Background: Mn2+ levels are lower in blood of diabetic and atherosclerosis patients.
Results: Mn2+ supplementation reduces monocyte adhesion in endothelial cells by down-regulating ROS, ICAM-1 expression, and MCP-1 secretion, and lowers blood levels of ICAM-1 and cholesterol in ZDF rats.
Conclusion: Mn2+ supplementation is beneficial in lowering markers of endothelial dysfunction.
Significance: Mn2+ supplementation can potentially prevent or delay progression of atherosclerosis.
Keywords: Adhesion, Diabetes, Endothelial Dysfunction, Manganese, Metabolism
Abstract
Endothelial dysfunction is a hallmark of increased vascular inflammation, dyslipidemia, and the development of atherosclerosis in diabetes. Previous studies have reported lower levels of Mn2+ in the plasma and lymphocytes of diabetic patients and in the heart and aortic tissue of patients with atherosclerosis. This study examines the hypothesis that Mn2+ supplementation can reduce the markers/risk factors of endothelial dysfunction in type 2 diabetes. Human umbilical vein endothelial cells (HUVECs) were cultured with or without Mn2+ supplementation and then exposed to high glucose (HG, 25 mm) to mimic diabetic conditions. Mn2+ supplementation caused a reduction in monocyte adhesion to HUVECs treated with HG or MCP-1. Mn2+ also inhibited ROS levels, MCP-1 secretion, and ICAM-1 up-regulation in HUVECs treated with HG. Silencing studies using siRNA against MnSOD showed that similar results were observed in MnSOD knockdown HUVECs following Mn2+ supplementation, suggesting that the effect of manganese on monocyte adhesion to endothelial cells is mediated by ROS and ICAM-1, but not MnSOD. To validate the relevance of our findings in vivo, Zucker diabetic fatty rats were gavaged daily with water (placebo) or MnCl2 (16 mg/kg of body weight) for 7 weeks. When compared with placebo, Mn2+-supplemented rats showed lower blood levels of ICAM-1 (17%, p < 0.04), cholesterol (25%, p < 0.05), and MCP-1 (28%, p = 0.25). These in vitro and in vivo studies demonstrate that Mn2+ supplementation can down-regulate ICAM-1 expression and ROS independently of MnSOD, leading to a decrease in monocyte adhesion to endothelial cells, and therefore can lower the risk of endothelial dysfunction in diabetes.
Introduction
Manganese is an essential micronutrient that serves as a cofactor for many enzyme systems. Metalloenzymes, or manganese-containing enzymes, such as arginase, pyruvate carboxylase, and manganese superoxide dismutase (MnSOD),2 require Mn2+ to function. MnSOD is the major mitochondrial antioxidant and is responsible for protecting the cell from reactive oxygen species (ROS) by scavenging mitochondrial superoxide (1). MnSOD acts by catalyzing the conversion of superoxide radicals (such as O2) to hydrogen peroxide, which is further metabolized to water by other antioxidant enzymes such as catalase and glutathione peroxidase (2). At low concentrations, Mn2+ ions have been shown to have antioxidant properties with the ability to scavenge superoxide and hydroxyl radicals (3). Several studies have reported that changes in dietary Mn2+ induced changes in MnSOD activity and that MnSOD activity was reduced in heart and livers of Mn2+-deficient animals (4–6). Previous studies report lower levels of Mn2+ in the plasma and lymphocytes of type 2 diabetic patients (7, 8) and in the heart and aortic tissue of patients with atherosclerosis when compared with those of healthy controls (9). Other studies have also shown beneficial effect of Mn2+ on lipid metabolism and a decrease in total serum cholesterol, aorta cholesterol, and regression of atherosclerosis following manganese supplementation in cholesterol-fed rabbits (10). The mechanisms by which Mn2+ can reduce cholesterol, however, are unknown. Also, a possible beneficial role of Mn2+ supplementation alone (without MnSOD) on vascular inflammation has never been investigated. Endothelial dysfunction and vascular inflammation, characterized by monocyte adhesion to endothelial cells and increased levels of MCP-1, ROS, and ICAM-1, are known to play a significant role in the development of atherosclerosis (11–13). This study examines the hypothesis that Mn2+ supplementation can prevent vascular inflammation and endothelial dysfunction in type 2 diabetes and that Mn2+ can have a beneficial role independently of MnSOD. Our results demonstrate that Mn2+ supplementation reduces ROS levels, MCP-1 secretion, ICAM-1 expression, and the adhesion of monocytes to endothelial cells. Furthermore, similar results were obtained in MnSOD knockdown human umbilical vein endothelial cells (HUVECs). Further studies in vivo showed that Mn2+ supplementation lowers blood levels of ICAM-1 and cholesterol in Zucker diabetic fatty rats. These in vitro and in vivo studies demonstrate that Mn2+ supplementation can lower markers of oxidative stress and endothelial dysfunction, such as monocyte adhesion to endothelial cells, ICAM-1, ROS, MCP-1, and cholesterol, thereby lowering the risk of endothelial dysfunction in diabetes. We also show for the first time that Mn2+ supplementation can have beneficial effects on endothelial cells independently of MnSOD.
EXPERIMENTAL PROCEDURES
Human Umbilical Vein Endothelial Cells
HUVECs were purchased from Lonza Walkersville Inc., Walkersville, MD. Cells were cultured in EGM-2 medium and 5% CO2, in a 37 °C humidified atmosphere, and grown to confluence in T75 flasks coated with gelatin. Experiments were performed within 24 h after reaching confluence, between passages 3 and 10. Cells were pretreated with Mn2+ (0, 5, and 10 μm as MnCl2) for 24 h followed by high glucose (HG, 25 mm) or normal glucose (7 mm) exposure for another 24 h. Many previous studies have reported glucose concentrations as high as 50 mm in the blood of patients with uncontrolled diabetes (25). It is true that blood glucose levels in patients are not likely to stay as high as 25 mm for 24 h. However, tissue damage in diabetic patients occurs over many years of countless hyperglycemic episodes. Thus, the glucose concentration of 25 mm used to mimic diabetes in this cell culture study does not seem unreasonable. We did not observe any effect of on Mn2+ on cell viability, similar to results from previous cell culture studies (14, 15).
Silencing Studies
SOD2 siRNA was purchased from Santa Cruz Biotechnology. For every transfection, 2 μl of transfection reagent (Lipofectamine from Invitrogen) was added to 100 μl of transfection medium (from Santa Cruz Biotechnology, serum-free). 100 nm SOD2 siRNA was added to the mix. Cells were trypsinized and then resuspended in transfection medium and plated to 60-mm dishes. Cells were incubated for 3–4 h at 37 °C. Normal medium was then added to the cells and incubated overnight at 37 °C. The next day, fresh medium was added, and the cells were treated for the experiment within the next 18–30 h.
MnSOD Activity Assay
Total SOD activity was assessed using the xanthine-xanthine oxidase and nitro blue tetrazolium (NBT) diformazan method as in Ref. 16. Xanthine oxidase is used to generate O2˙̄ and NBT reduction is used as an indicator of O2˙̄ production. SOD competes with NBT for O2˙̄; the percentage of inhibition of NBT reduction is a measure of the amount of SOD present. KCN was used to inhibit Cu/ZnSOD activity. Absorbance was measured at 560 nm to measure NBT reduction. Absorbance per minute was used to determine the percentage of inhibition of diformazan formation. 50% inhibition of NBT reduction equals to 1 unit of SOD activity.
ROS Assay
ROS levels were measured using the dihydrorhodamine 123 dye. Cells were incubated with the dye for 30 min after treatment (2 h HG instead of 24 h). Mean fluorescence was analyzed. After treatment, cells were washed once with PBS and then loaded with 30 μm dihydrorhodamine 123 in PBS with 10% FCS. The cells were incubated at 37 °C for 30 min in the dark and subsequently washed with PBS. The intensity of dihydrorhodamine 123 fluorescence in the supernatant was read at excitation and emission wavelengths of 485 and 528 nm, respectively, using a multidetection microplate reader (Synergy HT, Bio-Tek). The change in intracellular ROS level was plotted as mean fluorescence intensity.
Surface ICAM-1
Surface ICAM-1 was determined using flow cytometry. After treatment, cells were washed in FACS buffer (PBS without Mg2+ and Ca2+, with the addition of 10% fetal bovine serum and 0.1% sodium azide), centrifuged, resuspended in FACS buffer, and incubated for 1 h at 4 °C with FITC-conjugated anti-ICAM-1 antibody (Santa Cruz Biotechnology, sc-107) at a 1:100 dilution in the dark. The cells were then washed twice in washing buffer for FACS (PBS containing 1% BSA and 0.1% sodium azide) and resuspended in 0.3 ml of FACS buffer. In each experiment, a minimum of 15,000 cells was analyzed (per treatment condition) by FACSCalibur flow cytometer (BD Biosciences) equipped with multicolor analysis capability. Gates were set to exclude nonviable cells, cell debris, and cells of abnormal size and shape. Results were expressed as mean fluorescence intensity per 15,000 cells.
Western Blotting Analyses of Cell Lysates
Cells were lysed in radioimmunoprecipitation assay buffer (50 mm Tris pH 8, 150 mm NaCl, 1% Nonidet P-40, 0.5% deoxycholic acid, 0.1% SDS) supplemented with protease and phosphatase inhibitors (1 mm PMSF, 5 μg/ml leupeptin, 2 μg/ml aprotinin, 1 mm EDTA, 10 mm NaF, and 1 mm NaVO4). Lysates were subjected to mild sonication and centrifuged at 15,000 rpm (4 °C, 30 min), and the supernatants were collected. Total protein concentrations were determined by BCA assay (Pierce/Thermo Scientific). Equal amounts of protein from each group were loaded onto SDS-polyacrylamide gels after boiling for 5 min with or without β-mercaptoethanol as a reducing agent (ICAM-1 was determined in nonreducing conditions). The separated proteins were transferred to a nitrocellulose membrane, blocked with 1% BSA in TBS-T (0.25% Tween 20 in PBS), and incubated overnight at 4 °C with the respective primary antibodies, using 1:1000 dilutions. The next day, membranes were washed with TBS-T (8 min, four cycles) and incubated with secondary antibodies conjugated with horseradish peroxidase (HRP) in 5% nonfat milk for 30 min at room temperature. The membranes were again washed with TBS-T (8 min, 4 cycles), treated with chemiluminescence reagents for 2 min, and exposed to x-ray films developed through autoradiography. β-Actin or α-tubulin antibodies were used to assess the loading equality. Primary antibodies for MnSOD were purchased from Abcam, and antibodies for ICAM-1 were from Santa Cruz Biotechnology.
Human THP-1 Monocytes
Human THP-1 monocytes were purchased from the American Type Culture Collection (ATCC, Manassas, VA). Cells were cultured in RPMI 1640 medium supplemented with 10% (v/v) heat-inactivated fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 2 mm l-glutamine. The culture was maintained at 37 °C in a humidified atmosphere containing 5% CO2. For treatments, the cells were counted on a hemocytometer using trypan blue exclusion and adjusted to ∼1 × 106 cells/ml in complete medium. Cells were treated with HG and Mn2+ in the same manner as were HUVECs.
Monocyte-Endothelial Cells Adhesion Assay
Assay was performed as described previously (17). HUVECs were plated and allowed to grow to confluent monolayers. HUVECs were treated with different concentrations of Mn2+ (0–10 μm) for 24 h and then exposed to either HG or MCP-1 for another 24 h. Monocytes (THP-1) were loaded with 8 μm CellTracker Green (5-chloromethylfluorescein diacetate; Invitrogen) and then treated with concentrations of Mn2+ matching those of the HUVECs. After treatment, 2 × 106 monocytes were added to the endothelial monolayers and incubated at 37 °C for 30 min. The nonadherent cells were washed away with PBS, and adherent cells were lysed in 0.2% Triton X-100 for quantification. The fluorescent intensity of the monocytes added to the monolayer (input) as well as the nonadherent cells was measured at excitation 485 and emission 528 nm.
Murine 3T3L1 Fibroblast Cell Line
Adipocytes and murine 3T3L1 fibroblast cell line were obtained from the ATCC. Cells were cultured in high glucose DMEM medium containing 10% (v/v) FCS, 100 units/ml penicillin, 100 μg/ml streptomycin and maintained at 37 °C in an incubator containing 5% (v/v) CO2. 3 days after reaching confluence, cells were incubated in high glucose DMEM medium containing 10% (v/v) FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin and supplemented with 100 milliunits/ml insulin, 0.5 mm isobutylmethylxanthine, and 250 nm dexamethasone for 2 days to allow differentiation into adipocytes. Cells were then placed in the same medium containing insulin but lacking additional supplements for an additional 2 days. The medium was replaced every 2 days thereafter until more than 85% of the cells contained lipid droplets. 7–10 days after the induction of differentiation, 3T3L1 adipocytes were ready to be used in experiments (18). The cells were incubated with serum-free low glucose DMEM during the experimental incubation period. Cells were treated with HG and Mn2+ in the same manner as were HUVECs.
Animal Study
All procedures followed were in accordance with the ethical standards of the institution, and approval was obtained from the institutional Animal Welfare Committee. Male Zucker diabetic fatty (ZDF) rats were purchased at 5 weeks of age (200–220 g) from Charles River Laboratories (Wilmington, MA) and allowed 2 days for environmental and trainer handling acclimation. The rats were housed and labeled in individual cages. Rats were assigned into various groups by computer-generated randomization. Rats were fasted overnight and then weighed. The rats were tested for hyperglycemia by measuring their blood glucose concentration. Blood for blood glucose measurements was obtained via tail incision and measured using an Advantage Accu-chek glucometer (Roche Applied Science). At 6 weeks of age, rats were randomly divided into two groups: group A (diabetic controls) and group B (Mn2+-supplemented diabetic rats). Each rat was supplemented with the appropriate dose of Mn2+ or water daily for 7 weeks by oral gavage using 20-gauge feeding needles (Popper and Sons, New Hyde Park, NY). Group B was supplemented with a 16 mg/kg of body weight dose of Mn2+. Previous studies have used 0.001% Mn2+ in the diet as an adequate amount and 0.01% Mn2+ as supplementation (19, 20). Various studies have reported daily food consumption for ZDF rats to range between 35 and 50 g per day (21, 22). One study reported daily food consumption to be up to 66 g per day in ZDF rats used as their control group (23). Assuming that ZDF rats consume 50 g of food per day, 0.01% Mn2+ corresponds to 5 mg. Rats weigh on average 300 g; therefore, 5 mg of Mn2+ per rat equals 16 mg/kg of body weight. Body weights were monitored weekly to determine the dose of Mn2+ supplementation. We prepared a stock solution of 16 mg/ml MnCl2 and gavaged the rats according to weights, administrating 0.1 ml/100 g of body weight. Mn2+ content of the diet is 71 ppm, which equals to 71 mg/kg of diet and 3 mg/50 g of diet. Therefore, total supplementation in our experiment was 8 mg/rat, which is 0.016% Mn2+ supplementation in the diet.
The rats were maintained under standard housing conditions at 22 ± 2 °C with 12:12-h light/dark cycles and fed Purina 5008 lab chow diet (Charles River Laboratories). Food intake was monitored on two separate occasions during the 7-week period to assess consumption. At the end of 7 weeks, the rats were fasted overnight and then euthanized for analysis by exposure to isoflurane. Blood was collected into EDTA BD Vacutainer tubes via cardiac puncture with a 19.5-gauge needle. Plasma was isolated after centrifuging blood at 3000 rpm for 15 min at 4 °C. Immediately after blood collection, livers were perfused with cold saline, extracted, and stored in −70 °C freezers. A portion of blood from rats in each group was sent to the clinical laboratory of LSU Health Shreveport (located in the same building) for clinical tests to determine glucose, HbA1c, liver, and renal function and red blood cell counts.
MCP-1 and ICAM-1 Assay
Cytokine levels were determined using the sandwich ELISA kits from R&D Systems, Inc. (Minneapolis, MN) for MCP-1 and from Fisher Thermo Scientific for ICAM-1. All appropriate controls and standards as specified by the manufacturer's kit were used. Control samples were analyzed each time to check the variation from plate to plate on different days of analysis.
All chemicals were purchased from Sigma unless otherwise mentioned. Data were analyzed statistically using one-way analysis of variance between different groups using Sigma Plot 11 software (Systat Software Inc., San Jose, CA). A p value of less than 0.05 was considered significant.
RESULTS
Effects of Mn2+ Supplementation in Cell Culture
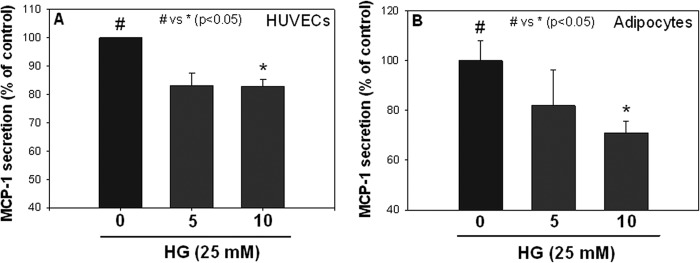
Fig. 1 shows the effects of Mn2+ pretreatment on MCP-1 secretion in endothelial cells and adipocytes exposed to high glucose. There was a significant reduction in MCP-1 secretion in both cell types in the presence of 10 μm Mn2+, indicating that the effect of Mn2+ on MCP-1 secretion inhibition is not specific to one cell type.
FIGURE 1.
Effects of manganese (Mn2+) supplementation on MCP-1 secretion in HUVECs (A) and 3T3L1 adipocytes (B). Values are ± S.E., n = 3, and expressed as a percentage of HG-treated cells.
Fig. 2 illustrates the effects of Mn2+ supplementation on monocyte adhesion to endothelial cells. Adhesion assays between HUVECs and THP-1 monocytes were performed after treating both cell types with and without Mn2+ (see “Experimental Procedures” for details). Mn2+ supplementation significantly inhibited monocyte adhesion to endothelial cells in both high glucose-treated and MCP-1-treated cells. These results suggest a potential beneficial effect of Mn2+ in reducing monocyte adherence to endothelial cells, a key event in the initiation of endothelial dysfunction.
FIGURE 2.
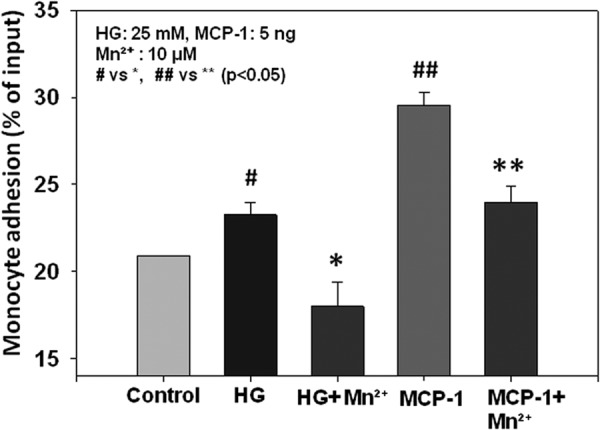
Effects of Mn2+ supplementation on monocyte (THP-1) adhesion to endothelial cells (HUVECs). Values are ± S.E., n = 3, and expressed as a percentage of input fluorescence.
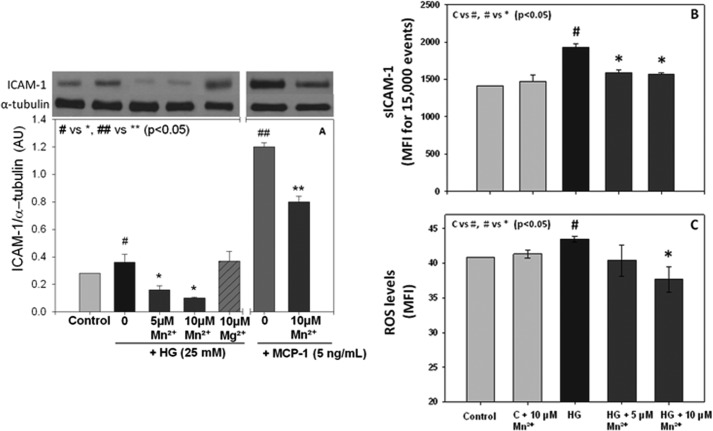
To determine what adhesion molecules mediate the reduction of monocyte adhesion to endothelial cells caused by Mn2+ supplementation, we measured total and surface ICAM-1 expression in HUVECs cultured with high glucose. Mn2+ supplementation caused a significant down-regulation of both total and surface ICAM-1 expression in endothelial cells (Fig. 3, A and B). The effect on ICAM-1 down-regulation was Mn2+-dose dependent. Magnesium (Mg2+) supplementation did not have any effect on total ICAM-1 regulation. Because Mn2+ caused a decrease in MCP-1 secretion and ICAM-1 expression, we then examined whether ICAM-1 regulation is associated with MCP-1 secretion in endothelial cells. Exogenous addition of MCP-1 per se also caused activation of ICAM-1 expression, and Mn2+ supplementation inhibited the MCP-1-induced up-regulation of ICAM-1 in endothelial cells. This suggests that inhibition of MCP-1 secretion caused by Mn2+ supplementation may play a role in down-regulation of ICAM-1 expression.
FIGURE 3.
Effects of Mn2+ supplementation on ICAM-1 total expression (A), surface expression (B), and ROS (C) in HUVECs. Quantification of band intensity is shown in arbitrary units (AU). Values are ± S.E., n = 3, expressed as mean fluorescence intensity (MFI) for surface ICAM-1. Values are ± S.E., n = 4 for ROS. C indicates control.
To further investigate the mechanisms by which Mn2+ supplementation reduces monocyte adhesion to endothelial cells and MCP-1 secretion, we measured ROS levels in HUVECs treated with or without Mn2+. Fig. 3C shows that Mn2+ supplementation inhibits the high glucose increase in ROS levels in a dose-dependent manner.
To evaluate the role of MnSOD in the regulation of ICAM-1 and ROS levels, we first measured MnSOD expression and activity. MnSOD expression was not different in endothelial cells treated with HG or MCP-1 with and without Mn2+ supplementation (data not shown). MnSOD activity was decreased in cells exposed to high glucose and increased in cells supplemented with Mn2+ and exposed to HG (Fig. 4).
FIGURE 4.
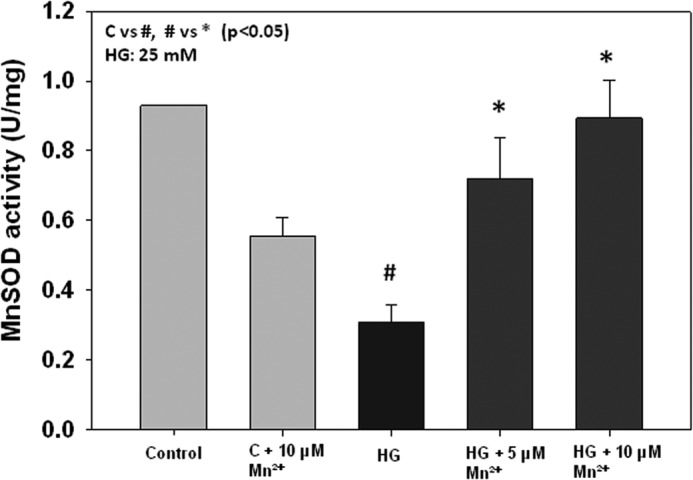
Effects of Mn2+ supplementation on MnSOD activity in HUVECs. MnSOD activity is expressed as units/mg of protein. Values are ± S.E., n = 3. C indicates control.
To further investigate the role of MnSOD, we knocked down its expression using siRNA before treating the cells with Mn2+. Fig. 5 shows the efficiency of our knockdown system, where MnSOD was knocked down about 70%, but the expression of the other SOD enzyme, Cu/ZnSOD, was unchanged in HUVECs. As shown in Fig. 6, monocyte adhesion to endothelial cells was still inhibited by Mn2+ supplementation in cells where MnSOD was knocked down. In addition, we still observed a down-regulation of ICAM-1 total and surface expression (Fig. 7, A and B), as well as a decrease in ROS levels (Fig. 7C), suggesting that the effects of Mn2+ supplementation on monocyte adhesion to endothelial cells are mediated by inhibition of ICAM-1 expression and ROS production, and not MnSOD. MnSOD activity was not detectable in HUVECs treated with MnSOD siRNA (data not shown). This is the first time that a role of Mn2+ is reported to have a beneficial effect on ICAM-1 and ROS levels in endothelial cells, without MnSOD.
FIGURE 5.

Effects of Mn2+ supplementation on MnSOD siRNA-transfected HUVECs on MnSOD and Cu/ZnSOD expression. Lane C indicates control.
FIGURE 6.
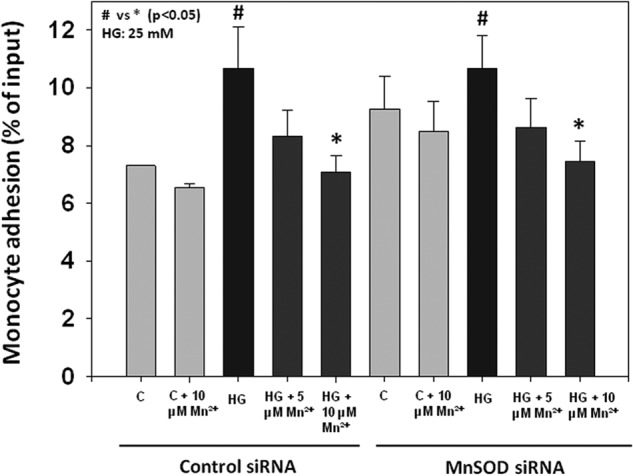
Effects of Mn2+ supplementation on monocyte (THP-1) adhesion to endothelial cells (HUVECs). Values are ± S.E., n = 3, and expressed as a percentage of input fluorescence. C indicates control.
FIGURE 7.
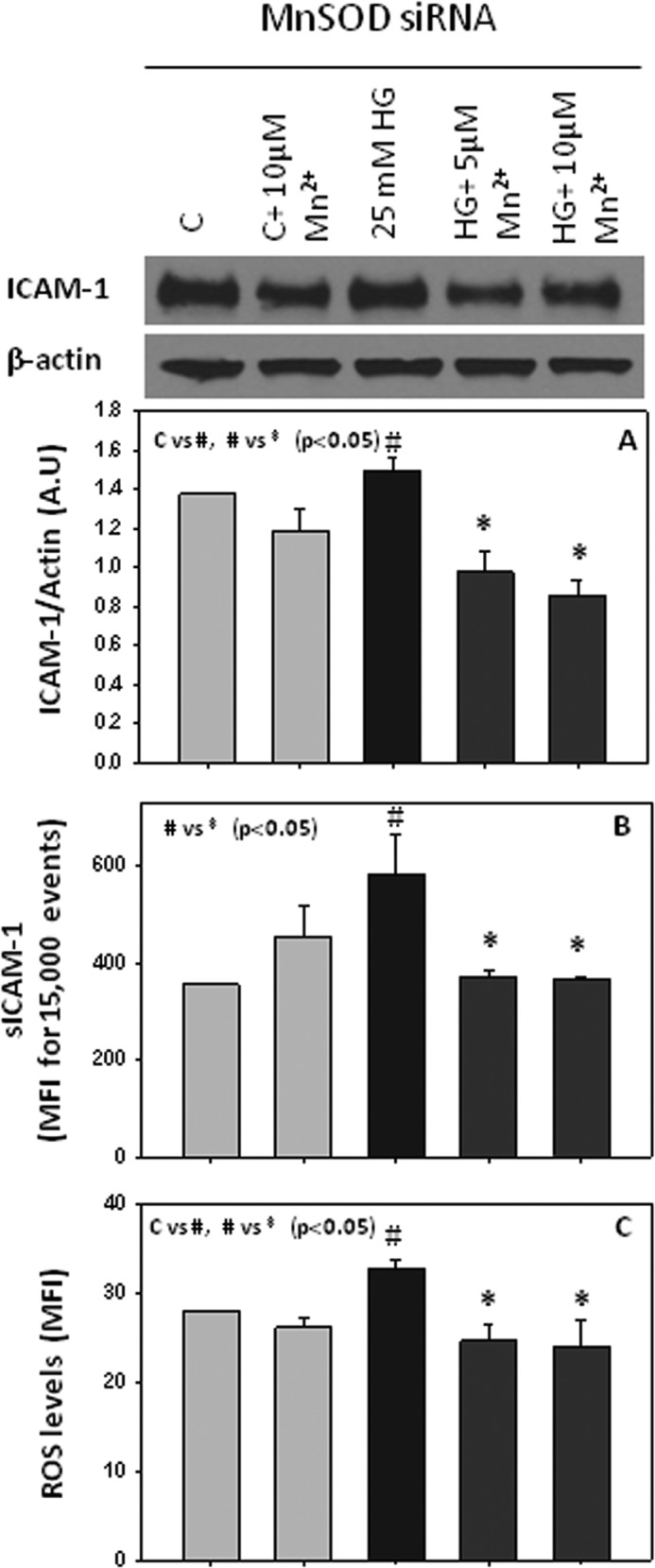
Effects of Mn2+ supplementation on ICAM-1 total expression (A), surface expression (B), and ROS (C) in HUVECs transfected with MnSOD siRNA. Values are ± S.E., n = 3. C indicates control. A. U., arbitrary units.
Effects of Mn2+ Supplementation in ZDF Rats
Elevated levels of ICAM-1 and lipids are a hallmark of endothelial cell dysfunction and are risk factors in the development of atherosclerosis. Fig. 8 shows that blood levels of ICAM-1 (A) and cholesterol (B) were significantly lower in Mn2+-supplemented when compared with placebo-supplemented ZDF rats. Fig. 8 also shows that blood levels of MCP-1 (C) and triglycerides (D) were lower but not statistically significant in Mn2+-supplemented rats when compared with placebo-supplemented rats. Further studies are needed on the effect of dose and duration of Mn2+ supplementation to determine its optimal efficacy in vivo. Nevertheless, these results demonstrate that our novel in vitro findings, that Mn2+ supplementation down-regulates ICAM-1 expression and progression of endothelial dysfunction, are validated in vivo.
FIGURE 8.
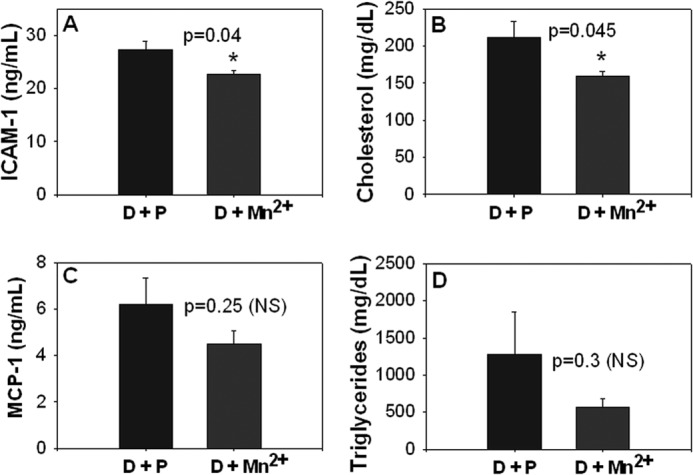
Effects of Mn2+ supplementation on plasma levels of ICAM-1 (A), cholesterol (B), MCP-1 (C), and triglycerides (D). Values are ± S.E. of placebo-supplemented rats (D + P, n = 6) and manganese-supplemented rats (D + Mn2++, n = 5).
There were no differences in body weight (345.6 g ± 7.6 versus 345 g ± 7.7), food consumption (42.4 g/day ± 2.6 versus 40.9 g/day ± 3.9), insulin (1.94 ± 0.46 versus 2.06 ± 0.36 ng/ml), blood glucose (278.4 ± 18 versus 323.3 ± 35.3 mg/dl), or glycated hemoglobin (HbA1, 17.6 ± 0.34 versus 16.5 ± 0.39%) in the Mn2+-supplemented group in comparison with the control diabetic group ZDF rats. Mn2+ supplementation did not affect hemoglobin, hematocrit, or RBC count, or markers of liver or kidney functions in diabetic rats, as assessed by alanine aminotransferase and aspartate aminotransferase, or creatinine levels.
DISCUSSION
Complications of atherosclerosis cause most morbidity and mortality in patients with diabetes (24). More than 25 million persons in the United States have at least one clinical manifestation of atherosclerosis (25). The key early event in the development of atherosclerosis is dysfunction of the endothelium, which is characterized by increased expression of cellular adhesion molecules, such as ICAM-1, and secretion of chemokines such as MCP-1. These events lead to the recruitment of monocytes to the arterial wall where they become macrophages and initiate chronic inflammation, leading to hyperlipidemia and atherosclerotic lesion development.
Various studies report blood concentrations of manganese between 0.15 and 7 μm (7, 8, 26, 27). Some studies have reported lower Mn2+ levels in lymphocytes (7), plasma, and hair samples (8) of type 2 diabetic patients and in the heart and aorta tissue of patients with atherosclerosis when compared with those of healthy controls (9). Manganese supplementation has been shown to cause a decrease in total serum cholesterol and aorta cholesterol and regression of atherosclerosis in cholesterol-fed rabbits (10). However, there is no evidence showing a direct beneficial role of Mn2+ supplementation on endothelial function and vascular inflammation, and the mechanisms by which Mn2+ can reduce cholesterol levels are unknown. MCP-1 is a chemokine that promotes the recruitment of monocytes and macrophages to the subendothelial cell layer. Deposition of lipids within these monocytes and macrophages then leads to development of atherosclerotic lesions. MCP-1 is also produced after induction of oxidative stress or growth factors by a variety of cell types, including monocytes, smooth muscle cells, and endothelial cells, and plays an important role in vascular inflammation and atherosclerotic lesion formation (28–31). Mn2+ is a potent antioxidant; it is the cofactor of the enzyme MnSOD, the main antioxidant enzyme in the mitochondria, and can also scavenge oxygen radicals itself. Various studies using Mn2+ link its effects with the function and role of MnSOD (4, 6).
This study demonstrates for the first time that Mn2+ supplementation down-regulates ICAM-1 expression, reduces ROS production, MCP-1 secretion, and monocyte adhesion in endothelial cells exposed to high glucose, and lowers blood levels of ICAM-1 and cholesterol in ZDF rats. In addition, our study provides a molecular mechanism for the beneficial effects of Mn2+ supplementation on lowering vascular inflammation markers in HUVECs and ZDF rats. We found that Mn2+ supplementation inhibits secretion of MCP-1 in endothelial cells and adipocyte cells and that the MCP-1-induced up-regulation of ICAM-1 expression and monocyte adhesion to endothelial cells can be inhibited with Mn2+ supplementation. Interestingly, Mn2+ supplementation also inhibited both the ICAM-1 down-regulation and the monocyte adhesion induced by exogenous MCP-1 treatment in endothelial cells. This demonstrates that the inhibition of MCP-1 secretion caused by Mn2+ supplementation may mediate the down-regulation of ICAM-1 expression and monocyte adhesion in endothelial cells.
Hyperglycemia is known to increase oxidative stress and glycation of protein (32–34). In this study, we observed that ROS, which was inhibited by Mn2+ supplementation, was increased in HG-treated HUVEC cells. Also, the decrease in MnSOD activity observed could be due to increase in oxidative stress or glycation of MnSOD. This study reports that Mn2+ supplementation increases MnSOD activity in HG-treated HUVECs. However, further investigation shows that the effect of Mn2+ on monocyte adhesion to endothelial cells is independent of MnSOD. Although activity of MnSOD increases in cells supplemented with Mn2+, similar effects of Mn2+ supplementation on monocyte adhesion to endothelial cells, inhibition of ICAM-1 and ROS levels, were observed in MnSOD knockdown cells, suggesting that MnSOD does not play a role in the decreased monocyte-endothelial cell adhesion in Mn2+-supplemented endothelial cells.
This study suggests that the effects of Mn2+ supplementation on monocyte adhesion to endothelial cells are mediated by the inhibition of ROS and ICAM-1 expression. This study also demonstrates that Mn2+ supplementation significantly lowers blood levels of ICAM-1 and cholesterol in ZDF rats. Thus, this study provides a novel molecular mechanism by which Mn2+ supplementation can prevent or delay endothelial dysfunction and atherosclerosis development using both cell culture and in vivo studies.
The findings reported in this study could have broader significance. In addition to atherosclerosis, adhesion molecules such as ICAM-1 are also implicated in the progression of infection (35–37). A recent study reported that manganese blocks intracellular trafficking of Shiga toxin and that manganese-supplemented mice were completely resistant to a lethal Shiga toxin challenge (38). Whether manganese inhibition of ICAM-1 expression occurs in other cell types and can play a beneficial role in preventing the progression of infection is not known and warrants investigation.
Acknowledgment
We thank Georgia Morgan for excellent editing of this manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant RO1 DK072433 through the NIDDK and the Office of Dietary Supplements. This work was also supported by the Malcolm Feist Endowed Chair in Diabetes.
- SOD
- superoxide dismutase
- ROS
- reactive oxygen species
- HUVEC
- human umbilical vein endothelial cell
- ZDF
- Zucker diabetic fatty
- HG
- high glucose
- NBT
- nitro blue tetrazolium.
REFERENCES
- 1. Fridovich I. (1997) Superoxide anion radical (O2˙̄), superoxide dismutases, and related matters. J. Biol. Chem. 272, 18515–18517 [DOI] [PubMed] [Google Scholar]
- 2. Anetor J. I., Asiribo O. A., Adedapo K. S., Akingbola T. S., Olorunnisola O. S., Adeniyi F. A. (2007) Increased plasma manganese, partially reduced ascorbate,1 and absence of mitochondrial oxidative stress in type 2 diabetes mellitus: implications for the superoxide uncoupling protein 2 (UCP-2) pathway. Biol. Trace Elem. Res. 120, 19–27 [DOI] [PubMed] [Google Scholar]
- 3. Hussain S., Ali S. F. (1999) Manganese scavenges superoxide and hydroxyl radicals: an in vitro study in rats. Neurosci. Lett. 261, 21–24 [DOI] [PubMed] [Google Scholar]
- 4. Paynter D. I. (1980) Changes in activity of the manganese superoxide dismutase enzyme in tissues of the rat with changes in dietary manganese. J. Nutr. 110, 437–447 [DOI] [PubMed] [Google Scholar]
- 5. Davis C. D., Ney D. M., Greger J. L. (1990) Manganese, iron, and lipid interactions in rats. J. Nutr. 120, 507–513 [DOI] [PubMed] [Google Scholar]
- 6. Zidenberg-Cherr S., Keen C. L., Lönnerdal B., Hurley L. S. (1983) Superoxide dismutase activity and lipid peroxidation in the rat: developmental correlations affected by manganese deficiency. J. Nutr. 113, 2498–2504 [DOI] [PubMed] [Google Scholar]
- 7. Ekmekcioglu C., Prohaska C., Pomazal K., Steffan I., Schernthaner G., Marktl W. (2001) Concentrations of seven trace elements in different hematological matrices in patients with type 2 diabetes as compared to healthy controls. Biol. Trace Elem. Res. 79, 205–219 [DOI] [PubMed] [Google Scholar]
- 8. Kazi T. G., Afridi H. I., Kazi N., Jamali M. K., Arain M. B., Jalbani N., Kandhro G. A. (2008) Copper, chromium, manganese, iron, nickel, and zinc levels in biological samples of diabetes mellitus patients. Biol. Trace Elem. Res. 122, 1–18 [DOI] [PubMed] [Google Scholar]
- 9. Volkov N. F. (1963) Cobalt, manganese, and zinc content in the blood of atherosclerosis patients. Fed. Proc. Transl. Suppl. 22, 897–899 [PubMed] [Google Scholar]
- 10. Bomb B. S., Kumawat D. C., Bomb P., Taly A. B., Bedi T., Bedi H. K. (1988) Effect of manganese on regression of atherosclerosis in cholesterol fed rabbits. J. Assoc. Physicians India 36, 149–150 [PubMed] [Google Scholar]
- 11. Peluso I., Morabito G., Urban L., Ioannone F., Serafini M. (2012) Oxidative stress in atherosclerosis development: the central role of LDL and oxidative burst. Endocr. Metab. Immune Disord. Drug Targets 12, 351–360 [DOI] [PubMed] [Google Scholar]
- 12. Panee J. (2012) Monocyte chemoattractant protein 1 (MCP-1) in obesity and diabetes. Cytokine 60, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rains J. L., Kanikarla-Marie P., Jain S. K. (2012) Hyperketonemia induces upregulation of LFA-1 in monocytes, which is mediated by ROS and p38 MAPK activation. Can. J. Physiol. Pharmacol. 90, 1642–1646 [DOI] [PubMed] [Google Scholar]
- 14. Malthankar G. V., White B. K., Bhushan A., Daniels C. K., Rodnick K. J., Lai J. C. (2004) Differential lowering by manganese treatment of activities of glycolytic and tricarboxylic acid (TCA) cycle enzymes investigated in neuroblastoma and astrocytoma cells is associated with manganese-induced cell death. Neurochem. Res. 29, 709–717 [DOI] [PubMed] [Google Scholar]
- 15. Isaac A. O., Kawikova I., Bothwell A. L., Daniels C. K., Lai J. C. (2006) Manganese treatment modulates the expression of peroxisome proliferator-activated receptors in astrocytoma and neuroblastoma cells. Neurochem. Res. 31, 1305–1316 [DOI] [PubMed] [Google Scholar]
- 16. Sun Y., Oberley L. W., Li Y. (1988) A simple method for clinical assay of superoxide dismutase. Clin. Chem. 34, 497–500 [PubMed] [Google Scholar]
- 17. Rains J. L., Jain S. K. (2011) Hyperketonemia increases monocyte adhesion to endothelial cells and is mediated by LFA-1 expression in monocytes and ICAM-1 expression in endothelial cells. Am. J. Physiol. Endocrinol. Metab. 301, E298–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Manna P., Jain S. K. (2011) Hydrogen sulfide and l-cysteine increase phosphatidylinositol 3,4,5-trisphosphate (PIP3) and glucose utilization by inhibiting phosphatase and tensin homolog (PTEN) protein and activating phosphoinositide 3-kinase (PI3K)/serine/threonine protein kinase (AKT)/protein kinase Cζ/λ (PKCζ/λ) in 3T3l1 adipocytes. J. Biol. Chem. 286, 39848–39859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Son E. W., Lee S. R., Choi H. S., Koo H. J., Huh J. E., Kim M. H., Pyo S. (2007) Effects of supplementation with higher levels of manganese and magnesium on immune function. Arch. Pharm. Res. 30, 743–749 [DOI] [PubMed] [Google Scholar]
- 20. Bae Y. J., Choi M. K., Kim M. H. (2011) Manganese supplementation reduces the blood cholesterol levels in ca-deficient ovariectomized rats. Biol. Trace Elem. Res. 141, 224–231 [DOI] [PubMed] [Google Scholar]
- 21. Metais C., Forcheron F., Abdallah P., Basset A., Del Carmine P., Bricca G., Beylot M. (2008) Adiponectin receptors: expression in Zucker diabetic rats and effects of fenofibrate and metformin. Metabolism 57, 946–953 [DOI] [PubMed] [Google Scholar]
- 22. Forcheron F., Basset A., Abdallah P., Del Carmine P., Gadot N., Beylot M. (2009) Diabetic cardiomyopathy: effects of fenofibrate and metformin in an experimental model – the Zucker diabetic rat. Cardiovasc. Diabetol. 8, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holm A. M., Johansen P. B., Ahnfelt-Rønne I., Rømer J. (2004) Adipogenic and orexigenic effects of the ghrelin-receptor ligand tabimorelin are diminished in leptin-signalling-deficient ZDF rats. Eur. J. Endocrinol. 150, 893–904 [DOI] [PubMed] [Google Scholar]
- 24. Beckman J. A., Creager M. A., Libby P. (2002) Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA 287, 2570–2581 [DOI] [PubMed] [Google Scholar]
- 25. Faxon D. P., Creager M. A., Smith S. C., Jr., Pasternak R. C., Olin J. W., Bettmann M. A., Criqui M. H., Milani R. V., Loscalzo J., Kaufman J. A., Jones D. W., Pearce W. H. (2004) Atherosclerotic Vascular Disease Conference: Executive Summary: Atherosclerotic Vascular Disease Conference proceeding for healthcare professionals from a special writing group of the American Heart Association. Circulation 109, 2595–2604 [DOI] [PubMed] [Google Scholar]
- 26. Walter R. M., Jr., Uriu-Hare J. Y., Olin K. L., Oster M. H., Anawalt B. D., Critchfield J. W., Keen C. L. (1991) Copper, zinc, manganese, and magnesium status and complications of diabetes mellitus. Diabetes Care 14, 1050–1056 [DOI] [PubMed] [Google Scholar]
- 27. Nahar Z., Azad M. A., Rahman M. A., Rahman M. A., Bari W., Islam S. N., Islam M. S., Hasnat A. (2010) Comparative analysis of serum manganese, zinc, calcium, copper, and magnesium level in panic disorder patients. Biol. Trace Elem. Res. 133, 284–290 [DOI] [PubMed] [Google Scholar]
- 28. Rollins B. J. (1997) Chemokines. Blood 90, 909–928 [PubMed] [Google Scholar]
- 29. Xing L., Remick D. G. (2007) Promoter elements responsible for antioxidant regulation of MCP-1 gene expression. Antioxid. Redox. Signal. 9, 1979–1989 [DOI] [PubMed] [Google Scholar]
- 30. Lefer D. J., Granger D. N. (1999) Monocyte rolling in early atherogenesis: Vital role in lesion development. Circ. Res. 84, 1353–1355 [DOI] [PubMed] [Google Scholar]
- 31. Egashira K. (2003) Molecular mechanisms mediating inflammation in vascular disease: Special reference to monocyte chemoattractant protein-1. Hypertension 41, 834–841 [DOI] [PubMed] [Google Scholar]
- 32. Jain S. K., McVie R., Duett J., Herbst J. J. (1989) Erythrocyte membrane lipid peroxidation and glycosylated hemoglobin in diabetes. Diabetes 38, 1539–1543 [DOI] [PubMed] [Google Scholar]
- 33. Jain S. K. (1989) Hyperglycemia can cause membrane lipid peroxidation and osmotic fragility in human red blood cells. J. Biol. Chem. 264, 21340–21345 [PubMed] [Google Scholar]
- 34. Jain S. K., Palmer M. (1997) The effect of oxygen radicals metabolites and vitamin E on glycosylation of proteins. Free Radic. Biol. Med. 22, 593–596 [DOI] [PubMed] [Google Scholar]
- 35. Bonazzi M., Cossart P. (2011) Impenetrable barriers or entry portals? The role of cell-cell adhesion during infection. J. Cell Biol. 195, 349–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Golias C., Batistatou A., Bablekos G., Charalabopoulos A., Peschos D., Mitsopoulos P., Charalabopoulos K. (2011) Physiology and pathophysiology of selectins, integrins, and IgSF cell adhesion molecules focusing on inflammation. A paradigm model on infectious endocarditis. Cell Commun. Adhes. 18, 19–32 [DOI] [PubMed] [Google Scholar]
- 37. Khan A. G., Pickl-Herk A., Gajdzik L., Marlovits T. C., Fuchs R., Blaas D. (2010) Human rhinovirus 14 enters rhabdomyosarcoma cells expressing ICAM-1 by a clathrin-, caveolin-, and flotillin-independent pathway. J. Virol. 84, 3984–3992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mukhopadhyay S., Linstedt A. D. (2012) Manganese blocks intracellular trafficking of shiga toxin and protects against Shiga toxicosis. Science 335, 332–335 [DOI] [PMC free article] [PubMed] [Google Scholar]