Abstract
Multiple lines of evidence support a role for curcumin in cancer chemoprevention. Nonetheless, despite its reported efficacy and safety profile, clinical translation of curcumin has been hampered by low oral bioavailability, requiring infeasible ‘mega’ doses for achieving detectable tissue levels. We have engineered a polymeric nanoparticle encapsulated formulation of curcumin (NanoCurc) to harness its full therapeutic potential. In the current study, we assessed the chemoprevention efficacy of NanoCurc administered via direct intraductal (i.duc) injection in a chemical carcinogen-induced rodent mammary cancer model. Specifically, Sprague–Dawley rats exposed to systemic N-methyl-N-nitrosourea were randomized to receive either oral free curcumin at a previously reported ‘mega’ dose (200mg/kg) or by direct i.duc injection of free curcumin or NanoCurc, respectively, each delivering 168 µg equivalent of curcumin per rodent teat (a ~20-fold lower dose per animal compared to oral administration). All three chemoprevention modalities resulted in significantly lower mammary tumor incidence compared with control rats; however, there was no significant difference in cancer incidence between the oral dosing and either i.duc arms. On the other hand, mean tumor size, was significantly smaller in the i.duc NanoCurc cohort compared with i.duc free curcumin (P < 0.0001), suggesting the possibility of better resectability for ‘breakthrough’ cancers. Reduction in cancer incidence was associated with significant decrease in nuclear factor -κB activation in the NanoCurc treated mammary epithelium explants, compared to either control or oral curcumin-administered rats. Our studies confirm the potential for i.duc NanoCurc as an alternative to the oral route for breast cancer chemoprevention in high-risk cohorts.
Introduction
Due to advances in our understanding of the genetic basis of breast cancer, ‘at-risk’ populations for this disease have now been identified, and the most significant of which are kindred harboring germline mutations in the breast/ovarian cancer genes BRCA1 and BRCA2 (1). Although these patients may not harbor a discrete neoplasm at the time of presentation, their lifetime risk for invasive cancer mandates aggressive intervention (2). Thus, incipient breast cancer prevention in high-risk populations has emerged as a challenge for these times. Few options for women at high risk of breast cancer are currently available for breast cancer prevention and may include bilateral prophylactic mastectomy, bilateral salpingo-oophorectomy and selective estrogen-response modulators such as tamoxifen or raloxifene. Unfortunately, bilateral prophylactic mastectomy and bilateral salpingo-oophorectomy can negatively impact on body image and sexuality, and selective estrogen-response modulators are associated with frequent bothersome side effects and a few rare, but potentially, life-threatening risks such as endometrial cancer or pulmonary embolism.
Since the majority of breast cancers originate in the epithelial cells lining the duct, we hypothesized that administration of agents directly into the breast ductal system (i.e. intraductal or i.duc) may result in eradication of premalignant disease and prevention of the development of invasive cancers. We have previously demonstrated in preclinical rodent models of chemical carcinogenesis that the i.duc administration of 4-hydroxytamoxifen and pegylated liposomal doxorubicin (Doxil®) is associated with a significant reduction in mammary tumor incidence, as well as growth inhibition of established tumors (3). Most recently, our group has completed the first clinical trial of i.duc pegylated liposomal doxorubicin in a small cohort of breast cancer patients, demonstrating the feasibility of the i.duc route in humans, as well as expanding the repertoire of chemotherapeutic agents that can be delivered via this route in the preclinical setting (4). Nonetheless, many of these agents have the potential for incidental adverse effects (including cutaneous toxicity at the injection site in the case of pegylated liposomal doxorubicin), mandating the continued search for safer compounds for chemoprevention of breast cancer.
Curcumin (diferuloyl methane) is a natural yellow-pigmented polyphenol extracted from the rhizome of turmeric (Curcuma longa), a plant indigenous to Southeast Asia (5). Curcumin has been used as an anti-inflammatory agent in traditional Indian Ayurvedic medicine for centuries (6). A large volume of published reports (numbering in several 100s) has established the anticancer and chemopreventive properties of curcumin (7,8). Systemic curcumin demonstrates potent antitumor effects against breast cancer, including inhibition of metastases in preclinical models (9–12). Equally important, free curcumin has been shown not to be cytotoxic to normal cells, including hepatocytes, mammary epithelial cells, kidney epithelial cells, lymphocytes and fibroblasts at the dosages required for therapeutic efficacy against cancer cell lines (13–15) ; these in vitro findings are underscored by the limited human clinical trials performed with oral curcumin, wherein doses up to 12g/day have had minimal adverse effects, even to the highly exposed gastrointestinal mucosa (16–19).
Despite the considerable promise that curcumin holds as an efficacious and safe compound for cancer therapy and chemoprevention, clinical usefulness of curcumin is diminished by its poor absorption, rapid metabolism and rapid systemic elimination, thus resulting in low systemic exposure (<1%) (20,21). For example, in a Phase I clinical trial, patients were required to partake 8g of free curcumin orally per day, in order to achieve detectable systemic levels; beyond 8g, the bulky volume of the drug was unacceptable to patients (19). As recently reviewed by us and others, oral consumption at clinically feasible doses may not furnish adequate tissue levels of curcumin necessary for effective cancer prevention and treatment (20,21). We have circumvented the pitfalls of poor aqueous solubility of curcumin by synthesizing a polymeric nanoparticle-encapsulated formulation of curcumin (NanoCurc), which demonstrates remarkably higher systemic bioavailability in plasma and tissues (including the brain) compared with free curcumin upon parenteral administration (22). For example, we recently reported that NanoCurc blocked tumor growth and metastases in orthotopic xenograft models of human pancreatic cancer, which was further accentuated upon combination with gemcitabine (22). These studies from our group, however, have mainly focused on established tumor settings, principally xenografts. The objective of the current study were 2-fold: (i) to evaluate the role of NanoCurc in a bona fide cancer chemoprevention model in the autochthonous organ, as exemplified by the N-methyl-N-nitrosourea (MNU)-induced chemical carcinogenesis model of rat mammary carcinoma (23,24); and (ii) to establish the i.duc route of administration as a feasible alternative for delivery of NanoCurc to the mammary epithelium, since this formulation is not amenable to oral delivery. We compared i.duc NanoCurc with two alternative prevention modalities: the first was oral free curcumin administered at previously reported ‘mega’ doses (200mg/kg) given orally and the second was i.duc free curcumin, with an equivalent curcumin amount administered per rodent teat as NanoCurc (both at ~20-fold lower dose compared with the oral free curcumin cohort). Our studies confirm that i.duc administration of curcumin, either as a free compound or as a nanoparticle encapsulated, attenuates mammary tumor incidence following MNU exposure at levels comparable with ‘mega’ oral dosing of free curcumin. In addition, however, NanoCurc results in significantly smaller tumor size for ‘breakthrough’ tumors (the bête noire of chemoprevention regimens), suggesting a greater potential for successful resection and cure than i.duc free curcumin. We propose that the combination of two novel approaches to improve the access of curcumin to carcinogen-exposed mammary epithelium—i.duc administration and nanoencapsulation—results in greater efficacy in breast cancer prevention and could be a promising avenue to pursue in at-risk populations.
Materials and methods
Animals
Female Sprague–Dawley rats, 3–4 weeks of age, were purchased from Harlan (Frederick, MD) and housed in a controlled environment with 12h light/dark cycle for at least 7 days before undergoing experimental procedures. The rats received a single intraperitoneal injection of 50mg/kg body weight of MNU at 5–6 weeks (3,4). Rats were allowed free access to standard laboratory food and water ad libitum. All animal experiments were performed with approval of the Animal Care and Use Committee of Johns Hopkins University School of Medicine.
Reagents for NanoCurc synthesis
Ultrapure curcumin (>99% diferuloylmethane) was purchased from Sabinsa Corporation; this source of curcumin had been used for both preclinical and clinical studies in the past (22,25,26). Monomers for NanoCurc synthesis, specifically N-isopropylacrylamide, vinylpyrrolidone and acrylic acid, were obtained from Sigma-Aldrich. Reagents for the polymerization step, including N,N′ methylene-bis-acrylamide, ammonium persulfate and ferrous sulfate, were also procured from Sigma.
Synthesis of NanoCurc
Polymer nanoparticles composed of N-isopropylacrylamide, vinylpyrrolidone and acrylic acid were synthesized through a free radical reaction, according to the detailed synthesis method we previously described (22,25,26). Briefly, predistilled monomers of N-isopropylacrylamide, VP and AA are mixed together in a molar ratio of 60:20:20, respectively, hence the acronym ‘NVA622’ for the resulting polymer. Polymerization was done for 24h at 30°C under an inert (nitrogen) atmosphere, using ammonium persulfate and ferrous sulfate as initiator and activator, respectively. After complete polymerization, the total aqueous solution of polymer was purified using dialysis and then lyophilized for postloading of curcumin, as described. Typically, a 10ml stock solution of polymeric nanoparticles (100mg) was slowly mixed with 150 µl of curcumin solution in chloroform (10mg/ml) and gently stirred and evaporated chloroform simultaneously. The resulting solution, corresponding to 1.5% (w/w) loading of curcumin in nanoparticles, was then snap frozen on a dry ice/acetone bath and lyophilized. The lyophilized NanoCurc powder was stored at 4°C until further use, whereupon simple reconstitution in an aqueous phase was required before i.duc administration.
Intraductal (i.duc) injection and oral administration of curcumin
Rats were anesthetized by isoflurane/oxygen inhalation. Keratin plugs were removed from the surface of the teat by rubbing gently with gauze soaked with alcohol, revealing the duct orifice. Mammary ducts were cannulated using a 1.0cm, 31G, blunt-ended needle (Hamilton, 7748-17) attached to a 0.1ml tuberculin syringe (3,4). Drug (100 µ l/teat, corresponding to 168 µg equivalent of curcumin, either as free drug or as nano-encapsulated) was injected slowly into the mammary gland while visualizing the opening under a dissecting microscope. Free curcumin for i.duc injection was administered dissolved in corn oil, whereas NanoCurc, which is soluble in aqueous media, was dissolved in phosphate-buffered saline. Each rat received the equivalent of 168 µg/teat (or ~2mg curcumin per injection cycle) through the i.duc route to all 12 mammary glands. I.duc injection was initiated at 14 days post-MNU exposure and repeated 4 weeks after the first injection in ‘Prevention Study 1’ (i.e. total of two injection cycles per rat), and at 1 and 2 weeks after the first injection in ‘Prevention Study 2’ (i.e. total of three injection cycles per rat). Finally, in ‘Prevention Study 2’, one cohort was also administered oral curcumin (200mg/kg via oral gavage, or the equivalent of 30–40mg of free curcumin dissolved in corn oil) at 14 days post-MNU exposure and at weeks 1 and 2 thereafter.
Pharmacokinetics of curcumin formulations in plasma and mammary tissues
Curcumin concentrations in the plasma were estimated by liquid chromatography-tandem mass spectrometry over the calibration range of 10–1000ng/ml, as previously described (22,25,26). Blood was obtained at 2, 4, 8 and 24h following i.duc injection (168 µg equivalent of curcumin, either as free drug or as nano-encapsulated formulation × 12 teats = 2016 µg of total curcumin/rat) or a comparable systemic dose (2016 µg of free curcumin dissolved in corn oil/rat, through the intraperitoneal route); a single dose of free curcumin (200mg/kg) in corn oil was also administered via oral gavage to determine the pharmacokinetics of this commonly used route of delivery. For each of the four variables (i.duc NanoCurc, i.duc free curcumin, i.p. free curcumin and oral gavage), three non-tumor bearing rats were used per arm. Two additional studies were performed for the i.duc NanoCurc formulation only: first, curcumin levels were assessed in the mammary tissues at 1, 2, 4 and 8h following injection (three individual mammary glands were harvested for each time point); second, in order to assess for ‘spillover’ into the contralateral breast, curcumin levels were assessed in the ipsilateral (injected) versus contralateral (non-injected) mammary glands (six glands per location), at 1h following injection.
For pharmacokinetic data, concentrations that were below the limits of quantitation were assigned a value of 5ng/ml (i.e. 1/2 the lower limit of quantitation or 10ng/ml). Mean plasma concentrations at each sampling point were calculated for curcumin. Pharmacokinetic variables were calculated from mean curcumin concentration-time data using noncompartmental methods as implemented in WinNonlin Professional version 5.3 (Pharsight Corp., Mountain View, CA). C max and T max were the observed values from the mean data. The Area Under Curve AUClastwas calculated using the linear trapezoidal method. The method of Bailer (27) was used to estimate the variance of AUClast based on the variance of the mean concentration at each time point. To determine whether there was a significant difference between exposures as expressed by AUC, a pairwise comparison was done using a Z test.
Tumor measurement and histopathology
The rats were palpated for the presence of tumors, and tumor size and body weight were measured at weekly intervals. The largest diameter (a) and the shortest dimension (b) perpendicular to (a) of the tumor was measured once a week. The tumor mass was calculated using the following formula: tumor mass (mm3) = (a × b 2)/2, as previously described (3). Mammary glands were dissected and whole mounts and paraffin-embedded sections were prepared. For whole mounts, mammary glands were prepared as previously described (3). Generation of paraffin-embedded sections and hematoxylin and eosin staining was performed according to standard procedures by the Reference Histology Laboratory at Johns Hopkins Hospital.
Immunohistochemistry
Immunohistochemistry was performed on formalin-fixed paraffin embedded tissue from two rats (one mammary gland each), using previously described techniques (22). Briefly, the slides were deparaffinized using xylene and hydrated by a graded series of ethanol washes. Antigen retrieval was accomplished by heating the slides in citrate buffer (pH 6.0) at 90°C for 20min. Endogenous peroxidase activity was quenched by 10-min incubation in 3% H2O2 and non-specific binding was blocked with 10% fetal bovine serum solution (Invitrogen, Carlsbad, CA) before incubation with the primary antibody. Chromogenic detection was enabled using the PowerVision + Poly-HRP IHC kit (Immunovision Technologies, Norwell, MA), following the manufacturer’s protocol. Slides were counterstained with Harris-hematoxylin solution. Primary antibodies utilized were anti-pp65 (rabbit anti-human phospho-Ser276 p65 antibody, monoclonal, Cell Signaling Technology, Danvers, MA); anti-Ki-67 (rabbit monoclonal, Cell Marque, Rocklin, CA); anti-cyclin D1 (rabbit monoclonal, Cell Signaling); anti-phospho-histone H3 (rabbit monoclonal, Epitomics, Inc. Burlingame, CA); and anti-cleaved caspase 3 (rabbit monoclonal, Cell Signaling). Quantification of signal was performed by evaluating 10 random high power fields (×40 magnification) on each slide (n = 2) and counting the total number of cells with positive labeling. Only nuclear localization of chromogenic signal was counted as positive.
Statistical analysis
Data are expressed as the mean ± standard error of the mean. Tumor-free survivals were estimated using the Kaplan–Meier method. Hazard ratios were estimated along with 95% confidence intervals via the Cox proportional hazard model. For the analysis of the tumor-free survival in glands, the within-animal correlation was taken into account by adjusting the standard errors for clustering of measurements collected on the glands of the same animal. For comparison of tumor volume data among the group, a mixed effects model was used to analyze these data with repeated measures, where correlations due to observation from the same gland were taken into account in modeling by assuming an exchangeable covariance structure and glands were nested within an animal. For immunohistochemical analysis, Poisson regression was used to model the clustered count data with respect to various antigens, which was performed with the generalized estimating equations method by specifying the exchangeable correlation structure for the counts in the same cluster (i.e. 10 random high power fields on each slide). To account for multiple testing, a significant difference is considered at P < 0.0083 based on Bonferroni adjustment.
Results
Pharmacokinetics of intraductal curcumin
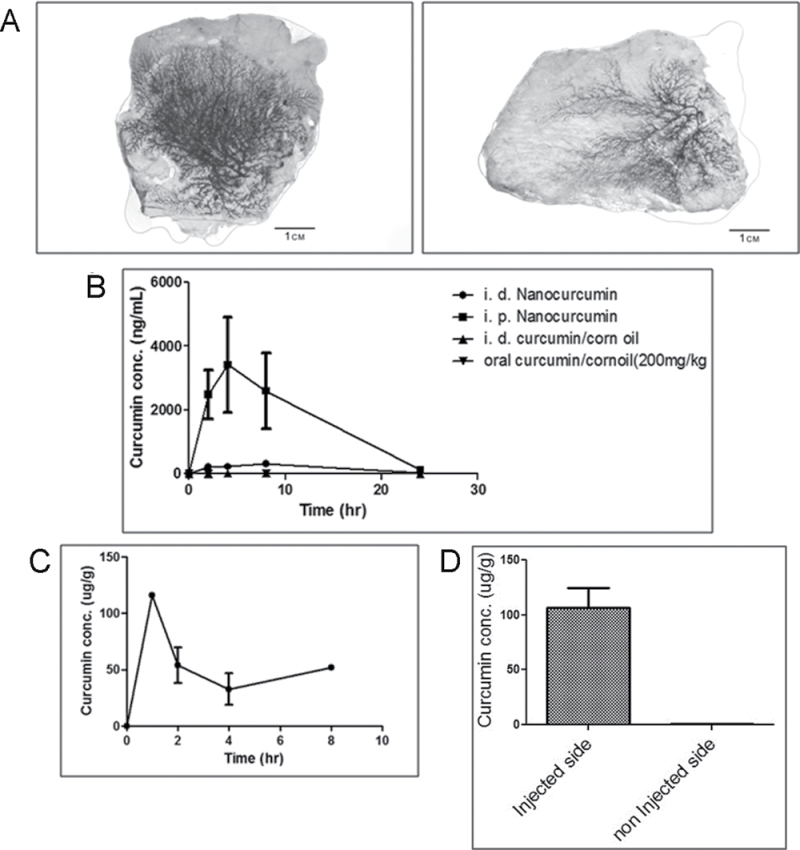
Our first goal was to determine if polymer nanoparticles administered through the i.duc route resulted in robust delivery of the encapsulated medicament to the entire mammary ductal tree. In order to visualize the extent of nanoparticle access, we encapsulated 0.5% crystal violet dye into the NVA622 nanoparticles and injected either nano-encapsulated or free dye through the i.duc route. As seen in Figure 1A, there was robust penetration by the nano-encapsulated crystal violet throughout the entire ductal tree, with no demonstrable evidence of spillage (panel on the left illustrates nanoparticle encapsulated dye and on the right free dye). We then assayed for plasma curcumin levels following intraductal versus systemic (intraperitoneal) injection of the agent. Specifically, we administered a single dose of i.duc NanoCurc or i.duc free curcumin (each at 168 µg/duct × 12 = 2016 µg), or the equivalent total amount as a single intraperitoneal injection of NanoCurc, in cohorts of three rats each. One cohort of rats received orally delivered free curcumin in corn oil (200mg/kg equivalent) as an additional control. Plasma drug levels peaked after 4h and were undetectable at 24h in all four cohorts (Figure 1B). The average (± standard deviation) C max following intraperitoneal injection, i.duc injection with NanoCurc, or i.duc injection with free curcumin was 923.0±431.4 (n = 3), 51.6±28.5 (n = 3) and 11.6±11.4 (n = 3; 2 undetectable) ng/ml, respectively. Total systemic exposure (AUClast) was reduced by ~2- to 4-fold when administered i.duc but was not significantly different due to the large variability noted following intraperitoneal administration (P > 0.05). These data support our prior observation that i.duc administration of therapeutics is associated with lower total and maximal systemic exposure, and hence, probably lower bystander adverse effects to visceral organs (3).
Fig. 1.
Distribution and pharmacokinetics of curcumin following i.duc injection. (A) The i.duc administration of 0.5% crystal violet, either encapsulated within NVA622 nanoparticles (left) or as free dye (right) results in robust distribution of dye throughout the mammary ductal tree in SD rats. Note that no demonstrable ‘spillover’ is observed outside the ductal tree. (B) To determine the pharmacokinetics of curcumin upon i.duc versus systemic injection, rats were administered i.duc NanoCurc (168 µg/duct × 12 ducts or 2016 µg/rat), i.duc free curcumin (168 µg/duct × 12 ducts or 2016 µg/rat), oral free curcumin (200mg/kg) in corn oil, or i.p. NanoCurc (2016 µg). Plasma was analyzed for curcumin concentration in blood samples taken 2, 4, 8 and 24h later by liquid chromatography-tandem mass spectrometry. The mean ± standard deviation is plotted for each time point with concentrations that were below the limits of quantitation (10ng/ml) as 5ng/mL. Compared with i.p. NanoCurc, the three other routes of administration result in minimal systemic curcumin levels. The plasma:tissue ratios for curcumin at 2h postinjection in the i.duc NanoCurc cohort was 0.0064, underscoring the minimal ‘spillover’. (C) Curcumin levels were assessed in the ipsilateral breast tissue at 1, 2, 4 and 8h following i.duc nanoCurc delivery (168 µg/duct). Following an initial spike at 1h, curcumin levels plateau and are readily detectable at 8h postinjection. Three independent mammary glands were assessed at each time. (D) Curcumin levels were determined in the ipsilateral injected mammary glands and contralateral (non-injected) glands at one hour postinjection (six individual mammary glands per side were examined). Compared with ipsilateral glands, curcumin is essentially non-detectable in the contralateral glands, reiterating minimal systemic spillover.
We also determined if curcumin was detectable within mammary tissues for an appreciable time following i.duc NanoCurc injection. In timed experiments performed at 1, 2, 4 and 8h following a single 168 µg/duct dosing (three ipsilateral injected mammary glands per time point), we identified a peak in tissue levels at 2h, followed by a plateau and detectable curcumin at 8h (Figure 1C). Of note, the plasma:tissue ratios for curcumin at 2h postinjection was 0.0064, underscoring that most of the injected formulation was retained within mammary tissues. Finally, we compared curcumin levels by liquid chromatography-tandem mass spectrometry in ipsilateral (injected) and contralateral (non-injected) mammary glands at 1h postinjection of i.duc NanoCurc and confirmed minimal ‘spillover’, with curcumin essentially restricted to the injected tissues (Figure 1D).
Intraductal administration of NanoCurc prevents mammary tumorigenesis in the MNU-induced carcinogenesis model
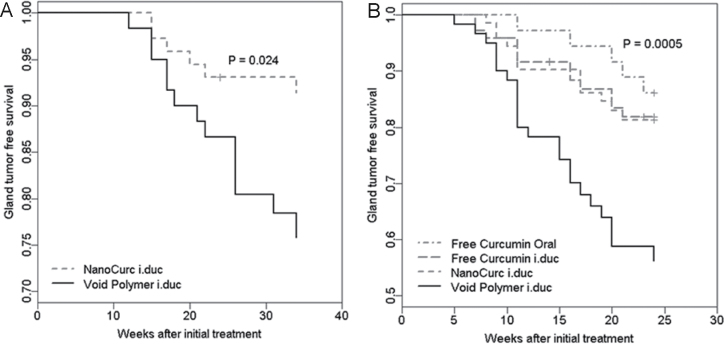
Prevention Study 1: i.duc NanoCurc versus i.duc void polymeric nanoparticle. In the initial chemoprevention study, we performed a two-arm experiment, simply comparing the efficacy of tumor reduction by i.duc NanoCurc versus void NVA622 polymer nanoparticles. Sprague–Dawley rats administered intraperitoneal MNU develop mammary ductal preneoplasia within 21 days and multiple palpable mammary tumors with a latency of 4–6 months. MNU-administered rats were randomized into two cohorts and were administered either NanoCurc dissolved in phosphate-buffered saline (equivalent to 168 µg of curcumin/teat) or void nanoparticle dissolved in phosphate-buffered saline (control) to each of the 12 teats per rat by i.duc injection. Two doses were administered, the first at day 14 post-MNU exposure and the second 4 weeks following the first treatment. Rats were subsequently observed for a total of 34 weeks post-MNU exposure. Treatment with i.duc NanoCurc demonstrated a significant reduction in mammary tumor incidence (6 tumors in 76 glands, 8%) compared with i.duc void nanoparticle treated rats (13 tumors in 60 glands, 22%) (P = 0.028, Table 1, Figure 2A). Notably, unlike that seen with i.duc pegylated liposomal doxorubicin, no evidence of cutaneous toxicity at the injection site (Supplementary Figure 1, available at Carcinogenesis Online) or significant differences in the average body weight of rats was observed in rats injected i.duc with void nanoparticles or NanoCurc.
Table I.
Risk of mammary tumor development in Prevention Study 1
| Treatment comparison | No. of tumors/glands treated | Hazard ratio | 95% Confidence inteval | P value |
|---|---|---|---|---|
| i.duc void polymer versus i.duc NanoCurc | 13/60 versus 6/72 | 2.88 | 1.12–7.42 | 0.028 |
Fig. 2.
Intraductal NanoCurc enhances tumor-free survival in the MNU-induced rat mammary tumor model. (A) Treatment of MNU-exposed rats with i.duc NanoCurc showed significant reduction in tumor incidence compared with i.duc void nanoparticle treated rats (negative control group). A total of 132 mammary glands were examined. Y-axis = Gland tumor free survival (%). (B) Both i.duc NanoCurc and i.duc free curcumin significantly protected animals against tumor formation compared with i.duc void nanoparticle (negative control). There was no significant difference in protection between two groups. Both i.duc NanoCurc and i.duc free curcumin showed no significant difference in prevention of mammary tumorigenesis compared with oral free curcumin (administered at 200mg/kg). A total of 240 mammary glands were examined. Y-axis = Gland tumor free survival (%).
Prevention Study 2: i.duc NanoCurc versus i.duc free curcumin versus oral free curcumin. In light of the results in Prevention Study 1, we expanded the chemoprevention trial to include two alternative modalities for comparison with i.duc NanoCurc—oral free curcumin and i.duc free curcumin, respectively. Carcinogen-administered rats were randomized into the three treatment cohorts, each of which received three treatment cycles—the first at day 14 following MNU exposure, and subsequently at weeks 1 and 2 following the 1st injection, at previously stated doses for each cohort. A fourth control cohort received i.duc void polymer at the specified time points. The rats were subsequently observed for a total of 24 weeks post-MNU exposure and palpated for the presence of mammary tumors at weekly intervals. As detailed in Table 2 and Figure 2B, all three treatment cohorts demonstrated a significant reduction in mammary tumor incidence compared with i.duc void nanoparticle (P < 0.0001 for i.duc NanoCurc, P = 0.020 for i.duc free curcumin and P = 0.007 for oral free curcumin). In contrast, no significant differences in tumor frequency were demonstrable between the three treatment groups, underscoring the premise that i.duc administration results in comparable efficacy of protection at a fraction of the systemic dose (in the current experiment, at 1/20th the dose of curcumin in the i.duc cohorts). Notably, despite the comparable frequency of ‘breakthrough’ tumors in both i.duc cohorts, we observed a significantly smaller mean tumor size at resection in the i.duc NanoCurc-treated rats versus animals receiving i.duc free curcumin (1672.7mm3 versus 11625.8mm3, P < 0.0001), an observation that leads us to believe that such tumors are more likely to be amenable to resection in NanoCurc-treated cohorts upon eventual clinical application.
Table II.
Risk of mammary tumor development in Prevention Study 2
| Treatment comparison | No. of tumors/glands treated | Hazard ratio | 95% Confidence interval | P value |
|---|---|---|---|---|
| i.duc void polymer versus oral free curcumin | 23/60 versus 5/36 | 3.95 | 1.47–10.6 | 0.007 |
| i.duc void polymer versus i.duc free curcumin | 23/60 versus 12/72 | 2.85 | 1.18–6.92 | 0.020 |
| i.duc void polymer versus i.duc NanoCurc | 23/60 versus 13/72 | 2.73 | 1.56–4.78 | < 0.0001 |
| i.duc free curcumin versus oral free curcumin | 12/72 versus 5/36 | 1.38 | 0.46–4.19 | 0.565 |
| i.duc NanoCurc versus oral free curcumin | 13/72 versus 5/36 | 1.45 | 0.62–3.37 | 0.394 |
| i.duc NanoCurc versus i.duc free curcumin | 13/72 versus 12/72 | 1.04 | 0.42–2.59 | 0.925 |
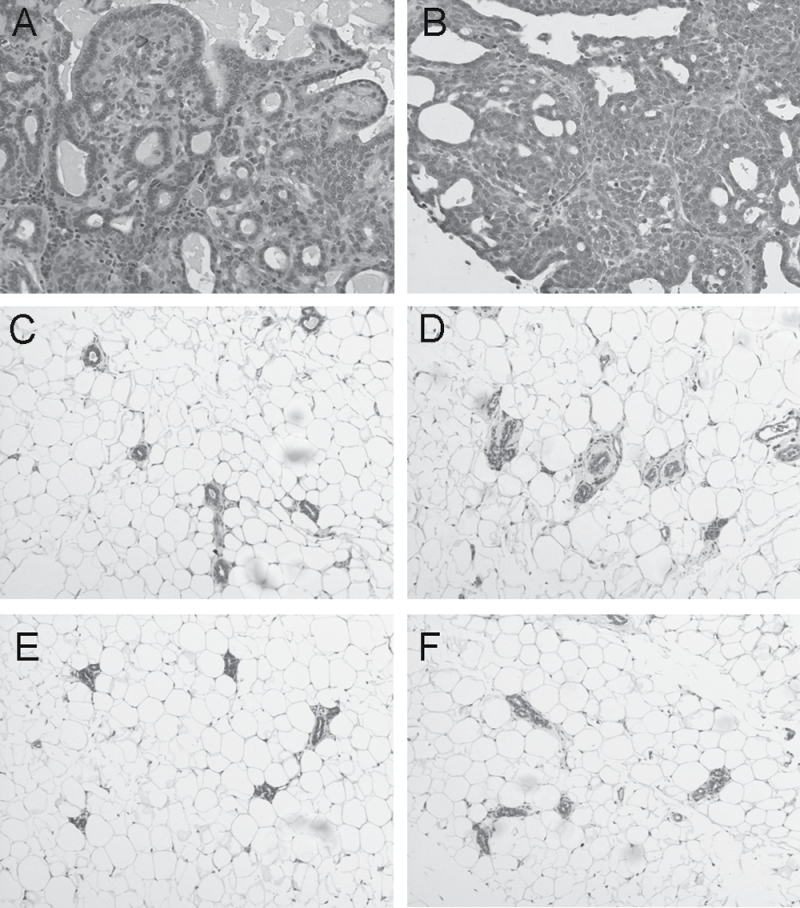
Curcumin preserves mammary architecture and attenuates activated NFκB signaling and proliferation in the native ductal epithelium. Alum carmine staining of whole mounts prepared from mammary glands of rats treated with either i.duc NanoCurc or i.duc free curcumin did not show significant differences in the numbers of main ducts or side branches, compared with control group (data not shown). The ‘breakthrough’ cancers that arose in the four arms were all tubuloalveolar adenocarcinomas (Figure 3A). NanoCurc breakthrough tumors also harbored some areas that were more solid or cribriform with less stroma (Figure 3B). Histological examination of non-tumor bearing areas from the resected mammary glands revealed some areas of hyperplasia, but very few inflammatory infiltrates. The minimal inflammatory infiltrates consisted of individual mononuclear cells including monocytes, lymphocytes and plasma cells and very rare neutrophils in periductal areas or in the surrounding mammary fat pad. The histology, consisting of mild, scattered mammary hyperplasia and minimal inflammatory infiltrates, was similar in all four groups at the cellular level in non-tumor areas of the mammary gland (Figure 3 C–F).
Fig. 3.
Histology of breakthrough tumors and non-involved mammary tissues. Breakthrough tumors arising in i.duc void polymer (A) and i.duc NanoCurc (B) are tubuloalveolar adenocarcinomas. NanoCurc breakthrough tumors also harbor some areas that are more solid or cribriform with less stroma. Comparable tumor histologies in i.duc free curcumin and oral free curcumin are not shown.
Areas of normal mammary gland from all four groups – (C) = i.duc free curcumin, (D) = i.duc NanoCurc, (E) = oral free curcumin, and (F) = i.duc void polymer – displayed minimal periductal infiltrates including mononuclear cells and rare neutrophils. The histology was similar in all four groups at the cellular level in non-tumor areas of the mammary gland.
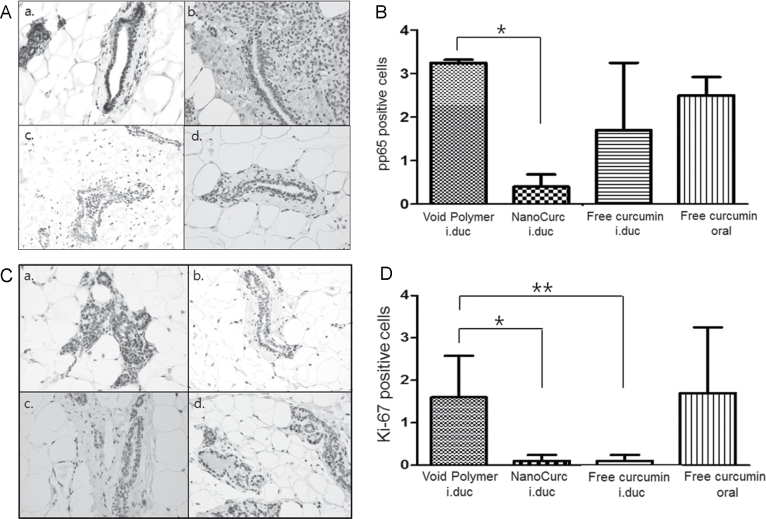
Finally, we examined potential pharmacodynamics parameters in the mammary epithelium, which might explain the underlying mechanism(s) of mammary tumor chemoprevention by curcumin. All of the studies were performed at the culmination of the chemoprevention trial as immunohistochemical analyses on formalin-fixed mammary glands. NFκB is a transcription factor that activates cell-survival pathways in cancer cells and renders them resistant to conventional cytotoxic agents (28,29). Multiple published studies have established that curcumin is a potent inhibitor of NFκB activity in cancer cells (11,30). NFκB activity was assessed by quantitative immunohistochemistry for nuclear localization of pp65 (phospho p65), since the latter is the principal subunit protein of NFκB. All three treatment groups demonstrated a reduction in nuclear pp65 levels in the mammary epithelium compared to control rats, although only the i.duc NanoCurc cohort reached statistical significance (Figure 4A and 4B, P = 0.0001). To assess proliferation of mammary epithelium in the glands following curcumin treatment, we performed immunohistochemistry using the well-established proliferation marker, nuclear Ki-67, which demonstrated a significant reduction in proliferation in mammary glands receiving either i.duc NanoCurc or i.duc free curcumin, compared with mammary glands treated with void polymer (P = 0.003) (Figure 4C and 4D). We also examined two additional parameters of cellular proliferation—cyclin D1 and phospho-histone H3 in the treatment cohorts, and neither showed robust correlation with Ki-67 data, likely reflecting somewhat distinct cell-cycle parameters assessed by each antigen (Table 3). Of note, the phospho-histone H3 levels (a marker of mitosis or M-phase in cycling cells) was most significantly downregulated (P < 0.0001) in the i.duc NanoCurc arm compared with void polymer, suggesting that the major effects of this formulation are through reduced cell division in the mammary epithelium. This was indirectly reiterated by the results obtained with immunohistochemistry analysis for cleaved caspase 3, an established marker of apoptosis, which was significantly increased in the i.duc free curcumin and oral free curcumin treated mammary glands compared with i.duc void polymer (P < 0.0001), but failed to reach statistical significance in the i.duc NanoCurc arm (Table 3). Thus, i.duc NanoCurc appears to have a more potent effect on cell proliferation than on cell survival, a feature that might be advantageous in preventing incidental toxicities in non-tumor bearing areas exposed to this formulation.
Fig. 4.
Curcumin attenuates activation of nuclear factor NFκB and reduces proliferation within the mammary epithelium. (A) Photomicrograph of nuclear pp65 labeling within the mammary epithelium in each treatment group- i.duc void polymer (a), i.duc NanoCurc (b), i.duc free curcumin (c), and oral free curcumin (d). Nuclear pp65 was assessed in the non-tumor bearing mammary epithelium at the culmination of the chemoprevention trial. (B) Immunohistochemistry for nuclear pp65 confirms downregulation in nuclear labeling in all three treatment cohorts compared to void polymer arm, with the i.duc NanoCurc-treated mammary glands demonstrating a significant decrease (P = 0.0001). The Y-axis designates number of cells with nuclear pp65 staining per high power field (×40), over an average of 10 randomly selected fields. (C) Immunohistochemistry was performed for nuclear MIB-1 (Ki-67) antigen expression, as a measure of cell proliferation. Photomicrograph of Ki-67 immunohistochemistry obtained from each treatment cohort—i.duc void polymer (a), i.duc NanoCurc (b), i.duc free curcumin (c) and oral free curcumin (d). (D) Quantification was done by counting number of cells expressing Ki-67 in ten randomly selected ×40 high power fields for each group. There is a significant reduction in Ki-67 labeling in i.duc NanoCurc and i.duc free curcumin groups compared with the control (void polymer) cohort.
Table III.
Immunohistochemical assessments of inflammation, proliferation and apoptosis in mammary tissue
| Comparison | P value by likelihood ratio test | ||||
|---|---|---|---|---|---|
| pp65 | Ki67 | CCND1 | pH3 | Caspase 3 | |
| i.duc void polymer versus i.duc NanoCurc | 0.0001 | 0.003 | 0.407 | <0.0001 | 0.880 |
| i.duc void polymer versus i.duc free curcumin | 0.067 | 0.003 | 0.764 | 0.086 | <0.0001 |
| i.duc void polymer versus oral free curcumin | 0.345 | 0.894 | 0.008 | 0.007 | <0.0001 |
| i.duc NanoCurc versus i.duc free curcumin | 0.051 | >0.999 | 0.302 | 0.093 | 0.0004 |
| i.duc NanoCurc versus oral free curcumin | 0.002 | 0.015 | 0.079 | <0.0001 | 0.004 |
| i.duc free curcumin versus oral free curcumin | 0.339 | 0.015 | 0.005 | 0.002 | 0.127 |
Nuclear localization of pp65 (phospho p65), the principal subunit protein of NFκB and a marker of inflammation, and proliferation markers: Cyclin D1 (CCND1), phospho-histone H3 (pH3) and Ki67 was significantly decreased, whereas cleaved caspase 3, a marker of apoptosis, was significantly increased in several treatment comparisons.
To account for multiple testing, a significant difference is considered at P < 0.0083 based on Bonferroni adjustment.
Discussion
For women in United States, breast cancer is the most common non-dermatologic cancer and the second leading cause of cancer death. In 2009, an estimated 192 370 new cases of breast cancer were diagnosed and an estimated 40 170 women died from breast cancer (31). Methods of breast cancer prevention in high risk women have historically been limited to surgical removal of breast. Bilateral mastectomy is an effective method for breast cancer prevention that results in a risk reduction of at least 90% (28). However, many women would prefer alternatives to such invasive procedures, and less-invasive methods for breast cancer prevention have been investigated. Tamoxifen, for more than a decade, and raloxifene more recently have been recommended for cancer reduction in women at increased risk for breast cancer (29,30). Although many women are eligible for intervention with tamoxifen or raloxifene, few women are treated with these drugs (32). This is because the efficacy of tamoxifen and raloxifene in preventing breast cancer is limited to estrogen receptor-positive tumors; in addition, there is increased risk from these medications for adverse medical conditions, including endometrial cancer, thromboembolic events and vasomotor effects (30,33,34); as well, the recommendations of dosing are different for pre and postmenopausal women, indicating that other methods of prevention must also be explored.
The chemopreventive properties of the plant phytochemical and dietary ingredient curcumin have been extensively investigated in several preclinical animal models, including in breast cancer (6). For example, Singletary et al. (35) showed that intraperitoneal administration of free curcumin at the dose of 100mg/kg or 200mg/kg significantly decreased the number of palpable mammary tumors and suppressed the production of mammary adenocarcinomas in Sprague–Dawley rats in a dimethylbenz(a)anthracence-induced model of mammary tumorigenesis. Similarly, Carroll et al. (36) reported that dimethylbenz(a)anthracence-induced and medroxy-progesterone acetate-accelerated mammary tumors can be delayed and tumor incidence decreased by intraperitoneal injection of free curcumin at a dose of 200mg/kg for 25 days (36). Nonetheless, a parenteral route for chemoprevention is not a long-term feasible approach in humans, and the doses utilized in rodents would translate into several grams of curcumin intake, further hampering clinical translation.
To improve the systemic bioavailability of curcumin, numerous approaches have been undertaken. These approaches involve, but are not limited to, the use of adjuvants such as piperine that interfere with glucuronidation (37), liposomal curcumin and phospholipid complexes (38,39), and the use of water-soluble structural analogs of curcumin (40). Our laboratory was one of the first to synthesize a nanoparticle formulation of curcumin in 2007 (41) and has since applied NanoCurc in spontaneously metastatic models of pancreatic cancer (22). Despite enabling widespread tissue distribution (including crossing the blood-brain-barrier) (26) and circumventing the need for noxious excipients, NanoCurc is not amenable to oral absorption. Therefore, NanoCurc is not suitable for chemoprevention using the oral route of administration.
In this study, we wed two innovative strategies devised by our group—the use of i.duc injection of chemotherapeutics and nano-encapsulation of curcumin enabling its solubility in aqueous media—in order to develop a novel approach toward chemoprevention of breast cancer. Although i.duc administration of agents is still in its infancy, there is an emerging acceptance among breast cancer clinicians for using this route of administration (42). The rationale for these clinical studies sprouts from preclinical data generated in 2006 in our laboratory using the MNU-induced mammary carcinoma model in rats (3) and more importantly, from the recent completion of a Phase I trial demonstrating feasibility of the i.duc route in patients (4). In our current study, we confirmed that i.duc administration of nano-encapsulated or the free curcumin significantly reduces the incidence of mammary tumors compared with control rats, and the protection effects are comparable with what is observed with administration of ‘mega’ amounts of the free drug (200mg/kg or 30–40mg per rat) through the oral route. Although the absolute frequency of ‘breakthrough’ tumors are not statistically distinct between i.duc NanoCurc and i.duc free curcumin cohorts, the former demonstrate significantly smaller size of tumors that do occur, supporting the contention that i.duc NanoCurc breakthrough tumors may be more resectable. Of note, whole mount and histopathological examination of the i.duc NanoCurc-treated breast tissues demonstrated no differences between treated and control groups in ductal histology and periductal inflammation, an important distinction from that observed using chemotherapeutics (3,4). This observation engenders the hope that patients receiving i.duc NanoCurc will have sufficient preservation of resident stem cells in the terminal ducto-lobular units to enable rapid repopulation of any ductal epithelium. The absence of histopathological abnormalities is of particular important as many at-risk patients are probably of reproductive age.
Multiple mechanisms of action have been proposed for the anticancer effects of curcumin, but none are probably as seminal as its inhibition of NFκB activity (43). NFκB is a critical transcription factor that is involved in a wide range of physiological and pathological cellular responses, from cell survival to chemoresistance to decreased apoptosis. In this study, we assessed NFκB activity using a surrogate quantitative measure of nuclear p65 localization and observed downregulation compared with control rats in all three treatments cohorts in Prevention Study 2, with the i.duc NanoCurc arm showing a statistically significant decrease. It is important to note that NFκB is not the only signaling pathway or molecule altered by curcumin, and Aggarwal and colleagues have elaborated a large number of intracellular targets such as cyclo-oxygenase COX-2, vascular endothelial growth factor, STAT3, and Akt, among others (43,44). This might explain why the oral free curcumin cohort demonstrated comparable reduction in tumor incidence despite the somewhat attenuated NFκB downregulation observed in mammary epithelium.
We also performed a pilot pharmacokinetic analysis in Sprague–Dawley rats administered i.duc curcumin (either NanoCurc or free curcumin dissolved in corn oil) and compared the circulating levels with that observed upon an equivalent dosing of NanoCurc intraperitoneally. These studies confirmed the prior observations from our group that agents administered through the i.duc route result in minimal peripheral spillover. This data should ameliorate concerns about potential systemic side effects on visceral organs from either curcumin (albeit a safe dietary ingredient) or the delivery vehicle itself (polymer nanoparticle). The lack of toxicity is further reiterated by the complete absence of local cutaneous side effects (excoriation, hair loss) as shown in Supplementary Figure 1, available at Carcinogenesis Online. Although these observations will need validation in clinical studies, the preclinical data provides encouraging signals to that effect.
In summary, this study suggests the feasibility of combining NanoCurc formulation with an i.duc approach as a novel strategy for chemoprevention of breast cancer in a well-established chemical carcinogenesis model. As a dietary ingredient used for centuries, curcumin provides a reasonable alternative to cytotoxic compounds as a ‘gentle’ agent for chemoprevention, likely amenable to repeat injections over a longer time frame. Additional studies are required to confirm the efficacy of this formulation in experimental models of carcinogenesis.
Supplementary material
Supplementary Figure 1 can be found at http://carcin.oxfordjournals.org/
Funding
National Institutes of Health (U54CA151838 to A.M.); National Cancer Institute (P50CA088843 to A.M and S.S.); Flight Attendants Medical Research Institute to A.M. and M.A.R.; the Analytical Pharmacology Core of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins National Institutes of Health (P30 CA006973); the Shared Instrument (1S10RR026824-01) to M.A.R.; the Breast Cancer Research Foundation to V.S.; National Center for Research Resources (UL1 RR 025005), a component of the National Institutes of Health and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of National Center for Research Resources or National Institutes of Health.
Supplementary Material
Acknowledgements
Conflict of Interest Statement: Dr. Maitra is a member of the scientific advisory board of SignPath Pharma, Inc., and any conflicts of interest under this arrangement are handled in accordance with the Johns Hopkins University Office of Policy Coordination (OPC) guidelines.
Disclosure
NanoCurc™ is a registered trademark of SignPath Pharmaceuticals, Inc., Quakerstown, Pennsylvania. Dr. Maitra is a member of the scientific advisory board of SignPath Pharma, Inc., and any conflicts of interest under this arrangement are handled in accordance with the Johns Hopkins University Office of Policy Coordination (OPC) guidelines. S.B. and A.M. have filed a patent application (U.S. 2008/0107749) that is relevant to the formulation described in this article. A report of invention to this effect has been filed with Johns Hopkins Technology Transfer and licensed by SignPath Pharma.
Glossary
Abbreviations
- i.duc
intraductal
- MNU
N-methyl-N-nitrosourea
References
- 1. Wooster R, et al. (2003). Breast and ovarian cancer N. Engl. J. Med. 348 2339–2347. [DOI] [PubMed] [Google Scholar]
- 2. Robson M, et al. (2007). Clinical practice. Management of an inherited predisposition to breast cancer N. Engl. J. Med. 357 154–162. [DOI] [PubMed] [Google Scholar]
- 3. Murata S, et al. (2006). Ductal access for prevention and therapy of mammary tumors Cancer Res. 66 638–645. [DOI] [PubMed] [Google Scholar]
- 4. Stearns V, et al. (2011). Preclinical and clinical evaluation of intraductally administered agents in early breast cancer Sci. Transl. Med. 3 106–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aggarwal B.B., et al. (2003). Anticancer potential of curcumin: preclinical and clinical studies Anticancer Res. 23 363–398. [PubMed] [Google Scholar]
- 6. Strimpakos A.S., et al. (2008). Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials Antioxid. Redox. Signal 10 511–545. [DOI] [PubMed] [Google Scholar]
- 7. Ravindran J, et al. (2009). Curcumin and cancer cells: how many ways can curry kill tumor cells selectively? AAPS. J. 11 495–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shishodia S, et al. (2007). Role of curcumin in cancer therapy Curr. Probl. Cancer 31 243–305. [DOI] [PubMed] [Google Scholar]
- 9. Aggarwal B.B., et al. (2005). Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice Clin. Cancer Res. 11 7490–7498. [DOI] [PubMed] [Google Scholar]
- 10. Labbozzetta M, et al. (2009). Curcumin as a possible lead compound against hormone-independent, multidrug-resistant breast cancer Ann. N. Y. Acad. Sci. 1155 278–283. [DOI] [PubMed] [Google Scholar]
- 11. Shao Z.M., et al. (2002). Curcumin exerts multiple suppressive effects on human breast carcinoma cells Int. J. Cancer 98 234–240. [DOI] [PubMed] [Google Scholar]
- 12. Rowe D.L., et al. (2009). Modulation of the BRCA1 protein and induction of apoptosis in triple negative breast cancer cell lines by the polyphenolic compound curcumin Breast Cancer (Auckl) 3 61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jiang M.C., et al. (1996). Curcumin induces apoptosis in immortalized NIH 3T3 and malignant cancer cell lines Nutr. Cancer 26 111–120. [DOI] [PubMed] [Google Scholar]
- 14. Syng-Ai C., et al. (2004). Effect of curcumin on normal and tumor cells: role of glutathione and bcl-2 Mol Cancer Ther 3 1101–1108. [PubMed] [Google Scholar]
- 15. Ramachandran C, et al. (1999). Differential sensitivity of human mammary epithelial and breast carcinoma cell lines to curcumin Breast Cancer Res. Treat. 54 269–278. [DOI] [PubMed] [Google Scholar]
- 16. Garcea G, et al. (2005). Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences Cancer Epidemiol. Biomarkers Prev. 14 120–125. [PubMed] [Google Scholar]
- 17. Sharma R.A., et al. (2004). Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance Clin. Cancer Res. 10 6847–6854. [DOI] [PubMed] [Google Scholar]
- 18.Lao C.D., et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern. Med. (2006);6:10.. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cheng A.L., et al. (2001). Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions Anticancer Res. 21 2895–2900. [PubMed] [Google Scholar]
- 20. Anand P, et al. (2007). Bioavailability of curcumin: problems and promises Mol. Pharm. 4 807–818. [DOI] [PubMed] [Google Scholar]
- 21. Bisht S, et al. (2009). Systemic delivery of curcumin: 21st century solutions for an ancient conundrum Curr. Drug Discov. Technol. 6 192–199. [DOI] [PubMed] [Google Scholar]
- 22. Bisht S, et al. (2010). Systemic administration of polymeric nanoparticle-encapsulated curcumin (NanoCurc) blocks tumor growth and metastases in preclinical models of pancreatic cancer Mol. Cancer Ther. 9 2255–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thompson H.J., et al. (2000). Classification of premalignant and malignant lesions developing in the rat mammary gland after injection of sexually immature rats with 1-methyl-1-nitrosourea J. Mammary Gland Biol. Neoplasia 5 201–210. [DOI] [PubMed] [Google Scholar]
- 24. Thompson H.J., et al. (1998). Temporal sequence of mammary intraductal proliferations, ductal carcinomas in situ and adenocarcinomas induced by 1-methyl-1-nitrosourea in rats Carcinogenesis 19 2181–2185. [DOI] [PubMed] [Google Scholar]
- 25. Bisht S, et al. (2011). A polymeric nanoparticle formulation of curcumin (NanoCurc) ameliorates CCl(4)-induced hepatic injury and fibrosis through reduction of pro-inflammatory cytokines and stellate cell activation Lab. Invest. 91 1383–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ray B., et al. (2011). Neuroprotective and neurorescue effects of a novel polymeric nanoparticle formulation of curcumin (NanoCurc) in the neuronal cell culture and animal model: implications for Alzheimer's disease J. Alzheimers Dis. 23 61–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bailer A.J. (1988). Testing for the equality of area under the curves when using destructive measurement techniques J. Pharmacokinet. Biopharm. 16 303–309. [DOI] [PubMed] [Google Scholar]
- 28. Hartmann L.C., et al. (1999). Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer N. Engl. J. Med. 340 77–84. [DOI] [PubMed] [Google Scholar]
- 29. Chlebowski R.T., et al. (1999). American Society of Clinical Oncology technology assessment on breast cancer risk reduction strategies: tamoxifen and raloxifene J. Clin. Oncol. 17 1939–1955. [DOI] [PubMed] [Google Scholar]
- 30. Visvanathan K, et al. (2009). American society of clinical oncology clinical practice guideline update on the use of pharmacologic interventions including tamoxifen, raloxifene, and aromatase inhibition for breast cancer risk reduction J Clin Oncol 27 3235–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jemal A, et al. (2010). Cancer statistics, 2010 CA Cancer J. Clin. 60 277–300 [DOI] [PubMed] [Google Scholar]
- 32. Waters E.A., et al. (2010). Prevalence of tamoxifen use for breast cancer chemoprevention among U.S. women Cancer Epidemiol. Biomarkers Prev. 19 443–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blaha P, et al. (2009). Breast cancer chemoprevention—a vision not yet realized Eur. J. Cancer Care (Engl) 18 438–446. [DOI] [PubMed] [Google Scholar]
- 34. Nelson H.D., et al. (2009). Systematic review: comparative effectiveness of medications to reduce risk for primary breast cancer Ann. Intern. Med. 151 703–715 W226–235. [DOI] [PubMed] [Google Scholar]
- 35. Singletary K, et al. (1996). Inhibition of 7,12-dimethylbenz[a]anthracene (DMBA)-induced mammary tumorigenesis and DMBA-DNA adduct formation by curcumin Cancer Lett 103. 137–141. [DOI] [PubMed] [Google Scholar]
- 36. Carroll C.E., et al. (2010). Curcumin delays development of medroxyprogesterone acetate-accelerated 7,12-dimethylbenz[a]anthracene-induced mammary tumors Menopause 17 178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shoba G, et al. (1998). Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers Planta Med. 64 353–356. [DOI] [PubMed] [Google Scholar]
- 38. Li L, et al. (2005). Liposome-encapsulated curcumin: in vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis Cancer 104 1322–1331. [DOI] [PubMed] [Google Scholar]
- 39. Maiti K, et al. (2007). Curcumin-phospholipid complex: Preparation, therapeutic evaluation and pharmacokinetic study in rats Int. J. Pharm. 330 155–163. [DOI] [PubMed] [Google Scholar]
- 40. Bao B, et al. (2012). Curcumin analogue CDF inhibits pancreatic tumor growth by switching on suppressor microRNAs and attenuating EZH2 expression Cancer Res., 72 335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bisht S, et al. Polymeric nanoparticle-encapsulated curcumin ("nanocurcumin"): a novel strategy for human cancer therapy. J. Nanobiotechnology. (2007);5:3.. doi: 10.1186/1477-3155-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jacobs L, et al. (2010). Intraductal therapy for the prevention of breast cancer Curr. Opin. Investig. Drugs 11 646–652. [PubMed] [Google Scholar]
- 43. Aggarwal B.B., et al. (2004). Suppression of the nuclear factor-kappaB activation pathway by spice-derived phytochemicals: reasoning for seasoning Ann. N. Y. Acad. Sci. 1030 434–441. [DOI] [PubMed] [Google Scholar]
- 44. Anand P, et al. (2008). Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature Biochem. Pharmacol. 76 1590–1611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.