Background: Methyl-CpG binding domain 4 (MBD4) is a DNA glycosylase that excises mismatched bases generated in methylated CpG sequences.
Results: We report the biochemical and structural properties of the methyl-CpG binding domain of MBD4 (MBDMBD4).
Conclusion: MBDMBD4 recognizes a wide range of 5-methylcytosine modifications via an extensive hydration network.
Significance: This study provides new insight into the structural mechanism of the broad base recognition that is unique to MBDMBD4.
Keywords: DNA Methylation, DNA Repair, DNA-Protein Interaction, Epigenetics, X-ray Crystallography, Methyl-CpG Binding Domain
Abstract
The methyl-CpG binding domain (MBD) protein MBD4 participates in DNA repair as a glycosylase that excises mismatched thymine bases in CpG sites and also functions in transcriptional repression. Unlike other MBD proteins, MBD4 recognizes not only methylated CpG dinucleotides (5mCG/5mCG) but also T/G mismatched sites generated by spontaneous deamination of 5-methylcytosine (5mCG/TG). The glycosylase activity of MBD4 is also implicated in active DNA demethylation initiated by the deaminase-catalyzed conversion of 5-methylcytosine to thymine. Here, we report the crystal structures of the MBD of MBD4 (MBDMBD4) complexed with 5mCG/5mCG and 5mCG/TG. The crystal structures show that the DNA interface of MBD4 has flexible structural features and harbors an extensive water network that supports its dual base specificities. Combined with the results of biochemical analyses, the crystal structure of MBD4 bound to 5-hydroxymethylcytosine further demonstrates that MBDMBD4 is able to recognize a wide range of 5-methylcytosine modifications through the unique water network. The versatile base recognition ability of MBDMBD4 implies multifunctional roles for MBD4 in the regulation of dynamic DNA methylation patterns coupled with deamination and/or oxidation of 5-methylcytosine.
Introduction
DNA methylation is the most prominent epigenetic modification in higher eukaryotic genomes (1, 2). In mammals, DNA methylation mainly occurs at the C5 position of symmetrically arranged cytosines in CpG dinucleotides, and plays essential roles in various cellular events such as gene repression, imprinting, X-chromosome inactivation, suppression of repetitive genomic elements, and carcinogenesis (3). Recent studies have shown that DNA methylation can be actively reversed and that its pattern is dynamically altered in mammalian cells (4–7). Although the underlying molecular mechanism is not fully understood, active DNA demethylation has been proposed to involve further oxidation or deamination of 5-methylcytosine (5mC)3 followed by base excision repair (6–13). Successive oxidation of 5mC to 5-hydroxymethylcytosine (hmC), 5-formylcytosine (foC), and 5-carboxylcytosine (caC) is catalyzed by TET proteins and, has attracted much attention as a crucial process in DNA demethylation. Furthermore, demethylation pathways are thought to involve the spontaneous or enzymatic deamination of 5mC or hmC and subsequent base excision repair of the mismatched thymine or 5-hydroxymethyluracil (hmU) base (6, 9, 11–13). Therefore, precise interpretation and regulation of the modification status of 5mC are required for various epigenetic events in cells.
MBD (methyl-CpG binding domain) proteins are archetypal mediators of DNA methylation marks. They recognize methyl-CpG sites (5mCG/5mCG) through a conserved MBD and recruit transcriptional repressors or chromatin modifiers to these sites (14). One of the MBD family proteins, MBD4 contains a C-terminal DNA glycosylase domain in addition to an N-terminal MBD domain. MBD4 is involved in DNA mismatch repair as a T/G or U/G mismatch glycosylase and also in transcriptional repression via its recruitment of Sin3A and HDAC1 (15, 16). The glycosylase activity of MBD4 specifically excises a mismatched thymine or hmU base generated by the deamination of 5mC or hmC in a CpG site; thus, MBD4 is thought to participate in both DNA repair in the context of CpG and DNA demethylation (14). The functional importance of MBD4 in maintaining genomic integrity has been demonstrated by an increased frequency of C to T transitions at CpG sites in MBD4−/− mice (17) and the finding that frequent MBD4 mutations in various human carcinomas are characterized by microsatellite instability (18). Moreover, MBD4 contributes to the stimuli-dependent active DNA demethylation of specific genomic loci together with thymine DNA glycosylase (TDG) (19).
Previous structural studies of MBD1, MBD2, and MeCP2 demonstrated how MBDs recognize only 5mCG/5mCG sites (20–22). However, in addition to the fully methylated CpG, the MBD domain of MBD4 binds to T/G mismatched base pairs that result from asymmetrical 5mC deamination of 5mCG/5mCG dinucleotides (16). Recent structural and biochemical studies of the glycosylase domain of MBD4 suggest that the specificity of full-length MBD4 for 5mCG/TG is provided by MBDMBD4 (23–25). The glycosylase domain recognizes the mismatched thymine or hmU base but not the adjacent 5mC/G base pair. Thus, the recognition of methylated DNA by MBDMBD4 appears to be indispensable for the multifunctional roles of MBD4 in the regulation and maintenance of DNA methylation patterns.
Here, we present the crystal structures of the MBD of MBD4 (MBDMBD4) in complex with a DNA fragment containing the 5mCG/5mCG site or its deamination product, 5mCG/TG. The structures reveal the unique flexible DNA interface of MBDMBD4 accompanied by an extensive water network. Our structural and biochemical data demonstrate that, in addition to 5mCG/5mCG and 5mCG/TG, the DNA interface of MBDMBD4 is able to accommodate hmC and its further oxidation or deamination products. We also determined the crystal structure of MBDMBD4 bound to a methylated CpG site containing hmC (5mCG/hmCG) and found that the water network at the DNA interface of MBDMBD4 can be finely tuned to accommodate various modified pyrimidine rings. Our structural and biochemical studies indicate the molecular basis of the broad base recognition ability of MBDMBD4, which underlies DNA methylation and gene regulation involving MBD4.
EXPERIMENTAL PROCEDURES
Protein Expression and Purification
A DNA fragment encoding MBDMBD4 (residues 69–136) was amplified by PCR and cloned into the bacterial expression vector pGEX4T-3 (GE Healthcare Biosciences), which was engineered for the expression of recombinant proteins with an N-terminal tandem fusion tag of glutathione S-transferase (GST) and small ubiquitin-like modifier-1 (SUMO-1). The GST-SUMO-1-MBDMBD4 fusion was overexpressed in Escherichia coli strain BL21(DE3). Cells were grown at 37 °C in Luria-Bertani (LB) medium containing 50 μg/ml of ampicillin, to an optical density of 0.5–0.6 at 660 nm, and then induced with 0.2 mm isopropyl β-d-thiogalactoside for 15 h at 18 °C. Cells were harvested by centrifugation, and lysed by sonication in 50 mm Tris-HCl, pH 8.0, buffer containing 300 mm NaCl, 1 mm dithiothreitol (DTT), 5% glycerol, 0.1% Triton X-100, and 1 mm phenylmethylsulfonyl fluoride. The clarified lysate was loaded onto glutathione-Sepharose 4 Fast Flow beads (GE Healthcare). GST-SUMO-1-fused MBDMBD4 was eluted from the beads with elution buffer containing 10 mm glutathione. The tag-free MBDMBD4 was prepared by SENP2 protease treatment, and was further purified by sequential column chromatography steps using HiTrap Heparin HP and HiLoad 16/60 Superdex 75 columns (GE Healthcare). Purified protein in the final elution buffer containing 10 mm Hepes-NaOH, pH 7.4, 150 mm NaCl, and 2 mm DTT was concentrated using an Amicon Ultra 3,000 cut-off membrane concentrator (Millipore). To introduce selenomethionine, Leu-116 of MBD4 was substituted with methionine. The selenomethionine containing MBDMBD4 was expressed in modified M9 medium (26). Purification of the selenomethionine-labeled L116M mutant was performed following the same procedure as that for the native protein.
Crystallization, Data Collection, and Structure Determination
MBDMBD4 at a concentration of 200–800 μm was mixed with each DNA fragment at a 1:1 molar ratio. Crystals of MBDMBD4 were obtained by a vapor diffusion method at 20 °C using PEG 10,000 or PEG 1500 as the precipitant. Details of crystallization conditions are listed in Table 1. MBDMBD4 bound to 14- and 11-bp oligomers containing 5mCG/TG were crystallized in orthorhombic C2221 and triclinic P1 forms, respectively. In the orthorhombic form, a complex of one protein and DNA is contained in an asymmetric unit, whereas the triclinic form comprises two protein molecules and one DNA oligomer. The complex of MBDMBD4 with 14-bp oligomer containing 5mCG/5mCG or 5mCG/hmCG was crystallized in a C2221 form. All crystals were flash frozen at 100 K in cryoprotectant containing 20% ethylene glycol. X-ray diffraction data sets were collected at a wavelength of 1.0000 Å on beamlines BL-5A, BL-17A, NE3A, and NW12 at Photon Factory (Tsukuba, Japan) and beamline BL-38 at SPring8 (Harima, Japan), and were processed with the program HKL2000 (27). The phases of the selenomethionine derivative MBDMBD4 L116M complexed with the 14-bp 5mCG/TG fragment were determined by the single wavelength anomalous dispersion method using the programs SOLVE and RESOLVE (28, 29). The initial model was built using the COOT program (30) and was refined against the native data using the PHENIX suite (31), thus yielding a crystallographic R factor of 18.8% and a free R factor of 22.4% to 2.0 Å. The triclinic form structure of the MBDMBD4-5mCG/TG complex and the structures of the MBDMBD4-5mCG/5mCG and MBDMBD4-5mCG/hmCG complexes were solved by a molecular replacement method using the orthorhombic form structure of MBDMBD4-5mCG/TG as the search model. The stereochemical quality of the final models was assessed using MolProbity (32). The sequence information of DNA fragments used for crystallization is summarized in Table 2. The crystallographic data, data collection statistics, and refinement statistics are summarized in Table 1. All structural figures were produced using PyMOL (43).
TABLE 1.
Crystallographic data and refinement statistics
| Crystal | 14 bp |
11 bp |
|||
|---|---|---|---|---|---|
| 5mCG/TG (SeMet) | 5mCG/TG | 5mCG/5mCG | 5mCG/hmCG | 5mCT/TG | |
| Crystallization condition | 12% PEG10,000 | 12% PEG10,000 | 7% PEG10,000 | 8% PEG1,500 | 2 mm Zn acetate |
| 0.1 m Na acetate, pH 4.4 | 0.1 m Na acetate, pH 4.4 | 0.1 m Na acetate, pH 4.4 | 0.1 m Na acetate, pH 3.9 | 0.1 m Na cacodylate | |
| 0.2 m NaCl | 0.2 m NaCl | 0.2 m NaCl | 0.1 m Na cacodylate, pH 5.4 | ||
| X-ray source | PF-NE3A | PF BL-5A | PF-NW12 | PF-BL17A | SPring8-BL38B1 |
| Wavelength (Å) | 0.97923 (peak) | 1.0000 | 1.0000 | 1.0000 | 1.0000 |
| Space group | C2221 | C2221 | C2221 | C2221 | P1 |
| Unit cell parameters (Å, °) | a = 88.752 | a = 89.074 | a = 89.182 | a = 88.693 | a = 30.324 |
| b = 97.588 | b = 94.989 | b = 93.829 | b = 97.758 | b = 33.781 | |
| c = 54.959 | c = 54.738 | c = 55.357 | c = 55.725 | c = 60.035 | |
| α = β = γ = 90 | α = β = γ = 90 | α = β = γ = 90 | α = β = γ = 90 | α = 75.317 | |
| β = 77.743 | |||||
| γ = 87.352 | |||||
| Resolution range (Å)a | 50–2.7 (2.8–2.7) | 50–2.0 (2.07–2.0) | 50–2.2 (2.28–2.2) | 50–2.19 (2.27–2.19) | 50–2.5 (2.59–2.5) |
| Total observations | 38,796 | 114,920 | 86,212 | 74,818 | 20,848 |
| Unique reflectionsa | 6,051 (363) | 16,062 (1591) | 12,048 (1192) | 11,517 (701) | 7,225 (531) |
| Multiplicitya | 6.4 (5.6) | 7.2 (7.3) | 7.2 (7.3) | 6.5 (4.1) | 2.9 (1.7) |
| Rmergea,b | 0.097 | 0.031 | 0.078 | 0.052 | 0.058 |
| (0.308) | (0.365) | (0.477) | (0.317) | (0.125) | |
| Completeness (%)a | 88.6 (54.0) | 99.7 (100) | 99.6 (99.9) | 89.6 (54.7) | 92.8 (67.4) |
| 〈I/σ(II)〉a | 12.7 (5.2) | 18.2 (4.3) | 12.4 (4.5) | 14.7 (4.1) | 16.3 (5.4) |
| Refinement | Resolution range (Å) | 32.5–2.00 | 28.3–2.20 | 34.7–2.40 | 33.0–2.53 |
| Rwork (%)c | 18.8 | 19.6 | 19.06 | 19.34 | |
| Rfree (%)c | 22.4 | 21.4 | 21.99 | 23.65 | |
| Root mean square deviations | Bond length (Å) | 0.018 | 0.006 | 0.004 | 0.007 |
| Bond angle (°) | 2.071 | 1.193 | 1.008 | 1.161 | |
| Ramachandran plot | Favored (%) | 100 | 100 | 98.36 | 99.18 |
| Allowed (%) | 100 | 100 | 100 | 100 | |
a Numbers in parentheses are the values for the highest resolution shell of each data set.
b Rmerge = Σh Σi|I(h)i − 〈I(h)〉|/Σh ΣiI(h)i, where I(h) is the intensity of reflection h, Σh is the sum of all measured reflections and Σi is the sum of i measurements of reflection.
c Rwork and Rfree = (Σhkl ‖Fo| −|Fc‖)/Σhkl |Fo|, where the free reflections (5% of the total used) were held aside for Rfree throughout refinement.
TABLE 2.
DNA sequences used in crystallization and binding assays
| Lower strand | Upper strand | |
|---|---|---|
| 11 bp 5mCG/TG | TCAC TG GATGT | ACATC 5mCG GTGA |
| 14 bp 5mCG/TG | GTC TG GTAGTGACT | GTCACTAC 5mCG GACA |
| 5mCG/hmUG | GTC hmUG GTAGTGACT | GTCACTAC 5mCG GACA |
| 5mCG/caCG | GTC caCG GTAGTGACT | GTCACTAC 5mCG GACA |
| 5mCG/5mCG | GTC 5mCG GTAGTGACT | GTCACTAC 5mCG GACA |
| 5mCG/hmCG | GTC hmCG GTAGTGACT | GTCACTAC 5mCG GACA |
| 5mCG/foCG | GTC foCG GTAGTGACT | GTCACTAC 5mCG GACA |
| hmCG/hmCG | GTC hmCG GTAGTGACT | GTCACTAC hmCG GACA |
| CG/CG | GTC CG GTAGTGACT | GTCACTAC CG GACA |
| 5mCG/CG | GTC CG GTAGTGACT | GTCACTAC 5mCG GACA |
| CG/TG | GTC TG GTAGTGACT | GTCACTAC CG GACA |
DNA Binding Assays
Isothermal titration calorimetry (ITC) measurements were performed on an iTC200 microcalorimeter (MicroCal, USA) at 25 °C. The protein solution was dialyzed to the ITC measurement buffer of 25 mm Hepes-NaOH, pH 7.4, containing 100 mm NaCl and 0.1 mm Tris(2-carboxyethyl)phosphine. Each annealed DNA duplex was dried and dissolved in ITC buffer. The DNA solution (10–20 μm) in a calorimetric cell was titrated with a 100–400 μm protein solution. Binding constants were calculated by fitting the data using the ITC data analysis module of Origin 7.0 (OriginLab). Competitive binding assays were also performed in the ITC buffer. The upper strand of the 14-bp 5mCG/5mCG DNA fragment was radioisotope labeled at the 5′ end with T4 polynucleotide kinase (TOYOBO, Japan) and [γ-32P]ATP (Muromachi Kagaku, Tokyo). The labeled strand was then mixed with a 1.2-fold amount of the complementary strand and annealed. The radioisotope-labeled 5mCG/5mCG fragment and MBD protein were mixed at concentrations of 1 and 3 μm, respectively. Subsequently, 0, 1, 2, or 4 μm nonlabeled competitor DNA fragment was added, and the solution was incubated for 30 min at 4 °C and analyzed using native gel electrophoresis. The DNA bands were visualized with a Fuji BAS-2000 phosphorimager. The DNA content of each band was quantified from the gel band density as a relative amount compared with total input DNA. A series of relative values were normalized against the control lane and plotted against the amount of competitor DNA. Curves for each experiment were fitted by a nonlinear, least square method using Morrison's equation (33).
Glycosylase Assays
Glycosylase assays were performed in a 10-μl reaction mixture containing 10 mm Tris-HCl, pH 8.0, 0.1 mm EDTA, and 0.1 mg/ml of BSA. The synthetic oligonucleotides, GGCTAAATACCTGGGCTXGAAGTGAACTGATTGCC, where X indicates T, hmU, caC, 5mC, hmC, or foC, was labeled by T4 polynucleotide kinase and [γ-32P]ATP, and annealed with complementary strand containing 5mC at the central CpG site. Each of the 32P radioisotope-labeled DNA duplexes at 40 or 400 nm were incubated with 200 or 2000 nm human TDG or mouse MBD4 at 37 °C for 1 h. Reactions were terminated by the addition of 10 μl of a reaction stop solution containing 0.2 m NaOH and 20 mm EDTA followed by incubation at 95 °C for 10 min. For the reaction using the foC-containing oligonucleotide, the incubation was carried out at 70 °C for 5 min to avoid the digestion under the alkaline conditions regardless of enzymatic activity. After addition of 60 μl of 10 m urea, 20 μl of each sample was subjected to electrophoresis in a 9 m urea, 20% PAGE and visualized with a Fuji BAS-2000 phosphorimager.
Accession Codes
Coordinates and structure factors have been deposited in the Protein Data Bank (PDB) under accession codes 3VXV, 3VXX, 3VYB, and 3VYQ.
RESULTS
Dual Binding Specificity of MBDMBD4 for 5mCG/5mCG and 5mCG/TG Sites
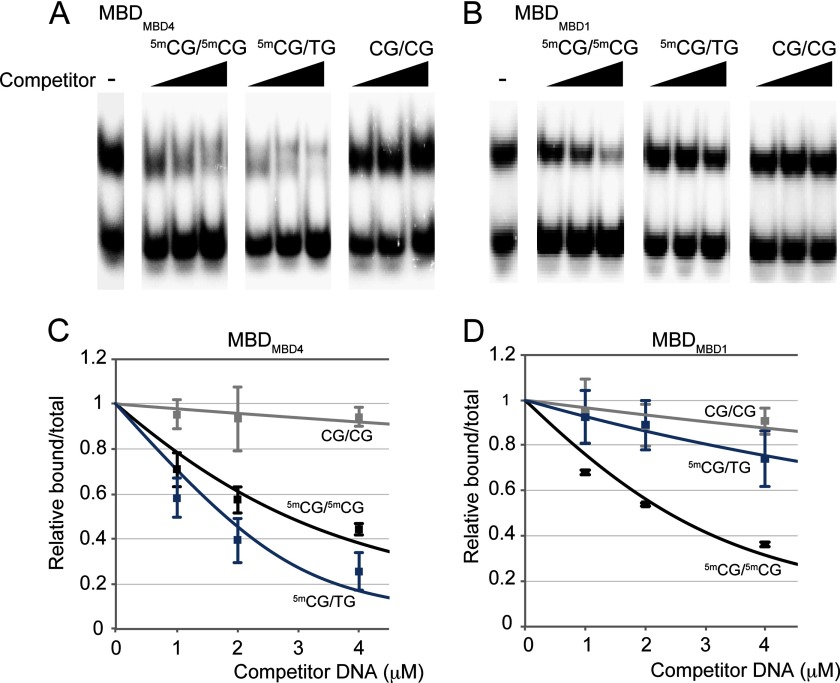
The DNA binding properties of mouse MBDMBD4 were examined quantitatively by ITC measurements using 14-bp double-stranded DNA oligomers containing a single CpG site in various modification or mismatch states (Tables 2 and 3). In agreement with previous reports (16), MBDMBD4 was tightly bound to 5mCG/TG with a dissociation constant (KD) of 98.8 nm, but did not interact with nonmethylated CG/CG or hemimethylated 5mCG/CG. The affinity of MBDMBD4 for the 5mCG/5mCG site (KD, 97.5 nm) was comparable with that for the 5mCG/TG site. However, MBDMBD1 exhibited a 5-fold greater affinity for the 5mCG/5mCG site (KD, 72.5 nm) over the 5mCG/TG site (KD, 458 nm). The binding specificities of MBDMBD4 and MBDMBD1 were also assessed by a competitive electrophoretic mobility shift assay (EMSA) in which the 32P-labeled 5mCG/5mCG oligomer bound to MBD competed with nonlabeled fragments. Both nonlabeled 5mCG/TG and 5mCG/5mCG fragments efficiently competed with the 5mCG/5mCG oligomer for binding to MBDMBD4, whereas the nonlabeled 5mCG/TG fragment did not abrogate the interaction between MBDMBD1 and the 5mCG/5mCG site (Fig. 1). Thus, MBDMBD4 is characterized as a unique MBD family protein based on its dual DNA binding ability, although the key residues for recognition of methylated CpG sites are almost completely conserved among MBDMBD1, MBDMBD2, and MBDMeCP2 (Fig. 2A).
TABLE 3.
Thermodynamic parameters obtained by ITC experiments
| DNA | MBD4 | MBD1 |
|---|---|---|
| 5mCG/5mCG | ||
| KD (nm) | 97.5 ± 76 | 72.5 ± 11 |
| ΔH (kJ/mol) | −7.69 ± 0.93 | −54.7 ± 5.1 |
| −TΔS (kJ/mol) | −32.8 ± 2.5 | 13.9 ± 5.3 |
| 5mCG/TG | ||
| KD (nm) | 98.8 ± 42 | 458 ± 92 |
| ΔH (kJ/mol) | −18.5 ± 1.7 | −46.7 ± 1.3 |
| −TΔS (kJ/mol) | −21.7 ± 0.71 | 10.4 ± 0.86 |
| 5mCG/hmCG | ||
| KD (nm) | 162 ± 58 | 1040 ± 422 |
| ΔH (kJ/mol) | −5.0 ± 1.3 | −49.5 ± 1.2 |
| −TΔS (kJ/mol) | −33.9 ± 2.3 | 15.1 ± 0.1 |
| CG/TG | ||
| KD (nm) | 213 ± 58 | 4025 ± 45 |
| ΔH (kJ/mol) | −11.5 ± 0.3 | −43.2 ± 1.0 |
| −TΔS (kJ/mol) | −26.7 ± 1.0 | 12.3 ± 1.1 |
| hmUG/5mCG | ||
| KD (nm) | 287 ± 78 | |
| ΔH (kJ/mol) | −15.0 ± 1.3 | Not performed |
| −TΔS (kJ/mol) | −22.3 ± 1.8 | |
| 5mCG/CG | ||
| KD (nm) | 3080 ± 830 | |
| ΔH (kJ/mol) | Not detected | −36.8 ± 0.9 |
| −TΔS (kJ/mol) | 5.2 ± 1.6 | |
| CG/CG | ||
| KD (nm) | 33,000 ± 6,900 | |
| ΔH (kJ/mol) | Not detected | −15.3 ± 1.9 |
| −TΔS (kJ/mol) | −10.3 ± 2.3 | |
FIGURE 1.
Binding of MBDMBD4 to 5mCG/5mCG and 5mCG/TG. A and B, representative autoradiographic images of competition assays for MBDMBD4 (A) and MBDMBD1 (B). Each competitor sequence is indicated above the lanes. The left panel in each section shows a control binding experiment in the absence of competitor DNA. C and D, each band was quantified relative to the total input DNA from the gel band density. The relative values were normalized against the control lane, and plotted against the amounts of competitor DNA. Each data point represents an average of three independent experiments using MBDMBD4 (C) or MBDMBD1 (D). Each solid line shows a curve fitted using Morrison's equation. An ∼1.5-fold excess of the nonlabeled 5mCG/5mCG fragment was required to obtain the same competitive effect as with nonlabeled 5mCG/TG, indicating that MBDMBD4 has a similar affinity for 5mCG/TG and 5mCG/5mCG sites.
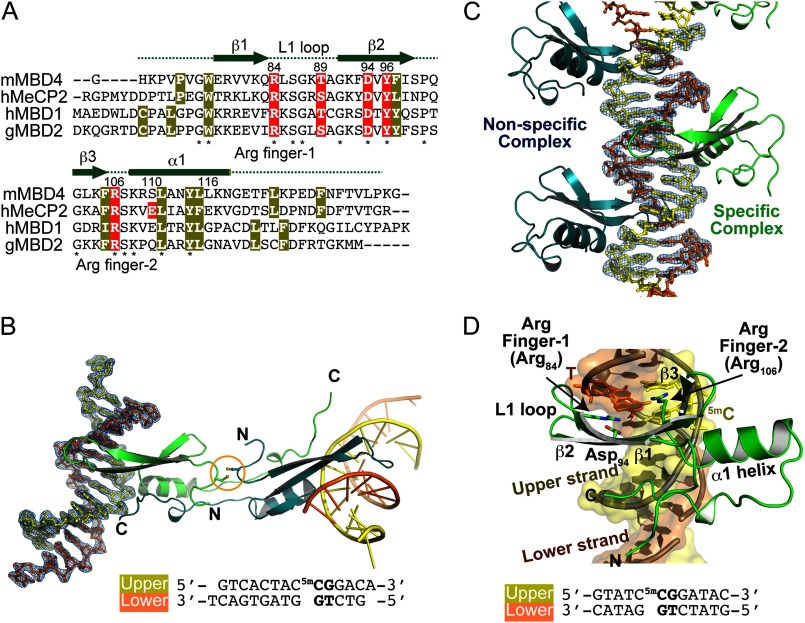
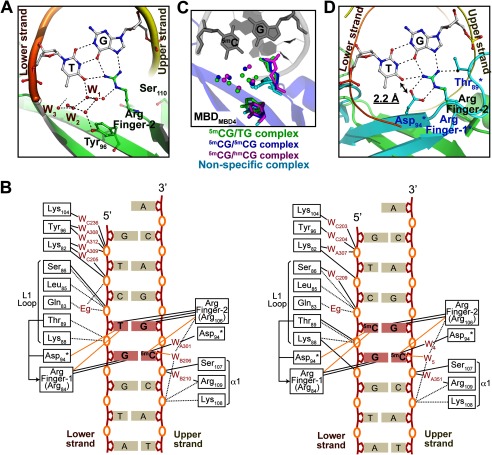
FIGURE 2.
Overall structure of MBDMBD4 complexed with DNA containing 5mCG/TG. A, sequence alignment of structurally known MBD domains; mouse MBD4 (mMBD4, 69–136 amino acids), human MeCP2 (hMeCP2, 88–167 amino acids), human MBD1 (hMBD1, 1–75 amino acids), and chicken MBD2 (gMBD2, 3–72 amino acids). Asterisks indicate the conserved residues among the MBD proteins. The hydrophobic core residues are highlighted in yellow. The residues highlighted in red are involved in recognition of methylated CpG base pairs. B and C, overall structures of MBDMBD4-5mCG/TG complexes. B, C2221 orthorhombic form; C, P1 triclinic form. The 2mFo − DFc electron density map for the DNA molecule contoured at 1.5 σ is shown in blue. The lower DNA strand containing a mismatched thymine is presented in orange, and the complementary upper strand is yellow. A disulfide bond linking two symmetry-related MBDs is indicated by an orange circle in B. In the P1 form, specific (green) and nonspecific (blue) MBDMBD4-DNA complexes are evident (C). D, specific binding of MBDMBD4 to 5mCG/TG. The triclinic P1 form structure of MBDMBD4 is shown as a green ribbon representation. Side chains of Arg-84, Arg-106, and Asp-94 are presented as stick models. The lower DNA strand containing a mismatched thymine is presented in orange, and the complementary upper strand is yellow. The 5mCG/TG base pairs are shown as stick models. The DNA sequences are indicated below in B and D.
Crystal Structures of MBDMBD4 Complexed with Methylated CpG and Its Deamination Product
To understand the structural basis of the unique DNA binding properties of MBD4, we solved the crystal structure of MBDMBD4 in complex with DNA fragments containing 5mCG/TG or 5mCG/5mCG sequences (Table 2). The crystal structures of the MBDMBD4-5mCG/TG complex were determined in orthorhombinc C2221 and triclinic P1 forms (Fig. 2, B and C, Table 1). In the C2221 form, the C-terminal parts of MBDMBD4 (residues 121–136) were exchanged between 2-fold symmetry-related molecules, resulting in a swapped dimer linked through a disulfide bond (Fig. 2B). However, dimer formation was not observed in our gel-filtration experiments (data not shown); therefore, the swapped dimer is interpreted as a crystallographic artifact. The C2221 form structure shares the core folding and the DNA binding interface with the P1 form despite the C-terminal swapping. In this report, the recognition of 5mCG/TG by MBDMBD4 is discussed based on the higher resolution structure of the C2221 form. The DNA strand containing a mismatched T is hereafter referred to as the “lower strand,” whereas the other strand is termed the “upper strand” (Fig. 2D).
Similar to other MBD family proteins, MBDMBD4 has an overall fold consisting of one α-helix (α1) and three β-strands (β1–3) (Fig. 2D) (20–22). The overall structures of MBDMBD4 and MBDMeCP2 are well superimposed with root mean square deviations of 1.59 Å for 54 equivalent Cα atoms. The T/G mismatch DNA fragment bound to MBDMBD4 adopts the canonical B-form conformation in both crystal structures. The 5mCG/TG site is recognized by MBDMBD4 in a major groove as previously observed in other MBD-5mCG/5mCG complexes (Fig. 2D) (20–22). Phosphate backbone recognition is also conserved among MBD family members. The positive end of the helix dipole from the α1 helix is placed in the major groove and capped by a phosphate group from the DNA backbone. Residues 85–89 in the L1 loop, which connect β1 and β2, also assist in holding the phosphate backbone via making extensive electrostatic contacts (Fig. 2D).
In the triclinic crystal structure, one of the MBDMBD4 molecules in an asymmetric unit binds to the 5mCG/TG site in a conserved manner, whereas the other protein molecule interacts with a joint region between two neighboring DNA fragments that are continuously linked through base stacking interactions (Fig. 2C). As described below, the latter protein-DNA interaction suggests a possible mode of nonspecific DNA binding for MBD4.
MBDMBD4 complexed with 5mCG/5mCG was crystallized in the C2221 form, and its structure was determined at 2.2 Å resolution (Table 1). The overall structure of the 5mCG/5mCG complex is almost identical to that observed in the orthorhombic crystal of the 5mCG/TG complex (root mean square deviation: 0.16 Å for 57 equivalent Cα atoms).
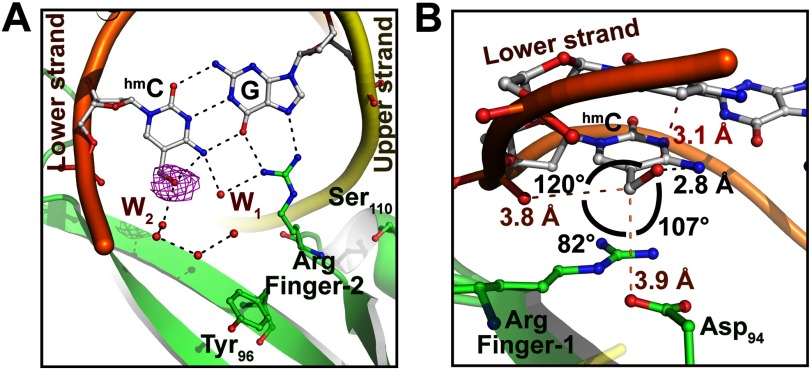
MBDMBD4 Recognizes the 5mCG/5mCG Sequence via Conserved Arginine Fingers
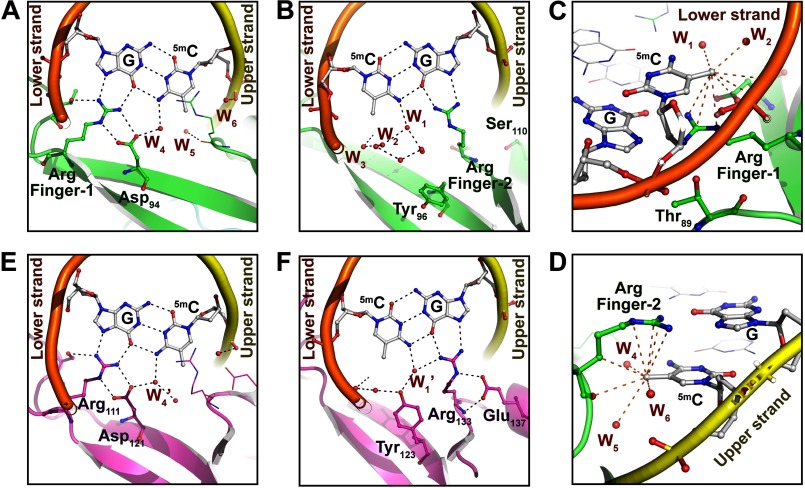
The overall 5mCG/5mCG recognition mode of MBDMBD4 is essentially analogous to that of MBDMeCP2, MBDMBD1, and MBDMBD2. Arg-84 and Arg-106, which are completely conserved in the MBD family (Fig. 2A), recognize symmetrically arranged guanine bases in the 5mCG/5mCG sequence in a manner similar to that of other MBD proteins (Fig. 2D). The Arg-84 and Arg-106 residues are hereafter termed Arg finger-1 and -2, respectively. A guanidino group of Arg finger-1 donates hydrogen bonds to the O6 and N7 atoms of the guanine base in the lower DNA strand, whereas Arg finger-2 recognizes the guanine base in the upper strand via an analogous hydrogen bonding pattern (Fig. 3, A and B). The aliphatic side chains of each arginine finger make van der Waals contacts with the 5-methyl group of the 5mC base adjacent to its interacting guanine (Fig. 3, C and D). Additionally, the main chain carbonyl group of Arg finger-2 forms a CHO hydrogen bond (3.8 Å) with the 5-methyl group of the 5mC base in the upper strand (Fig. 3D).
FIGURE 3.
Structural comparison of DNA binding surfaces of MBDMBD4 and MBDMeCP2. A and B, recognition of the 5mCG site by Arg finger-1 (A) or Arg finger-2 (B) of MBDMBD4. Hydration water molecules are represented as small red spheres. DNA backbone structures of the upper and lower strands are depicted as yellow and orange tubes, respectively. Black dotted lines indicate hydrogen bonds (<3.2 Å). C, recognition of the methyl group of 5mC in the lower strand by MBDMBD4. Orange dotted lines indicate nonbonded contacts with the 5-methyl group of 5mC (<4.2 Å). D, recognition of the 5-methyl group of the lower strand 5mC by MBDMBD4. The red spheres labeled as W1–6 in A–D represent the water molecules in the coordinate file of the MBDMBD4-5mCG/5mCG complex structure (PDB code 3VXX); W1, Wat-202 in chain C; W2, Wat-312 in chain A; W3, Wat-319 in chain A; W4, Wat301 in chain A; W5, Wat-214 in chain B; W6, Wat-202 in chain B. E and F, recognition of the 5mCG site by Arg finger-1 (E) or Arg finger-2 (F) of MBDMeCP2 (21). Black dotted lines indicate hydrogen bonds. W1′ and W4′ indicated in E and F correspond to the water molecules in the MeCP2-DNA complex structure (PDB code 3C2I), Wat-32 in chain B and Wat-183 in chain A, respectively.
In MBDMeCP2, the positions of both Arg fingers are stabilized through interactions with the conserved acidic residues (Fig. 3, E and F) (21). Similarly, the orientation of Arg finger-1 of MBDMBD4 is defined by its intramolecular interaction with a conserved acidic residue, Asp-94. The side chain carboxyl group of Asp-94 forms salt bridges with the guanidino group of Arg finger-1, resulting in an arginine side chain conformation suitable for recognition of the 5mCG sequence (Fig. 3A). Asp-94 also forms a CHO hydrogen bond (3.9 Å) with the 5-methyl group of the 5mC base in the lower strand (Fig. 3C). In contrast, Arg finger-2 lacks such an intramolecular lock because Glu-137 in MeCP2 is replaced by Ser-110 in MBD4 (Fig. 2A). Nevertheless, the position of Arg finger-2 bound to 5mCG/5mCG shows good superimposition with that in the MBDMeCP2-5mCG/5mCG complex (Fig. 3, B and F).
The Water Network in the MBDMBD4-DNA Interface
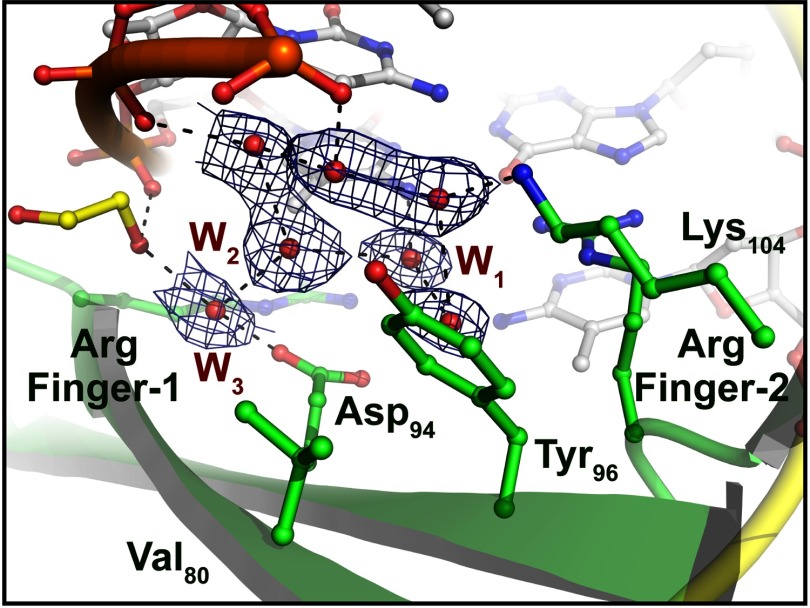
The most significant structural difference between MBDMBD4 and other MBD proteins is the orientation of the conserved tyrosine residue, Tyr-96, located on the DNA binding surface (Fig. 3B). The corresponding tyrosine residues of MBDMeCP2, MBDMBD1, and MBDMBD2 are oriented toward the 5mC base in the lower strand through hydrophobic interactions with the aliphatic side chains of their surrounding residues (20–22). In the crystal structure of the MBDMeCP2-DNA complex, the side chain of the corresponding residue, Tyr-123, recognizes the 5mC base via two water-mediated interactions (Fig. 3F) (21). Previous mutational analysis of MBD1 and MeCP2 suggested that the conserved Tyr residue is critical for DNA binding (20, 21). The side chain of Tyr-96 in MBDMBD4 is flipped out of the DNA interface and makes water-mediated interactions with the phosphate backbone of the lower DNA strand (Fig. 3B). The aromatic side chain is stabilized by a stacking interaction with the compact hydrophobic side chain of Val-80 (Fig. 4). Notably, despite the absence of the tyrosine hydroxyl group at the common position, the MBDMBD4-DNA interface retains the hydration water molecules involved in the recognition of the lower strand 5mC (Fig. 3, B and F). The water molecules, W1, W2, and W3, form van der Waals interactions with the 5-methyl group of the lower strand 5mC in a similar manner to that observed in the MBDMeCP2-DNA complex. Coordination of the three other water molecules (W4, W5, and W6 in the MBDMBD4-DNA complex) surrounding the upper strand 5mC base is also conserved in the MBDMBD4-DNA and MBDMeCP2-DNA complexes (Fig. 3, A and E). MBDMBD4 and MBDMeCP2 share the recognition scheme for the upper strand 5mC base involving a water molecule (W4 in the MBD4-5mCG/5mCG complex or W4′ in the MeCP2-DNA complex) that bridges the N4 atom of the base with the carboxyl group of the conserved Asp residue.
FIGURE 4.
The network structure of hydration water molecules within the interface of the MBDMBD4-5mCG/5mCG complex. The structure of the protein-DNA interface around Tyr-96 is magnified. The hydration water molecules around the lower strand 5mC are shown as red spheres with a 2mFo − DFc electron density map contoured at 1.0 σ. The ethylene glycol (EG) molecule is shown as a yellow stick model.
In the vacant space generated by the flipping of Tyr-96, the molecular water network is further extended at the MBDMBD4-DNA interface. For example, W1 forms a hydrogen-bonding network with other surrounding water molecules (Figs. 3B and 4), whereas its counterpart in the MBDMeCP2-DNA complex, W1′ in Fig. 3F, is fixed by hydrogen bonds with the Tyr-123 and Arg-133 residues of the protein. The hydrogen-bonding network within the DNA interface of MBDMBD4 is also maintained through water-mediated interactions between the phosphate groups of the DNA backbone and the side chains of Asp-94 and Lys-104 (Fig. 4). Thus, the DNA interface of MBDMBD4 contains more open space filled with ordered water molecules in comparison with other MBDs.
Recognition of 5mCG/TG by the Flexible DNA Binding Surface of MBDMBD4
The hydrogen bonding pattern of the T/G mismatched base pair in the MBDMBD4-5mCG/TG complex is identical to that observed in the crystal structure of a DNA oligomer with a T/G mismatch (PDB entry 113D) (34). The T/G mismatch still allows two hydrogen bonds to form between the bases, thus creating an overall shape similar to that in Watson-Crick base pairing. However, the mismatched thymine base is shifted 1–2 Å toward the major groove side of the DNA duplex (Fig. 5A). The base stacking interactions with neighboring pairs are unaffected by the mismatched pair (35), and the entire DNA binding mode common to MBDs is retained in the MBDMBD4-5mCG/TG complex.
FIGURE 5.
Recognition of 5mCG/TG by MBDMBD4. A, the structure of the T/G recognition site is shown in the same orientation as Fig. 3B. Hydration water molecules around the thymine base in the lower strand are shown as red spheres. Black dotted lines indicate hydrogen bonds (<3.2 Å). W1–3 represent the water molecules in the coordinate file of the MBDMBD4-5mCG/TG complex structure (PDB code 3VXV); W1, Wat-202 in chain C; W2, Wat-206 in chain C; W3, Wat-318 in chain A. B, diagrams of the interactions between MBDMBD4 and DNA in the 5mCG/TG (left panel) and 5mCG/5mCG (right panel) complexes. Indirect protein-DNA interactions only mediated by a single water molecule are included in the diagram. Solid and dotted black lines indicate hydrogen bonds (<3.2 Å) donated from the main chain and side chain atoms of MBDMBD4, respectively. Solid orange lines indicate nonbonded contacts with 5mC or mismatched T (<4.2 Å). Asp-94 (asterisk) is shown on both sides of the schematic DNA drawing for convenience. W and Eg represent ordered water molecules and an ethylene glycol molecule, respectively. In the right panel, W4 and W5 correspond to Wat-301 of chain A and Wat-214 of chain B in the MBDMBD4-5mCG/5mCG complex structure (PDB code 3VXX), respectively. C, comparison of the orientation of Arg finger-2 and the hydration water molecule network in the protein-DNA interfaces of the different complexes. Ribbon presentation represents the structure of the 5mCG/5mCG complex. D, model of MBDMBD4 bound to the 5mCG/TG sequence in the direction opposite to that observed in the crystal. The crystal structure of MBDMBD4 is shown as a green ribbon representation with a green stick model of Arg finger-2. The model in the reverse binding mode is shown in blue. A guanidino group of Arg finger-1 in the model structure is overlaid onto that of Arg finger-2 in the crystal structure. The model suggests steric hindrance between the side chain of Asp-94 and the mismatched thymine base in the reverse binding mode.
The guanine base in the T/G mismatch is recognized by Arg finger-2 through a hydrogen-bonding pattern analogous to that observed in the MBDMBD4-5mCG/5mCG complex (Fig. 5, A and B). However, in comparison with the 5mCG/5mCG complex, the side chain of Arg finger-2 is shifted by ∼0.8 Å to form an additional hydrogen bond with the protruding carbonyl group at the 4th position of the thymine ring (Fig. 5C). Except for the movement of Arg finger-2, there are no significant differences between the protein structures in the 5mCG/5mCG and 5mCG/TG complexes. The 5-methyl group of the thymine base is recognized via contacts with Arg finger-1, Asp-94, and water molecules as observed for the lower strand 5mC recognition in the 5mCG/5mCG complex (Fig. 5B). It is important to note that the water molecules in the protein-DNA interface are rearranged by local conformational changes upon binding to 5mCG/TG or 5mCG/5mCG (Fig. 5C).
The 5mC base of 5mCG/TG in the upper strand is also recognized by Arg finger-1 in the manner common to MBDs (Fig. 5B). Arg finger-2 retains the van der Waals contacts with the upper 5mC base via its aliphatic moiety despite its movement.
In contrast to Arg finger-2 of MBDMBD4, Arg finger-2 of MBDMBD1 or MBDMeCP2 is presumably incapable of recognizing the protruding mismatched base because its side chain is fixed by the interaction with conserved acidic residues (Fig. 3F) (20–22). Indeed, MBDMBD1 exhibited significantly weaker binding to 5mCG/TG compared with 5mCG/5mCG (Fig. 1; Table 3). Thus, the flexibility of Arg finger-2 provided by the lack of an intra-molecular lock appears to be indispensable for T/G mismatch recognition.
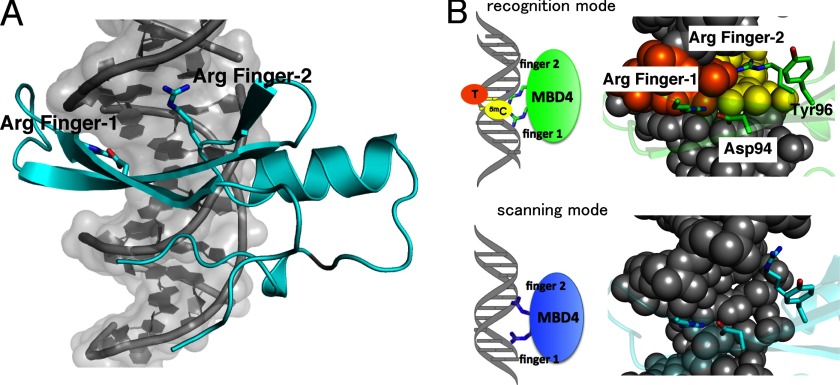
The Nonspecific DNA Binding Mode of MBDMBD4
The nonspecific DNA binding mode of MBD4 is observed in the crystal structure of the triclinic form of the MBD4-5mCG/TG complex (Fig. 2C). In the nonspecific complex, MBDMBD4 also binds to DNA via the major groove side. The phosphate backbone recognition scheme by the α1 helix and L1 loop is essentially identical to that in the specific complex (Fig. 6A).
FIGURE 6.
Structure of the nonspecific DNA complex of MBDMBD4. A, structure of the nonspecific complex observed in the P1 form. DNA molecule is presented as a surface model. The protein residues are shown as blue stick models. B, schematic model of recognition and scanning modes of MBDMBD4. Magnified views of the structure of Arg fingers in the nonspecific and the 5mCG/TG complexes are shown in right panels.
In the nonspecific complex, the dynamic movement of Arg finger-2 is of great interest; this movement takes place in the vacant space generated by the flipping of Tyr-96. Arg finger-2, which is directed toward the target base in the specific complexes, adopts a completely different conformation to form a hydrogen bond with an atom of the phosphate backbone (Fig. 6B) and, thereby reinforcing DNA duplex binding. The unique flexibility of Arg finger-2 in MBD4 presumably facilitates nonspecific DNA interaction, which implies a sliding mode prior to target recognition (Fig. 6B). In agreement with the structural observations, MBDMBD4 exhibited more highly significant binding to nonmodified CpG than MBDMBD1 in our electrophoretic mobility shift assay (data not shown).
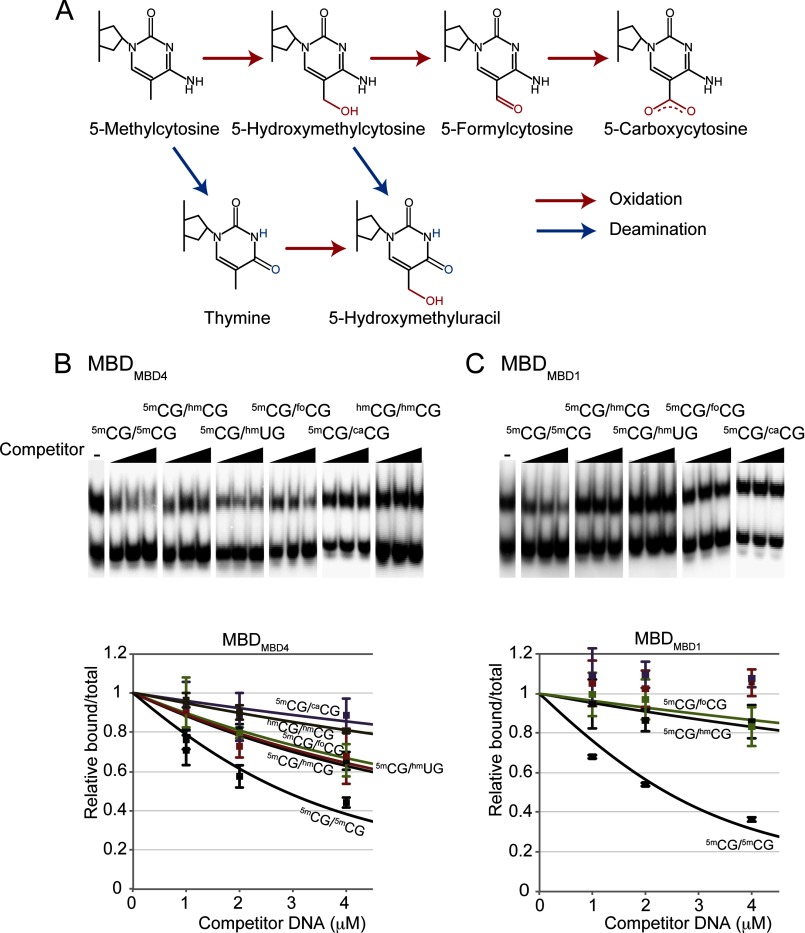
The DNA Binding Surface of MBD4 Tolerates Binding to Oxidation and Deamination Products of 5mC
The structural features of the protein-DNA interface suggest that MBDMBD4 has the ability to bind to modifications that are more bulky than the methyl group at the 5th position of cytosine. We therefore examined the binding of MBDMBD4 to a methylated CpG fragment containing hmC, hmU, foC, or caC (Fig. 7A). In a competitive EMSA, the nonlabeled 5mCG/hmCG, 5mCG/hmUG, and 5mCG/foCG fragments competed with a 32P-labeled 5mCG/5mCG duplex for binding to MBDMBD4. The affinity of MBDMBD4 for 5mCG/hmCG, 5mCG/hmUG, and 5mCG/foCG was estimated to be ∼2- or 3-fold weaker than its affinity for 5mCG/5mCG based on the data from the competitive EMSA and ITC binding assays (Fig. 7, B and D, and Table 3). However, the 5mCG/caCG and hmCG/hmCG fragments exhibited weaker binding to MBDMBD4 than the other modified nucleotides (Fig. 7B). In contrast, MBDMBD1 exhibited a tight specificity for 5mCG/5mCG (Fig. 7, C and E). The affinity of MBDMBD1 for 5mCG/hmCG (KD, 1.04 μm) was more than 10-fold weaker than that for 5mCG/5mCG (KD, 72.5 nm) (Table 3). Combined with the structural data, these findings suggest that MBDMBD4 is capable of binding to methylated CpG sequences that have undergone further asymmetric oxidative modification.
FIGURE 7.
Broad binding specificity of MBDMBD4. A, schematic representation of cytosine oxidation and deamination. B and C, DNA binding specificities of MBDMBD4 (B) and MBDMBD1 (C) analyzed by competitive electrophoretic mobility shift assay. Representative autoradiographic images of competitive assays with MBDMBD4 and MBDMBD1 are presented. The left panel of each section shows the control experiment in the absence of competitor. D and E, the relative values for each complex are plotted against the amount of competitor DNA. Each data point represents an average of three independent experiments using MBDMBD4 or MBDMBD1. Neither the 5mCG/caCG nor the 5mCG/hmUG fragment exhibited competitive effects on the MBDMBD1-5mCG/5mCG complex.
To achieve a better understanding of the structural basis of the versatile DNA binding ability of MBDMBD4, we determined its crystal structure at 2.4-Å resolution when bound to a 5mCG/hmCG fragment (Table 1). Hydroxylation of the 5-methyl group of 5mC does not perturb either the canonical hydrogen bonding pattern in the C/G base pair or the overall DNA binding mode of MBDMBD4 (Fig. 8A). An unambiguous electron density for the hydroxyl group of hmC suggests a confined rotational movement of the 5-hydroxymethyl moiety against the pyrimidine ring (Fig. 8A); intriguingly, the hydroxyl group makes an intra-base hydrogen bond with the amino group at the 4th position in addition to a hydrogen bond with a water molecule at the DNA interface. The 5-hydroxymethyl moiety also donates CHO hydrogen bonds to the carbonyl of Asp-94 and the phosphate group of the DNA backbone, which show tetrahedral coordination around the methyl carbon at the 5th position (Fig. 8B). Thus, the positional preference of the hydroxyl group is ensured by the intra-base hydrogen bond and the tetrahedral configuration around the methyl carbon despite the close contacts with the neighboring base on the 5′ side. The flexible DNA interface of MBDMBD4 is likely to have enough space to accommodate the hmU or foC base as well as hmC. In contrast, the relatively low affinity of MBDMBD4 for 5mCG/caCG is presumably caused by electrostatic repulsion between the 5-carboxyl group of the base and the side chain carboxyl of Asp-94.
FIGURE 8.
Structure of MBDMBD4 bound to 5mCG/hmCG. A, structure of the DNA interface in the MBDMBD4-5mCG/hmCG complex. The structure of the hmCG binding site is shown in the same orientation as Fig. 3B. The mFo − DFc simulated annealing omit map (>3.0 σ) for the hydroxyl group of the hmC base is shown as magenta mesh. Water molecules are represented as small red spheres. Black dotted lines indicate hydrogen bonds (<3.2 Å). W1 and W2 represent the water molecules in the PDB file of the MBDMBD4-5mCG/hmCG complex structure (PDB code 3VYB); W1, Wat-102 in chain C; W2, Wat-106 in chain C. B, the tetrahedral configuration around the carbon atom in the 5-hydroxymethyl group. The black, orange, and red dotted lines represent a hydrogen bond, a CHO hydrogen bond, and an unfavorable close contact, respectively.
DISCUSSION
The crystal structures of MBDMBD4 complexed with 5mCG/TG, 5mCG/5mCG, and 5mCG/hmCG provide new insight into the structural mechanism of the versatility of base recognition by MBD4. The broad base specificity of MBDMBD4 is implicated in heterochromatin localization and enzymatic activity of MBD4 associated with methylated DNA regions. In contrast to MBDMBD1, MBDMBD4 binds not only 5mCG/5mCG but also various modified pyrimidine rings including deamination and/or oxidation products of the 5mC base, such as 5mCG/TG, 5mCG/hmCG, 5mCG/hmUG, and 5mCG/foCG, in a methylated CpG site. MBDMBD4 shares an overall DNA recognition mode with other MBDs. The important role of the water molecules in target base recognition is highlighted by their conserved positions in the MBDMBD4-5mCG/5mCG and MBDMeCP2-5mCG/5mCG complexes (Fig. 3, A, B, E, and F) (21). However, local structural differences between MBDMBD4 and other MBDs have a large impact on the DNA binding properties of MBDMBD4. In particular, the structural features unique to MBDMBD4 around the conserved Tyr-96 and the Arg finger-2 provide plasticity in the DNA binding surface and allow versatile base recognition (Fig. 5C). As a consequence of the flipped Tyr-96 side chain of MBDMBD4, a more extensive water molecule network is established in its DNA interface compared with the MBDMeCP2-DNA surface. Intriguingly, the hydration water molecules responsible for the base recognition (W1, W2, and W3) are maintained at appropriate positions through the solvent network rather than through interactions with protein residues (Fig. 4). A comparison of the water structures around the lower strand target bases (5mC, mismatched T and hmC) highlighted the plasticity in the arrangement of the ordered water molecules in the DNA interface, in which the water-mediated hydrogen-bonding network of MBDMBD4 is finely tuned to accommodate each of the modified bases.
Compared with the lower strand target base recognition, the interface with the upper strand 5mC more strictly maintains the structural features conserved in other MBDs including the conformation of Arg finger-1, which is fixed by the aspartic acid and hydration water structure (Fig. 3, A and E). This structural feature obviously indicates that the symmetric oxidative modification of both 5mC bases in the CpG sequence perturbs MBDMBD4 binding. In fact, our DNA binding data combined with previously reported data demonstrate that neither MBD4 nor the other MBD proteins are capable of binding to the symmetrical hmCG/hmCG site (Fig. 7, B and C) (36, 37). MBDMBD4 does not make contact with bases other than the CpG sequence and are able to bind to the symmetric 5mCG/5mCG site equally in both directions as observed in the flipping motion of MBDMBD1 on its target DNA (38). In contrast, the tight recognition of 5mCG by the Arg finger-1 presumably prevents the flipping motion of MBDMBD4 on asymmetric target sequences, such as 5mCG/TG, 5mCG/hmCG, and 5mCG/hmUG (Fig. 5D).
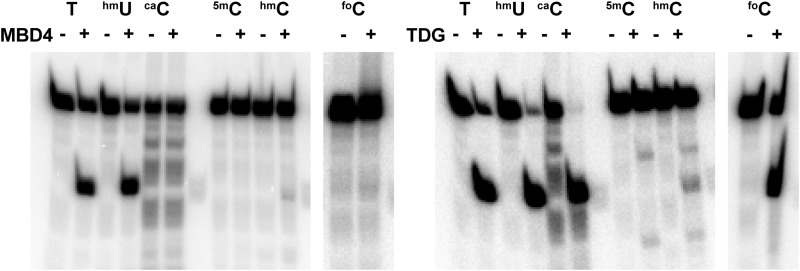
Despite the broad spectrum of MBDMBD4 binding targets, full-length MBD4 exhibits glycosylase activity only toward mismatched thymine and hmU bases (Fig. 9) (24, 39). The oxidative products of 5mC, such as hmC, foC, and caC, are not susceptible to digestion by MBD4, whereas TDG excises foC and caC (7, 10). These findings indicate a partial functional redundancy and a possible functional difference between MBD4 and TDG (7, 10). The glycosylase domain itself exhibits the substrate specificity for T/G or hmU/G mismatched bases regardless of the methylation status of the adjacent C/G base pair (23, 25). Therefore, the DNA binding of MBDMBD4 is presumably a prerequisite for the intrinsic glycosylase activity of MBD4 toward the mismatched bases generated in methylated CpG sites. Additionally, isolated MBDMBD4 has been shown to inhibit the catalytic activity of the glycosylase domain toward a single 5mCG/TG site in vitro (40), suggesting that the DNA substrate is transferred from MBDMBD4 to the glycosylase domain only in full-length MBD4. The unidirectional binding of MBDMBD4 to the 5mCG/TG or 5mCG/hmUG site could facilitate its synergetic action with the C-terminal glycosylase domain in DNA mismatch repair processes. It remains unclear whether the binding of MBDMBD4 to 5mC, hmC, or foC targets the glycosylase domain to neighboring 5mCG/TG or 5mCG/hmUG sites.
FIGURE 9.
Glycosylase activities of MBD4 and TDG. The glycosylase activity of the full-length MBD4 protein for mismatched, deamination, and/or oxidation products in the context of the 5mCG/5mCG sequence was assessed by NaOH cleavage of the resulting apyrimidinic site. We observed a significant digestion band for the strand containing either T or hmU in a mismatched wobble base. 5mC, hmC, foC, and caC bases, each of which forms canonical Watson-Crick base pairs, were not removed by MBD4, whereas, human TDG exhibited activity toward foC and caC in addition to T and hmU bases.
Intriguingly, the active DNA demethylation of the p15ink4b tumor suppressor gene triggered by the TGF-β/SMAD signaling pathway is accompanied by the accumulation of hmC bases, MBD4, TDG, and downstream base excision repair proteins (19). The versatile base recognition ability of MBDMBD4 demonstrated in our study may contribute to the stimuli-dependent accumulation of MBD4 at hydroxymethylated regions, which leads to erasure of DNA methylation marks. Further investigation of MBD4 protein complexes colocalized to hmC-rich regions will be crucial for fully understanding the functional roles of MBD4 in DNA demethylation pathways. Furthermore, recent studies have indicated that the hmC, foC, and caC bases have long lifetimes during preimplantation development (41, 42); thus they may function as bona fide epigenetic marks antagonistic to 5mC bases in vivo. MBD4 may recognize these bases independently of its glycosylase activity and act as a mediator via its multifunctional capabilities, although further investigation is necessary to fully understand the role of MBD4 in the biology of oxidized cytosine bases.
Acknowledgments
We thank Drs. N. Matsugaki, N. Igarashi, and Y. Yamada and Prof. S. Wakatsuki for data collection at Photo Factory, Tsukuba. The synchrotron radiation experiments at the BL38B1 were performed with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal No. 2010B1059).
This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology and the Japan Science and Technology Agency (MEXT) (to M. S.) and by JST, PRESTO (to M. A.) and the Global COE Program “International Center for Integrated Research and Advanced Education in Materials Science” (No. B-09) of MEXT of Japan, administered by the Japan Society for the Promotion of Science.
The atomic coordinates and structure factors (codes 3VXV, 3VXX, 3VYB, and 3VYQ) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- 5mC
- 5-methylcytosine
- hmC
- 5-hydroxymethylcytosine
- foC
- 5-formylcytosine
- caC
- 5-carboxylcytosine
- hmU
- 5-hydroxymethyluracil
- TDG
- thymine DNA glycosylase
- MBD
- methyl-CpG binding domain
- ITC
- isothermal titration calorimetry
- PDB
- Protein Data Bank.
REFERENCES
- 1. Bird A. (2002) DNA methylation patterns and epigenetic memory. Genes Dev. 16, 6–21 [DOI] [PubMed] [Google Scholar]
- 2. Li E. (2002) Chromatin modification and epigenetic reprogramming in mammalian development. Nat. Rev. Genet. 3, 662–673 [DOI] [PubMed] [Google Scholar]
- 3. Robertson K. D., Wolffe A. P. (2000) DNA methylation in health and disease. Nat. Rev. Genet. 1, 11–19 [DOI] [PubMed] [Google Scholar]
- 4. Métivier R., Gallais R., Tiffoche C., Le Péron C., Jurkowska R. Z., Carmouche R. P., Ibberson D., Barath P., Demay F., Reid G., Benes V., Jeltsch A., Gannon F., Salbert G. (2008) Cyclical DNA methylation of a transcriptionally active promoter. Nature 452, 45–50 [DOI] [PubMed] [Google Scholar]
- 5. Kangaspeska S., Stride B., Métivier R., Polycarpou-Schwarz M., Ibberson D., Carmouche R. P., Benes V., Gannon F., Reid G. (2008) Transient cyclical methylation of promoter DNA. Nature 452, 112–115 [DOI] [PubMed] [Google Scholar]
- 6. Cortellino S., Xu J., Sannai M., Moore R., Caretti E., Cigliano A., Le Coz M., Devarajan K., Wessels A., Soprano D., Abramowitz L. K., Bartolomei M. S., Rambow F., Bassi M. R., Bruno T., Fanciulli M., Renner C., Klein-Szanto A. J., Matsumoto Y., Kobi D., Davidson I., Alberti C., Larue L., Bellacosa A. (2011) Thymine DNA glycosylase is essential for active DNA demethylation by linked deamination-base excision repair. Cell 146, 67–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. He Y. F., Li B. Z., Li Z., Liu P., Wang Y., Tang Q., Ding J., Jia Y., Chen Z., Li L., Sun Y., Li X., Dai Q., Song C. X., Zhang K., He C., Xu G. L. (2011) Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science 333, 1303–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu S. C., Zhang Y. (2010) Active DNA demethylation. Many roads lead to Rome. Nat. Rev. Mol. Cell Biol. 11, 607–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guo J. U., Su Y., Zhong C., Ming G. L., Song H. (2011) Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell 145, 423–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maiti A., Drohat A. C. (2011) Thymine DNA glycosylase can rapidly excise 5-formylcytosine and 5-carboxylcytosine. Potential implications for active demethylation of CpG sites. J. Biol. Chem. 286, 35334–35338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bhutani N., Brady J. J., Damian M., Sacco A., Corbel S. Y., Blau H. M. (2010) Reprogramming towards pluripotency requires AID-dependent DNA demethylation. Nature 463, 1042–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Popp C., Dean W., Feng S., Cokus S. J., Andrews S., Pellegrini M., Jacobsen S. E., Reik W. (2010) Genome-wide erasure of DNA methylation in mouse primordial germ cells is affected by AID deficiency. Nature 463, 1101–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rai K., Huggins I. J., James S. R., Karpf A. R., Jones D. A., Cairns B. R. (2008) DNA demethylation in zebrafish involves the coupling of a deaminase, a glycosylase, and gadd45. Cell 135, 1201–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bogdanović O., Veenstra G. J. (2009) DNA methylation and methyl-CpG binding proteins. Developmental requirements and function. Chromosoma 118, 549–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kondo E., Gu Z., Horii A., Fukushige S. (2005) The thymine DNA glycosylase MBD4 represses transcription and is associated with methylated p16INK4a and hMLH1 genes. Mol. Cell. Biol. 25, 4388–4396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hendrich B., Hardeland U., Ng H. H., Jiricny J., Bird A. (1999) The thymine glycosylase MBD4 can bind to the product of deamination at methylated CpG sites. Nature 401, 301–304 [DOI] [PubMed] [Google Scholar]
- 17. Millar C. B., Guy J., Sansom O. J., Selfridge J., MacDougall E., Hendrich B., Keightley P. D., Bishop S. M., Clarke A. R., Bird A. (2002) Enhanced CpG mutability and tumorigenesis in MBD4-deficient mice. Science 297, 403–405 [DOI] [PubMed] [Google Scholar]
- 18. Riccio A., Aaltonen L. A., Godwin A. K., Loukola A., Percesepe A., Salovaara R., Masciullo V., Genuardi M., Paravatou-Petsotas M., Bassi D. E., Ruggeri B. A., Klein-Szanto A. J., Testa J. R., Neri G., Bellacosa A. (1999) The DNA repair gene MBD4 (MED1) is mutated in human carcinomas with microsatellite instability. Nat. Genet. 23, 266–268 [DOI] [PubMed] [Google Scholar]
- 19. Thillainadesan G., Chitilian J. M., Isovic M., Ablack J. N., Mymryk J. S., Tini M., Torchia J. (2012) TGF-β-dependent active demethylation and expression of the p15ink4b tumor suppressor are impaired by the ZNF217/CoREST complex. Mol. Cell, 46, 636–649 [DOI] [PubMed] [Google Scholar]
- 20. Ohki I., Shimotake N., Fujita N., Jee J., Ikegami T., Nakao M., Shirakawa M. (2001) Solution structure of the methyl-CpG binding domain of human MBD1 in complex with methylated DNA. Cell 105, 487–497 [DOI] [PubMed] [Google Scholar]
- 21. Ho K. L., McNae I. W., Schmiedeberg L., Klose R. J., Bird A. P., Walkinshaw M. D. (2008) MeCP2 binding to DNA depends upon hydration at methyl-CpG. Mol. Cell 29, 525–531 [DOI] [PubMed] [Google Scholar]
- 22. Scarsdale J. N., Webb H. D., Ginder G. D., Williams D. C. (2011) Solution structure and dynamic analysis of chicken MBD2 methyl binding domain bound to a target-methylated DNA sequence. Nucleic Acids Res. 39, 6741–6752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Manvilla B. A., Maiti A., Begley M. C., Toth E. A., Drohat A. C. (2012) Crystal structure of human methyl-binding domain IV glycosylase bound to abasic DNA. J. Mol. Biol. 420, 164–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hashimoto H., Liu Y., Upadhyay A. K., Chang Y., Howerton S. B., Vertino P. M., Zhang X., Cheng X. (2012) Recognition and potential mechanisms for replication and erasure of cytosine hydroxymethylation. Nucleic Acids Res., 40, 4841–4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moréra S., Grin I., Vigouroux A., Couvé S., Henriot V., Saparbaev M., Ishchenko A. A. (2012) Biochemical and structural characterization of the glycosylase domain of MBD4 bound to thymine and 5-hydroxymethyuracil-containing DNA. Nucleic Acids Res., 40, 9917–9926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Doublié S. (1997) Preparation of selenomethionyl proteins for phase determination. Methods Enzymol. 276, 523–530 [PubMed] [Google Scholar]
- 27. Otwinowski Z., Minor W. (1997) Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. A 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 28. Terwilliger T. C., Berendzen J. (1999) Automated MAD and MIR structure solution. Acta Crystallogr. D Biol. Crystallogr. 55, 849–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Terwilliger T. (2000) Maximum-likelihood density modification. Acta Crystallogr. D Biol. Crystallogr. 56, 965–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Emsley P., Cowtan K. (2004) COOT. Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 31. Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., Richardson J. S., Terwilliger T. C., Zwart P. H. (2010) PHENIX. A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lovell S. C., Davis I. W., Arendall W. B., 3rd, de Bakker P. I., Word J. M., Prisant M. G., Richardson J. S., Richardson D. C. (2003) Structure validation by Cα geometry. π, ψ and Cβ deviation. Proteins 50, 437–450 [DOI] [PubMed] [Google Scholar]
- 33. Morrison J. F. (1969) Kinetics of the reversible inhibition of enzyme-catalysed reactions by tight-binding inhibitors. Biochim. Biophys. Acta 185, 269–286 [DOI] [PubMed] [Google Scholar]
- 34. Hunter W. N., Brown T., Kneale G., Anand N. N., Rabinovich D., Kennard O. (1987) The structure of guanosine-thymidine mismatches in B-DNA at 2.5-Å resolution. J. Biol. Chem. 262, 9962–9970 [DOI] [PubMed] [Google Scholar]
- 35. Olson W. K., Bansal M., Burley S. K., Dickerson R. E., Gerstein M., Harvey S. C., Heinemann U., Lu X. J., Neidle S., Shakked Z., Sklenar H., Suzuki M., Tung C. S., Westhof E., Wolberger C., Berman H. M. (2001) A standard reference frame for the description of nucleic acid base-pair geometry. J. Mol. Biol. 313, 229–237 [DOI] [PubMed] [Google Scholar]
- 36. Jin S. G., Kadam S., Pfeifer G. P. (2010) Examination of the specificity of DNA methylation profiling techniques towards 5-methylcytosine and 5-hydroxymethylcytosine. Nucleic Acids Res. 38, e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Valinluck V., Tsai H. H., Rogstad D. K., Burdzy A., Bird A., Sowers L. C. (2004) Oxidative damage to methyl-CpG sequences inhibits the binding of the methyl-CpG binding domain (MBD) of methyl-CpG binding protein 2 (MeCP2). Nucleic Acids Res. 32, 4100–4108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Inomata K., Ohki I., Tochio H., Fujiwara K., Hiroaki H., Shirakawa M. (2008) Kinetic and thermodynamic evidence for flipping of a methyl-CpG binding domain on methylated DNA. Biochemistry 47, 3266–3271 [DOI] [PubMed] [Google Scholar]
- 39. Liu P., Burdzy A., Sowers L. C. (2003) Repair of the mutagenic DNA oxidation product, 5-formyluracil. DNA Repair 2, 199–210 [DOI] [PubMed] [Google Scholar]
- 40. Aziz M. A., Schupp J. E., Kinsella T. J. (2009) Modulation of the activity of methyl binding domain protein 4 (MBD4/MED1) whereas processing iododeoxyuridine generated DNA mispairs. Cancer Biol. Ther. 8, 1156–1163 [DOI] [PubMed] [Google Scholar]
- 41. Inoue A., Shen L., Dai Q., He C., Zhang Y. (2011) Generation and replication-dependent dilution of 5fC and 5caC during mouse preimplantation development. Cell Res. 21, 1670–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Inoue A., Zhang Y. (2011) Replication-dependent loss of 5-hydroxymethylcytosine in mouse preimplantation embryos. Science 334, 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. DeLano W. L. (2010) The PyMOL Molecular Graphics System, Schrödinger, LLC, New York [Google Scholar]