Abstract
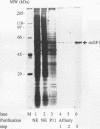
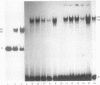
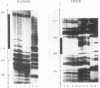
Contiguous deoxyguanosine residues (G strings) have been implicated in regulation of gene expression in several organisms via the binding of G-string factors. Regulation of expression of the chicken adult beta-globin gene may involve the interplay between binding of an erythrocyte-specific G-string factor, BGP1, and the stability of a positioned nucleosome (C. D. Lewis, S. P. Clark, G. Felsenfeld, and H. Gould, Genes Dev. 2:863-873, 1988). We have purified a 59.5-kDa nuclear protein (suGF1) from sea urchin embryos by DNA affinity chromatography. suGF1 has high binding affinity and specificity for oligo(dG).oligo(dC). The identity of the purified protein was confirmed by renaturation of sequence-specific DNA-binding activity from a sodium dodecyl sulfate-polyacrylamide gel slice and by Southwestern (DNA-protein) blotting. suGF1 binds in vitro to a G11 string present in the H1-H4 intergenic region of a sea urchin early histone gene battery. This suGF1 DNA recognition site occurs within a homopurine-homopyrimidine stretch previously shown to be incorporated into a positioned nucleosome core in vitro. DNase I footprinting shows that suGF1 protects the same base pairs on the promoter of the chicken beta A-globin gene as does BGP1. We show that a G-string cis-regulatory element of a sea urchin cell lineage-specific gene LpS1 (M. Xiang, S.-Y. Lu, M. Musso, G. Karsenty, and W. H. Klein, Development 113:1345-1355, 1991) also represents a high-affinity recognition site for suGF1. suGF1 may be a member of a family of G-string factors involved in the regulation of expression of unrelated genes during development of a number of different organisms.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barberis A., Superti-Furga G., Busslinger M. Mutually exclusive interaction of the CCAAT-binding factor and of a displacement protein with overlapping sequences of a histone gene promoter. Cell. 1987 Jul 31;50(3):347–359. doi: 10.1016/0092-8674(87)90489-2. [DOI] [PubMed] [Google Scholar]
- Barton M. C., Madani N., Emerson B. M. The erythroid protein cGATA-1 functions with a stage-specific factor to activate transcription of chromatin-assembled beta-globin genes. Genes Dev. 1993 Sep;7(9):1796–1809. doi: 10.1101/gad.7.9.1796. [DOI] [PubMed] [Google Scholar]
- Calzone F. J., Hög C., Teplow D. B., Cutting A. E., Zeller R. W., Britten R. J., Davidson E. H. Gene regulatory factors of the sea urchin embryo. I. Purification by affinity chromatography and cloning of P3A2, a novel DNA-binding protein. Development. 1991 May;112(1):335–350. doi: 10.1242/dev.112.1.335. [DOI] [PubMed] [Google Scholar]
- Clark S. P., Lewis C. D., Felsenfeld G. Properties of BGP1, a poly(dG)-binding protein from chicken erythrocytes. Nucleic Acids Res. 1990 Sep 11;18(17):5119–5126. doi: 10.1093/nar/18.17.5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey L., Roberts S. B., Heintz N. Purification of the human histone H4 gene-specific transcription factors H4TF-1 and H4TF-2. Genes Dev. 1988 Dec;2(12B):1700–1712. doi: 10.1101/gad.2.12b.1700. [DOI] [PubMed] [Google Scholar]
- Davis T. L., Firulli A. B., Kinniburgh A. J. Ribonucleoprotein and protein factors bind to an H-DNA-forming c-myc DNA element: possible regulators of the c-myc gene. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9682–9686. doi: 10.1073/pnas.86.24.9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson B. M., Lewis C. D., Felsenfeld G. Interaction of specific nuclear factors with the nuclease-hypersensitive region of the chicken adult beta-globin gene: nature of the binding domain. Cell. 1985 May;41(1):21–30. doi: 10.1016/0092-8674(85)90057-1. [DOI] [PubMed] [Google Scholar]
- Emerson B. M., Nickol J. M., Fong T. C. Erythroid-specific activation and derepression of the chick beta-globin promoter in vitro. Cell. 1989 Jun 30;57(7):1189–1200. doi: 10.1016/0092-8674(89)90056-1. [DOI] [PubMed] [Google Scholar]
- Hall D. J. Regulation of c-myc transcription in vitro: dependence on the guanine-rich promoter element ME1a1. Oncogene. 1990 Jan;5(1):47–54. [PubMed] [Google Scholar]
- Hentschel C. C., Birnstiel M. L. The organization and expression of histone gene families. Cell. 1981 Aug;25(2):301–313. doi: 10.1016/0092-8674(81)90048-9. [DOI] [PubMed] [Google Scholar]
- Hentschel C. C. Homocopolymer sequences in the spacer of a sea urchin histone gene repeat are sensitive to S1 nuclease. Nature. 1982 Feb 25;295(5851):714–716. doi: 10.1038/295714a0. [DOI] [PubMed] [Google Scholar]
- Hoffman E. K., Trusko S. P., Murphy M., George D. L. An S1 nuclease-sensitive homopurine/homopyrimidine domain in the c-Ki-ras promoter interacts with a nuclear factor. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2705–2709. doi: 10.1073/pnas.87.7.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson P. D., Felsenfeld G. A method for mapping intranuclear protein-DNA interactions and its application to a nuclease hypersensitive site. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2296–2300. doi: 10.1073/pnas.82.8.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. C., Jinno Y., Merlino G. T. Modulation of epidermal growth factor receptor proto-oncogene transcription by a promoter site sensitive to S1 nuclease. Mol Cell Biol. 1988 Oct;8(10):4174–4184. doi: 10.1128/mcb.8.10.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadonaga J. T., Carner K. R., Masiarz F. R., Tjian R. Isolation of cDNA encoding transcription factor Sp1 and functional analysis of the DNA binding domain. Cell. 1987 Dec 24;51(6):1079–1090. doi: 10.1016/0092-8674(87)90594-0. [DOI] [PubMed] [Google Scholar]
- Kadonaga J. T. Purification of sequence-specific binding proteins by DNA affinity chromatography. Methods Enzymol. 1991;208:10–23. doi: 10.1016/0076-6879(91)08004-2. [DOI] [PubMed] [Google Scholar]
- Karsenty G., de Crombrugghe B. Conservation of binding sites for regulatory factors in the coordinately expressed alpha 1 (I) and alpha 2 (I) collagen promoters. Biochem Biophys Res Commun. 1991 May 31;177(1):538–544. doi: 10.1016/0006-291x(91)92017-e. [DOI] [PubMed] [Google Scholar]
- Kefalas P., Gray F. C., Allan J. Precise nucleosome positioning in the promoter of the chicken beta A globin gene. Nucleic Acids Res. 1988 Jan 25;16(2):501–517. doi: 10.1093/nar/16.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi-Shigematsu T., Kohwi Y. Poly(dG)-poly(dC) sequences, under torsional stress, induce an altered DNA conformation upon neighboring DNA sequences. Cell. 1985 Nov;43(1):199–206. doi: 10.1016/0092-8674(85)90024-8. [DOI] [PubMed] [Google Scholar]
- Kohwi Y., Kohwi-Shigematsu T. Altered gene expression correlates with DNA structure. Genes Dev. 1991 Dec;5(12B):2547–2554. doi: 10.1101/gad.5.12b.2547. [DOI] [PubMed] [Google Scholar]
- Kohwi Y., Malkhosyan S. R., Kohwi-Shigematsu T. Intramolecular dG.dG.dC triplex detected in Escherichia coli cells. J Mol Biol. 1992 Feb 20;223(4):817–822. doi: 10.1016/0022-2836(92)90242-c. [DOI] [PubMed] [Google Scholar]
- Lewis C. D., Clark S. P., Felsenfeld G., Gould H. An erythrocyte-specific protein that binds to the poly(dG) region of the chicken beta-globin gene promoter. Genes Dev. 1988 Jul;2(7):863–873. doi: 10.1101/gad.2.7.863. [DOI] [PubMed] [Google Scholar]
- Lobanenkov V. V., Nicolas R. H., Adler V. V., Paterson H., Klenova E. M., Polotskaja A. V., Goodwin G. H. A novel sequence-specific DNA binding protein which interacts with three regularly spaced direct repeats of the CCCTC-motif in the 5'-flanking sequence of the chicken c-myc gene. Oncogene. 1990 Dec;5(12):1743–1753. [PubMed] [Google Scholar]
- McKeon C., Schmidt A., de Crombrugghe B. A sequence conserved in both the chicken and mouse alpha 2(I) collagen promoter contains sites sensitive to S1 nuclease. J Biol Chem. 1984 May 25;259(10):6636–6640. [PubMed] [Google Scholar]
- Patterton D., Hapgood J. suGF1 binds in the major groove of its oligo(dG).oligo(dC) recognition sequence and is excluded by a positioned nucleosome core. Mol Cell Biol. 1994 Feb;14(2):1410–1418. doi: 10.1128/mcb.14.2.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterton H. G., von Holt C. Negative supercoiling and nucleosome cores. I. The effect of negative supercoiling on the efficiency of nucleosome core formation in vitro. J Mol Biol. 1993 Feb 5;229(3):623–636. doi: 10.1006/jmbi.1993.1068. [DOI] [PubMed] [Google Scholar]
- Patterton H. G., von Holt C. Negative supercoiling and nucleosome cores. II. The effect of negative supercoiling on the positioning of nucleosome cores in vitro. J Mol Biol. 1993 Feb 5;229(3):637–655. doi: 10.1006/jmbi.1993.1069. [DOI] [PubMed] [Google Scholar]
- Postel E. H., Mango S. E., Flint S. J. A nuclease-hypersensitive element of the human c-myc promoter interacts with a transcription initiation factor. Mol Cell Biol. 1989 Nov;9(11):5123–5133. doi: 10.1128/mcb.9.11.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon E., Evans T., Welsh J., Efstratiadis A. Conformation of promoter DNA: fine mapping of S1-hypersensitive sites. Cell. 1983 Dec;35(3 Pt 2):837–848. doi: 10.1016/0092-8674(83)90116-2. [DOI] [PubMed] [Google Scholar]
- Simpson R. T. Nucleosome positioning: occurrence, mechanisms, and functional consequences. Prog Nucleic Acid Res Mol Biol. 1991;40:143–184. doi: 10.1016/s0079-6603(08)60841-7. [DOI] [PubMed] [Google Scholar]
- Tewari D. S., Cook D. M., Taub R. Characterization of the promoter region and 3' end of the human insulin receptor gene. J Biol Chem. 1989 Sep 25;264(27):16238–16245. [PubMed] [Google Scholar]
- Wells R. D., Collier D. A., Hanvey J. C., Shimizu M., Wohlrab F. The chemistry and biology of unusual DNA structures adopted by oligopurine.oligopyrimidine sequences. FASEB J. 1988 Nov;2(14):2939–2949. [PubMed] [Google Scholar]
- Wu T. C., Simpson R. T. Transient alterations of the chromatin structure of sea urchin early histone genes during embryogenesis. Nucleic Acids Res. 1985 Sep 11;13(17):6185–6203. doi: 10.1093/nar/13.17.6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang M., Lu S. Y., Musso M., Karsenty G., Klein W. H. A G-string positive cis-regulatory element in the LpS1 promoter binds two distinct nuclear factors distributed non-uniformly in Lytechinus pictus embryos. Development. 1991 Dec;113(4):1345–1355. doi: 10.1242/dev.113.4.1345. [DOI] [PubMed] [Google Scholar]
- von Holt C., Brandt W. F., Greyling H. J., Lindsey G. G., Retief J. D., Rodrigues J. D., Schwager S., Sewell B. T. Isolation and characterization of histones. Methods Enzymol. 1989;170:431–523. doi: 10.1016/0076-6879(89)70061-6. [DOI] [PubMed] [Google Scholar]