Abstract
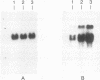
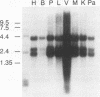
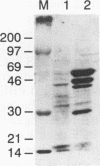
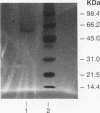
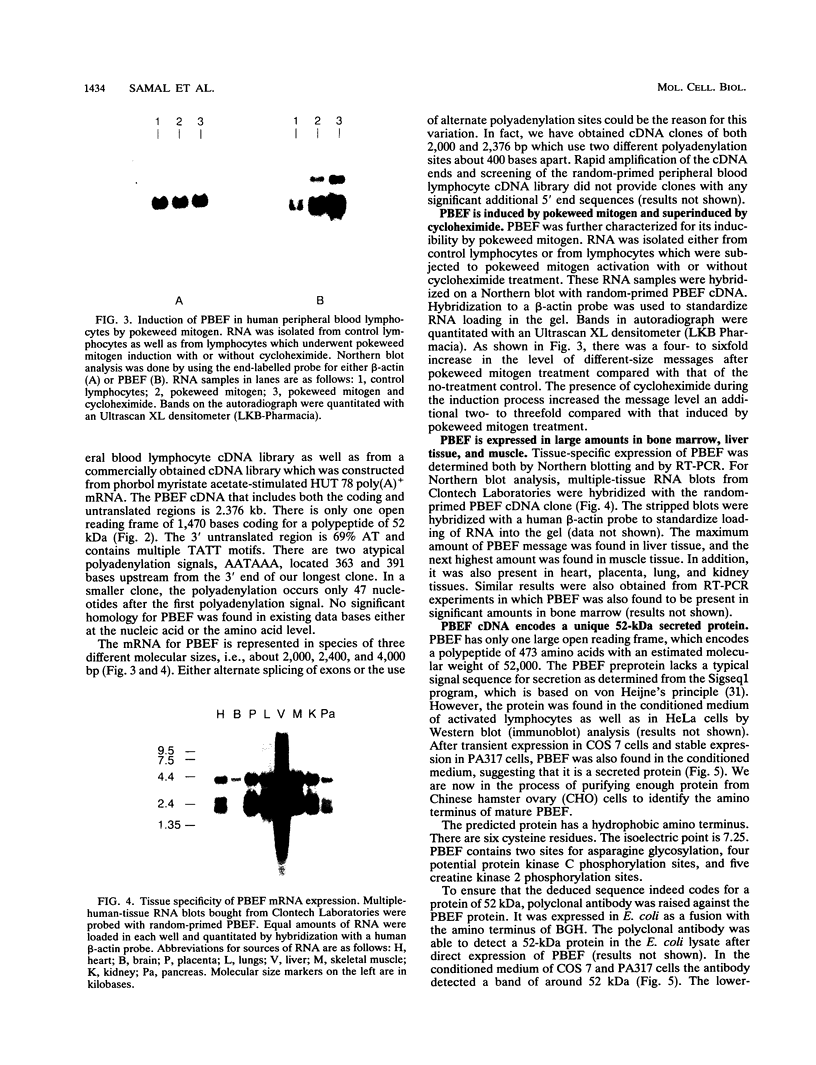
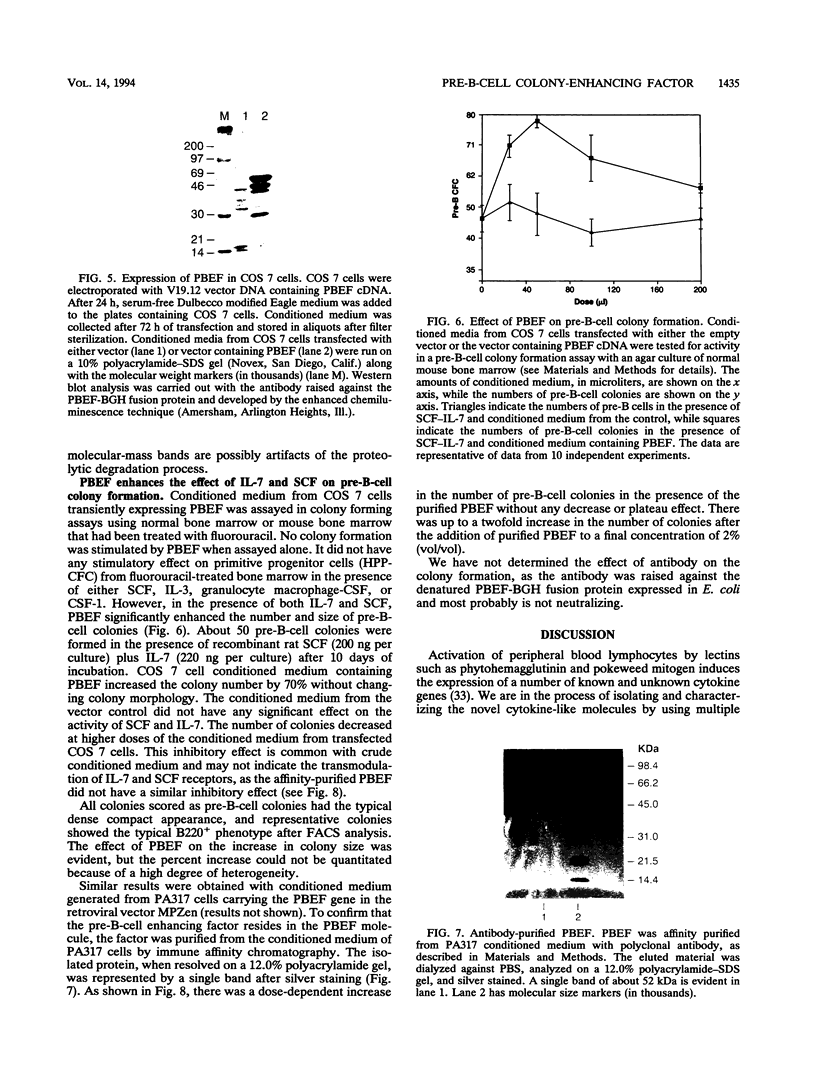
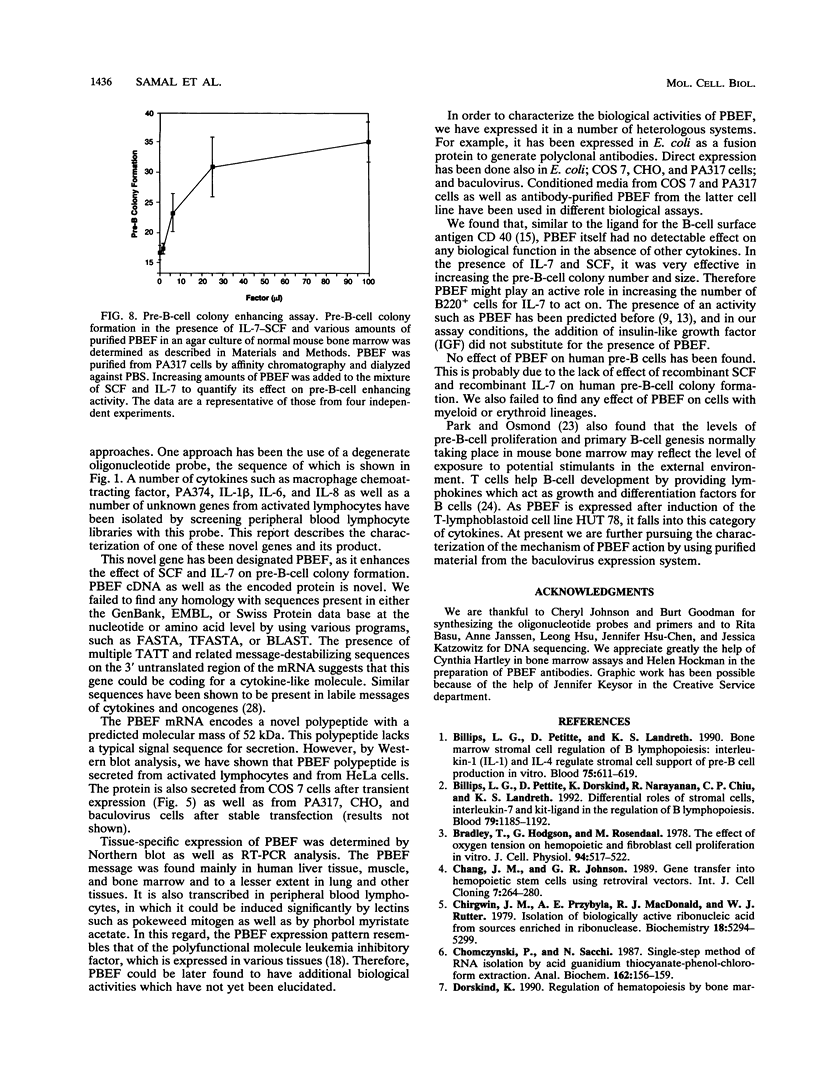
A novel gene coding for the pre-B-cell colony-enhancing factor (PBEF) has been isolated from a human peripheral blood lymphocyte cDNA library. The expression of this gene is induced by pokeweed mitogen and superinduced by cycloheximide. It is also induced in the T-lymphoblastoid cell line HUT 78 after phorbol ester (phorbol myristate acetate) treatment. The predominant mRNA for PBEF is approximately 2.4 kb long and codes for a 52-kDa secreted protein. The 3' untranslated region of the mRNA has multiple TATT motifs, usually found in cytokine and oncogene messages. The PBEF gene is mainly transcribed in human bone marrow, liver tissue, and muscle. We have expressed PBEF in COS 7 and PA317 cells and have tested the biological activities of the conditioned medium as well as the antibody-purified protein in different in vitro assays. PBEF itself had no activity but synergized the pre-B-cell colony formation activity of stem cell factor and interleukin 7. In the presence of PBEF, the number of pre-B-cell colonies was increased by at least 70% above the amount stimulated by stem cell factor plus interleukin 7. No effect of PBEF was found with cells of myeloid or erythroid lineages. These data define PBEF as a novel cytokine which acts on early B-lineage precursor cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billips L. G., Petitte D., Dorshkind K., Narayanan R., Chiu C. P., Landreth K. S. Differential roles of stromal cells, interleukin-7, and kit-ligand in the regulation of B lymphopoiesis. Blood. 1992 Mar 1;79(5):1185–1192. [PubMed] [Google Scholar]
- Billips L. G., Petitte D., Landreth K. S. Bone marrow stromal cell regulation of B lymphopoiesis: interleukin-1 (IL-1) and IL-4 regulate stromal cell support of pre-B cell production in vitro. Blood. 1990 Feb 1;75(3):611–619. [PubMed] [Google Scholar]
- Bradley T. R., Hodgson G. S., Rosendaal M. The effect of oxygen tension on haemopoietic and fibroblast cell proliferation in vitro. J Cell Physiol. 1978 Dec;97(3 Pt 2 Suppl 1):517–522. doi: 10.1002/jcp.1040970327. [DOI] [PubMed] [Google Scholar]
- Chang J. M., Johnson G. R. Gene transfer into hemopoietic stem cells using retroviral vectors. Int J Cell Cloning. 1989 Sep;7(5):264–280. doi: 10.1002/stem.5530070502. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dorshkind K., Landreth K. S. Regulation of B cell differentiation by bone marrow stromal cells. Int J Cell Cloning. 1992 Jan;10(1):12–17. doi: 10.1002/stem.5530100104. [DOI] [PubMed] [Google Scholar]
- Dorshkind K., Narayanan R., Landreth K. S. Regulatory cells and cytokines involved in primary B lymphocyte production. Adv Exp Med Biol. 1992;323:119–123. doi: 10.1007/978-1-4615-3396-2_15. [DOI] [PubMed] [Google Scholar]
- Dorshkind K. Regulation of hemopoiesis by bone marrow stromal cells and their products. Annu Rev Immunol. 1990;8:111–137. doi: 10.1146/annurev.iy.08.040190.000551. [DOI] [PubMed] [Google Scholar]
- Dorssers L., Postmes A. M. A simplified, orientation-specific cDNA cloning strategy. Nucleic Acids Res. 1987 Apr 24;15(8):3629–3629. doi: 10.1093/nar/15.8.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folz R. J., Gordon J. I. Computer-assisted predictions of signal peptidase processing sites. Biochem Biophys Res Commun. 1987 Jul 31;146(2):870–877. doi: 10.1016/0006-291x(87)90611-5. [DOI] [PubMed] [Google Scholar]
- Hirano T. Interleukin 6 (IL-6) and its receptor: their role in plasma cell neoplasias. Int J Cell Cloning. 1991 May;9(3):166–184. doi: 10.1002/stem.5530090303. [DOI] [PubMed] [Google Scholar]
- Landreth K. S., Narayanan R., Dorshkind K. Insulin-like growth factor-I regulates pro-B cell differentiation. Blood. 1992 Sep 1;80(5):1207–1212. [PubMed] [Google Scholar]
- Lee G., Namen A. E., Gillis S., Ellingsworth L. R., Kincade P. W. Normal B cell precursors responsive to recombinant murine IL-7 and inhibition of IL-7 activity by transforming growth factor-beta. J Immunol. 1989 Jun 1;142(11):3875–3883. [PubMed] [Google Scholar]
- Maliszewski C. R., Grabstein K., Fanslow W. C., Armitage R., Spriggs M. K., Sato T. A. Recombinant CD40 ligand stimulation of murine B cell growth and differentiation: cooperative effects of cytokines. Eur J Immunol. 1993 May;23(5):1044–1049. doi: 10.1002/eji.1830230510. [DOI] [PubMed] [Google Scholar]
- McNiece I. K., Langley K. E., Zsebo K. M. The role of recombinant stem cell factor in early B cell development. Synergistic interaction with IL-7. J Immunol. 1991 Jun 1;146(11):3785–3790. [PubMed] [Google Scholar]
- Metcalf D. Leukemia inhibitory factor--a puzzling polyfunctional regulator. Growth Factors. 1992;7(3):169–173. doi: 10.3109/08977199209046921. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Namen A. E., Lupton S., Hjerrild K., Wignall J., Mochizuki D. Y., Schmierer A., Mosley B., March C. J., Urdal D., Gillis S. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988 Jun 9;333(6173):571–573. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- Nelsen B., Sen R. Regulation of immunoglobulin gene transcription. Int Rev Cytol. 1992;133:121–149. doi: 10.1016/s0074-7696(08)61859-8. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y. H., Osmond D. G. Regulation of early precursor B cell proliferation in mouse bone marrow: stimulation by exogenous agents mediated by macrophages in the spleen. Cell Immunol. 1991 Jun;135(1):168–183. doi: 10.1016/0008-8749(91)90263-b. [DOI] [PubMed] [Google Scholar]
- Parker D. C. T cell-dependent B cell activation. Annu Rev Immunol. 1993;11:331–360. doi: 10.1146/annurev.iy.11.040193.001555. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesniaux V. F., Mayer P., Liehl E., Turner K., Goldman S. J., Fagg B. Review of a novel hematopoietic cytokine, interleukin-11. Int Rev Exp Pathol. 1993;34(Pt A):205–214. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Shields J. G., Kotowicz K., Callard R. E. Interleukin-4 stimulates immunoglobulin secretion by Epstein-Barr virus (EBV)-activated tonsillar B cells, and by EBV-transformed lymphoblastoid B cell lines without increasing cell division. Int J Clin Lab Res. 1992;22(2):95–99. doi: 10.1007/BF02591404. [DOI] [PubMed] [Google Scholar]
- Takatsu K. Interleukin-5. Curr Opin Immunol. 1992 Jun;4(3):299–306. doi: 10.1016/0952-7915(92)90080-x. [DOI] [PubMed] [Google Scholar]
- Zipfel P. F., Irving S. G., Kelly K., Siebenlist U. Complexity of the primary genetic response to mitogenic activation of human T cells. Mol Cell Biol. 1989 Mar;9(3):1041–1048. doi: 10.1128/mcb.9.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]