Abstract
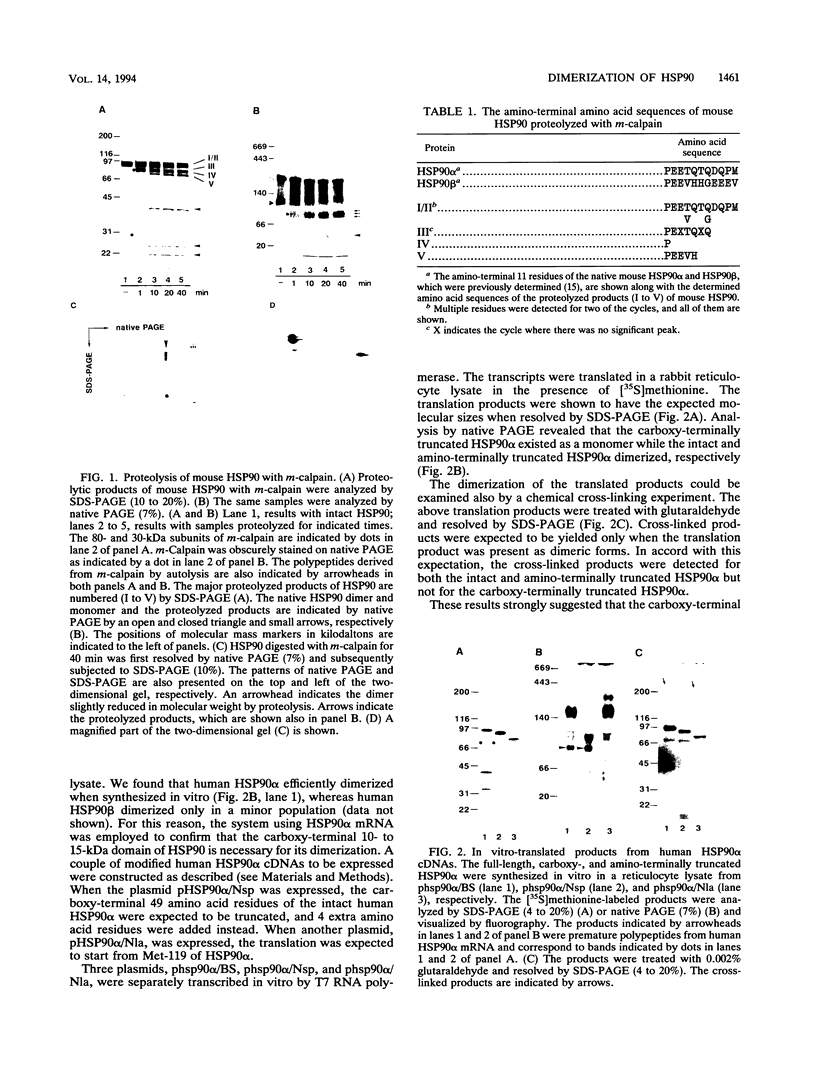
The majority of mouse HSP90 exists as alpha-alpha and beta-beta homodimers. Truncation of the 15-kDa carboxy-terminal region of mouse HSP90 by digestion with the Ca(2+)-dependent protease m-calpain caused dissociation of the dimer. When expressed in a reticulocyte lysate, the full-length human HSP90 alpha formed a dimeric form. A plasmid harboring human HSP90 alpha cDNA was constructed so that the carboxy-terminal 49 amino acid residues were removed when translated in vitro. This carboxy-terminally truncated human HSP90 alpha was found to exist as a monomer. In contrast, loss of the 118 amino acid residues from the amino terminus of human HSP90 alpha did not affect its in vitro dimerization. Introduction of an expression plasmid harboring the full-length human HSP90 alpha complements the lethality caused by the double mutations of two HSP90-related genes, hsp82 and hsc82, in a haploid strain of Saccharomyces cerevisiae. The carboxy-terminally truncated human HSP90 alpha neither formed dimers in yeast cells nor rescued the lethal double mutant.
Full text
PDF

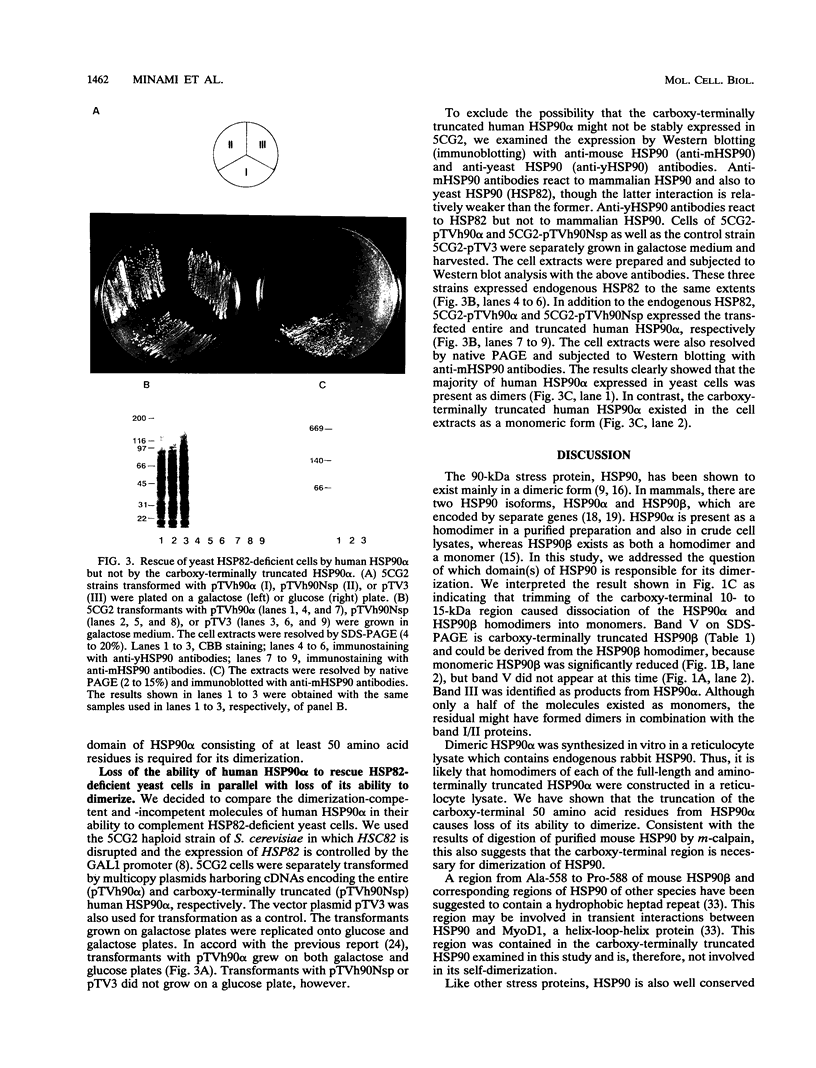


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borkovich K. A., Farrelly F. W., Finkelstein D. B., Taulien J., Lindquist S. hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol Cell Biol. 1989 Sep;9(9):3919–3930. doi: 10.1128/mcb.9.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnick E. H., Dalman F. C., Pratt W. B. Direct stoichiometric evidence that the untransformed Mr 300,000, 9S, glucocorticoid receptor is a core unit derived from a larger heteromeric complex. Biochemistry. 1990 Jan 16;29(2):520–527. doi: 10.1021/bi00454a028. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson E., Erikson R. L. The specific interaction of the Rous sarcoma virus transforming protein, pp60src, with two cellular proteins. Cell. 1981 Aug;25(2):363–372. doi: 10.1016/0092-8674(81)90055-6. [DOI] [PubMed] [Google Scholar]
- Dalman F. C., Bresnick E. H., Patel P. D., Perdew G. H., Watson S. J., Jr, Pratt W. B. Direct evidence that the glucocorticoid receptor binds to hsp90 at or near the termination of receptor translation in vitro. J Biol Chem. 1989 Nov 25;264(33):19815–19821. [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Hutchison K. A., Stancato L. F., Jove R., Pratt W. B. The protein-protein complex between pp60v-src and hsp90 is stabilized by molybdate, vanadate, tungstate, and an endogenous cytosolic metal. J Biol Chem. 1992 Jul 15;267(20):13952–13957. [PubMed] [Google Scholar]
- Inomata M., Nomoto M., Hayashi M., Nakamura M., Imahori K., Kawashima S. Comparison of low and high calcium requiring forms of the calcium-activated neutral protease (CANP) from rabbit skeletal muscle. J Biochem. 1984 Jun;95(6):1661–1670. doi: 10.1093/oxfordjournals.jbchem.a134779. [DOI] [PubMed] [Google Scholar]
- Koyasu S., Nishida E., Kadowaki T., Matsuzaki F., Iida K., Harada F., Kasuga M., Sakai H., Yahara I. Two mammalian heat shock proteins, HSP90 and HSP100, are actin-binding proteins. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8054–8058. doi: 10.1073/pnas.83.21.8054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeau M. C., Massol N., Herrick J., Faber L. E., Renoir J. M., Radanyi C., Baulieu E. E. P59, an hsp 90-binding protein. Cloning and sequencing of its cDNA and preparation of a peptide-directed polyclonal antibody. J Biol Chem. 1992 Mar 5;267(7):4281–4284. [PubMed] [Google Scholar]
- Lees-Miller S. P., Anderson C. W. The human double-stranded DNA-activated protein kinase phosphorylates the 90-kDa heat-shock protein, hsp90 alpha at two NH2-terminal threonine residues. J Biol Chem. 1989 Oct 15;264(29):17275–17280. [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Mendel D. B., Ortí E. Isoform composition and stoichiometry of the approximately 90-kDa heat shock protein associated with glucocorticoid receptors. J Biol Chem. 1988 May 15;263(14):6695–6702. [PubMed] [Google Scholar]
- Minami Y., Kawasaki H., Miyata Y., Suzuki K., Yahara I. Analysis of native forms and isoform compositions of the mouse 90-kDa heat shock protein, HSP90. J Biol Chem. 1991 Jun 5;266(16):10099–10103. [PubMed] [Google Scholar]
- Minami Y., Kawasaki H., Suzuki K., Yahara I. The calmodulin-binding domain of the mouse 90-kDa heat shock protein. J Biol Chem. 1993 May 5;268(13):9604–9610. [PubMed] [Google Scholar]
- Miyata Y., Yahara I. The 90-kDa heat shock protein, HSP90, binds and protects casein kinase II from self-aggregation and enhances its kinase activity. J Biol Chem. 1992 Apr 5;267(10):7042–7047. [PubMed] [Google Scholar]
- Moore S. K., Kozak C., Robinson E. A., Ullrich S. J., Appella E. Cloning and nucleotide sequence of the murine hsp84 cDNA and chromosome assignment of related sequences. Gene. 1987;56(1):29–40. doi: 10.1016/0378-1119(87)90155-7. [DOI] [PubMed] [Google Scholar]
- Moore S. K., Kozak C., Robinson E. A., Ullrich S. J., Appella E. Murine 86- and 84-kDa heat shock proteins, cDNA sequences, chromosome assignments, and evolutionary origins. J Biol Chem. 1989 Apr 5;264(10):5343–5351. [PubMed] [Google Scholar]
- Nemoto T., Ohara-Nemoto Y., Denis M., Gustafsson J. A. The transformed glucocorticoid receptor has a lower steroid-binding affinity than the nontransformed receptor. Biochemistry. 1990 Feb 20;29(7):1880–1886. doi: 10.1021/bi00459a031. [DOI] [PubMed] [Google Scholar]
- Nishida E., Koyasu S., Sakai H., Yahara I. Calmodulin-regulated binding of the 90-kDa heat shock protein to actin filaments. J Biol Chem. 1986 Dec 5;261(34):16033–16036. [PubMed] [Google Scholar]
- Oppermann H., Levinson W., Bishop J. M. A cellular protein that associates with the transforming protein of Rous sarcoma virus is also a heat-shock protein. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1067–1071. doi: 10.1073/pnas.78.2.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdew G. H., Whitelaw M. L. Evidence that the 90-kDa heat shock protein (HSP90) exists in cytosol in heteromeric complexes containing HSP70 and three other proteins with Mr of 63,000, 56,000, and 50,000. J Biol Chem. 1991 Apr 15;266(11):6708–6713. [PubMed] [Google Scholar]
- Picard D., Khursheed B., Garabedian M. J., Fortin M. G., Lindquist S., Yamamoto K. R. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990 Nov 8;348(6297):166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- Rebbe N. F., Ware J., Bertina R. M., Modrich P., Stafford D. W. Nucleotide sequence of a cDNA for a member of the human 90-kDa heat-shock protein family. Gene. 1987;53(2-3):235–245. doi: 10.1016/0378-1119(87)90012-6. [DOI] [PubMed] [Google Scholar]
- Rexin M., Busch W., Segnitz B., Gehring U. Tetrameric structure of the nonactivated glucocorticoid receptor in cell extracts and intact cells. FEBS Lett. 1988 Dec 5;241(1-2):234–238. doi: 10.1016/0014-5793(88)81068-8. [DOI] [PubMed] [Google Scholar]
- Rose A. B., Broach J. R. Propagation and expression of cloned genes in yeast: 2-microns circle-based vectors. Methods Enzymol. 1990;185:234–279. doi: 10.1016/0076-6879(90)85024-i. [DOI] [PubMed] [Google Scholar]
- Rose D. W., Wettenhall R. E., Kudlicki W., Kramer G., Hardesty B. The 90-kilodalton peptide of the heme-regulated eIF-2 alpha kinase has sequence similarity with the 90-kilodalton heat shock protein. Biochemistry. 1987 Oct 20;26(21):6583–6587. doi: 10.1021/bi00395a003. [DOI] [PubMed] [Google Scholar]
- Rose M. D., Broach J. R. Cloning genes by complementation in yeast. Methods Enzymol. 1991;194:195–230. doi: 10.1016/0076-6879(91)94017-7. [DOI] [PubMed] [Google Scholar]
- Sanchez E. R. Hsp56: a novel heat shock protein associated with untransformed steroid receptor complexes. J Biol Chem. 1990 Dec 25;265(36):22067–22070. [PubMed] [Google Scholar]
- Schowalter D. B., Sullivan W. P., Maihle N. J., Dobson A. D., Conneely O. M., O'Malley B. W., Toft D. O. Characterization of progesterone receptor binding to the 90- and 70-kDa heat shock proteins. J Biol Chem. 1991 Nov 5;266(31):21165–21173. [PubMed] [Google Scholar]
- Schuh S., Yonemoto W., Brugge J., Bauer V. J., Riehl R. M., Sullivan W. P., Toft D. O. A 90,000-dalton binding protein common to both steroid receptors and the Rous sarcoma virus transforming protein, pp60v-src. J Biol Chem. 1985 Nov 15;260(26):14292–14296. [PubMed] [Google Scholar]
- Shaknovich R., Shue G., Kohtz D. S. Conformational activation of a basic helix-loop-helix protein (MyoD1) by the C-terminal region of murine HSP90 (HSP84). Mol Cell Biol. 1992 Nov;12(11):5059–5068. doi: 10.1128/mcb.12.11.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. F., Sullivan W. P., Marion T. N., Zaitsu K., Madden B., McCormick D. J., Toft D. O. Identification of a 60-kilodalton stress-related protein, p60, which interacts with hsp90 and hsp70. Mol Cell Biol. 1993 Feb;13(2):869–876. doi: 10.1128/mcb.13.2.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai P. K., Albers M. W., Chang H., Faber L. E., Schreiber S. L. Association of a 59-kilodalton immunophilin with the glucocorticoid receptor complex. Science. 1992 May 29;256(5061):1315–1318. doi: 10.1126/science.1376003. [DOI] [PubMed] [Google Scholar]
- Tai P. K., Maeda Y., Nakao K., Wakim N. G., Duhring J. L., Faber L. E. A 59-kilodalton protein associated with progestin, estrogen, androgen, and glucocorticoid receptors. Biochemistry. 1986 Sep 9;25(18):5269–5275. doi: 10.1021/bi00366a043. [DOI] [PubMed] [Google Scholar]
- Wang K. K., Villalobo A., Roufogalis B. D. Calmodulin-binding proteins as calpain substrates. Biochem J. 1989 Sep 15;262(3):693–706. doi: 10.1042/bj2620693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Purification of the major mammalian heat shock proteins. J Biol Chem. 1982 Dec 25;257(24):14949–14959. [PubMed] [Google Scholar]
- Yamazaki M., Akaogi K., Miwa T., Imai T., Soeda E., Yokoyama K. Nucleotide sequence of a full-length cDNA for 90 kDa heat-shock protein from human peripheral blood lymphocytes. Nucleic Acids Res. 1989 Sep 12;17(17):7108–7108. doi: 10.1093/nar/17.17.7108. [DOI] [PMC free article] [PubMed] [Google Scholar]