Abstract
Aims
The channel function of the cardiac ryanodine receptor (RyR2) is modulated by calmodulin (CaM). However, the involvement of CaM in aberrant Ca2+ release in diseased hearts remains unclear. Here, we investigated the pathogenic role of defective CaM binding to the RyR2 in the channel dysfunction associated with heart failure.
Methods and results
The involvement of CaM in aberrant Ca2+ release was assessed in normal and pacing-induced failing canine hearts. The apparent affinity of CaM for RyR2 was considerably lower in failing sarcoplasmic reticulum (SR) compared with normal SR. Thus, the amount of CaM bound to RyR2 was markedly decreased in failing myocytes. Expression of the CaM isoform Gly-Ser-His-CaM (GSH-CaM), which has much higher binding affinity than wild-type CaM for RyR1, restored normal CaM binding to RyR2 in both SR and myocytes of failing hearts. The Ca2+ spark frequency (SpF) was markedly higher and the SR Ca2+ content was lower in failing myocytes compared with normal myocytes. The incorporation of GSH-CaM into the failing myocytes corrected the aberrant SpF and SR Ca2+ content to normal levels.
Conclusion
Reduced CaM binding to RyR2 seems to play a critical role in the pathogenesis of aberrant Ca2+ release in failing hearts. Correction of the reduced CaM binding to RyR2 stabilizes the RyR2 channel function and thereby restores normal Ca2+ handling and contractile function to failing hearts.
Keywords: Calmodulin, Ryanodine receptor, Sarcoplasmic reticulum, Calcium, Heart failure
1. Introduction
The cardiac Ca2+ release channel of the sarcoplasmic reticulum (SR), referred to as the ryanodine receptor (RyR2), is a huge scaffolding protein that is known to release a large amount of Ca2+ synchronously, producing the transient Ca2+ elevations required for muscle contraction on a beat-to-beat basis.1 In heart failure (HF), protein kinase A-dependent hyperphosphorylation and subsequent dissociation of RyR2-bound FKBP12.6,2,3 as well as Ca2+/calmodulin (CaM)-dependent protein kinase-II (CaMKII) activation, result in the leakage of Ca2+ through RyR2.4,5 We have reported that in pacing-induced HF, the interaction between the N-terminal domain (aa 1–600) and the central domain (aa 2000–2500) of RyR2, which acts as an implicit on/off switch (designated as the domain switch) for channel opening and closing, is aberrantly weak, resulting in Ca2+ leakage.6 We further demonstrated that oxidative stress is responsible for the defective inter-domain interaction and resultant Ca2+ leakage in failing hearts7 and that either K201 or dantrolene, which bind the 2114–2149 and 601–620 regions of RyR2, respectively, can correct the configuration of the domain switch from the aberrant unzipped state to a normal zipped state, thus restoring normal inter-domain interaction and correcting diastolic Ca2+ leakage.8,9
CaM is one of the accessory proteins of RyR2. One molecule of CaM binds to one subunit of RyR2 in the normal state.10 CaM binds to the 3583–3603 region of RyR2.11 Meissner and colleagues12 showed that RyR2-bound CaM was critical for normal muscle function and that CaM dissociation was involved in the pathogenesis of cardiac disorders. They generated a mouse with 3 amino acid substitutions in the CaM-binding domain (CaMBD) of RyR2 that abrogated the CaM binding to RyR2 and found that the mutant mouse developed hypertrophic cardiomyopathy with severely impaired contractile function and early death.12
In agreement with this report, we previously showed that RyR2-bound CaM, which acts essentially as an inhibitory modulator of channel gating over a wide [Ca2+] range, dissociates from RyR2 as a result of domain unzipping of the domain switch.13,14 Namely, the binding of CaM to RyR2 was markedly reduced in failing hearts13 or the catecholaminergic polymorphic ventricular tachycardia (CPVT)-type knock-in mouse model (R2474S),14 causing aberrant Ca2+ release and leading to HF and lethal arrhythmia. Thus, these findings suggest that aberrant unzipping of the domain switch in cardiac disorder, such as HF and CPVT, causes weakened CaM binding to the RyR2, which in turn causes leaky Ca2+ channels.
The CaMBD (3614–3643) of RyR1 has been reported to interact with the CaM-like domain (CaMLD: 4064–4210) of RyR1.15 The aa 4020–4166 segment of RyR2 is homologous to the CaMLD of RyR1. Recently, Ikemoto and colleagues16 showed that a Ca2+-dependent tight interaction between the CaMBD and the CaMLD (formation of an ‘activation link’) activates RyR channels. More recently, they demonstrated that CaM acts as a molecular ‘wedge’ to inhibit excessively tight formation of the CaMBD/CaMLD activation link in endothelin-1 (ET-1)-treated neonatal cardiomyocytes and thus prevents the hypertrophy that would otherwise develop in these cells.17 These results suggest that the excessively tight interaction between the CaMBD and the CaMLD results in the reduced CaM binding and an aberrant activation of the channel and diastolic Ca2+ leakage as seen in both HF and CPVT. Then, blockade of this excessively tight formation of the activation link will stabilize the channel in its closed state and will correct the channel leakage problem.
In the present study, we further investigated the underlying mechanism by which CaM dissociates from RyR2 in failing hearts and whether correction of the aberrant CaM–RyR2 interaction restores normal channel gating in failing RyR2.
2. Methods
For a detailed description, see also the expanded Materials and methods section in Supplementary material online.
2.1. Animal model
HF was induced in beagle dogs weighing 10–13 kg by continuous application of rapid ventricular pacing at 250 b.p.m. using an externally programmed miniature pacemaker (Taisho Biomed Instruments Co., Ltd) for 28 days, as described previously.3 Dogs were deeply anaesthetized before implantation of pacemaker or removal of hearts. At the end of the study, each animal was deeply anaesthetized and euthanized with an isoflurane and intravenous injection of sodium pentobarbital (50 mg/kg). This study conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). This study was approved by the Animal Ethics Committee of Yamaguchi University School of Medicine, and the care of the animals and the protocols used were in accordance with the guidelines laid down by the Animal Ethics Committee of Yamaguchi University School of Medicine.
2.2. Expression and purification of CaM and Gly-Ser-His-CaM
Human CaM cDNA was PCR amplified with oligonucleotide primers designated to include two restriction enzyme sites (the forward primer 5′-ACACAGGGGATCCCATATGGCTGAC-3′ and the reverse primer 5′-CAAGCTTGGCTCGAGTCACTTTGC-3′). The cDNA was inserted into a pGEX4T-1 vector. The expression vector was transformed into DH5α Escherichia coli (Nippongene). The strain was preincubated with Lysogeny Broth (LB) ampicillin for 16 h at 30°C followed by 2 h incubation with 10 times the volumes of LB ampicillin at 37°C.
2.3. Preparation of SR vesicles
SR vesicles were prepared from dog left ventricle (LV) muscle as previously described3 with a slight modification. Briefly, LV homogenate was centrifuged at 5500 g and the resultant supernatant fraction was purified by further extensive centrifugation (12 000 g followed by three cycles at 143 000 g). The final protein concentration was 10–20 mg protein/mL.
2.4. Peptides and peptide synthesis
The following RyR2 domain peptides were used: DPc10, which harbours a CPVT-type mutation site (R2474S), and DP4090–4123, both of which have been described previously.6,18
DPc10 (DP2460–2495): 2460-GFCPDHKAAMVLFLDRVYGIEVQDFLLHLLEVGFLP-2495
DP4090–4123: 4090-PAKDIGFNVAVLLTNLSEHMPNDTRLQTFLELAE-4123
Peptides were synthesized with an Applied Biosystems model 431A synthesizer using Fmoc (N-(9-fluorenyl)methoxycarbonyl) as the α-amino protecting group, as described previously.19 Boldface italic letter means the position of CPVT-type mutation site.
2.5. Site-directed fluorescent labelling of the DPc10-binding site in RyR2
Specific fluorescent labelling of RyR2 was performed with the fluorescent conformational probe sulfosuccinimidyl-2-(7-azido-4-methylcoumarin-3-acetamido)ethyl-1,3′-dithiopropionate (SAED, Pierce) with DPc10 as a site-specific carrier, as described previously.6,8
2.6. Preparation of isolated cardiomyocytes, measurement of cell shortening, and Ca2+ sparks
Cardiomyocytes were isolated from the canine LV as described previously.6 Briefly, a wedge of LV free wall was perfused with a collagenase-containing buffer solution and rod-shaped adult canine cardiomyocytes were prepared.
Ca2+ sparks were measured in saponin-permeabilized cardiomyocytes using Fluo-3 with a laser-scanning confocal microscope (LSM-510, Carl Zeiss).13 Ca2+ spark images were obtained in the presence of the CaMKII inhibitor KN-93 (1 µmol/L). Measurements of myocyte sarcomere shortening and intracellular Ca2+ were performed using fura-2 AM with cells stimulated by a field electric stimulator (IonOptix, MA, USA) by 0.5 Hz. Data were analysed with SparkMaster, an automated analysis program that enables rapid and reliable spark analysis.20
2.7. Binding of CaM to RyR2
We assessed the binding of CaM to RyR2 using a photoreactive crosslinker, sulfosuccinimidyl-6-[4′-azido-2′-nitrophenylamino]hexanoate (Sulfo-SANPAH, Pierce), as described previously.13 To assess direct binding ability of CaM to the RyR2 in cardiomyocytes, we added the exogenous CaM, fluorescently labelled with Alexa Fluor 633 (Molecular Probes, OR, USA), to the saponin-permeabilized cardiomyocytes. To determine the binding of endogenous CaM to the RyR2 in intact cardiomyocytes, we placed isolated cardiomyocytes on glass-based dishes, fixed with 4% paraformaldehyde in phosphate-buffered saline for 10 min, followed by immunostaining with a monoclonal mouse anti-CaM antibody and a polyclonal rabbit anti-RyR antibody.
2.8. Immunoblot analysis
Immunoblot analyses for cytological detection of CaM and RyR2 were performed using an anti-CaM antibody (Millipore, CA, USA), an anti-RyR antibody [mouse monoclonal C3-33 (Sigma) or rabbit polyclonal C-terminal Ab4963 (Sigma)], and an antibody against the RyR2 CaM-binding domain (3583–3603) (Sigma).
2.9. Measurement of the binding of exogenous CaM to RyR2 in saponin-permeabilized cardiomyocytes
Exogenous CaM was fluorescently labelled with Alexa Fluor 633 (Molecular Probes) as previously described13 and added to saponin-permeabilized normal and failing cardiomyocytes under the same conditions described for the Ca2+ spark measurements. The subcellular distribution of CaM was then quantified by densitometric measurement of CaM-Alexa fluorescence.
2.10. Measurement of the binding of endogenous CaM to RyR2 in intact cardiomyocytes
Isolated cardiomyocytes were fixed with 4% paraformaldehyde and permeabilized in 0.5% Triton X-100 and 1% bovine serum albumin. Co-localization of endogenous CaM and RyR2 was then detected by immunofluorescence.
2.11. Statistics
Unpaired t-tests were used for statistical comparison of data obtained under two different sets of conditions. Data are expressed as means ± standard deviations (SD). A P-value of <0.05 was accepted as statistically significant.
3. Results
3.1. Characteristics of normal and failing heart and cardiomyocytes
After 4 weeks, pacing LV chamber size was dilated and fractional shortening was reduced (see Supplementary material online, Table S1). Both the width and the length of the isolated failing cardiomyocytes were larger than those of normal cardiomyocytes (see Supplementary material online, Table S2). The number of cells yielded from one wedge of the heart was fewer in failing hearts [n=4: 155±28 (×103), P<0.05] than in normal hearts [n=4: 213±27 (×103)].
3.2. Wild-type CaM and Gly-Ser-His-CaM enhance CaMKII activity to similar extents
We tested the hypothesis (cf. Section 1) that restoring the CaM-binding stabilizes the RyR2 channel, inhibits Ca2+ leakage, and corrects myocyte dysfunction in failing hearts. For this purpose, we examined whether Gly-Ser-His-CaM (GSH-CaM), which was previously reported to show higher binding affinity than wild-type CaM (WT-CaM) for RyR1,21 also shows higher binding affinity for RyR2 and thereby corrects the defects in RyR2 function in failing hearts.
There are two major pathways by which CaM regulates RyR2 channel function. The first is the inhibition of the channel by a direct interaction between CaM and RyR2, which is the main subject of the present study (cf. Section 1). The other is the activation of the channel mediated by Ca2+/CaM activation of CaMKII-dependent phosphorylation of RyR2 at Ser2814.4 In order to compare the specific effects of direct binding of GSH-CaM and WT-CaM on RyR2 channel inhibition, we inhibited CaMKII activity in cardiomyocytes with the CaMKII inhibitor KN-93 (1 µmol/L) in the present study.
We also performed the assays in the absence of the inhibitor KN-93 to determine whether there is any qualitative difference in the effects of CaM and GSH-CaM on CaMKII-mediated RyR2 activation. As shown in Supplementary material online, Figure S1, the concentration dependence of CaMKII activation was indistinguishable between WT-CaM and GSH-CaM. This suggests that WT-CaM and GSH-CaM may have essentially the same affinity for CaMKII.
3.3. The affinity of GSH-CaM for RyR2 is higher than that of WT-CaM
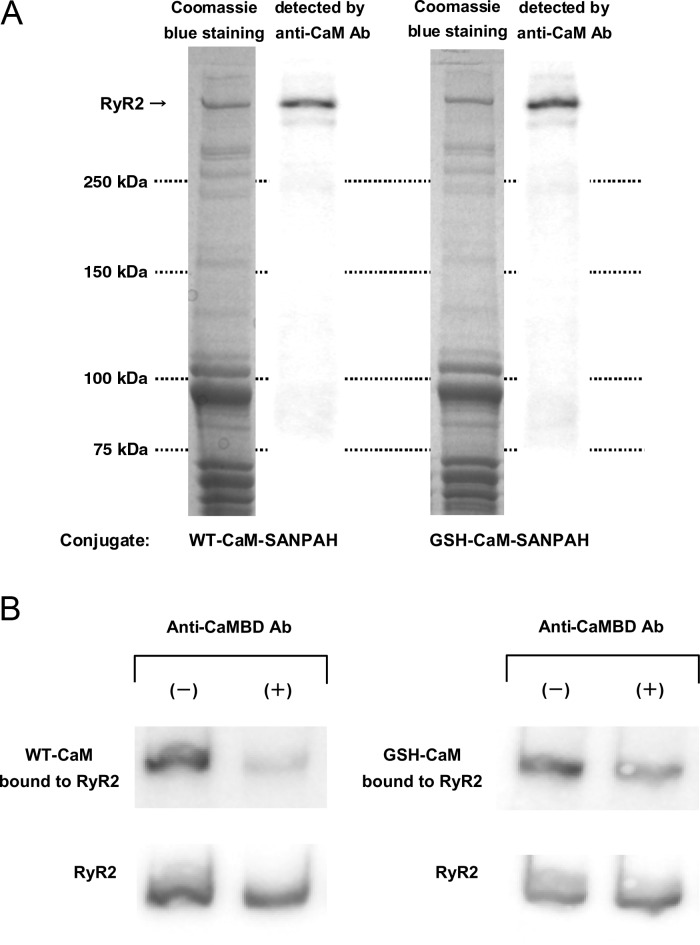
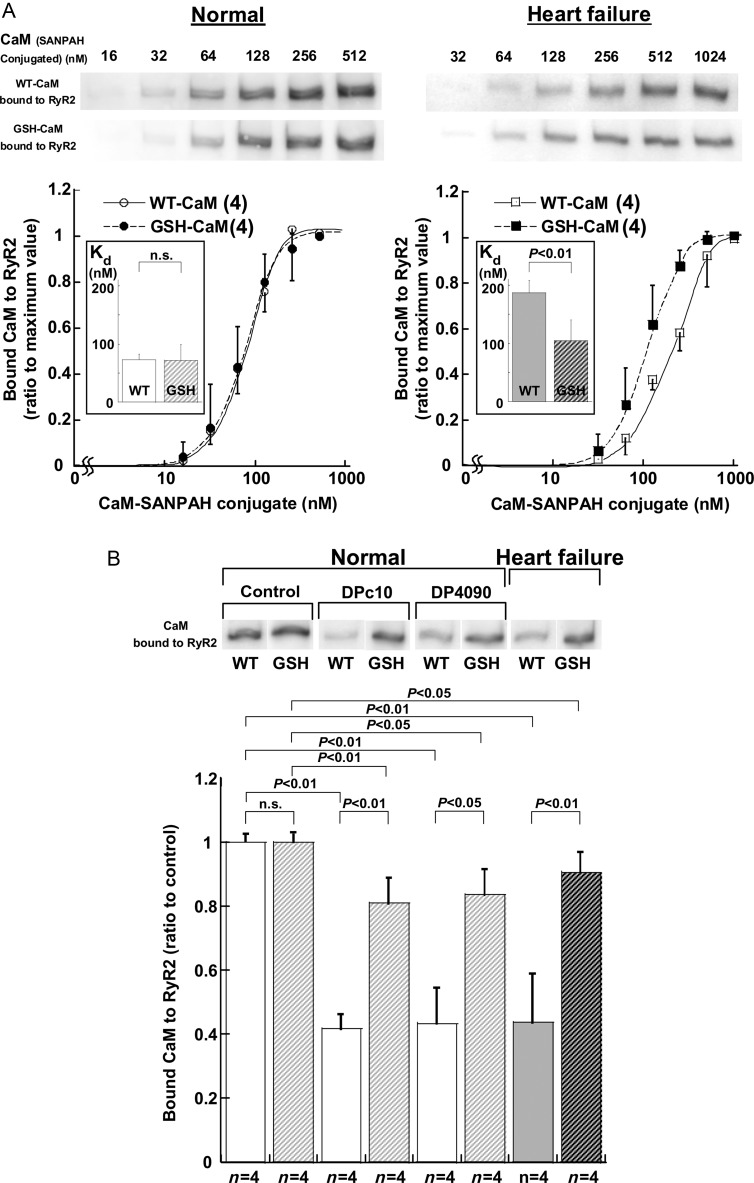
We evaluated the affinity of binding of WT-CaM and GSH-CaM to RyR2 by densitometric analysis of immunoblots of CaM cross-linked to RyR2. Regardless of whether WT-CaM or GSH-CaM was used, the anti-CaM antibody detected only RyR2 out of the many proteins in the SR, indicating that the binding of both WT-CaM and GSH-CaM is very specific to RyR2 (Figure 1A). The binding of either WT-CaM or GSH-CaM to RyR2 in the SR was markedly inhibited by the addition of an antibody raised against the CaMBD (3583–3603) (Figure 1B). This suggests that the 3583–3603 domain is indeed the major CaM/GSH-CaM-binding site of RyR2. Figure 2A shows that the apparent affinity of GSH-CaM for normal RyR2, which is estimated from the CaM concentration at half-maximal binding, is virtually indistinguishable from that of WT-CaM. In agreement with our previous finding,13 the apparent affinity of CaM for RyR2 was considerably reduced in failing SR compared with normal SR, as indicated by a significant right-shift of the concentration dependence of CaM binding to RyR2 from failing heart. The apparent affinity of GSH-CaM for RyR2 from the failing heart was, however, comparable with that of WT-CaM and GSH-CaM for RyR2 from normal SR (Figure 2A; see also Figure 2B, heart failure). Figure 2B shows that several previously reported probes that mimic pathological conditions also considerably reduce the binding of WT-CaM, but not GSH-CaM, to RyR2 from normal SR. Thus, DPc10, which mimics the aberrant unzipping of the domain switch induced by the R2474S CPVT-type mutation,6,22 and DP4090–4123,18 which mimics the CPVT-linked defective inter-domain interaction between the I domain (3772–4610)23 and its putative partner domain, significantly reduced the binding of WT-CaM but only slightly reduced the binding of GSH-CaM to RyR2 (Figure 2B).
Figure 1.
Cross-linking of CaM or GSH-CaM to the ryanodine receptor (RyR2). (A) Representative immunoblot of CaM or GSH-CaM cross-linked to RyR2. Note that the anti-CaM (or GSH-CaM) antibody detected only RyR2 among the many proteins in the SR. (B) Effect of the antibody against the CaM-binding domain (3583–3603; anti-CaMBD antibody) of RyR2 on CaM (or GSH-CaM) binding to RyR2.
Figure 2.
(A) CaM (or GSH-CaM) concentration dependence of the binding of CaM to normal RyR2 (top) and the summarized data (bottom). The immunoblot density of the CaM cross-linked to RyR2 was determined at various concentrations of CaM (or GSH-CaM)-SANPAH as indicated and expressed as the ratio to the maximum value obtained at 1 µmol/L CaM (or GSH-CaM). Each datum point per concentration represents mean ± SD of four SR preparations from four hearts, and the sigmoid concentration-dependent relationships for CaM binding was fitted by an equation: y = aKnxn/(1 + Knxn), and the EC50 values were calculated as 1/K. (Inset) Comparison of EC50. Paired t-test was employed to determine the statistical significance of EC50. The numerical value in the parenthesis means the number of concentration-dependent relationships for CaM binding. (B) Cross-linking of CaM or GSH-CaM (128 nmol/L) to RyR2 in DPc10 (30 µmol/L)- or DP4090–4123 (30 µmol/L)-treated SR vesicles from normal and failing hearts. Representative immunoblots of CaM bound to RyR2 (top) and summarized data (bottom) are shown. The immunoblot density of CaM cross-linked to RyR2 was measured and expressed as the ratio to the control. Data represent the means ± SD obtained from four SR preparations.
3.4. Binding of CaM but not GSH-CaM to RyR2 is inhibited by DPc10 and DP4090–4123 in isolated cardiomyocytes
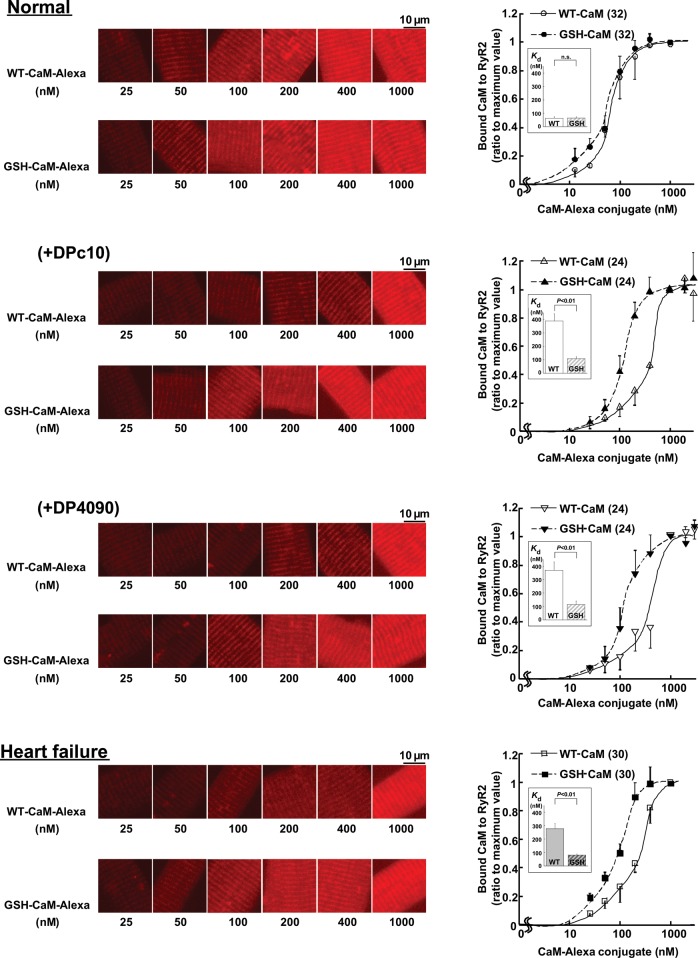
To assess whether the high-affinity binding of GSH-CaM to RyR2 under pathological conditions that we observed in the isolated SR is also preserved in cardiomyocytes, we examined the effect of DP4090–4123 or DPc10 on the binding affinities of CaM and GSH-CaM for RyR2 in cells. We evaluated the binding of Alexa-labelled WT-CaM or GSH-CaM to subcellular fractions of saponin-permeabilized cardiomyocytes (see Supplementary material online, Figure S2). Both WT-CaM-Alexa and GSH-CaM-Alexa co-localized with the anti-RyR2 immunostaining, suggesting that exogenous WT-CaM and GSH-CaM introduced into cardiomyocytes both bind to the RyR2 (see Supplementary material online, Figure S2). When we plotted the intensity of the Alexa fluorescence along the sarcomeres (see Section 2) as a function of the concentration of the introduced CaM-Alexa or GSH-CaM-Alexa in normal myocytes (Normal), the concentration dependence of GSH-CaM binding was slightly shifted towards the lower concentrations below 100 nmol/L and unchanged above 100 nmol/L relative to the dependence of WT-CaM binding (Figure 3). In the cardiomyocytes treated with either DPc10 or DP4090–4123, however, the concentration dependence of WT-CaM binding was greatly shifted towards higher concentrations, while the binding affinity of GSH-CaM appeared unchanged. These data suggest that defective inter-domain interaction (domain unzipping) of either the domain switch or the near C-terminal region [the CaMLD (4020–4166) and its partner domain] commonly reduces the binding of CaM to RyR2, while GSH-CaM can maintain normal binding to RyR2 even in the presence of either peptide. GSH-CaM, but not WT-CaM, bound normally to RyR2 in failing cardiomyocytes. These results are consistent with the data for CaM-SANPAH binding to RyR2 in the SR vesicles (Figure 2).
Figure 3.
The binding characteristics of exogenously introduced CaM in saponin-permeabilized cardiomyocytes. Delivery of various concentrations of CaM-Alexa or GSH-CaM-Alexa (left panel) and the summarized data (right panel) in normal cardiomyocytes and failing cardiomyocytes treated with RyR2 domain peptides (DPc10, 30 µmol/L, or DP4090–4123, 30 µmol/L). Either WT-CaM-Alexa or GSH-CaM-Alexa fluorescence was measured and expressed as the ratio to its maximum value. Each datum point per concentration represents mean ± SD of 8–10 cells from three to four hearts, and the sigmoid concentration-dependent relationships for CaM binding was fitted by an equation: y = aKnxn/(1 + Knxn), and the EC50 values were calculated as 1/K. (Inset) Comparison of EC50. Paired t-test was employed to determine the statistical significance of EC50. The numerical value in the parenthesis means the number of concentration-dependent relationships for CaM binding.
3.5. Increased CaM binding affinity corrects elevated Ca2+ spark frequency in saponin-permeabilized failing cardiomyocytes
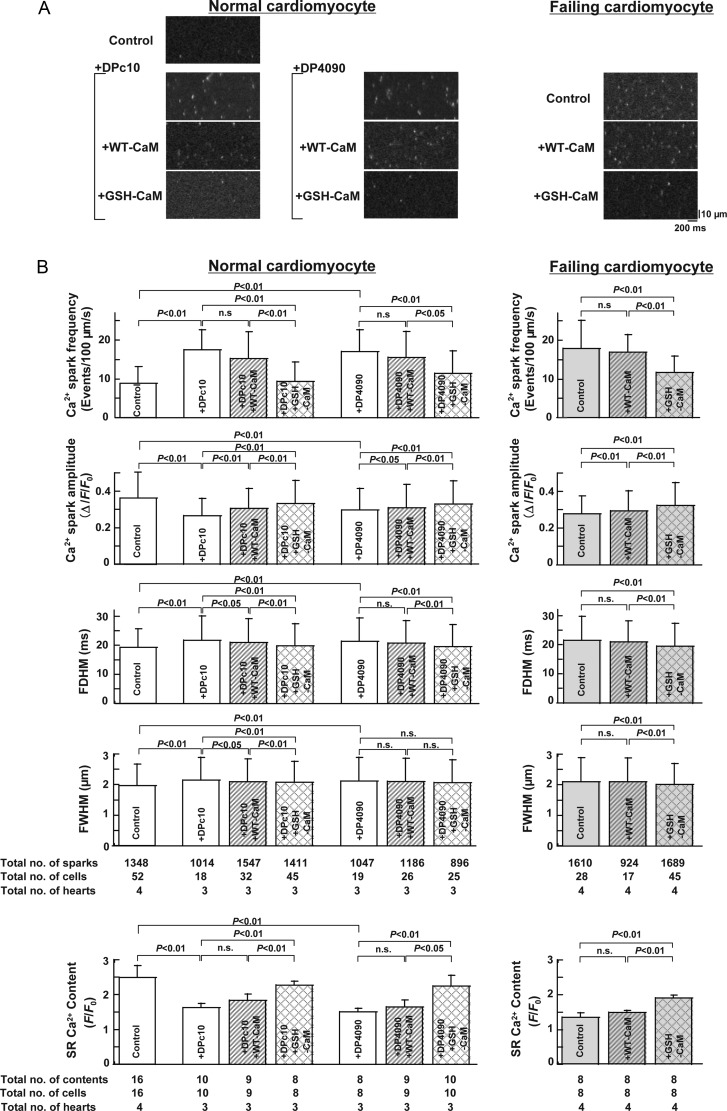
To examine whether correction of the defective interaction between CaM and RyR2 inhibits spontaneous Ca2+ release events, we measured the frequency of Ca2+ sparks in saponin-permeabilized cardiomyocytes (Figure 4). Both DPc10 and DP4090–4123 increased the Ca2+ spark frequency (SpF) in normal cardiomyocytes. Exogenous GSH-CaM, but not WT-CaM, blocked the increases in SpF induced by both peptides. The baseline Ca2+ SpF was significantly increased in failing cardiomyocytes. The addition of GSH-CaM, but not WT-CaM, blocked the increase in SpF. As shown in Figure 4B, both the full width at half-maximum (FWHM) and full duration at half-maximum (FDHM) increased under all pathological conditions (cardiomyocytes from failing hearts or normal myocytes treated with DPc10 or DP4090–4123), and GSH-CaM corrected both parameters to noticeable extents than did WT-CaM. In contrast to the greater spark frequencies observed under pathological conditions, the Ca2+ spark amplitudes were lower in both peptide-treated normal cardiomyocytes and failing cardiomyocytes, and GSH-CaM increased the Ca2+ spark amplitude to a greater extent than did WT-CaM.
Figure 4.
Effect of WT-CAM (or GSH-CaM) (200 nmol/L) on Ca2+ SpF and SR Ca2+ content in (saponin-permeabilized) normal cardiomyocytes and failing cardiomyocytes treated with RyR2 domain peptides (DPc10, 30 µmol/L, or DP4090–4123, 30 µmol/L). SR Ca2+ content was measured by adding 10 mmol/L caffeine. Ca2+ spark images were obtained in the presence of the CaMKII inhibitor KN-93 (1 µmol/L). (A) Representative images of Ca2+ sparks. (B) Summarized data of the FDHM, FWHM, Ca2+ spark amplitude, Ca2+ SpF, and SR Ca2+ content. Data represent the means ± SD of 8–32 cells from each of three to four hearts.
As shown in the bottom panel of Figure 4B, both peptides increased the SpF and decreased the SR Ca2+ content. GSH-CaM, but not WT-CaM, prevented the peptide-induced increase in SpF and decrease in SR Ca2+ content. Baseline SpF was increased and SR Ca2+ content decreased in failing cardiomyocytes, and both were restored to normal levels by GSH-CaM but not by WT-CaM.
3.6. GSH-CaM corrects defective Ca2+ transient and cell shortening in intact (‘non-permeabilized’) failing cardiomyocytes
3.6.1. Incorporation of WT-CaM or GSH-CaM into intact cardiomyocytes and effects of WT-CaM and GSH-CaM on peptide-treated normal cardiomyocytes and failing cardiomyocytes
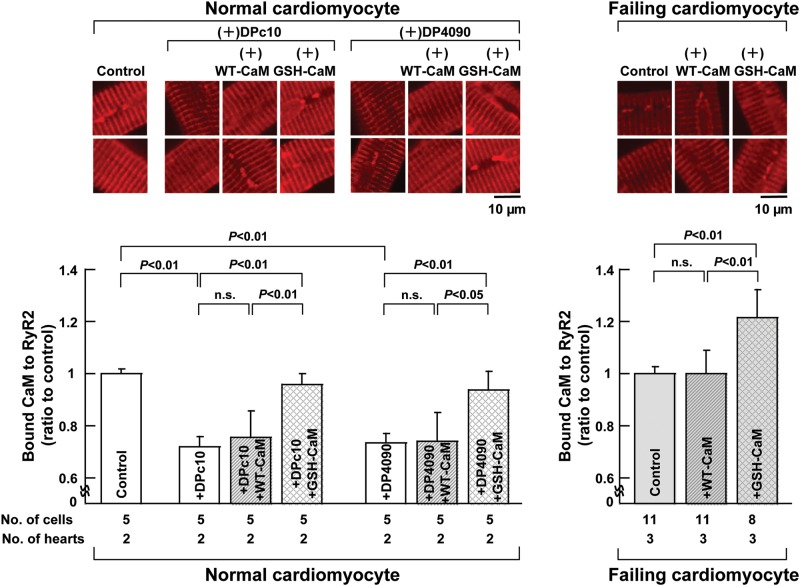
Incorporation of either WT-CaM or GSH-CaM into intact cardiomyocytes was successfully achieved by the use of a protein delivery kit (see Supplementary material online, Expanded Materials and Methods). The intracellular concentrations of GSH-CaM and CaM were 127 ± 38 nmol/L (n = 6 cells) and 131 ± 55 nmol/L (n = 7 cells), respectively (difference: statistically insignificant). As shown in Supplementary material online, Figure S3, CaM was detected along the sarcomeres by immunostaining and co-localized with RyR2. Both DPc10 and DP4090–4123 decreased the binding of endogenous CaM to RyR2. GSH-CaM, but not WT-CaM, restored normal binding to RyR2; a similar pattern was seen in failing cardiomyocytes, in which CaM-GSH, but not WT-CaM, restored CaM binding to RyR2 (Figure 5).
Figure 5.
Effect of incorporation of WT-CaM or GSH-CaM on the binding of CaM to RyR2. Representative images of the binding of CaM to RyR2 (top) and the summarized data (bottom) are shown. Data represent means ± SD of 5–11 cells from each of two to three hearts.
As shown in Supplementary material online, Figure S4, Ca SpF was increased and SR Ca2+ content was reduced in intact (non-permeabilized) failing cardiomyocytes. Importantly, the incorporation of GSH-CaM (by protein delivery kit), but not WT-CaM, restored normal levels in the intact non-permeabilized cardiomyocytes, as in the case of permeabilized myocytes. KN93 was without an effect on Ca2+ SpF and SR Ca2+ content in both intact (non-permeabilized) normal and failing cardiomyocytes (see Supplementary material online, Figure S5).
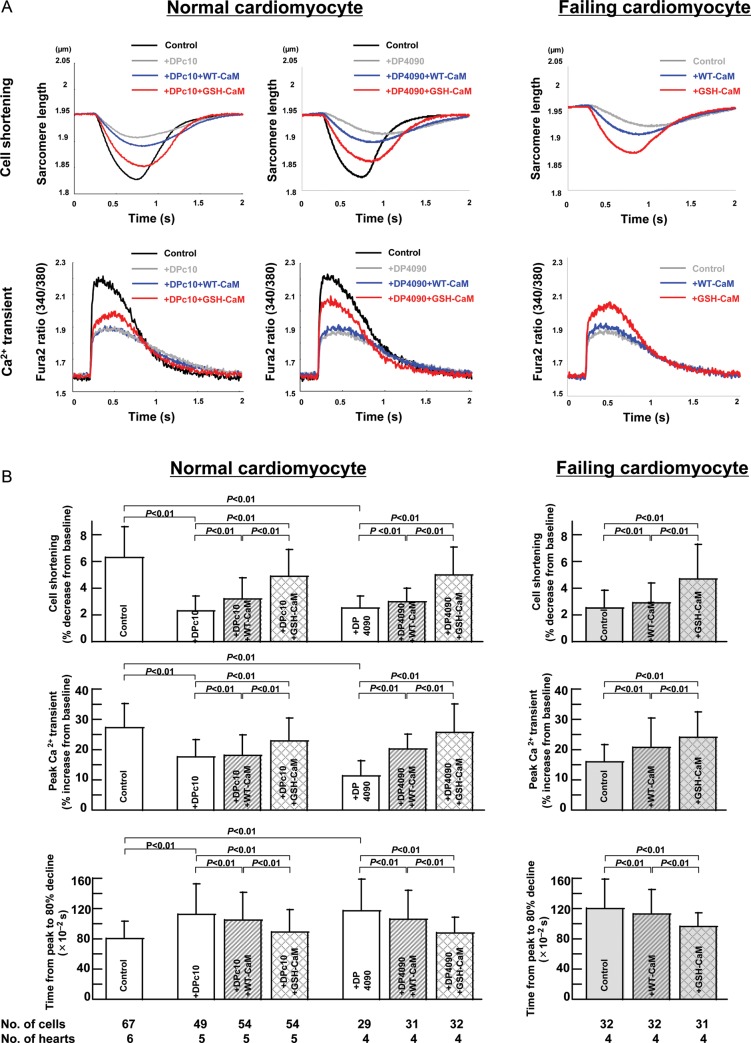
3.6.2. Effect of WT-CaM or GSH-CaM on Ca2+ transient and cell shortening in normal and failing cardiomyocytes
Figure 6B summarizes the characteristics of both Ca2+ transient and cell shortening in normal and failing cardiomyocytes. As shown, both Ca2+ transient and cell shortening were reduced in both DPc10- or DP4090–4123-treated normal cardiomyocytes and failing cardiomyocytes (Figure 6A). Incorporation of GSH-CaM but not WT-CaM improved both cell shortening and Ca2+ transient in DPc10- or DP4090–4123-treated normal cardiomyocytes and failing cardiomyocytes. KN93 was without the effect on Ca2+ transient and cell shortening in both intact (non-permeabilized) normal and failing cardiomyocytes (see Supplementary material online, Figure S6).
Figure 6.
Effect of CaM or GSH-CaM on Ca2+ transient and cell shortening in normal and failing cardiomyocytes. (A) Representative tracings of Ca2+ transient and cell shortening in normal and failing cardiomyocytes. (B) The characteristics of cell shortening and Ca2+ transient in normal and failing cardiomyocytes treated with RyR2 domain peptides (DPc10, 30 µmol/L, or DP4090–4123, 30 µmol/L).
To investigate the kinetic response of the contractile proteins to changes in [Ca2+], we plotted per cent cell shortening as a function of Ca2+ transient (see Supplementary material online, Figure S7). Although the area of the cell shortening-Ca2+ transient loop was reduced in DPc10- or DP4090–4123-added normal cardiomyocytes and failing cardiomyocytes, the slope during the deceleration phase of Ca2+ transient was virtually unchanged among all groups. This suggests that the dynamic Ca2+ sensitivity did not change by domain unzipping and subsequent Ca2+ leakage. GSH-CaM, but not WT-CaM, normally restored the area of the loop in DPc10- or DP4090–4123-added normal cardiomyocytes and failing cardiomyocytes.
4. Discussion
Accumulated evidence concerning the pathogenic mechanism of HF suggests that abnormal intracellular Ca2+ handling leads to cardiac dysfunction and lethal arrhythmia.24
The most important aspect of the present study is the finding that correction of the reduced CaM binding in the ‘destabilized’ RyR2 to a normal level restores normal channel gating and improves contractile function in failing hearts (see Supplementary material online, Figure S8). This indicates that increased binding of CaM serves as a ‘natural therapeutic agent,’ a hypothesis supported by the following evidence. GSH-CaM, which shows higher binding affinity than WT-CaM for RyR2, supported a normal level of CaM binding to RyR2 when added to CPVT-type defective RyR2 experimentally produced by DPc10 or DP4090–4123, where the binding affinity of WT-CaM is much reduced. Consequently, the increased CaM binding prevented diastolic Ca2+ leakage as indicated by the reduced frequency of spontaneous Ca2+ sparks. Similarly, the binding of GSH-CaM to RyR2 from failing cardiomyocytes restored normal spontaneous Ca2+ SpF, Ca2+ transient, and cell shortening.
The following important question then arises: what is the mechanism by which the RyR2-bound CaM controls RyR2 channel function? It has been postulated that the CaMLD/CaMBD interaction activates the Ca2+ channel, while competitive binding of CaM to the CaMBD suppresses channel activation and stabilizes the closed state of the channel (cf. Section 1).16 Under pathological conditions such as HF and CPVT, the CaMLD/CaMBD interaction becomes excessively tight, which overcomes the intrinsic affinity CaM for its binding to the CaMBD. According to this scheme, increasing the CaM binding affinity over the intrinsic level restores the ability of CaM to ‘wedge’ and disrupts the excessively tight channel activation interaction between the CaMLD and the CaMBD. In the present study, the introduction of GSH-CaM, which has a significantly higher affinity than WT-CaM for RyR2, permitted us to demonstrate that this previously postulated mechanism in fact operates. Importantly, we have further shown that dantrolene, which corrects defective unzipping, restored a normal level of binding of WT-CaM and normal RyR2 function in these disease models.13,14 These findings clearly indicate that the formation of an excessively tight CaMLD/CaMBD interaction, which causes aberrant channel activation, is mediated by unzipping (perhaps sustained unzipping) of the domain switch.
Ikemoto and colleagues17 recently showed that hypertrophic stimulus of neonatal cardiomyocytes with ET-1 induced dissociation of CaM from RyR2; this leads to a sequence of intracellular events including increased frequency of spontaneous Ca2+ transients and translocation of CaM, CaMKII, and N-FAT to the nucleus. They further demonstrated that an anti-CaMBD antibody, used as a ‘molecular wedge’ to disrupt the CaMBD/CaMLD interaction, prevented all of these ET-1-induced pathological intracellular events and consequently the development of hypertrophy.25 These data support the hypothesis that aberrant formation of the channel-activating interaction between the CaMBD and the CaMLD of RyR2 is an early key event leading to the development of hypertrophy in this cell model.
Thus, our recent and present findings suggest that unzipping of the domain switch and zipping of the CaMBD/CaMLD pair are coupled for the channel opening. According to George et al.,23 channel function is regulated by the interaction between RyR cytoplasmic and transmembrane domains via amino acids residues 3722–4610 (designated as I-domain), and leaky Ca2+ channels are characterized by unstable interactions between the I domain and the transmembrane channel domain. The present finding that DP4090–4123, a peptide corresponding to a part of the I-domain, activated the channel presumably by interfering with a tight interaction of the I-domain with the channel domain, causing their unzipping. Summarizing the accumulated pieces of information, we propose a general hypothesis that in the systolic phase of normal channels, unzipping of (A) the domain switch (the N-terminal domain/central domain pair), zipping (formation of a tight link) of (B) the CaMBD/CaMLD pair, and unzipping of (C) the I-domain/channel domain pair are conformationally coupled in an allosteric manner to activate the channel. In the diastolic phase of normal operation, reversed conformational changes take place in these three interacting pairs, again in a tightly coupled manner. In disease models, and peptide-treated mimicries of the pathological state, destabilization of the zipped state of regions A and C and formation of a tighter link in the region B take place again in a conformationally coupled manner in the diastolic state, causing erroneous channel activation and diastolic Ca2+ leakage. This scheme of conformational coupling suggests the possibility that correcting the problem in any of these three regions (A, B, and C) might correct the defective conformational state in the other regions. However, according to our preliminary data (not shown), the addition of GSH-CaM to SR from failing hearts or SR treated with DPc10, which restored normal CaM binding and normal channel closing, could not fully restore the normal zipped state of the domain switch. This suggests that in pathological conditions, the conformational coupling from the region B to the region A is loose or incomplete presumably owing to a sustained, or chronic, unzipped state of the domain switch, which can be restored to normal zipped state only by the agents such as dantrolene and K201 that are directed to the domain switch.
Although the pacing-induced HF model is widely recognized as a representative model of human HF because of the structural and functional similarity, whether we can extrapolate the present findings to other HF (e.g. post-myocardial infarction, ischaemic cardiomyopathy, hypertensive heart disease, etc.) must wait for future studies.
In conclusion, the defective inter-domain interaction between the N-terminal domain and the central domain within RyR2, coupled with a second defective inter-domain interaction between the CaMBD and the CaMLD, plays a key role in the pathogenic mechanism by which the channel is destabilized. The reduced binding of CaM to RyR2 caused by the defective inter-domain interactions predisposes failing hearts to aberrant Ca2+ release and lethal arrhythmia. Correction of the reduced CaM binding to the failing RyR2 can stabilize the channel function in the diseased hearts.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by grants-in-aid for scientific research from The Ministry of Education in Japan (grant nos 22659154 and 23390215 to M.Y., 23591082 to T.Y., and 23592256 to S.K.) and grant from the National Heart, Lung and Blood Institutes (HL072841 to N.I.).
Supplementary Material
References
- 1.Bers DM. Macromolecular complexes regulating cardiac ryanodine receptor function. J Mol Cell Cardiol. 2004;37:417–429. doi: 10.1016/j.yjmcc.2004.05.026. doi:10.1016/j.yjmcc.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 2.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. doi:10.1016/S0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 3.Yano M, Ono K, Ohkusa T, Suetsugu M, Kohno M, Hisaoka T, et al. Altered stoichiometry of FKBP12.6 versus ryanodine receptor as a cause of abnormal Ca2+ leak through ryanodine receptor in heart failure. Circulation. 2000;102:2131–2136. doi: 10.1161/01.cir.102.17.2131. doi:10.1161/01.CIR.102.17.2131. [DOI] [PubMed] [Google Scholar]
- 4.Guo T, Zhang T, Mestril R, Bers DM. Ca2+/calmodulin-dependent protein kinase II phosphorylation of ryanodine receptor does affect calcium sparks in mouse ventricular myocytes. Circ Res. 2006;99:398–406. doi: 10.1161/01.RES.0000236756.06252.13. doi:10.1161/01.RES.0000236756.06252.13. [DOI] [PubMed] [Google Scholar]
- 5.Maier LS, Zhang T, Chen L, DeSantiago J, Brown JH, Bers DM. Transgenic CaMKIIdeltaC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res. 2003;92:904–911. doi: 10.1161/01.RES.0000069685.20258.F1. doi:10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
- 6.Oda T, Yano M, Yamamoto T, Tokuhisa T, Okuda S, Doi M, et al. Defective regulation of interdomain interactions within the ryanodine receptor plays a key role in the pathogenesis of heart failure. Circulation. 2005;111:3400–3410. doi: 10.1161/CIRCULATIONAHA.104.507921. doi:10.1161/CIRCULATIONAHA.104.507921. [DOI] [PubMed] [Google Scholar]
- 7.Yano M, Okuda S, Oda T, Tokuhisa T, Tateishi H, Mochizuki M, et al. Correction of defective interdomain interaction within ryanodine receptor by antioxidant is a new therapeutic strategy against heart failure. Circulation. 2005;112:3633–3643. doi: 10.1161/CIRCULATIONAHA.105.555623. doi:10.1161/CIRCULATIONAHA.105.555623. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto T, Yano M, Xu X, Uchinoumi H, Tateishi H, Mochizuki M, et al. Identification of target domains of the cardiac ryanodine receptor to correct channel disorder in failing hearts. Circulation. 2008;117:762–772. doi: 10.1161/CIRCULATIONAHA.107.718957. doi:10.1161/CIRCULATIONAHA.107.718957. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi S, Yano M, Suetomi T, Ono M, Tateishi H, Mochizuki M, et al. Dantrolene, a therapeutic agent for malignant hyperthermia, markedly improves the function of failing cardiomyocytes by stabilizing interdomain interactions within the ryanodine receptor. J Am Coll Cardiol. 2009;53:1993–2005. doi: 10.1016/j.jacc.2009.01.065. doi:10.1016/j.jacc.2009.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodney GG, Williams BY, Strasburg GM, Beckingham K, Hamilton SL. Regulation of RyR1 activity by Ca2+ and calmodulin. Biochemistry. 2000;39:7807–7812. doi: 10.1021/bi0005660. doi:10.1021/bi0005660. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi N, Xu L, Pasek DA, Evans KE, Meissner G. Molecular basis of calmodulin binding to cardiac muscle Ca2+ release channel (ryanodine receptor) J Biol Chem. 2003;278:23480–23486. doi: 10.1074/jbc.M301125200. doi:10.1074/jbc.M301125200. [DOI] [PubMed] [Google Scholar]
- 12.Yamaguchi N, Takahashi N, Xu L, Smithies O, Meissner G. Early cardiac hypertrophy in mice with impaired calmodulin regulation of cardiac muscle Ca release channel. J Clin Invest. 2007;117:1344–1353. doi: 10.1172/JCI29515. doi:10.1172/JCI29515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ono M, Yano M, Hino A, Suetomi T, Xu X, Susa T, et al. Dissociation of calmodulin from cardiac ryanodine receptor causes aberrant Ca2+ release in heart failure. Cardiovasc Res. 2010;87:609–617. doi: 10.1093/cvr/cvq108. doi:10.1093/cvr/cvq108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu X, Yano M, Uchinoumi H, Hino A, Suetomi T, Ono M, et al. Defective calmodulin binding to the cardiac ryanodine receptor plays a key role in CPVT-associated channel dysfunction. Biochem Biophys Res Commun. 2010;394:660–666. doi: 10.1016/j.bbrc.2010.03.046. doi:10.1016/j.bbrc.2010.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiong L, Zhang JZ, He R, Hamilton SL. A Ca2+-binding domain in RyR1 that interacts with the calmodulin binding site and modulates channel activity. Biophys J. 2006;90:173–182. doi: 10.1529/biophysj.105.066092. doi:10.1529/biophysj.105.066092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gangopadhyay JP, Ikemoto N. Interaction of the Lys(3614)-Asn(3643) calmodulin-binding domain with the Cys(4114)-Asn(4142) region of the type 1 ryanodine receptor is involved in the mechanism of Ca2+/agonist-induced channel activation. Biochem J. 2008;411:415–423. doi: 10.1042/BJ20071375. doi:10.1042/BJ20071375. [DOI] [PubMed] [Google Scholar]
- 17.Gangopadhyay JP, Ikemoto N. Aberrant interaction of calmodulin with the ryanodine receptor develops hypertrophy in the neonatal cardiomyocyte. Biochem J. 2011;438:379–387. doi: 10.1042/BJ20110203. doi:10.1042/BJ20110203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tateishi H, Yano M, Mochizuki M, Suetomi T, Ono M, Xu X, et al. Defective domain–domain interactions within the ryanodine receptor as a critical cause of diastolic Ca2+ leak in failing hearts. Cardiovasc Res. 2009;81:536–545. doi: 10.1093/cvr/cvn303. doi:10.1093/cvr/cvn303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto T, Ikemoto N. Peptide probe study of the critical regulatory domain of the cardiac ryanodine receptor. Biochem Biophys Res Commun. 2002;291:1102–1108. doi: 10.1006/bbrc.2002.6569. doi:10.1006/bbrc.2002.6569. [DOI] [PubMed] [Google Scholar]
- 20.Picht E, Zima AV, Blatter LA, Bers DM. SparkMaster: automated calcium spark analysis with ImageJ. Am J Physiol. 2007;293:c1073–c1081. doi: 10.1152/ajpcell.00586.2006. doi:10.1152/ajpcell.00586.2006. [DOI] [PubMed] [Google Scholar]
- 21.Moore CP, Rodney G, Zhang JZ, Santacruz-Toloza L, Strasburg G, Hamilton SL. Apocalmodulin and Ca2+ calmodulin bind to the same region on the skeletal muscle Ca2+ release channel. Biochemistry. 1999;38:8532–8537. doi: 10.1021/bi9907431. doi:10.1021/bi9907431. [DOI] [PubMed] [Google Scholar]
- 22.Uchinoumi H, Yano M, Suetomi T, Ono M, Xu X, Tateishi H, et al. Catecholaminergic polymorphic ventricular tachycardia is caused by mutation-linked defective conformational regulation of the ryanodine receptor. Circ Res. 2010;106:1413–1424. doi: 10.1161/CIRCRESAHA.109.209312. doi:10.1161/CIRCRESAHA.109.209312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.George CH, Jundi H, Thomas NL, Scoote M, Walters N, Williams AJ, et al. Ryanodine receptor regulation by intramolecular interaction between cytoplasmic and transmembrane domains. Mol Biol Cell. 2004;15:2627–2638. doi: 10.1091/mbc.E03-09-0688. doi:10.1091/mbc.E03-09-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yano M, Ikeda Y, Matsuzaki M. Altered intracellular Ca2+ handling in heart failure. J Clin Invest. 2005;115:556–564. doi: 10.1172/JCI24159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gangopadhyay JP, Ikemoto N. Intracellular translocation of calmodulin and Ca2+/calmodulin-dependent protein kinase II during the development of hypertrophy in neonatal cardiomyocytes. Biochem Biophys Res Commun. 2010;396:515–521. doi: 10.1016/j.bbrc.2010.04.129. doi:10.1016/j.bbrc.2010.04.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.