Background: Analyses of pol II transcription are hampered by the difficulty of preparing an abundant functional preinitiation complex (PIC).
Results: We reconstituted milligram quantities of a complete 31-subunit PIC.
Conclusion: An intermediate comprising TBP, TFIIE, TFIIH, and DNA could be isolated and combined with TFIIB and pol II-TFIIF to generate the PIC.
Significance: The results enable definitive biochemical and structural studies of the transcription initiation machinery.
Keywords: Electron Microscopy (EM), RNA Polymerase II, Transcription, Transcription Initiation Factors, Yeast
Abstract
Whereas individual RNA polymerase II (pol II)-general transcription factor (GTF) complexes are unstable, an assembly of pol II with six GTFs and promoter DNA could be isolated in abundant homogeneous form. The resulting complete pol II transcription preinitiation complex (PIC) contained equimolar amounts of all 31 protein components. An intermediate in assembly, consisting of four GTFs and promoter DNA, could be isolated and supplemented with the remaining components for formation of the PIC. Nuclease digestion and psoralen cross-linking mapped the PIC between positions −70 and −9, centered on the TATA box. Addition of ATP to the PIC resulted in quantitative conversion to an open complex, which retained all 31 proteins, contrary to expectation from previous studies. Addition of the remaining NTPs resulted in run-off transcription, with an efficiency that was promoter-dependent and was as great as 17.5% with the promoters tested.
Introduction
The initiation of RNA polymerase II (pol II)4 transcription is a multistage process, most likely for the purpose of multifactorial control. It extends far beyond the formation of the first phosphodiester bond; a transcript of ∼25 nucleotides must be synthesized before a transition occurs from initiation to RNA chain elongation (1, 2). The proteins responsible for initiation, a set of general transcription factors (GTFs) and the polymerase, associate in a so-called preinitiation complex (PIC) (3, 4) and largely dissociate at every round of transcription. Evidence for a PIC was previously obtained with nuclear extract or with partially purified GTFs assembled on immobilized promoter DNA (5–9). Due to the poor efficiency of the reaction and trace amounts of the protein involved, detection was possible only by the synthesis of a radiolabeled transcript and by immunoblotting.
An abundant, homogeneous, and soluble PIC is required to elucidate the mechanisms of initiation and regulation of transcription. We sought to assemble a PIC with pure GTFs and pol II from the yeast Saccharomyces cerevisiae. Four GTFs (transcription factor (TF) IIA, TFIIB, the TATA box-binding protein (TBP), and TFIIE) were available in recombinant form, but the remaining GTFs (TFIIF and TFIIH) could be obtained only by isolation from yeast and presented technical difficulties. Pure TFIIF is as largely insoluble, and TFIIH, an 11-subunit complex, invariably dissociates upon isolation (10). The TFIIF problem was solved by identifying a detergent capable of solubilizing the protein without an effect on transcriptional activity. The instability of TFIIH was traced to a previously unrecognized subunit, termed Tfb6 (11), which provokes the dissociation of Ssl2, the helicase responsible for conversion of a closed (fully double-stranded) promoter to the open state (with ∼15 bp unwound in the form of a “transcription bubble”). Isolation of TFIIH from a tfb6 deletion strain of yeast resulted in a good yield of the complete 10-subunit protein, lacking only Tfb6 (therefore referred to here as TFIIH*) and active in transcription.
With all the GTFs in suitable form in hand, we investigated the assembly of a PIC and arrived at an efficient procedure for obtaining a stable functional complex. We found intermediates consistent with the emerging picture of PIC assembly in vivo. The results were also informative about the fate of the complex upon the initiation of transcription. The way is now open to structure determination of the PIC and to dissection of the initiation process.
EXPERIMENTAL PROCEDURES
Oligonucleotides
Short fragments of the HIS4 promoter DNA (HIS4(−81/+1) and HIS4(−81/+19)) were obtained by annealing equimolar amounts of complementary oligonucleotides (Integrated DNA Technologies). Other HIS4 promoter templates were obtained by restriction digestion of a concatemeric form as described below. HIS4 promoter DNA was amplified by PCR using two primers with EcoRV sites at both ends and was cloned into the pDrive vector (Qiagen). The plasmid construct was digested with EcoRI, and the promoter DNA fragment was purified and concentrated to 240 μg/ml using a Vivaspin 500 concentrator (5000 Mr cutoff; Vivascience). The fragment was self-ligated in 20 μl of ligation buffer with 2 units of T4 ligase (New England Biolabs) to obtain a concatemer (usually four to six copies). The concatemer DNA was purified by agarose gel electrophoresis and cloned into pUC18. The XL10-Gold strain (Stratagene) harboring the plasmid was grown in 2–6 liters of LB medium, and the plasmid was isolated using a plasmid Gigaprep kit (Qiagen). After restriction digestion with EcoRV, the plasmid DNA fragment (∼3.8 kbp) was precipitated by adding PEG 6000 and NaCl to final concentrations of 6–8% and 500 mm, respectively. The promoter DNA in the supernatant was extracted with phenol/chloroform/isoamyl alcohol (25:24:1) and chloroform/isoamyl alcohol (24:1) and precipitated with ethanol. The DNA pellet was resuspended in gel filtration buffer (10 mm HEPES (pH 7.6), 300 mm potassium acetate, and 5 mm DTT) and fractionated on a Superose 6 column (GE Healthcare), yielding 1–3 mg of fragment. Thus, non-native ATC and GAT were retained at the 5′- and 3′-ends (see Fig. 1A).
FIGURE 1.
Run-off transcription. A, the non-template strand sequence of the promoter HIS4(−96/+112) is shown. Non-native ATC and GAT were retained at the 5′- and 3′-ends, respectively. The in vitro transcription start sites are indicated by black arrows. B, the templates indicated above each lane were prepared as described under “Experimental Procedures”. HIS4 promoter DNA (2.5 pmol) was mixed with 3.7 pmol of TFIIB, 3.7 pmol of TFIIA, 2.5 pmol of TBP, 3.7 pmol of TFIIE, 1.5 pmol of Tfb6Δ-TFIIH (TFIIH*), and 1 pmol of pol II-TFIIF complex. Transcription was initiated by adding an equal volume of 2× transcription mixture containing 1.6 mm ATP, 1.6 mm GTP, 1.6 mm CTP, 40 μm UTP, and 0.083 μm [α-32P]UTP. C, requirement for each factor in a run-off transcription assay. The complete reaction was performed as described for B with HIS4(−96/+112) template DNA. Other reactions contained all of the factors except the component indicated over the lane. D, time course of run-off transcription. After PIC formation, transcription was initiated by the addition of all four NTPs and [α-32P]UTP, and reactions were terminated at 5, 15, 30, 45, or 60 min by the addition of stop buffer I. E, same as described for D for 60 min without [α-32P]UTP. After 60 min, [α-32P]UTP was added, and reactions were terminated at 65, 75, 90, 105, or 120 min by the addition of stop buffer I.
Run-off Transcription
DNA template (2.5 pmol) was mixed with 3.7 pmol of TFIIB, 3.7 pmol of TFIIA, 2.5 pmol of TBP, 3.7 pmol of TFIIE, 1.5 pmol of TFIIH*, and 1 pmol of pol II-TFIIF complex in 4 μl of buffer A (50 mm HEPES (pH 7.6), 300 mm potassium acetate, 5 mm DTT, and 5% glycerol). Following the addition of 6 μl of buffer B (50 mm HEPES (pH 7.6), 5 mm magnesium sulfate, 30 mm potassium acetate, and 5 mm DTT), the mixture was kept for 1 h at 4 °C. Transcription was initiated by adding an equal volume of 2× transcription mixture (1.6 mm ATP, 1.6 mm GTP, 1.6 mm CTP, 40 μm UTP, 0.083 μm [α-32P]UTP (2.5 μCi), 10 mm magnesium acetate, and 5 units of RNaseOUT) at 30 °C and stopped after 45 min by adding 185 μl of stop buffer I (10 mm Tris (pH 7.5), 300 mm sodium acetate (pH 5.5), 5 mm EDTA, 0.7% SDS, 0.1 mg/ml glycogen, and 0.013 mg/ml proteinase K). Transcripts were analyzed as described (12).
PIC Reconstitution on a Preparative Scale
All reconstitution experiments were performed on ice or at 4 °C with proteins purified as described previously (11, 13). Promoter DNA (0.5 nmol) was mixed with 0.75 nmol of TFIIB, 0.75 nmol of TFIIA, 0.5 nmol of TBP, 0.7 nmol of TFIIE, and 0.3 nmol of Tfb6Δ-TFIIH (TFIIH*) in 40 μl of buffer(500) (20 mm HEPES (pH 7.6), 5 mm DTT, 2 mm magnesium acetate, 5% glycerol, and the mm concentration of potassium acetate in parentheses). The protein mixture was dialyzed in steps of buffer(300), buffer(220), and buffer(150) for at least 4 h at each step and then combined with 0.25 nmol of pol II-TFIIF complex. The mixture was further dialyzed into buffer(100) and buffer(80) before loading on a 10–40% (v/v) glycerol gradient containing 20 mm HEPES (pH 7.6), 5 mm DTT, 2 mm magnesium acetate, and 80 mm potassium acetate. After centrifugation at 40,000 rpm in a Beckman SW 60 rotor for 9 h, the gradient was fractionated using a PGF Piston Gradient FractionatorTM (BioComp Instruments, Inc.). The fractions were kept at −80 °C without loss of transcriptional activity. For isolation of the open complex, the PIC was reconstituted in the same way; incubated with 1.6 mm ATP, 0.5 mm GTP, and 0.5 mm CTP for 15 min; and sedimented on a glycerol gradient containing 20 mm HEPES (pH 7.6), 5 mm DTT, 2 mm magnesium acetate, 80 mm potassium acetate, 1.6 mm ATP, 0.5 mm GTP, and 0.5 mm CTP. The pol II-TFIIF complex was omitted in the reconstitution of an intermediate complex, and glycerol gradient centrifugation was performed for 5 h at 60,000 rpm in a Beckman SW 60 rotor.
Exonuclease Footprinting
For determination of the downstream boundary of the PIC, the 5′-end of an upstream primer (5′-GGATATGACTATGAACAGTAG-3′) was labeled with [γ-32P]ATP using T4 polynucleotide kinase. HIS4(−96/+112) was amplified by PCR in a 1–2-ml reaction using the 32P-labeled upstream primer and downstream primer (5′-TATTCCATGAGGCCAGATC-3′) and purified by electrophoresis on a 2% agarose gel. The labeled DNA (1 pmol) was incubated for 1 h at room temperature with 2 pmol of TFIIB, 1.6 pmol of TFIIA, 1.1 pmol of TBP, 2.4 pmol of TFIIE, 1.5 pmol of TFIIH*, and 1.2 pmol of pol II-TFIIF complex in 8 μl of buffer A; combined with 12 μl of buffer B; and incubated for 1 h at 4 °C. The reconstituted PIC was combined with an equal volume of 2× NTP buffer (1.6 mm NTP(s) or 0.5 mm non-hydrolyzable analog ATPγS, 10 mm magnesium acetate, and 5 units of RNaseOUT) and incubated for 30 min at 30 °C. Exonuclease III digestion was performed with 200 units of exonuclease III (New England Biolabs) for 9 min at 30 °C and stopped by adding 185 μl of stop buffer II (10 mm Tris (pH 7.5), 300 mm sodium acetate (pH 5.5), 5 mm EDTA, 0.7% SDS, 0.1 mg/ml glycogen, 0.013 mg/ml proteinase K, and 0.5 mg/ml salmon sperm DNA (Invitrogen)). The products were precipitated with 650 μl of 100% ethanol and kept overnight at −20 °C. The DNA pellet was recovered by centrifugation at maximum speed for 1 h, dried at 37 °C, and resuspended in 10 μl of gel loading buffer (95% formamide, 0.02% bromphenol blue, 5 mm EDTA, and 0.025% xylene cyanol). The products were analyzed by denaturing 6% polyacrylamide gel electrophoresis and detected with a PhosphorImager.
Electron Microscopy
PICs were sedimented through 10–40% glycerol gradients (containing a gradient of glutaraldehyde from 0 to 0.1%) (14) for 9 h at 40,000 rpm in a Beckman SW 60 rotor. After dilution to 50–200 μg/ml, 2–3 μl were applied to continuous carbon-coated specimen grids (catalog number CF300-Cu, Electron Microscopy Sciences), washed with 2% uranyl acetate solution, blotted, and dried. Images were collected at a magnification of ×29,000 on a charge-coupled device (4096 × 4096 pixels, Gatan UltraScanTM 4000) under low-dose conditions (each exposure of ∼15–20 e−/Å−2) using an FEI Tecnai F20 microscope operating at 200 kV.
Psoralen Cross-linking of the PIC
The glutaraldehyde-fixed PIC was combined with psoralen (20 μg/ml) and irradiated with a 360-nm-long wavelength ultraviolet fluorescent lamp. DNA was denatured in the presence of glyoxal and spread for electron microscopy as described (15). Grids were scanned at a magnification of ×20,000 on a charge-coupled device (4096 × 4096 pixels, Gatan UltraScanTM 4000) with a JEOL 1230 electron microscope. The size of denatured bubbles was calculated from the average of the lengths of the two halves of the bubble.
C-terminal Domain (CTD) Phosphorylation
pol II (0.14 μm) was treated with 0.4 μm TFIIK (16) in 20 mm HEPES (pH 7.6), 7.5 mm magnesium acetate, 100 mm potassium acetate, 10 mm DTT, 5% glycerol, 0.1% 3-(decyldimethylammonio)propanesulfonate (Sigma), and 1 mm ATP for 1 h at room temperature. Reactions were stopped by adding EDTA and analyzed by SDS-PAGE.
RESULTS
Assembly and Isolation of Yeast pol II PIC
To identify a suitable DNA for assembly of a PIC, we performed transcription with a series of fragments of the HIS4 promoter and a mixture of pure transcription proteins (TFIIA, TFIIB, TBP, TFIIE, TFIIH*, and pol II-TFIIF complex). All fragments extending from −84 to +74 with respect to the first transcription start site at position +1 (Fig. 1A) yielded run-off transcripts of the expected lengths (Fig. 1B). A fragment truncated at position +50 failed to support transcription, consistent with a previous study that identified a requirement for promoter DNA extending at least 50 bp downstream from the transcription start site (17). All proteins except TFIIA were required for transcription (Fig. 1C), consistent with previous studies of transcription in vitro (12, 18). The amount of pol II-TFIIF complex was limiting, with all other proteins and DNA added in molar excess and saturating for activity; the yield of the reaction was 0.076 transcripts per pol II-TFIIF complex. Run-off transcription was ∼95% complete in 15 min and was limited to a single round (Fig. 1, D and E). Similar results were obtained with other promoters, except for variation in the level of transcription (Table 1).
TABLE 1.
Run-off transcription activity on different promoters
| Promoter | Transcriptional activity (mean ± S.E.)a |
|---|---|
| HIS4 | 0.076 ± 0.012 (n = 7) |
| SNR20 | 0.175 ± 0.03 (n = 3) |
| SNR14 | 0.041 ± 0.02 (n = 2) |
a The activity is defined as the mean number of transcripts per pol II.
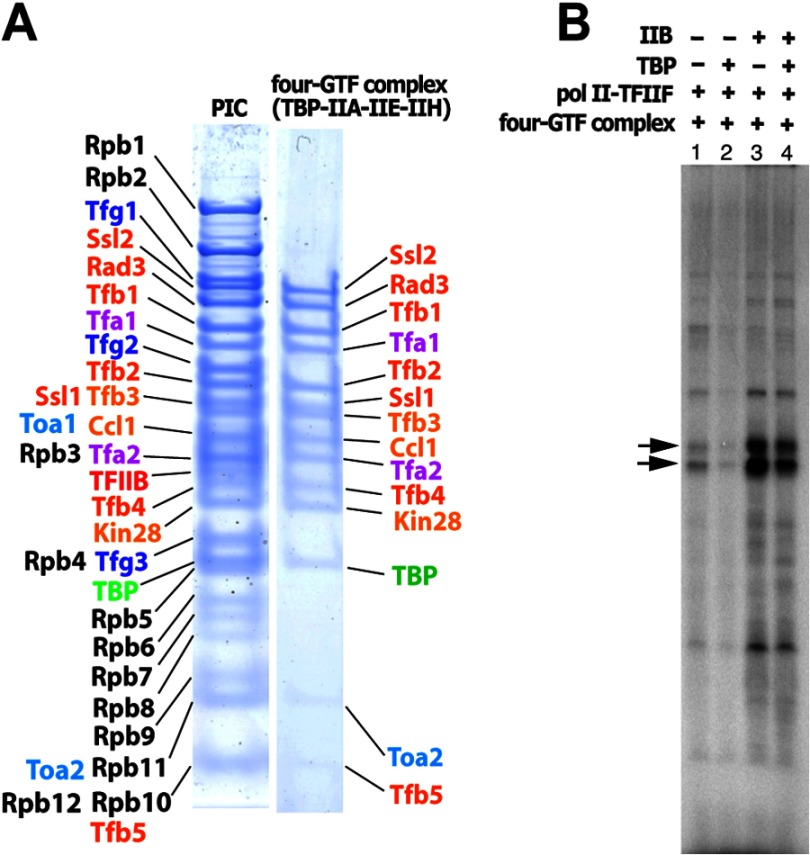
It was not possible to perform the reaction on a preparative scale by increasing the concentration of components, as a 10-fold increase resulted in precipitation, and no transcription was obtained (data not shown). A concentrated mixture was, however, soluble at elevated ionic strength. TFIIH* could be combined with excess TFIIA, TFIIB, TBP, TFIIE, and HIS4 promoter DNA fragment −96/+112 in 0.5 m potassium acetate and dialyzed to 0.15 m without precipitation. The resulting GTF-DNA complex was combined with the pol II-TFIIF complex, dialyzed to 0.1 m potassium acetate, and sedimented on a 10–40% glycerol gradient. A single peak in the center of the gradient (Fig. 2A, lanes 7 and 8) contained equimolar amounts of all transcription proteins (Fig. 2E), as shown by SDS-PAGE and densitometry, which resolved the 31 proteins in the PIC. The same results were obtained with a minimal DNA fragment (−81/+1) (Fig. 2E, lane 2).
FIGURE 2.
Isolation and characterization of the 31-subunit PIC. A, PIC was assembled with HIS4(−96/+112) and sedimented on a glycerol gradient, and fractions were analyzed by SDS-PAGE. B, run-off transcription. The peak fractions of the PIC from A were combined with an equal volume of 2× transcription mixture containing 1.6 mm ATP, 1.6 mm GTP, 1.6 mm CTP, 40 μm UTP, and 0.083 μm [α-32P]UTP, and the resulting transcripts were analyzed by gel electrophoresis. Arrows indicate the two major promoter-specific transcripts. C, run-off transcription using 1.5 pmol of the isolated PIC (lane 1) supplemented with 0.65 pmol of TFIIH* (lane 2), 1.3 pmol of TFIIE (lane 3), 1.0 pmol of TBP (lane 4), or 1.3 pmol of TFIIB (lane 5). D, run-off transcription of the isolated PIC (3 pmol; lane 1) supplemented with 2.2 pmol (lane 2) or 4.5 pmol (lane 3) of pol II-TFIIF was challenged with the shorter DNA fragment HIS4(−96/+74) (2.2 pmol). For lane 4, transcription was initiated by simple mixing of each component as described for Fig. 1B with 2.2 pmol of HIS4(−96/+112) and 2.2 pmol of HIS4(−96/+74). Black and red arrows indicate two major promoter-specific transcripts from HIS4(−96/+112) and HIS4(−96/+74), respectively. E, SDS-PAGE of peak glycerol gradient fractions (left), scanned and plotted (right). Lane 1, PIC assembled with HIS4(−96/+112); lane 2, PIC assembled with HIS4(−81/+1); lane 3; an internal standard in which equimolar amount of TFIIA, TFIIB, TBP, TFIIE, TFIIF, TFIIH, and pol II were combined. lane 4, mixture of TFIIA, TFIIB, and TBP; lane 5, mixture of TFIIH and TFIIE.
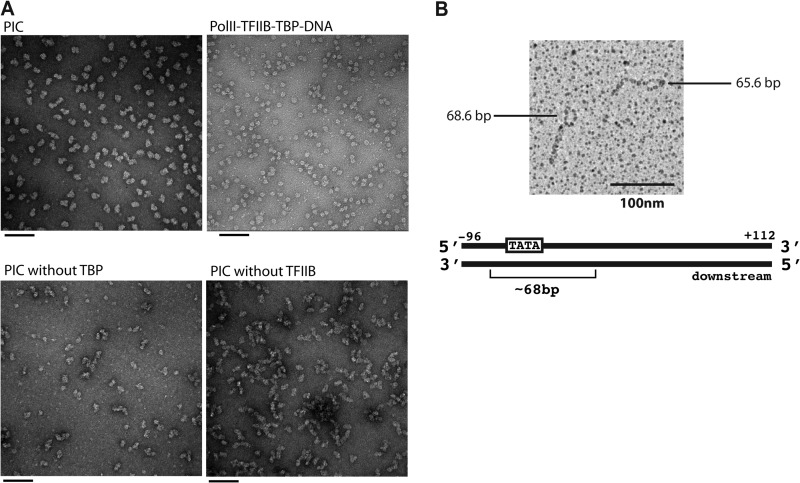
Electron microscopy provided support for the assembly of a complete PIC. Peak glycerol gradient fractions, embedded in stain, disclosed fields of particles virtually identical to one another except for differences in direction of view (Fig. 3A). The particles often appeared bipartite, with each part comparable in size to a pol II-TFIIB-TBP-DNA complex. No uniform particles were obtained when the experiment was repeated with the omission of any one of the GTFs.
FIGURE 3.
Electron microscopy analysis of the PIC. A, representative images of negatively stained particles: the complete 31-polypeptide PIC with HIS4(−81/+19) (upper left), the pol II-TFIIB-TBP-DNA complex (upper right), the PIC assembled without TBP (lower left), and the PIC assembled without TFIIB (lower right). Scale bars = 100 nm. B, analysis of the PIC on the HIS4 promoter by psoralen cross-linking of DNA. The PIC with HIS4(−96/+112) was exposed to an interstrand DNA cross-linking agent. The DNA was then deproteinized and spread under fully denaturing conditions. The micrograph reveals an ∼68-bp bubble on one end, where proteins blocked access of the cross-linker to the DNA.
The location of the PIC on the HIS4 promoter DNA fragment was mapped by reaction with psoralen, which cross-linked the two DNA strands (Fig. 3B). A region of DNA bound by protein was protected from cross-linking and appeared as a single-stranded bubble following denaturation. Analysis of the PIC in this way gave rise to a bubble at one end of the promoter fragment (Fig. 3B), in keeping with the location of the TATA box toward one end of the fragment. The contour length of the bubble corresponded to 67.6 ± 2.6 bp (average of 57 molecules) (Table 2) and increased by 10.9 bases (to 78.5 ± 5.0 bp) upon the addition of ATP, GTP, and CTP (average of 85 molecules), although the percentage of molecules with a bubble was much lower in this case (Table 2).
TABLE 2.
Analysis of the PIC on the HIS4 promoter by psoralen cross-linking of DNA
| Analyzed molecules | Molecules with a bubble | Bubble size (mean ± S.E.; nucleotides) | |
|---|---|---|---|
| No NTP (with fixation)a | 57 | 25 (43.8%) | 67.6 ± 2.62 |
| +ATP, GTP, and CTP (with fixation) | 85 | 10 (11.8%) | 78.5 ± 5.0 |
| DNA alone | 100 | 11 (11.0%) | 65.5 ± 5.34 |
a The PIC was fixed with glutaraldehyde.
The fully assembled PIC isolated by gradient sedimentation utilized the same start site as PICs obtained by simple mixing (Fig. 2B). The level of transcription, 0.106 ± 0.012 transcripts per PIC, was similar to levels obtained by simple mixing of pol II with all factors added in excess. There was no increase in transcription upon supplementation with additional TFIIB, TBP, TFIIE, or TFIIH*, further attesting to the completeness of the isolated PIC (Fig. 2C).
Further evidence that the PIC represents a stable entity, rather than a mixture capable of transcription with additional factors, came from a template challenge experiment. A PIC formed on HIS4(−96/+112) was transcribed in the presence of the shorter promoter fragment HIS4(−96/+74). Only the pair of run-off transcripts from the longer fragment was observed (Fig. 2D, lanes 1–3), but not the transcripts expected from the shorter fragment (lane 4).
Isolation of the GTF-DNA Intermediate
We investigated the intermediate formed in the first step of PIC assembly by sedimentation on a glycerol gradient. Two complexes were resolved, one containing equimolar amounts of four GTFs (TFIIA, TBP, TFIIE, and TFIIH*) as well as promoter DNA (Fig. 4A), and a second, slower sedimenting complex containing TFIIA, TBP, TFIIB, and promoter DNA. When supplemented with TFIIB and pol II-TFIIF, the four-GTF complex supported transcription (Fig. 4B), whereas little transcription was obtained when supplemented with the pol II-TFIIF complex alone, confirming the absence of TFIIB from the gradient-purified preparation. The four-GTF complex therefore provides a platform onto which pol II-TFIIF and TFIIB assemble to form the PIC.
FIGURE 4.
Isolation of the intermediate complex. A, HIS4(−92/+8) (0. 9 nmol) was mixed with TFIIB (0.6 nmol), TFIIA (0.6 nmol), TBP (0.6 nmol), TFIIE (0.6 nmol), and TFIIH (0.3 nmol) and sedimented on a glycerol gradient. SDS-PAGE of the 31-polypeptide PIC (left) and the intermediate complex containing TFIIA, TFIIE, TFIIH, and TBP (right) are shown. B, the intermediate complex was reconstituted with HIS4(−96/+112) and sedimented on a glycerol gradient. NTPs were added to initiate transcription along with the components indicated above the lanes.
Isolation of the Open Complex
The PIC assembled by simple mixing of all components at low concentration exhibited downstream and upstream barriers to digestion by exonuclease III at about positions −9 and −70, respectively, with respect to the first transcription start site (Fig. 5A). Upon the addition of ATP (but not the non-hydrolyzable analog ATPγS), the downstream barrier disappeared, and pauses or stops in digestion downstream (between +7 and +46) were intensified, indicative of essentially complete conversion from closed to open complexes. Open complex formation was dependent on the inclusion of TFIIE and TFIIH* in the PIC (Fig. 5). The shift in the downstream boundary of the PIC is consistent with the idea of “promoter scanning” proposed to explain the location of transcription start sites 40–120 bp downstream of the TATA box in yeast (19). There was no further change in the pattern of exonuclease III digestion upon the addition of GTP, CTP, and UTP, consistent with the small percentage of open complexes that give rise to run-off transcripts (∼7.6%, as noted above).
FIGURE 5.
Exonuclease III footprints of the PIC. A, the 5′-end of the non-template strand of HIS4(−93/+109) was labeled with 32P to determine the downstream boundary. The labeled DNA (1.0 pmol) was incubated with TFIIB (2.0 pmol), TFIIA (1.9 pmol), TBP (1.0 pmol), TFIIE (2.4 pmol), and TFIIH (1.0 pmol) with or without the nucleotides as indicated above the lanes, followed by treatment with exonuclease III (ExoIII) and gel electrophoresis. The positions of protected fragments are indicated on the right, and the positions of molecular markers are indicated on the left. The assay was performed in the presence (left) or absence (right) of TFIIE and TFIIH*. B, to determine the upstream boundary, the 5′-end of the template strand of HIS4(−132/+109) was labeled with 32P, incubated with the pol II and GTFs, and treated with 3′-exonuclease. The arrow indicates the upstream boundary of the DNA of the closed complex. The assay was performed in the presence (left) or absence (right) of TFIIE and TFIIH*.
The PIC in the presence of ATP (and also GTP and CTP) appeared in faster sedimenting fractions (Fig. 6, A and D). Following sedimentation on the glycerol gradient, the PIC retained its entire complement of 31 polypeptides (Fig. 6B). The largest subunit of pol II (Rpb1) was hyperphosphorylated (Fig. 6C), producing a quantitative mobility shift from the starting position (IIa) to that characteristic of a hyperphosphorylated state (IIo). Electron microscopy revealed a bipartite structure of the same size, regardless of the presence or absence of nucleotides (Fig. 6E).
FIGURE 6.
Isolation of PIC in the presence of nucleotides. A, the reconstituted PIC was subjected to glycerol gradient sedimentation in the presence of ATP, GTP, and CTP and analyzed by SDS-PAGE. B, SDS-PAGE of the PIC in the presence (right) or absence (left) of nucleotides. The peak glycerol gradient fraction was analyzed by 4–12% NuPAGE, followed by staining with Coomassie Blue. C, the PIC was analyzed as described for B on a 5% polyacrylamide gel (lanes 1 and 2). As a control, 0.14 μm pol II alone was treated with or without 0.4 μm TFIIK and analyzed by SDS-PAGE (lanes 3 and 4). The positions of the hyperphosphorylated Rpb1 subunit (IIo) and the unphosphorylated subunit (IIa) are indicated on the right. D, fractionation of the PIC in the absence or presence of nucleotides. E, representative images of the negatively stained particles of the PIC with HIS4(−96/+112) in the presence of ATP, GTP, and CTP. Scale bar = 100 nm.
DISCUSSION
The notable finding from this work is the isolation of an abundant homogeneous pol II transcription PIC. It was uncertain, even doubtful, that such a complex could be formed and that it would resist the rigors of isolation. In our experience, complexes of pol II with TFIIB, with TFIIF, and with DNA, dissociate during handling; we hoped, however, that a complex with all of the factors would be more stable than those with individual ones, and this hope was realized.
The procedure we developed for the assembly of the complete complex may resemble the pathway for PIC formation in vivo. An intermediate comprising TBP, TFIIE, TFIIH, and DNA could be isolated and then combined with TFIIB and pol II-TFIIF to generate the PIC. Support for the significance of this intermediate comes from studies of transcription reinitiation in vitro and in vivo. Following the addition of NTPs to a PIC formed with nuclear extract on immobilized promoter DNA, four GTFs (TFIIA, TFIID, TFIIE, and TFIIH), as well as Mediator, were retained on the DNA as revealed by immunoblot analysis. The addition of TFIIB, TFIIF, and pol II to the immobilized complex enabled transcription. The complex retained on the DNA was termed a reinitiation “scaffold” (7). A similar complex may be responsible for the identification by ChIP-ChIP analysis of genes enriched for TFIIH and Mediator (20). The assembly intermediate we have isolated may be regarded as a scaffold in the full sense of the word: it supports promoter DNA positioned with respect to pol II, following a path around pol II with little, if any, direct contact with the pol II surface.
Promoter-pol II interaction takes place upon the addition of ATP and open complex formation. We have shown that virtually all PICs undergo the transition to the open complex (Fig. 5), which can be re-isolated without loss of any of the 31 protein components (Fig. 6). In this respect, our findings differ from the unstable open complex obtained by others with immobilized templates. In the human system, PICs lose activity 1 min after the addition of ATP (21, 22), and the activity can be partially restored by additional TFIIE (8); in the yeast nuclear extract system, PICs incubated with ATP rapidly undergo dissociation of pol II, TFIIB, and TFIIF (7, 23). The loss of pol II is unexpected in light of studies demonstrating the high affinity of pol II for open promoter DNA. Indeed, if pol II is truly lost from the complex formed on immobilized DNA, it is unlikely to represent an intermediate on the pathway to transcription. We cannot explain this previous result of others, but the very stable open complex we have isolated, retaining all protein components, is demonstrably relevant, as shown by its capacity for transcription.
We have mapped the location and extent of the PIC on promoter DNA in both closed and open complexes by nuclease digestion and psoralen cross-linking. The barriers to exonuclease III digestion of the PIC around positions −70 to −9 are in good agreement with the observed size and location of the 68-bp psoralen bubble at the end of the DNA fragment and are consistent with previous protein-DNA cross-linking studies performed with yeast nuclear extract (24) and with a reconstituted human system (25). The TATA sequence, between positions −63 and −56, would be located off-center within the PIC, with an additional 40 bp lying within the PIC on the downstream side.
There was no loss of transcriptional activity of the PIC upon isolation. The level of transcription with the purified PIC was approximately the same as that obtained by direct mixing of pol II with excess GTFs but was much higher than that obtained by direct mixing of pol II and equimolar amounts of GTFs (data not shown), indicating that one or more of the GTFs was only partially active.
The level of transcription was promoter-dependent, ranging from 0.04 to 0.175 transcripts per PIC with the three promoters tested. In view of the homogeneity of the isolated PIC and the virtually complete transformation from closed to open complexes, it seems likely that the failure to achieve 100% transcription efficiency reflects some limitation(s) of the initiation process itself, in formation of the first phosphodiester bond, in events leading to a transcript length of ∼25 nucleotides, or in the subsequent transition from initiation to elongation. It is noteworthy that the observed initiation rate in vitro (on the order of 0.1/min) (Fig. 1, D and E) is much slower than the initiation rate in vivo, e.g. ∼0.15/s at an enhanced HIS3 promoter (26) and 0.25/s at the hsp70 promoter (27). The fraction of productive PICs and the initiation rate are doubtless influenced by other factors, such as activators and coactivators (28). There is now the possibility of separating inactive complexes from those engaged in transcription and determining the basis for the difference between them.
Acknowledgments
We thank colleagues in the Kornberg laboratory and F. Fazal, A. Meng, and S. Volker in the Steven Block laboratory at Stanford University for discussions and comments on this manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants GM36659 and AI21144 (to R. D. K.).
- pol II
- RNA polymerase II
- GTF
- general transcription factor
- PIC
- preinitiation complex
- TF
- transcription factor
- TBP
- TATA box-binding protein
- ATPγS
- adenosine 5′-O-(thiotriphosphate).
REFERENCES
- 1. Saunders A., Core L. J., Lis J. T. (2006) Breaking barriers to transcription elongation. Nat. Rev. Mol. Cell Biol. 7, 557–567 [DOI] [PubMed] [Google Scholar]
- 2. Wade J. T., Struhl K. (2008) The transition from transcriptional initiation to elongation. Curr. Opin. Genet. Dev. 18, 130–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Conaway R. C., Conaway J. W. (1993) General initiation factors for RNA polymerase II. Annu. Rev. Biochem. 62, 161–190 [DOI] [PubMed] [Google Scholar]
- 4. Kornberg R. D. (2007) The molecular basis of eukaryotic transcription. Proc. Natl. Acad. Sci. U.S.A. 104, 12955–12961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buratowski S., Hahn S., Guarente L., Sharp P. A. (1989) Five intermediate complexes in transcription initiation by RNA polymerase II. Cell 56, 549–561 [DOI] [PubMed] [Google Scholar]
- 6. Ranish J. A., Yudkovsky N., Hahn S. (1999) Intermediates in formation and activity of the RNA polymerase II preinitiation complex: holoenzyme recruitment and a postrecruitment role for the TATA box and TFIIB. Genes Dev. 13, 49–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yudkovsky N., Ranish J. A., Hahn S. (2000) A transcription reinitiation intermediate that is stabilized by activator. Nature 408, 225–229 [DOI] [PubMed] [Google Scholar]
- 8. Čabart P., Luse D. S. (2012) Inactivated RNA polymerase II open complexes can be reactivated with TFIIE. J. Biol. Chem. 287, 961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zawel L., Kumar K. P., Reinberg D. (1995) Recycling of the general transcription factors during RNA polymerase II transcription. Genes Dev. 9, 1479–1490 [DOI] [PubMed] [Google Scholar]
- 10. Svejstrup J. Q., Feaver W. J., LaPointe J., Kornberg R. D. (1994) RNA polymerase transcription factor IIH holoenzyme from yeast. J. Biol. Chem. 269, 28044–28048 [PubMed] [Google Scholar]
- 11. Murakami K., Gibbons B. J., Davis R. E., Nagai S., Liu X., Robinson P. J., Wu T., Kaplan C. D., Kornberg R. D. (2012) Tfb6, a previously unidentified subunit of the general transcription factor TFIIH, facilitates dissociation of Ssl2 helicase after transcription initiation. Proc. Natl. Acad. Sci. U.S.A. 109, 4816–4821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sayre M. H., Tschochner H., Kornberg R. D. (1992) Reconstitution of transcription with five purified initiation factors and RNA polymerase II from Saccharomyces cerevisiae. J. Biol. Chem. 267, 23376–23382 [PubMed] [Google Scholar]
- 13. Gibbons B. J., Brignole E. J., Azubel M., Murakami K., Voss N. R., Bushnell D. A., Asturias F. J., Kornberg R. D. (2012) Subunit architecture of general transcription factor TFIIH. Proc. Natl. Acad. Sci. U.S.A. 109, 1949–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kastner B., Fischer N., Golas M. M., Sander B., Dube P., Boehringer D., Hartmuth K., Deckert J., Hauer F., Wolf E., Uchtenhagen H., Urlaub H., Herzog F., Peters J. M., Poerschke D., Lührmann R., Stark H. (2008) GraFix: sample preparation for single-particle electron cryomicroscopy. Nat. Methods 5, 53–55 [DOI] [PubMed] [Google Scholar]
- 15. Cech T., Potter D., Pardue M. L. (1977) Electron microscopy of DNA cross-linked with trimethylpsoralen: a probe for chromatin structure. Biochemistry 16, 5313–5321 [DOI] [PubMed] [Google Scholar]
- 16. Feaver W. J., Svejstrup J. Q., Henry N. L., Kornberg R. D. (1994) Relationship of CDK-activating kinase and RNA polymerase II CTD kinase TFIIH/TFIIK. Cell 79, 1103–1109 [DOI] [PubMed] [Google Scholar]
- 17. Spangler L., Wang X., Conaway J. W., Conaway R. C., Dvir A. (2001) TFIIH action in transcription initiation and promoter escape requires distinct regions of downstream promoter DNA. Proc. Natl. Acad. Sci. U.S.A. 98, 5544–5549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dvir A., Garrett K. P., Chalut C., Egly J. M., Conaway J. W., Conaway R. C. (1996) A role for ATP and TFIIH in activation of the RNA polymerase II preinitiation complex prior to transcription initiation. J. Biol. Chem. 271, 7245–7248 [DOI] [PubMed] [Google Scholar]
- 19. Giardina C., Lis J. T. (1993) DNA melting on yeast RNA polymerase II promoters. Science 261, 759–762 [DOI] [PubMed] [Google Scholar]
- 20. Esnault C., Ghavi-Helm Y., Brun S., Soutourina J., Van Berkum N., Boschiero C., Holstege F., Werner M. (2008) Mediator-dependent recruitment of TFIIH modules in preinitiation complex. Mol. Cell 31, 337–346 [DOI] [PubMed] [Google Scholar]
- 21. Luse D. S., Kochel T., Kuempel E. D., Coppola J. A., Cai H. (1987) Transcription initiation by RNA polymerase II in vitro. At least two nucleotides must be added to form a stable ternary complex. J. Biol. Chem. 262, 289–297 [PubMed] [Google Scholar]
- 22. Cai H., Luse D. S. (1987) Transcription initiation by RNA polymerase II in vitro. Properties of preinitiation, initiation, and elongation complexes. J. Biol. Chem. 262, 298–304 [PubMed] [Google Scholar]
- 23. Liu Y., Kung C., Fishburn J., Ansari A. Z., Shokat K. M., Hahn S. (2004) Two cyclin-dependent kinases promote RNA polymerase II transcription and formation of the scaffold complex. Mol. Cell. Biol. 24, 1721–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miller G., Hahn S. (2006) A DNA-tethered cleavage probe reveals the path for promoter DNA in the yeast preinitiation complex. Nat. Struct. Mol. Biol. 13, 603–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim T. K., Ebright R. H., Reinberg D. (2000) Mechanism of ATP-dependent promoter melting by transcription factor IIH. Science 288, 1418–1422 [DOI] [PubMed] [Google Scholar]
- 26. Iyer V., Struhl K. (1996) Absolute mRNA levels and transcriptional initiation rates in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U.S.A. 93, 5208–5212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. O'Brien T., Lis J. T. (1991) RNA polymerase II pauses at the 5′ end of the transcriptionally induced Drosophila hsp70 gene. Mol. Cell. Biol. 11, 5285–5290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hahn S. (1998) Activation and the role of reinitiation in the control of transcription by RNA polymerase II. Cold Spring Harb. Symp. Quant. Biol. 63, 181–188 [DOI] [PubMed] [Google Scholar]