Abstract
Clostridium difficile infection (CDI) results in toxin-induced epithelial injury and marked intestinal inflammation. Fecal markers of intestinal inflammation correlate with CDI disease severity, but regulation of the inflammatory response is poorly understood. Previous studies demonstrated that C. difficile toxin TcdA activates p38 kinase in tissue culture cells and mouse ilium, resulting in interleukin-8 (IL-8) release. Here, we investigated the role of phosphorylated mitogen-activated protein kinase (MAPK)-activated protein kinase (MK2 kinase, pMK2), a key mediator of p38-dependent inflammation, in CDI. Exposure of cultured intestinal epithelial cells to the C. difficile toxins TcdA and TcdB resulted in p38-dependent MK2 activation. Toxin-induced IL-8 and GROα release required MK2 activity. We found that p38 and MK2 are activated in response to other actin-disrupting agents, suggesting that toxin-induced cytoskeleton disruption is the trigger for kinase-dependent cytokine response. Phosphorylated MK2 was detected in the intestines of C. difficile-infected hamsters and mice, demonstrating for the first time that the pathway is activated in infected animals. Furthermore, we found that elevated pMK2 correlated with the presence of toxigenic C. difficile among 100 patient stool samples submitted for C. difficile testing. In conclusion, we find that MK2 kinase is activated by TcdA and TcdB and regulates the expression of proinflammatory cytokines. Activation of p38-MK2 in infected animals and humans suggests that this pathway is a key driver of intestinal inflammation in patients with CDI.
INTRODUCTION
Clostridium difficile is among the most common hospital-associated infections, causing gastrointestinal disease which ranges from mild diarrhea to toxic megacolon, sepsis, and death; its incidence and case fatality rate are increasing (1–5). The principal C. difficile virulence factors are a pair of closely related large toxins known as TcdA (toxin A) and TcdB (toxin B). These secreted toxins bind to host cell receptors, are internalized, and then are transported into the cytoplasm, where they express their enzymatic activity. Specifically, both toxins are glucosyltransferases that target Rho GTPases (RhoA, Rac1, and Cdc42) (6). Glucosylation inactivates the GTPases, thereby disrupting signaling cascades, arresting cell cycle progression, and damaging cytoskeletal integrity (6). Immediate effects of cellular intoxication are increased fluid and electrolyte secretion from enterocytes and increased permeability of the intestinal mucosa. Within a few hours of toxin exposure, enterocytes become rounded and inflammation ensues (7, 8). Colonic injury in CDI is characterized by marked neutrophilic infiltration, which likely contributes to the local and systemic manifestations of CDI disease. Consistent with this notion, fecal lactoferrin (a marker of fecal leukocytes) and IL-8 (a neutrophil chemoattractant) are elevated in patients with severe disease (9). Furthermore, patients with specific interleukin-8 (IL-8) promoter polymorphisms are reported to be more susceptible to C. difficile disease (10, 11). Because the host inflammatory response is felt to be a major component of CDI disease manifestations, several novel approaches to mitigating inflammation in CDI are being explored (12–15).
In vitro and in vivo models indicate that TcdA and TcdB can directly induce inflammatory cytokine release. Indeed, inflammation occurs within the first few hours after direct toxin injection into a murine ileal loop model, and blocking the inflammatory cascade markedly attenuates mucosal injury (16, 17). Similarly, purified C. difficile toxins induce the release of inflammatory cytokines from cultured intestinal epithelial cells. In particular, IL-8, a neutrophil chemoattractant known to be regulated by the p38 pathway, is highly induced by toxin exposure in vitro (16–18). These findings suggest that p38 kinase is a critical early driver of the neutrophilic inflammatory response (16, 17).
Of the four main isoforms of p38 kinase, p38α is most widely distributed and is most implicated in cell-mediated inflammatory responses (19, 20). The kinase is activated by a number of extrinsic and intrinsic stimuli, including conditions that damage essential cellular components, like UV radiation and oxidative stress. Once activated, p38α can phosphorylate multiple downstream effectors. Depending on the stimulus or the cell type, these effectors affect proliferation, inflammation, or cell death. Indeed, TcdA is reported to cause p38-dependent necrosis of monocytes, apoptosis of colonocytes, and induction of IL-8, cyclooxygenase-2 and prostaglandin E2 synthesis in treated cells (16, 21, 22). In most circumstances, the major effector mediating p38-dependent inflammation is mitogen-activated protein kinase (MAPK)-activated protein kinase-2 (MK2), a member of the MK subfamily of calcium/calmodulin-dependent kinases. MK2 increases the expression of IL-8, tumor necrosis factor alpha (TNF-α), and other inflammatory cytokines (23–25). Activated MK2 phosphorylates many downstream targets, such as tristetraprolin (TTP), lymphocyte-specific protein tyrosine hydroxylase, and 5-lipoxygenase (24, 26). Among the best-studied MK2 targets is heat shock protein 27 (Hsp27). Upon MK2-induced phosphorylation, Hsp27 undergoes a conformational change, shifting from large multimers to dimers to affect chaperone function and interactions with the actin cytoskeleton (27, 28). Like p38 kinase, which is the target of several novel anti-inflammatory agents, MK2 kinase is a target of several anti-inflammatory drug discovery programs (29–34).
Here, we demonstrate that TcdA and TcdB cause p38-dependent activation of MK2 in toxin-exposed cells, colons of infected animals, and stools of humans with CDI. We describe that MK2 inhibition blocks toxin-induced cytokine release from toxin-exposed enterocytes. Inhibition of these kinases does not interfere with toxin activity but markedly attenuates the subsequent inflammatory cytokine release. Drugs that disrupt the actin cytoskeleton also cause p38- and MK2-dependent IL-8 secretion, suggesting that disruption of the actin network is the initiating event in C. difficile toxin-induced inflammation. Furthermore, we show that p38 and MK2 are activated in colons of infected animals and in human CDI patients. Our results indicate that toxin-induced MK2 activation drives C. difficile-associated inflammatory responses and suggest that MK2 inhibition may attenuate intestinal inflammation and thereby improve disease outcome in patients with CDI.
MATERIALS AND METHODS
Reagents and antibodies.
TcdA was purchased from List Laboratories (Campbell, CA). Recombinant TcdB was purified from Bacillus megaterium, the genome of which encodes full-length TcdB-(histidine)6 under the control of a xylose-inducible promoter (kindly provided by Lacy Borden) (35). B. megaterium was grown to an optical density at 600 nm (OD600) of ∼0.6 before addition of l-xylose (5 g/liter), which induced the expression of TcdB. After incubating for a further 3 h at 30°C, cells were pelleted and sonicated in lysis buffer (0.1% Triton X-100 in phosphate-buffered saline [PBS] containing protease inhibitor cocktail). The debris was pelleted, and the TcdB-His was purified by sequential nickel and gel filtration column chromatography. Aliquots were frozen at −80°C at a concentration of 0.4 mg/ml in phosphate-buffered saline until further use. SB203580, SP600125, latrunculin B, cytochalasin B, nocodazole, and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO). The MK2 inhibitor, PHA-781089, was a gift of Pfizer (36). Chemical inhibitors were diluted to 10 mM in DMSO and stored at −20°C until further use. Antibodies recognizing p38, phospho-p38, Hsp27, phospho-Hsp27, and total actin were purchased from Cell Signaling Technology (Danvers, MA), and monoclonal antibody recognizing the nonglucosylated form of Rac1 was from BD Transduction Labs (clone 102; 610650; San Jose, CA). Complete Mini protease inhibitor cocktail tablets were purchased from Roche. One tablet was dissolved in 15 ml of buffer during toxin purification and cell lysis. H29 (human colon adenocarcinoma; ATCC HTB-38), Caco2 (human colorectal adenocarcinoma; ATCC HTB-37), and T84 (human colorectal carcinoma; ATCC CCL-248) cells were obtained from the ATCC and were maintained at 37°C and 5% CO2 in Dulbecco's modified essential medium (DMEM) (Mediatech, Manassas, VA) supplemented with 10% fetal bovine serum (FBS) (HyClone, Rockford, IL) and 0.1% penicillin-streptomycin (Mediatech, Manassas, VA).
Western blotting.
Following experimental treatments, cells were lysed in lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin, 1× protease inhibitor cocktail). Colonic tissues excised from hamster or mice were prepared for protein immunoblotting by suspending tissue pieces in 10-fold volumes of lysis buffer and then homogenizing with a handheld tissue disruptor (Fisher Scientific, Pittsburgh, PA). After centrifuging to remove particulates, protein concentrations were determined and equalized by adding lysis buffer.
In each experiment, equal amounts of protein were mixed with SDS-PAGE loading buffer and then resolved on a 4 to 15% Tris-HCl gel (Bio-Rad Corp., Hercules, CA) before transfer to polyvinylidene difluoride (PVDF) membranes (Invitrogen Corp, Carlsbad, CA). Membranes were probed with corresponding primary antibodies (1:1,000) and anti-rabbit or anti-mouse alkaline phosphatase-conjugated secondary antibodies (Invitrogen Corp, Carlsbad, CA). Membranes were washed five times with wash buffer (WesternBreeze; Invitrogen) for 5 min each, twice with water for 5 min, and then developed using the WesternBreeze kit protocol (Invitrogen). For comparisons of multiple phosphoproteins, membranes were washed three times for 5 min in wash buffer (WesternBreeze) and reprobed with additional antibodies. Where indicated, equal fractions of lysates from the same experiment were run on separate gels and probed with the corresponding primary antibodies.
Phospho-MK2 EIA.
We developed a sandwich enzyme immunoassay (EIA) to quantify pMK2 in fecal samples. To prepare enzyme-linked immunosorbent assay (ELISA) plates, each well was coated with goat antibody against total MK2 (C-18; sc-6221; Santa Cruz Biotechnology, Santa Cruz, CA) at a concentration of 400 ng/ml in 100 μl 50 mM carbonate buffer, pH 9.5 (BD OptEIA kit; 550534; BD Biosciences, San Jose, CA). The following day, plates were blocked with 200 μl coating buffer (OptEIA kit) for at least 2 h. To process stool samples, approximately 100 mg fecal material was suspended in an equal volume of 1× lysis buffer containing phosphatase inhibitors (9803; Cell Signaling Technology) to which one tablet of Complete Mini protease inhibitor (Roche) per 15 ml was added. The samples were vortexed vigorously for 2 min and then centrifuged for 5 min at 10,000 × g to pellet debris. Supernatants were transferred to a new tube and used immediately for pMK2 detection or stored at −80°C until use. To detect pMK2 in processed fecal samples, 20 μl was added to 80 μl 1× lysis buffer per well, and the plates were incubated at 4°C for 24 h. After washing three times with wash buffer (OptEIA kit), rabbit anti-pMK2 (Thr222) antibody (sc-31675; Santa Cruz Biotechnology) was added at 200 ng/ml and incubated for 2 h at room temperature. After washing each well four times with 300 ml wash buffer (OptEIA kit), 100 μl of horseradish peroxidase (HRP)-conjugated anti-rabbit antibody diluted 1:5,000 was added per well and incubated for 1 h at room temperature. The wells were washed 6 times with 300 ml of wash buffer, and then 100 ml of peroxidase substrate was added (3,3′,5,5′-tetramethylbenzidine [TMB]; T8665; Sigma). The reaction was stopped after approximately 30 min at room temperature by the addition of 50 μl 2 M sulfuric acid, and the signal was read at 450 nm after subtracting background levels on an automated microtiter plate spectrophotometer. A standard curve using recombinant phosphorylated MK2 (American Research Products, Waltham, MA) was used to quantify the sample pMK2. A low positive internal control (75 to 100 pg/ml) was included on each microtiter plate run to measure reproducibility. Stools were also tested for IL-8 (Quantikine human CXCL8/IL-8; R&D Systems, Minneapolis, MN) and lactoferrin (IBD Scan; TechLab, Blackburn, VA) according to the manufacturer's instructions.
Cytotoxicity testing.
Stool or broth culture filtrates were tested for neutralizable cytotoxicity using HT-29 adenocarcinoma tissue-cultured cells (HTB 38; American Type Culture Collection) grown in microtiter plates with a TcdB positive control and compared to noninoculated cells (19). The antitoxin neutralizes TcdB-induced cytotoxicity (Clostridium difficile Toxin/Antitoxin kit; TechLab, Blacksburg, VA), and results were considered final after 48 h. This cell line is highly sensitive to C. difficile toxins (20, 21).
Culture PCR.
Stool samples were additionally cultured anaerobically for 48 h on taurocholate cefoxitin cycloserine mannitol agar (TCCMA), which is suitable for C. difficile vegetative and spore forms (22). TCCMA is a modification of commonly used C. difficile isolation agar TCCFA, with replacement of fructose by mannitol so as to limit the number of clostridial species (37). Characteristic colonies (consisting of anaerobic, large, Gram-positive rods) were then purified and subcultured to prereduced broth, and filtrates were tested for neutralizable cytotoxicity as described in the section on cytotoxicity testing. In addition, each isolate was tested for tcdB by suspending colonies in water and heating (95°C for 15 min), and the supernatant was subjected to PCR using published primers tcdB-1F (5′ GAAGGTGGTTCAGGTCATAC) and tcdB-1R (5′ CATTTTCTAAGCTTCTTAAACCTG) (23). The PCR products were visualized in ethidium-stained agarose gels. If the first PCR was negative, two other published primer sets were tested: TcdB-F (5′ AGCAGTTGAATATAGTGGTTTAGTTAGAGTTG) and TcdB-R (5′ CATGCTTTTTAGTTTCTGGATTGAA) (24) as well as TB1-F (5′ GAGCTGCTTCAATTGGAGAGA) and TB2-R (5′ GTAACCTACTTTCATAACACCAG) (25).
Hamster and murine C. difficile infections.
All animal protocols were approved by the Institutional Animal Care and Use Committee of the Washington University School of Medicine. Like humans, hamsters and mice become much more susceptible to C. difficile infection after antibiotic treatment. In the case of hamsters, a single intraperitoneal injection of clindamycin 2 days prior to infection renders the animals highly susceptible. In the case of mice, an antibiotic cocktail is utilized as described below. Six male Syrian golden hamsters, 5 to 6 weeks old (purchased from Harlan Laboratories, Indianapolis, IN), were given a single intraperitoneal injection of clindamycin (30 mg/kg) 1 day before challenge, which makes the animals much more susceptible to C. difficile infection (38). The following day, animals were gavaged with either 103 or 104 CFU of the highly virulent C. difficile strain NAP1. Thirty-six hours later, hamsters had moist stools, appeared ill, and were sacrificed by 100% carbon dioxide exposure. Colons were removed and processed for protein immunoblotting as described above.
Four male C57BL/6J mice, 6 to 8 weeks old (purchased from Jackson Laboratories, Bar Harbor, MN), were pretreated with antibiotics to render animals susceptible to C. difficile infection (kanamycin [0.4 mg/ml], gentamicin [0.035 mg/ml], colistin [850 U/ml], metronidazole [0.215 mg/ml], and vancomycin [0.045 mg/ml]) in drinking water for 5 days as previously described (39). Two days before infection, all mice were changed to regular autoclaved water lacking these antibiotics, and then 1 day before infection they received a single dose of clindamycin (30 mg/kg of body weight) intraperitoneally. Mice were gavaged on day 0 with 104 CFU of the NAP1 strain. Mice were sacrificed 48 h after infection, at which point they had developed loose stools, hunched appearance, and ruffled fur. Colons were immersed in OCT, snap-frozen in liquid nitrogen, and then sectioned with a cryotome. Specimens were fixed by immersion for 5 min in ice-cold acetone, air dried, and then rehydrated by immersing twice in PBS for 5 min each.
Slides were stained with antibodies against phospho-MK2 (3007; Cell Signaling Technology, Danvers MA) or CD45 (clone 30-F11; BioLegend, San Diego, CA) followed by fluorescently labeled secondary antibodies and then were mounted in media containing 4′,6-diamidino-2-phenylindole (DAPI). Slides were imaged by indirect immunofluorescence under 400× magnification using a Zeiss epifluorescence microscope, and images were captured using AxioVision software.
Human patients with C. difficile infection.
Loose stools submitted for C. difficile testing were obtained from 100 patients, aged 18 to 95 years and hospitalized at Barnes Jewish Hospital, St. Louis, MO, between 2010 and 2011. The use of deidentified samples precluded our ability to glean any knowledge of concurrent usage of anti-C. difficile antibiotics or anti-inflammatory medications. This component of the study was approved by the Institutional Review Board of Washington University School of Medicine. For the purposes of the current study, we defined C. difficile-positive samples as those that yielded a cytotoxin-positive C. difficile colony from culture. We did not exclude any samples and directly compared inflammatory markers from C. difficile cytotoxin-positive and -negative samples.
Statistical analyses.
Data were processed and analyzed using GraphPad Prism version 5.00 for Windows (GraphPad software). Comparisons of pMK2, IL-8, and lactoferrin levels to the presence of toxigenic C. difficile were made using a 2-tailed Wilcoxon signed rank test, and biomarker levels were compared to each other using Spearman correlations (StatView, SAS). Sensitivity, specificity, and positive and negative predictive values for pMK2 and IL-8 were determined after a cutoff was assigned using receiver operator characteristic curve (ROCC) analysis (GraphPad Prism 5); those for lactoferrin were determined using the manufacturer's recommendations. These and other statistical analyses are explained in the footnote to the table for respective variable comparisons, with P < 0.05 considered significant.
RESULTS
p38 and MK2 are activated in enterocytes and monocytes in response to TcdA and TcdB.
We wished to determine the ability of TcdB to activate p38 and MK2 in cultured human enterocytes. The data presented here demonstrate the effects of TcdB on HT-29 cells, which are derived from large intestinal epithelium and have been used as a model of proinflammatory gene expression (40). The data presented here focus on TcdB, because this toxin is generally thought to be most relevant to disease (41, 42). However, we have found that both TcdA and TcdB caused phosphorylation of p38 and MK2 in T84 and Caco-2 cells, which are also human intestinal cell lines (data not shown).
HT-29 cells were treated with TcdB at increasing concentrations (0 to 80 ng/ml TcdB) or for increasing amounts of time (0 to 360 min) (Fig. 1B). The cells were then lysed and subjected to SDS-PAGE followed by protein immunoblotting to detect phosphorylated p38 or phospho-Hsp27 as a specific marker of MK2 activation (36). We compared p38 and MK2 activation to nonglucosylated Rac1 signal. TcdB transfers a glucose moiety to Rac1, resulting in loss of reactivity with monoclonal antibody clone 102 (BD Transduction Laboratories), therefore it is a good indicator of the toxin effect on an intracellular target. Treatment of HT29 cells demonstrated that Rac1 reactivity began to decrease at 0.8 to 3 ng/ml TcdB (Fig. 1A). In contrast, p38 and Hsp27 were phosphorylated at toxin concentrations as low as 0.8 ng/ml and became maximal at approximately 16 ng/ml (Fig. 1A). These findings indicate that p38 and MK2 activation are very sensitive to intracellular toxin activity. Similarly, both kinases were activated within 60 min of toxin addition, when the Rac1 signal first began to fade, indicating that kinase activation occurs rapidly after toxin internalization (Fig. 1B). Similarly, TcdA causes phosphorylation of p38 and MK2 at the lowest concentrations affecting Rac1 glucosylation in T84 cells and with similarly rapid kinetics (see Fig. S1 in the supplemental material).
Fig 1.
p38 and MK2 are activated by toxin exposure. (A) HT-29 cells were exposed to TcdB at the indicated concentrations for 2 h. Cell lysates were subjected to SDS-PAGE and then immunoblotted for phospho-p38, phospho-Hsp27, or nonglucosylated Rac1. (B) HT-29 cells were treated with 20 ng/ml TcdB for the indicated times, and then cell lysates were collected and immunoblotting performed as described for panel A.
In summary, p38 and MK2 kinase activity are induced at the lowest toxin concentrations and the earliest time points at which TcdB has a measurable effect on HT29 cells.
Toxin-induced MK2 activation is p38 dependent.
We next confirmed that toxin-induced MK2 activation was attributable to p38 and not due to JNK, another kinase known to be activated by cellular stress. To demonstrate that p38 activity was necessary for MK2 phosphorylation and activation, HT-29 cells were incubated with kinase inhibitors starting 30 min prior to TcdB exposure. p38 inhibitor SB203580 and inhibitor III diminished TcdB-induced MK2 activation, as indicated by a marked decrease in phospho-Hsp27 in treated cells. However, the JNK kinase inhibitor SP600125 had no effect on TcdB-induced Hsp27 phosphorylation. Finally, the MK2-specific inhibitor PHA-781089 markedly diminished TcdB-induced MK2 activity. These results confirmed that TcdB-induced MK2 activity was p38 dependent. To exclude the possibility that this effect was caused by inhibition of toxin internalization or transport, we confirmed that neither inhibitor blocked TcdB effect on Rac1 glucosylation (Fig. 2). We extended these findings to show that TcdA induced p38-dependent MK2 activation in T84 cells, revealing that both toxins result in p38-dependent MK2 activation (see Fig. S2 in the supplemental material).
Fig 2.
Toxin-induced MK2 activation is p38 dependent. HT-29 cells were preincubated with either 0.1% DMSO, p38 inhibitor SB203580 (2.65 μM), p38 inhibitor III (0.5 μM), JNK kinase inhibitor SP600125 (25 μM), or MK2 inhibitor PHA-781089 (20 μM) for 30 min and then exposed to either no toxin or TcdB at 20 ng/ml for 2 h. Cell lysates were subjected to SDS-PAGE followed by immunoblotting for phospho-p38, phospho-Hsp27, or nonglucosylated Rac1.
p38 and MK2 inhibition blocks toxin-induced IL-8 and GROα release from HT-29 cells.
We next asked if p38 inhibition of toxin-induced cytokine production by cultured enterocytes is mediated through p38-dependent activation of MK2. IL-8 and GROα (also known as CXCL-1) are both neutrophil chemoattractants, binding to CXCR-2 receptors on neutrophils and macrophages, signaling their recruitment to the site of cytokine release. HT-29 cells were exposed to TcdB in the absence of inhibitor or in the presence of p38 inhibitor or a specific MK2 kinase inhibitor. The p38 inhibitor SB203580 efficiently blocked toxin-induced IL-8 and GROα secretion by HT-29 cells. MK2 kinase inhibitor PHA-781089 completely inhibited TcdB-induced IL-8 and GROα secretion induced by HT-29 cells (Fig. 3A and B). Similar effects on IL-8 secretion were seen when HT-29 cells were treated with TcdA in the presence or absence of p38 or MK2 kinase inhibitors (see Fig. S3 in the supplemental material). These results indicate that C. difficile toxin-induced inflammatory cytokine release from cultured enterocytes depends on activation of the p38-MK2 kinase cascade.
Fig 3.
Toxin-induced cytokine release is p38 and MK2 dependent. HT-29 cells were preincubated with p38 inhibitor SB203580 (2.65 μM) or MK2 inhibitor PHA-781089 (20 μM) for 30 min and then exposed to TcdB (20 ng/ml) for 12 h in the presence of the kinase inhibitors. The supernatants were harvested, and IL-8 concentration (A) or GROα concentration (B) was determined by ELISA (**, P < 0.01; ***, P < 0.001).
Neither p38 nor MK2 inhibition prevents toxin internalization or affects Rac1 glucosylation.
Having demonstrated that p38 or MK2 inhibition decreases IL-8 production, we next excluded the possibility that these kinases were required for toxin internalization or activity, particularly as p38 inhibition has been reported to affect intracellular transport of Shiga toxin (43). HT-29 cells were pretreated with either kinase inhibitor and then exposed to TcdB in the presence of kinase inhibitor. The cells rounded to the same extent and with the same kinetics independent of kinase inhibition (not shown). Furthermore, neither kinase inhibitor impaired toxin-induced Rac1 glucosylation, as demonstrated by loss of reactivity with monoclonal antibody 102, which is specific for the unmodified form. In contrast, bafilomycin A and ammonium chloride, both of which block endosome acidification and therefore toxin access to the cytoplasm, blocked Rac1 glucosylation (Fig. 4). Similar results were seen with the fibroblast-like HeLa cell line, where p38 or MK2 inhibition did not affect TcdB-mediated Rac1 glucosylation (see Fig. S4 in the supplemental material).
Fig 4.
p38 and MK2 inhibition do not interfere with toxin transport or activity. HT-29 cells were preincubated with either 0.1% DMSO, p38 inhibitor SB203580 (2.65 μM), MK2 inhibitor PHA-781089 (20 μM), bafilomycin (5 μM), or ammonium chloride (20 mM) for 30 min. TcdB then was added at a concentration of 20 ng/ml, and the cells were incubated for a further 2 h. Cell lysates were subjected to SDS-PAGE and immunoblotting for actin or nonglucosylated Rac1.
Actin-disrupting agents cause MK2 activation and IL-8 release.
The p38-MK2 kinase pathway is activated by diverse stimuli, including extrinsic and intrinsic cellular injuries (44, 45). For example, we previously demonstrated that cellular injury caused by Shiga toxin results in p38-MK2 kinase activation and IL-8 release (46). Shiga toxin damages the ribosome, impairing protein translation and ultimately causing cell death. C. difficile toxins have an entirely different mechanism of cellular injury. Specifically, TcdA and TcdB glucosylate and inactivate the small GTPases Rho, Rac, and CDC42. Among the resulting effects, one of the most striking outcomes is disruption of the actin cytoskeleton. We wondered whether cytoskeletal disruption could be a mechanism whereby the C. difficile toxins activate p38 and MK2 kinases and therefore directly tested the effects of actin- and microtubule-disrupting agents on p38 and MK2 kinase phosphorylation. HT-29 colonic epithelial cells were treated with toxin TcdB or small-molecule compounds that disrupt the actin cytoskeleton (latrunculin B or cytochalasin B) or that disperse microtubules (nocodazole). MK2 activation was assessed by detecting the phosphorylated form of MK2 substrate Hsp27. Cells exposed to actin-disrupting agents showed activation of MK2 at levels comparable to those caused by TcdB as assessed by Hsp27 phosphorylation, while the microtubule-disrupting agent nocodazole did not result in increased Hsp27 phosphorylation (Fig. 5A). Furthermore, actin disruption with cytochalasin B increased IL-8 secretion by HT-29 cells to an even greater extent than did TcdB, while the microtubule-disrupting agent nocodazole did not significantly increase IL-8 release. Pretreatment of cells with the highly specific MK2 inhibitor (36) completely abolished IL-8 secretion induced by toxin or actin disruption (Fig. 5B). These results suggest that disruption of the actin cytoskeleton is one means whereby the C. difficile toxins can activate p38 and MK2 and incite an inflammatory response.
Fig 5.
Actin-disrupting agents cause MK2 activation and IL-8 secretion. (A) HT-29 cells were treated for 2 h with TcdB (20 ng/ml), latrunculin (5 μM), cytochalasin B (25 μM), or nocodazole (10 μg/ml). Cell lysates were then subjected to SDS-PAGE followed by immunoblotting for actin or for phosphorylated Hsp27. The exposed film was then scanned, and band intensity was determined using ImageJ. (B) HT-29 cells were treated with TcdB (20 ng/ml), cytochalasin B (25 μM), or nocodazole (10 μg/ml) in the presence of either 0.1% DMSO, p38 inhibitor SB203580 (2.65 μM), or MK2 inhibitor PHA-781089 (20 μM) for 12 h. The supernatants were then removed, and IL-8 was detected by ELISA.
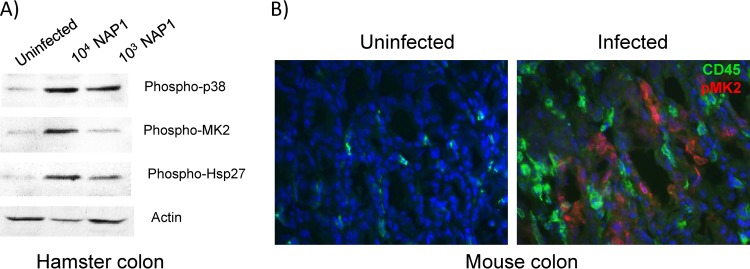
Phospho-MK2 is detected in tissues of infected animals.
We next determined if the p38-MK2 pathway was activated in vivo during C. difficile infection. Syrian golden hamsters have been used as a model for C. difficile infection. Pretreatment with a single dose of clindamycin renders the animals exquisitely susceptible to C. difficile. As has previously been reported, we found that infection of Syrian golden hamsters with 1,000 CFU of the NAP1 C. difficile strain resulted in 100% mortality (n = 6). As has been described previously, necropsy demonstrated a grossly enlarged and hemorrhagic cecum (47, 48). Histopathologic examination showed diffuse disruption of the epithelium, hemorrhage into the submucosal tissues, and a marked neutrophilic infiltrate (not shown). To determine if p38 and MK2 were activated during acute infection, hamsters were sacrificed 36 h after infection with either 103 or 104 CFU of the virulent NAP1 C. difficile strain. Compared to an uninfected animal, colons from hamsters infected with C. difficile had increases in phospho-p38, phospho-MK2, and the phosphorylated form of MK2 substrate Hsp27 (Fig. 6A). Phosphorylated p38 and MK2 were also detected in colons of infected mice by protein immunoblotting (not shown). Immunofluorescence staining demonstrated MK2 activation in intestinal epithelial cells. There was a brisk inflammatory infiltrate, as evidenced by the presence of CD45-positive cells, a marker for cells of hematopoietic origin, but these cells did not label with anti-pMK2 (Fig. 6B). These findings are consistent with the model that toxin-induced damage activates p38-MK2 in enterocytes, resulting in IL-8 release and subsequent inflammatory cell recruitment.
Fig 6.
C. difficile infection causes p38 and MK2 activation in hamster and murine colon. (A) The day after a single dose of clindamycin, hamsters were infected either with 104 or 103 CFU of C. difficile strain NAP1. Hamsters were sacrificed at 48 h after infection, their colons removed, and lysates subjected to SDS-PAGE followed by immunoblotting for phsopho-p38, phospho-MK2, phospho-Hsp27, or actin. (B) Mice were infected with C. difficile after 7 days of pretreatment with oral kanamycin, gentamicin, colistin, metronidazole, and vancomycin and a single dose of clindamycin 1 day prior to infection. Forty-eight h later the animals were sacrificed and colons labeled with antibodies against phospho-MK2 (red; Alexa Fluor 596) or CD45 (green; Alexa Fluor 488).
Phospho-MK2 is detected in fecal filtrates of patients with C. difficile infection.
Previous investigators demonstrated that fecal inflammatory markers were elevated in patients with CDI. In particular, fecal IL-8 and fecal lactoferrin were more highly elevated in patients with severe disease than in patients with mild disease (9). We investigated the possibility that the p38/MK2 pathway is similarly activated in patients with C. difficile infection. A convenience sample of 100 stool specimens submitted for C. difficile testing was tested for the presence of phospho-MK2, lactoferrin, and IL-8. C. difficile toxin immunoassay testing was performed in the clinical microbiology laboratory, and stools were frozen. Subsequently, the C. difficile status was verified in our laboratory by culture and in vitro toxin testing. Toxigenic C. difficile was detected in 15% of samples submitted for testing.
As described in Materials and Methods, our laboratory developed an enzyme immunoassay to detect phospho-MK2 and found the ELISA to be linear from 30 pg/ml to 5 ng/ml of pMK2 standard. The sensitivity of the ELISA compared well to detection by immunoblotting, which had a sensitivity of 150 pg/ml (data not shown). Using this assay and commercially available ELISAs, we quantitated pMK2, IL-8, and lactoferrin in stools submitted to the clinical microbiology laboratory.
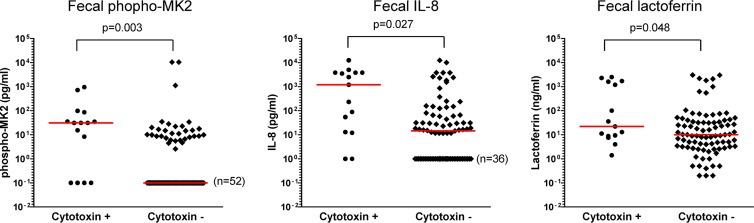
Although there was a large degree of variation between samples, pMK2, IL-8, and lactoferrin levels were significantly higher in filtrates from patients with stool positive for cytotoxigenic C. difficile than in samples from C. difficile-negative patients (P = 0.003, 0.012, and 0.048, respectively, by Wilcoxon signed rank test). Several of the C. difficile-negative patients had detectable levels of phospho-MK2, IL-8, or lactoferrin in the stool. These patients all had loose stools, which was the reason for sending their samples for C. difficile testing. Our results suggest that many of them had associated intestinal inflammation. Nevertheless, the degree of pMK2 elevation and the percentage of patients with detectable pMK2 were both greater in C. difficile-infected patients than in C. difficile-negative patients (Fig. 7). As an alternate method of comparing the samples, we set positive and negative cutoff values for each inflammatory parameter. A cutoff for pMK2 of >1 pg/ml was considered positive, revealing that 73% of C. difficile-positive samples and 38% of C. difficile-negative samples were positive for pMK2. Using a two-tailed Fisher's exact test, this difference was statistically significant (P = 0.021) (Table 1). Similarly, a cutoff value for IL-8 was set at 3.5 pg/ml based on the manufacturer's information, yielding a positive IL-8 result in 87% of C. difficile cytotoxin-positive and 58% of C. difficile-negative values (P = 0.043). Finally, using a lactoferrin cutoff of 7.25 μg/ml (as indicated by the manufacturer's information) yielded nonsignificant correlation between lactoferrin positivity and detectable cytotoxin (P = 0.134), being detected in 87% of C. difficile cytotoxin-positive samples and 63% of cytotoxin-negative samples.
Fig 7.
Median phospho-MK2, IL-8, and lactoferrin levels are higher in feces from C. difficile patients than in samples from C. difficile-negative patients. One hundred stool samples submitted for C. difficile testing to the clinical laboratory were analyzed for the presence of cytotoxin-producing C. difficile. From the same samples, phosphorylated MK2, IL-8, and lactoferrin levels were measured by ELISA, and each was compared to a standard curve. Median values are indicated by the thick black bar.
Table 1.
Biomarkers in patients with toxigenic C. difficile compared to patients with negative C. difficile
| Variable | No. (%) C. difficile positive (n = 15) | No. (%) C. difficile negative (n = 85) | P valuea |
|---|---|---|---|
| pMK2 (>1 pg/ml) | 11 (73) | 32 (38) | 0.021* |
| IL-8 (>3.5 pg/ml) | 13 (87) | 49 (58) | 0.043* |
| Lactoferrin (>7.25 μg/ml) | 13 (87) | 54 (63) | 0.134 |
Comparisons were done with two-tailed Fisher's exact test. *, Correlation is significant at the 0.05 level.
In summary, the analysis demonstrates that pMK2 levels are higher in fecal samples of C. difficile patients than in C. difficile-negative patients, and C. difficile patients were much more likely to have detectable pMK2 in their feces than C. difficile-negative patients, supporting a role for this kinase in toxin-induced intestinal inflammation.
DISCUSSION
Hospital-acquired Clostridium difficile infection is now considered a public health emergency in the United States, Canada, and Europe. Recently, C. difficile infection (CDI) incidence was reportedly 21% higher than methicillin-resistant Staphylococcus aureus infection as a cause of hospital-acquired infection and as common as hospital-wide bloodstream infections (49). Symptoms of CDI range from mild diarrhea to the formation of pseudomembranous lesions, toxic megacolon, sepsis, and death. Opportunistic CDI is most strongly associated with hospitalization and prolonged antibiotics use, as C. difficile can only colonize the gut if the normal intestinal microbiota is absent or disturbed. The full spectrum of CDI symptoms can be largely attributed to the production of its two toxins, toxin A (TcdA) and toxin B (TcdB), as nontoxigenic strains have been found to be avirulent in both human and animal models. Both toxins cause disruptions in the actin cytoskeleton and tight junctions of the host cell, thus compromising transepithelial resistance and the integrity of the epithelial barrier. Additionally, the toxins directly incite an inflammatory response in vitro (42) and in vivo (14, 50).
Current therapies for CDI are unsatisfactory. Only three antibiotics have demonstrated clinical efficacy. Metronidazole is recommended as the first-line agent in mild to moderate CDI (51). Treatment failures occur in 15 to 20% of cases (52). Oral vancomycin is recommended in severe disease or in cases of metronidazole failure (53, 54), but it fails to cure in up to 15% of cases (55). Fidaxomicin has just been approved as a third antibiotic for the treatment of CDI, but its global cure rate is only 77% (56). In addition, this drug is very costly, and its efficacy may have some variability depending on the C. difficile strain type. Regardless of therapy, patients remain symptomatic for an average of 5 days after starting antibiotics, and some will progress to severe or fulminant disease and die despite standard-of-care medical management (57). Progression and death from CDI may be due to an uncontrolled, toxin-driven inflammatory response, resulting in massive intestinal necrosis. The intense inflammatory response incited by C. difficile may be predominantly pathogenic, contributing to disease without significantly benefitting the host. In this regard, it is important to determine if the toxin-induced inflammatory response can be therapeutically modulated without impairing beneficial immune responses to the infection.
Biomarkers of inflammation, such as IL-8, a chemokine of neutrophils, and the neutrophil lactoferrin, have been assessed in the context of human CDI, where they are associated with more severe disease (9, 58–60). To date, cellular signaling molecules such as p38 and MK2 kinases have not been evaluated in patients with CDI. However, previous investigators found that p38 was a major regulator of inflammation and cell death induced by C. difficile, indicating that this pathway was essential to toxin-induced inflammation (17, 21, 22, 61). This kinase is widely expressed and regulates cellular response to injury. Its activation can result in proliferation, apoptosis, or inflammation depending on the stimulus and the cell type. p38 inhibitors have been explored as potential anti-inflammatory agents in a number of diseases; however, none of these compounds has yet to be licensed due to problems with efficacy or tolerability (62). For that reason, focus has shifted to MK2 kinase, a substrate of p38 which is more narrowly expressed and is felt to be more specifically involved in stress-induced inflammation (34, 63). pMK2, whose precursor is p38, belongs to the calcium-calmodulin kinase superfamily (64). After activation of p38 by a variety of stressors, pMK2 phosphorylates specific molecules which regulate the actin cytoskeleton and stabilize cytokine mRNA transcripts (34, 65, 66). mRNA transcripts for IL-8 and TNF-α can then mediate a number of downstream effects, including neutrophil chemotaxis, vasodilation, tissue edema, and cellular apoptosis. Several MK2 kinase inhibitors are currently in development for treatment of inflammatory diseases, and it is hoped that these compounds will retain the anti-inflammatory activity of p38 inhibitors while lacking the toxicities that may relate essential noninflammatory p38 activities (30, 34).
We find that TcdA and TcdB cause p38-dependent activation of MK2 and demonstrate that MK2 inhibition in vitro completely blocks toxin-induced IL-8 release from both enterocytes and monocytes despite the observation that MK2 inhibition does not affect the toxin-induced effects on the cell. These toxins inactivate the Rac and Rho family of small GTPases, resulting in disruption of the actin cytoskeleton. Treatment of cultured intestinal epithelial cells with actin- but not microtubule-disrupting agents also causes p38-MK2 activation and increased IL-8 production, suggesting that actin disassembly is the mechanism of inflammatory kinase activation in toxin-exposed cells. We demonstrate that the p38-MK2 pathway is activated in the intestines of infected animals. To determine whether pMK2 arose from damaged intestinal mucosa or from infiltrating myeloid cells, immunofluorescence microscopy was performed and demonstrated that pMK2 was detected in nonmyeloid cells. These findings are consistent with the notion that toxin-induced cellular injury results in activation of p38-MK2 in intestinal mucosa, resulting in increased inflammatory cytokine production and subsequent infiltration of pMK2-negative myeloid cells. Finally, we demonstrate that phosphorylated MK2 is present in the stools of infected patients, suggesting that the pathway is activated in at least a subset of individuals with CDI.
C. difficile toxin A (TcdA) is known to induce p38-dependent cytokine production when instilled directly into mouse ileum (17, 67). The present study is the first to demonstrate that MK2 is the downstream mediator of toxin-induced p38-dependent cytokine production. Moreover, this is the first study to demonstrate that the pathway is activated in C. difficile-infected animals and humans.
In human CDI patient samples, we found a significant association of pMK2 elevation with the presence of toxigenic C. difficile. There was considerable variability of all inflammatory markers among patients, and phospho-MK2, IL-8, and lactoferrin were detected in a significant fraction of patients without C. difficile infection. All of these patients had gastrointestinal symptoms. Inflammatory cytokine gene expression can be detected in fecal extracts from patients with other bacterial causes of enteritis (68, 69). Inflammatory cytokines and lactoferrin are also elevated in the feces of patients with active inflammatory bowel disease (9, 70, 71). Like inflammatory cytokine levels, fecal pMK2 may be a general indicator of intestinal inflammation. MK2 is activated by Shiga toxin and during influenza A virus infection of cultured cells (46). Whether the p38-MK2 pathway plays a more general role in pathogen-induced inflammation remains to be determined. Nevertheless, pMK2 elevation was significantly correlated with C. difficile infection in our unbiased collection of stool samples sent for C. difficile testing, indicating that this proinflammatory pathway is activated in the majority of patients with C. difficile infection.
Our results indicate that the p38-MK2 kinase pathway is activated during C. difficile infection and contributes to the host inflammatory response in patients with CDI. Phospho-MK2 may be a useful adjunctive biomarker of disease severity in patients with CDI, and by blocking toxin-induced cytokine release it may ultimately prove to be a useful adjunctive therapeutic target in C. difficile-infected patients with severe intestinal inflammation.
Supplementary Material
ACKNOWLEDGMENTS
Bacillus megaterium containing a plasmid encoding full-length (histidine)6-tagged TcdB was generously provided by Borden Lacy. Tissue section processing was performed at the Washington University School of Medicine DDRCC morphology core. Lactoferrin EIA kits were generously provided by TechLab.
Funding support was from institutional training grant T32-AI007172 and postdoctoral training grant UL1 RR024992 (L.D.B.). This work was supported by the Midwest Regional Centers for Excellence for Biodefense and Emerging Infectious Disease Research (U54-AI057160). D.B.H. received funding through a Washington University-Pfizer Biomedical Agreement to study the role of p38 and MK2 pathways in host response to C. difficile infection after these studies were completed.
L.D.B. and Z.H. report no conflicts of interest. E.R.D. receives research funding from Merck, Optimer, and Viropharma and is a consultant for Sanofi Pasteur, Optimer, Pfizer, and Merck.
Footnotes
Published ahead of print 21 December 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00186-12.
REFERENCES
- 1. Archibald LK, Banerjee SN, Jarvis WR. 2004. Secular trends in hospital-acquired Clostridium difficile disease in the United States, 1987–2001. J. Infect. Dis. 189:1585–1589 [DOI] [PubMed] [Google Scholar]
- 2. Loo VG, Poirier L, Miller MA, Oughton M, Libman MD, Michaud S, Bourgault AM, Nguyen T, Frenette C, Kelly M, Vibien A, Brassard P, Fenn S, Dewar K, Hudson TJ, Horn R, Rene P, Monczak Y, Dascal A. 2005. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N. Engl. J. Med. 353:2442–2449 [DOI] [PubMed] [Google Scholar]
- 3. Musher DM, Logan N, Mehendiratta V. 2006. Epidemic Clostridium difficile. N. Engl. J. Med. 354:1199–1203 [DOI] [PubMed] [Google Scholar]
- 4. Dubberke ER, Reske KA, Yan Y, Olsen MA, McDonald LC, Fraser VJ. 2007. Clostridium difficile–associated disease in a setting of endemicity: identification of novel risk factors. Clin. Infect. Dis. 45:1543–1549 [DOI] [PubMed] [Google Scholar]
- 5. Dubberke ER, Gerding DN, Classen D, Arias KM, Podgorny K, Anderson DJ, Burstin H, Calfee DP, Coffin SE, Fraser V, Griffin FA, Gross P, Kaye KS, Klompas M, Lo E, Marschall J, Mermel LA, Nicolle L, Pegues DA, Perl TM, Saint S, Salgado CD, Weinstein RA, Wise R, Yokoe DS. 2008. Strategies to prevent clostridium difficile infections in acute care hospitals. Infect. Control Hosp. Epidemiol. 29(Suppl. 1):S81–S92 [DOI] [PubMed] [Google Scholar]
- 6. Reinert DJ, Jank T, Aktories K, Schulz GE. 2005. Structural basis for the function of Clostridium difficile toxin B. J. Mol. Biol. 351:973–981 [DOI] [PubMed] [Google Scholar]
- 7. Ishida Y, Maegawa T, Kondo T, Kimura A, Iwakura Y, Nakamura S, Mukaida N. 2004. Essential involvement of IFN-gamma in Clostridium difficile toxin A-induced enteritis. J. Immunol. 172:3018–3025 [DOI] [PubMed] [Google Scholar]
- 8. Hippenstiel S, Soeth S, Kellas B, Fuhrmann O, Seybold J, Krull M, Eichel-Streiber C, Goebeler M, Ludwig S, Suttorp N. 2000. Rho proteins and the p38-MAPK pathway are important mediators for LPS-induced interleukin-8 expression in human endothelial cells. Blood 95:3044–3051 [PubMed] [Google Scholar]
- 9. Steiner TS, Flores CA, Pizarro TT, Guerrant RL. 1997. Fecal lactoferrin, interleukin-1beta, and interleukin-8 are elevated in patients with severe Clostridium difficile colitis. Clin. Diagn. Lab. Immunol. 4:719–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jiang ZD, DuPont HL, Garey K, Price M, Graham G, Okhuysen P, Dao-Tran T, LaRocco M. 2006. A common polymorphism in the interleukin 8 gene promoter is associated with Clostridium difficile diarrhea. Am. J. Gastroenterol. 101:1112–1116 [DOI] [PubMed] [Google Scholar]
- 11. Jiang ZD, Garey KW, Price M, Graham G, Okhuysen P, Dao-Tran T, LaRocco M, DuPont HL. 2007. Association of interleukin-8 polymorphism and immunoglobulin G anti-toxin A in patients with Clostridium difficile-associated diarrhea. Clin. Gastroenterol. Hepatol. 5:964–968 [DOI] [PubMed] [Google Scholar]
- 12. Castagliuolo I, Karalis K, Valenick L, Pasha A, Nikulasson S, Wlk M, Pothoulakis C. 2001. Endogenous corticosteroids modulate Clostridium difficile toxin A-induced enteritis in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 280:G539–G545 [DOI] [PubMed] [Google Scholar]
- 13. de Araujo Junqueira AF, Dias AA, Vale ML, Spilborghs GM, Bossa AS, Lima BB, Carvalho AF, Guerrant RL, Ribeiro RA, Brito GA. 2011. Adenosine deaminase inhibition prevents Clostridium difficile toxin A-induced enteritis in mice. Infect. Immun. 79:653–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ng J, Hirota SA, Gross O, Li Y, Ulke-Lemee A, Potentier MS, Schenck LP, Vilaysane A, Seamone ME, Feng H, Armstrong GD, Tschopp J, Macdonald JA, Muruve DA, Beck PL. 2010. Clostridium difficile toxin-induced inflammation and intestinal injury are mediated by the inflammasome. Gastroenterology 139:542–552 [DOI] [PubMed] [Google Scholar]
- 15. Jarchum I, Liu M, Lipuma L, Pamer EG. 2011. Toll-like receptor-5 stimulation protects mice from acute Clostridium difficile colitis. Infect. Immun. 80:2989–2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Warny M, Keates AC, Keates S, Castagliuolo I, Zacks JK, Aboudola S, Qamar A, Pothoulakis C, LaMont JT, Kelly CP. 2000. p38 MAP kinase activation by Clostridium difficile toxin A mediates monocyte necrosis, IL-8 production, and enteritis. J. Clin. Investig. 105:1147–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee JY, Park HR, Oh YK, Kim YJ, Youn J, Han JS, Kim JM. 2007. Effects of transcription factor activator protein-1 on interleukin-8 expression and enteritis in response to Clostridium difficile toxin A. J. Mol. Med. 85:1393–1404 [DOI] [PubMed] [Google Scholar]
- 18. Tixier E, Lalanne F, Just I, Galmiche JP, Neunlist M. 2005. Human mucosa/submucosa interactions during intestinal inflammation: involvement of the enteric nervous system in interleukin-8 secretion. Cell Microbiol. 7:1798–1810 [DOI] [PubMed] [Google Scholar]
- 19. Gaestel M, Mengel A, Bothe U, Asadullah K. 2007. Protein kinases as small molecule inhibitor targets in inflammation. Curr. Med. Chem. 14:2214–2234 [DOI] [PubMed] [Google Scholar]
- 20. Cargnello M, Roux PP. 2011. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 75:50–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim H, Kokkotou E, Na X, Rhee SH, Moyer MP, Pothoulakis C, Lamont JT. 2005. Clostridium difficile toxin A-induced colonocyte apoptosis involves p53-dependent p21(WAF1/CIP1) induction via p38 mitogen-activated protein kinase. Gastroenterology 129:1875–1888 [DOI] [PubMed] [Google Scholar]
- 22. Kim H, Rhee SH, Kokkotou E, Na X, Savidge T, Moyer MP, Pothoulakis C, LaMont JT. 2005. Clostridium difficile toxin A regulates inducible cyclooxygenase-2 and prostaglandin E2 synthesis in colonocytes via reactive oxygen species and activation of p38 MAPK. J. Biol. Chem. 280:21237–21245 [DOI] [PubMed] [Google Scholar]
- 23. Su X, Ao L, Zou N, Song Y, Yang X, Cai GY, Fullerton DA, Meng X. 2008. Post-transcriptional regulation of TNF-induced expression of ICAM-1 and IL-8 in human lung microvascular endothelial cells: an obligatory role for the p38 MAPK-MK2 pathway dissociated with HSP27. Biochim. Biophys. Acta 1783:1623–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Winzen R, Kracht M, Ritter B, Wilhelm A, Chen CY, Shyu AB, Muller M, Gaestel M, Resch K, Holtmann H. 1999. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 18:4969–4980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Funding AT, Johansen C, Gaestel M, Bibby BM, Lilleholt LL, Kragballe K, Iversen L. 2009. Reduced oxazolone-induced skin inflammation in MAPKAP kinase 2 knockout mice. J. Investig. Dermatol. 129:891–898 [DOI] [PubMed] [Google Scholar]
- 26. Ronkina N, Kotlyarov A, Dittrich-Breiholz O, Kracht M, Hitti E, Milarski K, Askew R, Marusic S, Lin LL, Gaestel M, Telliez JB. 2007. The mitogen-activated protein kinase (MAPK)-activated protein kinases MK2 and MK3 cooperate in stimulation of tumor necrosis factor biosynthesis and stabilization of p38 MAPK. Mol. Cell. Biol. 27:170–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zheng C, Lin Z, Zhao ZJ, Yang Y, Niu H, Shen X. 2006. MAPK-activated protein kinase-2 (MK2)-mediated formation and phosphorylation-regulated dissociation of the signal complex consisting of p38, MK2, Akt, and Hsp27. J. Biol. Chem. 281:37215–37226 [DOI] [PubMed] [Google Scholar]
- 28. Kotlyarov A, Yannoni Y, Fritz S, Laass K, Telliez JB, Pitman D, Lin LL, Gaestel M. 2002. Distinct cellular functions of MK2. Mol. Cell. Biol. 22:4827–4835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anderson DR, Meyers MJ, Kurumbail RG, Caspers N, Poda GI, Long SA, Pierce BS, Mahoney MW, Mourey RJ, Parikh MD. 2009. Benzothiophene inhibitors of MK2. Part 2: improvements in kinase selectivity and cell potency. Bioorg. Med. Chem. Lett. 19:4882–4884 [DOI] [PubMed] [Google Scholar]
- 30. Nayana RS, Bommisetty SK, Singh K, Bairy SK, Nunna S, Pramod A, Muttineni R. 2009. Structural analysis of carboline derivatives as inhibitors of MAPKAP K2 using 3D QSAR and docking studies. J. Chem. Infect. Model. 49:53–67 [DOI] [PubMed] [Google Scholar]
- 31. Xiong Z, Gao DA, Cogan DA, Goldberg DR, Hao MH, Moss N, Pack E, Pargellis C, Skow D, Trieselmann T, Werneburg B, White A. 2008. Synthesis and SAR studies of indole-based MK2 inhibitors. Bioorg. Med. Chem. Lett. 18:1994–1999 [DOI] [PubMed] [Google Scholar]
- 32. Schlapbach A, Feifel R, Hawtin S, Heng R, Koch G, Moebitz H, Revesz L, Scheufler C, Velcicky J, Waelchli R, Huppertz C. 2008. Pyrrolo-pyrimidones: a novel class of MK2 inhibitors with potent cellular activity. Bioorg. Med. Chem. Lett. 18:6142–6146 [DOI] [PubMed] [Google Scholar]
- 33. Keminer O, Kraemer J, Kahmann J, Sternberger I, Scheich C, Jungmann J, Schaert S, Winkler D, Ichihara O, Whittaker M, Ullmann D, Hesterkamp T. 2009. Novel MK2 inhibitors by fragment screening. Comb. Chem. High Throughput Screen. 12:697–703 [DOI] [PubMed] [Google Scholar]
- 34. Duraisamy S, Bajpai M, Bughani U, Dastidar SG, Ray A, Chopra P. 2008. MK2: a novel molecular target for anti-inflammatory therapy. Expert Opin. Ther. Targets 12:921–936 [DOI] [PubMed] [Google Scholar]
- 35. Pruitt RN, Chagot B, Cover M, Chazin WJ, Spiller B, Lacy DB. 2009. Structure-function analysis of inositol hexakisphosphate-induced autoprocessing in Clostridium difficile toxin A. J. Biol. Chem. 284:21934–21940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Anderson DR, Meyers MJ, Vernier WF, Mahoney MW, Kurumbail RG, Caspers N, Poda GI, Schindler JF, Reitz DB, Mourey RJ. 2007. Pyrrolopyridine inhibitors of mitogen-activated protein kinase-activated protein kinase 2 (MK-2). J. Med. Chem. 50:2647–2654 [DOI] [PubMed] [Google Scholar]
- 37. Bliss DZ, Johnson S, Clabots CR, Savik K, Gerding DN. 1997. Comparison of cycloserine-cefoxitin-fructose agar (CCFA) and taurocholate-CCFA for recovery of Clostridium difficile during surveillance of hospitalized patients. Diagn. Microbiol. Infect. Dis. 29:1–4 [DOI] [PubMed] [Google Scholar]
- 38. Sambol SP, Tang JK, Merrigan MM, Johnson S, Gerding DN. 2001. Infection of hamsters with epidemiologically important strains of Clostridium difficile. J. Infect. Dis. 183:1760–1766 [DOI] [PubMed] [Google Scholar]
- 39. Chen X, Katchar K, Goldsmith JD, Nanthakumar N, Cheknis A, Gerding DN, Kelly CP. 2008. A mouse model of Clostridium difficile-associated disease. Gastroenterology 135:1984–1992 [DOI] [PubMed] [Google Scholar]
- 40. Bruno MCE, Kaetzel CS. 2005. Long-term exposure of the HT-29 human intestinal epithelial cell line to TNF causes sustained up-regulation of the polymeric Ig receptor and proinflammatory genes through transcriptional and posttranscriptional methods. J. Immunol. 174:7278–7284 [DOI] [PubMed] [Google Scholar]
- 41. Lyras D, O'Connor JR, Howarth PM, Sambol SP, Carter GP, Phumoonna T, Poon R, Adams V, Vedantam G, Johnson S, Gerding DN, Rood JI. 2009. Toxin B is essential for virulence of Clostridium difficile. Nature 458:1176–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Voth DE, Ballard JD. 2005. Clostridium difficile toxins: mechanism of action and role in disease. Clin. Microbiol. Rev. 18:247–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Walchli S, Skanland SS, Gregers TF, Lauvrak SU, Torgersen ML, Ying M, Kuroda S, Maturana A, Sandvig K. 2008. The mitogen-activated protein kinase p38 links Shiga toxin-dependent signaling and trafficking. Mol. Biol. Cell 19:95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schieven GL. 2009. The p38alpha kinase plays a central role in inflammation. Curr. Top. Med. Chem. 9:1038–1048 [DOI] [PubMed] [Google Scholar]
- 45. Shiryaev A, Moens U. 2010. Mitogen-activated protein kinase p38 and MK2, MK3 and MK5: menage a trois or menage a quatre? Cell Signal. 22:1185–1192 [DOI] [PubMed] [Google Scholar]
- 46. Saenz JB, Li J, Haslam DB. 2010. The MAP kinase-activated protein kinase 2 (MK2) contributes to the Shiga toxin-induced inflammatory response. Cell Microbiol. 12:516–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ryden EB, Lipman NS, Taylor NS, Rose R, Fox JG. 1991. Clostridium difficile typhlitis associated with cecal mucosal hyperplasia in Syrian hamsters. Lab. Anim. Sci. 41:553–558 [PubMed] [Google Scholar]
- 48. Chang J, Rohwer RG. 1991. Clostridium difficile infection in adult hamsters. Lab. Anim. Sci. 41:548–552 [PubMed] [Google Scholar]
- 49. Miller BA, Chen LF, Sexton DJ, Anderson DJ. 2011. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect. Control Hosp. Epidemiol. 32:387–390 [DOI] [PubMed] [Google Scholar]
- 50. Savidge T. 2003. Toxin B is an inflammatory enterotoxin in human intestine. Gastroenterology 125:413–420 [DOI] [PubMed] [Google Scholar]
- 51. Ananthakrishnan AN. 2011. Clostridium difficile infection: epidemiology, risk factors and management. Nat. Rev. Gastroenterol. Hepatol. 8:17–26 [DOI] [PubMed] [Google Scholar]
- 52. Musher DM, Aslam S, Logan N, Nallacheru S, Bhaila I, Borchert F, Hamill RJ. 2005. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin. Infect. Dis. 40:1586–1590 [DOI] [PubMed] [Google Scholar]
- 53. Tedesco F, Markham R, Gurwith M, Christie D, Bartlett JG. 1978. Oral vancomycin for antibiotic-associated pseudomembranous colitis. Lancet ii:226–228 [DOI] [PubMed] [Google Scholar]
- 54. Pepin J. 2008. Vancomycin for the treatment of Clostridium difficile infection: for whom is this expensive bullet really magic? Clin. Infect. Dis. 46:1493–1498 [DOI] [PubMed] [Google Scholar]
- 55. Bartlett JG, Tedesco FJ, Shull S, Lowe B, Chang T. 1980. Symptomatic relapse after oral vancomycin therapy of antibiotic-associated pseudomembranous colitis. Gastroenterology 78:431–434 [PubMed] [Google Scholar]
- 56. Louie TJ, Miller MA, Mullane KM, Weiss K, Lentnek A, Golan Y, Gorbach S, Sears P, Shue YK, Group OPTCS. 2011. Fidaxomicin versus vancomycin for Clostridium difficile infection. N. Engl. J. Med. 364:422–431 [DOI] [PubMed] [Google Scholar]
- 57. McFarland LV. 2005. Alternative treatments for Clostridium difficile disease: what really works? J. Med. Microbiol. 54:101–111 [DOI] [PubMed] [Google Scholar]
- 58. Vaishnavi C, Thapa BR, Thennarasu K, Singh K. 2002. Faecal lactoferrin assay as an adjunct to Clostridium difficile diarrhoea. Indian J. Pathol. Microbiol. 45:69–73 [PubMed] [Google Scholar]
- 59. Vaishnavi C, Bhasin D, Kochhar R, Singh K. 2000. Clostridium difficile toxin and faecal lactoferrin assays in adult patients. Microbes Infect. 2:1827–1830 [DOI] [PubMed] [Google Scholar]
- 60. Miller JR, Barrett LJ, Kotloff K, Guerrant RL. 1994. A rapid test for infectious and inflammatory enteritis. Arch. Intern. Med. 154:2660–2664 [DOI] [PubMed] [Google Scholar]
- 61. Gerhard R, Tatge H, Genth H, Thum T, Borlak J, Fritz G, Just I. 2005. Clostridium difficile toxin A induces expression of the stress-induced early gene product RhoB. J. Biol. Chem. 280:1499–1505 [DOI] [PubMed] [Google Scholar]
- 62. Cohen S, Fleischmann R. 2010. Kinase inhibitors: a new approach to rheumatoid arthritis treatment. Curr. Opin. Rheumatol. 22:330–335 [DOI] [PubMed] [Google Scholar]
- 63. Ronkina N, Kotlyarov A, Gaestel M. 2008. MK2 and MK3–a pair of isoenzymes? Front. Biosci. 13:5511–5521 [DOI] [PubMed] [Google Scholar]
- 64. Gaestel M. 2006. MAPKAP kinases–MKs–two's company, three's a crowd. Nat. Rev. Mol. Cell Biol. 7:120–130 [DOI] [PubMed] [Google Scholar]
- 65. Jones SW, Brockbank SM, Clements KM, Le Good N, Campbell D, Read SJ, Needham MR, Newham P. 2009. Mitogen-activated protein kinase-activated protein kinase 2 (MK2) modulates key biological pathways associated with OA disease pathology. Osteoarthr. Cartilage 17:124–131 [DOI] [PubMed] [Google Scholar]
- 66. Park JK, Ronkina N, Hoft A, Prohl C, Menne J, Gaestel M, Haller H, Meier M. 2008. Deletion of MK2 signalling in vivo inhibits small Hsp phosphorylation but not diabetic nephropathy. Nephrol. Dial. Transplant. 23:1844–1853 [DOI] [PubMed] [Google Scholar]
- 67. Lee JY, Kim H, Cha MY, Park HG, Kim YJ, Kim IY, Kim JM. 2009. Clostridium difficile toxin A promotes dendritic cell maturation and chemokine CXCL2 expression through p38, IKK, and the NF-kappaB signaling pathway. J. Mol. Med. 87:169–180 [DOI] [PubMed] [Google Scholar]
- 68. Bennett WE, Jr, Gonzalez-Rivera R, Shaikh N, Magrini V, Boykin M, Warner BB, Hamvas A, Tarr PI. 2009. A method for isolating and analyzing human mRNA from newborn stool. J. Immunol. Methods 349:56–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bennett WE, Jr, Gonzalez-Rivera R, Puente BN, Shaikh N, Stevens HJ, Mooney JC, Klein EJ, Denno DM, Draghi AII, Sylvester FA, Tarr PI. 2010. Proinflammatory fecal mRNA and childhood bacterial enteric infections. Gut Microbes 1:209–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Archbald-Pannone L, Sevilleja JE, Guerrant R. 2010. Diarrhea, clostridium difficile, and intestinal inflammation in residents of a long-term care facility. J. Am. Med. Directors Assoc. 11:263–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rocha MF, Maia ME, Bezerra LR, Lyerly DM, Guerrant RL, Ribeiro RA, Lima AA. 1997. Clostridium difficile toxin A induces the release of neutrophil chemotactic factors from rat peritoneal macrophages: role of interleukin-1beta, tumor necrosis factor alpha, and leukotrienes. Infect. Immun. 65:2740–2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.