacting partners (68).
The cleft-like scaffold created by conserved residues on the concave side of the TPR moiety is considered to facilitate association with its interacting polypeptide by providing an amphipathic surface. IpgC captures the chaperone binding motif of IpaB in an extended conformation, which is stabilized by the conserved residues lining the cleft (9) (for details, see Fig. 2). The 65PELKAP70 region of IpaB is required for proper binding to IpgC. Comparison of the structures of the IpgC-IpaB and PcrH-PopD complexes revealed that both binding sequences are accommodated within their respective chaperone clefts in very similar fashions (7).
Other TPR-containing virulence factors.
P. aeruginosa requires the pilF gene product for Tfp biogenesis (69). Detailed analysis of the crystal structure of the P. aeruginosa PilF protein revealed a helical arrangement folded into six TPR units (8, 69). The TPR domain of P. aeruginosa PilF has been proposed to contain three possible interfaces that meet the criteria for protein-protein interaction (69), but a directly interacting partner has not yet been discovered.
M. tuberculosis needs a serine/threonine protein kinase G (PknG) for inhibition of bacterium degradation in lysosomes, thus promoting the survival of M. tuberculosis in macrophages (45). M. tuberculosis PknG contains a TPR domain (70) that is required for proper activity of the kinase (71). Protein-protein interactions mediated by the TPR domain in M. tuberculosis PknG remain to be explored.
Francisella and other pathogens such as P. gingivalis utilize putative TPR-containing proteins for their virulence mechanisms. F. tularensis needs PilF for the biogenesis of Tfp (35); pilF gene inactivation leads to significant defects in adherence to distinct types of cells and causes loss of virulence in a mouse model of infection (35). Other Francisella proteins, FTL_0205, ComL, and its ortholog FTT1244c, are important for a normal growth rate in different cell types (46–48). P. gingivalis expresses the TprA protein, which is likely required to transduce stress signals from the environment, leading to changes in the expression of proteins involved in the secretion system of the bacterium (44).
The positions of TPR motifs, as they have been predicted or structurally identified within the proteins discussed in this section, are depicted in Fig. 3.
SUMMARY AND CONCLUDING REMARKS
TPRs occur ubiquitously in organisms as diverse as bacteria and humans. The evolutionary conservation of the TPR motif suggests that it is fundamentally important for living organisms, functioning as a module for protein-protein interactions. TPR-containing proteins are involved in a variety of cellular functions, including those that participate in bacterial pathogenesis (3, 37). Using bioinformatics, a number of TPR-containing virulence factors have been predicted, stimulating investigation focused on molecular aspects of bacterial pathogenesis mediated by TPR motifs.
Of the TPR virulence factors proposed, class II chaperones of a bacterial TTSS of Y. pestis (LcrH), P. aeruginosa (PcrH), and Shigella (IpgC) have been experimentally shown to display TPR motifs. The roles of TPR motifs within these proteins have been specified, demonstrating that TPR motifs crucially determine the functioning of LcrH, PcrH, and IpgC by giving them the ability to bind and stabilize the respective translocator proteins in the bacterial cytoplasm (6, 7, 9, 34). Furthermore, a TPR domain enables class II chaperones to self-assemble homodimers (6, 7, 9, 68), an arrangement that is particularly required for the proper biological functioning of LcrH and IpgC (6, 9, 68). Besides class II chaperones of a TTSS, TPR-containing proteins have also been suggested to participate in virulence apparatuses of bacteria such as M. tuberculosis (PknG), F. tularensis (PilF, FTL_0205, ComL and its ortholog FTT1244c), P. gingivalis (TprA), and P. aeruginosa (PilF) (8, 44–48, 69, 72). Only limited experimental data on TPR motifs in these proteins are available. Therefore, these proteins represent interesting targets for further structural and/or functional studies that should be performed in order to obtain more information about the TPR domains involved in bacterial pathogenesis.
We believe that comprehensive knowledge of the structures and molecular functions of TPR domains in bacterial virulence factors could be generally applicable in drug design and in the development of attenuated live vaccines. For instance, it can be assumed that peptide modules that selectively block TPR-mediated protein-protein interactions required for bacterial pathogenesis could be utilizable in the discovery of a new class of antibacterial drugs. In cancer research, TPR modules that inhibit the interaction between Hsp90 and natural TPR-containing cochaperones of Hsp90 have already been designed, leading to the identification of new potential anticancer therapeutics (73, 74). Furthermore, several studies indicate that even a point gene mutation(s) encoding the conserved amino acids can disrupt the TPR fold, leading to failure of TPR-containing protein function(s) (6, 7, 34). A point mutation(s) thus can cause loss of virulence while preserving the expression of all immunogenic proteins. As long as there is no safe and functional vaccine (e.g., against tularemia [75]), such attenuated strains with a fully expressed repertoire of immunogenic proteins might be suitable candidates for the development of new live vaccines.
ACKNOWLEDGMENTS
We thank Jiri Damborsky for assistance with Fig. 1, depicting TPR motifs of PP5; Michael Kolbe for providing Fig. 2A and B; and finally Pavel Bostik for beneficial comments.
This work was supported by grants 160/50/15011 from the Grant Agency of Charles University, Prague, Czech Republic, and a Long-Term Organization Development plan from the Ministry of Defense of the Czech Republic.
Footnotes
Published ahead of print 21 December 2012
REFERENCES
- 1. Hirano T, Kinoshita N, Morikawa K, Yanagida M. 1990. Snap helix with knob and hole: essential repeats in S. pombe nuclear protein nuc2+. Cell 60:319–328 [DOI] [PubMed] [Google Scholar]
- 2. Sikorski RS, Boguski MS, Goebl M, Hieter P. 1990. A repeating amino acid motif in CDC23 defines a family of proteins and a new relationship among genes required for mitosis and RNA synthesis. Cell 60:307–317 [DOI] [PubMed] [Google Scholar]
- 3. Lamb JR, Tugendreich S, Hieter P. 1995. Tetratrico peptide repeat interactions: to TPR or not to TPR? Trends Biochem. Sci. 20:257–259 [DOI] [PubMed] [Google Scholar]
- 4. D'Andrea LD, Regan L. 2003. TPR proteins: the versatile helix. Trends Biochem. Sci. 28:655–662 [DOI] [PubMed] [Google Scholar]
- 5. Das AK, Cohen PW, Barford D. 1998. The structure of the tetratricopeptide repeats of protein phosphatase 5: implications for TPR-mediated protein-protein interactions. EMBO J. 17:1192–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Büttner CR, Sorg I, Cornelis GR, Heinz DW, Niemann HH. 2008. Structure of the Yersinia enterocolitica type III secretion translocator chaperone SycD. J. Mol. Biol. 375:997–1012 [DOI] [PubMed] [Google Scholar]
- 7. Job V, Mattei PJ, Lemaire D, Attree I, Dessen A. 2010. Structural basis of chaperone recognition of type III secretion system minor translocator proteins. J. Biol. Chem. 285:23224–23232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim K, Oh J, Han D, Kim EE, Lee B, Kim Y. 2006. Crystal structure of PilF: functional implication in the type 4 pilus biogenesis in Pseudomonas aeruginosa. Biochem. Biophys. Res. Commun. 340:1028–1038 [DOI] [PubMed] [Google Scholar]
- 9. Lunelli M, Lokareddy RK, Zychlinsky A, Kolbe M. 2009. IpaB-IpgC interaction defines binding motif for type III secretion translocator. Proc. Natl. Acad. Sci. U. S. A. 106:9661–9666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grove TZ, Cortajarena AL, Regan L. 2008. Ligand binding by repeat proteins: natural and designed. Curr. Opin. Struct. Biol. 18:507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blatch GL, Lassle M. 1999. The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. Bioessays 21:932–939 [DOI] [PubMed] [Google Scholar]
- 12. Goodarzi MO, Xu N, Cui J, Guo X, Chen YI, Azziz R. 2008. Small glutamine-rich tetratricopeptide repeat-containing protein alpha (SGTA), a candidate gene for polycystic ovary syndrome. Hum. Reprod. 23:1214–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sohocki MM, Bowne SJ, Sullivan LS, Blackshaw S, Cepko CL, Payne AM, Bhattacharya SS, Khaliq S, Qasim Mehdi S, Birch DG, Harrison WR, Elder FF, Heckenlively JR, Daiger SP. 2000. Mutations in a new photoreceptor-pineal gene on 17p cause Leber congenital amaurosis. Nat. Genet. 24:79–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grizot S, Fieschi F, Dagher MC, Pebay-Peyroula E. 2001. The active N-terminal region of p67phox. Structure at 1.8 A resolution and biochemical characterizations of the A128V mutant implicated in chronic granulomatous disease. J. Biol. Chem. 276:21627–21631 [DOI] [PubMed] [Google Scholar]
- 15. Tsukahara F, Urakawa I, Hattori M, Hirai M, Ohba K, Yoshioka T, Sakaki Y, Muraki T. 1998. Molecular characterization of the mouse mtprd gene, a homologue of human TPRD: unique gene expression suggesting its critical role in the pathophysiology of Down syndrome. J. Biochem. 123:1055–1063 [DOI] [PubMed] [Google Scholar]
- 16. Cliff MJ, Harris R, Barford D, Ladbury JE, Williams MA. 2006. Conformational diversity in the TPR domain-mediated interaction of protein phosphatase 5 with Hsp90. Structure 14:415–426 [DOI] [PubMed] [Google Scholar]
- 17. Scheufler C, Brinker A, Bourenkov G, Pegoraro S, Moroder L, Bartunik H, Hartl FU, Moarefi I. 2000. Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Cell 101:199–210 [DOI] [PubMed] [Google Scholar]
- 18. Zhang Y, Chan DC. 2007. Structural basis for recruitment of mitochondrial fission complexes by Fis1. Proc. Natl. Acad. Sci. U. S. A. 104:18526–18530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cortajarena AL, Regan L. 2006. Ligand binding by TPR domains. Protein Sci. 15:1193–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cortajarena AL, Kajander T, Pan W, Cocco MJ, Regan L. 2004. Protein design to understand peptide ligand recognition by tetratricopeptide repeat proteins. Protein Eng. Des. Sel. 17:399–409 [DOI] [PubMed] [Google Scholar]
- 21. Brinker A, Scheufler C, Von Der Mulbe F, Fleckenstein B, Herrmann C, Jung G, Moarefi I, Hartl FU. 2002. Ligand discrimination by TPR domains. Relevance and selectivity of EEVD-recognition in Hsp70 x Hop x Hsp90 complexes. J. Biol. Chem. 277:19265–19275 [DOI] [PubMed] [Google Scholar]
- 22. Eckert K, Saliou JM, Monlezun L, Vigouroux A, Atmane N, Caillat C, Quevillon-Cheruel S, Madiona K, Nicaise M, Lazereg S, Van Dorsselaer A, Sanglier-Cianferani S, Meyer P, Morera S. 2010. The Pih1-Tah1 cochaperone complex inhibits Hsp90 molecular chaperone ATPase activity. J. Biol. Chem. 285:31304–31312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krachler AM, Sharma A, Kleanthous C. 2010. Self-association of TPR domains: lessons learned from a designed, consensus-based TPR oligomer. Proteins 78:2131–2143 [DOI] [PubMed] [Google Scholar]
- 24. Lee JR, Lee SS, Jang HH, Lee YM, Park JH, Park SC, Moon JC, Park SK, Kim SY, Lee SY, Chae HB, Jung YJ, Kim WY, Shin MR, Cheong GW, Kim MG, Kang KR, Lee KO, Yun DJ, Lee SY. 2009. Heat-shock dependent oligomeric status alters the function of a plant-specific thioredoxin-like protein, AtTDX. Proc. Natl. Acad. Sci. U. S. A. 106:5978–5983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zeytuni N, Ozyamak E, Ben-Harush K, Davidov G, Levin M, Gat Y, Moyal T, Brik A, Komeili A, Zarivach R. 2011. Self-recognition mechanism of MamA, a magnetosome-associated TPR-containing protein, promotes complex assembly. Proc. Natl. Acad. Sci. U. S. A. 108:E480–E487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jínek M, Rehwinkel J, Lazarus BD, Izaurralde E, Hanover JA, Conti E. 2004. The superhelical TPR-repeat domain of O-linked GlcNAc transferase exhibits structural similarities to importin alpha. Nat. Struct. Mol. Biol. 11:1001–1007 [DOI] [PubMed] [Google Scholar]
- 27. Wu Y, Sha B. 2006. Crystal structure of yeast mitochondrial outer membrane translocon member Tom70p. Nat. Struct. Mol. Biol. 13:589–593 [DOI] [PubMed] [Google Scholar]
- 28. Main ER, Jackson SE, Regan L. 2003. The folding and design of repeat proteins: reaching a consensus. Curr. Opin. Struct. Biol. 13:482–489 [DOI] [PubMed] [Google Scholar]
- 29. Main ER, Xiong Y, Cocco MJ, D'Andrea L, Regan L. 2003. Design of stable alpha-helical arrays from an idealized TPR motif. Structure 11:497–508 [DOI] [PubMed] [Google Scholar]
- 30. Sonnhammer EL, Eddy SR, Durbin R. 1997. Pfam: a comprehensive database of protein domain families based on seed alignments. Proteins 28:405–420 [DOI] [PubMed] [Google Scholar]
- 31. Schultz J, Milpetz F, Bork P, Ponting CP. 1998. SMART, a simple modular architecture research tool: identification of signaling domains. Proc. Natl. Acad. Sci. U. S. A. 95:5857–5864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karpenahalli MR, Lupas AN, Soding J. 2007. TPRpred: a tool for prediction of TPR-, PPR- and SEL1-like repeats from protein sequences. BMC Bioinformatics 8:2 doi:10.1186/1471-2105-8-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bröms JE, Edqvist PJ, Forsberg A, Francis MS. 2006. Tetratricopeptide repeats are essential for PcrH chaperone function in Pseudomonas aeruginosa type III secretion. FEMS Microbiol. Lett. 256:57–66 [DOI] [PubMed] [Google Scholar]
- 34. Edqvist PJ, Bröms JE, Betts HJ, Forsberg A, Pallen MJ, Francis MS. 2006. Tetratricopeptide repeats in the type III secretion chaperone, LcrH: their role in substrate binding and secretion. Mol. Microbiol. 59:31–44 [DOI] [PubMed] [Google Scholar]
- 35. Chakraborty S, Monfett M, Maier TM, Benach JL, Frank DW, Thanassi DG. 2008. Type IV pili in Francisella tularensis: roles of pilF and pilT in fiber assembly, host cell adherence, and virulence. Infect. Immun. 76:2852–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chao J, Wong D, Zheng X, Poirier V, Bach H, Hmama Z, Av-Gay Y. 2010. Protein kinase and phosphatase signaling in Mycobacterium tuberculosis physiology and pathogenesis. Biochim. Biophys. Acta 1804:620–627 [DOI] [PubMed] [Google Scholar]
- 37. Pallen MJ, Francis MS, Futterer K. 2003. Tetratricopeptide-like repeats in type-III-secretion chaperones and regulators. FEMS Microbiol. Lett. 223:53–60 [DOI] [PubMed] [Google Scholar]
- 38. Mueller CA, Broz P, Cornelis GR. 2008. The type III secretion system tip complex and translocon. Mol. Microbiol. 68:1085–1095 [DOI] [PubMed] [Google Scholar]
- 39. Salomonsson EN, Forslund A-L, Forsberg A. 2011. Type IV pili in Francisella—a virulence trait in an intracellular pathogen. Front. Microbiol. 2:29 doi:10.3389/fmicb.2011.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bröms JE, Forslund A-L, Forsberg A, Francis MS. 2003. PcrH of Pseudomonas aeruginosa is essential for secretion and assembly of the type III translocon. J. Infect. Dis. 188:1909–1921 [DOI] [PubMed] [Google Scholar]
- 41. Mavris M, Page AL, Tournebize R, Demers B, Sansonetti P, Parsot C. 2002. Regulation of transcription by the activity of the Shigella flexneri type III secretion apparatus. Mol. Microbiol. 43:1543–1553 [DOI] [PubMed] [Google Scholar]
- 42. Ménard R, Sansonetti P, Parsot C. 1994. The secretion of the Shigella flexneri Ipa invasins is activated by epithelial cells and controlled by IpaB and IpaD. EMBO J. 13:5293–5302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Watson AA, Alm RA, Mattick JS. 1996. Identification of a gene, pilF, required for type 4 fimbrial biogenesis and twitching motility in Pseudomonas aeruginosa. Gene 180:49–56 [DOI] [PubMed] [Google Scholar]
- 44. Kondo Y, Ohara N, Sato K, Yoshimura M, Yukitake H, Naito M, Fujiwara T, Nakayama K. 2010. Tetratricopeptide repeat protein-associated proteins contribute to the virulence of Porphyromonas gingivalis. Infect. Immun. 78:2846–2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Walburger A, Koul A, Ferrari G, Nguyen L, Prescianotto-Baschong C, Huygen K, Klebl B, Thompson C, Bacher G, Pieters J. 2004. Protein kinase G from pathogenic mycobacteria promotes survival within macrophages. Science 304:1800–1804 [DOI] [PubMed] [Google Scholar]
- 46. Asare R, Abu Kwaik Y. 2010. Molecular complexity orchestrates modulation of phagosome biogenesis and escape to the cytosol of macrophages by Francisella tularensis. Environ. Microbiol. 12:2559–2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Qin A, Mann BJ. 2006. Identification of transposon insertion mutants of Francisella tularensis tularensis strain Schu S4 deficient in intracellular replication in the hepatic cell line HepG2. BMC Microbiol. 6:69 doi:10.1186/1471-2180-6-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dieppedale J, Sobral D, Dupuis M, Dubail I, Klimentova J, Stulik J, Postic G, Frapy E, Meibom KL, Barel M, Charbit A. 2011. Identification of a putative chaperone involved in stress resistance and virulence in Francisella tularensis. Infect. Immun. 79:1428–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cornelis GR, Wolf-Watz H. 1997. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol. Microbiol. 23:861–867 [DOI] [PubMed] [Google Scholar]
- 50. Navarro L, Alto NM, Dixon JE. 2005. Functions of the Yersinia effector proteins in inhibiting host immune responses. Curr. Opin. Microbiol. 8:21–27 [DOI] [PubMed] [Google Scholar]
- 51. Francis MS, Lloyd SA, Wolf-Watz H. 2001. The type III secretion chaperone LcrH co-operates with YopD to establish a negative, regulatory loop for control of Yop synthesis in Yersinia pseudotuberculosis. Mol. Microbiol. 42:1075–1093 [DOI] [PubMed] [Google Scholar]
- 52. Bröms JE, Edqvist PJ, Carlsson KE, Forsberg A, Francis MS. 2005. Mapping of a YscY binding domain within the LcrH chaperone that is required for regulation of Yersinia type III secretion. J. Bacteriol. 187:7738–7752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Finck-Barbançon V, Goranson J, Zhu L, Sawa T, Wiener-Kronish JP, Fleiszig SM, Wu C, Mende-Mueller L, Frank DW. 1997. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol. Microbiol. 25:547–557 [DOI] [PubMed] [Google Scholar]
- 54. Frithz-Lindsten E, Du Y, Rosqvist R, Forsberg A. 1997. Intracellular targeting of exoenzyme S of Pseudomonas aeruginosa via type III-dependent translocation induces phagocytosis resistance, cytotoxicity and disruption of actin microfilaments. Mol. Microbiol. 25:1125–1139 [DOI] [PubMed] [Google Scholar]
- 55. Yahr TL, Goranson J, Frank DW. 1996. Exoenzyme S of Pseudomonas aeruginosa is secreted by a type III pathway. Mol. Microbiol. 22:991–1003 [DOI] [PubMed] [Google Scholar]
- 56. Yahr TL, Vallis AJ, Hancock MK, Barbieri JT, Frank DW. 1998. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc. Natl. Acad. Sci. U. S. A. 95:13899–13904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kaufman MR, Jia J, Zeng L, Ha U, Chow M, Jin S. 2000. Pseudomonas aeruginosa mediated apoptosis requires the ADP-ribosylating activity of ExoS. Microbiology 146(Pt 10):2531–2541 [DOI] [PubMed] [Google Scholar]
- 58. Sundin C, Wolfgang MC, Lory S, Forsberg A, Frithz-Lindsten E. 2002. Type IV pili are not specifically required for contact dependent translocation of exoenzymes by Pseudomonas aeruginosa. Microb. Pathog. 33:265–277 [DOI] [PubMed] [Google Scholar]
- 59. Allmond LR, Karaca TJ, Nguyen VN, Nguyen T, Wiener-Kronish JP, Sawa T. 2003. Protein binding between PcrG-PcrV and PcrH-PopB/PopD encoded by the pcrGVH-popBD operon of the Pseudomonas aeruginosa type III secretion system. Infect. Immun. 71:2230–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schroeder GN, Hilbi H. 2008. Molecular pathogenesis of Shigella spp.: controlling host cell signaling, invasion, and death by type III secretion. Clin. Microbiol. Rev. 21:134–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Maurelli AT, Baudry B, d'Hauteville H, Hale TL, Sansonetti PJ. 1985. Cloning of plasmid DNA sequences involved in invasion of HeLa cells by Shigella flexneri. Infect. Immun. 49:164–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ménard R, Prevost MC, Gounon P, Sansonetti P, Dehio C. 1996. The secreted Ipa complex of Shigella flexneri promotes entry into mammalian cells. Proc. Natl. Acad. Sci. U. S. A. 93:1254–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ménard R, Sansonetti PJ, Parsot C. 1993. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 175:5899–5906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Parsot C, Ageron E, Penno C, Mavris M, Jamoussi K, d'Hauteville H, Sansonetti P, Demers B. 2005. A secreted anti-activator, OspD1, and its chaperone, Spa15, are involved in the control of transcription by the type III secretion apparatus activity in Shigella flexneri. Mol. Microbiol. 56:1627–1635 [DOI] [PubMed] [Google Scholar]
- 65. Zychlinsky A, Kenny B, Ménard R, Prevost MC, Holland IB, Sansonetti PJ. 1994. IpaB mediates macrophage apoptosis induced by Shigella flexneri. Mol. Microbiol. 11:619–627 [DOI] [PubMed] [Google Scholar]
- 66. Birket SE, Harrington AT, Espina M, Smith ND, Terry CM, Darboe N, Markham AP, Middaugh CR, Picking WL, Picking WD. 2007. Preparation and characterization of translocator/chaperone complexes and their component proteins from Shigella flexneri. Biochemistry 46:8128–8137 [DOI] [PubMed] [Google Scholar]
- 67. Kane CD, Schuch R, Day WA, Jr, Maurelli AT. 2002. MxiE regulates intracellular expression of factors secreted by the Shigella flexneri 2a type III secretion system. J. Bacteriol. 184:4409–4419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Barta ML, Zhang L, Picking WL, Geisbrecht BV. 2010. Evidence for alternative quaternary structure in a bacterial type III secretion system chaperone. BMC Struct. Biol. 10:21 doi:10.1186/1472-6807-10-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Koo J, Tammam S, Ku SY, Sampaleanu LM, Burrows LL, Howell PL. 2008. PilF is an outer membrane lipoprotein required for multimerization and localization of the Pseudomonas aeruginosa type IV pilus secretin. J. Bacteriol. 190:6961–6969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Scherr N, Honnappa S, Kunz G, Mueller P, Jayachandran R, Winkler F, Pieters J, Steinmetz MO. 2007. Structural basis for the specific inhibition of protein kinase G, a virulence factor of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 104:12151–12156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tiwari D, Singh RK, Goswami K, Verma SK, Prakash B, Nandicoori VK. 2009. Key residues in Mycobacterium tuberculosis protein kinase G play a role in regulating kinase activity and survival in the host. J. Biol. Chem. 284:27467–27479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cowley S, Ko M, Pick N, Chow R, Downing KJ, Gordhan BG, Betts JC, Mizrahi V, Smith DA, Stokes RW, Av-Gay Y. 2004. The Mycobacterium tuberculosis protein serine/threonine kinase PknG is linked to cellular glutamate/glutamine levels and is important for growth in vivo. Mol. Microbiol. 52:1691–1702 [DOI] [PubMed] [Google Scholar]
- 73. Cortajarena AL, Yi F, Regan L. 2008. Designed TPR modules as novel anticancer agents. ACS Chem. Biol. 3:161–166 [DOI] [PubMed] [Google Scholar]
- 74. Horibe T, Kohno M, Haramoto M, Ohara K, Kawakami K. 2011. Designed hybrid TPR peptide targeting Hsp90 as a novel anticancer agent. J. Transl. Med. 9:8 doi:10.1186/1479-5876-9-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Oyston PC, Quarry JE. 2005. Tularemia vaccine: past, present and future. Antonie Van Leeuwenhoek 87:277–281 [DOI] [PubMed] [Google Scholar]



