Abstract
Oral cannabinoids are taken for medicinal or recreational purposes, yet little is known about tolerance to their effects after high-dose extended exposure. The development of tolerance to effects of around-the-clock oral synthetic Δ9-tetrahydrocannabinol (THC) (20 mg every 3.5–6 h) was evaluated in 13 healthy male daily cannabis smokers residing on a secure research unit: 40 mg on Day 1; 100 mg on Days 2–4; 120 mg on Days 5–6. Systolic and diastolic blood pressure (BP), heart rate, and symptoms of subjective intoxication (100 mm visual-analogue scales, VAS) were assessed the morning of Day 1 (before any oral THC), and on Days 2, 4 and 6, every 30 min for 3 h after the first morning THC dose. Morning subjective intoxication ratings increased from Days 1 to 2, and then declined on Days 4 and 6. The morning THC dose increased intoxication ratings on Day 2, but had less effect on Days 4 and 6, a pattern consistent with tolerance. THC lowered BP and increased heart rate over the six days. Plasma THC and 11-OH-THC concentrations increased significantly over the first five days of dosing. Six days of around-the-clock, oral THC produced tolerance to subjective intoxication, but not to cardiovascular effects.
Introduction
Cannabis sativa (marijuana) is the most widely used illicit psychoactive drug in the world (1). Also, various formulations of cannabis or cannabinoids are legally prescribed for medicinal purposes in many countries (2–3). Numerous individuals are exposed to cannabinoids for extended periods, often on a daily basis, with resulting potential for developing tolerance to cannabinoid effects. However, there has been relatively little systematic study of cannabis or cannabinoid tolerance in humans.
Cannabinoids are most often smoked, but oral abuse also occurs (4, 5). Oral cannabinoids are also widely prescribed for medicinal purposes in some countries (3, 6–7). Oral synthetic Δ9-tetrahydrocannabinol (THC) (dronabinol, Marinol®) is approved by regulatory authorities for the treatment of nausea and vomiting in cancer chemotherapy patients and for appetite stimulation in patients with acquired immune deficiency syndrome (AIDS) and other wasting diseases. Typical dronabinol doses in the United States range from 2.5–40 mg daily, but the maximum approved dose (based on body surface area) is approximately 150 mg daily (8). Many patients take oral cannabinoids daily for weeks or months with persisting beneficial clinical effects, while limited anecdotal evidence suggests tolerance development to the euphoria and other subjective effects that are undesirable in the clinical setting.
Early studies of cannabis tolerance in humans found that continuous round-the-clock high THC doses for prolonged periods are required for tolerance to subjective and physiologic effects (9–10), rather than intermittent exposure, even to high doses (11, 12). In experienced male cannabis smokers, daily oral THC doses (10–30 mg every 4 h escalating to 210 mg/day over three to five days) produced tolerance to the acute subjective effects of a single oral THC dose after 11–16 days of dosing and to the acute heart rate and blood pressure effects after 9–12 days of dosing (13). In a later study by the same investigators, the intensity of acute subjective effects from a single 30 mg oral THC challenge dose diminished 50% after four days of 60–80 mg daily and 60–80% after ten days of 60–80 mg daily (11).
Later studies showed tolerance to the subjective effects of oral THC developing after 80–120 mg daily for four days (14). More recently, the same investigators found no tolerance to subjective effects over two weeks of 40 mg daily in cannabis smokers positive for the human immunodeficiency virus (HIV) (15).
This paper presents data on the development of tolerance to subjective and cardiovascular effects of oral THC over six days of round-the-clock, high-dose oral THC, obtained as part of a larger study on cannabis withdrawal (16). Plasma cannabinoid concentrations are also reported over this period to evaluate whether any observed tolerance was due to decreases in THC or 11-OH-THC concentrations, which might result in decreased activation of cannabinoid CB1 receptors.
Materials and Methods
Research protocols
The study was approved by the institutional review boards of the National Institute on Drug Abuse (NIDA) Intramural Research Program, the University of Maryland School of Medicine and the Maryland Department of Health and Mental Hygiene. All participants provided written informed consent when not acutely intoxicated or in withdrawal. For inclusion, participants were required to be 18–45 years old, to have smoked cannabis for at least the prior year and averaging daily use for at least three months before admission, to have used cannabis within 24 h of admission, to provide a urine specimen positive for cannabinoids in the 30 days before study entry, to have normal cardiac function and to have an IQ ≥85 (Wechsler Abbreviated Scale of Intelligence). Exclusion criteria included past or present clinically significant medical diseases that might interfere with safe study participation; history of psychosis or any current DSM-IV axis I disorder (other than cannabis, caffeine, or nicotine dependence, or simple phobia); current physical dependence on substances other than cannabis, nicotine or caffeine; history of clinically significant adverse events associated with cannabis intoxication or withdrawal, e.g., psychosis or seizure; ≥6 alcohol drinks/day ≥4 times/week in the month prior to study entry; sesame oil allergy; or current interest or participation in drug abuse treatment.
Participants were admitted to a secure research unit the evening before Day 1, 17.5–21 h before their first oral THC dose. The unit had 24-hour staffing, ensuring that subjects had no access to drugs except those provided in the study. Cigarette smoking was not allowed on the unit, but participants had limited time each day to smoke ad lib outdoors.
An escalating dose design was utilized. Oral synthetic THC (dronabinol, Marinol®; Unimed Pharmaceuticals, Marietta, GA) was administered in 20 mg capsules with increasing frequency (every 3.5–6 h) for total daily doses as follows: 40 mg on Day 1, 100 mg on Days 2–4 and 120 mg on Days 5 and 6 (Supplementary Table 1). The first 20 mg dose was administered on Day 1 at 15:00. This regimen attempted to standardize cannabis tolerance across these chronic daily cannabis smokers, while minimizing adverse events previously reported with 30 mg oral THC doses (14).
Table I.
Morning Subjective and Cardiovascular Responses to Prior Cumulative Dose of Oral THC in 10 Male Daily Cannabis Smokers
| Variable | Day 1 | Day 2 | Day 4 | Day 6 |
|---|---|---|---|---|
| Subjective | ||||
| Good Drug Effect* | 1.5 ± 2.7 | 17.7 ± 18.8‡ | 13.0 ± 16.4 | 8.2 ± 11.1§ |
| High† | 3.4 ± 7.2 | 17.7 ± 20.5‡ | 11.3 ± 16.7 | 8.3 ± 11.2 |
| Sedated | 1.6 ± 3.1 | 9.9 ± 18.4 | 3.0 ± 5.0 | 4.5 ± 9.5 |
| Stoned† | 1.3 ± 2.2 | 7.8 ± 11.0‡ | 5.0 ± 7.1 | 4.3 ± 6.0 |
| Stimulated | 3.4 ± 5.8 | 4.8 ± 8.0 | 5.4 ± 7.4 | 3.1 ± 5.2 |
| Cardiovascular | ||||
| Systolic BP (mm Hg)* | 129.1 ± 10.0 | 121.4 ± 10.5 | 120.4 ± 7.6 | 119.6 ± 6.5 |
| Diastolic BP (mm Hg)* | 74.5 ± 8.2 | 71.6 ± 6.2 | 63.1 ± 8.5 | 61.2 ± 8.9 |
| Heart rate (bpm)* | 59.6 ± 6.1 | 74.3 ± 13.4 | 68.8 ± 11.8 | 68.4 ± 12.8 |
Note: Responses were measured at 08:00 on Day 1 (after at least 15.5–18 h of cannabis abstinence; no prior THC administration) and just before morning THC dose on Days 2 (08:00, after 60 mg total THC), 4 (10:00, after 260 mg total THC) and 6 [10:00 (10:30 for cardiovascular variables), after 480 mg total THC]. Subjective responses were assessed with 100 mm visual-analogue scales defined as indicated. Values presented as mean ± SD.
*p < 0.05.
†p < 0.1 for change across the six days.
‡p < 0.05 for Day 1 versus Day 2 change.
§p < 0.05 for Day 2 versus Day 6 change.
Common symptoms of cannabis intoxication, drawn from the published literature (13–17), were assessed periodically with an 11-item battery [subjective effects scale (SES)] of 100 mm visual analogue scales (VASs) containing items typical of both intoxication and withdrawal to minimize cueing of participants: Good Drug Effect, High, Stoned, Stimulated, Sedated, Anxious, Depressed, Irritable, Restless, Craving for Cannabis and Angry/Aggressive. The VAS for Good Drug Effect, High, Stoned, and Sedated were considered measures of acute subjective effects (cannabis intoxication). The VAS for Stimulated was analyzed as a positive control because cannabis is considered a subjectively sedating rather than stimulating drug. VASs were anchored at the left with “not at all” and at the right with “most ever.” The score for each VAS was the number of mm the participant marked to the right of the left anchor point. The SES required approximately 3.5 min to complete and was administered twice daily (10:00 and 20:00), plus every 30 min for 3 h starting immediately after the morning (10:00, except 08:00 on Day 2) THC administration on Days 2, 4 and 6. Sitting systolic and diastolic blood pressure (BP) and pulse were measured daily at 08:00, 16:00 and 23:00, plus every 30 min for 3 h starting immediately after the morning (10:00, except 08:00 on Day 2) THC administration on Days 2, 4 and 6. A complete schedule of THC administration and SES, BP and pulse measurements is given in Supplementary Table 1.
Tolerance due to cumulative THC exposure was assessed in two ways. First, tolerance to the effect of the (preceding) cumulative THC dosing was assessed by comparing morning baseline (pre-THC dose) VAS scores and cardiovascular measures on Day 1 at 08:00 (no prior THC), Day 2 at 08:00 (60 mg prior THC), Day 4 at 10:00 (4 days of prior THC dosing), and Day 6 at 10:00 [10:30 for cardiovascular variables (6 days of prior THC)]. Within-subject comparisons among these time points were performed using SAS Proc Mixed (SAS Institute, Cary, NC) to fit a within-subject repeated-measures analysis of variance (ANOVA). Significant overall ANOVA F-tests were followed by post-hoc contrasts from this model comparing Days 6, 4 and 2 with Day 1; Days 6 and 4 with Day 2; and Day 6 with Day 4.
Second, tolerance to the acute subjective (VAS) and cardiovascular (heart rate, BP) effects of the morning THC dose were assessed by comparing the within-day early (first 3 h) time-course of response to the morning oral THC dose on Days 2, 4 and 6, using a within-subject repeated-measures ANOVA as before. The model was fitted as follows: responsedt = day + time + time × day, where responsedt = the change from the initial measurement on Day d at time t, and Day and time are categorical variables indicating the day and time within day. The primary effect of Day indicates a change in the average level of responses; the day × time interaction tests for a change in the time-course of THC response across days.
Venous blood (7 mL) was collected into lithium heparin Vacutainer® tubes on admission, twice before and every 30 min for 4 h after first dose, then daily at approximately 08:00 (Days 1 and 2) or 10:00 (Days 3–6) and 22:00 (Days 1–4) or 22:30 (Days 5–6) (Supplementary Table 1). Collection preceded dosing when both were scheduled at the same time. Blood was centrifuged and plasma separated within 2 h. Plasma was stored at 4°C (with one exception) and analyzed as soon as possible. Unconjugated cannabinoids were assayed within 10 ± 5 days. One participant's plasma was stored at –20°C for approximately 10 weeks due to laboratory relocation. Changes in analyte concentration over time were evaluated by two-way [study day × time of day (morning, evening)] within-subject repeated-measures ANOVA with Greenhouse-Geisser correction. Concentrations of THC (the primary psychoactive constituent of cannabis) and of combined THC + 11-OH-THC (the major pharmacologically active metabolite of THC) were evaluated separately.
Gas chromatography–mass spectrometry analysis
Unconjugated cannabinoids (THC and 11-OH-THC) were determined by a previously reported analytical method (18). Briefly, 1 mL 0.1 mol/L phosphate buffer (pH 6.8), deuterated internal standards and 1 mL plasma were mixed, proteins precipitated with 2 mL cold acetonitrile, and centrifuged. Solid-phase extraction (ZSTHC020®; United Chemical Technologies, Bristol, PA) was followed by derivatization with 25 µL BSTFA + 1% TMCS at 70°C for 40 min, and 3 µL were injected splitless onto a two-dimensional Agilent 6890 gas chromatograph–flame ionization detector (GC–FID)/5973 MSD mass spectrometer (2D-GC–MS) with a Deans switch (Agilent Technologies, Wilmington, DE) and cryotrap (Joint Analytical Systems, Marlton, NJ). Intra-assay and inter-assay imprecision were <11 and <14%, respectively, and analytical recoveries were 86–113% (18).
Results
Fourteen participants enrolled in the larger study (16), of whom one was discharged before receiving any medication because the study was terminated, one withdrew after one day of oral THC dosing for personal reasons, two withdrew on the fourth day of dosing (one due to premature ventricular contractions and one due to psychological reactions to THC) and 10 completed six days of dosing. All 13 participants [13 male, ten African-American, two white, one mixed race, mean ± standard deviation (SD) age 24.6 ± 3.7 years] who received THC are included in this study. These participants first smoked cannabis at 14.0 ± 2.4 years of age and began regular (at least weekly) smoking at age 15.6 ± 3.7 years. All but one participant reported at least 1,000 lifetime cannabis uses; eight reported at least 5,000 uses. All participants smoked cannabis joints and/or blunts (cannabis wrapped in tobacco leaves); five also self-administered hashish in the past. Seven participants reported lifetime experience with cannabis tolerance (needing to use more to get the same effect); five of these also reported the experience of cannabis withdrawal. At the time of study entry, participants averaged 5.5 ± 5.9 (median 3.5, range 1–24) joint-equivalents daily. At admission, all participants self-reported cannabis smoking in the prior 24 h, and all provided a positive cannabinoid urine test.
All 13 participants were cigarette smokers at some time in their lives; nine were daily cigarette smokers at the time of study entry, averaging 17.9 ± 18.8 (median 10, range 2–50) cigarettes daily. The remaining four participants abstained from smoking for four and six months and eight and 10 years prior to admission. All participants had ingested alcohol at some point in their lives. The 11 current drinkers averaged 12.1 ± 10.9 (median 12, range 0.25–32) standard drinks per week over the three months before study screening; two abstained for one month before study entry. Two participants reported current oral amphetamine use, averaging two pills each week. There was no other current self-reported illicit drug use, which was consistent with urine drug test results.
Morning baseline subjective ratings of good drug effect changed significantly over the six days of oral THC dosing (Table I) (F = 4.9, df = 3/26.2, p = 0.008 for Good Drug Effect). There was a trend towards increased ratings of Stoned (F = 2.9, df = 3/26.1, p = 0.056) and High (F = 2.5, df = 3/26.2, p = 0.09), whereas Sedated (F = 1.6, df = 3/26.3, p = 0.21) and Stimulated (F = 0.4, df = 3/26.2, p = 0.76) did not change. Ratings of Good Drug Effect (t = 3.6, p = 0.001), High (t = 2.6, p = 0.01) and Stoned (t = 2.9, p = 0.008) increased significantly from Days 1 to 2, presumably reflecting the intoxicating effects of 40 mg oral THC taken over the prior 17 h. Morning intoxication ratings declined by Day 4 and remained at similar levels on Day 6 (Table I) (t = 2.0, p = 0.05 for Day 2 versus Day 6 for Good Drug Effect; p-values 0.10–0.57 for all other pair-wise comparisons), despite continuing oral THC intake (420 mg over four days), suggesting early tolerance development.
On Day 2, 20 mg oral THC acutely increased ratings of High (t = 2.4, p = 0.04) (Table II). There was a trend toward the dissipation of this acute effect by Day 4 (Table II), after cumulative exposure to 260 mg oral THC, suggesting tolerance development (difference between Days 2 and 4: t = 1.9, p = 0.08). The acute intoxicating effect slightly increased on Day 6 (Table II), suggesting no further development of tolerance with an additional 220 mg of cumulative THC exposure.
Table II.
Subjective and Cardiovascular Response to a Single Oral Dose of THC (20 mg) with Increasing Cumulative Exposure to Oral THC in 10 Male Daily Cannabis Smokers
| Variable | Day 2 | Day 4 | Day 6 |
|---|---|---|---|
| Subjective | |||
| Good Drug Effect | 9.3 ± 15.6 | –2.0 ± 12.1 | 1.9 ± 10.3 |
| High | 12.0 ± 14.7* | –1.8 ± 8.4 | 5.5 ± 7.6 |
| Sedated | 1.0 ± 21.4 | –4.2 ± 7.5 | 2.3 ± 6.6 |
| Stoned | 3.6 ± 6.5 | –1.8 ± 4.8 | 0.7 ± 6.3 |
| Stimulated | 1.8 ± 5.1 | 1.9 ± 7.3 | 1.1 ± 4.4 |
| Cardiovascular | |||
| Systolic BP (mm Hg) | –0.9 ± 17.6 | –8.4 ± 18.8 | –3.4 ± 22.0 |
| Diastolic BP (mm Hg) | –10.3 ± 10.8* | –15.5 ± 9.8* | –14.8 ± 5.7* |
| Heart rate (bpm) | –0.7 ± 10.9 | –5.2 ± 21.2 | –12.8 ± 8.1 |
Note: Peak change from baseline over the 3 h following the morning THC dose was measured on Days 2 (08:00 baseline, after 60 mg total THC), 4 (10:00 baseline, after 260 mg total THC) and 6 (10:00 baseline, after 480 mg total THC). Subjective responses were assessed with 100 mm visual-analogue scales defined as indicated. Values presented as mean ± SD.
*p < 0.05 for peak change from baseline.
There was no significant acute effect of THC on Good Drug Effect (t = 1.6, p = 0.11), Stoned (t = 1.6, p = 0.14), Sedated (t = 0.14, p = 0.89) or Stimulated (t = 1.0, p = 0.33) on Day 2, so the development of tolerance for these parameters could not be evaluated.
Morning baseline cardiovascular parameters significantly changed over the six days of oral THC dosing (systolic BP: F = 3.6, df = 3/24.9, p = 0.03; diastolic BP: F = 6.9, df = 3/25.4, p = 0.001; heart rate: F = 5.4, df = 3/26.2, p = 0.005). Baseline systolic and diastolic BP decreased 9–10 mm Hg over the course of dosing (i.e., after Day 1) (Table I), the expected hypotensive cannabinoid effect (19). Baseline heart rate increased 9–14 bpm (Table I), the expected tachycardia following cannabinoids (19). No evidence of a decrease in these effects was observed between Days 4 and 6 (Table I), i.e., after an additional 220 mg cumulative exposure to oral THC, suggesting no development of tolerance for these cardiovascular measures.
On Day 2, 20 mg oral THC acutely decreased diastolic BP (Table II; t = 3.0, p = 0.01). Similar declines occurred on Days 4 and 6 (Table II). These were not significantly different from the Day 2 effect (Day 2 versus Day 4: t = 1.1, p = 0.27; Day 2 versus Day 6: t = 1.2, p = 0.26), suggesting no tolerance to the acute hypotensive effect of THC.
No significant acute effect of THC was observed on systolic BP (Table II; t = 0.16, p = 0.87) or heart rate (Table II; t = 0.20, p = 0.84) on Day 2, so the development of tolerance for these parameters could not be evaluated.
Plasma concentrations of THC (Figure 1A) and of combined THC + 11-OH-THC (Figure 1B) increased significantly over the first five days of dosing and were slightly lower on Day 6, although still higher than Day 1 (F = 2.6, df = 5, p = 0.04 and F = 4.5, df = 5, p = 0.002, respectively). Concentrations of both analytes were significantly greater in the evening than in the morning (F = 32.5, df = 1, p < 0.0001 and F = 40.9, df = 1, p < 0.0001, respectively) (Figure 1). This pattern did not change significantly for either analyte over the six days of dosing (F = 1.9, df = 5, p = 0.11 and F = 1.9, df = 5, p = 0.12, respectively).
Figure 1.
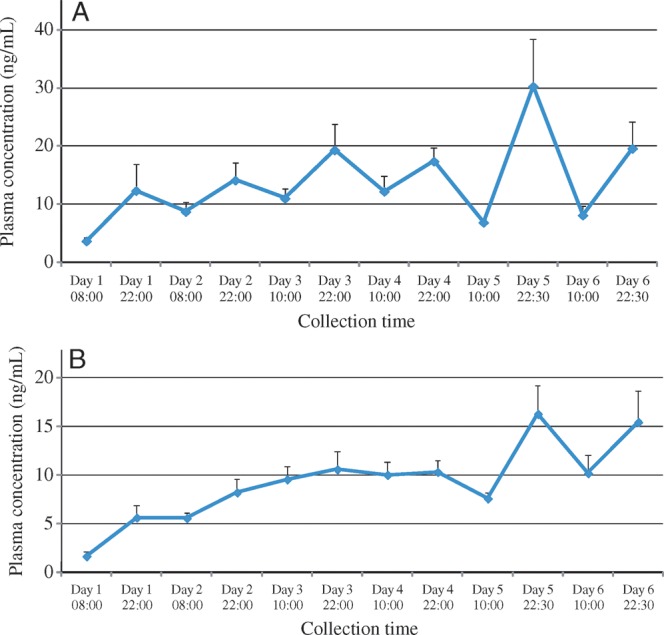
Time course of cannabinoid plasma concentrations over six days of around-the-clock oral THC administration: THC (A); 11-OH-THC (B). Peripheral venous blood was collected at indicated time points, and plasma was separated within 2 h, stored at 4°C (with one exception), and analyzed by GC–MS within 10 ± 5 days for unconjugated THC and 11-OH-THC. Vertical bars indicate SEM.
Discussion
This study found that substantial tolerance to the subjective intoxicating effects of oral THC developed after exposure to a total dose of 260 mg over four days. This is consistent with most, but not all, prior human studies. The one study that did not observe tolerance (15) administered oral THC doses (40 mg daily for two weeks) lower than those in other studies that showed tolerance, including by the same investigators (14). That study involved HIV+ subjects, but it is not known whether HIV status influences tolerance development.
The tolerance observed in this study was not attributable to decreasing concentrations of THC or its active metabolite 11-OH-THC, which might have resulted in decreased activation of the CB1 receptor. Rather, plasma concentrations of these analytes increased significantly in both the morning and evening over the first five days of dosing, before declining modestly from their peak on Day 6 (Figure 1).
In contrast to the subjective measures, this study found that no significant tolerance developed over six days to the hypotensive or tachycardic oral THC effects. One earlier study showed cardiovascular tolerance developing after 12 days of 180 mg daily oral THC (13). Failure to observe cardiovascular tolerance in this study may have been due to the lower THC doses (maximum 120 mg daily) and shorter duration of dosing (six days). Recent studies using THC doses and dosing durations more comparable to those in this study have not evaluated cardiovascular effects.
An alternative possible explanation for these findings is that this study observed differential dissipation of previously established tolerance from participants' cannabis self-administration in the community. This tolerance dissipated for subjective intoxication, but not for cardiovascular effects. This explanation is unlikely because, although it might explain the increased subjective intoxication ratings (and unchanged cardiovascular effects) over the first two days of oral THC dosing, it does not readily account for the decreased ratings over the subsequent four days.
Two studies comparing the acute response to a cannabis challenge in heavy versus occasional cannabis smokers also found a differential response to subjective versus cardiovascular effects, whereas a third study found no differential response. The first study showed that a 15 mg oral THC challenge elicited a significant subjective response in infrequent cannabis users, but a weaker response in frequent users (20). The same dose elicited a similar heart rate response in both groups. A second study found that smoking a single cannabis cigarette (approximately 20 mg THC) evoked significant subjective and heart rate responses in occasional cannabis smokers, but weaker responses in chronic smokers (21). Both groups showed a similar blood pressure response to the cannabis cigarette. A third study found no difference in heart rate or subjective high response to smoking a single cannabis cigarette (approximately 13 mg THC) in daily versus occasional cannabis smokers (22). The pattern of findings in the first two studies is consistent with tolerance to the subjective effects but no tolerance to the blood pressure effects of cannabis, similar to the pattern observed in the present study.
The mechanism of differential tolerance to THC's effects is unclear. Human studies with the CB1-receptor antagonist rimonabant indicated that both the subjective and cardiovascular effects of cannabis are mediated by activation of the CB1 receptor (17, 23, 24). Animal studies show that tolerance to different acute cannabinoid effects develops at different rates, which may be mediated by differential down-regulation or desensitization of CB1 receptors in various brain regions (25). A recent human PET study also found regional brain down-regulation of CB1 receptors in chronic cannabis smokers (26).
This study's findings suggest that patients taking oral THC for medicinal purposes are likely to develop tolerance to subjective intoxicating effects early in therapy. This may be beneficial, in that some patients without histories of prior recreational cannabis use experience these subjective effects as unpleasant, which may result in stopping the medication. Conversely, these findings suggest that patients may not quickly develop tolerance to the tachycardic and hypotensive effects of oral THC. Although the magnitude of these effects with oral THC is modest, this suggests that caution should be exercised when using more than minimal THC doses in patients with pre-existing cardiovascular disease.
This study has several limitations, including small sample size, relatively short duration of THC dosing, and no placebo comparison group. The absence of the latter means that the possibility cannot be excluded that changes observed over the course of the study were due to adaptations to admission to a hospital environment, rather than drug effects and tolerance to these effects. However, hospital admission is associated with short-term stress, anxiety and sleep disturbance (27), factors that seem unlikely to increase ratings of subjective intoxication. In addition, data were not collected at exactly the same time every morning, although the 2 h time difference between Day 2 and later days is too short an interval to allow significant influence by diurnal variation.
Conclusion
This is the first study in more than three decades to evaluate tolerance to both subjective and cardiovascular effects of oral THC in the same subjects. Finding differential tolerance to effects has clinical implications for individuals taking oral THC, whether recreationally or medicinally, and highlights the need for further research on pharmacodynamic mechanisms of tolerance to cannabinoids.
Acknowledgments
This work was funded by the Intramural Research Program, National Institutes of Health, National Institute on Drug Abuse (NIDA); NIDA Residential Research Support Services Contract HHSN271200599091CADB (PI: DK) to the Maryland Psychiatric Research Center (MPRC); and a Cooperative Research and Development Agreement between Sanofi-aventis and the National Institutes of Health. DB and CO-R are employees of Sanofi-aventis.
We thank the clinical staffs of the NIDA Intramural Research Program, the Johns Hopkins Behavioral Pharmacology Research Unit, and the MPRC Treatment Research Program for their work with study participants, and Dr. Garry Milman for performing the statistical analysis of cannabinoid concentrations and creating the figure.
References
- 1.United Nations Office on Drugs and Crime. Vienna, Austria: 2008. 2008 World Drug Report; p. 310. [Google Scholar]
- 2.Cotter J. Efficacy of crude marijuana and synthetic delta-9-tetrahydrocannabinol as treatment for chemotherapy-induced nausea and vomiting: A systematic literature review. Oncology Nursing Forum. 2009;36:345–352. doi: 10.1188/09.ONF.345-352. [DOI] [PubMed] [Google Scholar]
- 3.Turcotte D., Le Dorze J.-A., Esfahani F., Frost E., Gomori A., Namaka M. Examining the roles of cannabinoids in pain and other therapeutic indications: A review. Expert Opinion on Pharmacotherapy. 2010;11:17–31. doi: 10.1517/14656560903413534. [DOI] [PubMed] [Google Scholar]
- 4.André C., Jaber-Filho J.A., Bento R.M., Damasceno L.M., Aquino-Neto F.R. Delirium following ingestion of marijuana present in chocolate cookies. CNS Spectrums. 2006;11:262–264. doi: 10.1017/s1092852900020757. [DOI] [PubMed] [Google Scholar]
- 5.Chaudry H.R., Moss H.B., Bashir A, Suliman T. Cannabis psychosis following bhang ingestion. British Journal of Addiction. 1991;86:1075–1081. doi: 10.1111/j.1360-0443.1991.tb01874.x. [DOI] [PubMed] [Google Scholar]
- 6.Gorter RW, Butorac M., Cobian E.P., van der Sluis W. Medical use of cannabis in the Netherlands. Neurology. 2005;64:917–919. doi: 10.1212/01.WNL.0000152845.09088.28. [DOI] [PubMed] [Google Scholar]
- 7.Lynch M.E., Young J., Clark A.J. A case series of patients using medicinal marihuana for management of chronic pain under the Canadian marihuana medical access regulations. Journal of Pain and Symptom Management. 2006;32:497–501. doi: 10.1016/j.jpainsymman.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 8.Marinol. In Physicians desk reference, 60th edition. Thomson, Montvale, NJ. 2006:3334–3336. [Google Scholar]
- 9.Frank I.M., Lessin P.J., Tyrrell E.D., Hahn P.M., Szara S. Acute and cumulative effects of marihuana smoking in hospitalized subjects: A 36-day study. In: Braude M.C., Szara S, editors. New York, NY: Raven Press; 1976. pp. 673–680. The pharmacology of marihuana. [Google Scholar]
- 10.Mendelson J.H., Babor T.F., Kuehnle J.C., Rossi A.M., Bernstein J.G., Mello N.K., et al. Behavioral and biologic aspects of marijuana use. Annals of the New York Academy of Sciences. 1976;282:186–210. doi: 10.1111/j.1749-6632.1976.tb49899.x. [DOI] [PubMed] [Google Scholar]
- 11.Jones R.T., Benowitz N.L., Herning R.I. Clinical relevance of cannabis tolerance and dependence. Journal of Clinical Pharmacology. 1981;21:143S–152S. doi: 10.1002/j.1552-4604.1981.tb02589.x. [DOI] [PubMed] [Google Scholar]
- 12.Jones R.T., Benowitz N. The 30-day trip—Clinical studies of cannabis tolerance and dependence. In: Braude M.C., Szara S, editors. New York, NY: The pharmacology of marijuana, Raven Press; 1976. pp. 627–643. [Google Scholar]
- 13.Jones R.T., Benowitz N., Bachman J. Clinical studies of cannabis tolerance and dependence. Annals of the New York Academy of Sciences. 1976;282:221–239. doi: 10.1111/j.1749-6632.1976.tb49901.x. [DOI] [PubMed] [Google Scholar]
- 14.Haney M., Ward A.S., Comer S.D., Foltin R.W., Fischman M.W. Abstinence symptoms following oral THC administration to humans. Psychopharmacology. 1999;141:385–394. doi: 10.1007/s002130050848. [DOI] [PubMed] [Google Scholar]
- 15.Bedi G., Foltin R., Gunderson E., Rabkin J., Hart C., Comer S., et al. Efficacy and tolerability of high-dose dronabinol maintenance in HIV-positive marijuana smokers: A controlled laboratory study. Psychopharmacology. 2010;212:675–686. doi: 10.1007/s00213-010-1995-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorelick D.A., Goodwin R.S., Schwilke E., Schwope D.M., Darwin W.D., Kelly D.L., et al. Antagonist-elicited cannabis withdrawal in humans. Journal of Clinical Psychopharmacology. 2011;31:603–612. doi: 10.1097/JCP.0b013e31822befc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huestis M.A., Gorelick D.A., Heishman S.J., Preston K.L., Nelson R.A., Moolchan E.T., et al. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Archives of General Psychiatry. 2001;58:322–330. doi: 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- 18.Lowe R.H., Karschner E.L., Schwilke E.W., Barnes A.J., Huestis M.A. Simultaneous quantification of delta-9-tetrahydrocannabinol (THC), 11-hydroxy-delta-9-tetrahydrocannabinol (11-OH-THC), and 11-nor-delta-9-tetrahydrocannabinol-9-carboxylic acid (THCCOOH) in human plasma using two-dimensional gas chromatography, cryofocusing, and electron impact-mass spectrometry. Journal of Chromatography A. 2007;1163:318–327. doi: 10.1016/j.chroma.2007.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones R.T. Cardiovascular system effects of marijuana. Journal of Clinical Pharmacology. 2002;42:58S–63S. doi: 10.1002/j.1552-4604.2002.tb06004.x. [DOI] [PubMed] [Google Scholar]
- 20.Kirk J.M., de Wit H. Responses to oral delta-9-tetrahydrocannabinol in frequent and infrequent marijuana users. Pharmacology Biochemistry and Behavior. 1999;63:137–142. doi: 10.1016/s0091-3057(98)00264-0. [DOI] [PubMed] [Google Scholar]
- 21.Ponto L.L., O'Leary D.S., Koeppel J., Block R.I., Watkins G.L., Richmond J.C., et al. Effect of acute marijuana on cardiovascular function and central nervous system pharmacokinetics of [(15)O]water: Effect in occasional and chronic users. Journal of Clinical Pharmacology. 2004;44:751–766. doi: 10.1177/0091270004265699. [DOI] [PubMed] [Google Scholar]
- 22.Lindgren J.E., Ohlsson A., Agurell S., Hollister L., Gillespie H. Clinical effects and plasma levels of delta-9-tetrahydrocannabinol (delta-9-THC) in heavy and light users of cannabis. Psychopharmacology. 1981;74:208–212. doi: 10.1007/BF00427095. [DOI] [PubMed] [Google Scholar]
- 23.Huestis M.A., Boyd S.J., Heishman S.J., Preston K.L., Bonnet D., Le Fur G., et al. Single and multiple doses of rimonabant antagonize acute effects of smoked cannabis in male cannabis users. Psychopharmacology (Berlin) 2007;194:505–515. doi: 10.1007/s00213-007-0861-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorelick D.A., Heishman S.J., Preston K.L., Nelson R.A., Moolchan E.T., Huestis M.A. The cannabinoid CB1 receptor antagonist rimonabant attenuates the hypotensive effect of smoked marijuana in male smokers. American Heart Journal. 2006;151:751–754. doi: 10.1016/j.ahj.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez S., Cebeira M., Fernandez-Ruiz J. Cannabinoid tolerance and dependence: A review of studies in laboratory animals. Pharmacology Biochemistry and Behavior. 2005;81:300–318. doi: 10.1016/j.pbb.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 26.Hirvonen J., Goodwin R.S., Li C.T., Terry G.E., Zoghbi S.S., Morse C., et al. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Molecular Psychiatry. 2012;17:642–649. doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agnew H., Webb W., Williams R. The first night effect: An EEG study of sleep. Psychophysiology. 1966;2:263–266. doi: 10.1111/j.1469-8986.1966.tb02650.x. [DOI] [PubMed] [Google Scholar]


