Abstract
Objectives
We describe the outcomes of second-line drug resistance profiles and predict the efficacy of drugs for third-line therapy in patients monitored without the benefit of plasma HIV-1 RNA viral load (VL) or resistance testing.
Methods
We recruited 106 HIV-1-infected patients after second-line treatment failure in Mali. VL was determined by the Abbott RealTime system and the resistance by the ViroSeq HIV-1 genotyping system. The resistance testing was interpreted using the latest version of the Stanford algorithm.
Results
Among the 106 patients, 93 had isolates successfully sequenced. The median age, VL and CD4 cells were respectively 35 years, 72 000 copies/mL and 146 cells/mm3. Patients were exposed to a median of 4 years of treatment and to six antiretrovirals. We found 20% of wild-type viruses. Resistance to etravirine was noted in 38%, to lopinavir in 25% and to darunavir in 12%. The duration of prior nucleos(t)ide reverse transcriptase inhibitor exposure was associated with resistance to abacavir (P < 0.0001) and tenofovir (P = 0.0001), and duration of prior protease inhibitor treatment with resistance to lopinavir (P < 0.0001) and darunavir (P = 0.06).
Conclusion
Long duration of therapy prior to failure was associated with high levels of resistance and is directly related to limited access to VL monitoring and delayed switches to second-line treatment, precluding efficacy of drugs for third-line therapy. This study underlines the need for governments and public health organizations to recommend the use of VL monitoring and also the availability of darunavir and raltegravir for third-line therapies in the context of limited-resource settings.
Keywords: resistance, third-line, Africa
Introduction
In Mali, of ∼182 000 HIV-1-infected individuals, more than 33 592 were enrolled in the national antiretroviral therapy (ART) programme in December 2010. The majority of patients were receiving first-line regimens that included two nucleos(t)ide reverse transcriptase inhibitors (NRTIs) and one non-NRTI (NNRTI). The fixed-dose combination Triomune® (stavudine/lamivudine/nevirapine) is largely used in Mali as a first-line regimen1,2 Other available antiretroviral drugs for first-line therapy include zidovudine and efavirenz. It is estimated that <15% of patients are on a second-line regimen that includes two NRTIs combined with one ritonavir-boosted protease inhibitor (PI), as recommend by the WHO in 20093 and Malian antiretroviral guidelines in July 2010. The genetic barrier for resistance to NNRTIs is very low, requiring a high level of adherence for long-term suppression of plasma HIV-1 viral load (VL). Virological monitoring with VL and resistance testing is limited in Mali so far, thus there are many patients with detectable viraemia while on first- or second-line therapies. Detectable viraemia during treatment has important consequences, including the development of drug resistance mutations and the need to switch to another regimen, with considerable cost implications.4,5 If resources are available, VL monitoring every 6 months is now recommended, or targeted testing can be performed to confirm clinical or immunological failure.3 Virological monitoring, however, is still not feasible for the majority of patients on ART due to the absence of adequate laboratory facilities and the high cost of testing. HIV drug resistance genotyping is recommended at the population level for surveillance and monitoring in Mali for second-line failures only.3 There is thus a need to assess virological outcomes in routine care settings in Mali and other resource-limited countries in order to evaluate the effectiveness of antiretroviral programmes, to evaluate whether the second-line treatment recommended by WHO would still be effective and to inform the choice of third-line treatment. A study conducted in 2008 in Mali and Burkina Faso, West Africa, showed a 33% prevalence of thymidine analogue mutations (TAMs) with the use of a stavudine-based regimen, thereby limiting NRTI options for second-line treatment. The same study showed 55% of mutants carried Y181C and K103N, which confer resistance to first-generation NNRTIs. In 2011, a similar high prevalence of resistance to first-line therapy was reported in other West African settings, Togo and Benin.6,7 In collaboration with several partners [Northwestern University in Chicago, Pitié-Salpêtrière Hospital in Paris, ESTHER (a French agency for AIDS in developing countries), SOLTHIS (a non-governmental organization) and the National AIDS Program in Mali], a virology laboratory in Mali has been funded to monitor HIV drug resistance. The data presented here are the first generated by this laboratory.
In this study we aimed to determine the prevalence of drug resistance mutations among patients failing on second-line ART, to characterize the resistance genetic profiles and to evaluate the susceptibility to antiretrovirals, especially those that can be used as part of third-line therapy in resource-limited settings.
Patients and methods
Study setting
The study was conducted in Mali with the National AIDS Program (Cellule Sectorielle de Lutte contre le Sida du Ministère de la Santé). The SEREFO laboratory is a training and research centre in the Faculty of Medicine, Pharmacy and Dentistry at the University of Bamako. This laboratory was designated by the Ministry of Health to be the national reference laboratory for HIV drug resistance testing and VL testing in Mali. The patients were followed in different clinical centres, national public hospitals (Point-G Hospital, Gabriel Touré Hospital) or community centres (CESAC in Bamako and USAC Commune V) in Bamako and in other regions. First-line failure was determined according to the Malian national guidelines.8 The second-line regimen was selected by the clinician based on the guidelines, which recommended ritonavir-boosted lopinavir plus two NRTIs (including at least one of abacavir, didanosine or tenofovir). The NRTI selected was influenced by drug availability. All patients included in this study had been exposed to the three antiretroviral classes (NRTIs, NNRTIs and PIs) available in Mali and had virological failure, defined as VL ≥500 copies/mL after at least 12 months of second-line therapy.
Study population
Approximately 913 patients were receiving second-line ART in Mali at the time of this study. All patients receiving a second-line treatment regimen for at least 12 months in the different participant clinical centres in Bamako or in eight other regions of Mali were enrolled and tested for VL in the reference laboratory. If VL was ≥500 copies/mL, patients were selected for HIV-1 resistance testing and plasma samples were sent to the SEREFO resistance testing laboratory. We collected the following patient information: socio-demographic characteristics (sex and age), VL, CD4 cell count, treatment history (first-line regimen, switch date and second-line regimen) and the current treatment. After resistance testing, patient results were discussed in a multidisciplinary staff conference to propose an optimized third-line regimen for the patient.
HIV-1 genotyping
HIV-1 genotyping was performed using the Celera Diagnostics ViroSeq HIV-1 Genotyping System (version 2.0) according to the manufacturer's instructions with 0.6 mL of plasma. Sequence data were first analysed using the Sequence Analysis software and secondly by Celera Diagnostics ViroSeq HIV-1 Genotyping System software (version 2.8), which assembles sequence data from the primers into a contiguous sequence that can be inspected for the identification of drug resistance mutations.9 All operators at the SEREFO laboratory were trained and certified by the manufacturer and were proficient in running the ViroSeq assay in the last 2 years.
Drug resistance interpretation
The genotypic results were interpreted for each drug according to the 2011 version of the Stanford algorithm (http://hivdb.stanford.edu). Sequences classified as resistant or intermediate by the algorithm were considered resistant in our analyses. The cut-off used by the Stanford algorithm to define the intermediate resistance was 10–30. The full resistance cut-off was >30.
Statistical analysis
All the statistical analyses were performed using the Statview software. Simple descriptive statistics included medians and proportions, as appropriate. Student's t-test, the Wilcoxon rank-sum test and the χ2 test were applied as required. Univariate analyses were performed to look for factors associated with resistance to salvage antiretroviral drugs (abacavir, tenofovir, etravirine, lopinavir and darunavir). We performed a multivariate analysis to explore factors associated with resistance to darunavir.
Ethics
The study was approved by the national AIDS program in Mali (CSLS/MS) in collaboration with the Malian national ethics committee on health and life sciences for the protection of human subjects. Written or oral informed consent was obtained from all patients.
Results
Patient characteristics
We recruited 106 HIV-1-infected patients after second-line ART failure in Bamako and other regions in Mali. Among the 106 patients, sequences were obtained in 93 cases (88%). Three patients died during follow-up of the second-line ART after resistance testing. The sequenced patients' characteristics are shown in Table 1. The median VL was high at 72 000 copies/mL (4.86 log10 copies/mL; IQR 12 000–310 000) and the median CD4 cell count was 146 cells/mm3 (IQR 19–193). Sixty-seven percent of viruses were CRF02_AG. The other subtypes were CRF06_cpx 15%, CRF01_AE 6.5%, CRF19_cpx 4.3%, A-1 2.2%, F-2 1.1% and URF (unique recombinant form) 3.2%. All the patients were exposed to three different antiretroviral classes.
Table 1.
Socio-demographic, clinical and biological characteristics of study population with HIV-1 sequences (n = 93)
| Parameter | Value |
|---|---|
| Age, years median (IQR) | 35 (24–46) |
| Female (%) | 67 |
| Viral load, copies/mL median [IQR] | 72 000 (12 000–310 000) |
| HIV RNA copies/mL (%) | |
| <1000 | 4 |
| 1000–5000 | 13 |
| >5000 | 83 |
| CD4 count, cells/mm3 median (IQR) | 146 (67–193) |
| HIV subtype CRF02_AG (%) | 67 |
| Median duration of prior ART, years (IQR) | |
| NRTIs | 4 (2–6) |
| NNRTIs | 2 (1–3) |
| PIs | 2 (0–4) |
| Antiretrovirals | 4 (2–6) |
| Numbers of drug exposures including current treatment, median (IQR) | |
| ARVs | 6 (5–7) |
| NRTIs | 4 (3–5) |
| NNRTIs | 1 (0–1) |
| PIs | 1 (0–1) |
Exposure to ART in first- and second-line therapy
Patients were exposed to a median of six antiretrovirals and the details of exposure to the different drugs are illustrated in Figure 1. Patients were exposed to a median number of four NRTIs, one PI and one NNRTI. Sixty-eight percent of patients were exposed to the generic combination Triomune® (stavudine/lamivudine/nevirapine) for first-line ART, 17.6% to lamivudine/zidovudine/nevirapine and the remainder to other regimens. Eighty percent, 76% and 25% of patients were exposed to abacavir, ritonavir-boosted lopinavir and tenofovir, respectively, for second-line ART.
Figure 1.
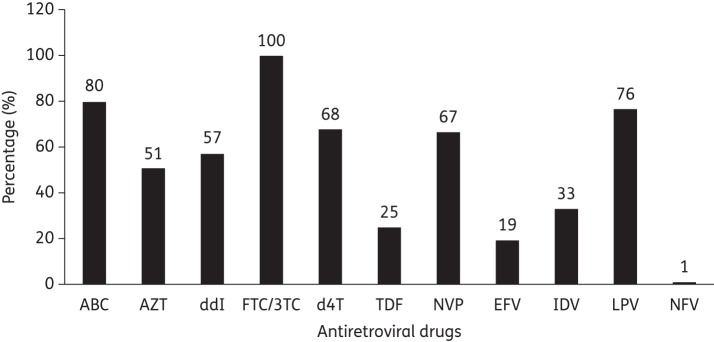
Previous treatment exposure of 93 patients failing to second-line antiretroviral therapy in 2011 in Mali. ABC, abacavir; AZT, zidovudine; ddI, didanosine; FTC, emtricitabine; 3TC, lamivudine; d4T, stavudine; TDF, tenofovir; NVP, nevirapine; EFV, efavirenz, IDV, indinavir; LPV, lopinavir; NFV, nelfinavir.
Genotypic resistance patterns after second-line ART failure in Mali
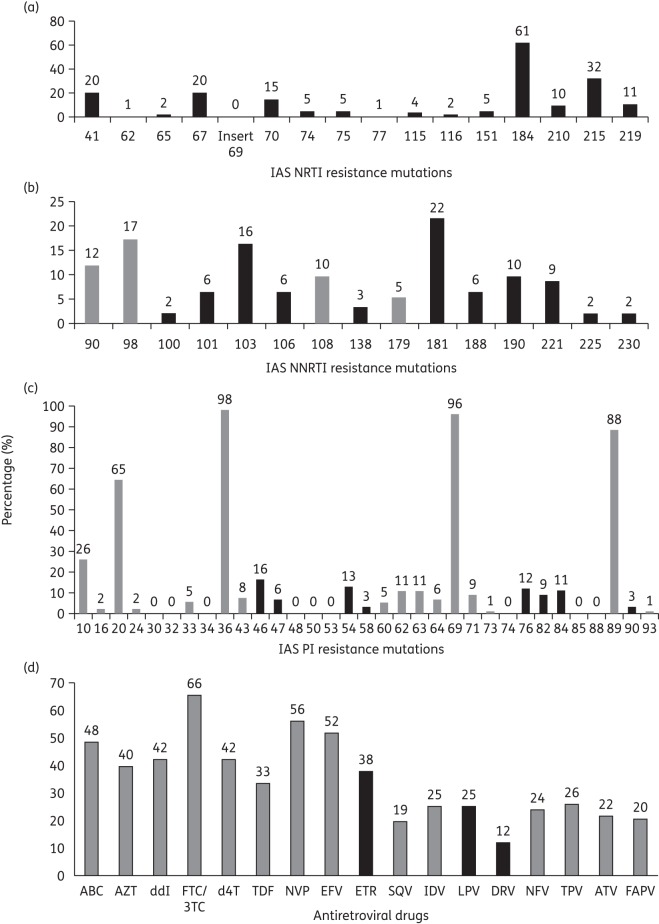
We found 20% of wild-type viruses. The prevalence of resistance mutations to NRTIs, NNRTIs and PIs is shown in Figure 2. The M184V mutation selected by lamivudine or emtricitabine was found in 61% of sequences. Thymidine analogue mutations (M41L, D67N, L210W and T215Y/F) were also frequently found: the mutation K70R was present in 15% of sequences and the K219Q/E in 11%. Five percent of patients harboured viruses with multiple NRTI resistance driven by the Q151M complex; no insertion at codon 69 was found. Two percent of patients harboured viruses with the K65R mutation (Figure 2a). The most prevalent resistance mutations to NNRTIs were Y181C/I/V (22%), K103N (16%), G190A/S (10%), H221Y (9%) and K101E/H/P, V106M and Y188L (6% each) (Figure 2b). Considering PI mutations, L76V selected by lopinavir/ritonavir was present in 12% of sequences. Other frequently observed major PI resistance mutations were M46I/L, I47V/A, I54M/L, Q58E, V82A/F/T/S, I84V and L90M, whereas G48V, N88D/S and D30N were not present in this study (Figure 2c).
Figure 2.
Prevalence of resistance mutations in patients failing a second-line antiretroviral therapy. Resistance mutations are those listed in the last version of the IAS drug resistance update in 2011. Major resistance mutations are in black and minor in grey. (a) IAS NRTI resistance mutations. (b) IAS NNRTI resistance mutations. (c) IAS PI resistance mutations. (d) Prevalence of resistance to antiretroviral drugs according to the latest version of the Stanford algorithm in 2011.
Drug resistance interpretations
The interpretation of resistance to NRTIs, NNRTIs and PIs according to the 2011 version of the Stanford algorithm is shown in Figure 2(d). For NRTIs, 66% of viruses were resistant to lamivudine/emtricitabine, 48% to abacavir, 42% to didanosine and stavudine, 40% to zidovudine and 33% to tenofovir. For NNRTIs, 56% were resistant to nevirapine, 52% to efavirenz and 38% to etravirine. For PIs, 25% were resistant to lopinavir and indinavir for each drug, 26% to tipranavir, 24% to nelfinavir, 22% to atazanavir, 20% to fosamprenavir, 19% to saquinavir and 12% to darunavir (Figure 2d). We also found 65% of viruses resistant to at least one NRTI, 60% to at least one NNRTI and 28% to at least one PI. The prevalence of viral strains resistant to all drugs in an antiretroviral class was 26% for NRTIs, 32% for NNRTIs and 11% for PIs. The prevalence of viruses resistant to all three drugs classes available in Mali was 8.6% (data not shown).
Factors associated with resistance to ART potentially used in a third-line regimen
Univariate analyses were performed to search for factors associated with resistance to different salvage antiretroviral drugs (abacavir, tenofovir, etravirine, lopinavir and darunavir). Duration of NRTI treatment was associated with resistance to abacavir (P < 0.0001) and tenofovir (P = 0.0001), while the duration of NNRTI treatment was associated with resistance to etravirine (P < 0.0001). The duration of PI treatment was associated with resistance to lopinavir (P < 0.0001) and darunavir (P = 0.06). Viral load was also associated with resistance to darunavir (P = 0.007), but not to lopinavir, abacavir, tenofovir or etravirine (all P ≥ 0.8). We detected an association between prior exposure to lopinavir and resistance to darunavir (P = 0.045).
The following variables were included in the final multivariate model: VL at failure, duration of PI treatment and the presence of L76V mutation. The two variables that were retained in the final multivariate model as independently associated with resistance to darunavir were the level of VL at failure (P = 0.06) and the presence of the L76V mutation (P = 0.003).
Discussion
We report here an analysis describing the prevalence of drug resistance mutations in 93 HIV-1-infected patients with VL ≥500 copies/mL after second-line highly active ART failure in Mali. We found 20% of patients who had a wild-type virus, which is probably due to a lack of adherence to treatment. Ferradini et al. in Cambodia showed only 7.7% (5/65) patients with HIV RNA detectable.10 We found resistance to at least one antiretroviral drug in ∼80% of patients using the latest version of the Stanford algorithm. At present, data on the resistance profiles after second-line treatment failure in Africa are very rare. Most of the resistance data available are from first-line failure and showed high levels of resistance to NNRTIs and high prevalence of TAMs, such as was reported in Togo and Benin.6,11 Interestingly, resistance prevalence following failure of second-line ART was slightly higher in other nationwide cross-sectional studies than our study. For example, 85% resistance to at least one drug was reported in France and Geneva in 200712 and 85% of children in the Central African Republic with 30 months of treatment harboured resistance to at least one drug.13 The prevalence was slightly lower in Iran, with 76% resistance to at least one drug.14 We could also estimate resistance to at least one NRTI in 65%, NNRTI in 60% and PI in 28% according to the latest version of the Stanford algorithm. Although the prevalence of resistance to at least one drug in Mali is lower compared with the results from developed countries, such as France and Switzerland, we urge caution in interpreting this because of differences in prior treatment experience and duration of ART exposure.12
The proportion of patients harbouring virus resistant to a drug class was 26% to all the NRTI family, 32% to NNRTIs and 11% to PIs. We found multi-class drug-resistant viruses (i.e. resistant to all the three antiretroviral classes available in Mali) in 8.6% (8/93). In the French national resistance network in 2009, Assoumou and colleagues found 10% of patients resistant to NRTI, 9.4% to NNRTI and 11.3% to PI class antiretrovirals.15 Triple-class resistance to NRTIs, NNRTIs and PIs was observed in 24 (53%) patients in the Indian cohort recently reported by Saravanan et al.16 The high prevalence of multidrug-resistant viruses in the present cohort is likely to have resulted in part from limited availability of biological monitoring (VL and resistance testing), leading to continuation of a failing regimen for a long time before switching to another regimen. The prevalence of resistance to potential drugs for second- or third-line therapy was 38% for etravirine, 25% to lopinavir and 12% to darunavir. Several studies have shown that the prevalence of NNRTI resistance in those failing first-generation NNRTI treatment is very high in Africa and threatens to compromise etravirine use in these settings.6,17 In Malawi, Hosseinipour et al.18,19 found 53% resistance to NNRTI after first-line failure. The high frequency of etravirine-resistant viruses is partly due to limited access to VL monitoring and very late switch to second-line therapies.20
The L76V mutation was found in 12% of patients in our study. This is an important finding since L76V can confer cross-resistance to PIs, such as lopinavir and darunavir,21 that can be used for second- or third-line therapy in resource-limited settings. Young and others found L76V in only 0.04% of a large cohort (20 501 sequences),22 and the mutation was associated with a 2- to 6-fold decrease in susceptibility to lopinavir, darunavir, amprenavir and indinavir and a 7- to 8-fold increase in susceptibility to atazanavir and saquinavir. The present prevalence of L76V is relatively high and this could be related to the type of PI used or the suboptimal activity of the NRTI backbone used in second-line therapy, perhaps making the regimen functional lopinavir monotherapy.23 Alternatively, it also may be due to the HIV subtype. However, this should be further investigated.
One limitation of our study is that we were not able to precisely determine the duration of viraemia because routine VL testing was not feasible; however, this makes our findings generalizable to other resource-limited settings. Another limitation is the absence of information about adherence to ART. Nevertheless, our results show a high prevalence of resistance to etravirine and lopinavir, as well as moderate resistance to darunavir. In our study, we analysed sequences with intermediate resistance according to the Stanford algorithm as resistant because affected drugs no longer exert full antiviral activity. It is important to have at least two fully active drugs in salvage regimens.3 In conclusion, while etravirine and darunavir became available through the national AIDS programme of Mali at the end of 2011, implementation of third-line therapy will be compromised considerably unless it is guided by viral load monitoring and resistance testing after second-line failure. The use of drugs such as raltegravir in the absence of viral load monitoring will probably lead to resistance to this compound as well and potentially compromise the future use of other integrase inhibitors.
Funding
This study was funded by ESTHER (Ensemble pour une Solidarité Hospitalière en Réseau) in Mali; the National AIDS Program in Mali (CSLS/MS), SOLTHIS (Solidarité Thérapeutique Hospitalière et Initiative contre le Sida) in Mali, ANRS (Agence Nationale de Recherche sur le SIDA) and The Northwestern University-United States National Institutes for Health/Fogarty International Center grant D43TW007995.
Transparency declarations
None to declare.
Author contributions
A. I. M., A. G. M. and V. C. designed the study and performed the statistical analyses. A. I. M. and A. G. M. wrote the first draft of the manuscript. All authors contributed to the interpretation of the results and to the final version of the manuscript. A. I. M and D. B. F. generated resistance data in the laboratory. M. C., F. D., M. Y. M. and H. A. T. were involved in patient recruitment, monitoring and outcomes. A. I. M. was the principal investigator of this study and had full access to all of the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgements
We thank all the study participants, Haidara Mohamed (Mali), Zaina Ait-Arkhoub (France), Alain Akonde (Mali), Louis Pizarro (France), Fatoumata Daou, Aboubacar Alassane Oumar and Aliou Balde (Mali), for their contribution, and the members of the HIV National Scientific Committee. This study was previously presented as a poster at the International AIDS Society conference in Rome (Italy) in July 2011 and as an oral presentation at the International Conference on AIDS and STIs in Africa (ICASA) 2011 in Addis Ababa (Ethiopia) in December 2011 (Abstract WEAA0301).
References
- 1.Germanaud D, Derache A, Traore M, et al. Level of viral load and antiretroviral resistance after 6 months of non-nucleoside reverse transcriptase inhibitor first-line treatment in HIV-1-infected children in Mali. J Antimicrob Chemother. 2010;65:118–24. doi: 10.1093/jac/dkp412. [DOI] [PubMed] [Google Scholar]
- 2.Marcelin AG, Jarrousse B, Derache A, et al. HIV drug resistance after the use of generic fixed-dose combination stavudine/lamivudine/nevirapine as standard first-line regimen. AIDS. 2007;21:2341–3. doi: 10.1097/QAD.0b013e328235a527. [DOI] [PubMed] [Google Scholar]
- 3.WHO. Rapid advice: antiretroviral therapy for HIV infection in adults and adolescents (30 November 2009) http://www.who.int/mediacentre/news/releases/2009/world_aids_20091130/fr/index.html. (01 July 2012, date last accessed)
- 4.El-Khatib Z, Ekstrom AM, Ledwaba J, et al. Viremia and drug resistance among HIV-1 patients on antiretroviral treatment: a cross-sectional study in Soweto, South Africa. AIDS. 2010;24:1679–87. doi: 10.1097/QAD.0b013e32833a097b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keiser O, Tweya H, Boulle A, et al. Switching to second-line antiretroviral therapy in resource-limited settings: comparison of programmes with and without viral load monitoring. AIDS. 2009;23:1867–74. doi: 10.1097/QAD.0b013e32832e05b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dagnra AY VN, Mensah A, Patassi A, et al. High prevalence of HIV-1 drug resistance among patients on first-line antiretroviral treatment in Lome, Togo. J Int AIDS Soc. 2011;14:30. doi: 10.1186/1758-2652-14-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monleau M, Faihum F, Affolabi D, et al. Antiretroviral drug resistance in HIV-1 infected patients receiving antiretroviral treatment in routine clinics in Cotonou, Benin. J AIDS HIV Res. 2011;3:114–20. [Google Scholar]
- 8.Ministry of Health, Mali. Politique et Protocoles de Prise en charge Antiretrovirale du VIH et du Sida. CSLS/HCNLS. 2010;3:1–84. [Google Scholar]
- 9.Eshleman SH, Hackett J, Jr, Swanson P, et al. Performance of the Celera Diagnostics ViroSeq HIV-1 Genotyping System for sequence-based analysis of diverse human immunodeficiency virus type 1 strains. J Clin Microbiol. 2004;42:2711–7. doi: 10.1128/JCM.42.6.2711-2717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferradini L, Ouk V, Segeral O, et al. High efficacy of lopinavir/r-based second-line antiretroviral treatment after 24 months of follow up at ESTHER/Calmette Hospital in Phnom Penh, Cambodia. J Int AIDS Soc. 2011;14:14. doi: 10.1186/1758-2652-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sylla M, Chamberland A, Boileau C, et al. Characterization of drug resistance in antiretroviral-treated patients infected with HIV-1 CRF02_AG and AGK subtypes in Mali and Burkina Faso. Antivir Ther. 2008;13:141–8. [PubMed] [Google Scholar]
- 12.Costagliola D, Descamps D, Assoumou L, et al. Prevalence of HIV-1 drug resistance in treated patients: a French nationwide study. J Acquir Immune Defic Syndr. 2007;46:12–8. doi: 10.1097/QAI.0b013e318074eb73. [DOI] [PubMed] [Google Scholar]
- 13.Charpentier C, Gody JMO, Moussa S, et al. Virological response and resistance profiles after 18 to 30 months of first- or second/third-line antiretroviral treatment: a cross-sectional evaluation in HIV-1-infected children living in the Central African Republic. AIDS Res Hum Retroviruses. 2012;28:87–94. doi: 10.1089/aid.2011.0035. [DOI] [PubMed] [Google Scholar]
- 14.Hamkar R, Mohraz M, Lorestani S, et al. Assessing subtype and drug-resistance-associated mutations among antiretroviral-treated HIV-infected patients. AIDS. 2010;24(Suppl 2):S85–91. doi: 10.1097/01.aids.0000386738.32919.67. [DOI] [PubMed] [Google Scholar]
- 15.Assoumou L, Descamps D, Yerly S, et al. Prevalence of HIV-1 drug resistance in treated patients with viral load >50 copies/mL in 2009: a French nationwide study. Antiviral Ther. 2010;15(Suppl 2):A185. [Google Scholar]
- 16.Saravanan S, Vidya M, Balakrishnan P, et al. Viremia and HIV-1 drug resistance mutations among patients receiving second-line highly active antiretroviral therapy in Chennai, southern India. Clin Infect Dis. 2012;54:995–1000. doi: 10.1093/cid/cir967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soria A, Porten K, Fampou-Toundji JC, et al. Resistance profiles after different periods of exposure to a first-line antiretroviral regimen in a Cameroonian cohort of HIV type-1-infected patients. Antivir Ther. 2009;14:339. [PubMed] [Google Scholar]
- 18.Hosseinipour MC, Kumwenda JJ, Weigel R, et al. Second-line treatment in the Malawi antiretroviral programme: high early mortality, but good outcomes in survivors, despite extensive drug resistance at baseline. HIV Med. 2010;11:510–8. doi: 10.1111/j.1468-1293.2010.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosseinipour MC, van Oosterhout JJ, Weigel R, et al. The public health approach to identify antiretroviral therapy failure: high-level nucleoside reverse transcriptase inhibitor resistance among Malawians failing first-line antiretroviral therapy. AIDS. 2009;23:1127–34. doi: 10.1097/QAD.0b013e32832ac34e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pujades-Rodríguez M, Balkan S, Arnould L, et al. Treatment failure and mortality factors in patients receiving second-line HIV therapy in resource-limited countries. JAMA. 2010:303–12. doi: 10.1001/jama.2010.980. [DOI] [PubMed] [Google Scholar]
- 21.Rhee SY, Taylor J, Fessel WJ, et al. HIV-1 protease mutations and protease inhibitor cross-resistance. Antimicrob Agents Chemother. 2010;54:4253–61. doi: 10.1128/AAC.00574-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young T, Parkin N, Stawiski E, et al. Prevalence, mutation patterns, and effects on protease inhibitor susceptibility of the L76V mutation in HIV-1 protease. Antimicrob Agents Chemother. 2010;54:4903–6. doi: 10.1128/AAC.00906-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delaugerre C, Flandre P, Chaix ML, et al. Protease inhibitor resistance analysis in the MONARK trial comparing first-line lopinavir-ritonavir monotherapy to lopinavir-ritonavir plus zidovudine and lamivudine triple therapy. Antimicrob Agents Chemother. 2009;53:2934–9. doi: 10.1128/AAC.01643-08. [DOI] [PMC free article] [PubMed] [Google Scholar]