Abstract
Working memory (WM) enables the online maintenance and manipulation of information and is central to intelligent cognitive functioning. Much research has investigated executive processes of WM in order to understand the operations that make WM “work.” However, there is yet little consensus regarding how executive processes of WM are organized. Here, we used quantitative meta-analysis to summarize data from 36 experiments that examined executive processes of WM. Experiments were categorized into 4 component functions central to WM: protecting WM from external distraction (distractor resistance), preventing irrelevant memories from intruding into WM (intrusion resistance), shifting attention within WM (shifting), and updating the contents of WM (updating). Data were also sorted by content (verbal, spatial, object). Meta-analytic results suggested that rather than dissociating into distinct functions, 2 separate frontal regions were recruited across diverse executive demands. One region was located dorsally in the caudal superior frontal sulcus and was especially sensitive to spatial content. The other was located laterally in the midlateral prefrontal cortex and showed sensitivity to nonspatial content. We propose that dorsal-“where”/ventral-“what” frameworks that have been applied to WM maintenance also apply to executive processes of WM. Hence, WM can largely be simplified to a dual selection model.
Keywords: central executive, cognitive control, executive function, fMRI, prefrontal cortex
Introduction
Many everyday activities require the ability to maintain and manipulate information in our mind. One salient example is mental arithmetic in which we store numbers in mind, shift our attention between subsets of numbers, draw upon long-term mathematical knowledge, and replace the operands with the solution. Collectively, the processes that sustain and transform information online are known as working memory (WM).
Since the inception of the notion of WM (Baddeley and Hitch 1974), a great deal of research has been targeted at understanding its component processes. Much of this research has been motivated by demonstrations of the centrality of WM throughout much of cognition. Variations in the capacity of WM have been shown to explain individual differences in reasoning, problem-solving, reading and language comprehension, and IQ (Daneman and Carpenter 1980; Carpenter et al. 1990; Just and Carpenter 1992; Daneman and Merikle 1996). Training WM capacity has been shown to increase intelligence (Jaeggi et al. 2008). Furthermore, impairments in WM underlie deficits in disorders, such as schizophrenia (Barch 2005), attention deficit/hyperactivity disorder (Martinussen et al. 2005), and Alzheimer’s disease (Baddeley et al. 1991). Hence, understanding WM has far-reaching implications.
The most influential model of WM comes from Baddeley and colleagues (Baddeley and Hitch 1974; Baddeley 2003; Repovs and Baddeley 2006). In its classic formulation, WM consists of separate storage buffers for verbal and visuospatial content, as well as executive processes that act upon the information in the storage buffers. Recent progress in cognitive neuroscience suggests that these storage buffers are not unique to WM per se but rely on the same mechanisms involved in representing information in perception, as well (Jonides et al. 2005, 2008; Postle 2006). These representational regions are thought to be lodged in posterior portions of cortex. What makes WM “work” are the executive processes of WM that sustain and transform posterior representations (Bledowski et al. 2009, 2010). Accordingly, a great deal of research has looked for ways to describe and organize executive processes (Smith and Jonides 1999; Miyake et al. 2000; Friedman and Miyake 2004; Bledowski et al. 2010). Much of this research has focused on the prefrontal cortex (PFC), but it is recognized that parietal regions are also critical to executive function (Collette et al. 2006). Detailed computational models further suggest that PFC interactions with the basal ganglia and thalamus are also important (Frank et al. 2001; O'Reilly and Frank 2006). How are these networks organized in the service of WM?
Organization of WM by Content
The description and organization of executive processes of WM is the topic of much debate. Part of this debate concerns organization by type of content. Pioneering research by Goldman-Rakic and colleagues suggested that a dorsal–ventral distinction between location-based “where” information and identity-based “what” information that has been well-established in posterior cortical regions (Ungerleider and Mishkin 1982) is carried through into the frontal lobes (for a review, see Levy and Goldman-Rakic 2000). According to this proposal, dorsal frontal and parietal regions mediate spatial WM while ventral frontal and temporal regions mediate object WM. Early human neuroimaging evidence was largely consistent with this proposal, at least with regard to maintenance, and this evidence further dissociated identity-based verbal content with identity-based object content. Such data implicated posterior aspects of the left inferior frontal gyrus (IFG; BA 44) and ventral aspects of left precentral gyrus (preCG) in verbal maintenance (i.e., the phonological loop), dorsal aspects of right premotor cortex in and near the caudal superior frontal sulcus (SFS) in spatial maintenance, and the right IFG in object maintenance (for a review, see Smith and Jonides 1999). Hence, both human and monkey research suggested that dorsal frontal regions are involved in location-based maintenance, while ventral frontal regions are involved in identity-based maintenance. The human research further suggested that identity-based maintenance may be distinguished for verbal and object content on the basis of laterality. However, these observations were largely qualitative. Research that followed tested these claims in more quantitative ways.
Quantitative meta-analyses of early functional neuroimaging studies produced mixed evidence for frontal content distinctions. A meta-analysis of 60 functional magnetic resonance imaging (fMRI) and PET studies found that although posterior regions of cortex clearly divided by type of content, there was only weak evidence of content selectivity in the PFC (Wager and Smith 2003). Ventral frontal regions did show somewhat of an identity preference along with a left-verbal/right-object laterality trend. However, in most cases, these impressions failed to reach significance. It is possible that the clustering method, which required the number of clusters to be specified in advance, may not have provided optimal sensitivity to detect regional differences in the PFC. A more recent meta-analysis of studies investigating the n-back task did provide some evidence of left PFC verbal dominance, dorsal premotor spatial dominance, and right PFC object dominance (Owen et al. 2005). However, given the complexities of the n-back task, it is difficult to know whether these differences reflected differences in storage or manipulation processes. More recent designs have afforded the ability to decompose WM tasks into component operations, thereby isolating maintenance processes associated with different forms of content (Sakai and Passingham 2003; Rama et al. 2004; Mohr et al. 2006; Sala and Courtney 2007). Results from these studies were largely consistent with the original observations of Smith and Jonides (1999). Taken together, there do appear to be more data in favor of distinct maintenance processes by type of content than against it (see also Curtis and D'Esposito 2003; Courtney 2004; Linden 2007).
Beyond maintenance processes, Baddeley’s model suggested that executive processes of WM are amodal, performing similar functions across different types of content (Baddeley and Hitch 1974; Baddeley 2003; Repovs and Baddeley 2006). Data from early fMRI studies suggested that executive processes of WM selectively activate the mid-dorsolateral prefrontal cortex (DLPFC) over and above simple maintenance (D'Esposito et al. 1999; Postle et al. 1999; Barde and Thompson-Schill 2002). These data led to the proposal that the mid-DLPFC may be the locus of domain-general executive processes (D'Esposito et al. 1998; Petrides 2000; Curtis and D'Esposito 2003). This proposal is somewhat difficult to test since different types of content are amenable to different sorts of operations (e.g., verbal material can be alphabetized and spatial material can be rotated). Given this challenge, Johnson et al. (2003, 2005) examined a minimal executive process, refreshing, across a variety of material types. Refreshing refers to mentally foregrounding a recently presented item. Across content types, refreshing elicited left lateralized PFC activations in the mid-DLPFC in the middle frontal gyrus (MFG) and ventrolateral PFC (VLPFC) in the IFG. However, left midlateral PFC activations, particularly in the IFG, were more consistent for verbal content. Moreover, the left caudal SFS was most responsive to refreshing spatial locations, while there was weak evidence for right rostral PFC preference for refreshing object information (Johnson et al. 2005). Notably, this network of content selectivity is quite similar to selectivity elicited by maintenance processes reviewed above. This is consistent with recent proposals that suggest that maintenance may in fact be decomposed into a series of repeated refresh-like operations (Jonides et al. 2008; Johnson MR and Johnson MK 2009). Given the relationship between refreshing and maintenance, systematic investigation of additional executive processes is needed to determine whether executive processes of WM are amodal or content-sensitive.
Unfortunately, aside from refreshing, there has been less systematic investigation of variations in a single executive process by type of content. Although we have previously attempted to summarize activations involved in resisting proactive interference, the paucity of nonverbal studies examining proactive interference resolution made it difficult to draw conclusions (Jonides and Nee 2006). However, we noted that, at least for verbal studies, the left IFG was a prominent locus of activation. Mixed results were found in studies using object content, while a single study that used spatial content found activations in the caudal SFS but not left IFG (Leung and Zhang 2004). Taken together, the convergence of maintenance, refreshing, and resisting proactive interference in the left IFG suggests that it may perform a general executive function for verbal content (Zhang et al. 2004; Nelson et al. 2009). There is also some indication of dorsal frontal selectivity for spatial content in and near the caudal SFS (Courtney et al. 1998). Whereas some studies indicate right PFC preferences for object content, the location and strength of this association has been somewhat variable perhaps because objects have more distributed representations than other types of content (Courtney 2004). Thus, there is tentative evidence for organization of executive processes of WM by content. However, systematic investigation across more putative functions is needed to solidify these distinctions.
In addition to distinctions among verbal, object, and spatial content, recent proposals suggest that the rostral–caudal axis of the frontal lobes is organized by abstraction of content (Koechlin et al. 2003; Koechlin and Jubault 2006; Badre 2008; Badre and D'Esposito 2009). Under these frameworks, more rostral portions of the PFC are thought to represent more abstract higher level content. This content is thought to reflect goals, rules, or contexts that constrain the processing of more caudal PFC regions. In a pair of studies, Sakai and Passingham (2003, 2006) demonstrated sustained activation in the rostral PFC in response to cues that signaled an upcoming WM task. These rostral PFC activations interacted with different content-specific caudal PFC regions depending upon the type of material to be held in WM in the upcoming task. These data are consistent with the notion that the rostral PFC establishes a cognitive set that prepares WM to encode particular types of information. So, task-contexts/rules might be considered another form of content stored in WM (Courtney 2004).
Organization of WM by Function
In addition to content, there are debates concerning divisions by function. Over the years, a variety of frameworks have been proposed to try to parcel executive processes in a sensible fashion (e.g., Smith and Jonides 1999; Miyake et al. 2000; Friedman and Miyake 2004; Nee et al. 2007a; Bledowski et al. 2010). Although there are several nuances associated with each proposal, there are a number of common themes. In all frameworks, there exist processes that shift attention between active representations (shifting), update what is actively maintained (updating), and prevent irrelevant information from becoming active (inhibition or interference resolution). Furthermore, there is at least some evidence that inhibition/interference resolution processes can be further subdivided (Friedman and Miyake 2004). For present purposes, one axis of this distinction concerns those processes that mitigate interference from the external environment (distractor resistance) and those that prevent interference from intrusive memories (intrusion resistance; Nee and Jonides 2008a; Nee and Jonides 2009). Whereas there have been some efforts to compare and contrast the neural underpinnings of these executive processes, tasks used to do so have not always required WM (e.g., Collette et al. 2005). This is an important concern since there is mounting evidence that PFC regions, and associated executive processes, are at least partly organized by level of abstraction (Koechlin et al. 2003; Koechlin and Jubault 2006; Badre 2008; Badre and D'Esposito 2009). So, comparing across levels of abstraction (e.g., task-switching vs. item-updating) may dissociate neural correlates by function or by abstraction of content. Hence, understanding the executive processes of WM necessitates comparisons within a single level of interest (e.g., on items within WM).
How to distinguish the various executive processes of WM is unclear. Part of the issue is that there have been few quantitative comparisons among executive WM functions. As a result, associations among and dissociations between processes have been based on largely qualitative judgments. For instance, in a comprehensive review, Courtney et al. (2007) suggested that updating, maintenance, shifting, and distractor resistance may all be associated with activations in mid-DLPFC. Whereas each function clearly implicates the lateral PFC, it is less clear that the same region is implicated across functions. For example, overlapping neural correlates of updating and maintenance were found in caudal aspects of the frontal lobe in the inferior frontal junction (IFJ; Roth et al. 2006), whereas activations related to distractor resistance were found in the anterior most aspects of the MFG (Sakai et al. 2002). Complicating the matter is that the same descriptive label (e.g., DLPFC) is often given to a wide range of activation foci (Courtney 2004; Brass et al. 2005) making it difficult to distinguish among subregions of the PFC. Hence, progress in understanding the organization of the frontal lobe can only be made through direct quantitative comparison. Ideally, such comparisons should be performed on the same samples (Nobre et al. 2004; Nee and Jonides 2008a, 2009; Roth et al. 2009). However, since comparing multiple functions in the same sample can be costly, meta-analysis can serve as a means to assess convergence and divergence between functions in an unbiased manner.
The Present Study
Here, we seek to foster understanding of executive processes of WM through quantitative meta-analysis of fMRI data. As a starting point, we characterize experiments by type of content (verbal, spatial, and object) and putative function (distractor resistance, intrusion resistance, shifting, and updating). Collectively, these functions are thought to be components of more complex functions of maintenance and manipulation (Johnson et al. 2003, 2005; Johnson MR and Johnson MK 2009). For example, maintenance is thought to consist of repeated shifts of the focus of attention (shifting) to keep representations active (Jonides et al. 2008). We focus on processes that act in the service of WM rather than those that act at motoric levels (e.g., response inhibition) or task levels (e.g., task-switching) in order to roughly equate level of abstraction. In trying to pick out component processes, we consider only event-related designs that focus on the epoch associated with the putative function of interest (i.e., we do note include block designs; Curtis and D'Esposito 2003). Moreover, complex tasks that are composed of a number of the above processes, such as the n-back task, were avoided. Meta-analyses concerning such designs and tasks have been reported elsewhere (Wager and Smith 2003; Owen et al. 2005). Finally, to avoid bias, we consider only experiments that report the results of whole-brain analyses. Through neural association and dissociation, we seek to refine our conceptualization of executive processes.
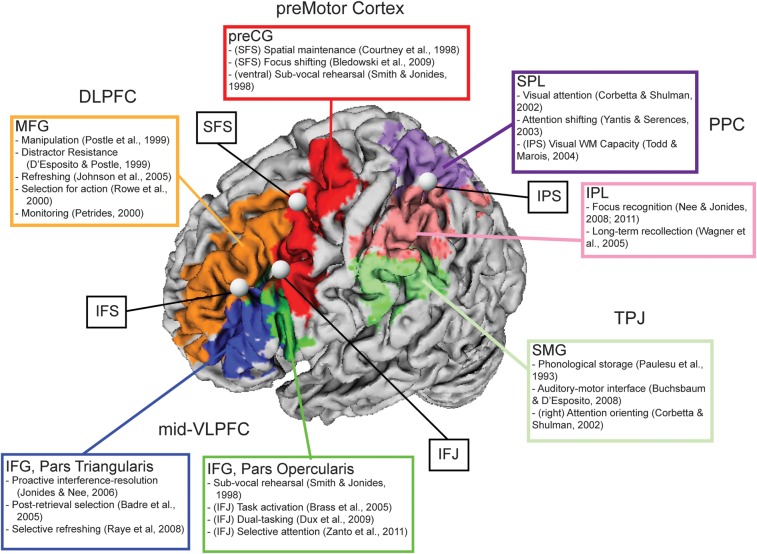
Since terminology is often inconsistent when different authors describe anatomical regions (Courtney 2004; Brass et al. 2005), we have provided a depiction of several areas of the brain that are relevant to the present investigation (Fig. 1). Regions are colored and denoted by gyral labels provided by the AAL atlas (Tzourio-Mazoyer et al. 2002). In addition, more abstract labels (e.g., DLPFC) are also roughly associated with different gyri (e.g., MFG). Such labels are commonly used when attempting to compare between species. Also indicated are some prominent sulcal and junction regions as well as some relevant literature associated with each region. This is by no means an exhaustive review nor do we intend to stamp each region with a particular function. Rather, it is meant to be a somewhat convenient starting point with which to reference and understand contributions of different regions. Finally, to preview: a number of experiments appear to converge in the inferior frontal sulcus (IFS) with activations extending into the MFG/DLPFC and IFG/VLPFC. We will use the term “mid-lateral PFC” to describe such activations as they are neither clearly dorsal nor ventral.
Figure 1.
Renderings of regions related to executive processes of WM. Colors denote gyral definitions derived from the AAL atlas (Tzourio-Mazoyer et al. 2002). Prominent sulcal and gyral regions are denoted with bubbles. Literature associated with each region is listed. More general anatomical labels are associated with one or more gyri: midventrolateral prefrontal cortex (VLPFC) = inferior frontal gyrus (IFG), pars triangularis and IFG, pars opercularis; dorsolateral PFC (DLPFC) = middle frontal gyrus (MFG); premotor cortex = precentral gyrus (preCG); posterior parietal cortex (PPC) = superior parietal lobule (SPL) and inferior parietal lobule (IPL); temporalparietal junction (TPJ) = supramarginal gyrus (SMG). Other abbreviations: inferior frontal sulcus (IFS); inferior frontal junction (IFJ); superior frontal sulcus (SFS); intraparietal sulcus (IPS).
Materials and Methods
Experiment Selection
Experiments were identified through searches of PubMed and Google Scholar and through referenced citations from the retrieved studies. Experiments were included only if they reported results from whole-brain group analyses based on healthy young adults in Talairach or Montreal Neurological Institute (MNI) space. In order to maximize precise isolation of component processes of interest, we included only experiments employing event-related designs with results from contrasts with a high-level control condition (e.g., contrasts against baseline or fixation were not included). Experiments meeting the above criteria were grouped into the following putative functions:
Distractor Resistance
Experiments that examined neural effects of filtering external distraction while either encoding or maintaining information in WM were classified as examining distractor resistance. A few experiments examined this function through functional connectivity analysis with seeds placed in posterior regions reflecting either the relevant or distracting information (Gazzaley et al. 2007; Zanto et al. 2010, 2011). Since results from these experiments appeared ostensibly similar to results from traditional univariate analysis in preliminary examinations, these experiments were pooled together. In total, 10 experiments with 145 foci were included under distractor resistance.
Intrusion Resistance
Experiments that examined neural effects of mitigating interference from intrusive memories were classified as examining intrusion resistance. In these experiments, a cue or probe could elicit the retrieval of task-irrelevant information. Included under this rubric were experiments that examined the resolution of proactive interference (e.g., Jonides and Nee 2006) and those that inhibited a cued associate from entering WM (i.e., the think/no-think task; Anderson and Levy 2009). Eight experiments with 74 foci were included under intrusion resistance.
Shifting
Experiments that examined neural effects of shifting the focus of attention in WM were classified as examining shifting. Most of these studies involved the refresh task in which a cue directs the subject to foreground a recently presented item (Johnson et al. 2005; Johnson MR and Johnson MK 2009). Also included was an experiment that referred to the process of “updating” the focus of attention (Bledowski et al. 2009). We make mention of this experiment to distinguish it from the process of updating the contents of WM described below. Critically, shifting is thought to leave the contents of WM unchanged while changing the focus of attention within those contents. Nine experiments with 90 foci were included under shifting.
Updating
Experiments that examined neural effects of updating the contents of WM were classified as updating. Critically, in these experiments, a cue directs subjects to change what is stored in WM. Generally, this involves removing some or all the items stored in WM and replacing them with new items. New content can be encoded from the environment or retrieved from long-term memory. In some of the experiments, a cue directed subjects to retain a “subset” of the items stored in WM (i.e., directed forgetting). Nine experiments with 152 foci were included under updating.
In addition to classification by function, we also classified experiments by content. We distinguished experiments that used verbal material (words, letters, and digits), from those using spatial material (locations and movement vector), from those using object material (faces, scenes, houses, drawings, colors, and unnamable objects). The sample included 17 verbal experiments with 215 foci, 8 spatial experiments with 142 foci, and 11 object experiments with 104 foci.
In total, our full sample included data from 36 experiments reporting 461 activation foci. Study names, classifications, stimuli, contrasts, and number of contributing foci are summarized in Table 1. Citation data for the included studies are reported in the Supplementary Material.
Table 1.
Experiment summary
| Study | Function | Content | Stimuli | Contrast | Number of foci | Selection |
| Clapp (2010) | Dist Res | Object | Faces/scenes | Ignore distractor–attend distractor | 2 | N |
| Gazzaley (2007) | Dist Res | Object | Faces/scenes | Attend–ignore (connectivity with PPA), passive–ignore (connectivity with PPA) | 24 | N |
| McNab (2008) | Dist Res | Spatial | Spatial arrays | Distraction (cue)–no distraction (cue) | 3 | Y |
| Nee (2009) | Dist Res | Verbal | Words/# | High distraction–low distraction | 31 | Y |
| Nobre (2004) | Dist Res | Spatial | Colored X’s | Precue–neutral cue | 34 | Y |
| Toepper (2010) | Dist Res | Spatial | Spatial arrays | BST–CBT | 2 | Y |
| Zanto (2010) | Dist Res | Object | Colored squares | Attend–ignore (connectivity with V4), ignore–attend (connectivity with V5) | 7 | Y |
| Zanto (2010) | Dist Res | Spatial | Moving squares | Attend–ignore (connectivity with V5) | 8 | Y |
| Zanto (2011) | Dist Res | Object | Colored squares | Attend–ignore (connectivity with V4) | 18 | Y |
| Zanto (2011) | Dist Res | Spatial | Moving squares | Attend–ignore (connectivity with V5) | 16 | Y |
| Anderson (2004) | Intr Res | Verbal | Words | No-think–think | 17 | N |
| Butler (2010) | Intr Res | Verbal | Words | No-think–think | 17 | N |
| Depue (2007) | Intr Res | Object | Faces/IAPS | No-think–think | 5 | N |
| Mecklinger (2003) | Intr Res | Verbal | Letters | Recent negative–nonrecent negative | 4 | Y |
| Mecklinger (2003) | Intr Res | Object | Unnamable objects | Recent negative–nonrecent negative | 1 | Y |
| Nee (2007) | Intr Res | Verbal | Letters | Recent negative–nonrecent negative | 14 | Y |
| Nee (2007) | Intr Res | Verbal | Letters | Suppress–control | 5 | Y |
| Nee (2008) | Intr Res | Verbal | Words | Suppress–control | 11 | Y |
| Bledowski (2009) | Shifting | Spatial | Spatial arrays | Update–no update | 8 | Y |
| Johnson (2003) | Shifting | Verbal | Words | Refresh − (read + repeat) | 2 | N |
| Johnson (2003) | Shifting | Object | Line-drawings | Refresh − (read + repeat) | 7 | N |
| Johnson (2003) | Shifting | Object | Patterns | Refresh − (read + repeat) | 8 | N |
| Raye (2002), Exp 1 | Shifting | Verbal | Words | Refresh–repeat | 7 | N |
| Raye (2002), Exp 2 | Shifting | Verbal | Words | Refresh–repeat | 14 | Y |
| Raye (2008) | Shifting | Verbal | Words | Refresh–repeat, Selective refresh–nonselective refresh | 9 | N/Y |
| Roth (2009) | Shifting | Verbal | Words | Refresh–read, refresh–update | 24 | N |
| Yi (2008) | Shifting | Object | Scenes | Refresh–repeat | 11 | N |
| Lepsien (2007) | Updating | Object | Faces/scenes | Switch–stay | 7 | Y |
| Leung (2007) | Updating | Spatial | Spatial grid | Parametric number of updates | 31 | N |
| Montojo (2008) | Updating | Verbal | Numbers/Math | Update–hold | 6 | N |
| Nee (2009) | Updating | Verbal | Words | Forget–rehearse | 18 | Y |
| Nobre (2004) | Updating | Spatial | Colored X’s | Retrocue–neutral cue | 9 | Y |
| Roth (2006) | Updating | Object | Faces/houses | Update–no update | 14 | N |
| Roth (2009) | Updating | Verbal | Words | Update–read, update–refresh | 11 | N |
| Sorqvist (2010) | Updating | Verbal | Numbers | Substitute–no substitute | 20 | Y |
| Zhang (2004) | Updating | Verbal | Letters | Forget–remember | 5 | Y |
Activation-Likelihood Estimation
Quantitative convergence maps were generated through activation-likelihood estimation (ALE) as implemented in GingerALE 2.1b (Eickhoff et al. 2009; Turkeltaub et al. 2012). For each experiment, foci were modeled as 3D Gaussian probability distributions capturing the spatial uncertainty associated with each focus. The width of the Gaussian was determined by empirical data and was a function of the number of subjects included in the experiment. Narrower widths were associated with larger samples owing to the greater spatial certainty associated with large samples (Eickhoff et al. 2009). Probabilities were joined using a revised algorithm that limits the contribution of foci that are nearby, so as not to overweight experiments that report cluster subpeaks (Turkeltaub et al. 2012). Using this method, each voxel was assigned the maximum probability associated with any one focus, which thereby associates a given voxel with the nearest contributing focus. Together, the voxel-wise probabilities formed a modeled activation map for a given experiment. ALE values were calculated by the voxel-wise union of the probabilities in the modeled activation maps. For each voxel, ALE values were compared against an empirical null distribution generated by randomly sampling voxels from each modeled activation map (i.e., a distribution generated from a random spatial association between experiments). The random sampling took into account the increased likelihood of identifying activation foci in gray matter and was repeated 5000 times to generate the null distribution. Voxel-wise P-values were computed by comparing a voxel’s ALE value to the sampled null distribution. ALE maps were then thresholded at P < 0.05 corrected for multiple comparisons by false discovery rate (FDR). Additionally, a 25-voxel (voxel-size = 2 × 2 × 2) extent criterion was employed.
Separate ALE maps were computed by content (verbal, spatial, and object) and function (distractor resistance, intrusion resistance, shifting, and updating). Content maps averaged across function and function maps averaged across content. Due to inequalities, a factorial design could not be employed. A global ALE map combining all experiments was also computed.
Valid conjunction analysis (Nichols et al. 2005) was used to determine convergence among ALE maps. For each voxel, a conjunction was deemed significant if all contributing ALE maps demonstrated significant activation in that voxel at the thresholds described above. An additional extent threshold of 20 contiguous voxels was employed.
Contrast ALE maps were computed using GingerALE 2.1b. First, voxel-wise differences in ALE values were calculated for the 2 contrasting maps. Then, all experiments contributing to either map were pooled and randomly divided into 2 groups of the same size as the original 2 sets. ALE values for these randomly assigned groups were calculated as above and the difference between the resultant ALE maps was measured. Repeating this process 5000 times generated a null distribution of the difference scores. For each voxel, the true difference was tested against the null distribution and the P-value was computed. The resultant contrast maps were thresholded at P < 0.05, FDR corrected, with a 25 voxel extent criterion.
Regions of Interest Analyses
ALE analyses were complemented with additional analyses within regions of interest (ROIs). These analyses sought to determine whether activations within an ROI differed in consistency by content and/or function. These analyses were based upon the number of experiments reporting foci within a given ROI and were performed only on ROIs that contained at least 5 contributing experiments. Analyses were performed in a 2-step fashion. First, χ2 tests of proportion were calculated separately for content and function. These tests determined whether the proportion of experiments finding activation in a given ROI differed. For example, we measured the proportion of verbal experiments, spatial experiments, and object experiments finding activation in the left IFG, pars triangularis. Based on the total proportion of experiments finding activation in this ROI, it could be determined whether any type of content differed from the expected proportion. This results in an omnibus test that determines whether a difference exists. Notably, since this test is based on proportions and not total counts, it is a suitable test when there are unequal numbers of experiments for each category.
For each ROI that demonstrated a significant χ2 proportion difference, logistic regression was performed to determine whether any one category predicted activation. Returning to our example, if left IFG, pars triangularis demonstrated a content difference, logistic regression was then performed to determine if verbal, spatial, or object content predicted activation in the region. ROIs demonstrating both a significant χ2 proportion difference and at least one significant predictor as determined by logistic regression are noted. Notably, a category does not necessarily need to be a positive predictor. Prediction could be demonstrated by the absence of activation from a particular category, as well.
ROIs were determined by both anatomical and functional means. Anatomical ROIs were created using the AAL atlas (Tzourio-Mazoyer et al. 2002) implemented in WFU pick atlas (Maldjian et al. 2003) focusing on frontal and parietal regions due to their presumed importance in executive processes. Separate anatomical ROIs were created for IFG—pars orbitalis, IFG—pars triangularis, IFG—pars opercularis, superior frontal gyrus, superior parietal lobule (SPL), inferior parietal lobule (IPL), precuneus, supramarginal gyrus (SMG), angular gyrus, preCG, anterior cingulate cortex (ACC), midanterior cingulate cortex (mACC), and the supplementary motor area. In addition, ROIs in the MFG were created by segmenting the MFG by Brodmann area maps provided by MRIcroN (http://www.mccauslandcenter.sc.edu/mricro/mricron/index.html). This led to the following MFG ROIs: MFG—BA 6, MFG—BA 9, MFG—BA 8, MFG—BA 46, and MFG—BA 10 (Supplementary Fig. 1). Separate ROIs were created for each hemisphere.
While anatomical ROIs provide unbiased ways to inspect different parts of the brain, they can also be coarse and cut across several functional zones. Hence, to complement the anatomical ROIs, 10 mm spherical ROIs were placed around each peak calculated from the ALE map that combined all experiments together. Such functional ROIs are particularly important for sulcal regions, which are typically not distinguished in anatomical ROI atlases.
Results
Results are described in terms of convergence. That is, the between-study consistency of finding activation in a particular location. While this measure is likely to rely somewhat on activation strength, which is the conventional metric for fMRI studies, it is not the same and should not strictly be interpreted as such.
ALE analysis of all 36 experiments combined is depicted in Figure 2 and described in Table 2. As expected, the network of executive processes of WM consisted primarily of medial and lateral frontal and parietal regions, although smaller occipitotemporal clusters were also present. The strongest points of convergence were the bilateral caudal SFS and SPL. There was also strong convergence in the intraparietal sulcus (IPS), preSMA extending ventrally into the dorsal ACC, bilateral preCG and the nearby IFJ, and left midlateral PFC extending from the IFS dorsally to the MFG and ventrally to the IFG.
Figure 2.
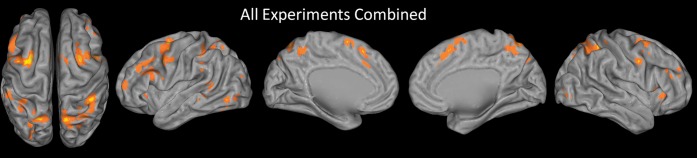
ALE map of all experiments combined. The sample included 36 experiments with 461 activation foci. Results are threshold at P < 0.05, corrected for multiple comparisons using false discovery rate and a 25 voxel extent criterion. Higher ALE values are depicted in yellow.
Table 2.
Significant clusters from all experiments combined and functional ROI analyses
| x | y | z | Number of voxel | BA | Region | Number of exp’s (36) | Number of verbal (17) | Number of spatial (8) | Number of object (11) | χ2 content | Number of Dist Res (10) | Number of Intr Res (8) | Number of Shift (9) | Number of Upd (9) | χ2 function | |
| Frontal | −28 | 0 | 58 | 1412 | 6, 8 | Left caudal SFS | 12 | 5 | 6* | 1 | 9.28 | 5 | 0 | 2 | 5 | 7.75 |
| −54 | 10 | 36 | 9 | Left caudal MFG | 10 | 5 | 2 | 3 | 0.05 | 2 | 1 | 3 | 4 | 2.62 | ||
| −42 | −4 | 42 | 6 | Left preCG | 9 | 5 | 4 | 0 | 6.51 | 3 | 2 | 1 | 3 | 1.39 | ||
| −52 | 2 | 42 | 6 | Left preCG | 8 | 4 | 2 | 2 | 0.16 | 1 | 0 | 3 | 4 | 6.36 | ||
| −50 | 20 | 28 | 45, 46 | Left IFS/MFG/IFG—tria | 9 | 4 | 2 | 3 | 0.05 | 1 | 2 | 3 | 3 | 1.87 | ||
| −44 | 28 | 28 | 46 | Left IFS/MFG | 7 | 5 | 0 | 2 | 3.02 | 1 | 1 | 2 | 3 | 1.97 | ||
| −52 | 12 | 22 | 44 | Left IFG—oper | 9 | 6 | 2 | 1 | 2.45 | 1 | 2 | 1 | 5* | 6.61 | ||
| −40 | 16 | 28 | 9 | Left IFS/IFG—oper | 6 | 5 | 0 | 1 | 4.04 | 0 | 3 | 2 | 1 | 4.90 | ||
| 0 | 18 | 50 | 442 | 8, 6 | preSMA | 12 | 6 | 3 | 3 | 0.27 | 4 | 1 | 3 | 4 | 2.26 | |
| −2 | 24 | 38 | 32 | dACC | 6 | 3 | 1 | 2 | 0.13 | 2 | 0 | 2 | 2 | 2.08 | ||
| 34 | 6 | 56 | 395 | 6, 8 | Right caudal SFS | 11 | 5 | 5* | 1 | 6.25 | 3 | 3 | 1 | 4 | 2.61 | |
| 48 | 4 | 38 | 253 | 9, 6 | Right IFJ | 9 | 7* | 2 | 0 | 6.04 | 2 | 2 | 1 | 4 | 2.87 | |
| −2 | 4 | 62 | 200 | 6 | Left preSMA | 7 | 5 | 1 | 1 | 2.08 | 1 | 1 | 1 | 4* | 4.81 | |
| 8 | 8 | 68 | 6 | Right preSMA | 5 | 4 | 1 | 0 | 3.11 | 2 | 1 | 1 | 1 | 0.44 | ||
| 36 | 28 | 2 | 190 | 47, 13 | Right IFG—orb/insula | 4 | 4 | 0 | 0 | — | 0 | 3 | 0 | 1 | — | |
| 44 | 18 | 4 | 45 | Right IFG—tria | 5 | 3 | 1 | 1 | 0.43 | 1 | 1 | 1 | 2 | 0.72 | ||
| 50 | 18 | −4 | 47 | Right IFG—orb | 6 | 3 | 1 | 2 | 0.13 | 2 | 0 | 2 | 2 | 2.08 | ||
| −38 | 52 | 10 | 82 | 10 | Left rostral MFG | 4 | 1 | 1 | 2 | — | 1 | 1 | 1 | 1 | — | |
| 40 | 32 | 24 | 51 | 46 | Right mid MFG | 4 | 1 | 1 | 2 | — | 1 | 2 | 0 | 1 | — | |
| 50 | 32 | 34 | 35 | 9, 46 | Right mid MFG | 4 | 4 | 0 | 0 | — | 0 | 2 | 0 | 2 | — | |
| −36 | 24 | 2 | 34 | 47, 13, 45 | Left IFG—orb/tria/insula | 5 | 4 | 0 | 1 | 2.82 | 1 | 2 | 1 | 1 | 1.07 | |
| 42 | 46 | 26 | 33 | 10, 46 | Right rostral MFG | 4 | 2 | 1 | 1 | — | 1 | 1 | 0 | 2 | — | |
| Parietal | 12 | −66 | 60 | 1248 | 7 | Right precuneus | 8 | 3 | 4* | 1 | 4.87 | 2 | 1 | 2 | 3 | 1.11 |
| 42 | −40 | 50 | 40 | Right IPS/IPL | 9 | 2 | 5* | 2 | 7.86 | 2 | 2 | 3 | 2 | 0.50 | ||
| 36 | −54 | 48 | 7, 40 | Right IPS/IPL | 8 | 5 | 2 | 1 | 1.64 | 2 | 2 | 1 | 3 | 1.35 | ||
| 16 | −60 | 54 | 7 | Right SPL/precuneus | 11 | 7 | 3 | 1 | 3.47 | 3 | 3 | 3 | 2 | 0.51 | ||
| 28 | −56 | 60 | 7 | Right SPL/IPS | 5 | 1 | 4* | 0 | 11.41 | 4* | 0 | 0 | 1 | 8.50 | ||
| 4 | −56 | 54 | 7 | Right precuneus | 5 | 1 | 3* | 1 | 4.85 | 3 | 1 | 0 | 1 | 3.69 | ||
| 18 | −72 | 50 | 7 | Right SPL/precuneus | 4 | 2 | 2 | 0 | — | 2 | 0 | 1 | 1 | — | ||
| 10 | −60 | 68 | 7 | Right precuneus | 5 | 2 | 3* | 0 | 5.57 | 1 | 0 | 1 | 3 | 4.32 | ||
| −22 | −64 | 54 | 555 | 7 | Left SPL/IPS | 7 | 3 | 4* | 0 | 7.46 | 2 | 2 | 1 | 2 | 0.60 | |
| −10 | −54 | 48 | 7 | Left precuneus | 4 | 2 | 2 | 0 | — | 1 | 1 | 0 | 2 | — | ||
| −12 | −72 | 46 | 7 | Left precuneus | 5 | 2 | 3* | 0 | 5.57 | 2 | 0 | 2 | 1 | 2.18 | ||
| −18 | −74 | 42 | 7 | Left SPL/precuneus | 5 | 3 | 2 | 0 | 2.80 | 1 | 1 | 1 | 2 | 0.72 | ||
| −10 | −70 | 62 | 7 | Left precuneus | 5 | 2 | 2 | 1 | 1.10 | 2 | 0 | 1 | 2 | 2.18 | ||
| −40 | −44 | 50 | 218 | 40 | Left IPS/IPL | 8 | 3 | 3 | 2 | 1.39 | 2 | 0 | 3 | 3 | 3.60 | |
| −34 | −38 | 42 | 40 | Left IPS/IPL | 5 | 3 | 2 | 0 | 2.80 | 1 | 0 | 3 | 1 | 4.32 | ||
| 26 | −66 | 38 | 112 | 7, 19, 39 | Right caudal IPS/SOG | 5 | 2 | 2 | 1 | 1.10 | 1 | 1 | 1 | 2 | 0.72 | |
| −56 | −38 | 48 | 57 | 40 | Left IPL | 4 | 1 | 1 | 2 | — | 0 | 0 | 3 | 1 | — | |
| −28 | −66 | 36 | 47 | 7, 19, 39 | Left caudal IPS/SOG | 5 | 4 | 1 | 0 | 3.11 | 0 | 1 | 2 | 2 | 2.67 | |
| −58 | −38 | 32 | 27 | 40 | Left SMG | 3 | 3 | 0 | 0 | — | 0 | 0 | 3 | 0 | — | |
| Other | −44 | −70 | −8 | 151 | 19, 37 | Left fusiform gyrus | 5 | 1 | 3* | 1 | 4.85 | 1 | 1 | 0 | 3 | 4.44 |
| −32 | −82 | 26 | 112 | 19 | Left MOG | 3 | 1 | 2 | 0 | — | 2 | 0 | 0 | 1 | — | |
| −32 | −84 | 22 | 19 | Left MOG | 3 | 1 | 2 | 0 | — | 2 | 0 | 0 | 1 | — | ||
| −60 | −50 | 10 | 43 | 22, 21 | Left STG/MTG | 7 | 2 | 1 | 4 | 2.90 | 1 | 1 | 3 | 2 | 1.97 | |
| −62 | −40 | −6 | 42 | 21, 22 | Left MTG/STG | 4 | 2 | 0 | 2 | — | 0 | 1 | 3 | 0 | — | |
| −8 | −22 | −10 | 39 | Midbrain | 4 | 1 | 2 | 1 | — | 1 | 0 | 0 | 3 | — | ||
| 40 | −78 | 2 | 30 | 19 | Right IOG | 5 | 2 | 3* | 0 | 5.57 | 1 | 2 | 0 | 2 | 2.93 | |
| −46 | −62 | −22 | 26 | 37 | Left fusiform gyrus | 3 | 3 | 0 | 0 | — | 0 | 1 | 1 | 1 | — |
Note: Coordinates reported in MNI space. Bold and italic denotes significant result. *Denotes significant positive logistic regression predictor. Abbreviations: IFG, inferior frontal gyrus; dACC, dorsal anterior cingulate cortex; orb, pars orbitalis; tria, pars triangularis; oper, pars opercularis; SOG, superior occipital gyrus; MOG, middle occipital gyrus; STG, superior temporal gyrus; MTG, middle temporal gyrus; IOG, inferior occipital gyrus.
ALE Analyses by Content
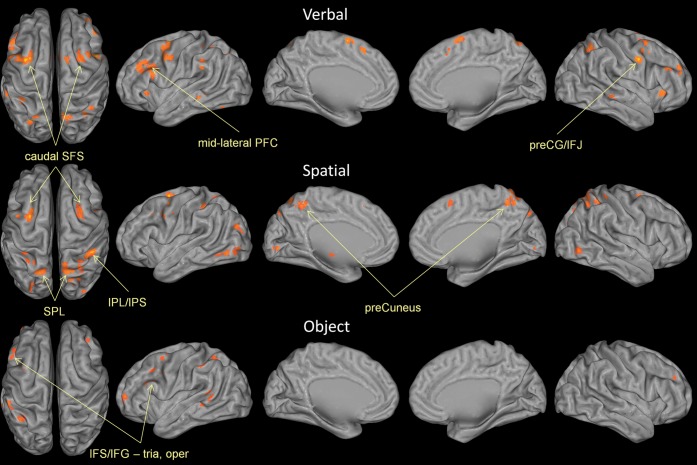
Analyses as a function of content sought to examine content-selective aspects of executive processes of WM. If content dissociations found in studies of WM maintenance are also present for executive processes of WM, we would expect verbal content to preferentially activate left VLPFC, spatial content to preferentially activate the caudal SFS, and object content to activate right VLPFC. By contrast, overlapping regions of consistent activation that do not show a content preference would provide support for a general executive function that is not tailored to the content of the representations on which the processes operate. Separate ALE analyses on verbal, spatial, and object content are depicted in Figure 3 and combined to demonstrate overlap in Figure 5. Full quantitative details can be found in Supplementary Table 1.
Figure 3.
ALE maps by content. Top: ALE map of all experiments using verbal content (letters, words, digits). Middle: ALE map of all experiments using spatial content (locations, movement vectors). Bottom: ALE map of all experiments using object content (faces, scenes, houses, drawings, colors, unnamable objects). Results are threshold at P < 0.05, corrected for multiple comparisons using false discovery rate and a 25 voxel extent criterion. Higher ALE values are depicted in yellow.
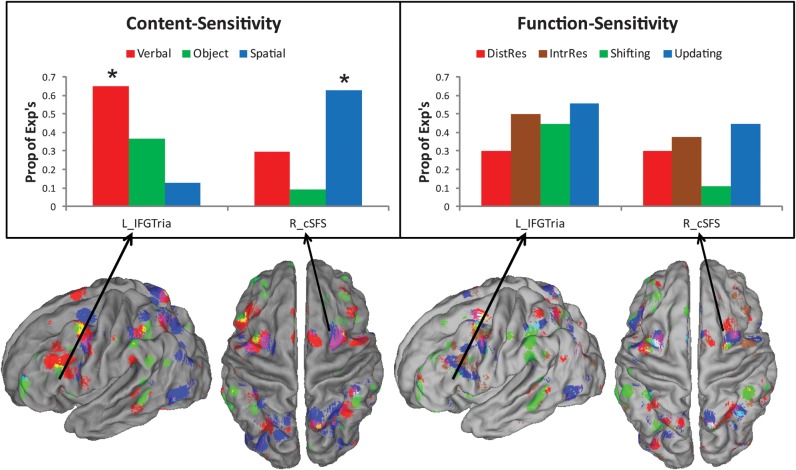
Figure 5.
Content sensitivity and functional generality of the left IFG, pars triangularis, and right caudal SFS. Top: Proportion of experiments reporting significant activation in the left IFG, pars triangularis (L_IFGTria), and right caudal SFS (R_cSFS) grouped by content (left) and function (right). Significant difference by content were found in both the left IFG, pars triangularis (χ2 = 6.42, P < 0.05) and right caudal SFS (χ2 = 6.25, P < 0.05). Follow-up logistic regression tests revealed that verbal content predicted activation in the left IFG, pars triangularis (t34 = 2.25, P < 0.05) while spatial content predicted activation in the right caudal SFS (t34 = 2.10, P < 0.05). By contrast, neither region was sensitive to differences by function (both P > 0.1). Bottom: Renderings by content (left) and function (right). Results are thresholded at P < 0.01 uncorrected for depiction of overlap and subthreshold convergence. Content key: red—verbal, green—object, blue—spatial, overlap—mix. Function key: red—distractor resistance, sienna—intrusion resistance, green—shifting, blue—updating, overlap—mix.
Verbal experiments showed somewhat of a left-sided dominance in the PFC, although many activations were bilateral. One of the most prominent sites of convergence was the left caudal SFS with a corresponding weaker peak in the right hemisphere. This was somewhat surprising given that this region has been most strongly associated with spatial WM (Courtney et al. 1998). There was the expected convergence in left mid-lateral PFC with corresponding less extensive convergence in the right hemisphere. Interestingly, the convergence in the left caudal SFS (max ALE value = 0.024) was numerically larger than the convergence in the left midlateral PFC (max ALE value = 0.016). Significant convergence was also found in bilateral preCG and the left IPL. The left IFG, preCG, and IPL network is similar to the neural underpinnings of the phonological loop (Smith and Jonides 1998, 1999; Smith et al. 1998).
As in verbal experiments, spatial experiments also appeared to have the most prominent focus in the caudal SFS and this convergence was also somewhat stronger in the left hemisphere. In contrast to the verbal ALE map, spatial studies produced more extensive activations in parietal cortex, particularly in the SPL and IPS, which is consistent with studies of spatial storage (Wager and Smith 2003). There was also nearly a complete absence of convergent activations in midlateral frontal cortex in the spatial ALE map, which is also consistent with studies of spatial storage. There were small clusters in the left posterior MFG and IFJ. Finally, the spatial analysis demonstrated some convergence in occipitotemporal regions not found in the verbal ALE map.
The analysis of object experiments revealed much less robust convergence compared with the verbal and spatial analyses. This may be partly attributable to the fact that there were fewer object foci than verbal and spatial foci. This may also be partly attributable to greater diversity in stimulus materials for the object experiments or that object representations were more distributed than locations or verbal representations (Courtney 2004). In contrast to documented right hemisphere dominance in object maintenance (Smith and Jonides 1999; Wager and Smith 2003), the object ALE map for executive processing in WM was very left-hemisphere dominant (485 significant voxels in the left hemisphere, 28 significant voxels in the right hemisphere). As in the verbal ALE map, there was significant convergence in left midlateral PFC, right MFG, and left IPS/IPL. In contrast to the verbal analysis, however, there was convergence in left rostral PFC (BA 10) and superior temporal sulcus. There was also an absence of convergence in the caudal SFS and SPL, which contrasted with both verbal and spatial analyses.
Consistency between content types was formally assessed using conjunction analysis (Nichols et al. 2005). Conjunction analyses revealed a number of consistencies between verbal and spatial experiments (Table 3). Both types of content produced overlapping activations in bilateral caudal SFS, bilateral preCG/IFJ, preSMA/dACC, and the SPL (Fig. 5). Overlap between verbal and object experiments did not pass our extent criterion, although there was subthreshold evidence of overlapping clusters in the left midlateral PFC (Fig. 5). Object and spatial maps demonstrated a small convergent cluster in the left posterior MFG. No region was consistently active for all 3 types of content.
Table 3.
ALE conjunctions and contrasts
| Conjunctions | X | Y | Z | Number of voxel | BA | Region |
| Content | ||||||
| Verbal and spatial | −30 | 0 | 58 | 76 | 6, 8 | Left caudal SFS |
| 30 | 4 | 54 | 94 | 6, 8 | Right caudal SFS | |
| −42 | 4 | 44 | 49 | 6 | Left preCG | |
| 48 | 6 | 36 | 36 | 6 | Right IFJ | |
| 0 | 16 | 52 | 23 | 8, 32 | preSMA/dACC | |
| 12 | −64 | 60 | 35 | 7 | Right SPL/precun | |
| Object and spatial | −52 | 12 | 36 | 20 | 9 | Left MFG |
| Function | ||||||
| Intr res and updating | 30 | 4 | 54 | 28 | 6, 8 | Right caudal SFS |
| Dist res and shifting | −28 | −2 | 58 | 22 | 6, 8 | Left caudal SFS |
| Dist res and updating | −28 | 2 | 56 | 98 | 6, 8 | Left caudal SFS |
| −54 | 10 | 36 | 34 | 9 | Left preCG | |
| −42 | −2 | 42 | 28 | 6 | Left preCG | |
| 48 | 6 | 38 | 34 | 6 | Right IFJ | |
| 14 | −66 | 60 | 24 | 7 | Right SPL/preCun | |
| Shifting and updating | −30 | −2 | 58 | 35 | 6, 8 | Left caudal SFS |
| −54 | 4 | 42 | 36 | 6 | Left preCG | |
| −38 | −44 | 50 | 26 | 40 | Left IPL/IPS | |
| Dist res and shifting and updating | −28 | −2 | 58 | 20 | 6, 8 | Left caudal SFS |
| Contrasts | X | Y | Z | Number of voxel | BA | Region |
| Content | ||||||
| Spatial > verbal | 30 | −54 | 60 | 127 | 7 | Right SPL/IPS |
| −24 | −2 | 58 | 116 | 6, 8 | Left caudal SFS | |
| 40 | −42 | 56 | 72 | 7, 40 | Right IPL/IPS | |
| 12 | −58 | 62 | 43 | 7 | Right SPL/preCun | |
| Spatial > object | −26 | −2 | 58 | 221 | 6, 8 | Left SFS |
| 22 | −56 | 60 | 295 | 7 | Right SPL/IPS | |
| −12 | −58 | 52 | 119 | 7 | Left precun | |
| 26 | 4 | 54 | 39 | 6, 8 | Right caudal SFS | |
| Function | ||||||
| Shifting > Intr res | −38 | −42 | 46 | 68 | 40 | Left SMG/IPL |
| −56 | −36 | 34 | 41 | 40 | Left SMG/IPL | |
| −56 | −38 | 48 | 119 | 40 | Left IPL | |
| Shifting > updating | −58 | −40 | 34 | 32 | 40 | Left SMG/IPL |
| Shifting > Dist res | −56 | −38 | 34 | 91 | 40 | Left SMG/IPL |
| −58 | −40 | 46 | 70 | 40 | Left IPL | |
| Updating > Intr res | −50 | 4 | 38 | 80 | 6, 9 | Left IFJ |
Finally, direct ALE contrasts assessed pair-wise differences between content types (Table 3). These contrasts revealed that spatial experiments more consistently activated left caudal SFS and the right SPL/IPS than experiments involving verbal or object content. Hence, although both verbal and spatial experiments activated the caudal SFS and SPL/IPS, spatial experiments did so significantly more reliably. No other contrasts produced significant results at our strict, corrected thresholds.
ROI Analyses by Content
Whole-brain ALE analyses were complemented by ROI analyses (for ROI descriptions, see Materials and Methods). These analyses afforded the ability to uncover differences by content that did not pass our strict whole-brain criteria. Analyses within functional ROIs are described in Table 2, while analyses within anatomical ROIs are summarized in Table 4. We describe significant results below.
Table 4.
Anatomical ROI analyses
| Region | Preference | χ2 | Logistic regression |
| Content | |||
| Left IFG—tria | Verbal | 6.424 | 2.249 |
| Left IFG—oper | Nonobject | 4.596 | −1.984 |
| Left preCG | Nonobject | 10.082 | −2.376 |
| Right preCG | Nonobject | 5.575 | −1.98 |
| Left SPL | Spatial | 8.122 | 2.558 |
| Right SPL | Spatial | 6.467 | 2.196 |
| Left MFG—BA 6 | Spatial | 6.155 | 2.026 |
| Function | |||
| Right IFG—tria | Intr res | 8.709 | 2.311 |
| Left IFG—oper | Updating | 7.02 | 2.163 |
| Left IPL | Shifting | 14.895 | 2.58 |
| Left SMG | Shifting | 13.7 | 2.853 |
| Left MFG—BA 46 | Shifting | 7.776 | 2.519 |
χ2 analyses revealed a significant difference in the proportion of experiments demonstrating activation in left IFG, pars triangularis (χ2 = 6.42, P < 0.05; Fig. 5). Follow-up logistic regression analyses revealed that experiments containing verbal content significantly predicted activation in this region (t34 = 2.25, P < 0.05). This result confirmed visual impressions from the ALE analyses that verbal content demonstrated more robust activation in left mid-VLPFC than other content types. Verbal selectivity was also found in a functional ROI in the right IFJ (48, 4, 38, BA 6; χ2 = 6.04, P < 0.05; logistic regression t34 = 1.99, P < 0.05).
Consistent with the contrast ALE analyses described above, spatial selectivity was found in the left (χ2 = 8.12, P < 0.05; logistic regression t34 = 2.56, P < 0.05) and right SPL (χ2 = 6.47, P < 0.05; logistic regression t34 = 2.20, P < 0.05) in addition to a number of parietal functional ROIs (for details, see Table 2). Also consistent with the ALE contrasts, spatial selectivity was found in left posterior MFG, BA 6 (χ2 = 6.15, P < 0.05; logistic regression t34 = 2.03, P < 0.05) and functional ROIs within left (χ2 = 9.28, P < 0.005; logistic regression t34 = 2.56, P < 0.05) and right caudal SFS (χ2 = 6.25, P < 0.05; logistic regression t34 = 2.10, P < 0.05; Fig. 5).
Finally, no region appeared to be positively associated with object content. However, 3 regions were negatively associated with object content. In other words, these regions were noted by the absence of activation for object content in contrast with consistent activation for verbal and spatial content. These regions included left IFG, pars opercularis, and the bilateral preCG.
ALE Analyses by Function
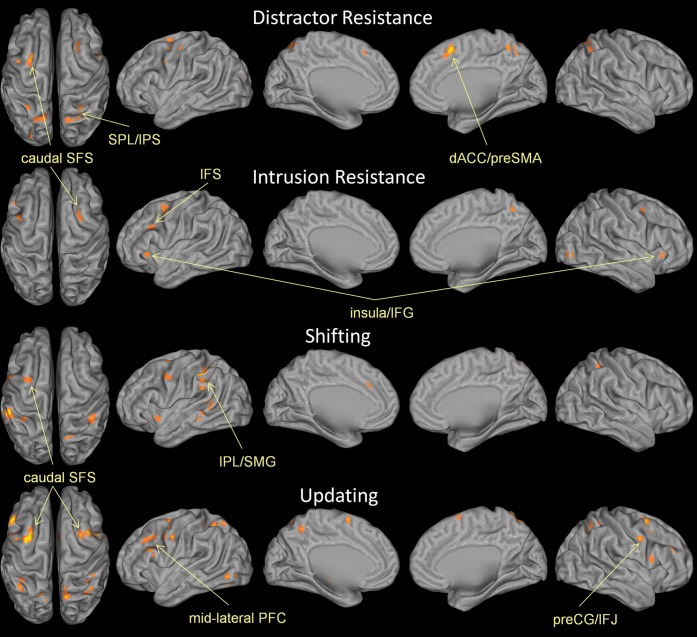
ALE analyses by function sought to investigate whether a priori-defined executive WM functions are associated with common or dissociable neural correlates. Separate ALE analyses on distractor resistance, intrusion resistance, shifting, and updating are depicted in Figure 4 while Figure 5 depicts all functions plotted together in a single rendering. Full quantitative details can be found in Supplementary Table 2.
Figure 4.
ALE maps by function. Distractor resistance involves preventing irrelevant external content from disrupting WM. Intrusion resistance involves preventing irrelevant memories from disrupting WM. Shifting involves changing the focus of attention within WM. Updating involves changing the content of what is stored in WM. See Materials and Methods for further details. Results are threshold at P < 0.05, corrected for multiple comparisons using false discovery rate and a 25 voxel extent criterion. Higher ALE values are depicted in yellow.
ALE analysis of distractor resistance revealed robust convergence in the left caudal SFS and right preSMA. Prominent parietal clusters were also present in the SPL, IPS, and preCuneus. Other than some small clusters in the left preCG/IFJ, there was a notable absence of activations in the lateral frontal surface. Overall, the distractor resistance ALE map looked qualitatively similar to the spatial content map suggesting perhaps that spatial mechanisms underlie the ability to filter out external distraction.
Intrusion resistance has been theorized to involve the left (Jonides and Nee 2006) and right mid-VLPFC (Anderson and Levy 2009). Consistent with these proposals, the strongest site of convergence for intrusion resistance was found in right VLPFC, including the insula and right IFG, pars triangularis with similar activations in the left hemisphere that extended more dorsally into the IFS (Supplementary Fig. 2). Additionally, convergent activations were found in the right caudal SFS, bilateral posterior MFG/preCG, right preCuneus, and left SPL/IPS.
Most of the experiments investigating the shifting function come from studies that used the refresh task (Johnson et al. 2003, 2005). Much of the literature examining this task implicates left midlateral PFC. Despite these suggestions, ALE analysis did not report significant convergence in left midlateral PFC, suggesting that activations are not consistently localized. The only significant left frontal clusters were found in the insula, preCG, dACC, and caudal SFS. Interestingly, there was strong convergence in inferior parietal regions including the bilateral IPL and left SMG, as well as left middle and superior temporal gyri. This suggests that shifting attention within WM is highly associated with activations in the left temporalparietal junction (TPJ).
Updating WM is thought to involve an interplay between the midlateral PFC and IFJ on the one hand and the dorsal attention system (caudal SFS, posterior parietal cortex) on the other (Roth et al. 2006). In this framework, lateral frontal regions are thought to reconfigure attentional priorities that are maintained by dorsal attention. Consistent with this proposal, updating WM was strongly associated with bilateral caudal SFS and left midlateral PFC. There was also strong convergence in the bilateral IFJ/preCG, bilateral IPS, and preSMA.
Conjunctions across and contrasts between functions are summarized in Table 3. Conjunction analyses revealed that across different putative executive functions, common activations are consistently found in the caudal SFS (Fig. 5). Intrusion resistance and updating produced overlapping clusters of activation in the right caudal SFS, while distractor resistance, shifting, and updating all produced overlapping clusters of activation in the left caudal SFS. These data highlight the prominence of the caudal SFS for diverse executive demands. Significant conjunctions were also found in posterior frontal regions including the left preCG for distractor resistance and shifting, as well as for shifting and updating, and in the right IFJ for distractor resistance and updating. Parietal conjunctions were found in the right SPL/preCuneus for distractor resistance and updating and in the left IPL/IPS for shifting and updating.
ALE contrasts revealed that activations in left inferior parietal regions including the IPL and SMG were more consistently related to shifting than other functions, as these regions were revealed in all pair-wise contrasts. The only other significant contrast result was greater consistency in the left IFJ for updating than intrusion resistance.
ROI Analyses by Function
Anatomical ROI results are summarized in Table 4 and functional ROI results are summarized in Table 2.
Distractor resistance predicted activations in a functional ROI in the right SPL in and around the IPS (28, −56, 60; χ2 = 8.50, P < 0.005; logistic regression t34 = 2.33, P < 0.05). However, as we reported above, spatial content also predicted activations in this region. When both spatial content and distractor resistance were entered as predictors in a logistic regression, spatial content (t33 = 2.04, P < 0.05), but not distractor resistance (t33 = 1.57, P = 0.12) predicted activation in the right SPL/IPS. Whereas the sample makes it somewhat difficult to fully disentangle contributions of spatial content and distractor resistance, it appears that the right SPL/IPS is more strongly related to the former.
Some of the authors of the present study have presumed a central role of left mid-VLPFC, particularly in pars triangularis, for intrusion resistance (Jonides and Nee 2006). Other authors have suggested the importance of right mid-VLPFC, relating the suppression of unwanted memories to the suppression of inappropriate motor responses (Anderson and Levy 2009). ROI analyses indicated that right, but not left IFG, pars triangularis was predictive of intrusion resistance (χ2 = 8.71, P < 0.005; logistic regression t34 = 2.31, P < 0.05; Supplementary Fig. 2). Nearly, half (16/36) of all experiments demonstrated activation in left IFG, pars triangularis with similar proportions of activation across all functions (Fig. 5; Supplementary Fig. 2). Hence, activation in left mid-VLPFC region appeared to be ubiquitous across all functions and did not favor any one function.
Consistent with the whole-brain ALE contrasts, ROI analyses of the left IPL and SMG demonstrated that activations in these regions were predicted by shifting (left IPL: χ2 = 14.90, P < 0.0005, logistic regression t34 = 2.58, P < 0.01; left SMG: χ2 = 13.7, P < 0.0005, logistic regression t34 = 2.85, P < 0.005). Interestingly, although we did not find significant convergence of activation in left midlateral PFC for shifting in ALE analysis, ROI analyses nevertheless suggested that left MFG, BA 46 was predictive of the shifting function (χ2 = 6.16, P < 0.05; logistic regression t34 = 2.03, P < 0.05). These data confirm the presumed role of left midlateral PFC in shifting (Johnson et al. 2005; Johnson MR and Johnson MK 2009) as well as highlight an understated, yet strong, role of inferior parietal cortex.
Finally, updating was found to predict activations in left IFG, pars opercularis (BA 44) in both anatomical (χ2 = 7.02, P < 0.01; logistic regression t34 = 2.16, P < 0.05) and functional ROIs (−52, 12, 22; χ2 = 6.61, P < 0.05; logistic regression t34 = 2.29, P < 0.05). Updating was also predictive of activations in a functional ROI in the left preSMA (−2, 4, 62; χ2 = 4.81, P < 0.05; logistic regression t34 = 2.04, P < 0.05).
Dorsal–Ventral Distinctions and Selection Demands
Several authors have proposed a dorsal/ventral distinction between executive processes and storage in WM (D'Esposito et al. 1998, 1999; Postle et al. 1999; Barde and Thompson-Schill 2002). By these proposals, executive processes of WM should be more prominently localized in the mid-DLPFC than the mid-VLPFC. In most of the ALE analyses, midlateral PFC activations were not definitively dorsal or ventral, as activations tended to cluster around the IFS. To explore further whether our activations favored mid-DLPFC over mid-VLPFC, we compared the proportion of experiments reporting activations in the mid-DLPFC (MFG BA 9 and BA 46) and mid-VLPFC (IFG, pars triangularis, and pars opercularis) separately by hemisphere. We found a significant difference in the left (χ2 = 5.57, P < 0.05) but not right (χ2 = 0.55, P > 0.45) hemisphere. The difference in the left hemisphere was driven by a greater number of experiments finding activation in the left mid-VLPFC (22/36) than left mid-DLPFC (12/36). Notably, this difference remained significant when considering only experiments using spatial content (χ2 = 4.27, P < 0.05), suggesting that the result is not entirely driven by verbalizable material.
The mid-DLPFC is thought to be particularly important when selecting among competing representations (Miller and Cohen 2001; Curtis and D'Esposito 2003; Courtney et al. 2007). In these contexts, the mid-DLPFC is thought to resolve competition through top-down biasing of task-relevant information. To investigate whether mid-DLPFC involvement is more prominent when selecting among competitors, we coded for the following situations: (1) experiments requiring distractor resistance when relevant and irrelevant content are simultaneously present, (2) experiments requiring intrusion resistance when a relevant item or context needs to be selected among competing items or contexts; (3) experiments requiring shifting attention when multiple items are held in WM; (4) experiments requiring updating WM when only a subset of the items in WM are changed. Twenty-one of the 36 experiments met these criteria (Table 1). Foci from these 21 experiments requiring selection are plotted in Figure 6 (high selection; red) along with the remaining 15 experiments that did not require selection (low selection; green).
Figure 6.
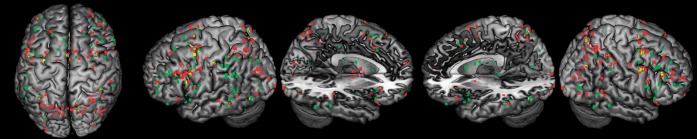
Plots of individual activation foci by selection demands. Activation foci from experiments requiring selection amidst competition (red; high selection) and selection without competition (green: low selection). Prominent clustering from experiments involving high selection can be seen in the left midlateral PFC along the IFS as well as in dorsal posterior parietal regions. Experiments involving low selection cluster prominently around inferior parietal cortex.
Comparing the prevalence of activations in left mid-DLPFC and mid-VLPFC for experiments requiring high selection, we found that once again, activations in the mid-VLPFC (16/21) were much more consistently reported than activations in the mid-DLPFC (5/21; = 11.52, P < 0.001). Whereas activations in the left mid-DLPFC did not differ as a function of selection demands (χ2 = 1.08, P > 0.25), activations in the left mid-VLPFC were reported at a significantly greater frequency when demands for selection were high (χ2 = 4.82, P < 0.05). Examination of the foci themselves revealed that many of the foci driving this result were situated in the IFS (Fig. 6). As a result, this analysis is highly sensitive to the dividing line drawn between mid-DLPFC and mid-VLPFC. So, competition appears to produce increased activation consistency in the left mid-lateral PFC in a region neither clearly dorsal nor ventral.
Another striking result revealed by this analysis was the division between activations in parietal cortex. Activations in dorsal aspects of the parietal cortex in the SPL and precuneus were nearly uniform from experiments with high selection demands. By contrast, activations in left inferior parietal regions, including the IPL and SMG, were nearly uniform from experiments with low selection demands. Direct contrasts of ALE maps of high and low selection confirmed these impressions: there was significantly greater convergence in the left SPL/precuneus in experiments with high than low selection demands (peak: −18, −68, 58) while the converse was true in the left IPL (peak: −58, −42, 46). Hence, a dorsal–ventral distinction by function of selection demands appeared to be more consistent in parietal cortex than the PFC.
Discussion
The present study examined whether different aspects of executive processes of WM can be dissociated by way of content and/or function using quantitative meta-analysis. The combination of all 36 experiments included in the analysis revealed a broad network of medial and lateral frontal and parietal regions involved in executive processing in WM. When experiments were distinguished by content there was evidence of dissociations. Although activations in the caudal SFS and SPL were common to most forms of content and function, they were particularly prominent when content was spatial. Activations in the mid-lateral PFC were primarily left lateralized and strongly associated with verbal content. Object content was also left lateralized and consistent in the mid-lateral PFC but demonstrated the weakest convergence throughout the brain. This is perhaps due to the fact that stimuli in object WM tasks are more varied than other forms of WM or that the representations of objects are more distributed thereby requiring more diverse and less consistent control processes (Courtney 2004). As a result, object content was not selectively predictive of activation in any region.
Functional dissociations were not as clear as taxonomies of executive function might suggest (Smith and Jonides 1999; Miyake et al. 2000; Friedman and Miyake 2004), although some differences did arise. Functionally, there was some evidence for an association between SPL/IPS and distractor resistance, although the association was stronger between this region and spatial content. Intrusion resistance was related to right IFG, pars triangularis, the right hemisphere homologue of the region previously thought to be most strongly associated with this function (Jonides and Nee 2006). There was mixed evidence that supported an association between the left mid-DLPFC and shifting (Johnson et al. 2003, 2005), but this was dwarfed by an extremely strong association between shifting and inferior parietal regions. Finally, posterior ventral frontal regions and the preSMA were associated with updating the contents of WM. These functional distinctions appeared to be minor compared with extensive convergence between functions in the caudal SFS and left mid-lateral PFC. We now discuss several prominent regions in turn.
Caudal SFS, SPL, and Spatial Attention
One of the most salient results was that the caudal SFS appeared to be the most consistent locus of activation for executive processes acting upon WM. Significant convergence in the caudal SFS was found across all 4 putative executive functions, as well as across 2 of 3 content types. In all of these cases save for one, activations were left lateralized. The lone exception was activation related to intrusion resistance that was significant in the right, but not left, caudal SFS. Content-wise, activations in the bilateral caudal SFS were strongly predicted by spatial material. This suggests that whatever general role the caudal SFS plays in WM, it has a spatial nature (Courtney et al. 1998).
Extensive research in both humans and monkeys has indicated that caudal dorsal regions of the PFC support top-down spatial visual attention (Kastner and Ungerleider 2000; Reynolds and Chelazzi 2004; Moore 2006). A meta-analysis of early neuroimaging studies of spatial attention reported strong clustering in and around the caudal SFS (Kastner and Ungerleider 2000). It has further been proposed that selective spatial attention processes are critical for both external attention and WM (Desimone and Duncan 1995; Kastner and Ungerleider 2000; Kane et al. 2001; Miller and Cohen 2001) and direct comparisons between the 2 have demonstrated close parallels (Awh and Jonides 2001; Awh et al. 2006; Tamber-Rosenau et al. 2011). Finally, detailed analysis has revealed that the caudal SFS regions involved in spatial WM can be distinguished from those involved in overt eye movements (Courtney et al. 1998). The consistent role of caudal SFS across the functions studied here and its particularly strong association with spatial content support the idea that this region is involved in spatial attention in WM.
Spatial attention may play a role in WM even when the content is not overtly spatial. One of the most consistent loci of activation in the ALE analysis of verbal experiments was the caudal SFS. The strongest contributions to this result came from 2 studies that compared external and internal attention. The first examined visual selection of words amid distraction (distractor resistance) and the selection of a subset of words from within WM (i.e., updating; Nee and Jonides 2009). The second study compared encoding a new visually presented item into WM (updating) with refreshing a recently presented item (i.e., shifting; Roth et al. 2009). The caudal SFS was recruited in all cases. In these studies, the external environment and internal memory may have been represented as different spaces among which the caudal SFS selected. This is consistent with recent demonstrations that the caudal SFS maintains attention even on locations in extraretinal space suggesting that spatial attention processes of this region can be allocated rather flexibly (Tark and Curtis 2009).
The caudal SFS often coactivates with the SPL during spatial attention (Kastner and Ungerleider 2000; Corbetta and Shulman 2002; Yantis and Serences 2003; Reynolds and Chelazzi 2004). Similar to the caudal SFS, the SPL demonstrated a preference for spatial content but was also activated across different functions (distractor resistance and updating) and content types (verbal and spatial). Previous research has suggested that medial portions of the SPL perform a domain general shifting function (Yantis and Serences 2003; Chiu and Yantis 2009; Tamber-Rosenau et al. 2011). Curiously, in the present data, shifting in WM did not consistently activate the SPL. This may suggest that whereas the caudal SFS appears to play a common role across perception and memory, the SPL may be more overtly related to the external environment (Courtney 2004; Nee and Jonides 2009; but see Tamber-Rosenau et al. 2011). Another possibility is that the SPL is only necessary when specific locations or features must be selected among competitors. Selective shifting was unnecessary in two-thirds of the shifting experiments examined here, which may have led to the inconsistent activation of the SPL. Of the 3 experiments requiring selective shifting, 2 of them reported activation in the bilateral SPL. While it is difficult to draw conclusions from such small numbers, future research may target whether the SPL is required only during shifting amidst competition.
We found evidence that activations in the SPL were predicted both by spatial content and by distractor resistance. When both content and function were entered as predictors, only spatial content predicted activation in the SPL, although a nonsignificant trend remained for distractor resistance. In a number of cases of distractor resistance, spatial attention is likely to play a role in filtering out distractors. This is especially important when both relevant and irrelevant items are simultaneously present. Notably, experiments examining distractor resistance that presented distractors in isolation did not demonstrate activation in this region. Hence, the SPL may be particularly important for distractor resistance when selective spatial attention is needed.
Prior evidence suggested that caudal SFS regions involved in maintaining spatial information are located just anterior to regions that are responsible for overtly directing gaze (Courtney et al. 1998). The caudal SFS regions found here closely match these previous reports in the left hemisphere (all experiments combined: −28, 0, 58 versus −32, −1, 48 in Courtney et al. 1998, translated into MNI), although the right-hemisphere activations may be just anterior (all experiments combined: 34, 6, 56 vs. 31, 1, 50 in Courtney et al. 1998). The spatial precision of the meta-analysis is somewhat limited, so strong claims cannot be made. However, it would be interesting to examine within a single study whether executive components of spatial attention in WM are identical to maintenance components or whether a fine gradation exists.
Executive Functions of Midlateral PFC
One of the earliest distinctions drawn from neuroimaging WM tasks was a dorsal–ventral split between executive processes on the one hand and simple maintenance on the other (D'Esposito et al. 1998). Several studies compared simple maintenance with the transformation or elaboration of the contents of WM. These studies converged on a strong association of mid-VLPFC with simple maintenance but not executive processes and mid-DLPFC with executive processes but not simple maintenance (D'Esposito et al. 1999; Postle et al. 1999; Wagner et al. 2001; Barde and Thompson-Schill 2002). This evidence appeared to be particularly prominent when comparing maintenance with manipulation. Whereas we attempted to investigate manipulation in the present study, of the 15 experiments we found that examined manipulation, only 3 matched our inclusion criteria, thereby precluding systematic analysis. Nevertheless, some of the functions studied here should be component processes of manipulation.
Considering all experiments together, there was little evidence for a mid-DLPFC executive preference. Activations clustered most prominently in the IFS, which is neither clearly dorsal nor ventral. When explicitly examining the proportion of experiments reporting foci in mid-DLPFC (MFG, BA 9, and 46) and mid-VLPFC (IFG, pars triangularis, and opercularis), there was evidence for a ventral preference in the left hemisphere. This was not solely due to contributions of experiments that used verbal content as consideration of only spatial experiments produced the same results. However, clustering in the mid-VLPFC was most prominent in the dorsal-most aspects with a relative absence of activations in inferior aspects of pars triangularis (rostral-VLPFC) and pars opercularis (caudal-VLPFC). These mid-VLPFC activations contrast somewhat with an early meta-analysis of maintenance versus executive demand where VLPFC activations were clustered more prominently in and around the anterior insula (D'Esposito et al. 1998). On the other hand, it is not clear that the activations found here correspond to the mid-DLPFC either as most of the activations clustered approximately 1 cm posterior to previous reports (D'Esposito et al. 1998). Perhaps, the IFS should be considered a region in its own right similar to current conceptions of the IFJ (Brass et al. 2005). For now, we will refer to this region as mid-lateral PFC as a compromise between mid-DLPFC and mid-VLPFC.
Activations in the left mid-lateral PFC were particularly pronounced when relevant information had to be selected amid competition. Badre et al. (2005) have suggested that the function of left mid-VLPFC is postretrieval selection. In this framework, anterior portions of the VLPFC retrieve information into WM and mid-VLPFC selects relevant representations among the retrieved contents. The sensitivity of left midlateral PFC to selection demands across a variety of functions fits well with this proposal. Previously, we had proposed a more specific role of selection of contextual information that assists in resolving proactive interference (Jonides and Nee 2006). Given that activations in the left mid-VLPFC were more broadly recruited across the 4 functions studied here, postretrieval selection appears to be a better fit.
Whereas most functions produced clustering in and around the IFS, there was evidence that shifting predicted activation in the mid-DLPFC (BA 46). This was the only function that demonstrated a greater proportion of activation in the DLPFC than VLPFC (Fig. 5). Two aspects of this result are striking. First, most of the experiments contributing to this result involved refreshing, which is in all likelihood the least effortful of all the processes studied here. Hence, it is doubtful that these activations could be explained on the basis of effort. Second, refreshing is not associated with an overt response. Some authors have suggested that BA 46 is involved in WM only insofar as information is selected for action (Rowe et al. 2000; Pochon et al. 2001). However, the lack of overt response associated with refreshing suggests that the selection involved is somewhat more general. Notably, when an item is selected for a response, it becomes the focus of attention. Similarly, refreshing foregrounds an item in WM in the focus of attention. Collectively, BA 46 may be involved in preferentially foregrounding a single item in WM.
An alternative explanation for the relationship between mid-DLPFC and shifting has to do with task selection. A notable aspect of most refreshing studies is that the refresh condition has an arbitrary cue to task mapping (e.g., a dot [•] symbol denotes refresh). Control conditions involve new items (e.g., words or pictures) that are read or viewed. As a result, contrasts of interest compare an arbitrary stimulus-to-task mapping (i.e., refreshing) with a natural stimulus-to-task mapping (e.g., reading). So, some of the neural activations to the refreshing task may have to do with task selection itself rather the process of foregrounding a representation (Courtney et al. 2007).
Right IFG and Inhibition
An interesting result was that intrusion resistance predicted activation in the right IFG. The right IFG has been strongly implicated in the inhibition of prepotent responses (Aron et al. 2004; Aron 2007). Our data suggest that it may also be involved in inhibiting irrelevant memories from entering WM (Anderson et al. 2004; Anderson and Levy 2009). The right IFG may interact with different subcortical structures in order to achieve either function. Aron (2007) has suggested that the right IFG sends top-down signals to the basal ganglia and subthalamic nucleus as a means to withhold inappropriate responses. Anderson et al. (2004) and Anderson and Levy (2009) have suggested that the right IFG downregulates the hippocampus in order to prevent inappropriate memories from entering mind. Hence, this region may perform a general purpose inhibitory function. Of note is that activations in the right IFG related to intrusion resistance were clustered most prominently along the insular surface rather than the lateral surface (Supplementary Fig. 2). Although the anterior insula has been associated with inhibition (Wager et al. 2005), it is also involved more generally in sustained task-related behavior (Dosenbach et al. 2008). Due to their close spatial proximity, the anterior insula and IFG have not always been distinguished making it somewhat difficult to dissociate their functionalities. More detailed investigation would be important to explore insular versus lateral distinctions.
Inferior Parietal Cortex and the Focus of Attention
The strongest functional distinction was the association of inferior parietal regions, including the IPL and SMG, with shifting. Preferential shifting-related activations were found for all pair-wise ALE contrasts, as well as ROI analyses. We have previously suggested that the IPL may be a source of top-down bias to foreground the representation in WM that is the focus of attention (Nee and Jonides 2008b, 2011). We hypothesized that part of this foregrounding process involves activating semantic and conceptual details related to the item in the focus of attention. In long-term memory, IPL activation accompanies recollection of specific details of an experience (Wagner et al. 2005). Therefore, the IPL may play a similar role throughout memory to highlight elaborative details. This may be accomplished through connections with anterior temporal regions that represent such content (Nee and Jonides 2008b). The relationship between the IPL and shifting is consistent with the idea that this region is prominently involved in shifting and elaborating information about the focus of attention within WM.
The SMG is situated inferior to the IPL where the parietal lobe meets the temporal lobe. Early neuroimaging studies attributed activations in this region to phonological storage (Paulesu et al. 1993) equating the region with Wernicke’s area. Buchsbaum and D'Esposito (2008) have suggested that this region is more generally involved as an auditory-motor interface. However, in the experiments studied here, phonological and auditory–motor interactions have typically been subtracted out by control conditions that involve reading or repeating words (Johnson et al. 2005). As a result, the activations found here may not directly pertain to phonological or auditory–motor aspects of WM. Another line of research has focused on homologous regions of the right hemisphere commonly referred to as the TPJ. The TPJ has been shown to be involved in attentional orienting, especially when salient external cues exogenously drive attention (Corbetta and Shulman 2002). It is possible that the activations found here represent a left-hemisphere WM homologue of such processes. In this case, the right TPJ may be involved in orienting external attention, whereas the left TPJ may be involved in orienting internal attention. Further research is needed to test this proposal.
Comparisons to Other Meta-analyses
In a previous meta-analysis of WM, Wager and Smith (2003) suggested that while spatial and nonspatial content diverged in posterior regions, there was limited evidence for content differences in the PFC. Whereas that study examined mostly block designs, here we restricted ourselves to event-related designs to hone in on component processes. It may be that such designs are more sensitive to dissociate PFC processes. Also, the clustering method, which required specifying the number of clusters in advance, may have produced clusters that were too coarse to identify PFC dissociations. More recently, Owen et al. (2005) performed ALE analysis on 24 experiments that used the n-back task. This study reported some evidence for verbal selectivity in mid-lateral PFC and spatial selectivity in the caudal SFS similar to the present study. However, given the nature of the n-back task, it was unclear whether those differences were attributable to storage or executive components of WM. Our results suggest that there may not be much of a distinction. One potential difference between our study and that of Owen et al. (2005), however, is that Owen et al. (2005) demonstrated greater convergence in the mid-DLPFC (BA 9, 46) than we did here. We did find evidence that shifting activated the mid-DLPFC. The mid-DLPFC activations found in the n-back task may be the result of shifting being an important component process.
It is also worthwhile to compare this study with meta-analyses of executive processes that act outside of WM. Previously, we performed a meta-analysis on 47 experiments that examined response inhibition (Nee et al. 2007a). Whereas response inhibition produced strongly right-lateralized activations in the mid-lateral PFC, the present study revealed a strongly left-lateralized network. In fact, direct comparisons between the 2 studies revealed no overlap in activations in the mid-lateral PFC, although both studies activated identical parts of the IFJ/preCG and dACC (Supplementary Fig. 3). Hence, executive functions that operate on WM may differ in laterality from those that operate on responses. Wager et al. (2004) performed a meta-analysis on a variety of switching functions (task, attribute, object, and location). Switching produced strong convergence in the bilateral IPS/SPL, right preCG, and dACC. Direct comparisons between the present study and the switching meta-analysis reveal overlapping activations in the left parietal cortex (IPS, IPL, and preCuneus) and dACC (Supplementary Fig. 3). The convergence of the executive processes of WM and switching in dorsal parietal cortex may be attributable to common attentional mechanisms between both processes (Chiu and Yantis 2009; Tamber-Rosenau et al. 2011). (A recent meta-analysis further dissociated switches of context/task-rules, responses, and visual stimuli [Kim et al. 2012]. This study found that context/task-rule switches preferentially engaged rostral PFC, while perceptual switches preferentially engaged areas in the caudal SFS and PPC. The caudal SFS and PPC regions are consistent with regions found presently that we have proposed to be involved in selective spatial attention. By contrast, the present study found limited engagement of the rostral PFC. These results suggest that context/task-rule switches preferentially activate regions anterior to regions subserving control over WM [see “WM: Dual-Selection++?” for more detailed discussion].). One notable commonality among all 3 of these meta-analyses is the presence of the dACC. There are a number of theories regarding the function of the dACC (Botvinick et al. 2001; Brown and Braver 2005; Carp et al. 2010; Alexander and Brown 2011; Grinband et al. 2011; Nee et al. 2011; Silvetti et al. 2011). Although it is outside the scope of this article to discuss them here, it is worth noting that many of the activations described here were not overtly response related. This suggests that the dACC performs a more general function than detecting response conflict (cf. Botvinick et al. 2001) and is recruited under a diverse set of executive demands (Duncan and Owen 2000; Dosenbach et al. 2008).
WM: Dual Selection++?
Of the myriad results documented here, 2 of the most prominent were the presence of caudal SFS in spatial selection and mid-lateral PFC in postretrieval selection. Reviews of earlier neuroimaging literature suggested similar regions as sources of maintenance in spatial WM and nonspatial WM (Smith and Jonides 1999; Curtis and D'Esposito 2003; Courtney 2004; Linden 2007). From the present study, it is unclear whether the regions found here are the same as the maintenance regions or just anterior to them (Courtney et al. 1998). However, the present results suggest that there does not appear to be a clear “central” executive. Instead, there appear to be 2 distinct selection mechanisms.
Dual selection hypotheses of frontal organization have frequently been proposed with regard to maintenance (Levy and Goldman-Rakic 2000; Mottaghy et al. 2002; Curtis and D'Esposito 2003; Courtney 2004; Sala and Courtney 2007). In these frameworks, dorsal PFC regions are thought to provide top-down biasing on the parietal “where” stream, whereas ventral PFC regions are thought to provide top-down biasing on the temporal “what” stream (Ungerleider and Mishkin 1982). Whereas there is some consensus for these proposals with regard to maintenance, there has been less evidence in favor of a similar organization of executive functions. Our data suggest, when considering only experiments with event-related designs and well-matched control conditions, executive processes of WM also appear to follow a dual selection principle. The strong association between the caudal SFS and spatial selection matches well with “where”-based selection. That both verbal and object content produced significant convergence in the left mid-lateral PFC, but spatial content did not, fits with “what” principles. Notably, the left mid-lateral PFC was also associated with postretrieval selection more generally, which applied to spatial content as well as verbal and object content. It may be the case that selection amidst competition in spatial WM requires a combination of “what”-based (“what” to select) and “where”-based (the actual spatial selection) mechanisms.
These results lead to the question of whether there is anything “special” with regard to executive control in WM. A burgeoning number of studies, putative functions, and terms would suggest there is. However, the present data suggest that both maintenance and executive control boil down to the act of selecting relevant representations. Selection serves to maintain information, protect it from irrelevant information, and update new information. Hence, much of the work of WM can be accomplished through a simple selection mechanism. A biased-competition model of selection can then simultaneously explain storage and executive aspects of WM (Desimone and Duncan 1995; Miller and Cohen 2001).
Some authors have proposed that the mid-DLPFC may be the locus of amodal executive processes of WM (Petrides 2000; Miller and Cohen 2001; Curtis and D'Esposito 2003; Mottaghy 2006). Others have suggested that instead, mid-DLPFC represents a different form of content: namely, contexts or rules that provide constraints for processing in more posterior PFC regions (Courtney 2004; Courtney et al. 2007). Mechanistically, both processes are quite similar. Under both frameworks, the mid-DLPFC is a source of top-down bias on posterior PFC regions involved in maintenance or perhaps selection more generally. The difference between these proposals is whether “activation” in the mid-DLPFC is related to a function (i.e., amodal executive) or a representation (i.e., a context or rule). By the representation account, the functional aspects of mid-DLPFC are embodied not in activation per se but in its connectivity (Courtney et al. 2007). There is some tentative support for the representational account. Sakai et al. (2002) examined activition in the mid-DLPFC, as well as the caudal SFS and IPS during a spatial WM task with distraction. They found that activation in the mid-DLPFC was sustained during correct but not error trials and that sustained activition in the mid-DLPFC predicted greater coupling between the caudal SFS and IPS. The authors interpreted this as evidence that the mid-DLPFC promotes distractor-resistant WM by mediating caudal PFC–parietal interactions. In a subsequent study, Rowe et al. (2007) examined fMRI activations in patients with damage to the mid-DLPFC and healthy controls during spatial and verbal WM tasks. Whereas both patients and healthy controls demonstrated normal activations in content-selective PFC and posterior regions during each WM task, patients showed reduced functional connectivity between task-related regions. As a result, these authors concluded that the mid-DLPFC is necessary to establish a cognitive set and does so by supporting connectivity between content-selective caudal PFC and posterior regions. Taken together, these data support the proposal that activation in the mid-DLPFC is related to the representation of context or rule information which, in turn, biases caudal PFC regions through connectivity.
Activations in the mid-DLPFC were largely absent in the data reviewed here, although there were some small mid-DLPFC clusters evident in the right hemisphere. The relative absence of mid-DLPFC activations may be due to the selection criteria that required that all contrasts include a high-level control condition. Hence, both experimental and control conditions may have been well matched for demands on context or rule representation and resulting contrasts subtracted this information out. Notable exceptions were the results from shifting. Whereas lateral PFC activations involved in shifting were not consistent enough in locale to produce a significant ALE result, nevertheless two-thirds of the experiments on shifting reported activation in left mid-DLPFC, BA 46. As we suggested above, in shifting studies that used the refresh task, the cue to refresh may have required greater retrieval of cue-to-task information. Hence, activation in the left mid-DLPFC for shifting may reflect the representation of higher level content. Another possibility is that shifting attention in WM requires a reorganization of the content of WM. Whereas functions such as updating and interference resolution can be accomplished by simple selection, shifting requires organizing WM such that one representation is highlighted over-and-above others. To accomplish this organization, the left mid-DLPFC may select the focus of attention while caudal PFC regions select and maintain more backgrounded representations. As a result, shifting the focus of attention in WM may require restructuring WM content in a way that is dissociable from other executive WM functions. Distinguishing between these possibilities would be an interesting avenue for future research.
Taken together, the preceding discussion suggests that WM might be characterized as a modified dual selection model. At the core, WM may consist of “what” and “where” selection mechanisms localized in the mid-lateral PFC and caudal SFS, respectively. These regions may represent attentional priority in order to accomplish selection. Anterior to these regions, mid-DLPFC, BA 46 may represent contextual or rule information that promotes selection in caudal PFC regions. These ideas are consistent with recent models of the hierarchical organization of the PFC (Koechlin et al. 2003; Koechlin and Jubault 2006; Badre and D'Esposito 2007, 2009; Badre 2008). One deviation is that hierarchical PFC models have not explicitly organized caudal PFC regions by type of content. Our data suggest that this is a critical distinction (see also O'Reilly 2010). Finally, hierarchical models of PFC organization have demonstrated that areas anterior to the mid-DLPFC in BA 10 represent even higher level content such as overarching goals. We did find some evidence of convergence in ventral portions of BA 10. However, only 4 experiments contributed to this result, making it difficult to investigate systematically. Again, such higher level goals may have been subtracted out in most contrasts included here. Whereas many of these ideas need further testing, we tentatively propose that WM can be characterized as dual selection + contexts/rules + goals (Dual Selection++).
The present study suggests a number of areas for future research. One such avenue is precise characterization of rostral PFC regions. A question that follows from the present data is whether rostral PFC regions also have a dorsal/ventral axis. It seems possible that higher level content may also be distinguished in more semantic (e.g., what to do) and spatial/action-based (e.g., how to do it) ways. Investigating such potential distinctions in rostral PFC regions would help to further illuminate the organization of the PFC. Another area needing future research is how to characterize activations in the IFS. It seems likely that authors have inconsistently attributed activations in the IFS to either the DLPFC or VLPFC. It may be possible that the IFS is a region distinct from both. Given its placement, the IFS may form a zone where dorsal and ventral information converge and form bound representations. Future research that carefully distinguishes activations in the lateral PFC will be needed to appropriately characterize the role of the IFS.
Relationship to Behavior
Much of the inspiration for positing multiple dissociable executive processes has come from behavioral evidence. Miyake et al. (2000) performed latent variable analysis on a variety of behavioral executive tasks to determine whether distinct factors could be dissociated. Their results suggested that updating, shifting, and inhibition could be distinguished (see also Friedman et al. 2006). A follow-up study further dissociated inhibition into distractor response inhibition (i.e., distractor resistance) and resistance to proactive interference (i.e., intrusion resistance; Friedman and Miyake 2004). Together, these studies provided behavioral evidence for the 4 distinct functions examined presently. Of note, however, is that the tasks studied by Miyake et al. (2000) differed in content. Shifting tasks involved switching between task-rules, updating tasks involved WM, and distractor resistance tasks involved control over responses. Furthermore, distractor-response inhibition and intrusion resistance may have been distinguished due to the fact that the former relied primarily on tasks requiring selective spatial attention (i.e., “where”-based) while the latter relied entirely on verbal tasks (i.e., “what”-based; Friedman and Miyake 2004). Hence, it is unclear whether the factors that were distinguished differed in type of control or type of representation being controlled. Our framework suggests the latter.
Is there evidence that behavioral measures correlate or dissociate when measured within content-type in WM? Our framework suggests that 2 tasks that share a common selection network should show correlated behavioral factors while 2 tasks that rely on distinct selection networks should show independent behavioral factors. To our knowledge, there has been little systematic investigation of this issue. Many of the tasks that were studied were designed specifically to assess neural processes and did not include meaningful behavioral metrics of the psychological process of interest. However, in our own research, we have found modest behavioral correlations between 2 different intrusion resistance tasks that were performed on verbal content (r = 0.39, P = 0.06; Nee et al. 2007b). This behavioral relationship would be expected based on neural overlap between both tasks in left mid-lateral PFC. In another study, we contrasted neural correlates of distractor resistance and updating (Nee and Jonides 2009). Whereas both functions were measured using verbal material (words), distractor resistance processes require selective spatial attention (“where”-based), while updating processes required item-based selection (“what”-based). Neurally, we found that distractor resistance loaded more strongly on dorsal “where”-based networks while updating loaded more strongly on lateral “what”-based networks. That study included 2 different behavioral markers of distractor resistance (“where”-based), a behavioral marker of updating (“what”-based) and a behavioral marker of intrusion resistance (also “what”-based). Although we did not report correlations due to low sample size (n = 18), data trends were consistent with our framework. Matched measures of distractor resistance and updating did not correlate (r = −0.01, P > 0.9) nor did matched measures of distractor resistance and intrusion resistance (r = −0.05, P > 0.8). By contrast, a nonsignificant positive relationship was found between 2 measures of distractor resistance (r = 0.29, P = 0.24), and nonsignificant negative relationship was found between updating and intrusion resistance (r = −0.33, P = 0.18). The latter is consistent with the idea that updating should proactively reduce intrusion resistance (Braver et al. 2007). While these data are by no means definitive, they do loosely support the idea of dissociable content-based selection networks.
Our framework suggests that when using behavioral factors to assess executive processes of WM, care should be taken to separately characterize tasks that require spatial selective attention processes (“where”-based), from those that require identity processes (“what”-based), as well as those that require higher level processes (“task” or “rule”-based). We propose that each type of process is instantiated by the representation of separable priorities. “Where”-based selection relies on spatial/location priorities, “what”-based selection relies on item priorities, and “task”-based selection relies on rule priorities. Updating, shifting, and inhibition can therefore be accomplished by changing the appropriate priorities. Behavioral tasks that measure different content-based selection mechanisms are therefore predicted to demonstrate low correlations while those that measure the same content-based selection mechanisms should correlate well. Hence, assessments of WM should take these dissociable selection networks into consideration.
Limitations
Whereas fMRI has strong spatial resolution, it has limited resolution in the temporal domain. Therefore, it is important to consider the limitations of our fMRI-based approach. We have argued for the presence of dissociable content-based networks based upon fMRI localization. Localization, however, is merely the first step in understanding the mechanistic underpinnings of WM and cognition more generally. More detailed analysis of temporal dynamics and network coherence will certainly be needed to characterize and model the executive processes of WM. It is possible that different putative executive functions are characterized by dissociable temporal dynamics within the spatial networks defined here. Such differences cannot be penetrated by the present approach and will be informed by EEG, local field potentials, and single-unit recordings. As a result, the Dual Selection++ model of WM will need further testing using a variety of methodologies.
Funding
Supported in part by R01 DA026457 (to J.W.B.) and the Intelligence Advanced Research Projects Activity (IARPA) via Department of the Interior (DOI) contract number D10PC20023. This research was also supported in part by the National Science Foundation under Grant BCS 0822748 (to J.J.) and in part by grant MH60655 from National Institute of Mental Health (to J.J.).
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Acknowledgments
The U.S. Government is authorized to reproduce and distribute reprints for Governmental purposes notwithstanding any copyright annotation thereon. The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of IARPA, DOI, or the U.S. Government. Conflict of Interest : None declared.
References
- Alexander WH, Brown JW. Medial prefrontal cortex as an action-outcome predictor. Nat Neurosci. 2011;14:1338–1344. doi: 10.1038/nn.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MC, Levy BJ. Suppressing unwanted memories. Curr Dir Psychol Sci. 2009;18:189–194. doi: 10.1177/0963721417689881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MC, Ochsner KN, Kuhl B, Cooper J, Robertson E, Gabrieli SW, Glover GH, Gabrieli JD. Neural systems underlying the suppression of unwanted memories. Science. 2004;303:232–235. doi: 10.1126/science.1089504. [DOI] [PubMed] [Google Scholar]
- Aron AR. The neural basis of inhibition in cognitive control. Neuroscientist. 2007;13:214–228. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Awh E, Jonides J. Overlapping mechanisms of attention and spatial working memory. Trends Cogn Sci. 2001;5:119–126. doi: 10.1016/s1364-6613(00)01593-x. [DOI] [PubMed] [Google Scholar]
- Awh E, Vogel EK, Oh SH. Interactions between attention and working memory. Neuroscience. 2006;139:201–208. doi: 10.1016/j.neuroscience.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch GJ. Working memory. In: Bower GH, editor. The psychology of learning and motivation. New York: Academic Press; 1974. pp. 47–89. [Google Scholar]
- Baddeley AD, Bressi S, Della Sala S, Logie R, Spinnler H. The decline of working memory in Alzheimer's disease. A longitudinal study. Brain. 1991;114(Pt 6):2521–2542. doi: 10.1093/brain/114.6.2521. [DOI] [PubMed] [Google Scholar]
- Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends Cogn Sci. 2008;12:193–200. doi: 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Badre D, D'Esposito M. Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. J Cogn Neurosci. 2007;19:2082–2099. doi: 10.1162/jocn.2007.19.12.2082. [DOI] [PubMed] [Google Scholar]
- Badre D, D'Esposito M. Is the rostro-caudal axis of the frontal lobe hierarchical? Nat Rev Neurosci. 2009;10:659–669. doi: 10.1038/nrn2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Barch DM. The cognitive neuroscience of schizophrenia. Annu Rev Clin Psychol. 2005;1:321–353. doi: 10.1146/annurev.clinpsy.1.102803.143959. [DOI] [PubMed] [Google Scholar]
- Barde LH, Thompson-Schill SL. Models of functional organization of the lateral prefrontal cortex in verbal working memory: evidence in favor of the process model. J Cogn Neurosci. 2002;14:1054–1063. doi: 10.1162/089892902320474508. [DOI] [PubMed] [Google Scholar]
- Bledowski C, Rahm B, Rowe JB. What “works” in working memory? Separate systems for selection and updating of critical information. J Neurosci. 2009;29:13735–13741. doi: 10.1523/JNEUROSCI.2547-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledowski C, Kaiser J, Rahm B. Basic operations in working memory: contributions from functional imaging studies. Behav Brain Res. 2010;214:172–179. doi: 10.1016/j.bbr.2010.05.041. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Brass M, Derrfuss J, Forstmann B, von Cramon DY. The role of the inferior frontal junction area in cognitive control. Trends Cogn Sci. 2005;9:314–316. doi: 10.1016/j.tics.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Braver TS, Gray JR, Burgess GC. Explaining the many varieties of working memory variation: dual mechanisms of cognitive control. In: Conway A, Jarrold C, Kane M, Miyake A, Towse J, editors. Variation in working memory. New York: Oxford University Press; 2007. pp. 76–106. [Google Scholar]
- Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307:1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, D'Esposito M. The search for the phonological store: from loop to convolution. J Cogn Neurosci. 2008;20:762–778. doi: 10.1162/jocn.2008.20501. [DOI] [PubMed] [Google Scholar]
- Carp J, Kim K, Taylor SF, Fitzgerald KD, Weissman DH. Conditional differences in mean reaction time explain effects of response congruency, but not accuracy, on posterior medial frontal cortex activity. Front Hum Neurosci. 2010;4:231. doi: 10.3389/fnhum.2010.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter PA, Just MA, Shell P. What one intelligence test measures: a theoretical account of the processing in the Raven Progressive Matrices Test. Psychol Rev. 1990;97:404–431. [PubMed] [Google Scholar]
- Chiu YC, Yantis S. A domain-independent source of cognitive control for task sets: shifting spatial attention and switching categorization rules. J Neurosci. 2009;29:3930–3938. doi: 10.1523/JNEUROSCI.5737-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collette F, Hogge M, Salmon E, Van der Linden M. Exploration of the neural substrates of executive functioning by functional neuroimaging. Neuroscience. 2006;139:209–221. doi: 10.1016/j.neuroscience.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Collette F, Van der Linden M, Laureys S, Delfiore G, Degueldre C, Luxen A, Salmon E. Exploring the unity and diversity of the neural substrates of executive functioning. Hum Brain Mapp. 2005;25:409–423. doi: 10.1002/hbm.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Courtney SM. Attention and cognitive control as emergent properties of information representation in working memory. Cogn Affect Behav Neurosci. 2004;4:501–516. doi: 10.3758/cabn.4.4.501. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Roth JK, Sala JB. A hierarchical biased-competition model of domain-dependent working memory maintenance and executive control. In: Osaka N, Logie R, D'Esposito M, editors. The cognitive neuroscience of working memory. Oxford: Oxford University Press; 2007. p. 369–383. [Google Scholar]
- Courtney SM, Petit L, Maisog JM, Ungerleider LG, Haxby JV. An area specialized for spatial working memory in human frontal cortex. Science. 1998;279:1347–1351. doi: 10.1126/science.279.5355.1347. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D'Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci. 2003;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: an event-related fMRI study. Brain Cogn. 1999;41:66–86. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Brain Res Cogn Brain Res. 1998;7:1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- Daneman M, Carpenter PA. Individual differences in working memory and reading. J Verb Learn Verb Behav. 1980;19:450–466. [Google Scholar]
- Daneman M, Merikle PM. Working memory and language comprehension: a meta-analysis. Psychon Bull Rev. 1996;3:422–433. doi: 10.3758/BF03214546. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Loughry B, O'Reilly RC. Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cogn Affect Behav Neurosci. 2001;1:137–160. doi: 10.3758/cabn.1.2.137. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A. The relations among inhibition and interference control functions: a latent-variable analysis. J Exp Psychol Gen. 2004;133:101–135. doi: 10.1037/0096-3445.133.1.101. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Corley RP, Young SE, Defries JC, Hewitt JK. Not all executive functions are related to intelligence. Psychol Sci. 2006;17:172–179. doi: 10.1111/j.1467-9280.2006.01681.x. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Rissman J, Cooney J, Rutman A, Seibert T, Clapp W, D'Esposito M. Functional interactions between prefrontal and visual association cortex contribute to top-down modulation of visual processing. Cereb Cortex. 2007;17(Suppl 1):i125–i135. doi: 10.1093/cercor/bhm113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinband J, Savitskaya J, Wager TD, Teichert T, Ferrera VP, Hirsch J. The dorsal medial frontal cortex is sensitive to time on task, not response conflict or error likelihood. Neuroimage. 2011;57:303–311. doi: 10.1016/j.neuroimage.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ. Improving fluid intelligence with training on working memory. Proc Natl Acad Sci U S A. 2008;105:6829–6833. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, Raye CL, Mitchell KJ, Greene EJ, Anderson AW. FMRI evidence for an organization of prefrontal cortex by both type of process and type of information. Cereb Cortex. 2003;13:265–273. doi: 10.1093/cercor/13.3.265. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Raye CL, Mitchell KJ, Greene EJ, Cunningham W, Sanislow CA. Using fMRI to investigate a component process of reflection: prefrontal correlates of refreshing a just-activated representation. Cogn Affect Behav Neurosci. 2005;5:339–361. doi: 10.3758/cabn.5.3.339. [DOI] [PubMed] [Google Scholar]
- Johnson MR, Johnson MK. Toward characterizing the neural correlates of component processes of cognition. In: Roesler F, Ranganath C, Roeder B, Kluwe RH, editors. Neuroimaging of human memory: linking cognitive processes to neural systems. New York: Oxford University Press; 2009. pp. 169–194. [Google Scholar]
- Jonides J, Nee DE. Brain mechanisms of proactive interference in working memory. Neuroscience. 2006;139:181–193. doi: 10.1016/j.neuroscience.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Jonides J, Lacey S, Nee D. Processes of working memory in mind and brain. Curr Dir Psychol Sci. 2005;14:2–5. [Google Scholar]
- Jonides J, Lewis RL, Nee DE, Lustig CA, Berman MG, Moore KS. The mind and brain of short-term memory. Annu Rev Psychol. 2008;59:193–224. doi: 10.1146/annurev.psych.59.103006.093615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Carpenter PA. A capacity theory of comprehension—individual-differences in working memory. Psychol Rev. 1992;99:122–149. doi: 10.1037/0033-295x.99.1.122. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Bleckley MK, Conway AR, Engle RW. A controlled-attention view of working-memory capacity. J Exp Psychol Gen. 2001;130:169–183. doi: 10.1037//0096-3445.130.2.169. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kim C, Cilles SE, Johnson NF, Gold BT. Domain general and domain preferential brain regions associated with different types of task switching: a meta-analysis. Hum Brain Mapp. 2012;33:130–142. doi: 10.1002/hbm.21199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Jubault T. Broca's area and the hierarchical organization of human behavior. Neuron. 2006;50:963–974. doi: 10.1016/j.neuron.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Leung HC, Zhang JX. Interference resolution in spatial working memory. Neuroimage. 2004;23:1013–1019. doi: 10.1016/j.neuroimage.2004.07.053. [DOI] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic PS. Segregation of working memory functions within the dorsolateral prefrontal cortex. Exp Brain Res. 2000;133:23–32. doi: 10.1007/s002210000397. [DOI] [PubMed] [Google Scholar]
- Linden DE. The working memory networks of the human brain. Neuroscientist. 2007;13:257–267. doi: 10.1177/1073858406298480. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Martinussen R, Hayden J, Hogg-Johnson S, Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:377–384. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Mohr HM, Goebel R, Linden DE. Content- and task-specific dissociations of frontal activity during maintenance and manipulation in visual working memory. J Neurosci. 2006;26:4465–4471. doi: 10.1523/JNEUROSCI.5232-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T. The neurobiology of visual attention: finding sources. Curr Opin Neurobiol. 2006;16:159–165. doi: 10.1016/j.conb.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Mottaghy FM. Interfering with working memory in humans. Neuroscience. 2006;139:85–90. doi: 10.1016/j.neuroscience.2005.05.037. [DOI] [PubMed] [Google Scholar]
- Mottaghy FM, Gangitano M, Sparing R, Krause BJ, Pascual-Leone A. Segregation of areas related to visual working memory in the prefrontal cortex revealed by rTMS. Cereb Cortex. 2002;12:369–375. doi: 10.1093/cercor/12.4.369. [DOI] [PubMed] [Google Scholar]
- Nee DE, Jonides J. Dissociable interference-control processes in perception and memory. Psychol Sci. 2008a;19:490–500. doi: 10.1111/j.1467-9280.2008.02114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, Jonides J. Neural correlates of access to short-term memory. Proc Natl Acad Sci U S A. 2008b;105:14228–14233. doi: 10.1073/pnas.0802081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, Jonides J. Common and distinct neural correlates of perceptual and memorial selection. Neuroimage. 2009;45:963–975. doi: 10.1016/j.neuroimage.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, Jonides J. Dissociable contributions of prefrontal cortex and the hippocampus to short-term memory: evidence for a 3-state model of memory. Neuroimage. 2011;54:1540–1548. doi: 10.1016/j.neuroimage.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci. 2007;7:1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- Nee DE, Jonides J, Berman MG. Neural mechanisms of proactive interference-resolution. Neuroimage. 2007;38:740–751. doi: 10.1016/j.neuroimage.2007.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, Kastner S, Brown JW. Functional heterogeneity of conflict, error, task-switching, and unexpectedness effects within medial prefrontal cortex. Neuroimage. 2011;54:528–540. doi: 10.1016/j.neuroimage.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JK, Reuter-Lorenz PA, Persson J, Sylvester CY, Jonides J. Mapping interference resolution across task domains: a shared control process in left inferior frontal gyrus. Brain Res. 2009;1256:92–100. doi: 10.1016/j.brainres.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Coull JT, Maquet P, Frith CD, Vandenberghe R, Mesulam MM. Orienting attention to locations in perceptual versus mental representations. J Cogn Neurosci. 2004;16:363–373. doi: 10.1162/089892904322926700. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC. The What and How of prefrontal cortical organization. Trends Neurosci. 2010;33:355–361. doi: 10.1016/j.tins.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly RC, Frank MJ. Making working memory work: a computational model of learning in the prefrontal cortex and basal ganglia. Neural Comput. 2006;18:283–328. doi: 10.1162/089976606775093909. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RS. The neural correlates of the verbal component of working memory. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Petrides M. The role of the mid-dorsolateral prefrontal cortex in working memory. Exp Brain Res. 2000;133:44–54. doi: 10.1007/s002210000399. [DOI] [PubMed] [Google Scholar]
- Pochon JB, Levy R, Poline JB, Crozier S, Lehericy S, Pillon B, Deweer B, Le Bihan D, Dubois B. The role of dorsolateral prefrontal cortex in the preparation of forthcoming actions: an fMRI study. Cereb Cortex. 2001;11:260–266. doi: 10.1093/cercor/11.3.260. [DOI] [PubMed] [Google Scholar]
- Postle BR. Working memory as an emergent property of the mind and brain. Neuroscience. 2006;139:23–38. doi: 10.1016/j.neuroscience.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, Berger JS, D'Esposito M. Functional neuroanatomical double dissociation of mnemonic and executive control processes contributing to working memory performance. Proc Natl Acad Sci U S A. 1999;96:12959–12964. doi: 10.1073/pnas.96.22.12959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rama P, Poremba A, Sala JB, Yee L, Malloy M, Mishkin M, Courtney SM. Dissociable functional cortical topographies for working memory maintenance of voice identity and location. Cereb Cortex. 2004;14:768–780. doi: 10.1093/cercor/bhh037. [DOI] [PubMed] [Google Scholar]
- Repovs G, Baddeley A. The multi-component model of working memory: explorations in experimental cognitive psychology. Neuroscience. 2006;139:5–21. doi: 10.1016/j.neuroscience.2005.12.061. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Annu Rev Neurosci. 2004;27:611–647. doi: 10.1146/annurev.neuro.26.041002.131039. [DOI] [PubMed] [Google Scholar]
- Roth JK, Serences JT, Courtney SM. Neural system for controlling the contents of object working memory in humans. Cereb Cortex. 2006;16:1595–1603. doi: 10.1093/cercor/bhj096. [DOI] [PubMed] [Google Scholar]
- Roth JK, Johnson MK, Raye CL, Constable RT. Similar and dissociable mechanisms for attention to internal versus external information. Neuroimage. 2009;48:601–608. doi: 10.1016/j.neuroimage.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RS, Passingham RE. The prefrontal cortex: response selection or maintenance within working memory? Science. 2000;288:1656–1660. doi: 10.1126/science.288.5471.1656. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Sakai K, Lund TE, Ramsoy T, Christensen MS, Baare WF, Paulson OB, Passingham RE. Is the prefrontal cortex necessary for establishing cognitive sets? J Neurosci. 2007;27:13303–13310. doi: 10.1523/JNEUROSCI.2349-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Passingham RE. Prefrontal interactions reflect future task operations. Nat Neurosci. 2003;6:75–81. doi: 10.1038/nn987. [DOI] [PubMed] [Google Scholar]
- Sakai K, Passingham RE. Prefrontal set activity predicts rule-specific neural processing during subsequent cognitive performance. J Neurosci. 2006;26:1211–1218. doi: 10.1523/JNEUROSCI.3887-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Rowe JB, Passingham RE. Active maintenance in prefrontal area 46 creates distractor-resistant memory. Nat Neurosci. 2002;5:479–484. doi: 10.1038/nn846. [DOI] [PubMed] [Google Scholar]
- Sala JB, Courtney SM. Binding of what and where during working memory maintenance. Cortex. 2007;43:5–21. doi: 10.1016/s0010-9452(08)70442-8. [DOI] [PubMed] [Google Scholar]
- Silvetti M, Seurinck R, Verguts T. Value and prediction error in medial frontal cortex: integrating the single-unit and systems levels of analysis. Front Hum Neurosci. 2011;5:75. doi: 10.3389/fnhum.2011.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Neuroimaging analyses of human working memory. Proc Natl Acad Sci U S A. 1998;95:12061–12068. doi: 10.1073/pnas.95.20.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Marshuetz C, Koeppe RA. Components of verbal working memory: evidence from neuroimaging. Proc Natl Acad Sci U S A. 1998;95:876–882. doi: 10.1073/pnas.95.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamber-Rosenau BJ, Esterman M, Chiu YC, Yantis S. Cortical mechanisms of cognitive control for shifting attention in vision and working memory. J Cogn Neurosci. 2011;23:2905–2919. doi: 10.1162/jocn.2011.21608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tark KJ, Curtis CE. Persistent neural activity in the human frontal cortex when maintaining space that is off the map. Nat Neurosci. 2009;12:1463–1468. doi: 10.1038/nn.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum Brain Mapp. 2012;33:1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of visual behavior. Cambridge (MA): MIT Press; 1982. pp. 549–586. [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage. 2004;22:1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Wager TD, Sylvester CY, Lacey SC, Nee DE, Franklin M, Jonides J. Common and unique components of response inhibition revealed by fMRI. Neuroimage. 2005;27:323–340. doi: 10.1016/j.neuroimage.2005.01.054. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Maril A, Bjork RA, Schacter DL. Prefrontal contributions to executive control: fMRI evidence for functional distinctions within lateral prefrontal cortex. Neuroimage. 2001;14:1337–1347. doi: 10.1006/nimg.2001.0936. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Yantis S, Serences JT. Cortical mechanisms of space-based and object-based attentional control. Curr Opin Neurobiol. 2003;13:187–193. doi: 10.1016/s0959-4388(03)00033-3. [DOI] [PubMed] [Google Scholar]
- Zanto TP, Rubens MT, Bollinger J, Gazzaley A. Top-down modulation of visual feature processing: the role of the inferior frontal junction. Neuroimage. 2010;53:736–745. doi: 10.1016/j.neuroimage.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Rubens MT, Thangavel A, Gazzaley A. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nat Neurosci. 2011;14:656–661. doi: 10.1038/nn.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JX, Feng CM, Fox PT, Gao JH, Tan LH. Is left inferior frontal gyrus a general mechanism for selection? Neuroimage. 2004;23:596–603. doi: 10.1016/j.neuroimage.2004.06.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.