Abstract
The Plasmodium falciparum circumsporozoite (CS) protein (CSP) is a major vaccine target for preventing malaria infection. Thus, developing strong and durable antibody and T cell responses against CSP with novel immunogens and potent adjuvants may improve upon the success of current approaches. Here, we compare four distinct full-length P. falciparum CS proteins expressed in Escherichia coli or Pichia pastoris for their ability to induce immunity and protection in mice when administered with long-chain poly(I·C) [poly(I·C)LC] as an adjuvant. CS proteins expressed in E. coli induced high-titer antibody responses against the NANP repeat region and potent CSP-specific CD4+ T cell responses. Moreover, E. coli-derived CS proteins in combination with poly(I·C)LC induced potent multifunctional (interleukin 2-positive [IL-2+], tumor necrosis factor alpha-positive [TNF-α+], gamma interferon-positive [IFN-γ+]) CD4+ effector T cell responses in blood, in spleen, and particularly in liver. Using transgenic Plasmodium berghei expressing the repeat region of P. falciparum CSP [Pb-CS(Pf)], we showed that there was a 1- to 4-log decrease in malaria rRNA in the liver following a high-dose challenge and ∼50% sterilizing protection with a low-dose challenge compared to control levels. Protection was directly correlated with high-level antibody titers but not CD4+ T cell responses. Finally, protective immunity was also induced using the Toll-like receptor 4 agonist glucopyranosyl lipid adjuvant-stable emulsion (GLA-SE) as the adjuvant, which also correlated with high antibody titers yet CD4+ T cell immunity that was significantly less potent than that with poly(I·C)LC. Overall, these data suggest that full-length CS proteins and poly(I·C)LC or GLA-SE offer a simple vaccine formulation to be used alone or in combination with other vaccines for preventing malaria infection.
INTRODUCTION
Malaria infection with Plasmodium falciparum causes more than 600,000 deaths annually as well as significant morbidity worldwide (1). A range of efforts to control and treat malaria include public health measures such as insecticide-treated bed nets, indoor residual spraying, and widespread usage of antimalarial drugs. Despite the impact of these approaches, the most cost-effective solution to prevent infection and to ultimately control the malaria endemic is to develop a vaccine.
Currently, the most advanced vaccine tested in humans against P. falciparum infection is RTS,S, which targets the circumsporozoite (CS) protein (CSP), the major and most abundant antigen expressed on the surfaces of infectious sporozoites. RTS,S given with the AS01 adjuvant (RTS,S/AS01) shows ∼30% protection against clinical disease and severe malaria (2, 3). Thus, while these first results in phase III trials with RTS,S/AS01 are encouraging, there may be additional approaches for further optimizing the breadth, potency, and duration of immunity against the CSP using different immunogens or more-potent adjuvants. In terms of antigen design, RTS,S is comprised of a truncated form of CSP containing the central repeat region, NANP, which is a target for antibody-mediated neutralization, as well as CD8+ and CD4+ T cell epitopes at the C-terminal end. This truncated CS protein is then fused to the hepatitis B virus surface antigen, creating an immunogenic particle. Therefore, using a more-full-length CSP, including the N-terminal end and the R1 region of CSP as well as the minor repeat region (NVDP), might favor broader antibody responses than against the NANP repeat region alone (4–8). Moreover, a full-length CSP may provide additional T cell epitopes, leading to increased breadth of cellular immunity, which could also enhance protection. Another approach is to enhance the humoral and cellular immune responses by altering the type of adjuvant given with the full-length-CSP-based protein vaccine.
Early studies in mice showed that protection was associated with high antibody titers (9–11). The next generation of malaria vaccines combined CSP with more-potent adjuvants, like Pseudomonas aeruginosa exotoxin A, monophospholipid A (MPL), mycobacterial cell wall skeleton, or squalene (Detox; Ribi Immunochem) (12–14), which resulted in high antibody titers; however, they failed to confer sufficient protective efficacy (15–17). In contrast, studies using irradiated sporozoites for vaccination have shown a critical requirement for gamma interferon (IFN-γ) and cellular immunity in mediating protection against malaria (18, 19). Accordingly, optimizing CD4+ T cell-derived IFN-γ production after RTS,S vaccination by altering the adjuvant formulation enhanced protection (20–22). Using the Toll-like receptor 4 (TLR4) ligand MPL and saponin (QS-21) in an oil-water emulsion (AS02) or liposome (AS01B or -E) formulation led to a strong antibody response and increased CD4+ T cell immunity compared to levels induced with older formulations with alum and MPL (21, 23, 24). Collectively, these data highlight the importance of adjuvant formulations in optimizing immunity and protection.
In this study, long-chain poly(I·C) [poly(I·C)LC] and the TLR4 agonist glucopyranosyl lipid adjuvant-stable emulsion (GLA-SE) were compared as adjuvants when they were administered with a number of CS proteins expressed in the yeast Pichia pastoris or in Escherichia coli, to which we refer as full length but which lack the glycosylphosphatidylinositol (GPI) signal region and contain various numbers of the NANP repeat region. Poly(I·C)LC is a unique formulation of long-chain double-stranded RNA, polyinosinic-poly(C), and carboxy methylcellulose that has extended in vivo activity compared to that of poly(I·C). Poly(I·C)LC mediates innate signaling through TLR3 and melanoma differentiation-associated protein 5 (MDA-5), leading to activation of dendritic cells and induction of interleukin 12 (IL-12) and type I interferons (IFNs) (25–27). In addition, poly(I·C) has been shown to promote T cell survival and enhance germinal-center formation through the generation of CD4+ T follicular helper (Tfh) cells (28). As an immune adjuvant, poly(I·C)LC has been shown to elicit strong humoral and cellular immunity when administered with a variety of protein- or dendritic cell-targeting vaccines in a number of mouse and nonhuman primate (NHP) studies (29–35).
GLA is a synthetic and therefore homogeneous variant of the TLR4 agonist lipid A, formulated in a stable oil-in-water emulsion (SE) (36). Strong Th1 immune responses induced by protein antigens in combination with GLA-SE have been observed in mouse models of tuberculosis (37), leishmaniasis (38), and influenza (39). Additionally, GLA-SE showed an adjuvant activity similar to or enhanced relative to that of MPL-SE (one of the adjuvant components in AS01) in various animal models, such as mice, guinea pigs, and nonhuman primates (40, 41).
Here, we compare four full-length P. falciparum CS proteins expressed in E. coli or in the yeast P. pastoris given with poly(I·C)LC or GLA-SE as an adjuvant and determine their relative immunogenicities and levels of protection using an in vivo mouse challenge model. Furthermore, one of the CS proteins and poly(I·C)LC was tested in NHPs. Together, these data provide a potential simple vaccine formulation for inducing potent and protective P. falciparum CSP responses.
MATERIALS AND METHODS
Animals.
Six- to 8-week-old C57BL/6 mice were obtained from The Jackson Laboratories (Bar Harbor, ME) and maintained in the Vaccine Research Center Animal Care Facility (Bethesda, MD) under pathogen-free conditions. Male, Indian rhesus macaques were stratified into comparable groups on the basis of age and weight. The animals were housed at the School of Medicine, Comparative Medicine and Veterinary Resources, University of Maryland. All experiments were conducted according to the guidelines of the National Research Council under protocols approved by the Institutional Animal Care and Use Committee at the National Institutes of Health.
Reagents.
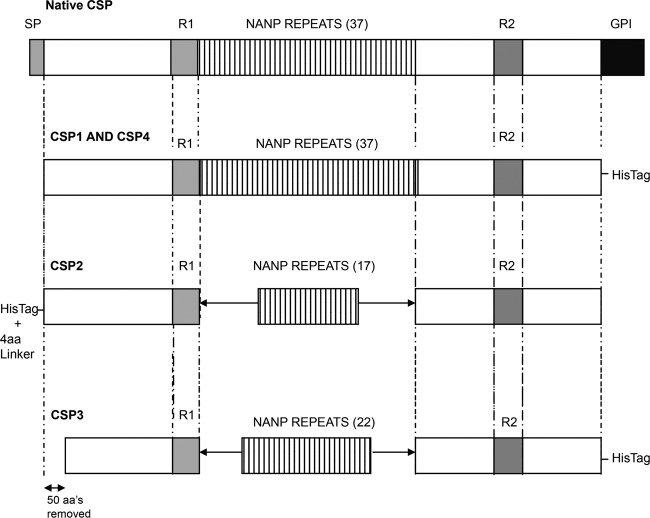
Four P. falciparum CS proteins were provided by the PATH Malaria Vaccine Initiative (MVI; Washington, DC) and provided to us in a blind manner (they were designated CSP1, CSP2, CSP3, and CSP4). These four proteins are called full length in the text, but they lack the GPI signal region and contain various numbers of the repeat region. After the immune studies were completed, it was revealed to us that two proteins were expressed in E. coli (CSP1 and CSP2) and two were expressed in P. pastoris (CSP3 and CSP4). CSP1 and CSP2 were provided to the MVI from Gennova (Pune, India) and WRAIR (Silver Spring, MD), respectively. Protein concentration was measured using a bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL). Endotoxin contaminants were determined using the Limulus amebocyte lysate (LAL) assay (Lonza, Walkersville, MD). If endotoxin levels exceeded 2 endotoxin units (EU)/ml, endotoxin was removed by two-phase extraction with Triton X-114. Small amounts of endotoxin was detected in the E. coli-derived proteins. Thus, all four proteins, including the two expressed in yeast, which had no detectable endotoxin, were treated with Triton X-114. All proteins used for immunization had final endotoxin levels below 1 EU/ml. After removal of endotoxin, protein concentration was determined to confirm that all groups of mice received the same amount of protein. P. falciparum CSP15-mer peptides overlapping by 11 amino acids (aa) and spanning the entire length of the protein (P. falciparum 3D7 strain) were synthesized by GenScript. Each batch of CSP was tested for accurate size using SDS-PAGE.
Antibodies.
The following anti-mouse antibodies for flow cytometry were purchased from BD Pharmingen: purified anti-CD28 (37.51), PerCP–Cy55–anti-CD3 (145-2C11), and Alexa 700–anti-CD4 (RM4-5). The following antibodies were purchased from BioLegend: allophycocyanin (APC)–Cy7–anti-CD8 (53-6.7). LIVE/DEAD fixable violet dead cell stain (ViViD) were purchased from Molecular Probes, and staining was performed as described by Perfetto et al. (42). Intracellular staining was performed according to the BD Cytofix/Cytoperm kit instructions using APC–anti-IFN-γ (XMG1.2), phycoerythrin (PE)–Cy7–anti-tumor necrosis factor alpha (anti-TNF-α) (MP6-XT22), and PE–anti-IL-2 (MQ1-17H12), which were purchased from BD Biosciences, and Alexa Fluor 488–anti-IL-10 (JES5-16E3), which was purchased from eBioscience.
For staining of nonhuman primate (NHP) cells, the following anti-human antibodies were purchased from BD Pharmingen: purified anti-CD49d (9F10), APC–Cy7–anti-CD3 (SP34-2), PE–Cy7–anti-TNF-α (MAb11), PE–anti-IL-2 (MQ1-17H12), PE–Cy5–anti-CD95 (DX2), and fluorescein isothiocyanate (FITC)–anti-IFN-γ (B27). PacificBlue–anti-CCR7 (TG8/CCR7) was purchased from BioLegend. Anti-CD45RA–R phycoerythrin-Texas Red (ECD) (clone 2H4LDH11LDB9) was purchased from Beckman Coulter, and Qdot605–anti-CD4 (S3.5) was purchased from Invitrogen. The conjugates Alexa 680–anti-CD28 (CD28.2) and Qdot655–anti-CD8 (RPA-T8) were produced in-house by the laboratory of Mario Roederer (NIAID, NIH, Bethesda, MD).
Immunizations.
C57BL/6 mice were immunized with 2 μg or 20 μg of CSP, with or without 50 μg poly(I·C)LC (Oncovir, Inc., Washington, DC) or 5 μg GLA-SE (Infectious Disease Research Institute, Seattle, WA) which are the optimal doses for these adjuvants. Naive mice or mice immunized with the adjuvant alone served as controls. The vaccines were administered subcutaneously (s.c.) in both hind footpads in a total volume of 50 μl per foot. Animals were immunized at weeks 0 and 3 or 0, 3, and 6.
Rhesus macaques were immunized with 100 μg of P. falciparum CSP mixed with 1 mg of poly(I·C)LC. A total volume of 1 ml was injected s.c. into the deltoid area of the upper arm. For boosting, the opposite arm was used. Animals were immunized at weeks 0, 5, and 16.
Analysis of CD4+ T cell responses.
Cells from mice were harvested from spleens at various times postvaccination, and single-cell suspensions (2 × 106 cells/well) from individual mice were incubated for 5 h with anti-CD28, 10 μg/ml Brefeldin A, and 2 μg/ml CSP peptide pool. Cells were stained with the viability dye LIVE/DEAD fixable violet dead cell stain (ViViD), CD4, and CD8, followed by intracellular staining for CD3, IFN-γ, IL-2, IL-10, and TNF-α using the BD Cytofix/Cytoperm kit according to the manufacturer's instructions. For intrahepatic-lymphocyte isolation, the liver was perfused with phosphate-buffered saline (PBS) before being processed, and lymphocytes were isolated by Percoll density centrifugation. Stimulation and staining were performed as described for splenocytes in this section.
For analysis of NHP peripheral blood mononuclear cells (PBMCs), cryopreserved cells were thawed and rested overnight at 37°C. The next day, cells (2 × 106 cells/well) were stimulated for 5 h with anti-CD49d and anti-CD28, 10 μg/ml Brefeldin A, and 2 μg/ml CSP peptide pool. Cells were stained in warm medium with CCR7, followed by surface staining with a LIVE/DEAD fixable aqua dead cell stain kit (Invitrogen), CD45RA, CD95, CD4, and CD8 in PBS. Intracellular cytokine staining was performed using IFN-γ, IL-2, CD3, and TNF-α with the BD Cytofix/Cytoperm kit according to the manufacturer's instructions. Cells were resuspended in 1% paraformaldehyde, acquired on a modified BD LSR II flow cytometer, and analyzed using FlowJo software (Tree Star), Pestle, and SPICE (Mario Roederer, NIAID, NIH).
Detection of CSP-specific antibodies.
Serum samples from immunized mice and NHPs were analyzed for IgG antibodies specific for the repeat region ([NANP]6 peptide) of P. falciparum CSP by the Malaria Serology Laboratory at WRAIR. Plates were read at 414 nm, and endpoint titers were calculated at an optical density (OD) of 1.0. For analysis of total CSP-specific IgG1 and IgG2a titers, enzyme-linked immunosorbent assay (ELISA) plates were coated with CSP1 protein at a concentration of 1 μg/ml and washed. Serially diluted serum samples from immunized or control mice were added in duplicate for 2 h. After being washed, samples were incubated with either anti-mouse IgG1-horseradish peroxidase (HRP) or anti-mouse IgG2a-HRP. Plates were then developed with 3,3′,5,5′-tetramethylbenzidine substrate-chromogen (Dako) and read at 450 nm. Midpoint 50% effective concentration (EC50) titers were calculated using 4PL curve fitting.
Immunofluorescent antibody (IFA) assay.
To determine whether serum bound to sporozoites, slides (Tekdon Inc.; poly-l-lysine coated) were coated with a 10-μl suspension of sporozoites of P. berghei expressing the repeat region of CSP from P. falciparum [Pb-CS(Pf)] at a concentration of 4 × 105 to 6 × 105 sporozoites/ml and air dried. Serum samples from immunized mice or NHPs were diluted in PBS-1% bovine serum albumin (BSA) prior to their addition to the slides (10 μl of sample) and then incubated for 30 min at room temperature. Slides were then washed with PBS-1% BSA, and 10 μl of a secondary-antibody solution [Alexa Fluor 488 F(ab′)2 fragment of goat anti-mouse IgG(H+L) (2 μg/ml; Invitrogen) and FITC-labeled goat anti-monkey IgG(H+L) (KPL) for mice and NHPs, respectively] was added for 30 min at room temperature; then slides were washed with PBS-1% BSA. Fluorescent sporozoites were visualized using an upright fluorescence microscope (Nikon Eclipse 90i). The lowest titer at which sporozoites were visualized was scored as positive. Binding of serum antibodies to sporozoites was scored as +++ (very good), ++ (good), + (weak), or − (no) binding.
Sporozoite challenge.
Mice were challenged intravenously (i.v.) with 1.5 × 104 transgenic Pb-CS(Pf) sporozoites, kindly provided by E. Nardin (10). Approximately 40 h later, mice were euthanized to assess parasite burden in livers. Parasite loads were determined by quantitative PCR (qPCR) for P. berghei 18S rRNA (43). For the assessment of blood-stage parasitemia, mice were challenged i.v. with 1 × 103 of the transgenic Pb-CS(Pf) sporozoites. Starting on day 4, blood smears were taken and observed under a microscope. Smears were fixed with methanol (for 30 s) before being stained with a 10% Giemsa stain solution (Sigma-Aldrich) for 15 min.
Serum transfer.
Serum was collected from individual mice 2 weeks after three immunizations with 20 μg CSP1 and poly(I·C)LC, and 0.5 ml was transferred i.v. immediately prior to challenge.
Isolation of PBMCs from NHPs.
PBMCs were isolated from fresh blood by Ficoll density centrifugation using LeucoSep tubes (Greiner Bio One) according to the manufacturer's instructions. After several washes, cells were cryopreserved.
Statistics.
The majority of the data and statistical analysis were created using Prism software (GraphPad) and a Mann-Whitney test. Differences were found to be significant when P was less than 0.05 or 0.01. Data are represented as means + standard deviations (SD) or as geometrical means, as indicated in the figure legends. Bar and pie charts of cytokine production were created using FlowJo software (Tree Star), Pestle, and SPICE (Mario Roederer, NIAID, NIH).
RESULTS
E. coli-derived CSP induces robust CD4+ T cell cytokine responses.
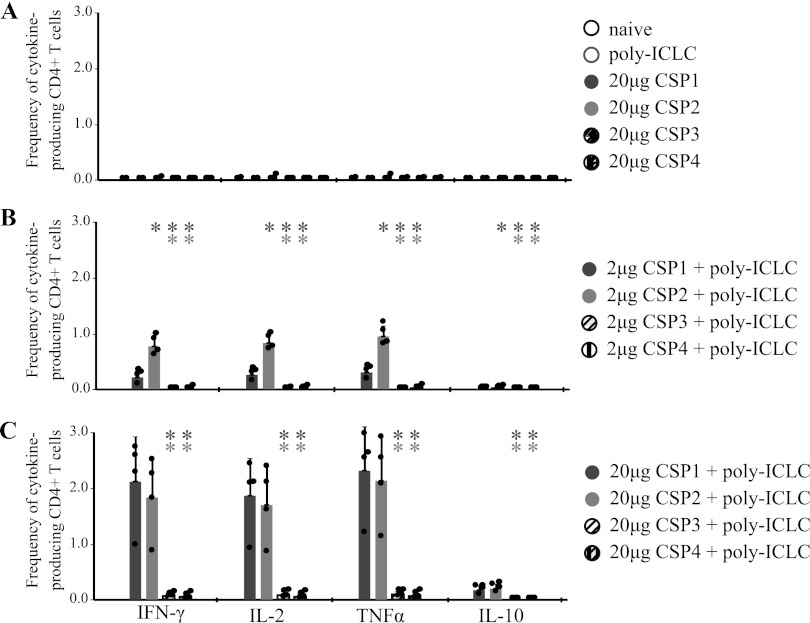
The goal of this blinded study was to compare the immunogenicities of four full-length CSPs (CSP1 to -4) in combination with different adjuvants. CSP1 and CSP2 were produced in E. coli, while CSP3 and CSP4 were expressed in the yeast P. pastoris. The amino acid sequences of CSP2 and CSP3, derived from the 3D7 strain of the malaria parasite, share strong homology at the amino- and carboxy-terminal regions but differ in their numbers of NANP repeats (Fig. 1). CSP1 and CSP4 are derived from the India strain IND637HDD1, contain all 37 NANP repeats, and share strong amino acid sequence homology. The 3D7 strain has a deletion in the N-terminal region (aa 91 to 97), whereas this region is intact in the India strain. We first selected poly(I·C)LC as an adjuvant based on its potency for generating T cell and antibody responses shown in prior studies by our lab and others (34, 35). C57BL/6 mice were immunized twice, 3 weeks apart, with 2 μg or 20 μg of CSP with or without 50 μg poly(I·C)LC. Four weeks after the second immunization, CD4+ T cell responses in the spleens were assessed by multiparameter flow cytometry. Mice immunized with poly(I·C)LC or any of the CSPs without adjuvant had no measurable CSP-specific CD4+ T cell cytokine responses (Fig. 2A). In contrast, 2 μg of CSP1 and CSP2 administered with poly(I·C)LC induced robust CD4+ T cell cytokine responses, producing IFN-γ, IL-2, and TNF-α (Fig. 2B). Such responses were increased ∼2- to 3-fold by using 20 μg of CSP with poly(I·C)LC (Fig. 2C). Remarkably, both CSPs produced in yeast (CSP3 and CSP4) failed to induce significant antigen-specific CD4+ T cell responses with poly(I·C)LC, even at the higher dose (20 μg).
Fig 1.
Graphic diagrams of the characteristics of four different CSPs. Full-length CSP consists of the signal peptide (SP), two highly conserved motifs at the N- and C-terminal ends of the protein, region 1 and region 2 plus (R1 and R2), tandem repeats (37 NANP repeats), and the GPI anchor signal (GPI). CSP1 and CSP2 were produced by expression in Escherichia coli, CSP3 and CSP4 by expression in P. pastoris.
Fig 2.
C57BL/6 mice (n = 4) were immunized twice, 3 weeks apart, with either 2 μg or 20 μg of various CSPs and 50 μg poly(I·C)LC. Naive mice and mice that received poly(I·C)LC alone or 20 μg of each protein alone served as controls. Four weeks later, T cells in spleens were analyzed for cytokine production by flow cytometry. Frequencies of CSP-specific IFN-γ-, IL-2-, TNF-α-, or IL-10-producing CD4+ T cells are shown for protein-only and control mice (A) and mice that received either 2 μg (B) or 20 μg (C) of CSP with poly(I·C)LC. Data are expressed as means + SD and are representative of two independent experiments. *, P < 0.05. The color of the asterisk indicates the group to which the value is being compared.
E. coli-derived CSP induces antibodies against the NANP repeat region.
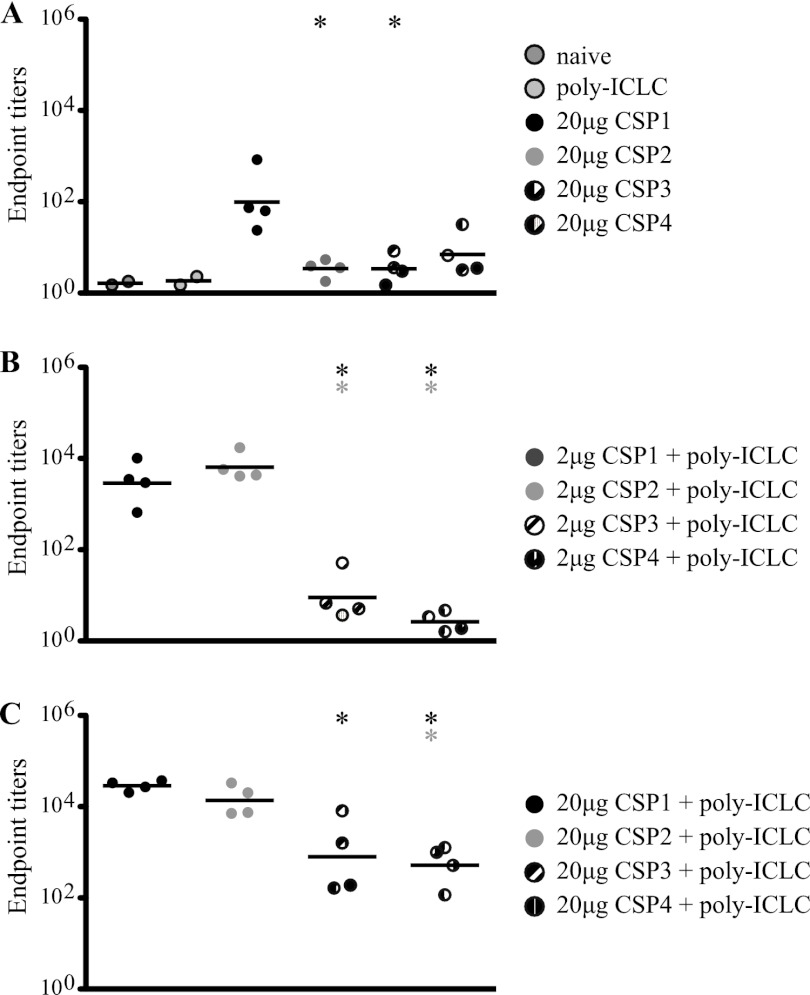
Since antibodies against CSP are critical for protection, we assessed responses against the NANP repeat region following immunization of all vaccine groups. Consistent with the data in Fig. 2, CSP1 and CSP2 vaccines produced in E. coli elicited significantly more potent antibody responses than did CSP3 and CSP4 expressed in yeast (Fig. 3A to C). Higher antibody titers were observed in mice that received the higher dose (20 μg) of CSP and poly(I·C)LC. Taken together, the E. coli-derived proteins were more potent for CSP-specific antibody and CD4+ T cell immunity in this study with poly(I·C)LC or with CpG as an adjuvant (data not shown). Therefore, CSP1 was selected for further testing.
Fig 3.
C57BL/6 mice (n = 4) were immunized as described in the legend of Fig. 2. At the time of tissue harvest, sera were collected and analyzed for CSP-specific IgG antibodies in protein-only and control mice (A) and mice that received either 2 μg (B) or 20 μg (C) of CSP with poly(I·C)LC. Antibodies were measured against part of the repeat region ([NANP]6). Data points are graphed as geometrical means of endpoint titers and are representative of two independent experiments. *, P < 0.05. The color of the asterisk indicates the group to which the value is being compared.
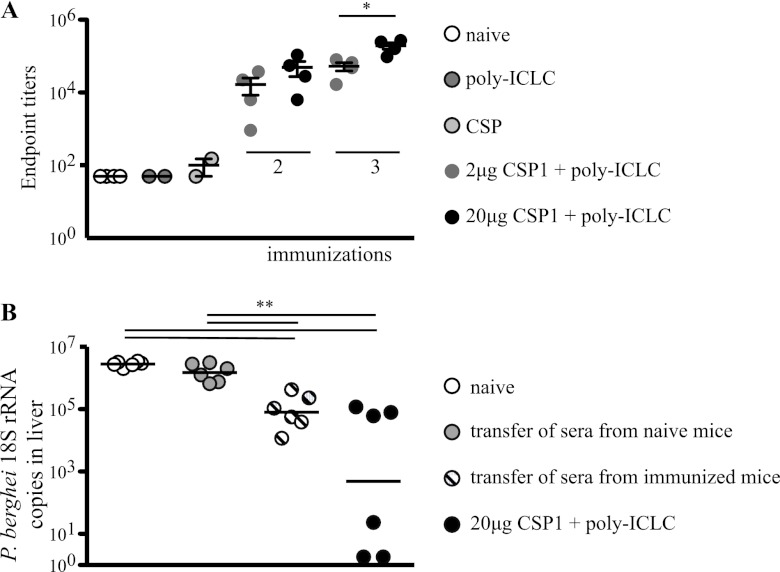
Protection requires three immunizations with CSP and poly(I·C)LC.
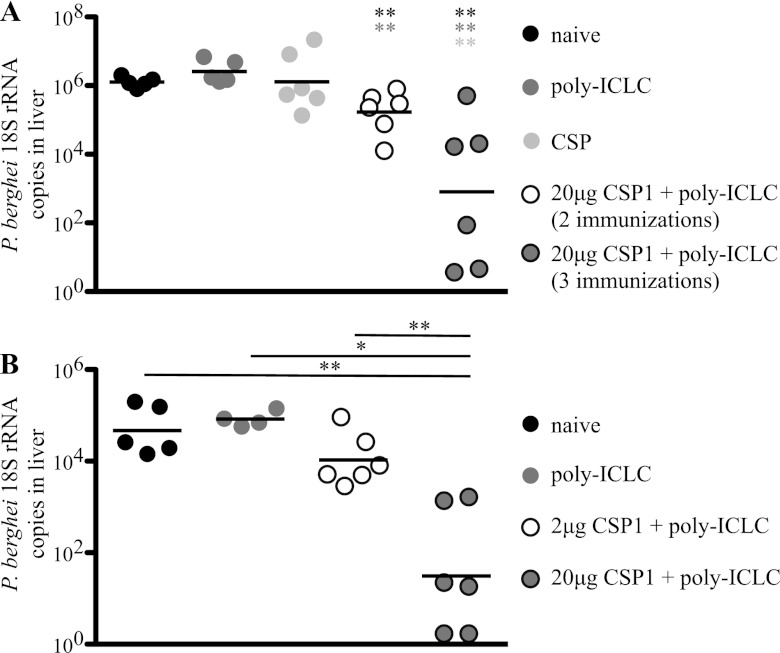
We next sought to establish the optimal antigen dose and number of immunizations required to induce protection against a challenge. Because mice are not susceptible to P. falciparum, we used a recombinant P. berghei parasite expressing the P. falciparum CSP repeat region [Pb-CS(Pf)] (10) as a challenge for our P. falciparum CSP-vaccinated mice. Mice were immunized two or three times at 3-week intervals with 20 μg of CSP1 and poly(I·C)LC. Four weeks after the last immunization, mice were challenged i.v. with 1.5 × 104 Pb-CS(Pf) parasites. Forty hours later, livers of infected mice were harvested and parasite-derived 18S rRNA was measured by real-time qPCR (RT-qPCR). Mice that received two immunizations with 20 μg of CSP1 and poly(I·C)LC showed a modest but significant (∼0.5 log) reduction (P < 0.01) of parasite-derived 18S rRNA levels compared to naive mice or mice that received poly(I·C)LC or CSP1 alone (Fig. 4A). Moreover, three immunizations with 20 μg CSP1 and poly(I·C)LC resulted in an ∼2- to 4-log reduction in 18S rRNA (Fig. 4A) compared to levels in mice immunized with protein alone, poly(I·C)LC alone, or CSP1 protein and poly(I·C)LC (two immunizations). A separate series of experiments sought to determine whether the dose of CSP1 influenced protection. We compared three immunizations with 2 or 20 μg of CSP1 and poly(I·C)LC. As shown in Fig. 4B, the higher dose of CSP1 provided a significant (P = 0.005) (∼2-log) reduction in parasite load in the livers of mice compared to those with the lower dose of CSP1. Overall, these data show that three immunizations with the higher dose (20 μg) of CSP1 and poly(I·C)LC provided significant reduction of parasite load against a high-dose i.v. challenge with sporozoites.
Fig 4.
C57BL/6 mice (n = 6) were immunized two or three times, 3 weeks apart, with 2 or 20 μg of CSP1 and 50 μg poly(I·C)LC. Naive mice and mice that received poly(I·C)LC alone or 20 μg of CSP1 alone served as controls. Four weeks later, mice were challenged with 1.5 × 104 Pb-CS(Pf) sporozoites. Parasite burden was determined 40 h postchallenge by counting the copies of 18S rRNA in the livers by RT-PCR. Shown are comparisons of levels of protective immunity in mice that received either two or three immunizations of 20 μg CSP1 and poly(I·C)LC (A) or three immunizations with 2 μg or 20 μg CSP1 and poly(I·C)LC (B). Data points are graphed as geometrical means and are representative of two independent experiments *, P < 0.05; **, P < 0.01. The color of the asterisk (A) or the length of the bar (B) indicates the group to which the value is being compared.
Antibody against CSP correlates with protection.
In the next series of experiments, we sought to determine the immunological correlates of protection following immunization with CSP1 and poly(I·C)LC. Prior studies in mice and humans showed that antibodies and CD4+ T cells play a role in protection (20, 22, 44–48). In Fig. 3, we show that two immunizations with 2 or 20 μg of CSP1 induced potent antibody responses against the NANP repeat region. To extend this analysis, we assessed CSP-specific antibody titers at both doses of CSP after two or three immunizations. As shown in Fig. 5A, the greatest antibody response was detected with the higher dose of CSP1 given three times, consistent with improved protection in this group (Fig. 4). To substantiate a direct role of antibodies in mediating protection, pooled serum from mice immunized three times with 20 μg CSP1 and poly(I·C)LC was transferred into naive recipients, and then the mice were challenged. As a negative control, serum from naive mice was transferred to naive mice. As a positive control, mice immunized three times with 20 μg of CSP1 and poly(I·C)LC were challenged at the same time. Naive mice that received serum from CSP1- and poly(I·C)LC-vaccinated animals showed an ∼1-log reduction in parasite burden compared to mice that received control serum or naive mice (Fig. 5B). However, this protection was not as robust as that seen in animals immunized with CSP1 and poly(I·C)LC. This could be due to dilution of transferred antibodies in the blood of recipient animals or to a contribution of vaccine-elicited CD4+ T cells absent in passively transferred animals. Nevertheless, these data show that humoral immunity is sufficient to confer some protection against this high-dose challenge.
Fig 5.
(A) C57BL/6 mice (n = 4 to 6) were immunized two or three times, 3 weeks apart, with 2 or 20 μg of CSP1 and 50 μg poly(I·C)LC. Naive mice and mice that received poly(I·C)LC alone or 20 μg of CSP1 alone served as controls. Two weeks later, sera were collected and CSP-specific IgG antibodies were determined. Data points are graphed as means + SD. *, P < 0.05. The values for all control groups are significantly lower than those for vaccine groups. (B) Serum from mice immunized three times with CSP1 and poly(I·C)LC or serum from naive mice was transferred into naive mice at the time of challenge with 1.5 × 104 Pb-CS(Pf) sporozoites. Vaccinated mice (CSP1 and poly(I·C)LC) served as a positive control. Forty hours postchallenge, parasite burden was determined by counting the copies of 18S rRNA in the livers by RT-PCR. Black bars show the geometrical means. **, P < 0.01. Results are representative of at least two independent experiments.
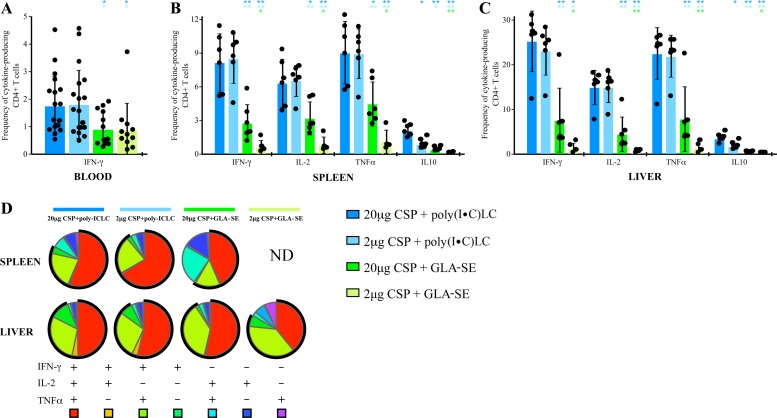
Poly(I·C)LC induces higher CD4+ Th1 cell immunity than GLA-SE.
The RTS,S vaccine has been formulated with the TLR4 ligand MPL and QS-21 in either a liposomal solution (AS01) or oil-in-water emulsion (AS02). Thus, to compare poly(I·C)LC (which signals through TLR3 and MDA-5) to an adjuvant with some commonality to AS02, we choose GLA-SE. This formulation consists of a synthetic form of the TLR4 agonist lipid A, which has been shown to provide adjuvant activity that is similar to, if not better than, that of MPL-SE (49).
Mice were immunized three times with 2 μg or 20 μg of CSP1 formulated with either poly(I·C)LC or GLA-SE, and the CD4+ T cell responses were analyzed in the blood, spleen, and liver 7 to 14 days after the third vaccination. At this peak time point, the frequencies of IFN-γ-producing CD4+ T cells in the blood (Fig. 6A) were similar when we compared the 2-μg and 20-μg doses of CSP1 protein using either poly(I·C)LC or GLA-SE as an adjuvant. However, CD4+ IFN-γ responses were significantly higher when poly(I·C)LC was used than when GLA-SE was used for both doses of CSP1 (Fig. 6A). Moreover, in spleen (Fig. 6B) and liver (Fig. 6C), CD4+ T cell responses were also significantly higher using poly(I·C)LC than using GLA-SE. Mice immunized with either adjuvant alone or CSP1 alone showed no detectable CD4+ T cell responses (data not shown). Lastly, we did not detect any CD8+ T cell responses (data not shown), consistent with the absence of a P. falciparum CSP-specific CD8+ T cell epitope in C57BL/6 mice.
Fig 6.
C57BL/6 mice (n = 6 to 18) were immunized three times, 3 weeks apart, with the indicated doses of CSP1 with either 50 μg poly(I·C)LC or 5 μg GLA-SE as the adjuvant. Mice that received poly(I·C)LC, GLA-SE, or CSP1 alone, mice that received CSP1 and SE, or naive mice served as controls (not shown). CD4+ T cells in blood, spleen, and liver were analyzed for cytokine production by flow cytometry. (A) Frequencies of CSP-specific IFN-γ-producing CD4+ T cells in the blood 7 days after the third immunization; (B, C) frequencies of CSP-specific IFN-γ-, IL-2-, TNF-α-, or IL-10-producing CD4+ T cells in the spleen (B) and liver (C) 2 weeks after three immunizations; (D) relative proportions of each individual combination of IFN-γ-, IL-2-, or TNF-α-producing cells in the spleen and liver 2 weeks after three immunizations. ND, not determined (because the frequency was too low). The black circle represents the percentage of CD4+ T cells that make IFN-γ. Bars show the means ± SD. *, P < 0.05; **, P < 0.01. All control groups had values below the limit of detection. The color of the asterisk indicates the group to which the value is being compared. Results are representative of two independent experiments.
To extend this analysis, we assessed the quality of the CD4+ T cell cytokine response based on the relative proportions of cells producing IL-2, IFN-γ, and TNF-α, either alone or simultaneously. The quality of the CD4+ T cell response may be an important metric because of evidence that multifunctional CD4+ T cells correlate with protection (20, 23, 50). As shown in Fig. 6D, ∼50% of the total cytokine-producing responses were multifunctional, producing IL-2, TNF-α, and IFN-γ, with another ∼25% making IFN-γ and TNF-α without IL-2. Thus, both adjuvants elicit the induction of CD4+ T cells that secrete two critical effector cytokines that may mediate parasite killing (51–53).
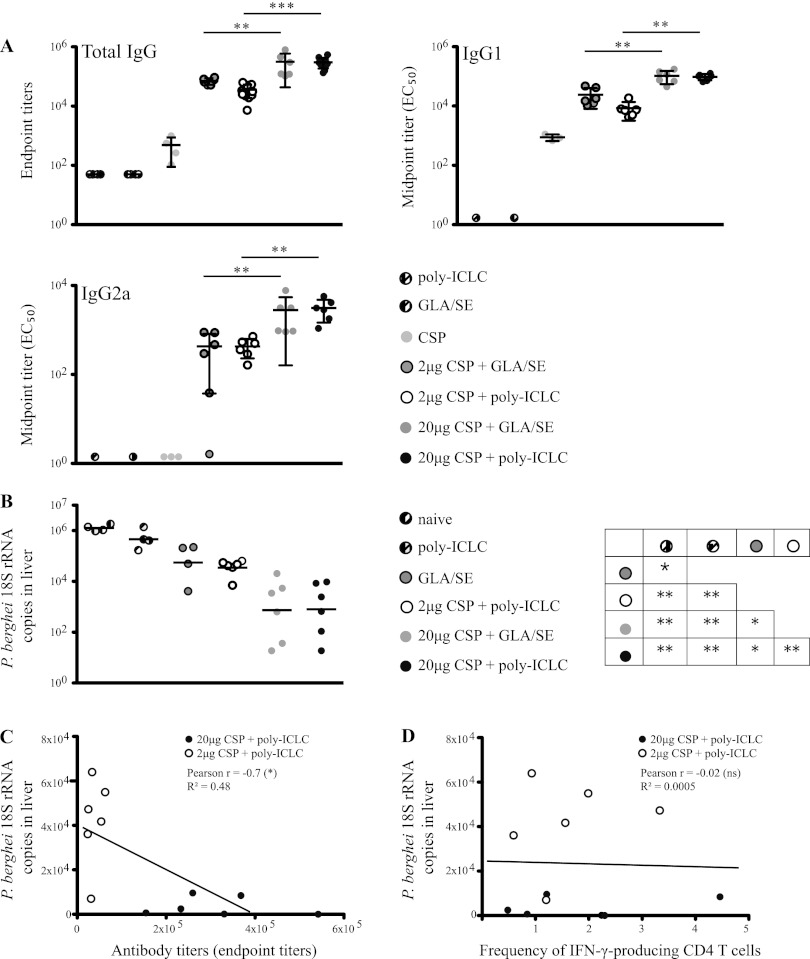
Poly(I·C)LC and GLA-SE confer comparable levels of protection correlating with antibody titers.
We next compared antibody responses induced with 2 or 20 μg of CSP1 and GLA-SE to poly(I·C)LC following three immunizations. In contrast to the magnitudes of CD4+ T cell cytokine responses, which were significantly different, the total titers of IgG against the NANP repeats measured with the two adjuvants were not significantly different (Fig. 7A, upper left). Of note, the lower dose (2 μg) of CSP1 consistently induced ∼2- to 5-fold-lower total antibody titers than the higher dose (20 μg) for both adjuvants. Similar results were seen after analyzing IgG1 and IgG2a antibody titers (Fig. 7A). In terms of protection, there was a 2- to 3-log reduction of 18S rRNA after challenge with Pb-CS(Pf) in mice immunized with 20 μg of CSP1 with poly(I·C)LC compared to that in naive mice, consistent with the results of previous experiments (Fig. 4). Immunization with 20 μg of CSP1 and GLA-SE resulted in similar levels of protection (Fig. 7B). Thus, in a setting of equivalent antibody titers, there was no significant difference in protection using poly(I·C)LC or GLA-SE as an adjuvant.
Fig 7.
C57BL/6 mice (n = 6) were immunized three times, 3 weeks apart, with the indicated doses of CSP1 together with either 50 μg poly(I·C)LC or 5 μg GLA-SE. Mice that received poly(I·C)LC, GLA-SE, or CSP1 (20 μg) alone or naive mice served as controls. (A) Two weeks after the third immunization, sera were collected and CSP-specific IgG, IgG1, and IgG2a antibodies were measured. Bars are graphed as means ± SD. All control groups showed significantly lower antibody titers than the vaccine groups. (B) Four weeks after the third immunization, mice were challenged with 1.5 × 104 Pb-CS(Pf) sporozoites. Forty hours postchallenge, parasite burden was determined by counting 18S rRNA copies in the livers by RT-PCR. Black bars show the geometrical means. The matrix (B) represents significances among various immunization groups. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (C) Correlation between antibody titers and parasite burden in the liver. (D) Correlation between frequency of IFN-γ-producing CD4+ T cells in the blood (day 7) and parasite burden in the liver. (C and D) As indicated, Pearson's r was calculated. Results are representative of at least two independent experiments.
To determine whether humoral or cellular immune responses correlated with protection, prechallenge antibody titers (Fig. 7C) and CD4+ T cell cytokine responses (Fig. 7D) were plotted against the level of protection achieved upon consecutive challenge. There was a highly significant correlation between CSP-specific antibody titers and the level of 18S rRNA measured in livers after parasite challenge (Pearson r = −0.7) (Fig. 7C). In contrast, there was no correlation between the frequency of IFN-γ-producing CD4+ T cells in the blood and the level protection after parasite challenge (Pearson r = −0.02) (Fig. 7D). Finally, to confirm that our vaccine-elicited CSP-specific antibodies had binding activity against the intact parasite, we used an IFA assay to visualize direct antibody binding to sporozoites (Table 1). Consistent with the CSP-specific antibody titers, poly(I·C)LC and GLA-SE induced strong and comparable IFA titers when they were administered with 20 μg of CSP1 protein. Taken together, these data show that poly(I·C)LC and GLA-SE differ in their capacities to induce CD4+/Th1 immune responses after immunization with CSP; however, they induce comparable antibody responses that correlate best with protection.
Table 1.
Antibodies in sera from mice that were immunized with a high dose of CSP1 and adjuvant showed a strong binding capacity to sporozoites in vitro
| Vaccine | Binding of serum antibodies to sporozoites as determined by fluorescence intensity at an antibody dilution ofa: |
|||||
|---|---|---|---|---|---|---|
| 1:300 | 1:900 | 1:2,700 | 1:8,100 | 1:24,300 | 1:72,900 | |
| Poly(I·C)LC | − | − | − | − | − | − |
| GLA-SE | − | − | − | − | − | − |
| CSP | + | +/− | − | − | − | − |
| 2 μg CSP + GLA-SE | ++ | ++ | + | + | − | − |
| 2 μg CSP + poly(I·C)LC | ++ | + | +/− | +/− | − | − |
| 20 μg CSP + GLA-SE | +++ | ++ | ++ | + | + | +/− |
| 20 μg CSP + poly(I·C)LC | +++ | +++ | ++ | + | + | +/− |
Serum samples that were collected 2 weeks after the third immunization (as described in the legend of Fig. 6) were pooled by group. The serum pools at different dilutions were incubated on slides coated with fixed sporozoites. The slides were washed and incubated with a secondary fluorescently labeled antibody. Finally, slides were analyzed under a microscope. +++, very good binding; ++, good binding; +, weak binding; −, no binding. Results are representative of two independent experiments.
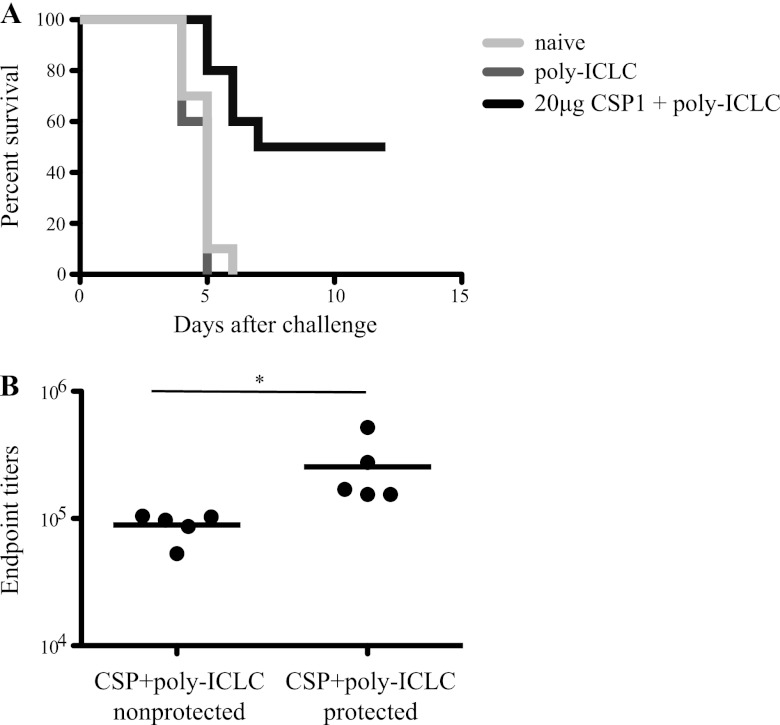
While reduction in parasite load in the liver following a high-dose i.v. challenge may have important clinical consequences by reducing the prepatent period and time to infection (20, 54, 55), the most important and clearest clinical outcome is sterilizing immunity. Thus, mice vaccinated with CSP1 and poly(I·C)LC were i.v. challenged with a low dose of 1 × 103 Pb-CS(Pf) sporozoites and monitored daily by blood smear to establish the onset of infection. As shown in Fig. 8, all naive mice and mice immunized with poly(I·C)LC alone developed parasitemia within 5 days. In contrast, 5 out of 10 mice immunized with CSP1 and poly(I·C)LC did not develop parasitemia during the 15 days of follow-up. Also, the prepatent period was significantly delayed (P < 0.05) in mice that received CSP1 and poly(I·C)LC (5.8 days) compared to mice that received poly(I·C)LC alone or naive animals (4.6 days and 4.8 days, respectively) (Fig. 8A; Table 2). Analysis of prechallenge sera from mice vaccinated with CSP1 and poly(I·C)LC revealed that protected mice from this group displayed significantly higher antibody titers than the nonprotected mice from this same vaccine group (Fig. 8B). These data are consistent with human trials showing that a very high level of antibody is required for sterilizing immunity (20, 56).
Fig 8.
C57BL/6 mice (n = 10 per group) were immunized three times, 3 weeks apart, with 20 μg of CSP1 together with 50 μg poly(I·C)LC as the adjuvant. Mice that received poly(I·C)LC or naive mice served as controls. (A) Five weeks later, mice were challenged i.v. with 1 × 103 Pb-CS(Pf) sporozoites. Starting on day 4, daily blood smears were taken and analyzed under a microscope. Kaplan-Meier plots show the time to detection of parasites in the blood for each vaccine group after the challenge. (B) Sera collected 3 weeks before challenge were analyzed for CSP-specific IgG antibodies. Endpoint titers are grouped by whether mice went on to be protected from challenge. *, P < 0.05. Results are representative of two independent experiments.
Table 2.
Vaccination with CSP1 and poly(I·C)LC prevents 50% of mice from developing parasitemia after a low-dose sporozoite challengea
| Immunization | No. of infected/no. of challenged mice | Prepatent period (days) | Protection (%) |
|---|---|---|---|
| 20 μg CSP + poly(I·C)LC | 5/10 | 5.8 | 50 |
| Poly(I·C)LC | 10/10 | 4.6 | 0 |
| None (naive mice) | 10/10 | 4.8 | 0 |
C57BL/6 mice (n = 10/group) were immunized three times, 3 weeks apart, with 20 μg CSP1 together with 50 μg poly(I·C)LC as the adjuvant. Mice that received poly(I·C)LC only or naive mice served as controls. Five weeks after the last immunization, mice were challenged i.v. with 1 × 103 Pb-CS(Pf) sporozoites. Starting at day 4, daily blood smears were taken and observed under a microscope to look for the onset of parasitemia. Results are representative of two independent experiments.
CSP and poly(I·C)LC induce humoral and cellular responses in NHPs.
Based on the results from the mouse studies, we determined whether CSP1 and poly(I·C)LC could induce potent antibody and CD4+ T cell responses in NHPs. NHPs are a more useful animal model than mice for testing vaccine adjuvants because of greater similarities to humans, with respect to innate immune pathways and TLR expression. Rhesus macaques received either two or three immunizations of 100 μg CSP1 and poly(I·C)LC, and CD4+ T cell and antibody responses were assessed at different time points after immunization. For CD4+ T cell cytokine responses, a batch analysis was done on frozen PBMCs from various time points. As shown in Fig. S1 in the supplemental material, as with the mouse data in Fig. 2, the frequency of CSP-specific cytokine-producing CD4+ T cells is increased in NHPs that received three immunizations rather than only two immunizations (see Fig. S1A and B in the supplemental material), with a substantial portion of multifunctional cells secreting IFN-γ, IL-2, and TNF-α or IFN-γ and TNF-α (Fig. S1C and D). There were also robust CSP antibody titers that were maximal after two immunizations with CSP1 and poly(I·C)LC. Waning antibody titers could be boosted with a third immunization but did not exceed the levels that were reached after two immunizations (Fig. S1E and F). Moreover, in the IFA assay, sera from animals that received three immunizations contained antibodies with a stronger binding capacity than those from animals that received two immunizations (see Table S1 in the supplemental material). Therefore, we conclude that the CSP1 protein and poly(I·C)LC are strongly immunogenic in NHPs.
DISCUSSION
Currently, the RTS,S vaccine administered with AS01 is the most advanced malaria vaccine, providing ∼30 to 50% protection in humans (2, 3). The recent results of the pivotal phase III clinical efficacy trial were a major breakthrough in establishing that a protein/particle-based vaccine with a well-formulated adjuvant system could achieve some protective efficacy against a parasite infection and confirms this approach as a first step toward developing a successful malaria vaccine. In the study presented here, we compared various full-length CS proteins for their ability to induce strong immunity and protection. Such proteins were provided by four partners, in partnership with MVI, to enable direct comparison and down-selection of one or more proteins for further development, thereby providing a potentially simpler formulation than RTS,S/AS01 with comparable or improved efficacy. The data presented here using poly(I·C)LC as an adjuvant show a striking difference between the immunogenicities elicited by E. coli-derived CSP and yeast-derived CSP. The relatively low responses to the yeast-derived protein were surprising, since RTS,S (as well as other successful protein-based vaccines) is produced in yeast (57) and one of the yeast-derived proteins used in this study when given with Montanide did elicit potent antibody titers in mice (data not shown), showing that at least for antibody responses, the yeast-derived protein was immunogenic. We speculate that differences in the amino acid sequences or mannosylation sites of CSPs expressed in yeast and E. coli may have accounted for the limited immunogenicity. Indeed, one of the yeast-derived proteins induced higher antibody and CD4+ T cell responses when the same amino acid sequence was expressed in E. coli (data not shown). However, modifications in the mannosylation sites did not significantly alter the immunogenicity of yeast-expressed CSP (data not shown). Therefore, the mechanistic basis for the differences in immunogenicity of yeast- and E. coli-expressed CSPs used in this experiment is not entirely clear. In view of these results, CSP1 was used with the two adjuvants, poly(I·C)LC and GLA-SE, for the remainder of the studies.
Both poly(I·C)LC and GLA-SE elicited strong and comparable levels of CSP-specific IgG antibody titers, which conferred similar degrees of protection against high-dose sporozoite challenge. However, poly(I·C)LC induced a substantially higher frequency of CSP-specific CD4+ T cell cytokine responses than GLA-SE. Moreover, such responses were comprised of a large percentage of multifunctional cells secreting IFN-γ, IL-2, and TNF-α, which have been proposed to contribute to antimalarial protection after RTS,S/AS01 vaccination (20, 23). Of note, while vaccination with CSP (P. vivax) and GLA-SE in mice has been shown to induce a robust CD4+ T cell effector response (58), the same vaccine induced monofunctional (IL-2 only) CD4+ T cell responses in NHPs (49). In contrast, we show that CSP and poly(I·C)LC induced robust multifunctional Th1 CD4+ T cell responses in both mice and NHPs. These data highlight potential differences in the magnitudes and quality of CD4+ T cell responses induced in mice and NHPs and are consistent with the results of other studies showing poly(I·C) or poly(I·C)LC to be an especially potent adjuvant in NHPs for such responses (34, 59). Finally, it is notable that despite the dramatic differences in the CD4+/Th1 responses between poly(I·C)LC and GLA-SE in mice, the antibody titers were similar. These data highlight the possibility that adjuvants can differentially mediate effects on antibody and Th1 immunity, which is currently being investigated.
In terms of correlates of protection, both antibody and CD4+ T cells have been described to occur in mice or humans with CSP vaccines. Here, we show that the antibody titers using poly(I·C)LC or GLA-SE strongly correlate with protection (Fig. 7C). Moreover, serum transfer from CSP-immunized animals reduced the parasite burden after high-dose i.v. challenge. Thus, antibodies are necessary and sufficient in this model. Of note, protection was best with the higher dose of CSP (20 μg), which induced an ∼1-log increase in antibody titer compared to that induced by the lower dose of protein (2 μg). It is notable that, in humans, 25 μg or 50 μg of RTS,S induced antibody titers better than and comparable to those induced by 10 μg, suggesting some dose effect similar to what we observed in this study (60). This suggests that there may be a threshold for the amount of CSP-specific antibody required to provide or increase protection. Indeed, when we analyzed antibody titers from protected and nonprotected animals (Fig. 7C and 8B), there was a threshold for the amount of antibody required to mediate protection after low- or high-dose challenge. This is consistent with data from humans in which anti-CSP antibody titers needed to be above 40 EU/ml (56) to induce protection. In addition, we show that nonprotected mice had a significantly delayed prepatent period compared to the control animals (Table 2). Accordingly, studies using RTS,S and different adjuvants in humans also show that the prepatent period can be delayed. At present, it still is not clear whether this delay might play a role in improving the clinical outcome of a malaria infection (20, 54, 55).
Regarding the role of cellular immunity in mediating protection, previous studies have shown that CD4+ T cells specific for P. yoelii CSP eliminate infected hepatocytes in vitro and, when adoptively transferred, mediate protection in vivo (61, 62). The protective role of CD4+ effector cells against malaria was further substantiated in other murine and human malaria models (22, 63, 64). In data not shown, antibody depletion of CD4+ T cells in mice immunized with CSP1 and poly(I·C)LC at the time of sporozoite challenge resulted in only modestly reduced protection. Hence, in our model, the data support a far more critical role for CSP antibodies in mediating protection than for CD4+ effector T cells. Nevertheless, human studies using the AS02 or AS01 adjuvant showing that improved CD4+ T cell responses were associated with improved protection after challenge with P. falciparum (20, 22) provide evidence for the importance of these T cells. Therefore, the ability of poly(I·C)LC to elicit such potent CD4+ T cells in blood, liver, and spleen may be of importance in humans through a variety of mechanisms. Indeed, IFN-γ has been shown to inhibit the development of liver-stage malaria in various animal models (52, 65). That would provide a potential advantageous role for poly(I·C)LC, based on the high frequency of those cells detected in the livers of immunized mice.
There is also strong evidence for cytotoxic CD8+ T cells in mediating protective immunity against liver-stage malaria (18, 46, 66, 67). As our study used a strain of mice in which there was no major histocompatibility complex (MHC) class I recognition of P. falciparum CSP peptides, we did not detect any CD8+ T cell responses. However, we and others have previously shown that type I IFN is essential for mediating cross-priming of protein in mice (68, 69), highlighting the potential advantage of poly(I·C)LC over other adjuvants (25). Nevertheless, while adjuvants that induce type I IFN should be preferred to induce CD8+ T cell responses upon protein vaccination, virus-based vaccines seem preferable if robust CD8+ T cell immunity is required. Improving how the protein is formulated and targeting it to specific dendritic cell subsets may ultimately improve the capacity of such vaccines to consistently and efficiently induce CD8+ T cells.
In conclusion, we show that a full-length P. falciparum CS protein when combined with poly(I·C)LC or GLA-SE induces potent immunity and protection in mice. Moreover, poly(I·C)LC is an effective adjuvant for eliciting such responses in NHPs, providing a predictive model for what might occur in humans (20, 21, 70, 71). It is possible that the increased breadth of CSP responses with a full-length CSP (compared to the CSP responses with truncated RTS,S) and/or the enhanced CD4+ Th1 immunity that would potentially be elicited by poly(I·C)LC would enhance protection and/or durability. Whether a full-length protein vaccine with an adjuvant would substantially improve the outcome over the current platforms of RTS,S/AS01 and RTS,S/AS02, which have the advantages of being particles with well-formulated adjuvants and of being safe and scalable, remains a question for further development. Our study shows that a protein platform can provide effective protection against malaria. The key question for future studies is whether this protection will also provide a prolonged durability compared to that of the RTS,S vaccine.
Supplementary Material
ACKNOWLEDGMENTS
We thank P. A. Darrah for critically reading the manuscript and D. Berry and C. J. Redmond for technical support. Also we thank S. Reed for providing GLA-SE and the Malaria Serology Laboratory at WRAIR for their help with the serum samples. The proteins were provided through agreements between PATH Malaria Vaccine Initiative (MVI) and its partners.
We thank MVI for funding and facilitating this comparative study by identifying and accessing the proteins and ensuring agreement of all partners on the final study design. We thank Gennova Biopharmaceuticals and WRAIR for providing the proteins CSP1 and CSP2, respectively.
Footnotes
Published ahead of print 28 December 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01108-12.
REFERENCES
- 1.WHO 2011. World malaria report 2011. World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/world_malaria_report_2011/en/ [Google Scholar]
- 2.Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, Conzelmann C, Methogo BGNO, Doucka Y, Flamen A, Mordmüller B, Issifou S, Kremsner PG, Sacarlal J, Aide P, Lanaspa M, Aponte JJ, Nhamuave A, Quelhas D, Bassat Q, Mandjate S, Macete E, Alonso P, Abdulla S, Salim N, Juma O, Shomari M, Shubis K, Machera F, Hamad AS, Minja R, Mtoro A, Sykes A, Ahmed S, Urassa AM, Ali AM, Mwangoka G, Tanner M, Tinto H, D'Alessandro U, Sorgho H, Valea I, Tahita MC, Kaboré W, Ouédraogo S, Sandrine Y, Guiguemdé RT, Ouédraogo JB, Hamel MJ, Kariuki S, Odero C, Oneko M, Otieno K, Awino N, Omoto J, Williamson J, Muturi-Kioi V, Laserson KF, Slutsker L, Otieno W, Otieno L, Nekoye O, Gondi S, Otieno A, Ogutu B, Wasuna R, Owira V, Jones D, Onyango AA, Njuguna P, Chilengi R, Akoo P, Kerubo C, Gitaka J, Maingi C, Lang T, Olotu A, Tsofa B, Bejon P, Peshu N, Marsh K, Owusu-Agyei S, Asante KP, Osei-Kwakye K, Boahen O, Ayamba S, Kayan K, Owusu-Ofori R, Dosoo D, Asante I, Adjei G, Adjei G, Chandramohan D, Greenwood B, Lusingu J, Gesase S, Malabeja A, Abdul O, Kilavo H, Mahende C, Liheluka E, Lemnge M, Theander T, Drakeley C, Ansong D, Agbenyega T, Adjei S, Boateng HO, Rettig T, Bawa J, Sylverken J, Sambian D, Agyekum A, Owusu L, Martinson F, Hoffman I, Mvalo T, Kamthunzi P, Nkomo R, Msika A, Jumbe A, Chome N, Nyakuipa D, Chintedza J, Ballou WR, Bruls M, Cohen J, Guerra Y, Jongert E, Lapierre D, Leach A, Lievens M, Ofori-Anyinam O, Vekemans J, Carter T, Leboulleux D, Loucq C, Radford A, Savarese B, Schellenberg D, Sillman M, Vansadia P, RTS,S Clinical Trials Partnership 2011. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N. Engl. J. Med. 365:1863–1875 [DOI] [PubMed] [Google Scholar]
- 3.The RTS,S Clinical Trials Partnership 2012. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N. Engl. J. Med. 367:2284–2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi YP, Udhayakumar V, Alpers MP, Povoa MM, Oloo AJ, Ruebush TK, II, Lal AA. 1993. Natural antibody responses against the non-repeat-sequence-based B-cell epitopes of the Plasmodium falciparum circumsporozoite protein. Infect. Immun. 61:2425–2433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bongfen SE, Ntsama PM, Offner S, Smith T, Felger I, Tanner M, Alonso P, Nebie I, Romero JF, Silvie O, Torgler R, Corradin G. 2009. The N-terminal domain of Plasmodium falciparum circumsporozoite protein represents a target of protective immunity. Vaccine 27:328–335 [DOI] [PubMed] [Google Scholar]
- 6.Rathore D, Nagarkatti R, Jani D, Chattopadhyay R, de la Vega P, Kumar S, McCutchan TF. 2005. An immunologically cryptic epitope of Plasmodium falciparum circumsporozoite protein facilitates liver cell recognition and induces protective antibodies that block liver cell invasion. J. Biol. Chem. 280:20524–20529 [DOI] [PubMed] [Google Scholar]
- 7.Coppi A, Pinzon-Ortiz C, Hutter C, Sinnis P. 2005. The Plasmodium circumsporozoite protein is proteolytically processed during cell invasion. J. Exp. Med. 201:27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coppi A, Natarajan R, Pradel G, Bennett BL, James ER, Roggero MA, Corradin G, Persson C, Tewari R, Sinnis P. 2011. The malaria circumsporozoite protein has two functional domains, each with distinct roles as sporozoites journey from mosquito to mammalian host. J. Exp. Med. 208:341–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zavala F, Tam JP, Barr PJ, Romero PJ, Ley V, Nussenzweig RS, Nussenzweig V. 1987. Synthetic peptide vaccine confers protection against murine malaria. J. Exp. Med. 166:1591–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Persson C, Oliveira GA, Sultan AA, Bhanot P, Nussenzweig V, Nardin E. 2002. Cutting edge: a new tool to evaluate human pre-erythrocytic malaria vaccines: rodent parasites bearing a hybrid Plasmodium falciparum circumsporozoite protein. J. Immunol. 169:6681–6685 [DOI] [PubMed] [Google Scholar]
- 11.Nussenzweig V, Nussenzweig RS. 1989. Rationale for the development of an engineered sporozoite malaria vaccine. Adv. Immunol. 45:283–334 [DOI] [PubMed] [Google Scholar]
- 12.Fries LF, Gordon DM, Schneider I, Beier JC, Long GW, Gross M, Que JU, Cryz SJ, Sadoff JC. 1992. Safety, immunogenicity, and efficacy of a Plasmodium falciparum vaccine comprising a circumsporozoite protein repeat region peptide conjugated to Pseudomonas aeruginosa toxin A. Infect. Immun. 60:1834–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fries LF, Gordon DM, Richards RL, Egan JE, Hollingdale MR, Gross M, Silverman C, Alving CR. 1992. Liposomal malaria vaccine in humans: a safe and potent adjuvant strategy. Proc. Natl. Acad. Sci. U. S. A. 89:358–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffman SL, Edelman R, Bryan JP, Schneider I, Davis J, Sedegah M, Gordon D, Church P, Gross M, Silverman C. 1994. Safety, immunogenicity, and efficacy of a malaria sporozoite vaccine administered with monophosphoryl lipid A, cell wall skeleton of mycobacteria, and squalane as adjuvant. Am. J. Trop. Med. Hyg. 51:603–612 [DOI] [PubMed] [Google Scholar]
- 15.Sherwood JA, Copeland RS, Taylor KA, Abok K, Oloo AJ, Were JB, Strickland GT, Gordon DM, Ballou WR, Bales JD, Wirtz RA, Wittes J, Gross M, Que JU, Cryz SJ, Oster CN, Roberts CR, Sadoff JC. 1996. Plasmodium falciparum circumsporozoite vaccine immunogenicity and efficacy trial with natural challenge quantitation in an area of endemic human malaria of Kenya. Vaccine 14:817–827 [DOI] [PubMed] [Google Scholar]
- 16.Sherwood JA, Oster CN, Adoyo-Adoyo M, Beier JC, Gachihi GS, Nyakundi PM, Ballou WR, Brandling-Bennett AD, Schwartz IK, Were JB. 1991. Safety and immunogenicity of a Plasmodium falciparum sporozoite vaccine: boosting of antibody response in a population with prior natural exposure to malaria. Trans. R. Soc. Trop. Med. Hyg. 85:336–340 [DOI] [PubMed] [Google Scholar]
- 17.Brown AE, Singharaj P, Webster HK, Pipithkul J, Gordon DM, Boslego JW, Krinchai K, Su-archawaratana, Wongsrichanalai PC, Ballou WR. 1994. Safety, immunogenicity and limited efficacy study of a recombinant Plasmodium falciparum circumsporozoite vaccine in Thai soldiers. Vaccine 12:102–108 [DOI] [PubMed] [Google Scholar]
- 18.Schofield L, Villaquiran J, Ferreira A, Schellekens H, Nussenzweig R, Nussenzweig V. 1987. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature 330:664–666 [DOI] [PubMed] [Google Scholar]
- 19.Weiss WR, Sedegah M, Beaudoin RL, Miller LH, Good MF. 1988. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc. Natl. Acad. Sci. U. S. A. 85:573–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kester KE, Cummings JF, Ofori-Anyinam O, Ockenhouse CF, Krzych U, Moris P, Schwenk R, Nielsen RA, Debebe Z, Pinelis E, Juompan L, Williams J, Dowler M, Stewart VA, Wirtz RA, Dubois Lievens M-CM, Cohen J, Ballou WR, Heppner DG, RTS,S Vaccine Evaluation Group 2009. Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J. Infect. Dis. 200:337–346 [DOI] [PubMed] [Google Scholar]
- 21.Mettens P, Dubois PM, Demoitié Bayat M-AB, Donner Bourguignon M-NP, Stewart VA, Heppner DG, Garçon N, Cohen J. 2008. Improved T cell responses to Plasmodium falciparum circumsporozoite protein in mice and monkeys induced by a novel formulation of RTS,S vaccine antigen. Vaccine 26:1072–1082 [DOI] [PubMed] [Google Scholar]
- 22.Sun P, Schwenk R, White K, Stoute JA, Cohen J, Ballou WR, Voss G, Kester KE, Heppner DG, Krzych U. 2003. Protective immunity induced with malaria vaccine, RTS,S, is linked to Plasmodium falciparum circumsporozoite protein-specific CD4+ and CD8+ T cells producing IFN-gamma. J. Immunol. 171:6961–6967 [DOI] [PubMed] [Google Scholar]
- 23.Lumsden JM, Schwenk RJ, Rein LE, Moris P, Janssens M, Ofori-Anyinam O, Cohen J, Kester KE, Heppner DG, Krzych U. 2011. Protective immunity induced with the RTS,S/AS vaccine is associated with IL-2 and TNF-α producing effector and central memory CD4 T cells. PLoS One 6:e20775 doi:10.1371/journal.pone.0020775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Regules JA, Cummings JF, Ockenhouse CF. 2011. The RTS,S vaccine candidate for malaria. Expert Rev. Vaccines 10:589–599 [DOI] [PubMed] [Google Scholar]
- 25.Longhi MP, Trumpfheller C, Idoyaga J, Caskey M, Matos I, Kluger C, Salazar AM, Colonna M, Steinman RM. 2009. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J. Exp. Med. 206:1589–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Bon A, Schiavoni G, D'Agostino G, Gresser I, Belardelli F, Tough DF. 2001. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity 14:461–470 [DOI] [PubMed] [Google Scholar]
- 27.Le Bon A, Thompson C, Kamphuis E, Durand V, Rossmann C, Kalinke U, Tough DF. 2006. Cutting edge: enhancement of antibody responses through direct stimulation of B and T cells by type I IFN. J. Immunol. 176:2074–2078 [DOI] [PubMed] [Google Scholar]
- 28.Ma Y, Ross AC. 2009. Toll-like receptor 3 ligand and retinoic acid enhance germinal center formation and increase the tetanus toxoid vaccine response. Clin. Vaccine Immunol. 16:1476–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ichinohe T, Watanabe I, Ito S, Fujii H, Moriyama M, Tamura Takahashi S-IH, Sawa H, Chiba J, Kurata T, Sata T, Hasegawa H. 2005. Synthetic double-stranded RNA poly(I:C) combined with mucosal vaccine protects against influenza virus infection. J. Virol. 79:2910–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amos SM, Pegram HJ, Westwood JA, John LB, Devaud C, Clarke CJ, Restifo NP, Smyth MJ, Darcy PK, Kershaw MH. 2011. Adoptive immunotherapy combined with intratumoral TLR agonist delivery eradicates established melanoma in mice. Cancer Immunol. Immunother. 60:671–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Llopiz D, Dotor J, Zabaleta A, Lasarte JJ, Prieto J, Borrás-Cuesta F, Sarobe P. 2008. Combined immunization with adjuvant molecules poly(I:C) and anti-CD40 plus a tumor antigen has potent prophylactic and therapeutic antitumor effects. Cancer Immunol. Immunother. 57:19–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui Z, Qiu F. 2006. Synthetic double-stranded RNA poly(I:C) as a potent peptide vaccine adjuvant: therapeutic activity against human cervical cancer in a rodent model. Cancer Immunol. Immunother. 55:1267–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahonen CL, Doxsee CL, McGurran SM, Riter TR, Wade WF, Barth RJ, Vasilakos JP, Noelle RJ, Kedl RM. 2004. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J. Exp. Med. 199:775–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flynn BJ, Kastenmüller K, Wille-Reece U, Tomaras GD, Alam M, Lindsay RW, Salazar AM, Perdiguero B, Gomez CE, Wagner R, Esteban M, Park CG, Trumpfheller C, Keler T, Pantaleo G, Steinman RM, Seder R. 2011. Immunization with HIV Gag targeted to dendritic cells followed by recombinant New York vaccinia virus induces robust T-cell immunity in nonhuman primates. Proc. Natl. Acad. Sci. U. S. A. 108:7131–7136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stahl-Hennig C, Eisenblätter M, Jasny E, Rzehak T, Tenner-Racz K, Trumpfheller C, Salazar AM, Uberla K, Nieto K, Kleinschmidt J, Schulte R, Gissmann L, Müller M, Sacher A, Racz P, Steinman RM, Uguccioni M, Ignatius R. 2009. Synthetic double-stranded RNAs are adjuvants for the induction of T helper 1 and humoral immune responses to human papillomavirus in rhesus macaques. PLoS Pathog. 5:e1000373 doi:10.1371/journal.ppat.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson RC, Fox CB, Dutill TS, Shaverdian N, Evers TL, Poshusta GR, Chesko J, Coler RN, Friede M, Reed SG, Vedvick TS. 2010. Physicochemical characterization and biological activity of synthetic TLR4 agonist formulations. Colloids Surf. B Biointerfaces 75:123–132 [DOI] [PubMed] [Google Scholar]
- 37.Windish HP, Duthie MS, Ireton G, Lucas E, Laurance JD, Bailor RH, Coler RN, Reed SG. 2011. Protection of mice from Mycobacterium tuberculosis by ID87/GLA-SE, a novel tuberculosis subunit vaccine candidate. Vaccine 29:7842–7848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gomes R, Teixeira C, Oliveira F, Lawyer PG, Elnaiem DE, Meneses C, Goto Y, Bhatia A, Howard RF, Reed SG, Valenzuela JG, Kamhawi S. 2012. KSAC, a defined Leishmania antigen, plus adjuvant protects against the virulence of L. major transmitted by its natural vector Phlebotomus duboscqi. PLoS Negl. Trop. Dis. 6:e1610 doi:10.1371/journal.pntd.0001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coler RN, Baldwin SL, Shaverdian N, Bertholet S, Reed SJ, Raman VS, Lu X, DeVos J, Hancock K, Katz JM, Vedvick TS, Duthie MS, Clegg CH, Van Hoeven N, Reed SG. 2010. A synthetic adjuvant to enhance and expand immune responses to influenza vaccines. PLoS One 5:e13677 doi:10.1371/journal.pone.0013677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bertholet S, Goto Y, Carter L, Bhatia A, Howard RF, Carter D, Coler RN, Vedvick TS, Reed SG. 2009. Optimized subunit vaccine protects against experimental leishmaniasis. Vaccine 27:7036–7045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bertholet S, Ireton GC, Ordway DJ, Windish HP, Pine SO, Kahn M, Phan T, Orme IM, Vedvick TS, Baldwin SL, Coler RN, Reed SG. 2010. A defined tuberculosis vaccine candidate boosts BCG and protects against multidrug-resistant Mycobacterium tuberculosis. Sci. Transl. Med. 2:53ra74 doi:10.1126/scitranslmed.3001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perfetto SP, Chattopadhyay PK, Lamoreaux L, Nguyen R, Ambrozak D, Koup RA, Roederer M. 2006. Amine reactive dyes: an effective tool to discriminate live and dead cells in polychromatic flow cytometry. J. Immunol. Methods 313:199–208 [DOI] [PubMed] [Google Scholar]
- 43.Bruña-Romero O, Hafalla JC, González-Aseguinolaza G, Sano G, Tsuji M, Zavala F. 2001. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int. J. Parasitol. 31:1499–1502 [DOI] [PubMed] [Google Scholar]
- 44.Tsuji M, Romero P, Nussenzweig RS, Zavala F. 1990. CD4+ cytolytic T cell clone confers protection against murine malaria. J. Exp. Med. 172:1353–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Potocnjak P, Yoshida N, Nussenzweig RS, Nussenzweig V. 1980. Monovalent fragments (Fab) of monoclonal antibodies to a sporozoite surface antigen (Pb44) protect mice against malarial infection. J. Exp. Med. 151:1504–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodrigues MM, Cordey AS, Arreaza G, Corradin G, Romero P, Maryanski JL, Nussenzweig RS, Zavala F. 1991. CD8+ cytolytic T cell clones derived against the Plasmodium yoelii circumsporozoite protein protect against malaria. Int. Immunol. 3:579–585 [DOI] [PubMed] [Google Scholar]
- 47.Rénia L, Grillot D, Marussig M, Corradin G, Miltgen F, Lambert PH, Mazier D, Del Giudice G. 1993. Effector functions of circumsporozoite peptide-primed CD4+ T cell clones against Plasmodium yoelii liver stages. J. Immunol. 150:1471–1478 [PubMed] [Google Scholar]
- 48.Reece WHH, Pinder M, Gothard PK, Milligan P, Bojang K, Doherty T, Plebanski M, Akinwunmi P, Everaere S, Watkins KR, Voss G, Tornieporth N, Alloueche A, Greenwood BM, Kester KE, Mcadam KPWJ, Cohen J, Hill AVS. 2004. A CD4(+) T-cell immune response to a conserved epitope in the circumsporozoite protein correlates with protection from natural Plasmodium falciparum infection and disease. Nat. Med. 10:406–410 [DOI] [PubMed] [Google Scholar]
- 49.Lumsden JM, Pichyangkul S, Srichairatanakul U, Yongvanitchit K, Limsalakpetch A, Nurmukhambetova S, Klein J, Bertholet S, Vedvick TS, Reed SG, Sattabongkot J, Bennett JW, Polhemus ME, Ockenhouse CF, Howard RF, Yadava A. 2011. Evaluation of the safety and immunogenicity in rhesus monkeys of a recombinant malaria vaccine for Plasmodium vivax with a synthetic Toll-like receptor 4 agonist formulated in an emulsion. Infect. Immun. 79:3492–3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moorthy VS, Ballou WR. 2009. Immunological mechanisms underlying protection mediated by RTS,S: a review of the available data. Malar. J. 8:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mendis KN, Naotunne TD, Karunaweera ND, Del Giudice G, Grau GE, Carter R. 1990. Anti-parasite effects of cytokines in malaria. Immunol. Lett. 25:217–220 [DOI] [PubMed] [Google Scholar]
- 52.Vigario AM, Belnoue E, Cumano A, Marussig M, Miltgen F, Landau I, Mazier D, Gresser I, Renia L. 2001. Inhibition of Plasmodium yoelii blood-stage malaria by interferon alpha through the inhibition of the production of its target cell, the reticulocyte. Blood 97:3966–3971 [DOI] [PubMed] [Google Scholar]
- 53.Depinay N, Franetich JF, Gruner AC, Mauduit M, Chavatte JM, Luty AJ, van Gemert GJ, Sauerwein RW, Siksik JM, Hannoun L, Mazier D, Snounou G, Renia L. 2011. Inhibitory effect of TNF-alpha on malaria pre-erythrocytic stage development: influence of host hepatocyte/parasite combinations. PLoS One 6:e17464 doi:10.1371/journal.pone.0017464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kester KE, McKinney DA, Tornieporth N, Ockenhouse CF, Heppner DG, Hall T, Krzych U, Delchambre M, Voss G, Dowler MG, Palensky J, Wittes J, Cohen J, Ballou WR. 2001. Efficacy of recombinant circumsporozoite protein vaccine regimens against experimental Plasmodium falciparum malaria. J. Infect. Dis. 183:640–647 [DOI] [PubMed] [Google Scholar]
- 55.Kester KE, McKinney DA, Tornieporth N, Ockenhouse CF, Heppner DG, Jr, Hall T, Wellde BT, White K, Sun P, Schwenk R, Krzych U, Delchambre M, Voss G, Dubois MC, Gasser RA, Jr, Dowler MG, O'Brien M, Wittes J, Wirtz R, Cohen J, Ballou WR. 2007. A phase I/IIa safety, immunogenicity, and efficacy bridging randomized study of a two-dose regimen of liquid and lyophilized formulations of the candidate malaria vaccine RTS,S/AS02A in malaria-naive adults. Vaccine 25:5359–5366 [DOI] [PubMed] [Google Scholar]
- 56.Olotu A, Lusingu J, Leach A, Lievens M, Vekemans J, Msham S, Lang T, Gould J, Dubois Jongert M-CE, Vansadia P, Carter T, Njuguna P, Awuondo KO, Malabeja A, Abdul O, Gesase S, Mturi N, Drakeley CJ, Savarese B, Villafana T, Lapierre D, Ballou WR, Cohen J, Lemnge MM, Peshu N, Marsh K, Riley EM, von Seidlein L, Bejon P. 2011. Efficacy of RTS,S/AS01E malaria vaccine and exploratory analysis on anti-circumsporozoite antibody titres and protection in children aged 5-17 months in Kenya and Tanzania: a randomised controlled trial. Lancet Infect. Dis. 11:102–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cohen J, Nussenzweig V, Nussenzweig R, Vekemans J, Leach A. 2010. From the circumsporozoite protein to the RTS, S/AS candidate vaccine. Hum. Vaccin. 6:90–96 [DOI] [PubMed] [Google Scholar]
- 58.Lumsden JM, Nurmukhambetova S, Klein JH, Sattabongkot J, Bennett JW, Bertholet S, Fox CB, Reed SG, Ockenhouse CF, Howard RF, Polhemus ME, Yadava A. 2012. Evaluation of immune responses to a Plasmodium vivax CSP-based recombinant protein vaccine candidate in combination with second-generation adjuvants in mice. Vaccine 30:3311–3319 [DOI] [PubMed] [Google Scholar]
- 59.Tewari K, Flynn BJ, Boscardin SB, Kastenmueller K, Salazar AM, Anderson CA, Soundarapandian V, Ahumada A, Keler T, Hoffman SL, Nussenzweig MC, Steinman RM, Seder RA. 2010. Poly(I:C) is an effective adjuvant for antibody and multi-functional CD4+ T cell responses to Plasmodium falciparum circumsporozoite protein (CSP) and αDEC-CSP in non human primates. Vaccine 28:7256–7266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bojang KA, Olodude F, Pinder M, Ofori-Anyinam O, Vigneron L, Fitzpatrick S, Njie F, Kassanga A, Leach A, Milman J, Rabinovich R, McAdam KP, Kester KE, Heppner DG, Cohen JD, Tornieporth N, Milligan PJ. 2005. Safety and immunogenicity of RTS,S/AS02A candidate malaria vaccine in Gambian children. Vaccine 23:4148–4157 [DOI] [PubMed] [Google Scholar]
- 61.Schwenk RJ, Richie TL. 2011. Protective immunity to pre-erythrocytic stage malaria. Trends Parasitol. 27:306–314 [DOI] [PubMed] [Google Scholar]
- 62.Rénia L, Marussig MS, Grillot D, Pied S, Corradin G, Miltgen F, Del Giudice G, Mazier D. 1991. In vitro activity of CD4+ and CD8+ T lymphocytes from mice immunized with a synthetic malaria peptide. Proc. Natl. Acad. Sci. U. S. A. 88:7963–7967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singh B, Cabrera-Mora M, Jiang J, Moreno A. 2012. A hybrid multistage protein vaccine induces protective immunity against murine malaria. Infect. Immun. 80:1491–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oliveira GA, Kumar KA, Calvo-Calle JM, Othoro C, Altszuler D, Nussenzweig V, Nardin EH. 2008. Class II-restricted protective immunity induced by malaria sporozoites. Infect. Immun. 76:1200–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferreira A, Schofield L, Enea V, Schellekens H, van der Meide P, Collins WE, Nussenzweig RS, Nussenzweig V. 1986. Inhibition of development of exoerythrocytic forms of malaria parasites by gamma-interferon. Science 232:881–884 [DOI] [PubMed] [Google Scholar]
- 66.Romero P, Maryanski JL, Corradin G, Nussenzweig RS, Nussenzweig V, Zavala F. 1989. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature 341:323–326 [DOI] [PubMed] [Google Scholar]
- 67.Schmidt NW, Butler NS, Harty JT. 2009. CD8 T cell immunity to Plasmodium permits generation of protective antibodies after repeated sporozoite challenge. Vaccine 27:6103–6106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tough DF, Borrow P, Sprent J. 1996. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science 272:1947–1950 [DOI] [PubMed] [Google Scholar]
- 69.Kastenmüller K, Wille-Reece U, Lindsay RWB, Trager LR, Darrah PA, Flynn BJ, Becker MR, Udey MC, Clausen BE, Igyarto BZ, Kaplan DH, Kastenmüller W, Germain RN, Seder RA. 2011. Protective T cell immunity in mice following protein-TLR7/8 agonist-conjugate immunization requires aggregation, type I IFN, and multiple DC subsets. J. Clin. Invest. 121:1782–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stewart VA, McGrath SM, Walsh DS, Davis S, Hess AS, Ware LA, Kester KE, Cummings JF, Burge JR, Voss G, Delchambre M, Garçon N, Tang DB, Cohen JD, Heppner DG. 2006. Pre-clinical evaluation of new adjuvant formulations to improve the immunogenicity of the malaria vaccine RTS,S/AS02A. Vaccine 24:6483–6492 [DOI] [PubMed] [Google Scholar]
- 71.Stewart VA, Walsh DS, McGrath SM, Kester KE, Cummings JF, Voss G, Delchambre M, Garçon N, Cohen JD, Heppner DG. 2006. Cutaneous delayed-type hypersensitivity (DTH) in a multi-formulation comparator trial of the anti-falciparum malaria vaccine candidate RTS,S in rhesus macaques. Vaccine 24:6493–6502 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.