Abstract
Linker of nucleoskeleton and cytoskeleton (LINC) complexes span the double membrane of the nuclear envelope (NE) and physically connect nuclear structures to cytoskeletal elements. LINC complexes are envisioned as force transducers in the NE, which facilitate processes like nuclear anchorage and migration, or chromosome movements. The complexes are built from members of two evolutionary conserved families of transmembrane (TM) proteins, the SUN (Sad1/UNC-84) domain proteins in the inner nuclear membrane (INM) and the KASH (Klarsicht/ANC-1/SYNE homology) domain proteins in the outer nuclear membrane (ONM). In the lumen of the NE, the SUN and KASH domains engage in an intimate assembly to jointly form a NE bridge. Detailed insights into the molecular architecture and atomic structure of LINC complexes have recently revealed the molecular basis of nucleo-cytoskeletal coupling. They bear important implications for LINC complex function and suggest new potential and as yet unexplored roles, which the complexes may play in the cell.
Keywords: LINC complex, SUN domain, KASH domain, nuclear envelope, mechanotransduction, nuclear membrane spacing, spermatogenesis, nuclear pore complex, spindle pole body, membrane fusion
The LINC Between the Nucleus and the Cytoskeleton
The NE separates the nucleoplasm from the cytoplasm in eukaryotic cells and generates the spatial and functional compartmentalization essential for the maintenance and processing of genetic information. The cellular role of the NE, however, extends far beyond its function as a physical barrier. As an integral element of cellular architecture, the NE impacts on cytoskeleletal structure and chromatin organization, and NE components regulate gene expression, signal transduction and cell cycle progression.1,2 The physiological role of the NE becomes evident in a number of genetic diseases, referred to as nuclear envelopathies or laminopathies, which are caused by mutations in genes of NE proteins. Nuclear envelopathies commonly manifest in dystrophic, degenerative or premature aging syndromes. In most cases, pathogenesis is still poorly understood, but it likely involves both structural and regulatory dysfunction of the NE.3
The NE is a double membrane associated with various protein complexes. The INM and the ONM enclose the perinuclear space (PNS), an evenly-spaced luminal compartment continuous with the lumen of the endoplasmic reticulum (ER). The NE is bridged by two types of elaborate, multi-subunit protein complexes, namely nuclear pore complexes (NPCs) and LINC complexes, both of which are highly conserved throughout evolution.1,2 NPCs decorate the sites of fusion between the INM and the ONM and generate aqueous pores in the NE, which guide the selective exchange of molecules between the nucleoplasm and the cytoplasm.4
LINC complexes establish the physical connection between the nucleus and the cytoskeleton. They are built from SUN and KASH domain proteins, TM proteins of the INM and the ONM, respectively. In the nucleoplasm, SUN domain proteins engage in a network of INM proteins, chromatin and the nuclear lamina, an intermediate filament mesh that lines and supports the INM in metazoan cells. In the cytoplasm, KASH domain proteins bind to cytoskeletal elements, like actin, intermediate filaments and microtubule motors. The hallmark of the LINC complex, however, lies in the lumen of the NE, where the conserved SUN and KASH domains interact. Thereby, the complexes bridge the NE and connect the structural elements of the nucleus and the cytoskeleton. SUN-KASH complexes are thought to serve as handles in the NE, which bear or transmit both cytoskeleton and chromatin-derived forces.5-7
Consistently, functions of LINC complexes involve mechanical action and crosstalk between the two sides of the NE, such as during nuclear anchorage and migration,5,7 or in meiotic chromosome movements.6 Based on their central position in cellular architecture and function, SUN and KASH domain proteins are essential for development, differentiation and reproduction.
The Molecular Architecture of the LINC Complex
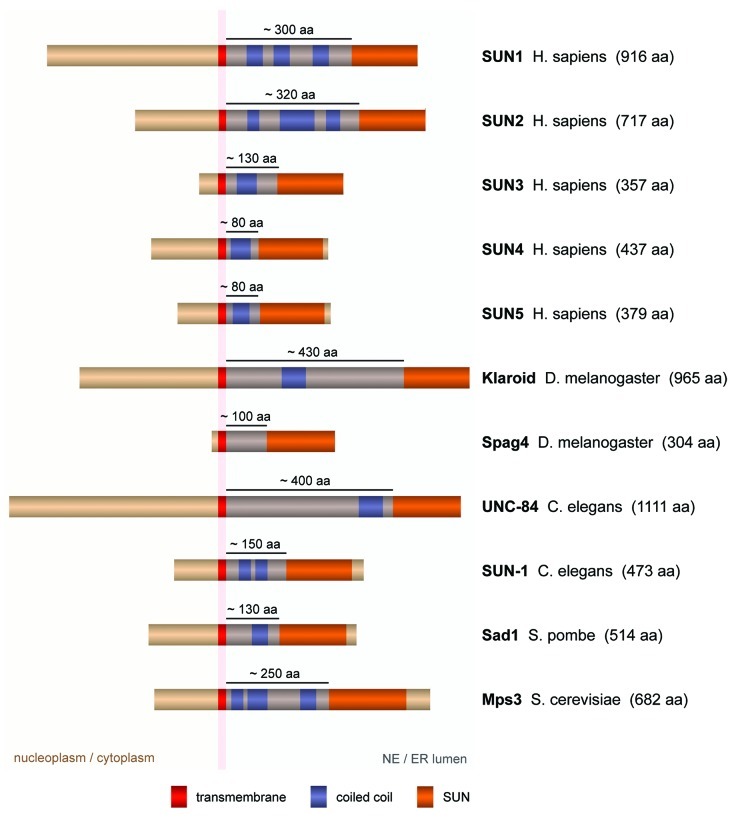
It has long been suggested that SUN domain proteins form oligomers. This idea was based on the prediction of coiled coil elements preceding the C-terminal SUN domain in the luminal part of all typical SUN homologs (Figs. 1 and 2) ), as well as on the oligomerization of the coiled coil region of human SUN1 in vitro. An organization of SUN domain proteins as homo- or heterodimers had been widely assumed.5-7
Figure 1. Typical SUN domain proteins. Typical SUN domain proteins contain one TM region (red). Their N-terminal portions localize in the nucleo/cytoplasm, while their C-terminal portions, consisting of a coiled coil region (blue) and the conserved SUN domain (orange), are exposed to the lumen of the NE/ER. TM regions, coiled coils and SUN domains are indicated based on bioinformatic predictions (TMHMM2, parcoil2, prosite), literature and visual comparison of homologs. Note that additional hydrophobic stretches or potential TM regions are present in most SUN domain proteins, but have been omitted from the scheme for clarity. Further, although only short coiled coil elements are predicted (solid blue), it is conceivable that the entire luminal region preceding the SUN domain forms one continuous coiled coil (blue shadow, length indicated).
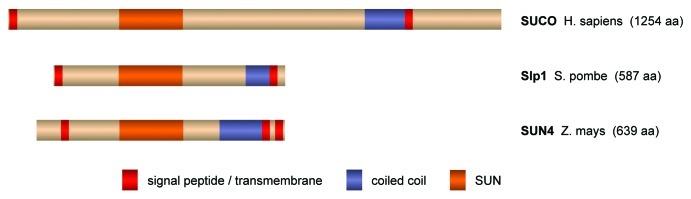
Figure 2. Atypical SUN domain proteins. Besides the typical SUN domain proteins, an unusual second subfamily exists. This subfamily is also highly conserved throughout evolution and includes, for instance, human SUCO/C1ORF9, Saccharomyces cerevisiae Slp1 (SUN-like protein 1) and several plant homologs like Zea mays SUN4. Interestingly, ZmSUN4 has recently been shown to concentrate in the nuclear periphery in maize.60 The domain structure of this type of SUN domain proteins is strikingly different to the conventional one. The SUN domain is located in the middle of the protein instead of at the C terminus and is followed—and not as usual preceded—by a putative coiled coil region. Further, the proteins are potentially membrane-anchored at both their N and C termini and might thus form NE bridges independently of binding partners. Presently, it is unclear if these atypical SUN domain proteins interact with KASH-like peptides and whether they function in force transduction, NE spacing or membrane fusion. Most likely, however, their distinct domain structure, i.e., the inverse order of SUN domain, coiled coils and membrane anchor, might reflect a unique role of these proteins in the NE. TM regions, coiled coils and SUN domains were predicted as in Figure 1. A novel type of SUN domain proteins in the nuclear envelope.
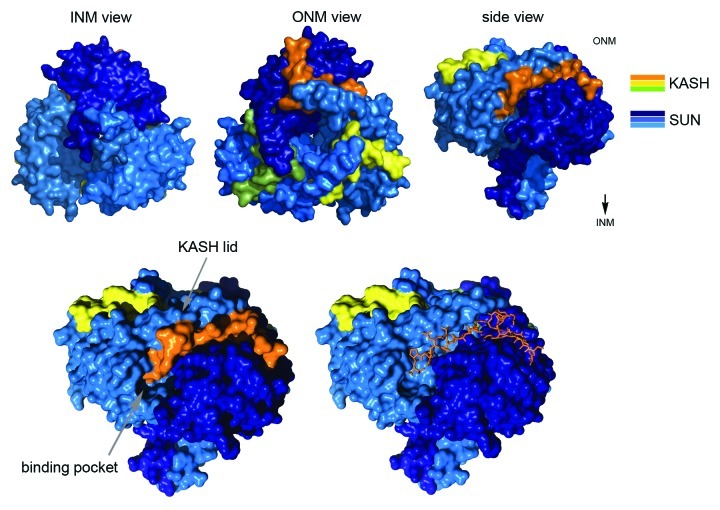
The first crystal structures of a SUN domain protein, human SUN2, therefore bore some surprise: SUN2 is a trimer (Fig. 3).8,9 The shape of the complex resembles a lollipop, with the three SUN domains forming a globular head, from which the N-terminal extensions of the domains emanate as a helical stalk. The stalk folds into an unusual, right-handed, trimeric coiled coil. A conserved salt bridge between the stalk and the SUN domains holds the two elements in a rigid orientation and may serve to organize the spatial arrangement of the globular head. In fact, the trimeric organization of SUN2 is the essential prerequisite for the assembly of LINC complexes. Isolated SUN domains lacking their helical extensions are monomeric in solution and are deficient in recruiting KASH domain proteins both in vitro and in vivo.8
Figure 3. The SUN-KASH complex. LINC complexes are hexamers. Three KASH peptides (yellow, orange, green) bind to the subunit interfaces of a SUN domain trimer (shades of blue), where they are tightly anchored by the binding pocket and the KASH lid of the SUN domain. Presentations were generated for the human SUN2/KASH2 complex (PDB 4XDS).
The significance of SUN trimerization is immediately evident in the mode of SUN-KASH interaction. KASH domain proteins are tail-anchored proteins that contain a short luminal portion of 20–30 residues, the KASH peptide, which directly contacts SUN.10,11 Crystal structures of SUN2 in complex with the KASH peptides of either nesprin-1 or -2 have revealed the molecular details of their interaction (Fig. 3). SUN and KASH associate with 3:3 stoichiometry. Importantly, the KASH peptides are accommodated at the interfaces of adjacent SUN domain subunits, clearly illustrating how their binding sites can be shaped only by the trimeric arrangement of SUN.8 Note that this principle mode of SUN-KASH binding, as first reported by Sosa et al.,8 has recently been confirmed by Wang et al.12
The KASH peptides act as the molecular glue of the LINC complex (Fig. 3). Each peptide is buried in an extended binding groove at the interface of two subunits and engages in multiple contacts with both neighboring SUN domains. The C terminus of the peptide is anchored in a tailor-made binding pocket on the SUN domain with its terminal carboxyl group forming hydrogen bonds to conserved binding pocket side chains. A stretch of three conserved proline residues in the terminal PPPX motif of the peptide helps to orient the carboxyl into the binding pocket. SUN-KASH interactions do not tolerate any interference with this terminal anchorage mechanism as evident from both in vitro and in vivo binding studies.8
The central part of the KASH peptide is clamped between the “KASH lid,” a β-hairpin extension of the SUN domain and the core of the neighboring SUN subunit (Fig. 3). The peptide backbone and the 'KASH lid' form a joint β-sheet, and hydrophobic peptide side chains in this region are buried in deep binding clefts formed between the SUN domain subunits. Besides the terminal binding pocket, the “KASH lid” adds the second essential and conserved contribution to SUN-KASH interaction.8
In addition, many metazoan LINC complexes seem to contain a customized safety lock. Complexes of human SUN1 or SUN2 and nesprins are covalently linked via intermolecular disulfide bonds. The bonds are formed between a cysteine residue in the N-terminal, membrane-facing part of the KASH peptide and a cysteine on the SUN domain. At both positions, cysteines are conserved throughout a range of metazoan homologs suggesting that the covalent linkage has been preserved during evolution. However, the disulfide bond does not seem to be required for the association of SUN and KASH domain proteins per se.8 Rather, it may represent a backup mechanism to prevent the disruption of complexes under peak force loads. Alternatively, the covalent linkage of SUN and KASH may serve to regulate the dynamics of SUN-KASH association or the turnover of the proteins.
The LINC Complex and Force Transduction
The extensive network of non-covalent and covalent contacts between SUN and KASH domains, in combination with the binding avidity gained by the association of three KASH peptides with each SUN trimer, suggests a highly stable complex. The hexameric assembly appears, in fact, ideally suited to bear and transmit forces on the NE.
During processes like nuclear migration or chromosome movement, however, LINC complexes likely do not act as individual units, but organize into higher-order structures. The most striking description of a regular, higher-order assembly of LINC components stems from fibroblasts studied in wounded cell monolayers. In preparation for cell migration, fibroblasts polarize by rearward movement of their nucleus, which is dependent on retrograde actin flow and its coupling to the NE via LINC complexes. During this nuclear movement, SUN2 and nesprin-2—normally distributed homogenously throughout the NE11,13—were shown to organize into linear arrays aligned with actin cables, which were termed TM actin-associated nuclear (TAN) lines.14
Another deviation from the usual homogenous NE distribution of mammalian LINC components is observed in meiotic cells. A complex between SUN1 and the germ cell-specific KASH domain protein KASH5 seems to be dedicated to a role during the prophase of meiosis.15,16 The LINC complex serves to tether the telomeres of the meiotic chromosomes to the NE, which is thought to facilitate meiotic prophase-specific chromosome movements and to assist the pairing and recombination of the homologs.15 During this process, SUN1 and KASH5 were shown to cluster at the sites of telomere attachment to the NE.15,16 In contrast to SUN1, SUN2 is dispensable for the progression of meiosis.15 Nevertheless, SUN2 is expressed in meiotic cells and has been detected at telomere attachment sites,17 indicating that it may participate in the formation of meiosis-specific higher-order LINC assemblies.
It is well conceivable that LINC complexes form permanent or transient higher-order structures also in other cell types, which could so far not be resolved by conventional imaging techniques. Their organization may be established by oligomerization of KASH domain proteins,18 or may be mediated via nucleo- or cytoskeletal interaction partners, such as actin filaments19-21 or the nuclear lamina.10,22 Higher-order structures of LINC complexes may be flexible and adapted to dedicated functions, as suggested for TAN line formation.14 Importantly, networks of SUN and KASH domain proteins in the NE could potentiate the force load, which the complexes can withstand. LINC complexes in the NE might thus function in nucleo-cytoskeletal force transduction analogous to integrins in the plasma membrane, which assemble in focal adhesions to transmit forces between the extracellular matrix and the cytoskeleton.23
The mechanical coupling between the nucleus and the cytoplasm can directly be assessed by analyzing changes in nuclear structure and shape upon the application of forces to either the cytoplasm or to the cell surface.24 A role of LINC complexes in the mechanical coupling of cellular compartments has been demonstrated in several experimental setups, supporting the idea that nucleo-cytoskeletal force transduction is a general principle of LINC complex function25,26 Elucidating the contributions of the characteristic molecular features of the SUN-KASH assembly in dedicated assays may in future provide insight into the mechanisms of intracellular force transduction and the basis of fundamental cellular processes.
The LINC Complex and Nuclear Membrane Shape
The physiological role of the LINC complex, however, may go far beyond these relatively well-characterized tasks. The molecular architecture of the complex, together with its integral position in the NE, suggests important functions in membrane shaping and organization. By spanning the double membrane of the NE, assemblies of SUN and KASH domain proteins may be critical to generate and/or maintain the characteristic shape of the nuclear membrane system. Indeed, observations by electron microscopy indicate that the even spacing of the nuclear membranes is lost upon the co-depletion of SUN1 and SUN2 from HeLa cells, giving rise to dilations of the ONM and irregular expansions of the PNS.10
LINC complexes may in fact serve as the molecular rulers, which set the distance between the INM and the ONM.8 When the luminal region of SUN2 preceding the SUN domain is extrapolated as a continuous and extended, trimeric coiled coil, it reaches a length of ~45 nm.27 Together with the globular SUN domain assembly, the entire luminal portion of SUN2 would thus span a distance of ~48 nm, fitting strikingly well the observed spacing of the nuclear membranes in mammalian cells (Fig. 4).28 Importantly, domain organization and size of the luminal parts are conserved between SUN1 and SUN2 (Fig. 1). Although it cannot be excluded that they simply represent adaptions to a given membrane distance, the existing experimental evidence clearly points toward an active and collaborative role of LINC complexes in shaping the mammalian NE.8,10
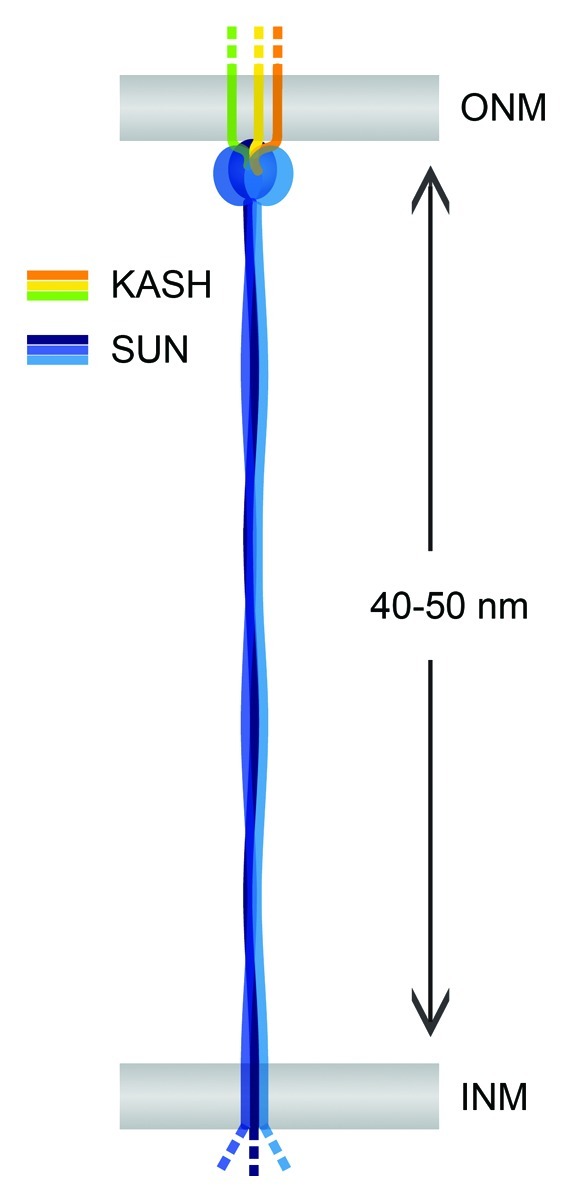
Figure 4. The LINC complex as a nuclear membrane spacer. SUN and KASH domain proteins (blue and yellow) are TM proteins of the INM and the ONM, respectively. SUN-KASH complexes may serve as nuclear membrane spacers and determine the regular shape of the NE. Observed INM-ONM distances in mammalian cells fit well the luminal sizes of both SUN1 and SUN2 given that their coiled coil regions form continuous and extended structures. Analogously, other LINC complexes might determine the spacing of the nuclear membranes in different cell types or species.
But is this function limited to SUN1 and SUN2? Or may SUN domain homologs in other cell types and organisms fulfill a similar task? Besides SUN1 and SUN2, the mammalian genome encodes at least three further SUN domain proteins (Fig. 1). In sharp contrast to the ubiquitous expression of the former,10 SUN3–5 are highly tissue-specific and almost exclusively found in male germ cells.10,29-31 SUN3 was shown to localize to the NE of developing spermatids, where it accumulates in specific regions in the posterior part of the nucleus.29 Its localization corresponds to the contact region between the NE and the manchette, a spermatid-specific, calyx-like microtubule structure that is thought to assist the elongation and compaction of the nucleus during sperm head formation.32 SUN3-containing LINC complexes were suggested to tether the manchette microtubules to the NE, potentially required for the remodeling of the sperm nucleus.29
Besides SUN3, the fourth mammalian SUN domain family member, known as SUN4 or SPAG4, has also been detected in spermatid-specific microtubule structures.33 Its D. melanogaster homolog was shown to focus in microtubule- and centriole-associated regions of the NE during spermatogenesis and was required for the attachment of the spermatid's centriole to the nucleus.34 Interestingly, early electron microscopic studies on both rodent and fly spermatids have described dense material, or even rod-like structures, between microtubules and the NE,35,36 which might from our current point of view well be interpreted as LINC complexes.
Although the overall domain architecture is shared between all typical SUN domain homologs, the sperm-specific family members SUN3–5 differ from SUN1 and 2 in some critical aspects. They contain sequence variations in the KASH lid, and the cysteine involved in disulfide-bonding to KASH is not conserved.8 Most strikingly, however, the coiled coil regions in the luminal portions of SUN3–5 are significantly shorter than the ones of their “larger brothers”—80–130 amino acids (aa) compared with ~300 aa in both SUN1 and SUN2 (Fig. 1). Might a close proximity between the INM and the ONM in the sperm nucleus be intended? In electron micrographs of the manchette region, the NE indeed seems narrow—with a width of ~25 nm as estimated from comparison with neighboring microtubules.35 Close membrane coupling might in fact provide particular stability and rigidity to the NE at the posterior pole of the sperm nucleus. It might assist nuclear remodeling during sperm development, or might enable the nucleus of mature spermatozoa to withstand potentially immense cytoskeletal forces generated during sperm movement.
The potential function of LINC complexes in NE organization and membrane spacing may be conserved throughout the evolution of eukaryotes. C. elegans and D. melanogaster possess SUN domain homologs with similar molecular properties and sub-cellular localizations as mammalian SUN1/2 (Fig. 1),34,37,38 which would thus be well suited to determine NE structure in these organisms. Yeast, however, poses a challenge to the hypothesis. In contrast to the homogenous distribution of most metazoan SUN and KASH domain proteins in the NE, LINC components in both S. cerevisiae and S. pombe cluster at the spindle pole bodies (SPBs) during vegetative growth.39-43 Nevertheless, a minor pool of both SUN and KASH domain proteins appears to be dispersed throughout the NE in both budding and fission yeast,43-46 which may well be sufficient to tether the INM and the ONM together and to generate the homogenous shape of the nuclear membrane system.
Interestingly, the lengths of the luminal coiled coil regions differ significantly not only between mammalian SUN domain homologs, but also between family members from metazoans and yeast (Fig. 1). While the coiled coils of S. cerevisiae Mps3 are with ~250 aa only slightly shorter than the ones of SUN1 and SUN2, S. pombe Sad1 contains only ~130 aa between its TM helix and the C-terminal SUN domain. To our knowledge, no direct measurements of INM-ONM distances in yeast have been published. We therefore attempted to estimate values from electron micrographs acquired under conditions optimized for the preservation of nuclear membrane structure,47,48 which obtained INM-ONM distances of ~20 nm for both S. cerevisiae and S. pombe. This would fit well with the predicted length of the Sad1 coiled coil region, but be too narrow to accommodate an extended coiled coil for Mps3. This discrepancy sheds some doubt on the suggested role of LINC complexes in NE spacing in yeasts. Yet, it is presently unclear whether the entire luminal region preceding the SUN domain adopts a continuous coiled coil structure in all SUN domain proteins.
Taken together, SUN domain proteins may serve as molecular rulers to set the distance between the INM and ONM in a wide range of eukaryotes. Moreover, the composition of LINC complexes might be adapted in certain cell types, or even within specific regions of the NE, to modulate membrane spacing for dedicated functions. Clearly, more systematic analyses of NE structure in different cell types and organisms, and directed experimental approaches will be required to verify a role of LINC complexes in nuclear membrane spacing. Expression and localization patterns of SUN domain proteins and correlations between the lengths of their coiled coil regions and INM-ONM distances may uncover new facets of intra-cellular membrane shaping.
The LINC Complex and Membrane Fusion
Fundamental cellular processes may rely on the proper structural organization of the nuclear membrane system. The coupling of the nuclear membranes may be particularly important for membrane remodeling and fusion events, such as the breakdown and reformation of the NE during open mitosis in metazoan cells,49 or the assembly of new NPCs during interphase.50 Interphase NPC biogenesis occurs by insertion of new NPCs into the intact double membrane of the NE. A fusion event between the INM and the ONM is thus required to form the aqueous pore.50 Membrane fusion, in general, necessitates a very close proximity of two membranes51—which at the sites of NPC insertion into the NE might be assisted by LINC complexes that sew INM and ONM together. In line with this, human SUN1 was found to locally cluster around NPCs and to be required for the equal distribution of NPCs in the NE.52 Moreover, it has been proposed that SUN1 assists NPC biogenesis specifically during interphase.53 In vitro studies revealed that the formation of aqueous pores in the NE was prohibited in the absence of SUN1, suggesting a function in the membrane fusion step of NPC biogenesis. Consistently, the assembly of NPCs during NE reformation at the end of mitosis, which could in principle mechanistically differ from interphase assembly and must not necessarily require membrane fusion,50 was unaffected by SUN1 depletion. Transient interactions between the luminal portions of SUN1 and the TM nucleoporin POM121 were suggested to facilitate the fusion between the INM and the ONM, but such mechanism remains to be validated experimentally.53
Similar to NPCs, the SPBs of yeast are gigantic, multi-subunit protein complexes inserted into the double membrane of the NE.54 SPBs are the main microtubule-organizing centers of yeast cells, and their integration into the NE is critical for the formation of the intra-nuclear spindle during mitosis.55 In S. cerevisiae, SPBs are inserted into the NE as a late step of their duplication in G1 and remain embedded throughout the cell cycle,55 whereas SPBs of S. pombe become integrated into the NE only temporarily during mitosis.56 Also the insertion of SPBs thus necessitates the fusion between the INM and the ONM and likely poses strict requirements on nuclear membrane structure.54
Strikingly, LINC complexes are once again found in the center of action. SUN and KASH domain proteins are integral components of SPBs in both budding and fission yeast,39-43 and their importance for SPB structure and function is well established.40-42,57,58 The S. cerevisiae SUN domain protein Mps3 has been implicated specifically in the membrane insertion step of SPB duplication. Mps3 was found to influence the lipid composition of the nuclear membranes, potentially by recruiting lipid-modifying enzymes to the NE, which was proposed to modulate physical membrane properties required for SPB insertion.57 For a detailed discussion on the connections between NPC and SPB insertion in yeast, and on potential roles of S. cerevisiae Mps3 in these processes, we would like to refer to a recent extra view by Jaspersen and Ghosh.54
How might fusion of INM and ONM for insertion of NPCs53 and SPBs57 depend on LINC complexes mechanistically? As an underlying principle, LINC complexes may simply generate and/or maintain NE structure, in particular the even spacing of the nuclear membranes. Their molecular architecture—the extended coiled coil region in combination with the robustly coupled SUN-KASH assembly8—renders the complexes ideally suited for this task. Or might LINC complexes even play a more direct role? Although purely speculative at this point, it is conceivable that SUN-KASH complexes actively mediate membrane fusion. Their molecular architecture bears surprising similarity with membrane fusion complexes from enveloped viruses.59 Both LINC and viral fusion complexes are trimeric protein assemblies, which—at least at certain steps during the fusion process—are anchored in two opposing lipid bilayers. Influenza hemagglutinin and LINC complexes further share their composition of extended, trimeric coiled coils and globular head domains.8,59 Similar to viral fusion complexes, SUN-KASH assemblies might thus employ conformational changes, potentially coupled to association/dissociation cycles, to induce nuclear membrane fusion. However, it is currently unclear whether the extended luminal coiled coil regions of SUN domain proteins are indeed capable of undergoing such changes. Clearly, much remains to be discovered with respect to structure, function and dynamics of these versatile LINCs in the NE.
Glossary
Abbreviations:
- NE
nuclear envelope
- INM
inner nuclear membrane
- ONM
outer nuclear membrane
- PNS
perinuclear space
- ER
endoplasmic reticulum
- NPC
nuclear pore complex
- LINC
linker of nucleoskeleton and cytoskeleton
- SUN
Sad1/UNC-84
- KASH
Klarsicht/ANC-1/SYNE homology
- TM
transmembrane
- SPB
spindle pole body
Footnotes
Previously published online: www.landesbioscience.com/journals/nucleus/article/23387
References
- 1.Shimi T, Butin-Israeli V, Goldman RD. The functions of the nuclear envelope in mediating the molecular crosstalk between the nucleus and the cytoplasm. Curr Opin Cell Biol. 2012;24:71–8. doi: 10.1016/j.ceb.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stewart CL, Roux KJ, Burke B. Blurring the boundary: the nuclear envelope extends its reach. Science. 2007;318:1408–12. doi: 10.1126/science.1142034. [DOI] [PubMed] [Google Scholar]
- 3.Worman HJ, Ostlund C, Wang Y. Diseases of the nuclear envelope. Cold Spring Harb Perspect Biol. 2010;2:a000760. doi: 10.1101/cshperspect.a000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grossman E, Medalia O, Zwerger M. Functional architecture of the nuclear pore complex. Annu Rev Biophys. 2012;41:557–84. doi: 10.1146/annurev-biophys-050511-102328. [DOI] [PubMed] [Google Scholar]
- 5.Burke B, Roux KJ. Nuclei take a position: managing nuclear location. Dev Cell. 2009;17:587–97. doi: 10.1016/j.devcel.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 6.Fridkin A, Penkner A, Jantsch V, Gruenbaum Y. SUN-domain and KASH-domain proteins during development, meiosis and disease. Cell Mol Life Sci. 2009;66:1518–33. doi: 10.1007/s00018-008-8713-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Starr DA, Fridolfsson HN. Interactions between nuclei and the cytoskeleton are mediated by SUN-KASH nuclear-envelope bridges. Annu Rev Cell Dev Biol. 2010;26:421–44. doi: 10.1146/annurev-cellbio-100109-104037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sosa BA, Rothballer A, Kutay U, Schwartz TU. LINC complexes form by binding of three KASH peptides to domain interfaces of trimeric SUN proteins. Cell. 2012;149:1035–47. doi: 10.1016/j.cell.2012.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Z, Du X, Cai Z, Song X, Zhang H, Mizuno T, et al. Structure of Sad1-UNC84 homology (SUN) domain defines features of molecular bridge in nuclear envelope. J Biol Chem. 2012;287:5317–26. doi: 10.1074/jbc.M111.304543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, et al. Coupling of the nucleus and cytoplasm: role of the LINC complex. J Cell Biol. 2006;172:41–53. doi: 10.1083/jcb.200509124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padmakumar VC, Libotte T, Lu W, Zaim H, Abraham S, Noegel AA, et al. The inner nuclear membrane protein Sun1 mediates the anchorage of Nesprin-2 to the nuclear envelope. J Cell Sci. 2005;118:3419–30. doi: 10.1242/jcs.02471. [DOI] [PubMed] [Google Scholar]
- 12.Wang W, Shi Z, Jiao S, Chen C, Wang H, Liu G, et al. Structural insights into SUN-KASH complexes across the nuclear envelope. Cell Res. 2012;22:1440–52. doi: 10.1038/cr.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodzic DM, Yeater DB, Bengtsson L, Otto H, Stahl PD. Sun2 is a novel mammalian inner nuclear membrane protein. J Biol Chem. 2004;279:25805–12. doi: 10.1074/jbc.M313157200. [DOI] [PubMed] [Google Scholar]
- 14.Luxton GW, Gomes ER, Folker ES, Vintinner E, Gundersen GG. Linear arrays of nuclear envelope proteins harness retrograde actin flow for nuclear movement. Science. 2010;329:956–9. doi: 10.1126/science.1189072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding X, Xu R, Yu J, Xu T, Zhuang Y, Han M. SUN1 is required for telomere attachment to nuclear envelope and gametogenesis in mice. Dev Cell. 2007;12:863–72. doi: 10.1016/j.devcel.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Morimoto A, Shibuya H, Zhu X, Kim J, Ishiguro K, Han M, et al. A conserved KASH domain protein associates with telomeres, SUN1, and dynactin during mammalian meiosis. J Cell Biol. 2012;198:165–72. doi: 10.1083/jcb.201204085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitt J, Benavente R, Hodzic D, Höög C, Stewart CL, Alsheimer M. Transmembrane protein Sun2 is involved in tethering mammalian meiotic telomeres to the nuclear envelope. Proc Natl Acad Sci U S A. 2007;104:7426–31. doi: 10.1073/pnas.0609198104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mislow JM, Holaska JM, Kim MS, Lee KK, Segura-Totten M, Wilson KL, et al. Nesprin-1alpha self-associates and binds directly to emerin and lamin A in vitro. FEBS Lett. 2002;525:135–40. doi: 10.1016/S0014-5793(02)03105-8. [DOI] [PubMed] [Google Scholar]
- 19.Padmakumar VC, Abraham S, Braune S, Noegel AA, Tunggal B, Karakesisoglou I, et al. Enaptin, a giant actin-binding protein, is an element of the nuclear membrane and the actin cytoskeleton. Exp Cell Res. 2004;295:330–9. doi: 10.1016/j.yexcr.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Q, Skepper JN, Yang F, Davies JD, Hegyi L, Roberts RG, et al. Nesprins: a novel family of spectrin-repeat-containing proteins that localize to the nuclear membrane in multiple tissues. J Cell Sci. 2001;114:4485–98. doi: 10.1242/jcs.114.24.4485. [DOI] [PubMed] [Google Scholar]
- 21.Zhen YY, Libotte T, Munck M, Noegel AA, Korenbaum E. NUANCE, a giant protein connecting the nucleus and actin cytoskeleton. J Cell Sci. 2002;115:3207–22. doi: 10.1242/jcs.115.15.3207. [DOI] [PubMed] [Google Scholar]
- 22.Haque F, Mazzeo D, Patel JT, Smallwood DT, Ellis JA, Shanahan CM, et al. Mammalian SUN protein interaction networks at the inner nuclear membrane and their role in laminopathy disease processes. J Biol Chem. 2010;285:3487–98. doi: 10.1074/jbc.M109.071910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roca-Cusachs P, Iskratsch T, Sheetz MP. Finding the weakest link: exploring integrin-mediated mechanical molecular pathways. J Cell Sci. 2012;125:3025–38. doi: 10.1242/jcs.095794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lombardi ML, Zwerger M, Lammerding J. Biophysical assays to probe the mechanical properties of the interphase cell nucleus: substrate strain application and microneedle manipulation. J Vis Exp. 2011;55:e3087. doi: 10.3791/3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anno T, Sakamoto N, Sato M. Role of nesprin-1 in nuclear deformation in endothelial cells under static and uniaxial stretching conditions. Biochem Biophys Res Commun. 2012;424:94–9. doi: 10.1016/j.bbrc.2012.06.073. [DOI] [PubMed] [Google Scholar]
- 26.Lombardi ML, Jaalouk DE, Shanahan CM, Burke B, Roux KJ, Lammerding J. The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton. J Biol Chem. 2011;286:26743–53. doi: 10.1074/jbc.M111.233700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Testa OD, Moutevelis E, Woolfson DN. CC+: a relational database of coiled-coil structures. Nucleic Acids Res. 2009;37(Database issue):D315–22. doi: 10.1093/nar/gkn675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Voeltz GK, Prinz WA. Sheets, ribbons and tubules - how organelles get their shape. Nat Rev Mol Cell Biol. 2007;8:258–64. doi: 10.1038/nrm2119. [DOI] [PubMed] [Google Scholar]
- 29.Göb E, Schmitt J, Benavente R, Alsheimer M. Mammalian sperm head formation involves different polarization of two novel LINC complexes. PLoS One. 2010;5:e12072. doi: 10.1371/journal.pone.0012072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarnasky H, Gill D, Murthy S, Shao X, Demetrick DJ, van der Hoorn FA. A novel testis-specific gene, SPAG4, whose product interacts specifically with outer dense fiber protein ODF27, maps to human chromosome 20q11.2. Cytogenet Cell Genet. 1998;81:65–7. doi: 10.1159/000014990. [DOI] [PubMed] [Google Scholar]
- 31.Xing XW, Li LY, Liu G, Fu JJ, Tan XJ, Lu GX. Identification of a novel gene SRG4 expressed at specific stages of mouse spermatogenesis. Acta Biochim Biophys Sin (Shanghai) 2004;36:351–9. doi: 10.1093/abbs/36.5.351. [DOI] [PubMed] [Google Scholar]
- 32.Kierszenbaum AL, Rivkin E, Tres LL. Molecular biology of sperm head shaping. Soc Reprod Fertil Suppl. 2007;65:33–43. [PubMed] [Google Scholar]
- 33.Shao X, Tarnasky HA, Lee JP, Oko R, van der Hoorn FA. Spag4, a novel sperm protein, binds outer dense-fiber protein Odf1 and localizes to microtubules of manchette and axoneme. Dev Biol. 1999;211:109–23. doi: 10.1006/dbio.1999.9297. [DOI] [PubMed] [Google Scholar]
- 34.Kracklauer MP, Wiora HM, Deery WJ, Chen X, Bolival B, Jr., Romanowicz D, et al. The Drosophila SUN protein Spag4 cooperates with the coiled-coil protein Yuri Gagarin to maintain association of the basal body and spermatid nucleus. J Cell Sci. 2010;123:2763–72. doi: 10.1242/jcs.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russell LD, Russell JA, MacGregor GR, Meistrich ML. Linkage of manchette microtubules to the nuclear envelope and observations of the role of the manchette in nuclear shaping during spermiogenesis in rodents. Am J Anat. 1991;192:97–120. doi: 10.1002/aja.1001920202. [DOI] [PubMed] [Google Scholar]
- 36.Tokuyasu KT. Dynamics of spermiogenesis in Drosophila melanogaster. IV. Nuclear transformation. J Ultrastruct Res. 1974;48:284–303. doi: 10.1016/S0022-5320(74)80083-3. [DOI] [PubMed] [Google Scholar]
- 37.Kracklauer MP, Banks SM, Xie X, Wu Y, Fischer JA. Drosophila klaroid encodes a SUN domain protein required for Klarsicht localization to the nuclear envelope and nuclear migration in the eye. Fly (Austin) 2007;1:75–85. doi: 10.4161/fly.4254. [DOI] [PubMed] [Google Scholar]
- 38.Tapley EC, Ly N, Starr DA. Multiple mechanisms actively target the SUN protein UNC-84 to the inner nuclear membrane. Mol Biol Cell. 2011;22:1739–52. doi: 10.1091/mbc.E10-08-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miki F, Kurabayashi A, Tange Y, Okazaki K, Shimanuki M, Niwa O. Two-hybrid search for proteins that interact with Sad1 and Kms1, two membrane-bound components of the spindle pole body in fission yeast. Mol Genet Genomics. 2004;270:449–61. doi: 10.1007/s00438-003-0938-8. [DOI] [PubMed] [Google Scholar]
- 40.Jaspersen SL, Martin AE, Glazko G, Giddings TH, Jr., Morgan G, Mushegian A, et al. The Sad1-UNC-84 homology domain in Mps3 interacts with Mps2 to connect the spindle pole body with the nuclear envelope. J Cell Biol. 2006;174:665–75. doi: 10.1083/jcb.200601062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hagan I, Yanagida M. The product of the spindle formation gene sad1+ associates with the fission yeast spindle pole body and is essential for viability. J Cell Biol. 1995;129:1033–47. doi: 10.1083/jcb.129.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jaspersen SL, Giddings TH, Jr., Winey M. Mps3p is a novel component of the yeast spindle pole body that interacts with the yeast centrin homologue Cdc31p. J Cell Biol. 2002;159:945–56. doi: 10.1083/jcb.200208169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muñoz-Centeno MC, McBratney S, Monterrosa A, Byers B, Mann C, Winey M. Saccharomyces cerevisiae MPS2 encodes a membrane protein localized at the spindle pole body and the nuclear envelope. Mol Biol Cell. 1999;10:2393–406. doi: 10.1091/mbc.10.7.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bupp JM, Martin AE, Stensrud ES, Jaspersen SL. Telomere anchoring at the nuclear periphery requires the budding yeast Sad1-UNC-84 domain protein Mps3. J Cell Biol. 2007;179:845–54. doi: 10.1083/jcb.200706040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.King MC, Drivas TG, Blobel G. A network of nuclear envelope membrane proteins linking centromeres to microtubules. Cell. 2008;134:427–38. doi: 10.1016/j.cell.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horigome C, Okada T, Shimazu K, Gasser SM, Mizuta K. Ribosome biogenesis factors bind a nuclear envelope SUN domain protein to cluster yeast telomeres. EMBO J. 2011;30:3799–811. doi: 10.1038/emboj.2011.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murray S. High pressure freezing and freeze substitution of Schizosaccharomyces pombe and Saccharomyces cerevisiae for TEM. Methods Cell Biol. 2008;88:3–17. doi: 10.1016/S0091-679X(08)00401-9. [DOI] [PubMed] [Google Scholar]
- 48.McDonald K. Cryopreparation methods for electron microscopy of selected model systems. Methods Cell Biol. 2007;79:23–56. doi: 10.1016/S0091-679X(06)79002-1. [DOI] [PubMed] [Google Scholar]
- 49.Güttinger S, Laurell E, Kutay U. Orchestrating nuclear envelope disassembly and reassembly during mitosis. Nat Rev Mol Cell Biol. 2009;10:178–91. doi: 10.1038/nrm2641. [DOI] [PubMed] [Google Scholar]
- 50.Doucet CM, Hetzer MW. Nuclear pore biogenesis into an intact nuclear envelope. Chromosoma. 2010;119:469–77. doi: 10.1007/s00412-010-0289-2. [DOI] [PubMed] [Google Scholar]
- 51.Risselada HJ, Grubmüller H. How SNARE molecules mediate membrane fusion: recent insights from molecular simulations. Curr Opin Struct Biol. 2012;22:187–96. doi: 10.1016/j.sbi.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 52.Liu Q, Pante N, Misteli T, Elsagga M, Crisp M, Hodzic D, et al. Functional association of Sun1 with nuclear pore complexes. J Cell Biol. 2007;178:785–98. doi: 10.1083/jcb.200704108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Talamas JA, Hetzer MW. POM121 and Sun1 play a role in early steps of interphase NPC assembly. J Cell Biol. 2011;194:27–37. doi: 10.1083/jcb.201012154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jaspersen SL, Ghosh S. Nuclear envelope insertion of spindle pole bodies and nuclear pore complexes. Nucleus. 2012;3:226–36. doi: 10.4161/nucl.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jaspersen SL, Winey M. The budding yeast spindle pole body: structure, duplication, and function. Annu Rev Cell Dev Biol. 2004;20:1–28. doi: 10.1146/annurev.cellbio.20.022003.114106. [DOI] [PubMed] [Google Scholar]
- 56.Ding R, West RR, Morphew DM, Oakley BR, McIntosh JR. The spindle pole body of Schizosaccharomyces pombe enters and leaves the nuclear envelope as the cell cycle proceeds. Mol Biol Cell. 1997;8:1461–79. doi: 10.1091/mbc.8.8.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Friederichs JM, Ghosh S, Smoyer CJ, McCroskey S, Miller BD, Weaver KJ, et al. The SUN protein Mps3 is required for spindle pole body insertion into the nuclear membrane and nuclear envelope homeostasis. PLoS Genet. 2011;7:e1002365. doi: 10.1371/journal.pgen.1002365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winey M, Goetsch L, Baum P, Byers B. MPS1 and MPS2: novel yeast genes defining distinct steps of spindle pole body duplication. J Cell Biol. 1991;114:745–54. doi: 10.1083/jcb.114.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harrison SC. Viral membrane fusion. Nat Struct Mol Biol. 2008;15:690–8. doi: 10.1038/nsmb.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murphy SP, Simmons CR, Bass HW. Structure and expression of the maize (Zea mays L.) SUN-domain protein gene family: evidence for the existence of two divergent classes of SUN proteins in plants. BMC Plant Biol. 2010;10:269. doi: 10.1186/1471-2229-10-269. [DOI] [PMC free article] [PubMed] [Google Scholar]