Abstract
Animal models of infective endocarditis (IE) induced by high-grade bacteremia revealed the pathogenic roles of Staphylococcus aureus surface adhesins and platelet aggregation in the infection process. In humans, however, S. aureus IE possibly occurs through repeated bouts of low-grade bacteremia from a colonized site or intravenous device. Here we used a rat model of IE induced by continuous low-grade bacteremia to explore further the contributions of S. aureus virulence factors to the initiation of IE. Rats with aortic vegetations were inoculated by continuous intravenous infusion (0.0017 ml/min over 10 h) with 106 CFU of Lactococcus lactis pIL253 or a recombinant L. lactis strain expressing an individual S. aureus surface protein (ClfA, FnbpA, BCD, or SdrE) conferring a particular adhesive or platelet aggregation property. Vegetation infection was assessed 24 h later. Plasma was collected at 0, 2, and 6 h postinoculation to quantify the expression of tumor necrosis factor (TNF), interleukin 1α (IL-1α), IL-1β, IL-6, and IL-10. The percentage of vegetation infection relative to that with strain pIL253 (11%) increased when binding to fibrinogen was conferred on L. lactis (ClfA strain) (52%; P = 0.007) and increased further with adhesion to fibronectin (FnbpA strain) (75%; P < 0.001). Expression of fibronectin binding alone was not sufficient to induce IE (BCD strain) (10% of infection). Platelet aggregation increased the risk of vegetation infection (SdrE strain) (30%). Conferring adhesion to fibrinogen and fibronectin favored IL-1β and IL-6 production. Our results, with a model of IE induced by low-grade bacteremia, resembling human disease, extend the essential role of fibrinogen binding in the initiation of S. aureus IE. Triggering of platelet aggregation or an inflammatory response may contribute to or promote the development of IE.
INTRODUCTION
Staphylococcus aureus is currently the major etiological agent of infective endocarditis (IE) (1). The pathogenesis of S. aureus-induced IE has been shown to involve a series of complex interactions between a variety of bacterial surface adhesins, proteins of the extracellular matrix, platelets, and inflammation. These events lead to the formation of vegetations at the injured cardiac valve, followed by bacterial embedment in the nascent vegetation and proliferation of vegetations (1, 2).
Major S. aureus cell wall-associated adhesins include the fibrinogen-binding proteins clumping factor A and B (ClfA and ClfB) and fibronectin-binding proteins A and B (FnbpA and FnbpB) (3). These surface proteins bind to fibrinogen and/or fibronectin and trigger platelet activation and aggregation through interaction with specific platelet membrane receptors (4–9). Additional important members of cell wall-anchored proteins are the Sdr proteins (for serine-aspartate repeats), which include SdrC, SdrD, and SdrE (10). Among them, SdrE has been identified previously as unable to interact with both fibrinogen and fibronectin but capable of inducing platelet aggregation (11).
Support for the comprehension of the pathogenesis of S. aureus IE comes predominantly from studies in animal models. For instance, it has been demonstrated that the incidence of experimental IE was reduced in rats inoculated with ClfA-negative or FnbpA-negative S. aureus strains (12, 13). Moreover, it has been shown that after individual expression of ClfA and FnbpA in the nonpathogenic bacterium Lactococcus lactis, fibrinogen binding was pivotal in promoting IE, while fibronectin binding was necessary for persistent valve colonization and endothelial invasion (14, 15). FnbpA has also been demonstrated to lead to adhesion and internalization of S. aureus into endothelial cells in vitro and to contribute to inflammation by the production of proinflammatory cytokines, such as interleukin 1β (IL-1β) and IL-6 (16–20). SdrE also contributes to valve infection, but to a lesser extent than ClfA or FnbpA (21).
A major limitation of these studies on S. aureus pathogenesis is that they were performed in IE models where infection was induced by bolus inoculation of large numbers of bacteria (between 105 and 107 CFU). Such inocula resulted in massive, high-grade bacteremia, which is incomparably greater than the spontaneous or procedure-induced bacteremia occurring in humans. Indeed, in humans, S. aureus IE possibly follows cumulative low-grade bacteremia, which could result from a colonized site (22), from injection of impure material in cases of intravenous (i.v.) drug abuse, or from more or less prolonged low-grade staphylococcal discharges from an infected intravascular device, rather than from transient high-grade bacteremia (23).Thus, the question is whether the results obtained with the bolus model are fully relevant to human IE.
We have recently established a new model of experimental IE in rats where valve infection was induced by continuous injection of bacteria at a very slow pace (24). The results showed that low-grade continuous infusion (over at least 10 h) was as infective as traditional high-grade bolus injection (24). This newly developed model of IE is expected to be closer to the human situation than the bolus inoculation model.
In the present work, we used the low-grade continuous-infusion model and recombinant L. lactis strains expressing individual S. aureus surface proteins to further investigate the roles of adhesion to fibrinogen, adhesion to fibronectin, and platelet aggregation in the initiation of IE.
(Parts of the present study were presented at the 50th Interscience Conference on Antimicrobial Agents and Chemotherapy [ICAAC], Boston, MA, 12 to 15 September 2010, and to the 22th European Congress of Clinical Microbiology and Infectious Diseases [ECCMID], London, United Kingdom, 31 March to 3 April 2012.)
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The L. lactis strains used in this study are well-described recombinant strains of the nonvirulent Lactococcus lactis subsp. cremoris strain 1363 expressing individual S. aureus proteins in their surface, i.e., the L. lactis ClfA, L. lactis FnbpA, L. lactis BCD (expressing a truncated form of FnbpA with only the fibronectin-binding domain [BCD], lacking the fibrinogen-binding domain [A]), and L. lactis SdrE strains (14, 15, 21). These strains possess fibrinogen and/or fibronectin adhesion and platelet aggregation properties, individually or in combination, allowing the investigation of the specific role of each factor in the initiation of IE. L. lactis pIL253, expressing only the erythromycin resistance determinant and lacking adhesive and aggregation properties, was used as a control mutant strain. All lactococci were grown at 30°C without shaking in M17 broth medium (Difco; Becton Dickinson, Sparks, MD) or on M17 agar plates supplemented with 0.5% glucose and 5 μg/ml erythromycin. Bacterial stocks were kept at −80°C in liquid medium supplemented with 20% (vol/vol) glycerol.
Bacterial adhesion to solid-phase fibrinogen or fibronectin.
The ability of L. lactis strains to bind to surface-adsorbed fibrinogen (Sigma, Buchs, Switzerland) or fibronectin (Sigma) was determined by using a 96-well plate assay, and the adherence score was calculated as described previously (25).
Platelet preparation.
Whole blood was drawn from healthy donors, who had not taken nonsteroidal anti-inflammatory drugs during the previous 10 days, into citrate-containing tubes. Platelet-rich plasma (PRP) was prepared by centrifugation of blood at room temperature at 150 × g for 10 min. The blood that remained after the removal of PRP was centrifuged at 630 × g for 10 min to obtain platelet-poor plasma (PPP). The PPP was used to adjust the PRP to a final concentration of 2 × 1011 platelets/ml, as used in routine clinical hematology laboratories. Because PRP already contains physiological concentrations of fibrinogen but not of fibronectin, the PRP suspension was supplemented with fibronectin to a final concentration of 50 μg/ml (5).
Quantification of aggregation.
The ability of L. lactis strains to induce platelet aggregation in vitro was evaluated using two distinct methods: (i) aggregometry, as used in routine clinical hematology laboratories, and (ii) fluorescence microscopy under conditions of high shear stress, to closely mimic the situation in arteries.
Aggregometry was performed as described previously (26). Twenty microliters of L. lactis (final concentration, 1 × 108 CFU/ml in Tyrode's buffer) was added to 180 μl of PRP in siliconized flat-bottom cuvettes. Platelet aggregation was monitored by an increase in light transmission at 37°C with stirring at 120 rpm using a LABiTec Apact 4004 aggregometer (LABiTec GmbH, Ahrensburg, Germany). The light transmission of PRP without added bacteria and the light transmission of PPP were defined as 0% and 100% light transmission, respectively. Platelets were also tested with 10 μl ADP, used as a positive control. Aggregation was recorded for 40 min. Strains were compared with regard to the interval between the addition of organisms to the PRP suspension and the onset of the aggregation response (lag time) and the number of platelets (expressed as a percentage) that were aggregated in the presence of bacteria (maximal aggregation). Three independent assays were performed.
Platelet aggregation under high shear rates (800 s−1) was analyzed by fluorescence microscopy, by a modification of a previously described method (27), in μ-Slide VI0.4 flow chambers (ibidi GmbH, Martinsried, Germany). Because previous studies have shown that L. lactis failed to adhere to the plastic surface of the flow chamber, probably due to the surface charge, chambers were prepared to generate appropriate charges and to ensure the binding of bacteria, as described previously (27). Briefly, flow chambers were submersed in 100 μl of 1% Triton X-100 (Sigma) for 30 min and were washed twice in distilled H2O. The chambers were then sterilized using 95% ethanol and were allowed to air dry. In order to produce a covalently bound amino group on the plastic surface, the slides were covered with a fresh 2% solution of 3-aminopropyltriethoxysilane (Sigma) in dry acetone for 5 s, rinsed twice in distilled H2O, and dried overnight at 42°C. A 100 μl solution of L. lactis (108 cells in Tyrode's buffer) was applied to the chambers and was allowed to adhere for 18 h at 4°C. The flow chambers were then washed once with saline to remove any unbound bacteria. The interaction of platelets with bound L. lactis was studied by infusing PRP, labeled with 0.5 ng/ml of DiOC6 (Sigma), at a shear rate of 800 s−1, for 15 min. To assess nonspecific platelet adhesion, a suspension of PRP was perfused over chambers containing no bacteria. The flow rate was created using a programmable infusion pump (Pump 44; Harvard Apparatus, Inc., South Natick, MA), and platelet aggregation was visualized under a Leica DMI400 B automated inverted microscope (Leica AG, Nidau, Switzerland). Pictures were taken every 5 s up to 300 s using a Leica AF6000 camera. Platelet aggregation was analyzed using ImageJ software (Wayne Rasband, National Institutes of Health, Bethesda, MD), by considering the percentage of surface coverage to be the percentage of platelet aggregation.
Studies of the inhibition of platelet aggregation.
To analyze the effects of aggregation inhibitors, platelets were incubated for 10 min prior to the addition of bacteria with aspirin (50 μg/ml), an inhibitor of cyclooxygenase 1, or with abciximab (10 μg/ml), an antagonist of the glycoprotein IIb/IIIa (GPIIb/IIIa) platelet receptor for fibrinogen and fibronectin (5).
Animal model of endocarditis.
All animal experiments were carried out according to Swiss regulations (authorization 879.8). Catheter-induced aortic vegetations were produced in female Wistar rats, and an intravenous (i.v.) line, connected to a programmable infusion pump (Pump 44; Harvard Apparatus), was inserted into the jugular vein to deliver the inocula, as described previously (28, 29).
Bacteria were grown overnight, recovered by centrifugation, washed, and adjusted to the desired inoculum size (106 CFU) in saline. The inoculum size was confirmed by colony counts on blood agar plates. This inoculum was chosen because it permitted a clear differentiation between infection with recombinant L. lactis and infection with the nonvirulent L. lactis strain pIL253 in previous studies (14, 15, 21). Animals were inoculated 24 h after catheterization, via the infusion pump, by either of two distinct protocols. In protocol 1, animals received an i.v. bolus (1 ml in 1 min) of 106 CFU. In protocol 2, animals received the same total absolute numbers of bacteria as in protocol 1, but the bacteria were delivered progressively at a pace of 0.0017 ml/min over 10 h. During this period, no growth of the original inoculum used for challenge was observed, as checked in preliminary experiments.
To evaluate bacteremia, 1 ml of blood was drawn into heparin-containing tubes from a random subset of 3 to 5 animals per group. Samples were taken just before inoculation and either 1 min and 2 h (bolus injection) or 2 h and 6 h (continuous infusion) after the start of inoculation (24). Serial dilutions of blood were spread onto M17 agar plates, and colonies were enumerated after incubation for 48 h at 30°C. The sensitivity of the assay was 1 CFU/ml of blood.
Rats were sacrificed 24 h after the end of inoculation. Quantitative valve cultures were performed as described previously (24, 30). This method permitted the detection of 2 log10 CFU/g of vegetation.
Determination of cytokines in plasma.
Levels of tumor necrosis factor (TNF), IL-1α, IL-1β, IL-6, and IL-10 were measured in the plasma of animals inoculated with either L. lactis pIL253, the L. lactis ClfA strain, or the L. lactis FnbpA strain by using the Bio-Plex Rat Cytokine assay (Bio-Rad, Hercules, CA) according to the manufacturer's indications. Blood was collected into heparinized tubes before inoculation and 2 h and 6 h postinoculation and was centrifuged at 10,000 rpm for 5 min. Plasma was preserved at −80°C until analysis. The lower detection limit for TNF, IL-1α, IL-1β, IL-6, and IL-10 was 1 pg/ml.
Statistical analysis.
The adherence scores of the different bacteria and their abilities to trigger platelet aggregation were compared by the unpaired t test. Plasma cytokine values were compared by one-way analysis of variance (ANOVA) with Tukey's correction for multiple comparisons. The percentages of vegetation infection in each inoculation group were analyzed descriptively by Fisher's exact test. We complemented the descriptive approach with a logistic regression model in order to determine the role of fibrinogen adherence, fibronectin adherence, and platelet aggregation in the risk of infection. Note that in the presence of these three confounding factors, a full factorial design would require 23 (i.e., 8) groups per inoculation method, whereas only 5 groups were examined in this study. As a consequence, some confounding remains, and it is not possible to identify all the main effects and interaction terms in the model. For instance, since all strains featuring fibrinogen adherence (Fg) also induced platelet aggregation (PlAgg), it is not possible to identify the main effect of Fg on the risk of infection (instead of Fg alone, we use the term Fg + PlAgg). Therefore, we considered Fg + PlAgg, PlAgg, and fibronectin adherence (Fn) as the main effects in the logistic model and looked for the presence of an interaction between Fg + PlAgg and Fn [(Fg + PlAgg) × Fn]. Data were either analyzed separately or pooled in a single data set. In the modeling of pooled data, a bolus term interacting with all the terms mentioned above was also included in order to capture any difference between the two inoculation methods. All statistical analyses were conducted with R software for statistical computing (31).
RESULTS
Bacterial adhesion to immobilized fibrinogen and fibronectin.
L. lactis pIL253 showed no binding to fibrinogen (adherence score, 0.01 ± 0.01) or to fibronectin (adherence score, 0.27 ± 0.02). The adherence scores for the recombinant strains indicated that, compared to strain pIL253, L. lactis expressing ClfA was able to bind to fibrinogen (13.55 ± 0.79; P < 0.001); L. lactis expressing full-length FnbpA was able to bind to both fibrinogen and fibronectin (8.37 ± 3.91 and 7.60 ± 2.73, respectively; P ≤ 0.02); and the L. lactis BCD strain, expressing the fibrinogen-truncated form of FnbpA, failed to bind to fibrinogen (0.15 ± 0.07) but maintained the adhesion to fibronectin (3.07 ± 0.26; P < 0.001). L. lactis expressing SdrE failed to bind to either fibrinogen (0.03 ± 0.03) or fibronectin (0.15 ± 0.04). These results were consistent with those described previously and confirmed the phenotype of each isolate (11, 14, 15).
Effects of S. aureus surface proteins expressed in L. lactis on platelet aggregation.
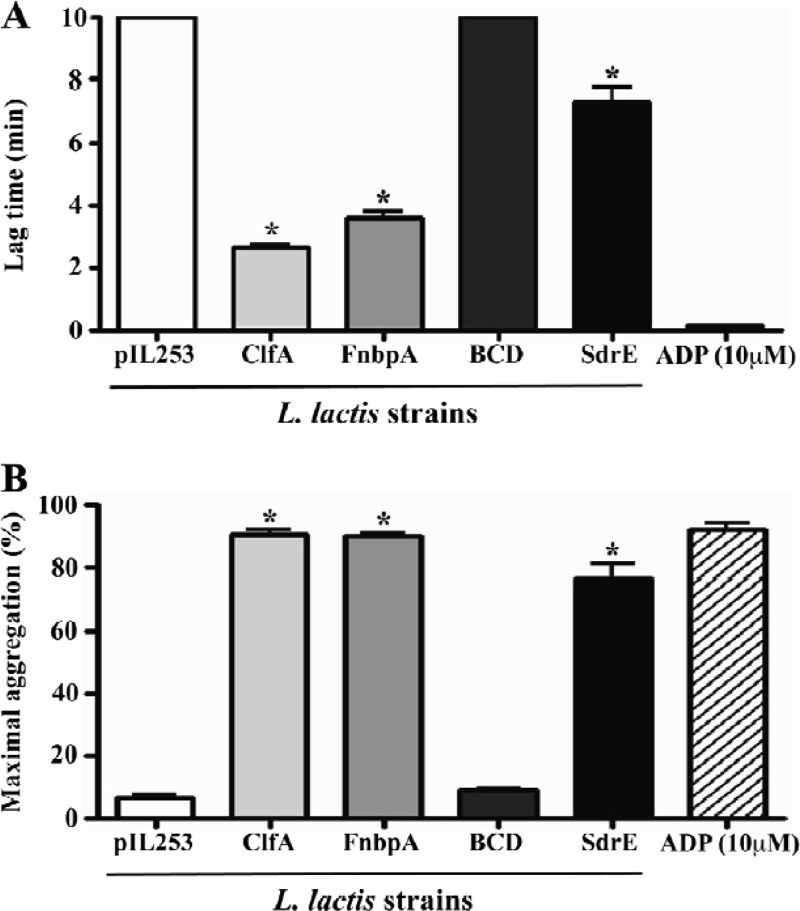
The platelet aggregation results measured by light transmission are shown in Fig. 1. No platelet aggregation induced by L. lactis pIL253 was observed. The expression of ClfA or FnbpA in L. lactis led to a significantly shorter platelet aggregation time (lag times, 2.66 ± 0.13 and 3.62 ± 0.36 min, respectively [P < 0.05]) (Fig. 1A). L. lactis expressing SdrE stimulated platelet aggregation with a longer lag time (7.33 ± 0.82 min) than that for L. lactis expressing ClfA or FnbpA, a result similar to that published previously (11). In contrast, the L. lactis BCD strain, expressing the truncated form of FnbpA lacking the fibrinogen-binding domain, did not promote platelet activation within 40 min after the addition of the bacterial inoculum to PRP. Maximal aggregation assessed after 40 min (Fig. 1B) showed a pattern opposite that for lag time. It remained almost unchanged in strains pIL253 and BCD, while, in contrast, it was close to ADP-induced maximum aggregation in strains expressing ClfA, FnbpA, or SdrE. L. lactis-induced platelet aggregation was inhibited (no aggregation within 40 min) by both aspirin (50 μg/ml) and abciximab (10 μg/ml), suggesting that this was true platelet aggregation (data not shown).
Fig 1.
L. lactis-induced platelet aggregation measured by light transmission aggregometry. Results are expressed as means ± standard deviations for three independent assays. (A) Lag time (i.e., the time from the addition of bacteria to the platelets to the first signs of aggregation). Lag times of 10 min indicate that there was no aggregation even when the time was extended to 40 min. (B) Maximal aggregation (i.e., the maximum extent of aggregation over 40 min). Asterisks indicate a significant difference (P < 0.05) from the results for L. lactis pIL253 (by an unpaired t test).
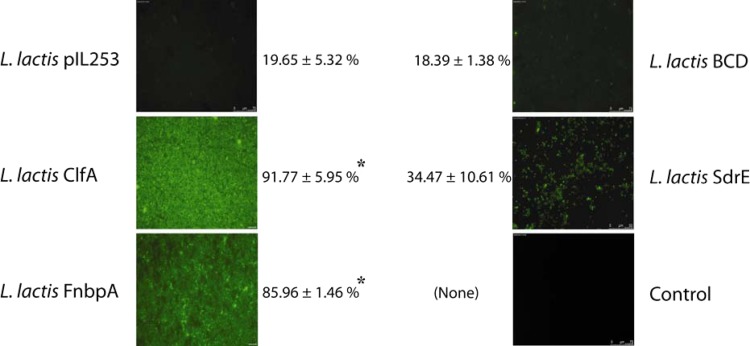
The results for platelet aggregation by L. lactis under high-shear conditions are depicted in Fig. 2. As observed by the aggregometry method, the L. lactis ClfA, FnbpA, and SdrE strains induced aggregation within significantly shorter times (1, 1, and 3 min, respectively) than the L. lactis BCD strain or L. lactis pIL253 (>5 min) (P < 0.05) and presented significantly higher percentages of aggregation. No platelet aggregation was observed on non-bacterium-covered chambers (control).
Fig 2.
Ability of L. lactis to trigger platelet aggregation under high-shear conditions (rate, 800 s−1), determined by using μ-Slide VI0.4 flow chambers. Images were taken 3 min after platelet perfusion over immobilized bacteria (i.e., the longest lag time for aggregating strains) and are representative fields for 3 independent experiments that yielded similar results. The percentage of chamber surface coverage (platelet aggregation) is given next to each image. For the control, a suspension of PRP was perfused over a chamber without adherent bacteria. Asterisks indicate a significant difference (P < 0.001) from the result for pIL253 (by an unpaired t test).
In vivo studies.
Bolus inoculation of 106 CFU of L. lactis was followed by bacteremia of 5 × 103 to 9 × 103 CFU/ml 1 min after inoculation. In comparison, bacteremia levels during continuous infusion had median values of 10 CFU (range, 1 to 24 CFU/ml), which were similar in all the L. lactis groups. Thus, the level of bacteremia was 375 to 5,000 times greater after bolus administration than during continuous inoculation.
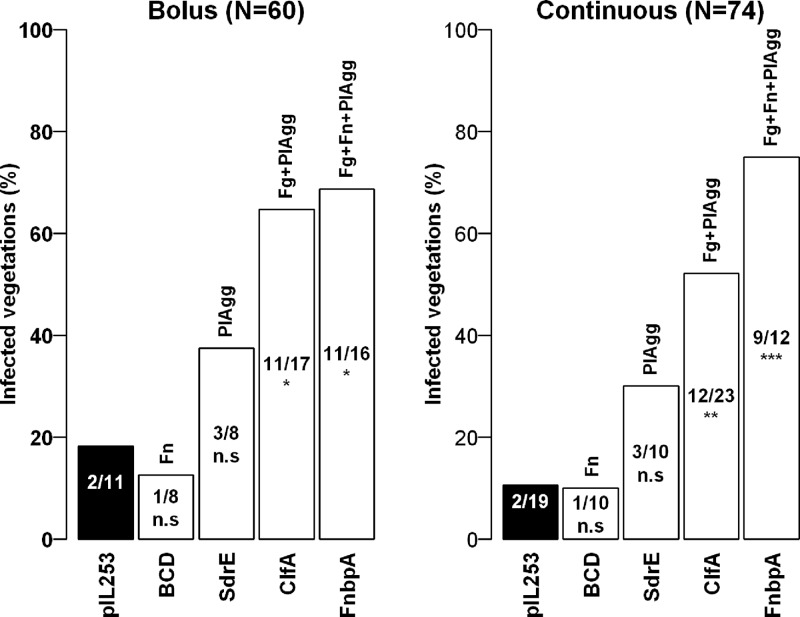
Figure 3 depicts the contributions of ClfA, FnbpA, BCD, and SdrE expressed in L. lactis to the initiation of IE. Descriptive analysis showed that the percentages of infection for the ClfA and FnbpA groups were significantly higher than for the L. lactis pIL253 control group. Both platelet aggregation (PlAgg), observed with L. lactis SdrE, and PlAgg plus fibrinogen adherence (Fg), observed with L. lactis ClfA, favored the occurrence of endocarditis, although there were not enough data for the SdrE group to generate a significant result by Fisher's exact test, which remains rather conservative. The data also suggest that fibronectin adherence (Fn) may further promote infection when it occurs in association with fibrinogen adherence and platelet aggregation, as in the L. lactis FnbpA strain, but not when it is present alone, as in the L. lactis BCD strain. Again, however, comparison of the FnbpA and ClfA strains by Fisher's test failed to detect any significant difference (P > 0.281). It is remarkable that the patterns of infection in the bolus and continuous infusion models appear similar, although the levels of circulating bacteria in the continuous infusion model are much lower.
Fig 3.
Experimental endocarditis induced by bolus or continuous-infusion inoculation. Rats with catheter-induced aortic vegetations were challenged either with an i.v. bolus (1 ml in 1 min) of 106 CFU or with the same total absolute numbers of L. lactis delivered as a continuous infusion of 0.0017 ml/min over 10 h. Bars indicate the percentages of infected vegetations for each inoculation method. The recombinant strains of L. lactis are given under the bars, and the factors expressed by these strains are given above the bars. Fg, fibrinogen binding; Fn, fibronectin binding; PlAgg, platelet aggregation. *, P < 0.05; **, P < 0.01; ***, P < 0.001; n.s., not significant (by Fisher's exact test).
The logistic regression model for the probability of infection was used in subsequent statistical analysis. Because for each strain no significant differences could be observed between the two inoculation methods (P, >0.525 by Fisher's exact test), data were pooled. This model did reveal that the interaction term (Fg + PlAgg) × Fn was not significant, either with separate data or with pooled data (P > 0.449); hence, it was dropped from the model. All interactions involving the bolus term were also dropped. Table 1 presents parameter estimates and odds ratios (OR) for a reduced model that includes only the additive effects of PlAgg, Fn + PlAgg, and Fn, as well as an average bolus effect. Recall that parameters in a logistic regression model are to be interpreted as the logarithm of the OR [log(OR)] comparing a group of interest with a control group (which in our context refers to animals inoculated with L. lactis pIL253 using continuous inoculation). The reduced model (Table 1) confirms that the bolus injection and continuous-infusion models exhibit essentially the same infection pattern, as suggested by the nonsignificant bolus term. On the other hand, PlAgg alone or in association with Fg increases the OR of infection to 4.03 (P = 0.045) or 11.94 (P < 0.001), respectively. Note that we gain statistical power in the modeling, which translates into a significant effect of PlAgg on the risk of infection (P = 0.045), while this was not detected by Fisher's test comparing the odds of infection in the L. lactis SdrE and L. lactis pIL253 groups. Finally, Fn appears relatively neutral (P = 0.414) with respect to the risk of infection.
Table 1.
Coefficients, odds ratios, and P values for the logistic regression modela
| Parameter | Bolus inoculation |
Continuous infusion |
Pooled data |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Log(OR) | OR | P | Log(OR) | OR | P | No. of infected vegetations/total | Log(OR) | OR | P | |
| Control (intercept)b | −1.69 | 0.19 | 0.014 | −2.45 | 0.09 | <0.001 | −2.22 | 0.11 | <0.001 | |
| Bolus | 0.30 | 1.35 | 0.459 | |||||||
| PlAgg | 1.18 | 3.24 | 0.241 | 1.61 | 4.98 | 0.099 | 6/18 | 1.39 | 4.03 | 0.045 |
| Fg + PlAgg | 2.37 | 10.6 | 0.001 | 2.63 | 13.8 | <0.001 | 23/40 | 2.48 | 11.9 | <0.001 |
| Fn | 0.03 | 1.03 | 0.964 | 0.72 | 2.05 | 0.269 | 2/18 | 0.37 | 1.45 | 0.414 |
An OR larger than 1 indicates a higher risk (odds) of infection, while an OR smaller than 1 indicates a lower risk of infection, in the group of interest than in the control group. A significant result refers to a log(OR) significantly different from zero, or an OR significantly different from 1. P values of <0.05 are shown in boldface.
The intercept refers to the logarithm of the odds in the control group (L. lactis pIL253). Values for the intercept are expressed as log(odds) and odds.
Production of cytokines.
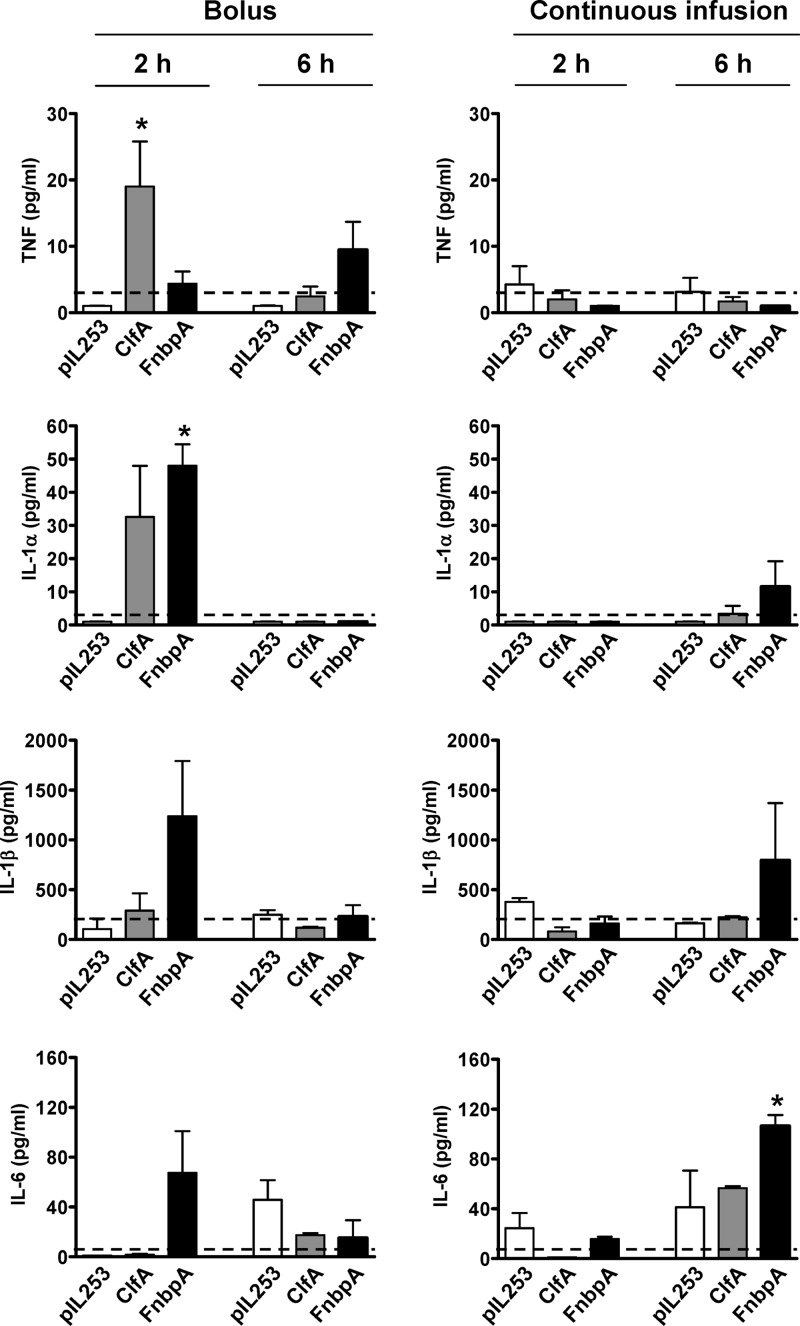
Concentrations of TNF, IL-1α, IL-1-β, IL-6, and IL-10 in plasma were quantified by Luminex 2 h and 6 h after bolus and continuous-infusion inoculation of rats with L. lactis pIL253, L. lactis expressing ClfA, and L. lactis expressing FnbpA. Cytokines were produced at different levels depending on the experimental conditions. TNF, IL-1α, and IL-6 were produced at low levels, and IL-1β at high levels (up to the ng/ml range), in rats infected with the L. lactis ClfA and FnbpA strains (Fig. 4).
Fig 4.
Levels of TNF, IL-1α, IL-1β, and IL-6 in the plasma of rats 2 h and 6 h after the initiation of inoculation with L. lactis pIL253, L. lactis expressing ClfA, or L. lactis expressing FnbpA by either bolus (left) or low-grade continuous infusion (right). Horizontal dashed lines represent background values at 0 h. Results are expressed as means ± standard errors of the means for three values per group. Asterisks indicate a significant difference (P < 0.05) from cytokine production in L. lactis pIL253 as determined by ANOVA with Tukey's multiple-comparison test.
Some interesting trends were observed, although intergroup differences in cytokine concentrations were most often not statistically significant because of interindividual variability and the relatively small number of animals tested (3 in each group). The production of TNF, IL-1α, IL-1-β, IL-6, and IL-10 (Fig. 4; also data not shown) peaked earlier following bolus inoculation (2 h) than following continuous-infusion inoculation (6 h) of the L. lactis ClfA and FnbpA strains. This likely results from a high but transient presence of bacteria in the blood following bolus injection, compared with the low but constant level of bacteria in the blood after continuous inoculation.
Following continuous inoculation, TNF and IL-1α were close to basal levels or undetectable in all groups for the whole period studied. IL-1β and IL-6 levels, which were close to basal levels in all groups at 2 h, strongly increased at 6 h in rats infected with L. lactis expressing FnbpA (795.4 ± 573.2 and 106.5 ± 8.7 pg/ml, respectively) and were higher than those in rats infected with L. lactis pIL253 (162.5 ± 9.16 and 41.2 ± 29.4 pg/ml) or L. lactis expressing ClfA (223.1 ± 10.8 and 56.6 ± 1.6 pg/ml). Plasma IL-10 levels did not differ among the L. lactis groups at either 2 h (360 to 461 pg/ml) or 6 h (441 to 638 pg/ml), indicating that the ability to induce IL-10 secretion was independent of the adherence properties of L. lactis in this model (data not shown).
DISCUSSION
The ability to bind fibrinogen and/or fibronectin, and to trigger platelet aggregation, has been shown to be a major determinant in the pathogenesis of S. aureus IE. This was demonstrated in experimental endocarditis models using S. aureus protein-defective mutants or, more convincingly, employing L. lactis recombinants individually expressing the fibrinogen-binding protein ClfA or the fibronectin-binding protein FnbpA (12, 13, 32, 33). In these studies, experimental IE was induced in animals by artificial bolus inoculation of high bacterial numbers, leading to transient high-grade bacteremia. However, S. aureus IE in humans may possibly result from recurrent low-grade bacteremia rather than from transient high-grade bacteremia (22, 23).
In the present study, we used a newly developed rat model of experimental IE by low-grade bacteremia, resembling human low-grade bacteremia, to explore further the individual contributions of fibrinogen binding, fibronectin binding, and platelet aggregation to the initiation of S. aureus IE in a more realistic situation. A panel of recombinant L. lactis strains expressing individual S. aureus cell wall proteins, to circumvent their redundancy of function, was used.
Several interesting observations can be made. First, the incidence of experimental IE (expressed as a percentage) by low-grade bacteremia was similar to that resulting from bolus inoculation, supporting the use of this new model for the study of pathogenic factors. Second, these results strengthen earlier observations on the essential role of adherence to fibrinogen in the initiation of IE and confirm that the interaction with fibronectin alone is not sufficient to induce the disease. This is supported by an increased rate of infection by L. lactis expressing either ClfA or FnbpA and the absence of valve infection by L. lactis expressing BCD, the truncated form of FnbpA lacking fibrinogen-binding activity. A limitation of our study using recombinant L. lactis expressing S. aureus-derived factors is that it is not always feasible to generate strains with specific combinations of factors. For instance, it is not possible to induce fibrinogen adherence without also inducing platelet aggregation. As a consequence, a full factorial design is impossible to set up, and some confounding remains within the main effects. Despite this limitation, platelet aggregation, alone or in combination with fibrinogen adherence, was shown to increase the risk of infection significantly, since a higher rate of IE was observed with L. lactis expressing SdrE or ClfA, respectively.
In vitro results also suggest that fibrinogen is the main mediator of S. aureus platelet aggregation. Indeed, the L. lactis ClfA and FnbpA strains, both binding to fibrinogen, triggered platelet aggregation, while the L. lactis BCD strain, expressing the fibrinogen-truncated form of FnbpA, did not. The absence of in vitro platelet aggregation by L. lactis expressing BCD is in apparent contradiction of previous findings by Fitzgerald et al. (5), where BCD, expressed in L. lactis NZ9800, supported platelet aggregation (5). The reasons for this discrepancy remain unknown. It is possible that BCD is expressed in L. lactis pIL253 at a lower level than in L. lactis NZ9800. This could, in turn, result in a longer time to platelet aggregation, since this process requires a minimum level of protein expressed on the surfaces of the cells (7). Heying et al. (34) have also observed that L. lactis expressing BCD binds significantly less fibronectin than L. lactis expressing FnbpA, thus likely contributing to decreased interaction with platelets.
Although binding to fibronectin is not a prerequisite for valve infection, the ability to adhere to fibronectin in addition to attaching to fibrinogen (i.e., by FnbpA) contributed further to infection and to disease severity, as suggested both by a slighter increase in the rate of valve infection and by an elevated systemic inflammatory response. Indeed, although we analyzed only a small number of animals, which can limit the evaluation of the results, it is interesting the high levels of the proinflammatory cytokines IL-1β and IL-6 in plasma following both bolus and continuous-infusion inoculation with L. lactis expressing FnbpA, although bolus inoculation increased cytokine levels earlier than did continuous inoculation. FnbpA was previously shown to induce the production of IL-6 by endothelial cells in vitro (19, 35) and to be a major player responsible for both the proinflammatory and the procoagulant endothelium responses in vivo during S. aureus endovascular infections (15, 18, 36). The increase in the level of IL-1β is in line with the findings of previous studies showing that fibronectin induces IL-1β gene transcription and increases IL-1β protein secretion in vitro (37). FnbpA was shown to trigger the uptake of L. lactis (and S. aureus) by endothelial cells (16, 18, 38). Collectively, these results compared well with the elevated levels of IL-1β and IL-6 observed after internalization of S. aureus by endothelial cells in vitro (20) and in patients with S. aureus IE (39–41). Previous studies by Dankert el al. indicated that IL-1α production could favor Streptococcus mitis IE in rabbits, in a model of IE induced by injection of a large bacterial inoculum (42). The results of the present study support the possibility that an enhanced inflammatory response may indeed promote the development of IE, as observed after inoculation with the L. lactis FnbpA strain.
In conclusion, the present study demonstrates that IE is equally inducible by either low-grade or bolus bacteremia. The low-grade model appears to be a reliable system for realistic assessment of the roles of bacterial pathogenic factors in IE and strengthens the critical role of ClfA and platelet aggregation in IE initiation. These data may have implications for the design of appropriate prevention strategies for S. aureus IE in a context closer to the human situation. Antibiotics are unlikely to solve the problem of IE prophylaxis for humans in a setting of recurrent low-level bacteremia. Thus, other approaches should be explored. Active and passive immunization against ClfA has already been attempted in humans based on some protection obtained against S. aureus infections in different animal models (43–45). However, no single vaccine was effective in clinical trials (46). Antiplatelet intervention using a combination of aspirin and ticlopidine to prevent experimental S. aureus endocarditis in rabbits was tested previously (47), but this strategy was not totally successful. The major drawback of this study could be the use of the classical high-inoculum infection model of IE. It is likely that the antiplatelet prophylaxis was overwhelmed by the high-grade bacteremia. Due to the important role of platelets in IE initiation and development, the use of antiplatelet drugs warrants further examination in the context of a low-grade bacteremia model of IE. This approach, if effective, could lead to the development of global IE prevention strategies, since both staphylococci and streptococci, the most frequent IE pathogens, induce this infection through interaction with platelets.
ACKNOWLEDGMENTS
This work was supported by the Swiss National Science Foundation (grant 310030-125325).
We thank Anne Angelillo-Scherrer and Christiane Gerschheimer, of the Service and Central Laboratory of Hematology, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland, for help with aggregometry.
Footnotes
Published ahead of print 17 December 2012
REFERENCES
- 1.Que YA, Moreillon P. 2011. Infective endocarditis. Nat. Rev. Cardiol. 8:322–336 [DOI] [PubMed] [Google Scholar]
- 2.Moreillon P, Que YA, Bayer AS. 2002. Pathogenesis of streptococcal and staphylococcal endocarditis. Infect. Dis. Clin. North Am. 16:297–318 [DOI] [PubMed] [Google Scholar]
- 3.Foster TJ, Hook M. 1998. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 6:484–488 [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald JR, Foster TJ, Cox D. 2006. The interaction of bacterial pathogens with platelets. Nat. Rev. Microbiol. 4:445–457 [DOI] [PubMed] [Google Scholar]
- 5.Fitzgerald JR, Loughman A, Keane F, Brennan M, Knobel M, Higgins J, Visai L, Speziale P, Cox D, Foster TJ. 2006. Fibronectin-binding proteins of Staphylococcus aureus mediate activation of human platelets via fibrinogen and fibronectin bridges to integrin GPIIb/IIIa and IgG binding to the FcγRIIa receptor. Mol. Microbiol. 59:212–230 [DOI] [PubMed] [Google Scholar]
- 6.Loughman A, Fitzgerald JR, Brennan MP, Higgins J, Downer R, Cox D, Foster TJ. 2005. Roles for fibrinogen, immunoglobulin and complement in platelet activation promoted by Staphylococcus aureus clumping factor A. Mol. Microbiol. 57:804–818 [DOI] [PubMed] [Google Scholar]
- 7.Miajlovic H, Loughman A, Brennan M, Cox D, Foster TJ. 2007. Both complement- and fibrinogen-dependent mechanisms contribute to platelet aggregation mediated by Staphylococcus aureus clumping factor B. Infect. Immun. 75:3335–3343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siboo IR, Cheung AL, Bayer AS, Sullam PM. 2001. Clumping factor A mediates binding of Staphylococcus aureus to human platelets. Infect. Immun. 69:3120–3127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeaman MR, Sullam PM, Dazin PF, Norman DC, Bayer AS. 1992. Characterization of Staphylococcus aureus-platelet binding by quantitative flow cytometric analysis. J. Infect. Dis. 166:65–73 [DOI] [PubMed] [Google Scholar]
- 10.Josefsson E, McCrea KW, Ni Eidhin D, O'Connell D, Cox J, Hook M, Foster TJ. 1998. Three new members of the serine-aspartate repeat protein multigene family of Staphylococcus aureus. Microbiology 144(Part 12):3387–3395 [DOI] [PubMed] [Google Scholar]
- 11.O'Brien L, Kerrigan SW, Kaw G, Hogan M, Penades J, Litt D, Fitzgerald DJ, Foster TJ, Cox D. 2002. Multiple mechanisms for the activation of human platelet aggregation by Staphylococcus aureus: roles for the clumping factors ClfA and ClfB, the serine-aspartate repeat protein SdrE and protein A. Mol. Microbiol. 44:1033–1044 [DOI] [PubMed] [Google Scholar]
- 12.Kuypers JM, Proctor RA. 1989. Reduced adherence to traumatized rat heart valves by a low-fibronectin-binding mutant of Staphylococcus aureus. Infect. Immun. 57:2306–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreillon P, Entenza JM, Francioli P, McDevitt D, Foster TJ, Francois P, Vaudaux P. 1995. Role of Staphylococcus aureus coagulase and clumping factor in pathogenesis of experimental endocarditis. Infect. Immun. 63:4738–4743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piroth L, Que YA, Widmer E, Panchaud A, Piu S, Entenza JM, Moreillon P. 2008. The fibrinogen- and fibronectin-binding domains of Staphylococcus aureus fibronectin-binding protein A synergistically promote endothelial invasion and experimental endocarditis. Infect. Immun. 76:3824–3831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Que YA, Haefliger JA, Piroth L, Francois P, Widmer E, Entenza JM, Sinha B, Herrmann M, Francioli P, Vaudaux P, Moreillon P. 2005. Fibrinogen and fibronectin binding cooperate for valve infection and invasion in Staphylococcus aureus experimental endocarditis. J. Exp. Med. 201:1627–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massey RC, Kantzanou MN, Fowler T, Day NP, Schofield K, Wann ER, Berendt AR, Hook M, Peacock SJ. 2001. Fibronectin-binding protein A of Staphylococcus aureus has multiple, substituting, binding regions that mediate adherence to fibronectin and invasion of endothelial cells. Cell. Microbiol. 3:839–851 [DOI] [PubMed] [Google Scholar]
- 17.Palmqvist N, Foster T, Fitzgerald JR, Josefsson E, Tarkowski A. 2005. Fibronectin-binding proteins and fibrinogen-binding clumping factors play distinct roles in staphylococcal arthritis and systemic inflammation. J. Infect. Dis. 191:791–798 [DOI] [PubMed] [Google Scholar]
- 18.Sinha B, Herrmann M. 2005. Mechanism and consequences of invasion of endothelial cells by Staphylococcus aureus. Thromb. Haemost. 94:266–277 [DOI] [PubMed] [Google Scholar]
- 19.Soderquist B, Alriksson I, Kallman J, Kihlstrom E. 2006. The influence of adhesive and invasive properties of Staphylococcus aureus defective in fibronectin-binding proteins on secretion of interleukin-6 by human endothelial cells. APMIS 114:112–116 [DOI] [PubMed] [Google Scholar]
- 20.Yao L, Bengualid V, Lowy FD, Gibbons JJ, Hatcher VB, Berman JW. 1995. Internalization of Staphylococcus aureus by endothelial cells induces cytokine gene expression. Infect. Immun. 63:1835–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Widmer E, Que YA, Entenza JM, Moreillon P. 2006. New concepts in the pathophysiology of infective endocarditis. Curr. Infect. Dis. Rep. 8:271–279 [DOI] [PubMed] [Google Scholar]
- 22.von Eiff C, Becker K, Machka K, Stammer H, Peters G. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. N. Engl. J. Med. 344:11–16 [DOI] [PubMed] [Google Scholar]
- 23.Saginur R, Suh KN. 2008. Staphylococcus aureus bacteraemia of unknown primary source: where do we stand? Int. J. Antimicrob. Agents 32(Suppl. 1):S21–S25 [DOI] [PubMed] [Google Scholar]
- 24.Veloso TR, Amiguet M, Rousson V, Giddey M, Vouillamoz J, Moreillon P, Entenza JM. 2011. Induction of experimental endocarditis by continuous low-grade bacteremia mimicking spontaneous bacteremia in humans. Infect. Immun. 79:2006–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ythier M, Entenza JM, Bille J, Vandenesch F, Bes M, Moreillon P, Sakwinska O. 2010. Natural variability of in vitro adherence to fibrinogen and fibronectin does not correlate with in vivo infectivity of Staphylococcus aureus. Infect. Immun. 78:1711–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kerrigan SW, Jakubovics NS, Keane C, Maguire P, Wynne K, Jenkinson HF, Cox D. 2007. Role of Streptococcus gordonii surface proteins SspA/SspB and Hsa in platelet function. Infect. Immun. 75:5740–5747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerrigan SW, Clarke N, Loughman A, Meade G, Foster TJ, Cox D. 2008. Molecular basis for Staphylococcus aureus-mediated platelet aggregate formation under arterial shear in vitro. Arterioscler. Thromb. Vasc. Biol. 28:335–340 [DOI] [PubMed] [Google Scholar]
- 28.Fluckiger U, Moreillon P, Blaser J, Bickle M, Glauser MP, Francioli P. 1994. Simulation of amoxicillin pharmacokinetics in humans for the prevention of streptococcal endocarditis in rats. Antimicrob. Agents Chemother. 38:2846–2849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heraief E, Glauser MP, Freedman LR. 1982. Natural history of aortic valve endocarditis in rats. Infect. Immun. 37:127–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Entenza JM, Caldelari I, Glauser MP, Francioli P, Moreillon P. 1997. Importance of genotypic and phenotypic tolerance in the treatment of experimental endocarditis due to Streptococcus gordonii. J. Infect. Dis. 175:70–76 [DOI] [PubMed] [Google Scholar]
- 31.R Core Team 2012. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org [Google Scholar]
- 32.Que YA, Francois P, Haefliger JA, Entenza JM, Vaudaux P, Moreillon P. 2001. Reassessing the role of Staphylococcus aureus clumping factor and fibronectin-binding protein by expression in Lactococcus lactis. Infect. Immun. 69:6296–6302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullam PM, Bayer AS, Foss WM, Cheung AL. 1996. Diminished platelet binding in vitro by Staphylococcus aureus is associated with reduced virulence in a rabbit model of infective endocarditis. Infect. Immun. 64:4915–4921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heying R, van de Gevel J, Que YA, Piroth L, Moreillon P, Beekhuizen H. 2009. Contribution of (sub)domains of Staphylococcus aureus fibronectin-binding protein to the proinflammatory and procoagulant response of human vascular endothelial cells. Thromb. Haemost. 101:495–504 [PubMed] [Google Scholar]
- 35.Shinji H, Yosizawa Y, Tajima A, Iwase T, Sugimoto S, Seki K, Mizunoe Y. 2011. Role of fibronectin-binding proteins A and B in in vitro cellular infections and in vivo septic infections by Staphylococcus aureus. Infect. Immun. 79:2215–2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heying R, van de Gevel J, Que YA, Moreillon P, Beekhuizen H. 2007. Fibronectin-binding proteins and clumping factor A in Staphylococcus aureus experimental endocarditis: FnBPA is sufficient to activate human endothelial cells. Thromb. Haemost. 97:617–626 [PubMed] [Google Scholar]
- 37.Graves KL, Roman J. 1996. Fibronectin modulates expression of interleukin-1β and its receptor antagonist in human mononuclear cells. Am. J. Physiol. 271:L61–L69 [DOI] [PubMed] [Google Scholar]
- 38.Sinha B, Francois PP, Nusse O, Foti M, Hartford OM, Vaudaux P, Foster TJ, Lew DP, Herrmann M, Krause KH. 1999. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin α5β1. Cell. Microbiol. 1:101–117 [DOI] [PubMed] [Google Scholar]
- 39.Alter P, Hoeschen J, Ritter M, Maisch B. 2002. Usefulness of cytokines interleukin-6 and interleukin-2R concentrations in diagnosing active infective endocarditis involving native valves. Am. J. Cardiol. 89:1400–1404 [DOI] [PubMed] [Google Scholar]
- 40.Rawczynska-Englert I, Hryniewiecki T, Dzierzanowska D. 2000. Evaluation of serum cytokine concentrations in patients with infective endocarditis. J. Heart Valve Dis. 9:705–709 [PubMed] [Google Scholar]
- 41.Watkin RW, Harper LV, Vernallis AB, Lang S, Lambert PA, Ranasinghe AM, Elliott TS. 2007. Pro-inflammatory cytokines IL6, TNF-α, IL1β, procalcitonin, lipopolysaccharide binding protein and C-reactive protein in infective endocarditis. J. Infect. 55:220–225 [DOI] [PubMed] [Google Scholar]
- 42.Dankert J, van der Werff J, Joldersma W, Zaat SA. 2006. Interleukin 1α increases the susceptibility of rabbits to experimental viridans streptococcal endocarditis. Infect. Immun. 74:947–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Josefsson E, Hartford O, O'Brien L, Patti JM, Foster T. 2001. Protection against experimental Staphylococcus aureus arthritis by vaccination with clumping factor A, a novel virulence determinant. J. Infect. Dis. 184:1572–1580 [DOI] [PubMed] [Google Scholar]
- 44.Tuchscherr LP, Buzzola FR, Alvarez LP, Lee JC, Sordelli DO. 2008. Antibodies to capsular polysaccharide and clumping factor A prevent mastitis and the emergence of unencapsulated and small-colony variants of Staphylococcus aureus in mice. Infect. Immun. 76:5738–5744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vernachio JH, Bayer AS, Ames B, Bryant D, Prater BD, Syribeys PJ, Gorovits EL, Patti JM. 2006. Human immunoglobulin G recognizing fibrinogen-binding surface proteins is protective against both Staphylococcus aureus and Staphylococcus epidermidis infections in vivo. Antimicrob. Agents Chemother. 50:511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verkaik NJ, van Wamel WJ, van Belkum A. 2011. Immunotherapeutic approaches against Staphylococcus aureus. Immunotherapy 3:1063–1073 [DOI] [PubMed] [Google Scholar]
- 47.Nicolau DP, Tessier PR, Nightingale CH. 1999. Beneficial effect of combination antiplatelet therapy on the development of experimental Staphylococcus aureus endocarditis. Int. J. Antimicrob. Agents 11:159–161 [DOI] [PubMed] [Google Scholar]