Abstract
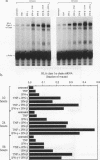
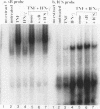
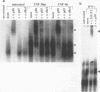
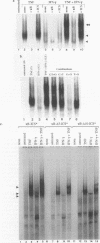
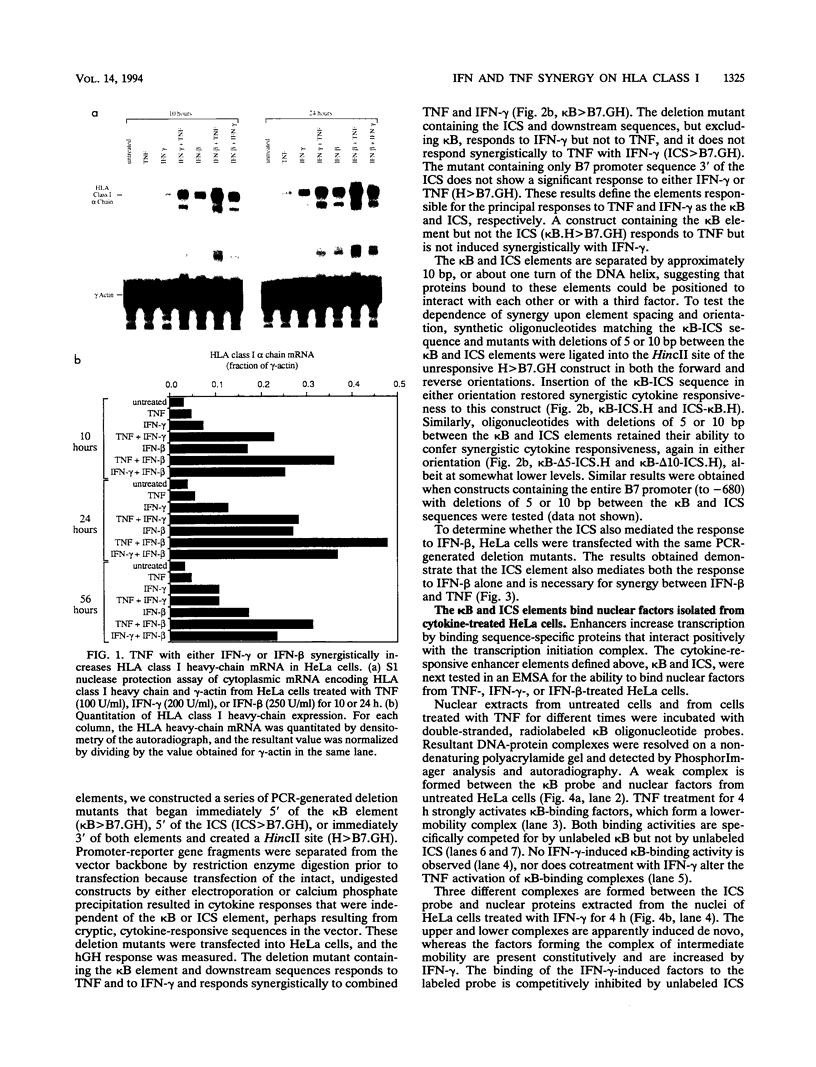
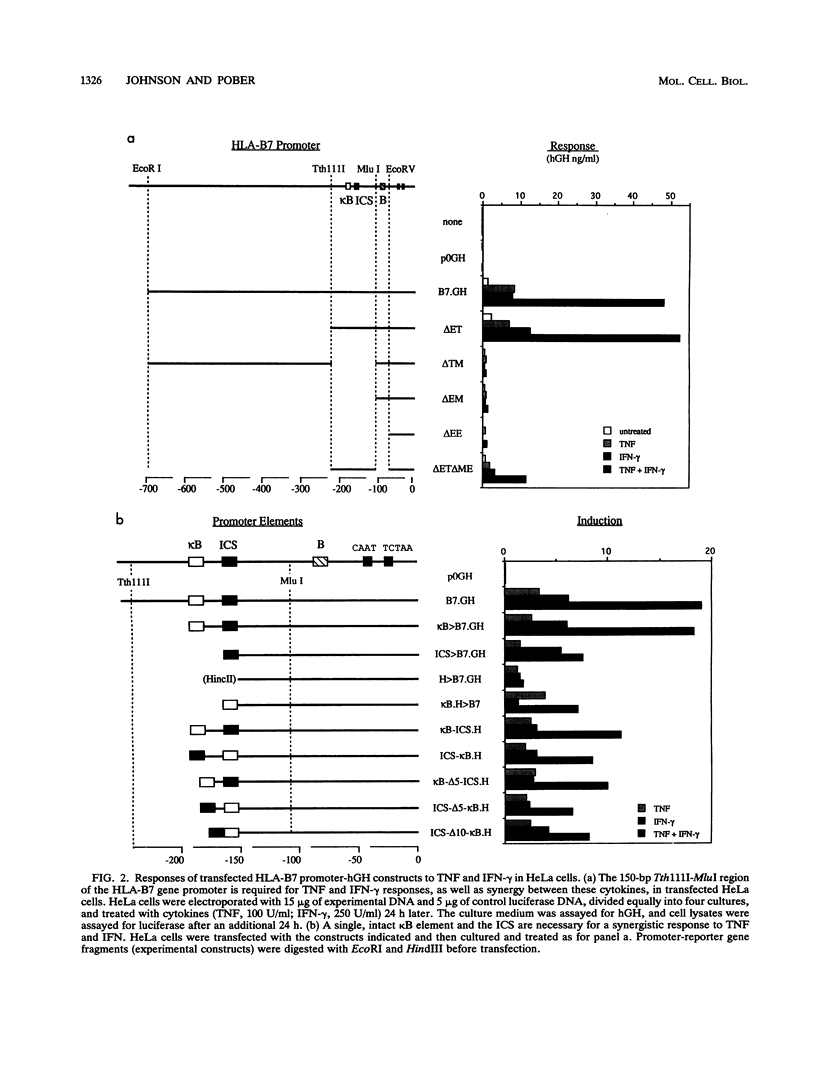
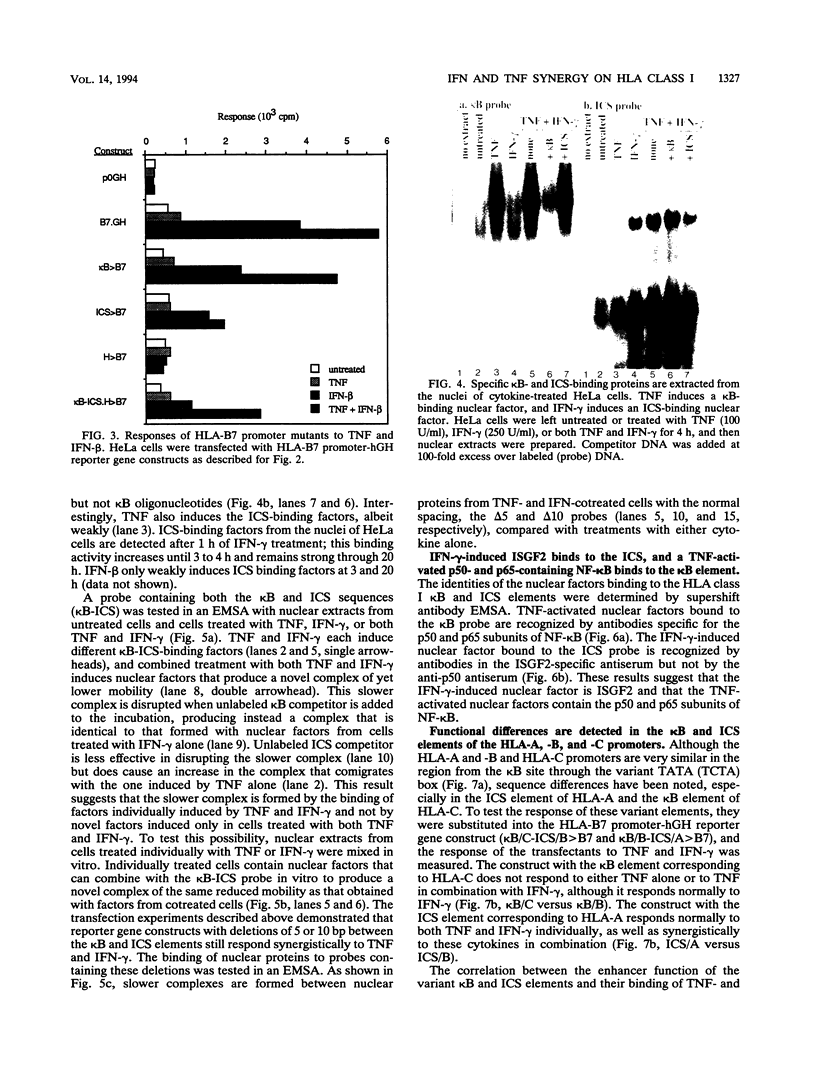
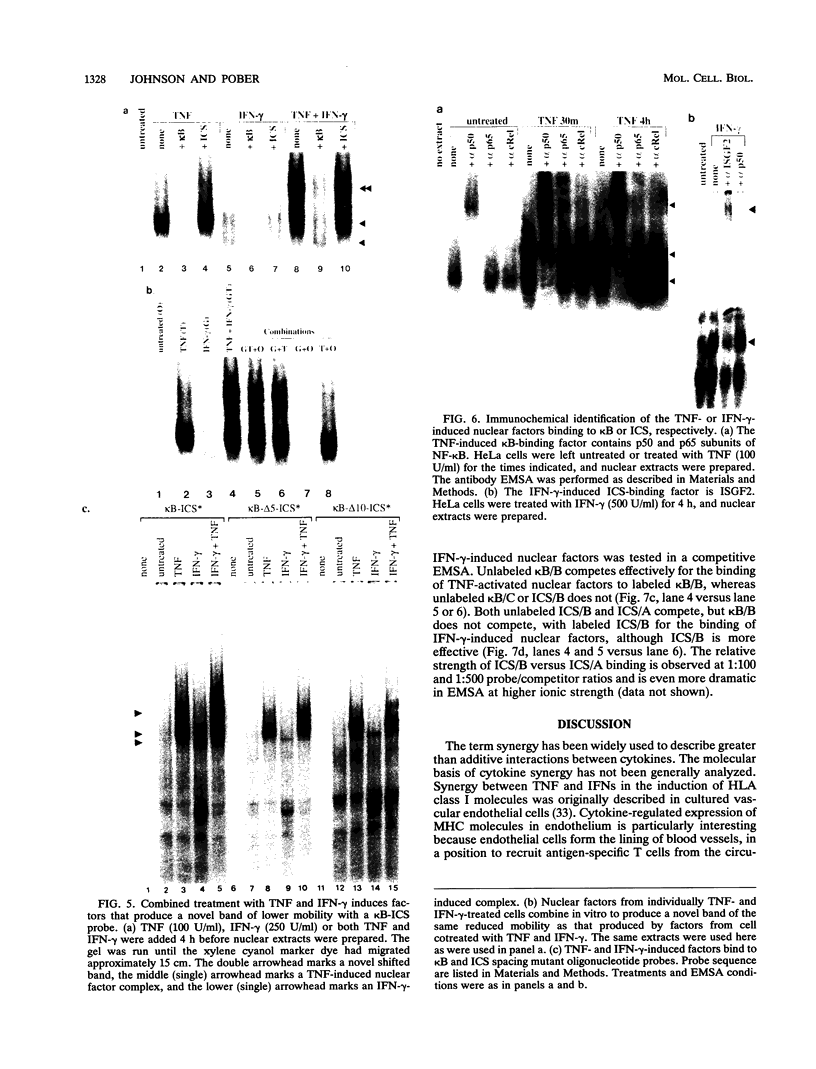
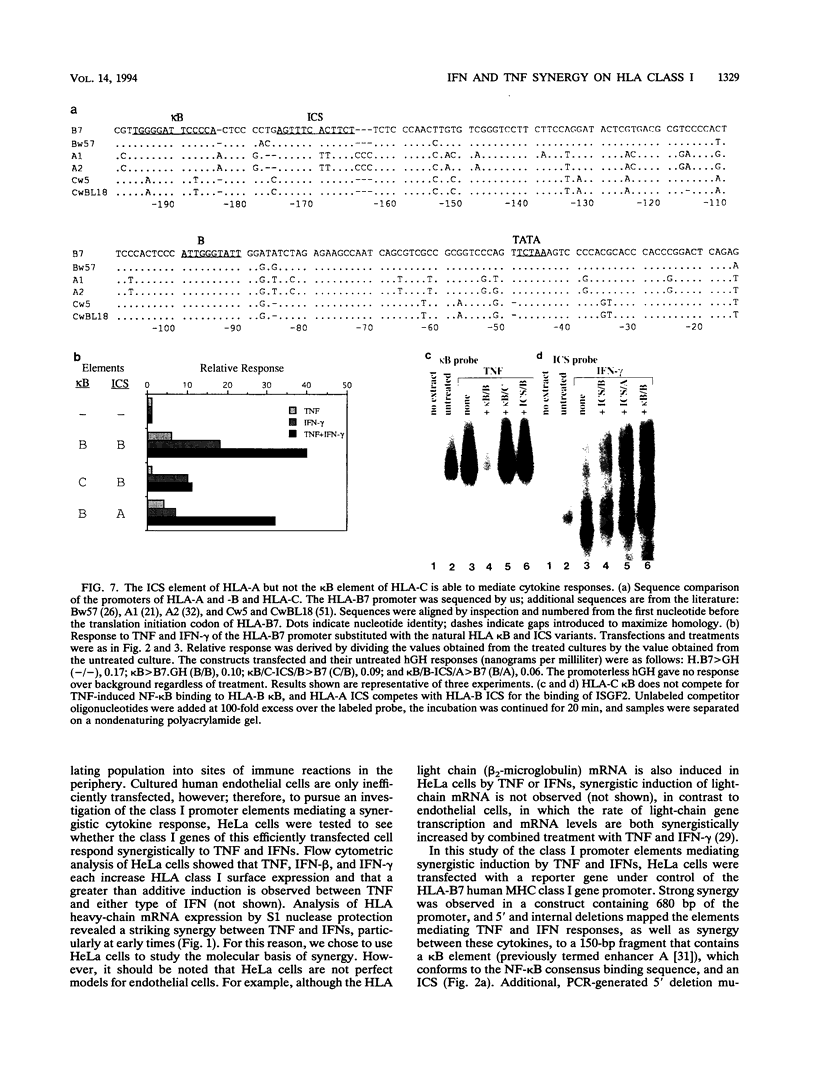
The cytokines tumor necrosis factor (TNF), beta interferon (IFN-beta), and IFN-gamma increase major histocompatibility complex class I molecule expression. A greater than additive (i.e., synergistic) induction of class I heavy-chain mRNA is observed in HeLa cells treated with TNF in combination with either type of IFN. To define the cis-acting elements mediating cytokine synergy, the promoter of a human major histocompatibility complex class I heavy-chain gene (HLA-B7) was placed in front of a reporter gene and transfected into HeLa cells. Deletion analysis mapped the elements required for synergy to a 40-bp region containing a kappa B-like element, which is necessary for the response to TNF, and an interferon consensus sequence (ICS), which is necessary for the responses to IFNs. When the orientation of these elements was reversed or their normal 20-bp spacing was reduced by 5 or 10 bp, i.e., one half or one full turn of the DNA helix, essentially equivalent responses were obtained, suggesting that these parameters are not critical. In electromobility shift assays, a p50-containing NF-kappa B nuclear factor from TNF-treated cells binds kappa B-containing probes, and ISGF-2 from IFN-gamma-treated cells binds ICS-containing probes. A probe containing both the kappa B and ICS elements (kappa B-ICS) forms a novel complex with nuclear factors isolated from cells treated with both TNF and IFN-gamma; this complex also forms when nuclear factors from individually cytokine-treated cells are mixed in vitro. The natural variant ICS found in HLA-A responds to IFN-gamma and can mediate synergy with TNF. However, the variant kappa B found in HLA-C does not respond to TNF, nor can it mediate synergy between TNF and IFN-gamma. These observations suggest that synergy between TNF and IFNs in the induction of HLA class I gene expression results from the sum of individual interactions of cytokine-activated enhancer-binding factors with the transcription initiation complex.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender B. S., Croghan T., Zhang L., Small P. A., Jr Transgenic mice lacking class I major histocompatibility complex-restricted T cells have delayed viral clearance and increased mortality after influenza virus challenge. J Exp Med. 1992 Apr 1;175(4):1143–1145. doi: 10.1084/jem.175.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanar M. A., Baldwin A. S., Jr, Flavell R. A., Sharp P. A. A gamma-interferon-induced factor that binds the interferon response sequence of the MHC class I gene, H-2Kb. EMBO J. 1989 Apr;8(4):1139–1144. doi: 10.1002/j.1460-2075.1989.tb03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchet O., Bourge J. F., Zinszner H., Israel A., Kourilsky P., Dausset J., Degos L., Paul P. Altered binding of regulatory factors to HLA class I enhancer sequence in human tumor cell lines lacking class I antigen expression. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3488–3492. doi: 10.1073/pnas.89.8.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bours V., Franzoso G., Azarenko V., Park S., Kanno T., Brown K., Siebenlist U. The oncoprotein Bcl-3 directly transactivates through kappa B motifs via association with DNA-binding p50B homodimers. Cell. 1993 Mar 12;72(5):729–739. doi: 10.1016/0092-8674(93)90401-b. [DOI] [PubMed] [Google Scholar]
- Brenner D. A., O'Hara M., Angel P., Chojkier M., Karin M. Prolonged activation of jun and collagenase genes by tumour necrosis factor-alpha. Nature. 1989 Feb 16;337(6208):661–663. doi: 10.1038/337661a0. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. W., Vasavada H. A., Ganguly S., Weissman S. M. Identification of cis sequences controlling efficient position-independent tissue-specific expression of human major histocompatibility complex class I genes in transgenic mice. Mol Cell Biol. 1991 Jul;11(7):3564–3572. doi: 10.1128/mcb.11.7.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow K. L., Hogan M. E., Schwartz R. J. Phased cis-acting promoter elements interact at short distances to direct avian skeletal alpha-actin gene transcription. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1301–1305. doi: 10.1073/pnas.88.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Hayakawa H., Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987 Feb 11;15(3):1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comb M., Mermod N., Hyman S. E., Pearlberg J., Ross M. E., Goodman H. M. Proteins bound at adjacent DNA elements act synergistically to regulate human proenkephalin cAMP inducible transcription. EMBO J. 1988 Dec 1;7(12):3793–3805. doi: 10.1002/j.1460-2075.1988.tb03264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. H., Yewdell J. W., Eisenlohr L. C., Johnson P. R., Bennink J. R. Antigen presentation requires transport of MHC class I molecules from the endoplasmic reticulum. Science. 1990 Feb 9;247(4943):715–718. doi: 10.1126/science.2137259. [DOI] [PubMed] [Google Scholar]
- Decker T., Lew D. J., Mirkovitch J., Darnell J. E., Jr Cytoplasmic activation of GAF, an IFN-gamma-regulated DNA-binding factor. EMBO J. 1991 Apr;10(4):927–932. doi: 10.1002/j.1460-2075.1991.tb08026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey A., Thornton A. M., Lonergan M., Weissman S. M., Chamberlain J. W., Ozato K. Occupancy of upstream regulatory sites in vivo coincides with major histocompatibility complex class I gene expression in mouse tissues. Mol Cell Biol. 1992 Aug;12(8):3590–3599. doi: 10.1128/mcb.12.8.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Martin P. L., Shastry B. S., Roeder R. G. Eukaryotic gene transcription with purified components. Methods Enzymol. 1983;101:582–598. doi: 10.1016/0076-6879(83)01039-3. [DOI] [PubMed] [Google Scholar]
- Driggers P. H., Elenbaas B. A., An J. B., Lee I. J., Ozato K. Two upstream elements activate transcription of a major histocompatibility complex class I gene in vitro. Nucleic Acids Res. 1992 May 25;20(10):2533–2540. doi: 10.1093/nar/20.10.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duh E. J., Maury W. J., Folks T. M., Fauci A. S., Rabson A. B. Tumor necrosis factor alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5974–5978. doi: 10.1073/pnas.86.15.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. L., Stark G. R. alpha-Interferon-induced transcription of HLA and metallothionein genes containing homologous upstream sequences. Nature. 1985 Apr 18;314(6012):637–639. doi: 10.1038/314637a0. [DOI] [PubMed] [Google Scholar]
- Fujita T., Reis L. F., Watanabe N., Kimura Y., Taniguchi T., Vilcek J. Induction of the transcription factor IRF-1 and interferon-beta mRNAs by cytokines and activators of second-messenger pathways. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9936–9940. doi: 10.1073/pnas.86.24.9936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly S., Vasavada H. A., Weissman S. M. Multiple enhancer-like sequences in the HLA-B7 gene. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5247–5251. doi: 10.1073/pnas.86.14.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Gifford A. M., Riviere L. R., Tempst P., Nolan G. P., Baltimore D. Cloning of the p50 DNA binding subunit of NF-kappa B: homology to rel and dorsal. Cell. 1990 Sep 7;62(5):1019–1029. doi: 10.1016/0092-8674(90)90276-k. [DOI] [PubMed] [Google Scholar]
- Girdlestone J., Milstein C. Differential expression and interferon response of HLA class I genes in thymocyte lines and response variants. Eur J Immunol. 1988 Jan;18(1):139–143. doi: 10.1002/eji.1830180121. [DOI] [PubMed] [Google Scholar]
- Girdlestone J. Nucleotide sequence of an HLA-A1 gene. Nucleic Acids Res. 1990 Nov 25;18(22):6701–6701. doi: 10.1093/nar/18.22.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakem R., Le Bouteiller P., Jezo-Bremond A., Harper K., Campese D., Lemonnier F. A. Differential regulation of HLA-A3 and HLA-B7 MHC class I genes by IFN is due to two nucleotide differences in their IFN response sequences. J Immunol. 1991 Oct 1;147(7):2384–2390. [PubMed] [Google Scholar]
- Harada H., Fujita T., Miyamoto M., Kimura Y., Maruyama M., Furia A., Miyata T., Taniguchi T. Structurally similar but functionally distinct factors, IRF-1 and IRF-2, bind to the same regulatory elements of IFN and IFN-inducible genes. Cell. 1989 Aug 25;58(4):729–739. doi: 10.1016/0092-8674(89)90107-4. [DOI] [PubMed] [Google Scholar]
- Hochschild A., Ptashne M. Interaction at a distance between lambda repressors disrupts gene activation. Nature. 1988 Nov 24;336(6197):353–357. doi: 10.1038/336353a0. [DOI] [PubMed] [Google Scholar]
- Isamat M., Girdlestone J., Milstein C. Nucleotide sequence of an HLA-Bw57 gene. Nucleic Acids Res. 1990 Nov 25;18(22):6702–6702. doi: 10.1093/nar/18.22.6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel A., Kimura A., Fournier A., Fellous M., Kourilsky P. Interferon response sequence potentiates activity of an enhancer in the promoter region of a mouse H-2 gene. Nature. 1986 Aug 21;322(6081):743–746. doi: 10.1038/322743a0. [DOI] [PubMed] [Google Scholar]
- Israël A., Le Bail O., Hatat D., Piette J., Kieran M., Logeat F., Wallach D., Fellous M., Kourilsky P. TNF stimulates expression of mouse MHC class I genes by inducing an NF kappa B-like enhancer binding activity which displaces constitutive factors. EMBO J. 1989 Dec 1;8(12):3793–3800. doi: 10.1002/j.1460-2075.1989.tb08556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. R., Pober J. S. Tumor necrosis factor and immune interferon synergistically increase transcription of HLA class I heavy- and light-chain genes in vascular endothelium. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5183–5187. doi: 10.1073/pnas.87.13.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieran M., Blank V., Logeat F., Vandekerckhove J., Lottspeich F., Le Bail O., Urban M. B., Kourilsky P., Baeuerle P. A., Israël A. The DNA binding subunit of NF-kappa B is identical to factor KBF1 and homologous to the rel oncogene product. Cell. 1990 Sep 7;62(5):1007–1018. doi: 10.1016/0092-8674(90)90275-j. [DOI] [PubMed] [Google Scholar]
- Kimura A., Israël A., Le Bail O., Kourilsky P. Detailed analysis of the mouse H-2Kb promoter: enhancer-like sequences and their role in the regulation of class I gene expression. Cell. 1986 Jan 31;44(2):261–272. doi: 10.1016/0092-8674(86)90760-9. [DOI] [PubMed] [Google Scholar]
- Koller B. H., Orr H. T. Cloning and complete sequence of an HLA-A2 gene: analysis of two HLA-A alleles at the nucleotide level. J Immunol. 1985 Apr;134(4):2727–2733. [PubMed] [Google Scholar]
- Lapierre L. A., Fiers W., Pober J. S. Three distinct classes of regulatory cytokines control endothelial cell MHC antigen expression. Interactions with immune gamma interferon differentiate the effects of tumor necrosis factor and lymphotoxin from those of leukocyte alpha and fibroblast beta interferons. J Exp Med. 1988 Mar 1;167(3):794–804. doi: 10.1084/jem.167.3.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo M. J., Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989 Jul 28;58(2):227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- Ljunggren H. G., Stam N. J., Ohlén C., Neefjes J. J., Höglund P., Heemels M. T., Bastin J., Schumacher T. N., Townsend A., Kärre K. Empty MHC class I molecules come out in the cold. Nature. 1990 Aug 2;346(6283):476–480. doi: 10.1038/346476a0. [DOI] [PubMed] [Google Scholar]
- Miyamoto M., Fujita T., Kimura Y., Maruyama M., Harada H., Sudo Y., Miyata T., Taniguchi T. Regulated expression of a gene encoding a nuclear factor, IRF-1, that specifically binds to IFN-beta gene regulatory elements. Cell. 1988 Sep 9;54(6):903–913. doi: 10.1016/s0092-8674(88)91307-4. [DOI] [PubMed] [Google Scholar]
- Palombella V. J., Maniatis T. Inducible processing of interferon regulatory factor-2. Mol Cell Biol. 1992 Aug;12(8):3325–3336. doi: 10.1128/mcb.12.8.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins N. D., Schmid R. M., Duckett C. S., Leung K., Rice N. R., Nabel G. J. Distinct combinations of NF-kappa B subunits determine the specificity of transcriptional activation. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1529–1533. doi: 10.1073/pnas.89.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine R., Decker T., Kessler D. S., Levy D. E., Darnell J. E., Jr Purification and cloning of interferon-stimulated gene factor 2 (ISGF2): ISGF2 (IRF-1) can bind to the promoters of both beta interferon- and interferon-stimulated genes but is not a primary transcriptional activator of either. Mol Cell Biol. 1990 Jun;10(6):2448–2457. doi: 10.1128/mcb.10.6.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M., Gann A. A. Activators and targets. Nature. 1990 Jul 26;346(6282):329–331. doi: 10.1038/346329a0. [DOI] [PubMed] [Google Scholar]
- Reich N. C., Darnell J. E., Jr Differential binding of interferon-induced factors to an oligonucleotide that mediates transcriptional activation. Nucleic Acids Res. 1989 May 11;17(9):3415–3424. doi: 10.1093/nar/17.9.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid L. E., Brasnett A. H., Gilbert C. S., Porter A. C., Gewert D. R., Stark G. R., Kerr I. M. A single DNA response element can confer inducibility by both alpha- and gamma-interferons. Proc Natl Acad Sci U S A. 1989 Feb;86(3):840–844. doi: 10.1073/pnas.86.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis L. F., Harada H., Wolchok J. D., Taniguchi T., Vilcek J. Critical role of a common transcription factor, IRF-1, in the regulation of IFN-beta and IFN-inducible genes. EMBO J. 1992 Jan;11(1):185–193. doi: 10.1002/j.1460-2075.1992.tb05041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C., Fu X. Y., Improta T., Aebersold R., Darnell J. E., Jr Proteins of transcription factor ISGF-3: one gene encodes the 91-and 84-kDa ISGF-3 proteins that are activated by interferon alpha. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7836–7839. doi: 10.1073/pnas.89.16.7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz M. L., Baeuerle P. A. The p65 subunit is responsible for the strong transcription activating potential of NF-kappa B. EMBO J. 1991 Dec;10(12):3805–3817. doi: 10.1002/j.1460-2075.1991.tb04950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüle R., Muller M., Kaltschmidt C., Renkawitz R. Many transcription factors interact synergistically with steroid receptors. Science. 1988 Dec 9;242(4884):1418–1420. doi: 10.1126/science.3201230. [DOI] [PubMed] [Google Scholar]
- Sen G. C., Lengyel P. The interferon system. A bird's eye view of its biochemistry. J Biol Chem. 1992 Mar 15;267(8):5017–5020. [PubMed] [Google Scholar]
- Shirayoshi Y., Burke P. A., Appella E., Ozato K. Interferon-induced transcription of a major histocompatibility class I gene accompanies binding of inducible nuclear factors to the interferon consensus sequence. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5884–5888. doi: 10.1073/pnas.85.16.5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava R., Duceman B. W., Biro P. A., Sood A. K., Weissman S. M. Molecular organization of the class I genes of human major histocompatibility complex. Immunol Rev. 1985 Jul;84:93–121. doi: 10.1111/j.1600-065x.1985.tb01127.x. [DOI] [PubMed] [Google Scholar]
- Stoscheck C. M. Quantitation of protein. Methods Enzymol. 1990;182:50–68. doi: 10.1016/0076-6879(90)82008-p. [DOI] [PubMed] [Google Scholar]
- Tibensky D., Delovitch T. L. Promoter region of HLA-C genes: regulatory elements common to and different from those of HLA-A and HLA-B genes. Immunogenetics. 1990;32(3):210–213. doi: 10.1007/BF02114976. [DOI] [PubMed] [Google Scholar]
- Townsend A., Elliott T., Cerundolo V., Foster L., Barber B., Tse A. Assembly of MHC class I molecules analyzed in vitro. Cell. 1990 Jul 27;62(2):285–295. doi: 10.1016/0092-8674(90)90366-m. [DOI] [PubMed] [Google Scholar]
- Yoshie O., Schmidt H., Lengyel P., Reddy E. S., Morgan W. R., Weissman S. M. Transcripts of human HLA gene fragments lacking the 5'-terminal region in transfected mouse cells. Proc Natl Acad Sci U S A. 1984 Feb;81(3):649–653. doi: 10.1073/pnas.81.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]