Background: Estrogen modulates synaptic plasticity in the hippocampus.
Results: GPR30 (an estrogen-sensitive GPCR) localizes to dendritic spines, interacts with PSD-95, and can associate with other GPCRs. PSD-95 increases GPR30 membrane levels.
Conclusion: GPR30 localization to dendritic spines would allow for estrogen modulation at synapses.
Significance: This is the first report to demonstrate a GPR30 association with other post-synaptic proteins.
Keywords: Estrogen, G-Protein-coupled Receptors (GPCR), Neuroendocrinology, Protein-Protein Interactions, Steroid Hormone Receptor, CRHR1, GPR30, PDZ Domain, PSD-95, Dendritic Spine
Abstract
The estrogen 17β-estradiol (E2) modulates dendritic spine plasticity in the cornu ammonis 1 (CA1) region of the hippocampus, and GPR30 (G-protein coupled estrogen receptor 1 (GPER1)) is an estrogen-sensitive G-protein-coupled receptor (GPCR) that is expressed in the mammalian brain and in specific subregions that are responsive to E2, including the hippocampus. The subcellular localization of hippocampal GPR30, however, remains unclear. Here, we demonstrate that GPR30 immunoreactivity is detected in dendritic spines of rat CA1 hippocampal neurons in vivo and that GPR30 protein can be found in rat brain synaptosomes. GPR30 immunoreactivity is identified at the post-synaptic density (PSD) and in the adjacent peri-synaptic zone, and GPR30 can associate with the spine scaffolding protein PSD-95 both in vitro and in vivo. This PSD-95 binding capacity of GPR30 is specific and determined by the receptor C-terminal tail that is both necessary and sufficient for PSD-95 interaction. The interaction with PSD-95 functions to increase GPR30 protein levels residing at the plasma membrane surface. GPR30 associates with the N-terminal tandem pair of PDZ domains in PSD-95, suggesting that PSD-95 may be involved in clustering GPR30 with other receptors in the hippocampus. We demonstrate that GPR30 has the potential to associate with additional post-synaptic GPCRs, including the membrane progestin receptor, the corticotropin releasing hormone receptor, and the 5HT1a serotonin receptor. These data demonstrate that GPR30 is well positioned in the dendritic spine compartment to integrate E2 sensitivity directly onto multiple inputs on synaptic activity and might begin to provide a molecular explanation as to how E2 modulates dendritic spine plasticity.
Introduction
17β-Estradiol (E2)2 is the predominant circulating estrogen and modulates dendritic spine plasticity in the hippocampus (1). This modulation is due in part to the non-genomic actions by E2 in hippocampal neurons. These non-genomic actions of E2 are often localized within the dendritic spines and are likely to underlie the neuronal remodeling observed in E2-driven synaptic plasticity as well as how E2 might influence responses to other synaptic inputs (2).
Recently, the E2-sensitive G protein-coupled receptor (GPCR) GPR30 (GPCR estrogen receptor 1 (GPER1)) has been identified and characterized in breast cancer cell lines (3, 4) as well as in multiple tissue types (5). Importantly, GPR30-immunoreactivity (IR) has been detected in the mammalian brain (6), including in E2-sensitive regions such as the hypothalamus (7) and in the hippocampal formation (8–10).
At the subcellular level, GPR30-IR has been identified with the plasma membrane (10, 11) as well as with intracellular structures such as the endoplasmic reticulum (12). Because GPR30 regulates non-genomic actions that are relevant to modulating synaptic function (13–15), it is important to establish if GPR30-IR can be identified within the dendritic spine structures and to identify the molecular mechanisms involved in such subcellular positioning.
Analysis of the GPR30 amino acid sequence reveals that the GPR30 C-terminal (CT) domain presents a Class I post-synaptic density-95 (PSD)-95/Disc large tumor suppressor (Dlg1)/Zonula occludens (ZO)-1 (PDZ) ligand (16, 17). However, the function of this GPR30-CT tail domain has not yet been determined (18). Other GPCRs that contain similar CT PDZ ligand sequences have been described previously with the capacity to associate with PDZ-containing proteins such as the dendritic spine scaffolding protein PSD-95 (19).
If GPR30 is capable of a specific interaction with PSD-95, then this interaction would explain how GPR30 might be targeted to the PSD. Furthermore, because PSD-95 scaffolding functions to assemble larger receptorsome complexes at the synapse (20, 21), a GPR30 association with PSD-95 would suggest that GPR30 might be able to interact with other proteins at the synapse as well.
Here, we identify GPR30-IR within the dendritic spine of CA1 neurons and at the PSD, and we determine that GPR30 can interact specifically with PSD-95. Functionally, the GPR30 interaction with PSD-95 increased the amount of GPR30 protein that can be isolated from the plasma membrane. This interaction with PSD-95 is also dependent on the GPR30-CT, which is necessary and sufficient for PSD-95 binding capacity. We further demonstrate that GPR30 is able to associate with other receptors that can be found in the dendritic spine.
These studies place GPR30 in the dendritic spine and demonstrate that GPR30 has the potential to associate with dendritic spine protein complexes that would integrate E2 sensitivity with diverse neuromodulating inputs to the synapse.
EXPERIMENTAL PROCEDURES
Animals and Tissue Preparation
Adult female Sprague-Dawley rats (225–250 g upon arrival; ∼60 days old) were from Charles River Laboratories and housed in groups of three with ad libitum access to food and water. All methods were approved by the Weill Medical College of Cornell University Institutional Animal Care and Use Committee (IACUC) as well as by The Rockefeller University IACUC and conform to the National Institutes of Health guidelines. Estrous cycle phases were determined as reported previously (22). Animals at both pro- and diestrus were used in the immunohistochemistry studies. Rats were deeply anesthetized with sodium pentobarbital (150 mg/kg), and brains were fixed by perfusion with 3.75% acrolein and 2% paraformaldehyde in 0.1 m phosphate buffer (pH 7.6). Brains were removed from the skull and coronally blocked, then post-fixed in 2% paraformaldehyde in phosphate buffer. Sections (40 μm thick) were prepared on a vibratome (Leica) and stored in cryoprotectant until processed later for immunohistochemistry. For whole brain isolation, animals were decapitated, and the hippocampi were dissected and rapidly frozen.
Isolation of Hippocampal Membrane Proteins
Plasma membrane protein fractions from the adult rat hippocampus were isolated and prepared by sucrose density ultracentrifugation as previously described (23). Briefly, dissected female rat hippocampi were frozen at −80 °C until needed. Tissue was weighed and homogenized in nine volumes of homogenization buffer (0.32 m sucrose, 2 mm EDTA, 2 mm EGTA, 20 mm HEPES; protease and phosphatase inhibitors were added fresh). Samples were centrifuged for 10 min at 500 × g to remove the nuclear fraction, and supernatants were centrifuged at 31k × g for 10 min at 4 °C. The pellet, which includes the plasma membrane fraction, was resuspended in hypo-osmotic buffer (5 mm Tris-HCl (pH 7.4)) and lysed on ice for 30 min. The lysate was gently combined with 48% sucrose to a final concentration of 34% sucrose. To this, 28.5% sucrose was layered followed with 0.32 m sucrose. Samples were then ultracentrifuged in a swinging bucket rotor at 60k × g for 110 min at 4 °C. The plasma membrane fraction settles at the 34–28.5% interface and was removed. The fraction was pelleted, resuspended in 1× PBS (pH 7.4), and stored at −80 °C. A280 spectrophotometry (NanoDrop) was used to determine protein concentration.
Electron Microscopy and Ultra-structural Analysis
Electron microscopy (EM) and ultra-structural analyses were as previously described (22). Briefly, single-labeled immuno-peroxidase activity was processed first for immuno-histochemistry using an avidin-biotin complex (Vector Laboratories). Ultrathin sections (70–72 nm thick) from the stratum radiatum of the CA1 region were prepared on a UCT ultratome (Leica), counterstained, and then examined with a Tecnai Biotwin transmission electron microscope (FEI), and images were captured with a digital camera (Advanced Microscopy Techniques). Immuno-peroxidase labeling was distinguished as an electron-dense reaction product precipitate. Profiles were identified using morphological criteria previously established (24). Micrograph panels of dendritic spine profiles were assembled with Photoshop software (Adobe Systems, Inc).
Cells
COS-7 cells and MCF-7 cells were maintained in DMEM (Invitrogen) supplemented with 10% fetal bovine serum (FBS, Sigma) and 1% PenStrep (Invitrogen). For transfections, COS-7 cells were plated in antibiotic-free DMEM with 10% FBS and transfected with Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. Rat brain synaptosome preparations (Upstate-Millipore) correspond to the particulate fraction that is collected by the centrifugation of nuclei-free rat brain microsomal membrane homogenates and are routinely tested by the manufacturer for post-synaptic membrane proteins. According to the manufacturer, the protocol was similar to other known protocols for synaptosomally enriched protein lysates (25), as rat brain tissue was homogenized in 0.25 m sucrose and then centrifuged at 6k × g to remove the nuclei and other large cellular debris. The supernatant was then centrifuged again at 45k × g to pellet microsomal and synaptosomal proteins.
Antibodies
Rabbit anti-GPR30 (C-term) polyclonal antibody was from MBL International. GPR30 antibody specificity was demonstrated by immunoblotting on both endogenous protein material as well as exogenous protein material expressed in heterologous cells (data not shown).
Anti-Myc rabbit monoclonal antibody (mAb), anti-HA mouse mAb, peroxidase conjugated anti-HA mouse mAb, rabbit anti-FLAG polyclonal antibody, and rabbit anti-β-actin polyclonal antibody are from Cell Signaling Technology. Anti-glutathione S-transferase (GSTπ) rabbit polyclonal antibody was from MBL International. EZview agarose-conjugated Protein A, EZview agarose-conjugated rabbit anti-Myc polyclonal antibody, and EZview agarose-conjugated mouse anti-HA mAb were from Sigma.
Mouse anti-PSD-95 mAb was from NeuroMabs (UC Davis/National Institutes of Health NeuroMab Facility). Rabbit anti-PSD-95 mAb (clone D74D3) was from Cell Signaling Technology.
Plasmid Constructs
The rat GPR30 coding sequence (cds) and the murine GPR30 cds were cloned by RT-PCR from rat and mouse brain first strand cDNA preparations, respectively. The rat GPR30 cds with the endogenous stop codon intact was subcloned into (ppt)3xFLAG(FL)-myc-CMV25 (Sigma) to generate the expression construct (ppt)3xFL-rGPR30. The mouse GPR30 cds with the endogenous stop codon intact was subcloned into pDisplay (Invitrogen) to generate (Igκ)HA-mGPR30, which contains the Igκ leader sequence. A point mutation designed in PCR subcloning primers was used to mutate the terminal valine (V) codon into an alanine (A) codon in the mouse GPR30 cds, and this mutant full-length GPR30 cds was also subcloned into pDisplay to generate (Igκ)HA-mGPR30375A.
A single HA epitope tag was inserted into pEGFP-C1 (Clontech) to generate the vector HA-eGFP-C1. The mouse GPR30 C-terminal tail cds was subcloned into the vector HA-eGFP-C1 to generate the expression construct HA-eGFP-mGPR30(CT). Point mutations were designed in PCR primers to generate the expression construct HA-eGFP-mGPR30(CT)375A, where the terminal V codon was again mutated into an A codon.
The cds to the N terminus of neuronal NOS (nNOS), amino acids 1–159, was subcloned into pCMV-HA (Clontech) to generate the construct HA-nNOS(159). The rat PSD-95 cds and the rat spinophilin (SPL) coding sequences were cloned by RT-PCR from a rat brain cDNA preparation and then subcloned into pCMV-Myc (Clontech) to generate Myc-tagged PSD-95 protein (Myc-PSD95) and Myc-SPL, respectively. The rat PSD-95 cds was also subcloned into pCMV-HA to generate HA-PSD95 and into p3xFLAG-CMV-7.1 (Sigma) to generate 3xFL-PSD95.
The rat PSD-95 N-terminal tandem PDZ domains PDZ1 and PDZ2 (amino acids 59–303) and the third PDZ domain of PSD-95 (amino acids 307–446) were isolated by PCR and subcloned into pCMV-Myc to generate the expression constructs Myc-PDZ(1 + 2) and Myc-PDZ(3), respectively. The eGFP cds was subcloned by PCR from pEGFP-N1 (Clontech) and into p3xFLAG-CMV-7.1 to generate the expression construct 3xFL-eGFP.
The rat progestin membrane receptor-β (PMRβ) expression construct (26) was kindly provided by Dr. Carlos Stocco (Yale University). The rat corticotropin releasing hormone receptor-1 (CRHR1) with an internal FLAG epitope tag insert (27) was kindly provided by Dr. Greti Aguilera (National Institutes of Health). The human 5-HT1a serotonin receptor (5HT1aR) was purchased from the Missouri S&T UMR cDNA Resource Center.
The PMRβ, FL-CRHR1, and 5HT1aR cds were each subcloned into the vector (ppt)3xFL-myc-CMV25 to generate the expression constructs (ppt)3xFL-rPMRβ, (ppt)4xFL-rCRHR1, and (ppt)3xFL-human 5HT1aR, respectively. Additionally, the rat CRHR1 CT tail was isolated by RT-PCR. The CRHR1-CT cds was then subcloned into the pGFLEX mammalian expression vector (ATCC) for chimeric expression with an N-terminal GSTπ tag. This resulted in the expression construct GSTπ-CRHR1(CT). All expression constructs described above were first validated by bidirectional sequencing and then tested for exogenous protein expression by immunoblotting against the epitope tag or by the recombinant antigen.
Co-immunoprecipitation
48 h post-transfection, whole cell lysates were prepared in an n-dodecyl-β-maltoside (DDM) detergent and cholesterol hemisuccinate (CHS)-based lysis buffer (50 mm Tris, 150 mm NaCl, 1% DDM, 0.1% CHS, 10% glycerol, protease inhibitors). DDM and CHS were both purchased from Anatrace-Affymetrix.
Briefly, cells were washed once in ice-cold PBS and then lysed in 1× DDM-CHS lysis buffer on ice for 10 min. Lysates were scrape-collected and rotated for an additional 20 min and then centrifuged for 30 min at 21k × g to clarify. Clarified lysates were precleared by with agarose-conjugated IgG (Sigma) of the appropriate species background. Equal volumes per sample of clarified and cleared lysates were then used for co-immunoprecipitation (IP) assays with overnight rotations including EZview red agarose-conjugated antibodies (Sigma) that had been pre-equilibrated in lysis buffer.
After IP, the agarose-conjugated antibodies were washed stringently 5 times in wash buffer (50 mm Tris, 150 mm NaCl, 1% DDM, 0.05% CHS, 10% glycerol). Pelleted antibodies were then resuspended in an equal volume of 2× Laemmli sample buffer (Bio-Rad) with β-mercaptoethanol (Sigma) to a final concentration of 5% to elute proteins and frozen before analyses. All co-IP procedures were performed in a 4 °C cold room.
To determine any in vivo interactions, plasma membrane fractions collected from female adult rat hippocampi by sucrose density gradient ultracentifugation as described above was incubated overnight with either anti-PSD-95 antibody or with mouse IgG alone. Briefly, 150 μg of total protein was resuspended in DDM-CHS lysis buffer to a final volume of 1.5 ml. This volume was then split in half, and 10 μg of mouse anti-PSD-95 mAb or 10 μg mouse IgG was added to either split. Samples were incubated with gentle rotations at 4 °C for 4 h, then 30 μl of EZview Red-conjugated Protein A was added to each tube. Incubations continued overnight, and samples were washed and eluted as described above for recombinant protein interactions.
Immunoblotting
Whole cell lysates, synaptosomal lysates, and recombinant GPR30 co-immunoprecipitation samples were heated at 37 °C for 5 min. Membrane fraction GPR30 co-immunoprecipitation samples were heated at 42 °C for 10 min. Extended incubation times at 37 °C or 42 °C, higher temperatures over 50 °C, or multiple freeze-thaws on samples proved to be detrimental to any potential GPR30 Western blot, as extensive denatured banding patterns resulted; 37 °C incubation was optimal.3
Proteins were resolved on 4–20% gradient SDS-PAGE gels (Bio-Rad) and transferred to Immobilon-P PVDF membranes (Millipore). Membranes were blocked and then incubated in primary antibody overnight at 4 °C. For HA-tag immunoblotting, the primary antibody used was a peroxidase-conjugated anti-HA mouse mAb (Cell Signaling Technology), and no secondary antibody was used. For all other immunoblots, peroxidase-conjugated goat anti-rabbit IgG secondary antibody (Jackson ImmunoResearch) was used. Visualization was achieved by West Femto enhanced chemiluminescence (Pierce). For visualizing the rat hippocampus co-immunoprecipitations, the CleanBlot reagent (Pierce) was used. Films were scanned by VueScan software (Hamrick Software), and images were assembled with Photoshop software.
Surface Protein Biotinylation
For GPR30 and PSD-95, COS-7 cells were transfected as indicated. 24 h post-transfection, the media were replaced with serum-free and phenol red-free DMEM. 48 h post-transfection and at least 24 h post-serum starvation, the cells were treated as indicated with either 10 nm E2 or with diluent control (DMSO, final concentration 0.0001%) at 37 °C. 10 min post-treatment, the cells were moved to 4 °C and washed twice with ice-cold PBS. The cells were then incubated for 60 min at 4 °C with 1 mg/ml membrane-impermeable EZlink Sulfo-NHS-SS-Biotin (ThermoScientific Pierce) to biotinylate all surface proteins. The cells were then washed twice with ice-cold 100 mm glycine in PBS to quench excess biotin followed by one wash with PBS alone. The cells were lysed with DDM-CHS lysis buffer (supplemented as above with fresh protease and phosphatase inhibitors), scrape-collected, and then pelleted to clarify and remove cellular debris (as above, all at 4 °C). After protein concentrations were determined, an aliquot of each biotinylated sample was reserved, and the clarified lysates were affinity-precipitated overnight at 4 °C with EZview Red agarose-conjugated streptavidin (Sigma). The agarose conjugates were washed 5 times stringently in lysis buffer and then resuspended in Laemmli sample buffer that was supplemented with 5% β-mercaptoethanol to elute. Eluted proteins were analyzed by Western blotting, and ECL-visualized bands were scanned and quantified by densitometry (GelEval software, FrogDance).
Protein levels were represented as the comparative amount of GPR30 normalized by the amount of surface GPR30 isolated from untreated GPR30-transfected COS-7 cells (which was by default defined as 1.00 for each replicate). Statistical significance (p < 0.05) was determined by unpaired T-Test (InStat software, GraphPad).
RESULTS
GPR30 Immunoreactivity Is Identified in the Dendritic Spine
GPR30 is an E2-sensitive GPCR that is expressed in the hippocampus. To identify the subcellular localization for GPR30 in vivo and to determine if GPR30 is positioned in rat CA1 hippocampal neurons, GPR30 IR was analyzed by post-embedding EM (Fig. 1).
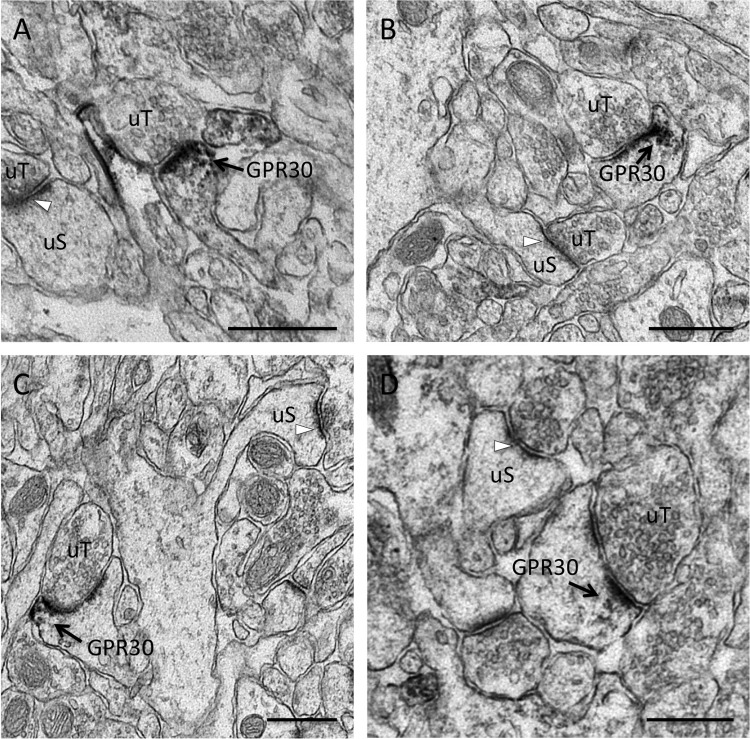
FIGURE 1.
GPR30 immunoreactivity in CA1 dendritic spine profiles. Shown are representative electron micrographs of GPR30 immunolabeling from the adult female rat CA1 hippocampus. Using pre-embedding electron microscopy, GPR30-labeled dendritic spines (GPR30) are identified by diffuse granular immuno-peroxidase reaction product and are indicated by black arrows. Unlabeled spines (uS) with unlabeled PSDs (white arrows) and unlabeled pre-synaptic terminals (uT) also are indicated. GPR30 IR is evident in multiple spine profile types, including thin spines (A), mushroom-shaped spines (B), cupped spines (C), and spines with perforated PSDs (D). GPR30-IR localized both at the PSD as well as in the peri-synaptic zone of the PSD. Panel A, at proestrus. Panels B–D, at diestrus. Bars, 500 nm.
GPR30-IR was observed in the dendritic spine compartment in vivo and in close proximity to the electron-dense PSD structure. GPR30-IR was identified in a subset of dendritic spines as some spine profiles remained unlabeled, suggesting that E2 does not act in all dendritic spines via GPR30. GPR30-IR was detected in animals at both proestrus (Fig. 1A) and at diestrus (Fig. 1, B–D).
GPR30-IR was also identified in various types of spine profiles as based on ultra-structural morphology, including thin spines (Fig. 1A), mushroom-shaped spines (Fig. 1B), concaved-shaped spines (Fig. 1C), and dendritic spines with perforated PSDs (Fig. 1D). Furthermore, immuno-peroxidase activity for GPR30-IR was evident not only along the PSD but often in the peri-synaptic zone adjacent to the PSD. Taken together, these results suggest that GPR30 labeling can be found at synapses in spines across the maturation profile (28) and across the estrus cycle as well as in specific regions within the dendritic spine compartment known for lateral protein trafficking and endocytic activity (29).
GPR30 Protein from the Rat Brain Is Co-isolated Along with Synaptosomal Proteins
In parallel with these immuno-histologic findings, microsomal membrane preparations consisting of synaptosomal proteins were also immunoblotted for GPR30 protein (Fig. 2A, upper blot). These preparations contain many synaptosomal proteins and were positive for actin (Fig. 2A, lower blot) and for the dendritic spine proteins PSD-95 and Spinophilin (Fig. 2B).
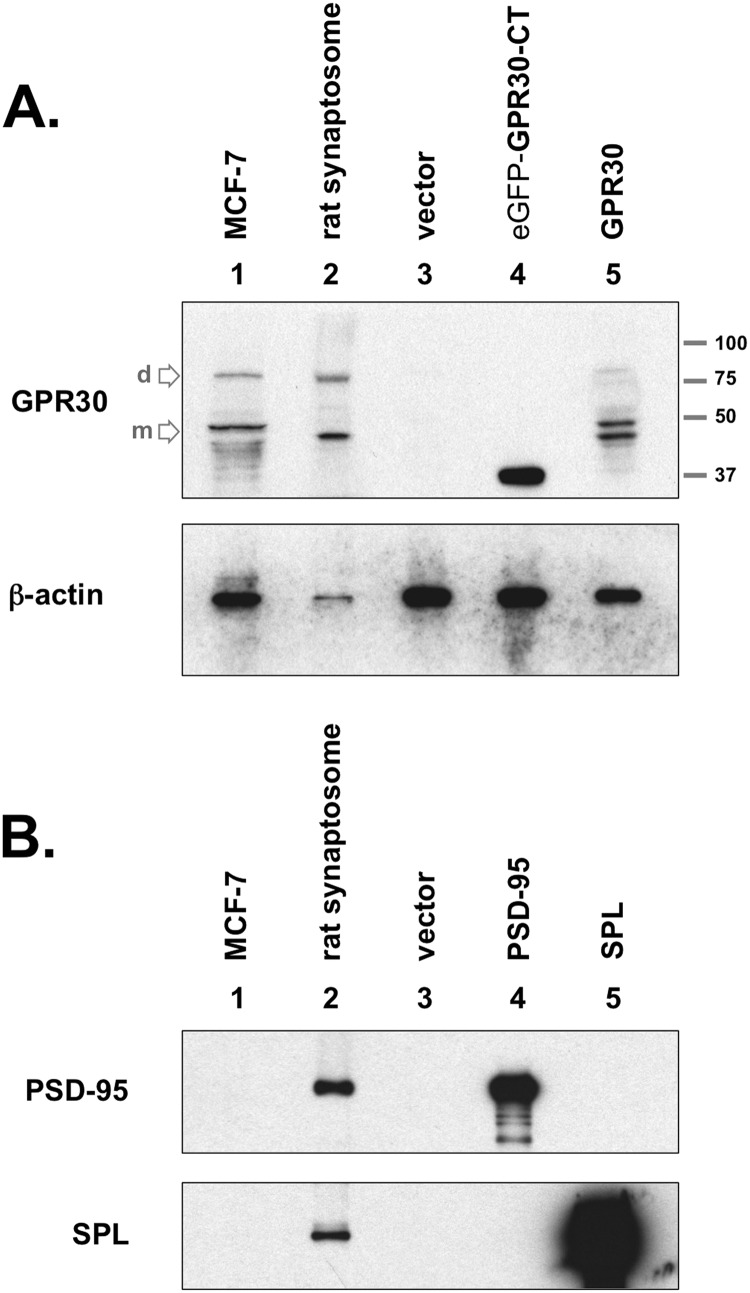
FIGURE 2.
GPR30 protein expression in the rat brain. A, upper blot, GPR30 was detected in crude rat brain synaptosome preparations (lane 2). COS-7 cells did not express GPR30, as GPR30 was not detected in empty vector-transfected COS-7 whole cell lysates (lane 3). GPR30 (C-terminal-specific) antibody recognizes the C-terminal domain of GPR30 (GPR30-CT), which was subcloned for chimeric expression with eGFP (lane 4). The rat full-length GPR30 protein are also detected when exogenously expressed in COS-7 cells (lane 5). MCF-7 whole cell lysate was used as a positive GPR30 antibody control (lane 1). The primary lower molecular weight GPR30 band is the monomeric protein state (m), and the upper secondary band at approximately twice the apparent molecular weight is likely the dimeric state (d) for the receptor. The apparent molecular weight ladder is shown on right. Lower blot, the same lysates were immunoblotted (IB) for actin protein content. Both blots are representative of five independent experiments. B, rat brain preparations contain synaptosomal proteins, including the PSD proteins PSD-95 and SPL. MCF-7 whole cell lysate (lane 1), rat brain synaptosome lysate (lane 2), or COS-7 whole cell lysates (lane 3, empty vector; lane 4, Myc-PSD95 transfected; lane 5, Myc-SPL transfected) were immunoblotted for PSD-95 protein (upper blot) or for SPL protein (lower blot). Blots are representative of three independent experiments.
GPR30 protein was identified in MCF-7 whole cell lysate and in the synaptosomal preparations (Fig. 2A, lanes 1 and 2) but not in COS-7 whole cell lysate (Fig. 2A, lane 3). When transfected with an expression construct encoding for the GPR30 C terminus (GPR30-CT), COS-7 cell lysates were GPR30-CT antigen-positive (Fig. 2A, lanes 4 and 5). When full-length GPR30 was detected, a predominant band was visualized at ∼40–50 kDa in apparent molecular mass but also a secondary band that migrated at approximately twice the apparent molecular mass (80–90 kDa). This secondary band potentially represents a stable, homodimeric structure for GPR30.
GPR30 Associates with PSD-95
PSD-95 is a scaffolding protein that is enriched in the PSD of the dendritic spine (30). As a primary scaffolding component of the dendritic spine, PSD-95 serves to organize receptors and networked protein complexes to coordinate specific synaptic responses to incoming stimuli (20, 31). Post-synaptic GPCRs are often assembled into larger receptosome complexes via specific protein-protein interactions with PSD-95 at the PSD.
GPR30-IR within the dendritic spine and at the PSD in vivo (Fig. 1) indicates that GPR30 localizes with PSD-95 and suggests that a protein-protein interaction may exist between GPR30 and with protein scaffolds at the PSD.
Amino acid analysis of the GPR30 protein sequence reveals that the C-terminal tail of GPR30 contains a canonical class I PDZ domain binding sequence, -SSAV (17, 32), although the function of this carboxylate sequence has not yet been determined. If this terminal sequence of the GPR30 receptor can specifically interact with PDZ-containing proteins such as PSD-95, then this protein-protein interaction would biochemically substantiate the subcellular localization of GPR30 within the dendritic spine. To determine whether the full-length GPR30 protein associates with PSD-95 in a cellular context, co-IP assays were employed with a series of epitope-tagged constructs expressed in COS-7 cells.
Because the N-terminal amino acids of nNOS are known to bind to PSD-95 (33), nNOS(159), consisting of the first 159 amino acids of nNOS, was used as a positive PSD-95 co-IP control. The non-neural protein eGFP was used as a nonspecific, negative co-IP control. A hemagglutinin (HA) epitope tag was applied to the N terminus of nNOS(159) and to eGFP as well as to the murine GPR30 protein.
HA-nNOS(159), HA-eGFP, and HA-GPR30 were then each co-transfected in COS-7 cells with Myc-tagged PSD-95 (Myc-PSD95). HA-nNOS(159) and HA-GPR30 were both able to co-immunoprecipitate Myc-PSD95, but HA-eGFP (or the HA empty vector) were not (Fig. 3, lanes 1–4).
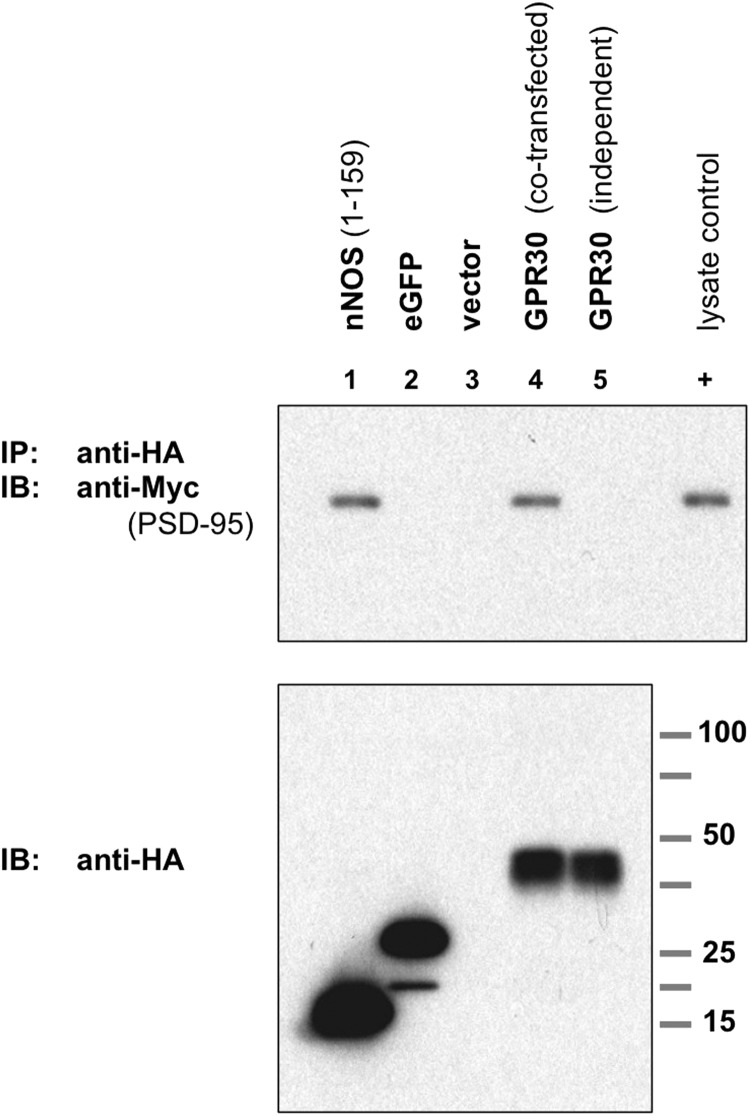
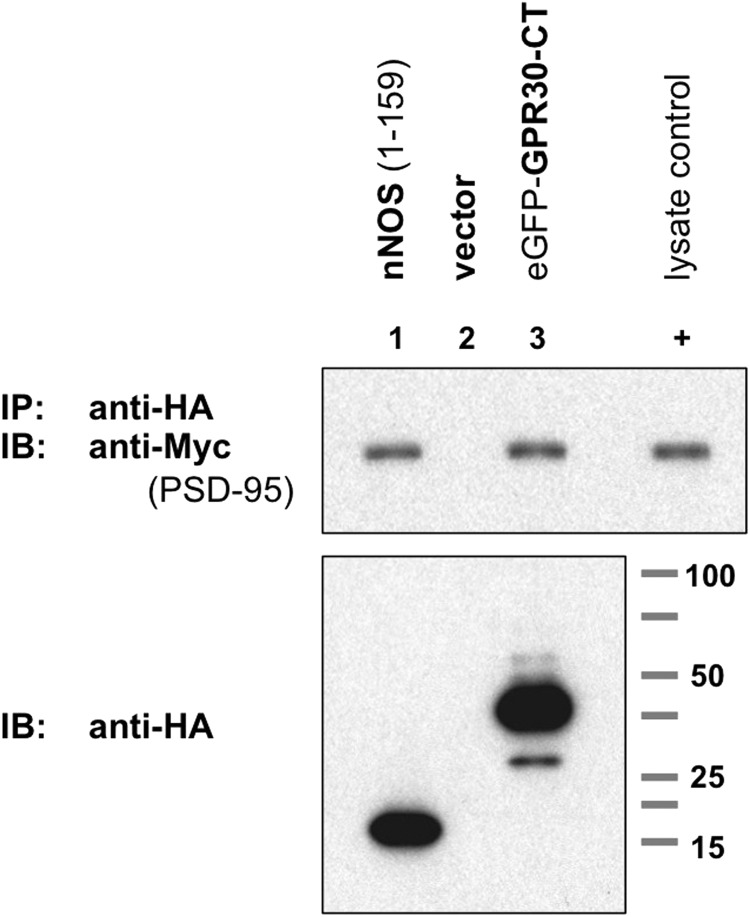
FIGURE 3.
GPR30 associates with PSD-95 and requires co-expression. Upper blot, PSD-95 co-IP with GPR30 is shown. COS-7 cells were transiently co-transfected to express Myc-PSD95 plus the lane-indicated HA-tagged proteins. The N-terminal 159 amino acids (1–159) of nNOS, HA-nNOS-(1–159) (lane 1), HA-eGFP (lane 2), empty HA vector (lane 3), and HA-tagged murine GPR30 (lane 4) are shown. Additionally, cells were transfected independently with HA-GPR30 and Myc-PSD95, and whole cell lysates from both transfections were combined before immunoprecipitation. HA-tagged proteins were immunoprecipitated with an anti-HA antibody, and PSD-95 protein was detected by immunoblot (IB) with an anti-Myc antibody. HA-nNOS-(1–159) served as a PSD-95 co-IP-positive control, and HA-eGFP served as a PSD-95 co-IP negative control. When co-expressed in the same cell, GRP30 was able to associate with and to co-immunoprecipitate PSD-95 (lane 4), but when expressed independently, GPR30 was unable to co-immunoprecipitate PSD-95 (lane 5). 0.1% of whole cell lysate from Myc-PSD95-transfected COS-7 cells was also run as a positive Myc-epitope control (+). Lower blot, HA-tagged proteins in whole cell lysates. Blots are representative of five independent experiments.
Additionally, HA-GPR30 and Myc-PSD95 were individually transfected into COS-7 cells, and the resulting whole cell lysates were then combined. When GPR30 was expressed independently of PSD-95, GPR30 could not co-immunoprecipitate with PSD-95 in mixed lysates (Fig. 3, lane 5). These results suggest that the full-length GPR30 protein is able to associate with PSD-95 in vitro and that GPR30 must be co-expressed with PSD-95 within the same cell for GPR30 to interact properly with PSD-95.
GPR30 Interacts with PSD-95 in Vivo
To validate the interaction between recombinant GPR30 and PSD-95 in vivo, membrane proteins from the adult female rat hippocampus were isolated by sucrose density gradient and ultracentrifugation (23). These fractions were then used for PSD-95 co-immunoprecipitations (Fig. 4). The plasma membrane fractions were incubated with either PSD-95 antibody (Fig. 4, lane 2) or with no antibody (Fig. 4, lane 1). PSD-95 protein from the rat hippocampus was also able to co-immunoprecipitate with the GPR30 receptor, suggesting that this physical interaction at the membrane level also occurs in vivo.
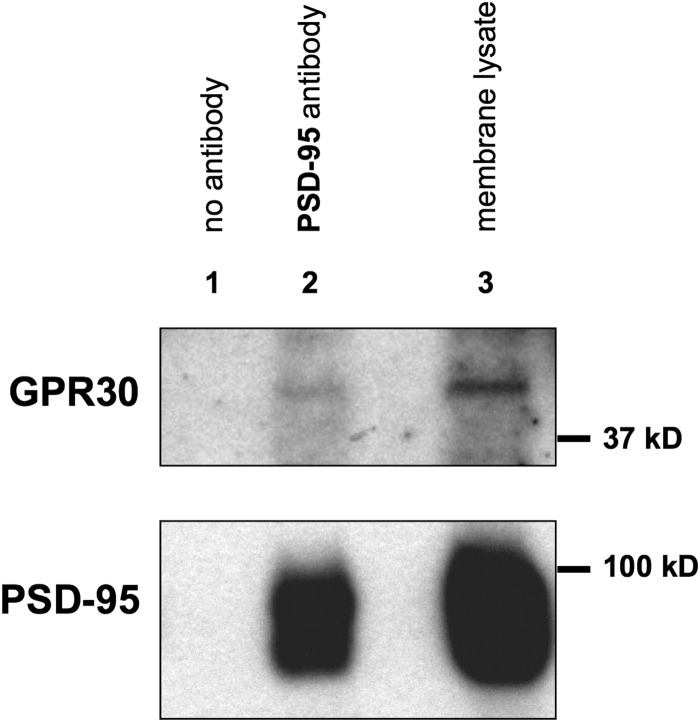
FIGURE 4.
GPR30 receptor co-immunoprecipitates in vivo with PSD-95 protein from membrane preparations isolated from the adult rat hippocampus. Whole hippocampi were dissected from adult female rats, and hippocampal membrane fractions were isolated by sucrose density gradients and high speed ultracentrifugation. Membrane lysates were incubated with either anti-PSD-95 antibody (lane 2) or with mouse IgG only (lane 1, no antibody). Immunoprecipitates were then immunoblotted with anti-GPR30 antibody (upper blot) or with anti-PSD-95 antibody (lower blot). Likewise, whole membrane lysate (1% of total protein amount used in the immunoprecipitation step in the upper blot, 0.1% total protein amount in the lower blot) was run alongside each immunoprecipitation (lane 3). GPR30 receptor co-immunoprecipitated with PSD-95 (lane 2) but not with IgG alone (lane 1). Blots are representative of three independent experiments.
The GPR30 C-terminal Tail Is Sufficient and Necessary for PSD-95 Binding Capacity
Because a PDZ ligand motif is found at the GPR30-CT, it is important to determine if the GPR30-CT is sufficient for the observed PSD-95 interaction.
GPR30-CT was expressed as a C-terminal chimeric addition to the HA-tagged eGFP protein, and when co-expressed with PSD-95, the GPR30-CT chimeric was sufficient for PSD-95 co-IP (Fig. 5, lane 3). In contrast, the eGFP protein alone did not co-immunoprecipitate with PSD-95 (Fig. 3, lane 2).
FIGURE 5.
The C-terminal tail of GPR30 is sufficient for PSD-95 association. Upper blot, PSD-95 co-immunoprecipitated with the GPR30 C-terminal tail (CT). Cells were co-transfected with Myc-PSD95 plus HA-tagged nNOS-(1–159) (lane 1), empty HA vector (lane 2), or HA-tagged eGFP-GPR30-CT, where the GPR30-CT is chimerically expressed with HA-eGFP (eGFP-GPR30-CT, lane 3). Anti-HA IP followed by anti-Myc immunoblot (IB) demonstrates that the GPR30-CT is sufficient for PSD-95 co-IP. 0.1% of whole cell lysate from Myc-PSD95-transfected COS-7 cells was also run as a positive Myc-epitope control (+). Lower blot, HA-tagged proteins in whole cell lysates are shown. Blots are representative of five independent experiments.
The specificity of a C-terminal PDZ ligand sequence is largely determined by the residues at the carboxylate 0 or −2 position (34–36), and mutations at either of these positions can disrupt the protein-protein interaction with the PDZ domain.
To determine if the GPR30 interaction is dependent on the CT, a single point mutation at the terminal GPR30 carboxylate residue was introduced. The terminal valine residue at amino acid 375 was mutated to an alanine residue (375A) on both the full-length construct as well as the abbreviated GPR30-CT chimeric.
These mutations were co-expressed with PSD-95 alongside the wild-type (wt) receptor equivalents. Both GPR30 wt and eGFP-GPR30-CT wt were able to associate with PSD-95 (Fig. 6, lanes 1 and 3), but GPR30 375A and eGFP-GPR30-CT 375A were not (Fig. 6, lanes 2 and 4).
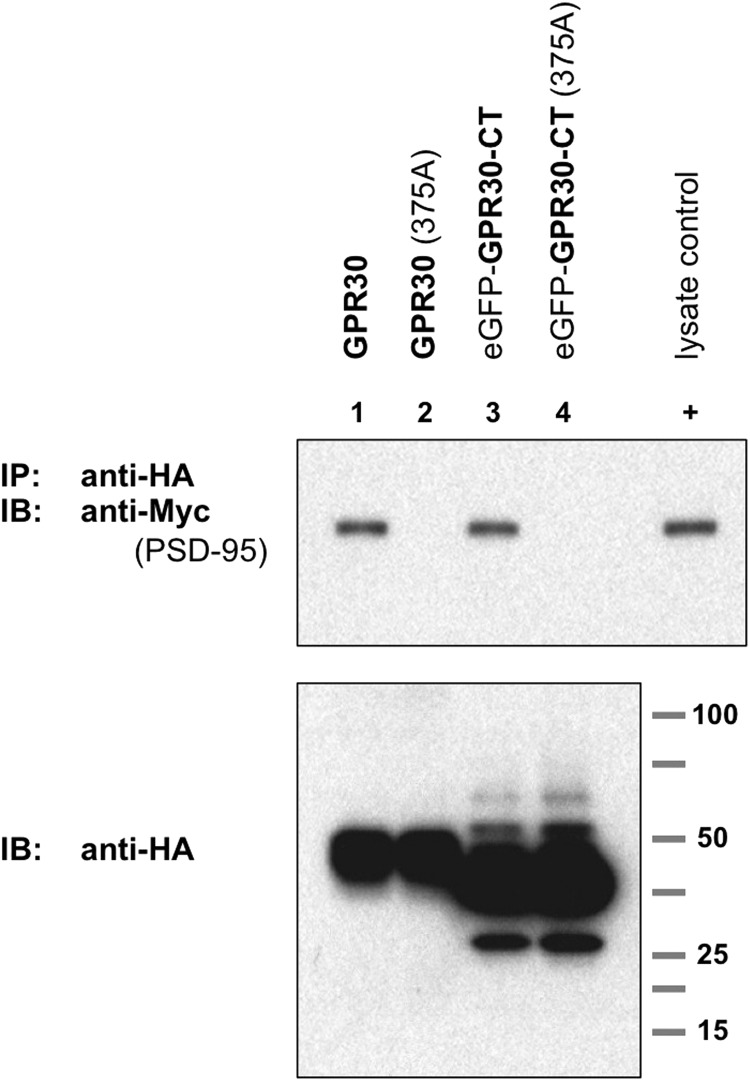
FIGURE 6.
The C-terminal tail of GPR30 is necessary for PSD-95 association. The terminal carboxylate residue in GPR30 is valine at position 375. In both the full-length GPR30 protein as well as the GPR30-CT protein, this terminal residue was mutated into an alanine residue (375A) to disrupt the PDZ domain ligand sequence. Upper blot, COS-7 cells were co-transfected for Myc-PSD95 expression plus the indicated HA-tagged proteins: lane 1, full-length wild-type GPR30; lane 2, full-length GPR30 (375A); lane 3, eGFP-GPR30-CT (wt); lane 4, eGFP-GPR30 (375A). GPR30 (wt) and eGFP-GPR30-CT(wt) were able to co-immunoprecipitate PSD-95 protein (lanes 1 and 3), but GPR30 (375A) and eGFP-GPR30-CT (375A) with disrupted PDZ ligand sequences were unable to co-immunoprecipitate PSD-95 protein (lanes 2 and 4). 0.1% of whole cell lysate from Myc-PSD95-transfected COS-7 cells was also run as a positive Myc-epitope control (+). Lower blot, HA-tagged proteins in whole cell lysates are shown. Blots are representative of five independent experiments. IB, immunoblot.
These results suggest that the GPR30-CT is not only sufficient for interacting with PSD-95 but is also necessary for any interaction with PSD-95, as a single point mutation of the terminal carboxylate residue of either the full-length GPR30 protein or the GPR30-CT eliminated the ability of GPR30 to associate with PSD-95.
GPR30 Interacts Specifically with the Tandem PDZ Domains in PSD-95
PDZ domains are protein-protein interaction domains that are often components of large, multi-domain scaffolding proteins such as PSD-95 (37).
PSD-95 contains three PDZ domains. The first two PDZ domains (PDZ(1 + 2)) are located near the N terminus of PSD-95 and are positioned in tandem proximity to one another. The third PDZ domain PDZ(3) is positioned independently within PSD-95.
The tandem PDZ orientation provides a means by which PSD-95 can cluster or stabilize dimeric receptor targets (38, 39). PDZ(3) also has the capacity to interact with certain GPCR C-termini, but this allosteric interaction may in part be regulated by PSD-95 phosphorylation (40–43), suggesting that the different PDZ domains confer different allosteric functions. Therefore, it is important to identify which PDZ domain(s) can interact with GPR30. To determine which PDZ domain(s) of PSD-95 is associated with GPR30, Myc-tagged PSD-95 and Myc-tagged PDZ domains were used for reverse co-IP assays.
HA-tagged eGFP-GPR30-CT was co-transfected with either Myc-eGFP, Myc-PSD95, Myc-PDZ(1 + 2), or Myc-PDZ(3). Full-length PSD-95 was able to associate with GPR30-CT (Fig. 7A, lane 3). PDZ(1 + 2) was able to associate with GPR30-CT (Fig. 7A, lane 4), but PDZ(3) did not (Fig. 7A, lane 5). The negative controls Myc-eGFP and empty vector also demonstrated no interaction with GPR30-CT (Fig. 7A, lanes 1 and 2). The same pattern of GPR30 co-IP was observed with full-length GPR30 as well, where the full-length GPR30 interacted with PSD-95 via the PDZ ligand, but the full-length GPR30375A mutant could not (Fig. 7B, lanes 3 and 4).
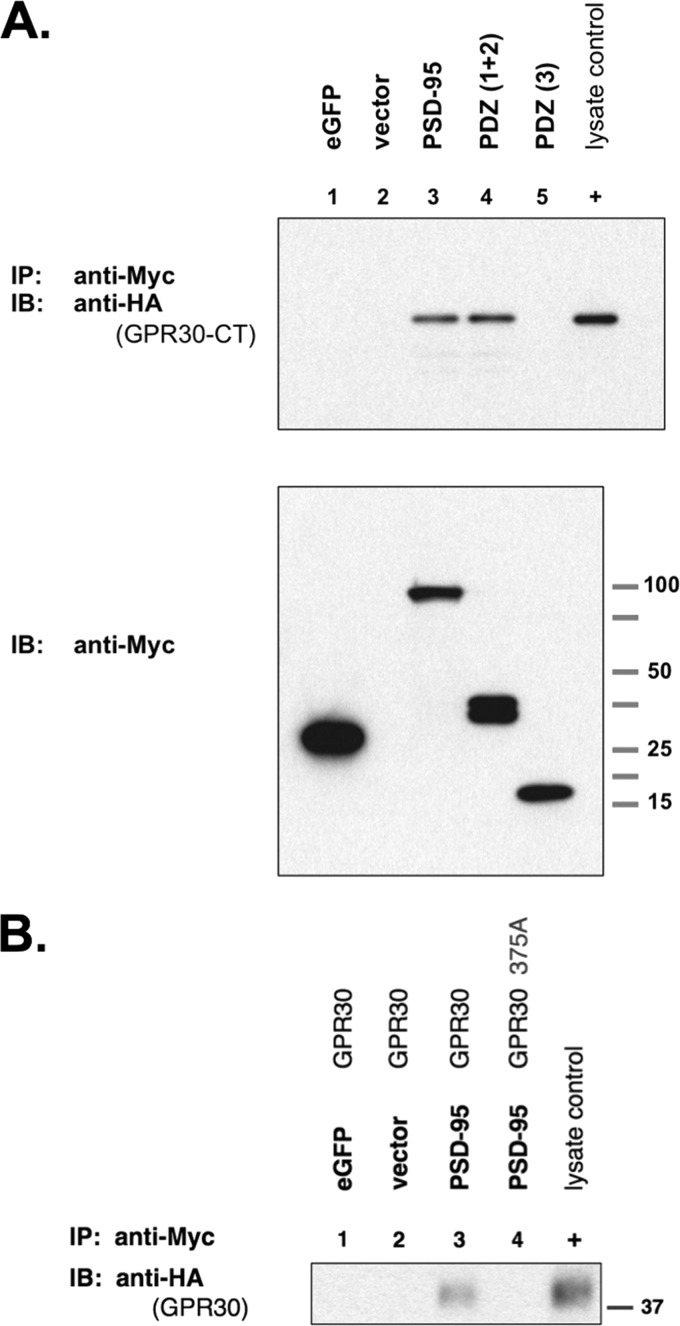
FIGURE 7.
PSD-95 associates with GPR30 via the N-terminal tandem PDZ domains. A, upper blot, GPR30 co-IP with PSD-95 is shown. COS-7 cells were co-transfected with HA-tagged eGFP-GPR30-CT plus the following Myc-tagged proteins: lane 1, eGFP; lane 2, empty Myc vector; lane 3, PSD-95; lane 4, the first two tandem PDZ domains of PSD-95, PDZ(1 + 2); lane 5, the third PDZ domain of PSD-95, PDZ(3). Whole cell lysates were collected, and Myc-tagged proteins were immunoprecipitated with an anti-Myc antibody. HA-tagged eGFP-GPR30-CT was immunoblotted with an anti-HA antibody. PSD-95 and PDZ(1 + 2) were able to co-immunoprecipitate the GPR30-CT (lanes 3 and 4), but the third PDZ(3) domain did not associate with GPR30-CT (lane 5). 0.1% of whole cell lysate from HA-eGFP-GPR30-CT-transfected COS-7 cells was also run as a positive HA-epitope control (+). Lower blot, Myc-tagged proteins in whole cell lysates are shown. Blots are representative of five independent experiments. B, full-length GPR30 can co-immunoprecipitate with PSD-95. COS-7 cells were co-transfected as indicated (lanes 1–4) with either Myc-tagged eGFP, Myc-vector, or Myc-tagged PSD-95 plus either HA-tagged full-length GPR30 or HA-tagged full-length GPR30375A mutant. Additionally, whole cell lysates prepared from HA-GPR30-transfected COS-7 cells were used as a positive lysate control (+) for immunoblotting. Myc-tagged proteins were immunoprecipitated from lysates with agarose-conjugated anti-Myc antibody, and the precipitates were then immunoblotted with a peroxidase-conjugated anti-HA antibody. Full-length GPR30 was able to co-immunoprecipitate with PSD-95 (lane 3), whereas the mutant full-length GPR30375A did not (lane 4). IB, immunoblot.
As GPR30 can associate with PSD-95 in vitro (Fig. 3) and in vivo (Fig. 4), and as PSD-95 can likewise associate with GPR30, these results would suggest that the protein-protein interaction between the GPR30 and PSD-95 is specific. Furthermore, that GPR30-CT selectively targets the tandem PDZ(1 + 2) domains suggests GPR30 may exist as either a homodimeric or a heterodimeric partner at the PSD.
GPR30 Can Potentially Associate with Other Post-synaptic GPCRs
In vitro, GPR30 demonstrated a potential for homdimerization, as a secondary band that resolved at twice the apparent molecular weight molecular weight for monomeric GPR30 was detected by immunoblotting (Fig. 2). If GPR30 has the GPCR potential to homodimerize, then it is also possible that GPR30 might heterodimerize with other GPCRs as well (44, 45).
Based on known neuroendocrine modulations by estrogens in the hippocampus (46–51), a small panel of select FLAG-tagged GPCR targets was generated as an initial step to determine if GPR30 can heterodimerically associate with other GPCRs. These included the PMRβ, CRHR1, and the 5HT1aR.
HA-GPR30 was co-expressed with each receptor and then HA was immunoprecipitated for FLAG immunoblotting. GPR30 was able to associate with FLAG-tagged PSD-95, which was used as a positive control (Fig. 8A, lane 1), but GPR30 did not associate with FLAG-tagged eGFP, which was used as a negative control (Fig. 8A, lane 2), nor did it non-specifically associate with any endogenous proteins (Fig. 8A, lane 3).
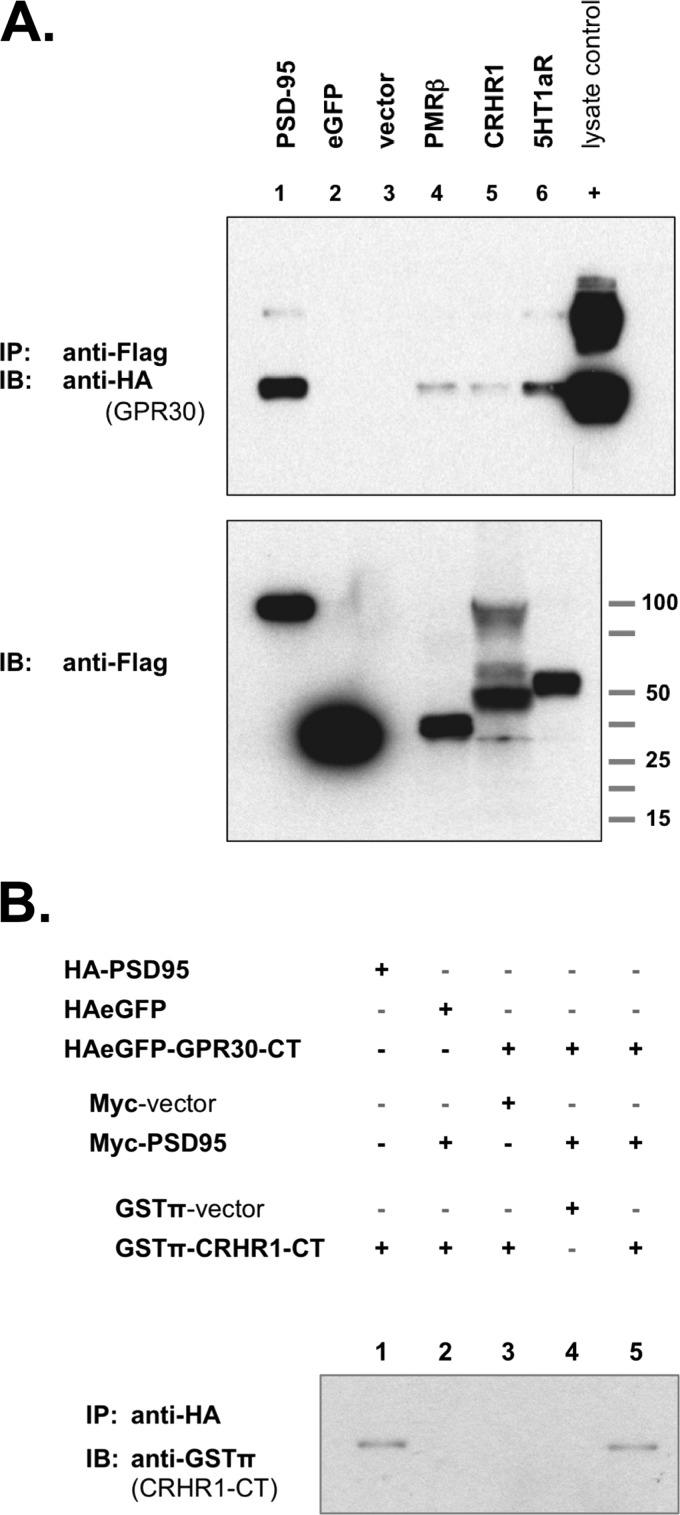
FIGURE 8.
GPR30 associates with other GPCRs that have been previously identified in post-synaptic structures. A, upper blot, GPCR co-immunoprecipitates with GPR30. COS-7 cells were co-transfected with HA-tagged GPR30 plus the following FLAG-tagged expression constructs: lane 1, PSD-95; lane 2, eGFP; lane 3, empty FLAG vector; lane 4, PMRβ; lane 5, CRHR1, lane 6, 5HT1aR. FLAG-tagged proteins were precipitated with anti-FLAG antibody, and GPR30 protein was detected by immunoblot with anti-HA antibody. All three GPCRs could co-immunoprecipitate with GPR30 protein. 0.1% of whole cell lysate from HA-GPR30-transfected COS-7 cells was also run as a positive HA-epitope control (+). Lower blot, FLAG-tagged proteins in whole cell lysates are shown. Blots are representative of seven independent experiments. B, PSD-95 coupled GPR30-CT with CRHR1-CT. The C-terminal tail of the rat CRHR1 receptor was chimerically linked to GSTπ in a GSTπ-CRHR1-CT expression construct. COS-7 cells were transfected as indicated, and lysates were collected for precipitating HA-tagged proteins. When GPR30-CT was co-expressed with PSD-95 and with CRHR1-CT, GPR30-CT co-immunoprecipitated with CRHR1-CT (lane 5). In the absence of either GPR30-CT (lane 2) or PSD-95 (lane 3), this co-IP capability is lost. GPR30-CT coupling to CRHR1-CT is specific, as GPR30-CT and PSD-95 together cannot co-immunoprecipitate GSTπ alone (lane 4). As a control, PSD-95 alone can co-immunoprecipitate CRHR1-CT (lane 1). IB, immunoblot.
GPR30 was able to associate with PMRβ (Fig. 8A, lane 4), with CRHR1 (Fig. 8A, lane 5), and with 5HT1aR (Fig. 8A, lane 5). When transfected independently and lysates were combined, GPR30 was unable to associate with any of the receptors (data not shown). Additionally, when GPR30-CT was co-expressed with PSD-95 and the C-terminal domain of CRHR1 (CRHR1-CT), GPR30-CT can co-immunoprecipitate with CRHR1-CT (Fig. 8B).
The rat CRHR1-CT domain was chimerically expressed as a fusion with GSTπ. When PSD-95 were absent, GPR30-CT was unable to co-immunoprecipitate with CRHR1-CT (Fig. 8B, lane 3). GPR30-CT association with CRHR1-CT was specific, as GPR30-CT was unable to associate with GSTπ in the presence of PSD-95 (Fig. 8B, lane 4), and GPR30-CT could co-immunoprecipitate with CRHR1-CT only when co-expressed with PSD-95 (Fig. 8B, lane 5).
These data suggest that GPR30 has the potential not only to homodimerize with itself but also has the potential to heterodimerize with other GPCRs. Moreover, although PSD-95 may not be required for full-length GPR30 to associate with full-length CRHR1, PSD-95 appears to couple the GPR30-CT to the CRHR1-CT. This would suggest that PSD-95 might stabilize a heterodimeric GPCR partnering between GPR30 and CRHR1 via the receptor C-terminal tails.
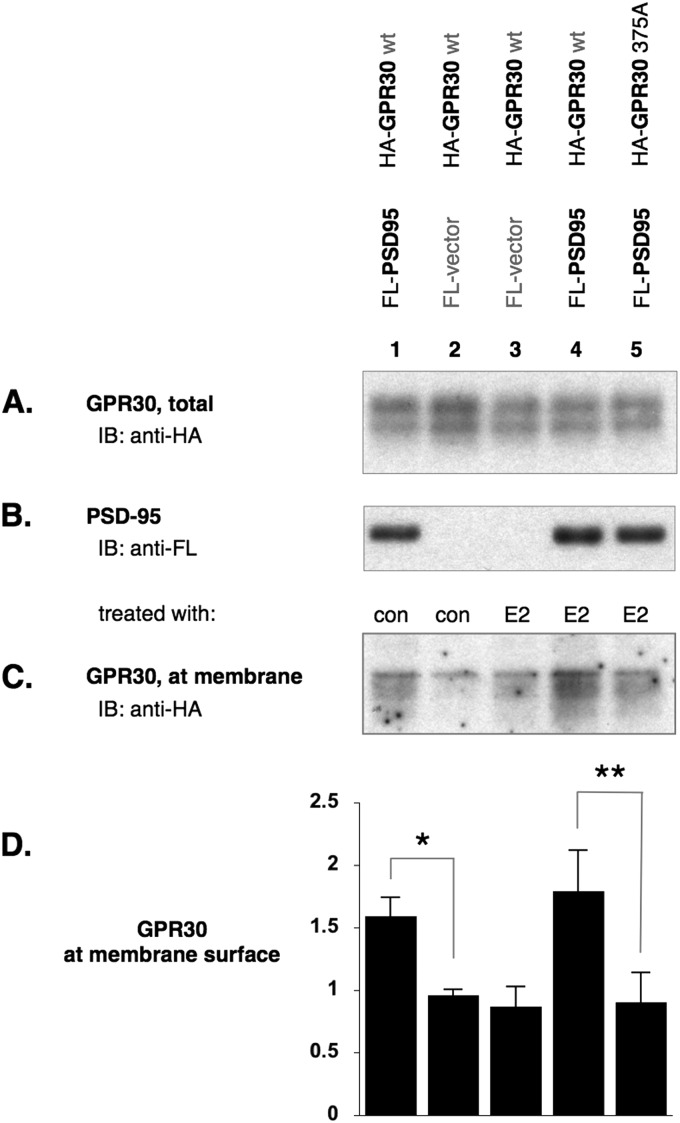
PSD-95 Association Increases the Total Amount of GPR30 Protein at the Plasma Membrane
PSD-95 demonstrated a specific interaction with GPR30, PSD-95 associated with GPR30 in plasma membrane preparations from the hippocampus, and PSD-95 was able to couple the C termini of GPR30 and CRHR1. Therefore, additional functionality was addressed and how PSD-95 affected GPR30 membrane localization was determined. Based on the ability of PSD-95 to influence GPCR internalization (52), it was hypothesized that PSD-95 might also affect GPR30 internalization from the cell surface.
COS-7 cells were transfected to express GPR30 with or without PSD-95. Transfected cells were also treated with E2 or diluent control. Total protein at the cell surface was biotinylated, and biotinylated proteins were streptavidin-precipitated then immunoblotted for recombinant GPR30 protein levels. When compared with untreated COS-7 cells transfected with only GPR30, the addition of PSD-95 increased the plasma membrane levels of GPR30 protein (Fig. 9, lane 1). When PSD-95 was absent, plasma membrane-localized GPR30 levels were reduced to control levels regardless of agonist stimulus (Fig. 9, lanes 2 and 3). The addition of PSD-95 maintained the elevated levels of membrane-localized GPR30, also regardless of agonist stimulus (Fig. 9, lanes 1 and 4). This maintained level was lost when GPR30 could no longer associate with PSD-95 (Fig. 9, lane 5). These data would suggest that one functional significance of a GPR30 and PSD-95 association is that PSD-95 increases the surface residency of GPR30 or reduces the internalization of GPR30 from the plasma membrane.
FIGURE 9.
PSD-95 increases the amount of GPR30 residing at the plasma membrane. COS-7 cells were co-transfected and treated as indicated (A and C, diluent control (con), estrogen agonist (E2)). Surface proteins were biotinylated and affinity-precipitated with agarose-streptavidin. A, shown is an immunoblot (IB) on HA-tagged proteins before streptavidin affinity precipitation, therefore detecting the total amounts of GPR30 (biotinylated surface and non-biotinylated intracellular) per lysate. B, shown is an immunoblot on FLAG (FL-)tagged PSD-95 co-expression with HA-tagged GPR30. C, shown is an immunoblot for GPR30 on streptavidin affinity precipitation surface proteins. PSD-95 co-expression increased the amount of HA-tagged receptor available for cell surface biotinylation (lane 1 versus, lane 2, control-treated). In the absence of PSD-95 (lane 2 versus 3), agonist treatment had no effect to the level of GPR30 at the plasma membrane. Likewise, agonist treatment had no effect to the elevated level of GPR30 at the plasma membrane when PSD-95 was co-expressed (lanes 1 versus 4). When the C terminus was mutated (375A) and the ability of GPR30 to associate with PSD-95 was abolished, PSD-95 co-expression has no effect on the membrane levels of GPR30. D, shown is a graphics representation of panel C where GPR30 protein levels at the plasma membrane surface are expressed relative to untreated COS-7 cells co-transfected with GPR30 and PSD-95 (not shown). n = 3 independent experiments, ±S.D. *, statistically significant, p < 0.03. **, statistically significant, p < 0.02.
DISCUSSION
PSD-95 and GPR30 Association
In this study we sought to investigate the subcellular localization and the initial allosteric interactions of the E2-sensitive GPCR, GPR30. The data presented here identify PSD-95 as a novel, allosteric binding partner for GPR30 both in vitro and in vivo.
PSD-95 is a scaffolding protein that is highly enriched in the PSD of excitatory synapses, and the PSD-95 binding capacity of GPR30 may begin to explain the GPR30-IR pattern that we observed in the hippocampus. Namely, GPR30 labeling has been previously identified in the hippocampus and in hippocampal neurons (6, 7, 10), and as shown in ultra-structural analyses here, GPR30 protein was detectable in the dendritic spine compartment at the PSD in vivo, and GPR30 labeling was detected in PSD-95-positive synaptosomal lysates.
PSD-95 interacts with GPR30 protein, and the PSD-95 binding capacity of GPR30 is highly specific. The CT domain of GPR30 binds to the N-terminal tandem pair of PDZ domains of PSD-95 but does not bind to the third PDZ domain, suggesting that GPR30 may potentially dimerize with other GPCRs to be found in the dendritic spine. As shown here, GPR30 was able to associate with the progesterone-, corticotropin-, and serotonin-sensitive GPCRs. This PSD-95 binding capacity of the GPR30 receptor suggests that E2 sensitivity is structurally integrated into the post-synaptic compartment of CA1 hippocampal neurons.
GPR30 Receptor and Estrogen Sensitivity in the Hippocampus
E2 governs several aspects of the central nervous system (CNS), including cognitive performance, behavior, mood, stress responses, and aging (53).
The hippocampus is a particularly E2-sensitive brain region, and one example of how E2 can govern hippocampal synaptic plasticity is by increasing the dendritic spine density in an N-methyl-d-aspartate receptor-dependent manner (54). The molecular mechanisms that couple E2 effects to spine synaptic plasticity are not well understood but based on recent studies (2) are believed to involve non-genomic actions (55, 56).
E2 can impact synaptic structure by rapidly and transiently altering the actin cytoskeletal network to promote spinogenesis and to advance the dendritic spine maturation profile in hippocampal neurons (57, 58). Such non-genomic E2 effects can also have significant cellular and physiologic consequences as well, as membrane-initiated E2 stimuli can impact neuronal excitability, neurotransmitter release, and neuronal survival (59).
Alongside our previous findings on the nonnuclear localization of estrogen receptor α (ERα) and ERβ in dendritic spines (60, 61), these data on GPR30 and spine localization may begin to provide an additional explanation on how E2 might modulate synaptic plasticity and non-genomic hormone effects in the hippocampus.
GPR30 Interaction with Other GPCRs
The identification of GPR30 in spine structures as found here would provide a subcellular explanation as to how E2 could directly modulate the synaptic responses to other stimuli. GPR30 could potentially dimerize with other post-synaptic receptors (62, 63) in the hippocampus and thereby contribute to sex differences (64) observed in higher brain functions. If GPR30 can dimerize with other GPCRs, then GPR30 might not act independently but rather collaboratively (65) with other cell type-specific allosteric partners. A GPR30 interaction might allow for the sex hormone estrogen to influence how a hippocampal neuron responds to the various incoming stimuli.
In the dendritic spine, PSD-95 is responsible for assembling different receptors with allosteric partners at the PSD, and PSD-95 is necessary for maintaining the organization of protein complexes within the PSD. Several GPCRs are known to associate with the PDZ domains of PSD-95 via the receptor CT tail, including the β-adrenergic receptor, which associates with the PDZ(3) domain (52), and the somatostatin receptor, which associates with the dimer stabilizing PDZ(1 + 2) domain (66). By targeting the PDZ(1 + 2) domain, GPR30 may dimerize with other PDZ-targeting receptors as well.
For example, progesterone and E2 have antagonist effects in the CNS, and progesterone is able to diminish E2-stimulated spine formation faster than the removal of E2 alone (67). The PMRβ progestin receptor is expressed almost exclusively in the brain (26), and if heterodimeric GPCR pairings can co-modulate one another, then a heterodimeric relationship with GPR30 may explain how PMRβ might offset GPR30 function.
We have demonstrated that CRHR1-IR is in dendritic spines of CA1 neurons (22), and E2 is involved is sex differences seen in stress-related changes in hippocampal-dependent behavior and cognition (48). Although GPR30 is associated with CRHR1 in vitro, the CRHR1-CT also contains a PDZ ligand sequence (-STAV) and can also co-immunoprecipitate with PSD-95. GPR30 co-localizes with CRH (47), and future studies, therefore, should establish and expand on a heterodimeric function between GPR30 and CRHR1.
Likewise, GPR30 and 5HT1aR provide an example of a possible heterodimeric function, as E2 attenuates serotonergic responses in the CNS (68). 5HT1aR-IR is identified in the dendritic spine (69), and GPR30 co-localizes with 5HT1aR in the hypothalamus (47). In the hippocampus, E2 can attenuate 5HT1aR binding, and E2 attenuates 5HT1aR signaling via GPR30 (70). Additional studies are needed to address the functionality of any direct interaction between these two receptors.
PSD-95 Interaction Functions to Increase the Amount of GPR30 at the Plasma Membrane
The interaction between GPR30 and PSD-95 is functionally significant, as PSD-95 is able to inhibit GPR30 internalization from the plasma membrane. This finding is similar to other reports on GPCRs that interact with PSD-95 that also demonstrate PSD-95 is able to suppress the internalization of various GPCRs from the plasma membrane (52, 71, 72). The interaction between GPR30 and PSD-95 might inhibit receptor internalization and thereby increase GPR30 protein levels at the membrane surface by potentially multiple mechanisms.
First, PSD-95 is a palmitoylated protein (73), and such lipid modifications alter the protein structure and increases the hydrophobicity of proteins, causing them to adhere to cellular membranes. PSD-95, therefore, could recruit and hold associated proteins such as GPR30 directly to the plasma membrane due to the specific lipid palmitoyl modifications that would anchor the scaffold and any accompanying proteins to the membrane (74, 75). Likewise, as suggested (52), PSD-95 can scaffold together other proteins to form larger protein complexes that are possibly resistant to internalization. Lastly, PSD-95 association to the cytosolic portions of GPR30 (4, 76, 77) might sterically block the access of other cytosolic proteins that are important for receptor internalization, such as β-arrestins (78). GPR30, however, has been recently shown to undergo clathrin-mediated and potentially β-arrestin-independent constitutive endocytosis instead (79).
This atypical mechanism of receptor internalization can occur with other neuronal GPCRs located at glutamatergic synapses, including the metabotropic glutamate receptors, and it is believed to provide basal cell functions such as regulating neuronal activity and maintaining neuronal polarity (80, 81). Dysregulation of such internalization mechanisms is associated with neurologic disorders including Alzheimer disease (82).
Constitutive internalization is also a cellular mechanism used to regulate a receptor population and distribution at the cell surface. Therefore, constitutive internalization would provide an ongoing, active means to modulate the sensitivities of individual synapses to the incoming stimuli (83, 84).
Unlike many other GPCRs that have been shown to be internalized by a β-arrestin-dependent and agonist-stimulated mechanism, GPR30 is ubiquitinated at the cell surface and has a short half-life, instead being constantly targeted back to the trans-Golgi network for degradation (79, 85). A continuous removal of surface GPR30 could curb any undesirable non-genomic responses to estrogen that would be initiated at the plasma membrane.
The co-expression with, and the interaction between GPR30 and PSD-95 via PDZ binding appear to increase the amount of GPR30 at the plasma membrane surface. The presence or absence of agonist did not appear to affect the level of GPR30 in the plasma membrane, and this observation is consistent with the previous report describing an agonist-independent internalization of GPR30 receptor (79).
A possible explanation for this observation is that GPCRs that bind to PDZ domain-containing proteins can delay constitutive internalization via an association with a subset of clathrin-coated pits, prolonging the surface residence time of the GPCR (86) and targeting the GPCR for select endosomal destinations (87) such as the trans-Golgi network.
This PDZ-dependent mechanism would, however, appear to also include β-arrestin (86), and one contrasting study has in fact demonstrated that GPR30 is involved in an agonist-stimulated recruitment of β-arrestin to the plasma membrane (88). This discrepancy in GPR30 internalization would be similar to the neuronal GPCR metabotropic glutamate receptor 1a, which also utilizes both β-arrestin-dependent as well as -independent mechanisms at the synapse for constitutive internalization (89, 90).
Although it is possible that plasma membrane localization is not required for GPR30-mediated signaling (91, 92) because the E2 agonist is a readily membrane-permeable steroid hormone, by increasing the surface residence time at the plasma membrane, the association between GPR30 and PSD-95 would increase the potential opportunities for GPR30 to interact functionally (6, 47, 70, 93, 95) with other receptors in the dendritic spine.
Moreover, the two mutually supportive lines of evidence presented here also support the suggestion that both endogenous and recombinant GPR30 protein can exist at the plasma membrane (18, 96); first, by isolated plasma membrane preparations from the rat hippocampus in vivo and, second, by the biotinylation of surface proteins in vitro. Previous confocal microscopy studies on cultured cells have questioned the localization of GPR30 at the plasma membrane (97, 98), but the evidence demonstrated here would suggest that GPR30 protein indeed can be localized at the plasma membrane.
The allosteric interaction between GPR30 and PSD-95 provides a molecular means underpinning the dendritic spine localization of GPR30 observed in vivo. The PSD-95 binding capacity of GPR30 suggests that the receptor is biochemically networked into the post-synaptic structure, allowing E2 to participate in the neuromodulation of synaptic plasticity and transmission directly via non-genomic action as part of a supramolecular complex. The spatiotemporal availability of allosteric components assembling with GPR30 may provide an explanation as to how estrogen can modulate brain function, and different spatiotemporal protein assemblies surrounding GPR30 may explain how estrogen introduces different responses in different brain regions during development and aging (51, 94).
Acknowledgments
We thank Dr. Carlos Stocco and Dr. Greti Aguilera for permission to use their plasmid expression constructs. We are very grateful to Dr. Lawrence Reagan for instruction and for generously providing material for the ultracentrifuged membrane preparations.
This work was supported, in whole or in part, by National Institutes of Health Grants AG016765 and NS007080 (to B. S. Mc) and DA08259 and HL098351 (to T. A. M.).
K. T. Akama, unpublished data.
- E2
- 17β-estradiol
- GPCR
- G protein-coupled receptor
- ER
- estrogen receptor
- DDM
- n-dodecyl-β-maltoside
- CHS
- cholesterol hemisuccinate
- IR
- immunoreactivity
- IP
- immunoprecipitation
- PSD
- post-synaptic density
- PDZ
- (PSD)-95/Disc large tumor suppressor (Dlg1)/Zonula occludens (ZO)-1
- CT
- C-terminal
- FL
- FLAG
- nNOS
- neuronal NOS
- SPL
- spinophilin
- eGFP
- enhanced green fluorescent protein
- CRHR1
- corticotropin releasing hormone receptor-1
- PMRβ
- progestin membrane receptor-β
- 5HT1aR
- 5-hydroxy-tryptophan-1a receptor
- cds
- coding sequence
- mAb
- monoclonal antibody.
REFERENCES
- 1. McEwen B. S. (2001) Invited review. Estrogens effects on the brain. Multiple sites and molecular mechanisms. J. Appl. Physiol. 91, 2785–2801 [DOI] [PubMed] [Google Scholar]
- 2. Srivastava D. P., Woolfrey K. M., Woolfrey K., Jones K. A., Shum C. Y., Lash L. L., Swanson G. T., Penzes P. (2008) Rapid enhancement of two-step wiring plasticity by estrogen and NMDA receptor activity. Proc. Natl. Acad. Sci. U.S.A. 105, 14650–14655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thomas P., Pang Y., Filardo E. J., Dong J. (2005) Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 146, 624–632 [DOI] [PubMed] [Google Scholar]
- 4. Carmeci C., Thompson D. A., Ring H. Z., Francke U., Weigel R. J. (1997) Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics 45, 607–617 [DOI] [PubMed] [Google Scholar]
- 5. Prossnitz E. R., Barton M. (2011) The G-protein-coupled estrogen receptor GPER in health and disease. Nat. Rev. Endocrinol 7, 715–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hazell G. G., Yao S. T., Roper J. A., Prossnitz E. R., O'Carroll A. M., Lolait S. J. (2009) Localization of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J. Endocrinol. 202, 223–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brailoiu E., Dun S. L., Brailoiu G. C., Mizuo K., Sklar L. A., Oprea T. I., Prossnitz E. R., Dun N. J. (2007) Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J. Endocrinol. 193, 311–321 [DOI] [PubMed] [Google Scholar]
- 8. Matsuda K., Sakamoto H., Mori H., Hosokawa K., Kawamura A., Itose M., Nishi M., Prossnitz E. R., Kawata M. (2008) Expression and intracellular distribution of the G protein-coupled receptor 30 in rat hippocampal formation. Neurosci. Lett. 441, 94–99 [DOI] [PubMed] [Google Scholar]
- 9. Hammond R., Nelson D., Gibbs R. B. (2011) GPR30 co-localizes with cholinergic neurons in the basal forebrain and enhances potassium-stimulated acetylcholine release in the hippocampus. Psychoneuroendocrinology 36, 182–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Funakoshi T., Yanai A., Shinoda K., Kawano M. M., Mizukami Y. (2006) G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem. Biophys. Res. Commun. 346, 904–910 [DOI] [PubMed] [Google Scholar]
- 11. Filardo E., Quinn J., Pang Y., Graeber C., Shaw S., Dong J., Thomas P. (2007) Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology 148, 3236–3245 [DOI] [PubMed] [Google Scholar]
- 12. Revankar C. M., Cimino D. F., Sklar L. A., Arterburn J. B., Prossnitz E. R. (2005) A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307, 1625–1630 [DOI] [PubMed] [Google Scholar]
- 13. Cuesto G., Enriquez-Barreto L., Caramés C., Cantarero M., Gasull X., Sandi C., Ferrús A., Acebes Á., Morales M. (2011) Phosphoinositide-3-kinase activation controls synaptogenesis and spinogenesis in hippocampal neurons. J. Neurosci. 31, 2721–2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Q., Liu L., Pei L., Ju W., Ahmadian G., Lu J., Wang Y., Liu F., Wang Y. T. (2003) Control of synaptic strength, a novel function of Akt. Neuron 38, 915–928 [DOI] [PubMed] [Google Scholar]
- 15. Abel T., Nguyen P. V. (2008) Regulation of hippocampus-dependent memory by cyclic AMP-dependent protein kinase. Prog. Brain Res. 169, 97–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bezprozvanny I., Maximov A. (2001) Classification of PDZ domains. FEBS Lett. 509, 457–462 [DOI] [PubMed] [Google Scholar]
- 17. Hung A. Y., Sheng M. (2002) PDZ domains. Structural modules for protein complex assembly. J. Biol. Chem. 277, 5699–5702 [DOI] [PubMed] [Google Scholar]
- 18. Mizukami Y. (2010) In vivo functions of GPR30/GPER-1, a membrane receptor for estrogen. From discovery to functions in vivo. Endocr. J. 57, 101–107 [DOI] [PubMed] [Google Scholar]
- 19. Kreienkamp H. J. (2002) Organization of G-protein-coupled receptor signalling complexes by scaffolding proteins. Curr. Opin. Pharmacol. 2, 581–586 [DOI] [PubMed] [Google Scholar]
- 20. Chen X., Nelson C. D., Li X., Winters C. A., Azzam R., Sousa A. A., Leapman R. D., Gainer H., Sheng M., Reese T. S. (2011) PSD-95 is required to sustain the molecular organization of the postsynaptic density. J. Neurosci. 31, 6329–6338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gomperts S. N. (1996) Clustering membrane proteins. It's all coming together with the PSD-95/SAP90 protein family. Cell 84, 659–662 [DOI] [PubMed] [Google Scholar]
- 22. Williams T. J., Akama K. T., Knudsen M. G., McEwen B. S., Milner T. A. (2011) Ovarian hormones influence corticotropin releasing factor receptor colocalization with δ- opioid receptors in CA1 pyramidal cell dendrites. Exp. Neurol. 230, 186–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Piroli G. G., Grillo C. A., Hoskin E. K., Znamensky V., Katz E. B., Milner T. A., McEwen B. S., Charron M. J., Reagan L. P. (2002) Peripheral glucose administration stimulates the translocation of GLUT8 glucose transporter to the endoplasmic reticulum in the rat hippocampus. J. Comp. Neurol. 452, 103–114 [DOI] [PubMed] [Google Scholar]
- 24. Peters A., Palay S. L., Webster H. d. (1991) The Fine Structure of the Nervous System: Neurons and Their Supporting Cells, 3rd ed., Oxford University Press, New York [Google Scholar]
- 25. Yamamura H. I., Snyder S. H. (1973) High affinity transport of choline into synaptosomes of rat brain. J. Neurochem. 21, 1355–1374 [DOI] [PubMed] [Google Scholar]
- 26. Cai Z., Stocco C. (2005) Expression and regulation of progestin membrane receptors in the rat corpus luteum. Endocrinology 146, 5522–5532 [DOI] [PubMed] [Google Scholar]
- 27. Young S. F., Griffante C., Aguilera G. (2007) Dimerization between vasopressin V1b and corticotropin releasing hormone type 1 receptors. Cell. Mol. Neurobiol. 27, 439–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hering H., Sheng M. (2001) Dendritic spines. Structure, dynamics, and regulation. Nat. Rev. Neurosci. 2, 880–888 [DOI] [PubMed] [Google Scholar]
- 29. Rácz B., Blanpied T. A., Ehlers M. D., Weinberg R. J. (2004) Lateral organization of endocytic machinery in dendritic spines. Nat. Neurosci. 7, 917–918 [DOI] [PubMed] [Google Scholar]
- 30. Xu W. (2011) PSD-95-like membrane associated guanylate kinases (PSD-MAGUKs) and synaptic plasticity. Curr. Opin. Neurobiol. 21, 306–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Keith D., El-Husseini A. (2008) Excitation control. Balancing PSD-95 function at the synapse. Front. Mol. Neurosci. 1, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kalyoncu S., Keskin O., Gursoy A. (2010) Interaction prediction and classification of PDZ domains. BMC Bioinformatics 11, 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Christopherson K. S., Hillier B. J., Lim W. A., Bredt D. S. (1999) PSD-95 assembles a ternary complex with the N-methyl-d-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J. Biol. Chem. 274, 27467–27473 [DOI] [PubMed] [Google Scholar]
- 34. Gee S. H., Quenneville S., Lombardo C. R., Chabot J. (2000) Single-amino acid substitutions alter the specificity and affinity of PDZ domains for their ligands. Biochemistry 39, 14638–14646 [DOI] [PubMed] [Google Scholar]
- 35. Lee H. J., Zheng J. J. (2010) PDZ domains and their binding partners. Structure, specificity, and modification. Cell Commun. Signal. 8, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Songyang Z., Fanning A. S., Fu C., Xu J., Marfatia S. M., Chishti A. H., Crompton A., Chan A. C., Anderson J. M., Cantley L. C. (1997) Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science 275, 73–77 [DOI] [PubMed] [Google Scholar]
- 37. Kim E., Sheng M. (2004) PDZ domain proteins of synapses. Nat. Rev. Neurosci. 5, 771–781 [DOI] [PubMed] [Google Scholar]
- 38. Long J. F., Tochio H., Wang P., Fan J. S., Sala C., Niethammer M., Sheng M., Zhang M. (2003) Supramodular structure and synergistic target binding of the N-terminal tandem PDZ domains of PSD-95. J. Mol. Biol. 327, 203–214 [DOI] [PubMed] [Google Scholar]
- 39. Wang W., Weng J., Zhang X., Liu M., Zhang M. (2009) Creating conformational entropy by increasing interdomain mobility in ligand binding regulation. A revisit to N-terminal tandem PDZ domains of PSD-95. J. Am. Chem. Soc. 131, 787–796 [DOI] [PubMed] [Google Scholar]
- 40. Razandi M., Pedram A., Merchenthaler I., Greene G. L., Levin E. R. (2004) Plasma membrane estrogen receptors exist and functions as dimers. Mol. Endocrinol. 18, 2854–2865 [DOI] [PubMed] [Google Scholar]
- 41. Sheng M., Sala C. (2001) PDZ domains and the organization of supramolecular complexes. Annu. Rev. Neurosci. 24, 1–29 [DOI] [PubMed] [Google Scholar]
- 42. Zhang J., Petit C. M., King D. S., Lee A. L. (2011) Phosphorylation of a PDZ domain extension modulates binding affinity and interdomain interactions in postsynaptic density-95 (PSD-95) protein, a membrane-associated guanylate kinase (MAGUK). J. Biol. Chem. 286, 41776–41785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Petit C. M., Zhang J., Sapienza P. J., Fuentes E. J., Lee A. L. (2009) Hidden dynamic allostery in a PDZ domain. Proc. Natl. Acad. Sci. U.S.A. 106, 18249–18254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Prinster S. C., Hague C., Hall R. A. (2005) Heterodimerization of g protein-coupled receptors. Specificity and functional significance. Pharmacol. Rev. 57, 289–298 [DOI] [PubMed] [Google Scholar]
- 45. Lohse M. J. (2010) Dimerization in GPCR mobility and signaling. Curr. Opin. Pharmacol. 10, 53–58 [DOI] [PubMed] [Google Scholar]
- 46. Foy M. R., Baudry M., Akopian G. K., Thompson R. F. (2010) Regulation of hippocampal synaptic plasticity by estrogen and progesterone. Vitam. Horm. 82, 219–239 [DOI] [PubMed] [Google Scholar]
- 47. Xu H., Qin S., Carrasco G. A., Dai Y., Filardo E. J., Prossnitz E. R., Battaglia G., Doncarlos L. L., Muma N. A. (2009) Extra-nuclear estrogen receptor GPR30 regulates serotonin function in rat hypothalamus. Neuroscience 158, 1599–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shors T. J., Chua C., Falduto J. (2001) Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J. Neurosci. 21, 6292–6297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brinton R. D., Thompson R. F., Foy M. R., Baudry M., Wang J., Finch C. E., Morgan T. E., Pike C. J., Mack W. J., Stanczyk F. Z., Nilsen J. (2008) Progesterone receptors. Form and function in brain. Front. Neuroendocrinol. 29, 313–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Le Saux M., Di Paolo T. (2005) Changes in 5-HT1A receptor binding and G-protein activation in the rat brain after estrogen treatment. Comparison with tamoxifen and raloxifene. J. Psychiatry Neurosci. 30, 110–117 [PMC free article] [PubMed] [Google Scholar]
- 51. McEwen B. S. (2010) Stress, sex, and neural adaptation to a changing environment. Mechanisms of neuronal remodeling. Ann. N.Y. Acad. Sci. 1204, E38–E59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hu L. A., Tang Y., Miller W. E., Cong M., Lau A. G., Lefkowitz R. J., Hall R. A. (2000) β1-Adrenergic receptor association with PSD-95. Inhibition of receptor internalization and facilitation of β1-adrenergic receptor interaction with N-methyl-d-aspartate receptors. J. Biol. Chem. 275, 38659–38666 [DOI] [PubMed] [Google Scholar]
- 53. Morrison J. H., Brinton R. D., Schmidt P. J., Gore A. C. (2006) Estrogen, menopause, and the aging brain. How basic neuroscience can inform hormone therapy in women. J. Neurosci. 26, 10332–10348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Woolley C. S., McEwen B. S. (1994) Estradiol regulates hippocampal dendritic spine density via an N-methyl-d-aspartate receptor-dependent mechanism. J. Neurosci. 14, 7680–7687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McEwen B. S., Milner T. A. (2007) Hippocampal formation. Shedding light on the influence of sex and stress on the brain. Brain Res. Rev. 55, 343–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Prossnitz E. R., Maggiolini M. (2009) Non-genomic signaling by steroids. Mol. Cell. Endocrinol. 308, 1–2 [DOI] [PubMed] [Google Scholar]
- 57. Kramár E. A., Chen L. Y., Rex C. S., Gall C. M., Lynch G. (2009) Estrogen's place in the family of synaptic modulators. Mol. Cell. Pharmacol. 1, 258–262 [PMC free article] [PubMed] [Google Scholar]
- 58. Li C., Brake W. G., Romeo R. D., Dunlop J. C., Gordon M., Buzescu R., Magarinos A. M., Allen P. B., Greengard P., Luine V., McEwen B. S. (2004) Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc. Natl. Acad. Sci. U.S.A. 101, 2185–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Roepke T. A., Ronnekleiv O. K., Kelly M. J. (2011) Physiological consequences of membrane-initiated estrogen signaling in the brain. Front. Biosci. 16, 1560–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Milner T. A., Ayoola K., Drake C. T., Herrick S. P., Tabori N. E., McEwen B. S., Warrier S., Alves S. E. (2005) Ultrastructural localization of estrogen receptor β immunoreactivity in the rat hippocampal formation. J. Comp. Neurol. 491, 81–95 [DOI] [PubMed] [Google Scholar]
- 61. Romeo R. D., McCarthy J. B., Wang A., Milner T. A., McEwen B. S. (2005) Sex differences in hippocampal estradiol-induced N-methyl-d-aspartic acid binding and ultrastructural localization of estrogen receptor-α. Neuroendocrinology 81, 391–399 [DOI] [PubMed] [Google Scholar]
- 62. Brady A. E., Limbird L. E. (2002) G protein-coupled receptor interacting proteins. Emerging roles in localization and signal transduction. Cell. Signal. 14, 297–309 [DOI] [PubMed] [Google Scholar]
- 63. Luttrell L. M. (2006) Transmembrane signaling by G protein-coupled receptors. Methods Mol. Biol. 332, 3–49 [DOI] [PubMed] [Google Scholar]
- 64. McCarthy M. M., Arnold A. P. (2011) Reframing sexual differentiation of the brain. Nat. Neurosci. 14, 677–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Levin E. R. (2009) G protein-coupled receptor 30. Estrogen receptor or collaborator? Endocrinology 150, 1563–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Christenn M., Kindler S., Schulz S., Buck F., Richter D., Kreienkamp H. J. (2007) Interaction of brain somatostatin receptors with the PDZ domains of PSD-95. FEBS Lett. 581, 5173–5177 [DOI] [PubMed] [Google Scholar]
- 67. Woolley C. S., McEwen B. S. (1993) Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J. Comp. Neurol. 336, 293–306 [DOI] [PubMed] [Google Scholar]
- 68. Rybaczyk L. A., Bashaw M. J., Pathak D. R., Moody S. M., Gilders R. M., Holzschu D. L. (2005) An overlooked connection. Serotonergic mediation of estrogen-related physiology and pathology. BMC Womens Health 5, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Riad M., Garcia S., Watkins K. C., Jodoin N., Doucet E., Langlois X., el Mestikawy S., Hamon M., Descarries L. (2000) Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J. Comp. Neurol. 417, 181–194 [PubMed] [Google Scholar]
- 70. Rossi D. V., Dai Y., Thomas P., Carrasco G. A., DonCarlos L. L., Muma N. A., Li Q. (2010) Estradiol-induced desensitization of 5-HT1A receptor signaling in the paraventricular nucleus of the hypothalamus is independent of estrogen receptor-β. Psychoneuroendocrinology 35, 1023–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jugloff D. G., Khanna R., Schlichter L. C., Jones O. T. (2000) Internalization of the Kv1.4 potassium channel is suppressed by clustering interactions with PSD-95. J. Biol. Chem. 275, 1357–1364 [DOI] [PubMed] [Google Scholar]
- 72. Xia Z., Gray J. A., Compton-Toth B. A., Roth B. L. (2003) A direct interaction of PSD-95 with 5-HT2A serotonin receptors regulates receptor trafficking and signal transduction. J. Biol. Chem. 278, 21901–21908 [DOI] [PubMed] [Google Scholar]
- 73. Ho G. P., Selvakumar B., Mukai J., Hester L. D., Wang Y., Gogos J. A., Snyder S. H. (2011) S-Nitrosylation and S-palmitoylation reciprocally regulate synaptic targeting of PSD-95. Neuron 71, 131–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hsueh Y. P., Kim E., Sheng M. (1997) Disulfide-linked head-to-head multimerization in the mechanism of ion channel clustering by PSD-95. Neuron 18, 803–814 [DOI] [PubMed] [Google Scholar]
- 75. Fukata M., Fukata Y., Adesnik H., Nicoll R. A., Bredt D. S. (2004) Identification of PSD-95 palmitoylating enzymes. Neuron 44, 987–996 [DOI] [PubMed] [Google Scholar]
- 76. Kim K. M., Caron M. G. (2008) Complementary roles of the DRY motif and C-terminus tail of GPCRS for G protein coupling and β-arrestin interaction. Biochem. Biophys. Res. Commun. 366, 42–47 [DOI] [PubMed] [Google Scholar]
- 77. Bonini J. A., Anderson S. M., Steiner D. F. (1997) Molecular cloning and tissue expression of a novel orphan G protein-coupled receptor from rat lung. Biochem. Biophys. Res. Commun. 234, 190–193 [DOI] [PubMed] [Google Scholar]
- 78. Prossnitz E. R. (2004) Novel roles for arrestins in the post-endocytic trafficking of G protein-coupled receptors. Life Sci. 75, 893–899 [DOI] [PubMed] [Google Scholar]
- 79. Cheng S. B., Quinn J. A., Graeber C. T., Filardo E. J. (2011) Down-modulation of the G-protein-coupled estrogen receptor, GPER, from the cell surface occurs via a trans-Golgi-proteasome pathway. J. Biol. Chem. 286, 22441–22455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Xu Z. Q., Zhang X., Scott L. (2007) Regulation of G protein-coupled receptor trafficking. Acta Physiol (Oxf) 190, 39–45 [DOI] [PubMed] [Google Scholar]
- 81. Lasiecka Z. M., Winckler B. (2011) Mechanisms of polarized membrane trafficking in neurons. Focusing in on endosomes. Mol. Cell. Neurosci. 48, 278–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. McGough I. J., Cullen P. J. (2011) Recent advances in retromer biology. Traffic 12, 963–971 [DOI] [PubMed] [Google Scholar]
- 83. Marchese A., Paing M. M., Temple B. R., Trejo J. (2008) G protein-coupled receptor sorting to endosomes and lysosomes. Annu. Rev. Pharmacol. Toxicol. 48, 601–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hanyaloglu A. C., von Zastrow M. (2008) Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu. Rev. Pharmacol. Toxicol. 48, 537–568 [DOI] [PubMed] [Google Scholar]
- 85. Bonifacino J. S., Rojas R. (2006) Retrograde transport from endosomes to the trans-Golgi network. Nat. Rev. Mol. Cell Biol. 7, 568–579 [DOI] [PubMed] [Google Scholar]
- 86. Puthenveedu M. A., von Zastrow M. (2006) Cargo regulates clathrin-coated pit dynamics. Cell 127, 113–124 [DOI] [PubMed] [Google Scholar]
- 87. Haucke V. (2006) Cargo takes control of endocytosis. Cell 127, 35–37 [DOI] [PubMed] [Google Scholar]
- 88. Sandén C., Broselid S., Cornmark L., Andersson K., Daszkiewicz-Nilsson J., Mårtensson U. E., Olde B., Leeb-Lundberg L. M. (2011) G protein-coupled estrogen receptor 1/G protein-coupled receptor 30 localizes in the plasma membrane and traffics intracellularly on cytokeratin intermediate filaments. Mol. Pharmacol. 79, 400–410 [DOI] [PubMed] [Google Scholar]
- 89. Dale L. B., Bhattacharya M., Seachrist J. L., Anborgh P. H., Ferguson S. S. (2001) Agonist-stimulated and tonic internalization of metabotropic glutamate receptor 1a in human embryonic kidney 293 cells. Agonist-stimulated endocytosis is β-arrestin1 isoform-specific. Mol. Pharmacol. 60, 1243–1253 [DOI] [PubMed] [Google Scholar]
- 90. Pula G., Mundell S. J., Roberts P. J., Kelly E. (2004) Agonist-independent internalization of metabotropic glutamate receptor 1a is arrestin- and clathrin-dependent and is suppressed by receptor inverse agonists. J. Neurochem. 89, 1009–1020 [DOI] [PubMed] [Google Scholar]
- 91. Prossnitz E. R., Arterburn J. B., Smith H. O., Oprea T. I., Sklar L. A., Hathaway H. J. (2008) Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu. Rev. Physiol. 70, 165–190 [DOI] [PubMed] [Google Scholar]
- 92. Calebiro D., Nikolaev V. O., Persani L., Lohse M. J. (2010) Signaling by internalized G-protein-coupled receptors. Trends Pharmacol. Sci. 31, 221–228 [DOI] [PubMed] [Google Scholar]
- 93. Kelly M. J., Rønnekleiv O. K. (2009) Control of CNS neuronal excitability by estrogens via membrane-initiated signaling. Mol. Cell. Endocrinol. 308, 17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Dumitriu D., Rapp P. R., McEwen B. S., Morrison J. H. (2010) Estrogen and the aging brain. An elixir for the weary cortical network. Ann. N.Y. Acad. Sci. 1204, 104–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hammond R., Gibbs R. B. (2011) GPR30 is positioned to mediate estrogen effects on basal forebrain cholinergic neurons and cognitive performance. Brain Res. 1379, 53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Maggiolini M., Picard D. (2010) The unfolding stories of GPR30, a new membrane-bound estrogen receptor. J. Endocrinol. 204, 105–114 [DOI] [PubMed] [Google Scholar]
- 97. Olde B., Leeb-Lundberg L. M. (2009) GPR30/GPER1. searching for a role in estrogen physiology. Trends Endocrinol. Metab. 20, 409–416 [DOI] [PubMed] [Google Scholar]
- 98. Langer G., Bader B., Meoli L., Isensee J., Delbeck M., Noppinger P. R., Otto C. (2010) A critical review of fundamental controversies in the field of GPR30 research. Steroids 75, 603–610 [DOI] [PubMed] [Google Scholar]