Background: Mechanism of enhanced Wnt/β-catenin activation in breast cancer (BCa) is not fully characterized.
Results: SOX9 was highly expressed in basal-like BCa. SOX9 maintained and enhanced LRP6 and TCF4 transcription and Wnt/β-catenin activation in vitro and in vivo.
Conclusion: SOX9 supports a positive feedback loop to sustain Wnt/β-catenin signal.
Significance: The results reveal a new mechanism of Wnt/β-catenin pathway activation in BCa.
Keywords: Breast Cancer, T-cell Factor (TCF), Transcription Regulation, Wnt Pathway, Wnt Signaling, LRP6, SOX9, TCF4
Abstract
Gene expression profiling has identified breast cancer (BCa) subtypes, including an aggressive basal-like (BL) subtype. The molecular signals underlying the behavior observed in BL-BCa group are largely unknown, although recent results indicate a prevalent increase in Wnt/β-catenin activity. Our immunohistochemistry study confirmed that SOX9, one of the BL-BCa signature genes, was expressed by most BL-BCa, and its expression correlated with indicators of poor prognosis. Importantly, BCa gene expression profiling strongly associated SOX9 with the expression of Wnt/β-catenin pathway components, LRP6 and TCF4. In cancer cell lines, SOX9 silencing reduced cell proliferation and invasion, LRP6 and TCF4 transcription, and decreased Wnt/β-catenin activation. SOX9 expression was also increased by Wnt, indicating that SOX9 is at the center of a positive feedback loop that enhances Wnt/β-catenin signaling. Consistently, SOX9 overexpression in BCa cell lines and transgenic SOX9 expression in breast epithelium caused increased LRP6 and TCF4 expression and Wnt/β-catenin activation. These results identify SOX9-mediated Wnt/β-catenin activation as one of the molecular mechanisms underlying aberrant Wnt/β-catenin activity in BCa, especially in the BL-BCa subgroup.
Introduction
Breast cancer (BCa)4 comprises a remarkably diverse group of diseases in terms of presentation, morphology, and biological or clinical behavior. Gene expression profiling has been used to examine BCa heterogeneity and identified sets of genes (signatures) whose expression can be used to classify BCa into multiple “intrinsic subtypes,” each associated with a different survival rate. The subgroups include luminal subtypes, a subtype with high expression of the HER2 proto-oncogene (Her2), and a basal-like subtype (BL) (1, 2). The BL subgroup (comprising between 17 and 37% of human BCa) is poorly differentiated and is enriched for tumors lacking hormone receptors or HER2 overexpression, so it does not respond to targeted therapies available for receptor-positive cancers (reviewed in Refs. 3 and 4). This subgroup is associated with an early age of onset, a distinct pattern of metastasis, and short times to relapse and disease progression, reflecting its aggressive nature.
The critical signal pathways responsible for the BL phenotype and its aggressive behavior are largely unknown, but recent data support a role for the Wnt/β-catenin signaling pathway (5–7). Activation of the canonical Wnt/β-catenin pathway involves stabilization of cytoplasmic β-catenin through the binding of Wnt ligands to their cell surface receptors, the Frizzled family receptors and the low-density lipoprotein receptor-related protein 5 (LRP5) or LRP6. In the absence of Wnt ligands, cytoplasmic β-catenin is phosphorylated by a multiprotein degradation complex that marks it for ubiquitination and degradation by the proteasome. Wnt ligand binding inhibits this complex, allowing the stabilized β-catenin to accumulate in the cytoplasm, to translocate to the nucleus, and to coactivate with the T-cell factor/lymphoid enhancer binding factor (TCF/LEF) transcription factors, which regulate crucial target genes that promote cell proliferation, differentiation, and development of multiple tissues including breast (8). Significantly, enhanced cytoplasmic and nuclear β-catenin staining has been reported in human BCa, indicating that aberrant activation of this pathway may contribute to mammary carcinogenesis (9–11). Moreover, recent reports indicate that the BL-subtype in particular is enriched for tumors with increased levels of LRP6 and cytoplasmic and nuclear β-catenin staining (5–7, 12, 13). Although the Wnt/β-catenin pathway may be activated in BCa, mutations that activate this pathway frequently found in other cancers (including APC truncation, stabilizing mutations in β-catenin, or Axin loss found in colon cancers) are rarely detected in human BCa (7, 14–16). Therefore, the underlying causes of aberrant Wnt/β-catenin activation in BCa, and its correlation with the BL subgroup, remain unexplained.
SOX9 (Sry-related HMG box 9) is a member of the BL signature genes used in classifying BCa subgroups (1, 2, 17). SOX9 belongs to the SOX family of transcription factors that share a homologous high-mobility group (HMG) box DNA binding domain and regulate many developmental processes (18). SOX9 mutations are the cause of the human autosomal dominant disease campomelic dysplasia, which is characterized by extreme cartilage and bone malformation, frequent XY sex reversal, and multiple defects in other organs, supporting SOX9 as a key mediator of fate determination in developmental processes (19, 20). The identified targets of SOX9 include particular collagen genes during chondrogenesis and the Mullerian inhibiting substance during male sex differentiation (21, 22). However, SOX transcription factors function in a context-dependent manner (23), and the role of SOX9 and its regulated genes in normal and neoplastic breast has not been fully determined. A recent report indicated that SOX9, in cooperation with Slug, plays a critical role in supporting mammary epithelial stem cells and enhancing BCa cell metastasis (24).
In this study, we show that SOX9 protein is expressed at intermediate or high levels in the majority of BL subgroup BCa. Significantly, SOX9 expression in BCa was positively correlated with expression of LRP6 and TCF4, which are two major components of the canonical Wnt/β-catenin pathway. We show that SOX9 regulates LRP6 and TCF4 expression and supports Wnt/β-catenin activity. Moreover, we observe an increase in LRP6 and TCF4 levels and in the cytoplasmic and nuclear β-catenin staining in transgenic mice overexpressing SOX9 in mammary epithelium. These data indicate that SOX9-dependent Wnt/β-catenin pathway activation may contribute to BCa pathobiology, particularly in the majority of BL-BCa expressing high levels of SOX9.
EXPERIMENTAL PROCEDURES
Cell Lines and Reagents
MCF-7, T47D, Au656, SKβR3, HCC1937, MDA-MB231, and 293T cells were from ATCC. The MCF10A-DCIS.com, SUM149, and SUM1315 were from Asterand. The cells were maintained under conditions recommended by the providers. Recombinant mouse Wnt3A and Dkk1 were from R&D System.
Tissue Microarrays and Immunohistochemical Analyses
A cohort of 129 patients with invasive BCa and their sub-classification by gene expression array were described previously (25, 26). Tissue microarrays were prepared from archived tissue blocks containing representative tumor tissues of 114 cases of this cohort (26, 27). SOX9 expression was detected by standard immunohistochemical methods using a SOX9-specific antibody (O9-1) as described (28). The SOX9 expression in tumor cells was blindly scored by a study pathologist (Xin Yuan) and was categorized according to the percentage of the tumor cells showing distinctively positive nuclear staining: <2% (SOX9 negative), between 2–30% (SOX9 intermediate), and >30% (SOX9 high). The Fishers Exact Test was used to statistically analyze the association of the SOX9 immunostaining score among BCa subtypes, p53 staining patterns, and Bloom-Richardson tumor grades. The antibodies used in the immunohistochemical analyses of BCa xenografts or mouse mammary tissues are detailed in the supplemental data.
Meta-analysis of Gene Expression
SOX9 mRNA expression levels (relative expression units) of the 114 cases were determined from the Affymetrix U133 plus 2.0 gene expression array data (NCBI GEO accession no. GSE5460) using dChip software (29). The analysis of variance function in dChip identified gene probes with significant correlation to two SOX9 probes (202936_s_at and 202935_s_at) using the p < 1e−6 as a cut-off (The p value is testing the null hypothesis that the correlation is 0). The selected genes were ranked according to the correlation coefficient.
Immunoblotting
The analyses were performed as described in Ref. 30. The primary antibodies are listed in the supplement data.
Transfection and Viral Infection
The SOX9 transactivation reporter, Col 2α1 4X48, was a gift from B. de Crombrugghe (University of Texas M. D. Anderson, Houston, TX), and the pTCF4 promoter reporter was a gift from K. Engeland (Universitat Leipzig, Leipzig, Germany). The 8× Topflash was acquired from Addgene (plasmid 12456), which was made by Ajamete Kaykas in the Moon laboratory (31). The LRP6-pCS2 was a gift from X. He (Children's Hospital, Boston, MA) (32). The cells were transfected with mixtures of DNA and Lipofectamine 2000 (Invitrogen). Cells were analyzed for luciferase activity using the Dual-Luciferase measurement system (Promega).
The siRNA transfection and retrovirus-mediated shRNA transduction were described (28, 30). Lentiviruses expressing SOX9-specific or luciferase shRNA were generated by transfecting 293T cells with hairpin-pLKO.1 plasmids, together with packaging plasmids. The virus-containing culture media were collected every 24 h for 3 days. The HCC1937 cells were incubated with the lentivirus-containing culture media plus 4 μg/ml polybrene for 48 h and then selected for 72 h in 1.5 μg/ml puromycin. The pLKO.1 luciferase hairpin plasmid was a gift from Gregory Finn (Beth Israel Deaconess Medical Center, Boston, MA) and the pLKO.1 SOX9 lentiviral plasmids were purchased from Open Biosystems (shSOX9-L1: RHS3979–9587794).
Real-time RT-PCR
Methods are described in Ref. 30. The primer/probes sets are detailed in the supplemental data.
Cell Proliferation and Invasion
50,000 cells were plated in each well of a 12-well plate, and the cells were counted on the next (day 0) and following days (days 1 and 2).
Cell invasion was measured using the QCMTM 24-well collagen-based cell invasion assay kid (Millipore). 15,000 cells were seeded in a well insert, and after 48 h, the invaded cells were stained and directly counted.
ChIP
The experiment was performed as described in Ref. 33. The following anti-SOX9 antibodies were used: Ab-1, sc-20095; Ab-2, sc-17341 (Santa Cruz Biotechnology) and Ab-3, O9-1. The primer sequences are detailed in the supplemental data.
Tetracycline-inducible MCF7 Cell Lines
The 3×FLAG-SOX9 fragment was cloned between the BamHI and EcoRI sites of pLVX-Tight-Puro vector (Clontech), which was cotransfected with pLVX-Tet-On Advanced (Clontech) into 293T cells to generate lentiviruses as described above. The inducible SOX9-expressing MCF7 cell lines were generated by lentiviral infection, followed by selection with puromycin (1 μg/ml) and G418 (1 mg/ml).
Transgenic Mice
A 3×FLAG-SOX9 cDNA fragment was cloned into the HindIII-EcoRV site of pTet-splice vector, which contains seven copies of the Tet operator (TetO, Invitrogen). To generate TetO-SOX9 transgenic mice, the XhoI-NotI fragment containing the TetO-3xFLAG-SOX9 and SV40 intron/polyadenylation signal was gel purified and injected into the pro-nuclei of fertilized mouse eggs at the Beth Israel Deaconess Transgenic Core Facility. The founders and their offspring mice were genotyped by PCR. The MMTV-tetracycline transactivator (tTA) mice (34) were kindly provided by M. Kelliher (University of Massachusetts). The sequences of the genotyping primers are listed in the supplemental data.
RESULTS
SOX9 Protein Expression in BCa
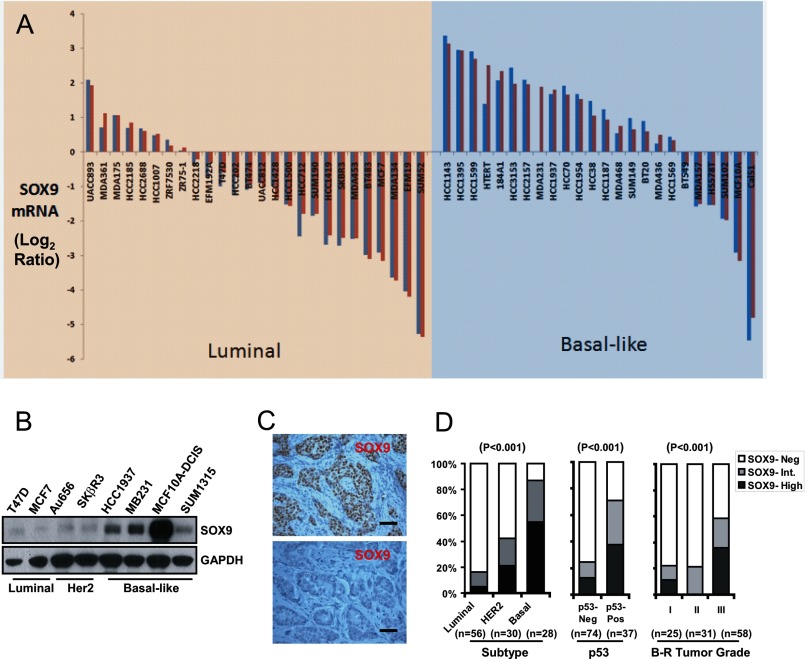
SOX9 is one of the signature genes of the BL-BCa based on mRNA expression array analyses (1, 2). We first compared SOX9 mRNA expression in various BCa cell lines of the luminal or the BL subgroups, based on a published gene expression data set (35). SOX9 expression was much more prevalent in the BL subgroup, compared with its levels in the luminal subgroup (Fig. 1A). We next examined SOX9 protein expression in a series of BCa cell lines that were sub-typed according to the above and another published gene expression-profiling classifications (35, 36). SOX9 levels were higher within the BL subgroup (HCC 1937, MDA-MB231, MCF10A-DCIS.com, and SUM 1315) compared with HER2 (Au656 and SKβR3) or luminal (T47D and MCF7) subtypes (Fig. 1B). Immunohistochemistry was then used to assess SOX9 protein expression in vivo in previously sub-typed BCa tissue microarrays (25, 26). Among these tumors, SOX9 immunoreactivity ranged from absent (<2%; Fig. 1C, lower panel), to intermediate (2–30%) and high level expression (> 30% of tumor cells positive; Fig. 1C, upper panel). SOX9 protein expression was statistically different among the BCa subtypes, with the BL subgroup containing the highest percentage of SOX9 positive (intermediate or high) samples (86%), followed by the Her 2 (43%) and the luminal subgroups (18%) (Fig. 1D, left panel).
FIGURE 1.
SOX9 expression in BCa. A, a waterfall chart of SOX9 expression in various BCa cell lines was adapted from a published database (35). The SOX9 mRNA expression level is shown as the log2 ratio of R/G, where R is the red probe signal of RNA from each individual cell line and G is the green probe signal of pooled RNA from 11 human cell lines. SOX9 mRNA levels measured by one or two independent probes were plotted. B, immunoblotting of SOX9 in BCa cell lines subgrouped according to published gene profiling, with GAPDH as a protein loading control. C, representative immunohistochemical staining of SOX9 in primary BCa tissue microarrays, demonstrating SOX9 high (upper panel) or negative (bottom panel) staining patterns. Bars, 50 μm. D, the percentage of SOX9-negative (Neg), intermediate (Int.), and high cases were compared among BCa subtypes (left panel); between negative or positive p53 immunostaining groups (middle panel); or among Bloom-Richardson (B-R) tumor grade groups (right panel). The p values of the difference analysis (Fisher's Exact Test) are indicated.
Among all cases, SOX9 staining was significantly associated with positive p53 immunostaining, an indicator of p53 mutation and consequently impaired p53 activity and a marker of poor prognosis. SOX9 was expressed in 71% of the p53 positive samples, compared with 25% of the p53 negative cases (Fig. 1D, middle panel). Furthermore, SOX9 expression varied significantly among BCa of different pathological grades, with expression in 57% of the high grade (Bloom-Richardson grade III) compared with 21 and 22% in intermediate and low grades, respectively (Bloom-Richardson grade II or I, Fig. 1D, right panel). These results indicate that SOX9 is prevalently expressed in BL-BCa and is associated with more aggressive diseases, although larger data sets are needed to determine whether SOX9 is a predictive biomarker of aggressive behavior independent of subtyping.
SOX9 Regulates LRP6 and TCF4 Expression in BCa Cells
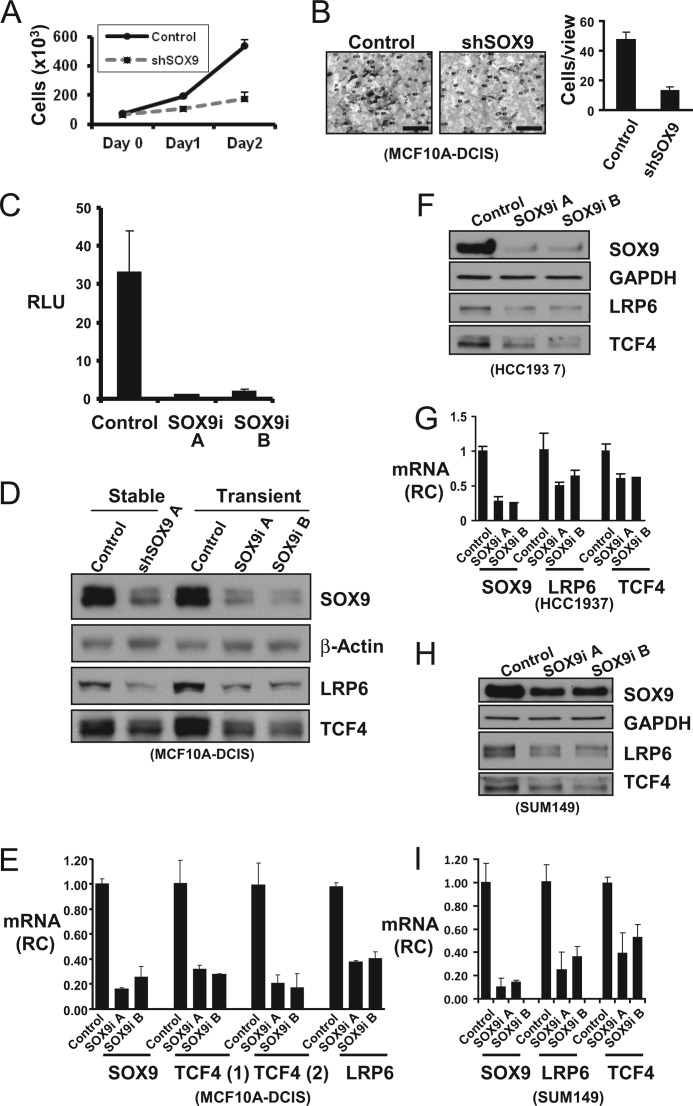
We first examined the biological functions of SOX9 in BCa cell lines. We examined the effect of SOX9 down-regulation in MCF10A DCIS.com (MCF10A-DCIS) cells, which express the highest level of endogenous SOX9 protein among the examined cell lines (Fig. 1B). MCF10A-DCIS is a BCa line established by in vitro adaptation of xenografts generated by v-Ras transformed MCF10A cells (38). Stable SOX9 silencing (shown in Fig. 2D) using retrovirus-transduced shRNA reduced cell proliferation and invasion (Fig. 2A, B).
FIGURE 2.
SOX9 supports cell proliferation, invasion, and LRP6 and TCF4 expression in BL-BCa cell lines. A, cellular proliferation measured by direct counting was plotted for control or SOX9-specific shRNA (shSOX9) expressing MCF10A-DCIS cells. B, cell invasion through collagen gel was measured at 48 h post-seeding. Representative staining of the invaded cells were shown in the left panels (bars, 100 μm). The average number of invaded cells per high power view (400×) was shown in the right panel. C, SOX9 transcriptional activity in MCF10A-DCIS cells was assessed by transfection of a SOX9 transcription reporter plasmid (Col2a1 4 × 48-luc) together with two SOX9-specific siRNA (SOX9i A and B) or a non-targeting control siRNA (Control), and a control Renilla luciferase reporter. The ratio of firefly to Renilla luciferase activity (relative light units, RLU) was measured 72 h after transfection. D, MCF10A-DCIS cells were infected with retroviruses expressing either SOX9-specific shRNA (shSOX9 A) or control shRNA and selected with puromycin for 72 h. Alternatively, the MCF10A-DCIS cells were transfected with SOX9-specific siRNA (SOX9i A or B) or control, and the cell lysates were collected at 72 h post-transfection. SOX9, LRP6, and TCF4 were immunoblotted, with β-actin as a protein loading control. E, total RNA from siRNA transfected MCF10A-DCIS cells were collected at 72 h post-transfection, and the RNA levels of SOX9, LRP6, and TCF4 (using two distinct primer/probe sets) were measured by quantitative real time RT-PCR. Relative change (RC) is calculated by comparing to control siRNA transfected cells. F and G, HCC1937 were transfected with control or SOX9-specific siRNA. The protein and mRNA levels were measured at 72 h after transfection. H and I, SUM149 cells were examined similarly as in F and G. Statistical analysis was performed with Student's t test (n = 3). Error bars, S.E.
Being a transcription factor, SOX9 is likely to carry out its biological functions in BCa through regulating the expression of its target genes. We examined genes whose mRNA levels were positively correlated with SOX9 expression among the BCa samples. The top ranked gene was TCF4 (detected by multiple probes with correlation coefficients of 0.51–0.63), whereas another gene strongly associated with SOX9 was LRP6 (correlation coefficient of 0.57) (Table 1). Interestingly, TCF4 and LRP6 are critical components of the canonical Wnt/β-catenin pathway, where LRP6 is a co-receptor for Wnt and TCF4 is a major TCF family member that co-activates with nuclear β-catenin. The correlation would be consistent with SOX9 being regulated by the Wnt/β-catenin pathway (30, 37). However, this would also be consistent with SOX9 functioning upstream of the Wnt/β-catenin pathway by regulating expression of its key components.
TABLE 1.
The SOX9-correlated genes in BCa
The gene expression arrays for 114 BCa samples were analyzed, and the genes whose expression levels were significantly associated with SOX9 (p < 1 × 10−6) were ranked according to the correlation coefficient (correlation). Genes with a correlation coefficient ≥0.50 were listed.
| Accession no. | Correlation | p value | |
|---|---|---|---|
| TCF4 | AI375916 | 0.63 | 4.30 × 10−14 |
| TCF4 | AI703074 | 0.62 | 1.72 × 10−13 |
| TCF4 | AV721430 | 0.58 | 1.83 × 10−11 |
| LRP6 | NM002336 | 0.57 | 2.97 × 10−11 |
| COL27A1 | AK021957 | 0.56 | 1.14 × 10−10 |
| GATA 6 | D87811 | 0.55 | 1.55 × 10−10 |
| CITED4 | AI858001 | 0.53 | 1.08 × 10−9 |
| CRIM1 | AW243081 | 0.53 | 1.39 × 10−9 |
| TCF4 | AA664011 | 0.52 | 2.13 × 10−9 |
| BAP28 | NM018072 | 0.52 | 3.85 × 10−9 |
| COL27A1 | AI949136 | 0.52 | 3.92 × 10−9 |
| TCF4 | AI949687 | 0.52 | 4.52 × 10−9 |
| TCF4 | AJ270770 | 0.51 | 5.61 × 10−9 |
| COL27A1 | AU145229 | 0.51 | 9.90 × 10−9 |
| BAP28 | NM018072 | 0.50 | 1.19 × 10−8 |
| NF I/X | AI817698 | 0.50 | 1.21 × 10−8 |
| TLR5 | AF051151 | 0.50 | 1.88 × 10−8 |
| CRIM1 | BG546884 | 0.50 | 2.03 × 10−8 |
SOX9 in MCF10A-DCIS cells was transcriptionally active, as indicated by the transactivation of a transfected SOX9 reporter carrying multiple SOX9-specific enhancer elements from the Col2a1 gene (39), which was markedly reduced after cotransfection of two independent SOX9 siRNAs (Fig. 2C). To directly test whether LRP6 or TCF4 were SOX9-regulated, we used immunoblotting to first confirm the down-regulation of SOX9 protein in a stable MCF10A-DCIS line expressing a SOX9-specific shRNA (stable) or by transient transfection of two SOX9 siRNAs, compared with their respective controls (Fig. 2D). Importantly, LRP6 and TCF4 protein levels were also reduced in response to stable or transient SOX9 down-regulation. Moreover, LRP6 and TCF4 mRNA levels, as measured by quantitative real-time RT-PCR, were similarly reduced in response to SOX9 silencing, indicating that SOX9 is regulating LRP6 and TCF4 at the transcriptional level (Fig. 2E).
SOX9 regulation of LRP6 and TCF4 expression was further validated using SOX9 siRNAs in two other BL-BCa cell lines, HCC1937 and SUM149. In each cell line, SOX9 down-regulation led to reduction of LRP6 and TCF4, both at the protein (Fig. 2, F and H) and mRNA levels (Fig. 2, G and I). Together, these results strongly support a SOX9 function in maintaining LRP6 and TCF4 levels in BCa.
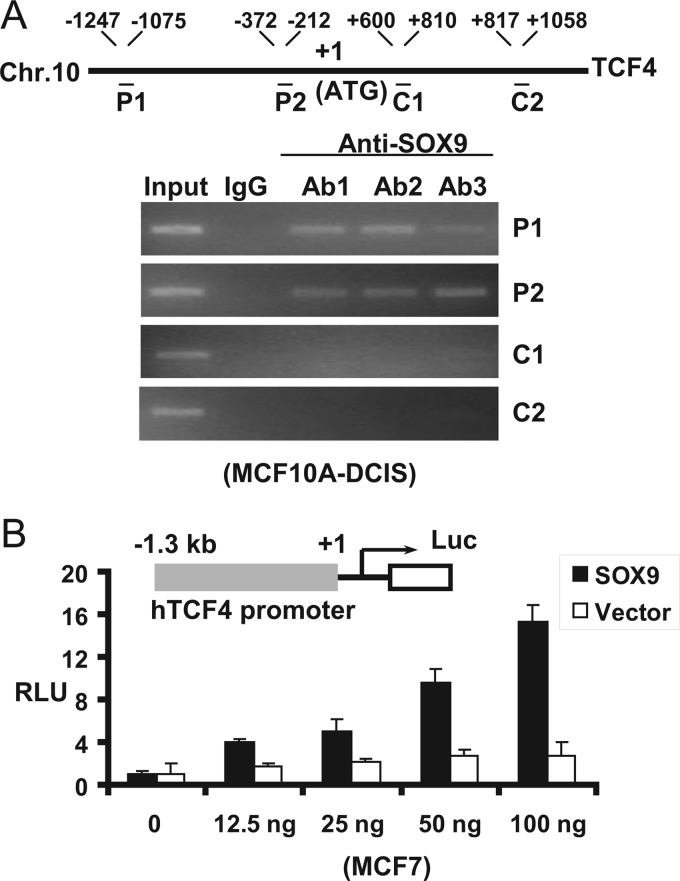
SOX9 Associates with the TCF4 Promoter
To further characterize the molecular basis for SOX9 regulation of TCF4 expression, we performed ChIP assays in MCF10A-DCIS cells to examine SOX9 interaction with the TCF4 immediate promoter region (directly or through chromatin looping with distal enhancers). Using three independent SOX9-specific antibodies, we detected binding of endogenous SOX9 to the TCF4 gene promoter region (detected by the P1 and P2 primer pairs) but not to the exon or intron regions using two primer pairs (C1 and C2) (Fig. 3A). SOX9 transactivation of the TCF4 promoter was further examined by transiently transfecting a TCF4 reporter containing 1.3 kb of the TCF4 promoter sequence (TCF4 1.3 kb-Luc) (40) into MCF7 with increasing amounts of a SOX9 expression vector, which resulted in a dose-dependent transactivation of the reporter (Fig. 3B). Although further studies are needed to pinpoint the precise SOX9 binding sites, these results strongly support the conclusion that SOX9 is a direct transcriptional regulator of the TCF4 gene. Similar studies of the LRP6 gene did not show evidence of SOX9 binding to the promoter region (data not shown), suggesting that SOX9 may bind to distant sites or indirectly influence LRP6 transcription.
FIGURE 3.
SOX9 transcriptionally activates TCF4 by binding to the TCF4 promoter. A, ChIP assay using three independent SOX9-specific antibodies (Ab1–3) was performed in MCF10A-DCIS cells. P1 and P2 are PCR fragments of the promoter region, whereas C1 and C2 are PCR fragments of exon or intron region (numbered from the translation initiation site). B, MCF7 cells were transfected with a TCF4 promoter-driven firefly luciferase reporter and a CMV-driven Renilla luciferase reporter together with increasing quantities of SOX9 expression vector or empty vector control. The luciferase activities were assessed at 72 h posttransfection. RLU, relative light unit (the ratio of firefly over Renilla luciferase light units; n = 3). Error bars show S.E.
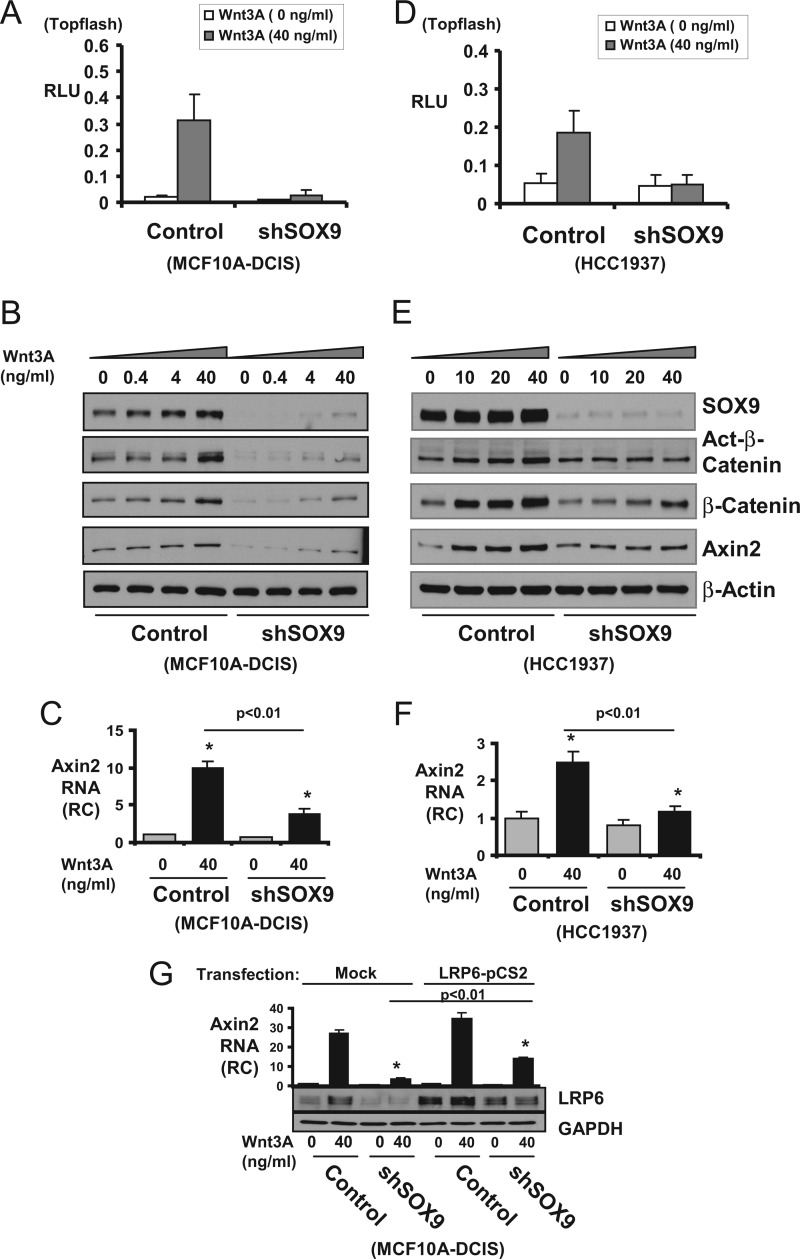
SOX9 Regulates Wnt/β-Catenin Pathway Activation
To determine whether endogenous SOX9 was required for Wnt/β-catenin pathway activation, we next examined the effect of SOX9 down-regulation on cellular responses to Wnt stimulation. SOX9 regulation of Wnt signaling was first demonstrated by the markedly reduced Wnt 3A stimulation of a β-catenin/TCF4-regulated reporter (Topflash) after SOX9 silencing in two BL-BCa cell lines (MCF10A-DCIS and HCC1937; Fig. 4, A and D). In control cells, Wnt3A caused a dose-dependent increase in the protein levels of active and total β-catenin, as well as of Axin2 (a canonical Wnt/β-catenin pathway downstream target protein) (Fig. 4, B and E). It is noteworthy that SOX9 protein levels also increased in response to Wnt3A, consistent with previously published data that SOX9 is a downstream target of the Wnt signal (30, 37). In contrast, the Wnt3A stimulation of β-catenin and Axin2 protein accumulation was diminished by SOX9 loss in SOX9 shRNA cells. Wnt3A stimulation of the Axin2 mRNA expression was also similarly hampered by SOX9 down-regulation (Fig. 4, C and F). We next tested whether exogenous LRP6 expression can rescue the Wnt/β-catenin activation in SOX9 shRNA cells. Transient transfection of a LRP6 expression vector partially rescued Wnt3A stimulated Axin2 mRNA expression in the shSOX9 cells to about half of the level in mock transfected control cells, further supporting that SOX9-mediated Wnt signaling is at least in part mediated through its regulation of LRP6 (Fig. 4G). Overall, these results indicate that SOX9 is both upstream and downstream of Wnt/β-catenin, resulting in the formation of a SOX9-regulated positive feedback loop that can enhance Wnt/β-catenin signaling.
FIGURE 4.
SOX9 enhances Wnt3A stimulation of Wnt/β-catenin activity. A and D, MCF10A-DCIS (retroviral transduced) or HCC1937 (lentiviral transduced) cells stably expressing control or SOX9-targeted shRNA were transfected with Topflash-firefly-luc together with CMV-Renilla-luc reporters for 72 h. The cells were treated with mouse Wnt3A during the last 8 h. The activity was presented as relative light unit (RLU) with firefly corrected for Renilla luciferase activity. B and E, MCF10A-DCIS or HCC1937 cells stably expressing control or SOX9-targeted shRNA were treated with mouse Wnt3A for 8 h, and the cell lysates were immunoblotted for SOX9, active (Act-) or total β-catenin and Axin2. C and F, cells were similarly treated as described in B and E. Total RNA was collected, and Axin2 mRNA was measured by quantitative real time RT-PCR. RC, relative change compared with the level in untreated control cell. G, MCF10A-DCIS cells expressing control or SOX9-specific shRNA (shSOX9) were transfected with either mock (pcDNA3) or LRP6 expression vectors (LRP6-pCS2) for 48 h. Carrier or Wnt3A (40 ng/ml) were added during the last 8 h. The LRP6 protein levels were measured by immunoblotting (bottom panels), and Axin2 mRNA levels were measured by quantitative real time RT-PCR. Statistical analysis was performed with Student's t test (n = 3). Error bars, S.E.
SOX9 Overexpression Induced LRP6 and TCF4 Expression and Enhanced Wnt/β-Catenin Pathway Activation
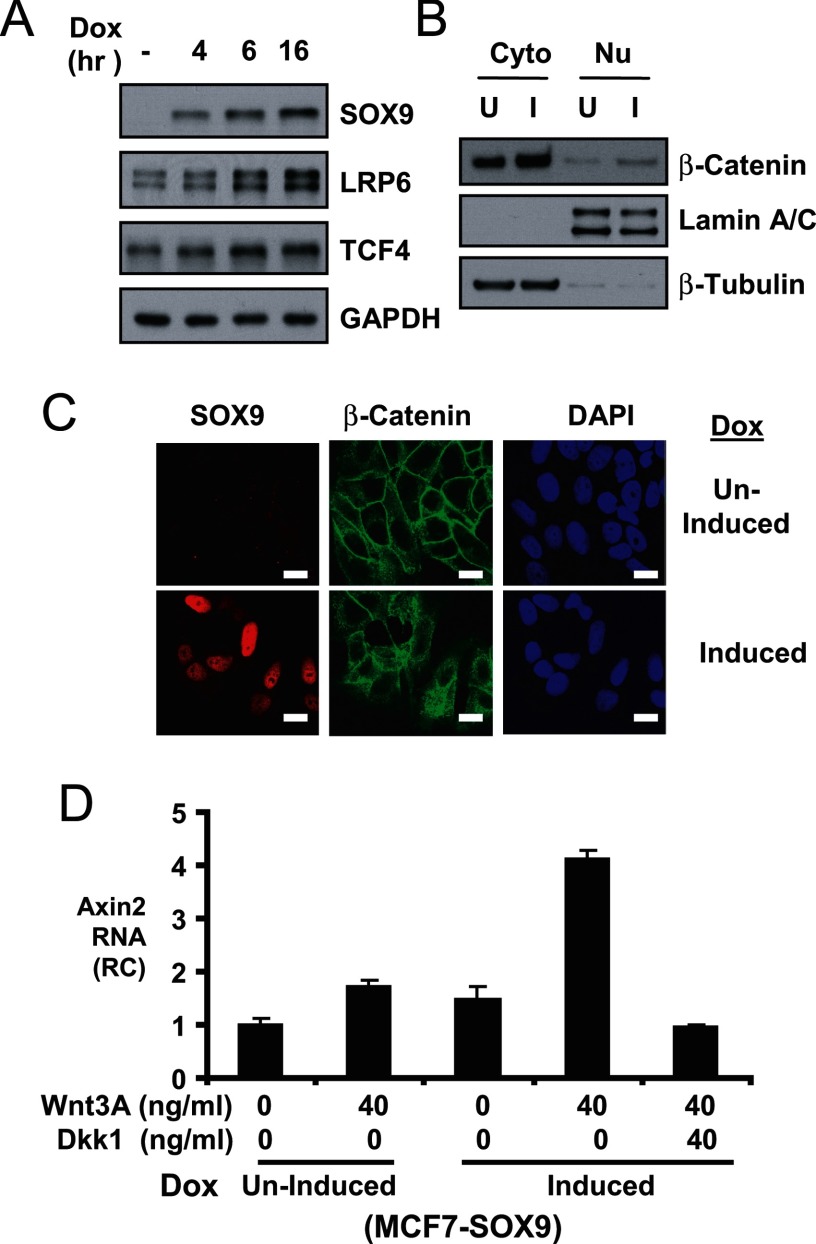
We next addressed whether SOX9 overexpression in cells expressing low levels of endogenous SOX9 was sufficient to induce LRP6 or TCF4 expression and to enhance Wnt/β-catenin pathway activation. For this purpose, we generated MCF7 BCa cell lines with tetracycline-regulated expression of exogenous FLAG-tagged SOX9. The exogenous SOX9 is increased in response to doxycycline (Fig. 5A). Importantly, LRP6 and TCF4 proteins were also increased correspondingly, supporting that SOX9 is an upstream activator of these genes.
FIGURE 5.
SOX9 in vitro overexpression leads to increased LRP6 and TCF4 expression and enhanced Wnt/β-catenin signaling. A, inducible SOX9 overexpressing MCF7 cells (MCF7-SOX9) were induced to express SOX9 with 30 ng/ml doxycycline for indicated times and blotted for SOX9, LRP6, and TCF4. GAPDH was also blotted for protein loading. B, MCF7-SOX9 were treated with 0 (uninduced, U) or 30 ng/ml doxycycline (induced, I) for 6 h. Both uninduced and induced cells were treated with mouse Wnt3A (40 ng/ml) during the last hour. Cell fractionation analysis and immunoblotting of β-catenin are shown. Lamin A/C and β-tubulin were blotted to demonstrate the effective separation of nuclear (Nu) and cytoplasmic (Cyto) fractions. C, the uninduced and Induced cells were also examined by SOX9 and β-catenin immunofluorescence analysis with a confocal microscope. 4′,6-diamidino-2-phenylindole (DAPI) was used to stain nuclei. Error bars, 20 μm. D, MCF7-SOX9 cells were treated without/with 30 ng/ml doxycycline (uninduced or induced) for 24 h. The cells were then treated with Wnt3A or Dkk1 as indicated during the last 8 h. The Axin2 mRNA levels were measured by quantitative real time RT-PCR.
We next examined SOX9 effect on Wnt3A stimulated β-catenin cytoplasmic and nuclear redistribution in these cells with induced SOX9 expression. The 6-h-induced or uninduced MCF7-SOX9 cells were treated with Wnt3A during the last hour. Measured by cell fractionation, both cytoplasmic and nuclear β-catenin was accumulated at higher levels in the SOX9-induced cells compared with uninduced cells (Fig. 5B). Immunofluorescence staining further demonstrated an increased Wnt3A-stimulated cytoplasmic and nuclear β-catenin accumulation in the induced MCF7-SOX9 cells (Fig. 5C). Wnt3A-stimulated Axin2 mRNA expression was also increased in induced versus uninduced MCF7-SOX9 cells, and the induced Axin2 level was suppressed by concomitant treatment with a LRP6 inhibitor, Dkk1 (Fig. 5D). Together, these results show that SOX9 is capable of enhancing LRP6 and TCF4 expression and boosting Wnt/β-catenin activation.
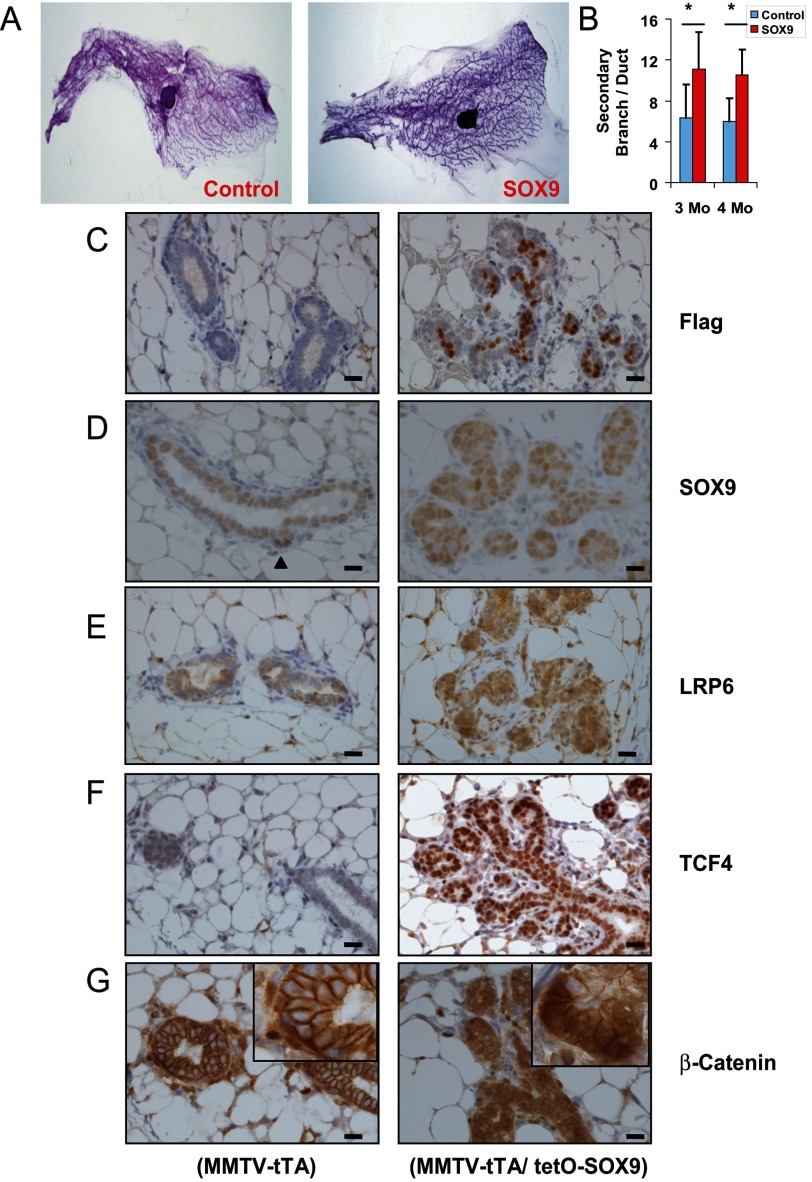
Transgenic Expression of SOX9 in Mammary Epithelium Increases Ductal Branching, LRP6 and TCF4 Expression, and Wnt/β-catenin Activity
Wnt signaling has been implicated in playing an important role during mammary development (reviewed in Ref. 41). To assess the SOX9 effect on Wnt signaling in normal breast, we generated mice with a TetO-regulated 3xFLAG-SOX9 transgene. These were crossed with mice expressing an MMTV-directed tTA to obtain double transgenic mice that constitutively express SOX9 in the absence of tetracycline (Tet-off). In 3- or 4-month-old virgin mouse mammary glands, the MMTV/SOX9 double transgenic mice showed modest, but significant increase in the number of secondary branching (Fig. 6, A and B). Expression of the FLAG-tagged SOX9 transgene was evident in a subset of the epithelial cells by anti-FLAG immunostaining (Fig. 6C). SOX9 immunostaining showed increased SOX9 expression in a similar fraction of epithelial cells (Fig. 6D). Interestingly, expression of endogenous SOX9 in control MMTV-tTA alone mice was higher in a small number of cells, especially in areas that appeared to be undergoing ductal branching (arrowhead in Fig. 6D, left panel). LRP6, TCF4, as well as cytoplamic and nuclear β-catenin levels were increased in the epithelium of double transgenic mice compared with the single transgenic mice (Fig. 6, E–G). These data showed that SOX9 can increase LRP6 and TCF4 in vivo and can enhance Wnt/β-catenin signaling and ductal proliferation in non-neoplastic breast.
FIGURE 6.
SOX9 in vivo overexpression leads to increased mammary dutal branching, increased LRP6 and TCF4 expression, and enhanced Wnt/β-catenin signaling. A, whole mount staining of 4th pair mammary tissues of 4-month-old wild type FVB (control) or MMTV-rTA/TetO-SOX9 (SOX9) mice. B, the secondary branching was counted under low power view (40×). Two mice from control or SOX9 groups were examined in either age group. In the 3-month-old groups (3 Mo), a total 39 and 37 ducts were examined in the control and SOX9 group, respectively. Similarly, 39 and 35 ducts were examined in the 4-month-old group (4 Mo). Student's t test (two-sided) was performed to determine statistical significance (*, p < 0.01). C–G, mammary tissues from 4-month-old single (MMTV-tTA) or double (MMTV-tTA/TetO-SOX9) transgenic virgin mice were examined by immunohistochemistry for FLAG-tagged SOX9 transgene (C), SOX9 (D), LRP6 (E), TCF4 (F) and β-catenin (G). Arrowhead in D indicates increased endogenous SOX9 expression at a site that appears to be branching. Insets, higher power views. Error bars, 20 μm.
DISCUSSION
SOX9 is one of the signature genes that define the BL subgroup of BCa (1, 2), but the molecular functions of SOX9 and many other BL signature genes have not been fully established in normal breast or in BCa. We found that SOX9 protein was expressed at intermediate or high levels in the majority of BL BCa and in a smaller proportion of HER2 and luminal subtypes and that its expression was associated with indicators of more aggressive disease. Significantly, SOX9 strongly correlates with LRP6 and TCF4, critical components of the canonical Wnt/β-catenin pathway. Although SOX9 expression was increased by Wnt/β-catenin pathway activation in BCa cells, we found that SOX9 also functions upstream to increase expression of LRP6 and TCF4 and showed that Tcf4 is a direct SOX9-regulated gene. SOX9-dependent Wnt/β-catenin pathway activation was demonstrated in vitro by loss of Wnt-stimulated Wnt/β-catenin activation after SOX9 silencing. Moreover, we found increased LRP6 and TCF4 expression and Wnt/β-catenin activation, as well as increased ductal branching, in transgenic mice overexpressing SOX9 in mammary epithelium. These data reveal a positive feedback loop between SOX9 and the Wnt/β-catenin signaling pathway.
The role of Wnt signaling in mammary morphogenesis is suggested by the differential expression of Wnt ligands at distinctive development stages and at different locations within the mammary ductal tree, supporting potential non-redundant Wnt functions in tissue patterning and homeostasis (42–45). Knock-out of Wnt4, Lrp5, or Lrp6 genes severely block mammary ductal branching and terminal bud formation (12, 46, 47). Conversely, increased Wnt signaling through ectopic expression of ΔN89-β-catenin (a constitutively active β-catenin) or LRP6 can induce side branching in virgin mice, similar to that seen in early pregnancy (48, 49). Our finding of increased ductal branching in virgin SOX9 transgenic mice is consistent with its mediation of Wnt signal activation.
Enhanced Wnt signaling has also been implicated in increasing mammary stem cells, especially through loss-of-function studies of LRP5/6 in vivo and Wnt-mediated long term clonal expansion of mammary stem cells in culture (12, 24, 47, 50, 51). SOX9 has similarly been implicated in supporting stem cells in small intestine and hair follicle (52–54). Recent lineage tracing studies found that SOX9 marks the adult stem cell population that contributes to self-renewal and repair of the liver, exocrine pancreas, and intestine (55). Therefore, we suggest that SOX9 may also participate in normal breast homeostasis and renewal during puberty, estrous cycling, and pregnancy, probably taking part in mammary stem cell maintenance by controlling LRP6 and TCF4 expression and supporting Wnt/β-catenin activity.
Interestingly, β-catenin can also directly interact with SOX9, so this positive feedback system may reach equilibrium when high levels of SOX9 result in sequestration of nuclear β-catenin and decreased β-catenin/TCF4 activity (56, 57). Indeed, SOX9 in chondrocytes has been reported to suppress nuclear β-catenin activity. In addition, SOX9 may have distinct biological activities in certain cell or tissue contexts due to differences in the spectrum of SOX9-regulated genes. With respect to BCa, previous studies have shown that retinoic acid can strongly induce SOX9 expression in luminal subtype cells expressing lower basal SOX9, but not in BL type cells with high basal SOX9 expression. Significantly, SOX9 induction contributes to the retinoic acid-mediated cell differentiation and growth suppression in luminal BCa cells, with one mechanism appearing to be SOX9-stimulated expression of a transcriptional repressor, HES-1 (58, 59).
Mutations in Axin, Apc, and β-catenin mediate Wnt/β-catenin pathway activation in certain types of human cancers such as colon and hepatocellular cancers (60), but these mutations are typically absent in human breast tumors. Nonetheless, aberrant nuclear and cytoplasmic localization of β-catenin in human BCa specimens has been observed, with the BL subtype in particular being enriched for tumors with higher expression of LRP6 and cytoplasmic and nuclear β-catenin (5, 6, 61). The results of this study indicate that aberrant or persistent expression of SOX9 may be an event in carcinogenesis that renders cells hyper-reactive to physiological Wnt signals by up-regulating LRP6 and TCF4 expression. Conversely, as SOX9 expression is also increased by Wnt/β-catenin signaling, aberrant Wnt activation may be an initiating event driving Wnt/β-catenin activity and SOX9 expression. In any case, this study indicates that SOX9 expression identifies a subset of tumors with increased Wnt/β-catenin pathway activation and aggressive behavior and may be predictive of responsiveness or resistance to particular therapeutics. It is reasonable to predict that newly developed Wnt/β-catenin pathway-targeted therapies will be more effective in the subgroup of BCa expressing high levels of SOX9 and its regulated Wnt/β-catenin activity.
Supplementary Material
Acknowledgments
We thank P. Berta (Institut de Genetique Humane) for providing the pcDNA3-SOX9; M. Wegner (Technische Universitat Munchen) for providing the 09-1 SOX9-specific antibody; K. Engeland (Universitat Leipzig) for providing the pTCF4-promoter reporter, B. de Crombrugghe (University of Texas MD Anderson) for providing the Col 2α1 4X48 reporter construct; X. He (Children's Hospital) for providing the LRP6-pCS2 vector; and M Kelliher (University of Massachusetts) for providing the MMTV-tTA mice. We thank Q. Wang (Dana Farber Cancer Institute) for advice on ChIP assay and G. Finn (Beth Israel Deaconess Medical Center) for providing reagents of shRNA lentivirus preparation. We thank O. Kocher and S. Schnitt (Beth Israel Deaconess Medical Center) for pathological expertise and Z. Fang for technical assistance.
This work was supported by National Institutes of Health Grant R01 DK079962 (to X. Y.); the Dana-Farber/Harvard SPORE in Prostate Cancer Grant P50 CA 090381 (to S. P. B.) and Dana-Farber/Harvard SPORE in Breast Cancer Grant CA089393 (to A. L. R.), the Breast Cancer Research Foundation (to A. L. R.), and Department of Defense Postdoctoral Award W81XWH-08-1-0160 (to H. W.).

This article contains supplemental Methods, table, and data.
- BCa
- breast cancer
- BL
- basal-like
- HMG
- high-mobility-group
- LRP
- low-density lipoprotein receptor-related protein
- TCF/LEF
- T-cell factor/lymphoid enhancer binding factor
- SOX9
- Sry-related HMG box 9
- TetO
- tetracycline operator
- tTA
- tetracycline transactivator
- MMTV
- murine mammary tumor virus.
REFERENCES
- 1. Perou C. M., Sørlie T., Eisen M. B., van de Rijn M., Jeffrey S. S., Rees C. A., Pollack J. R., Ross D. T., Johnsen H., Akslen L. A., Fluge O., Pergamenschikov A., Williams C., Zhu S. X., Lønning P. E., Børresen-Dale A. L., Brown P. O., Botstein D. (2000) Molecular portraits of human breast tumours. Nature 406, 747–752 [DOI] [PubMed] [Google Scholar]
- 2. Sørlie T., Perou C. M., Tibshirani R., Aas T., Geisler S., Johnsen H., Hastie T., Eisen M. B., van de Rijn M., Jeffrey S. S., Thorsen T., Quist H., Matese J. C., Brown P. O., Botstein D., Lønning P.E., Børresen-Dale A. L. (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. U.S.A. 98, 10869–10874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rakha E., Reis-Filho J. S. (2009) Basal-like breast carcinoma: from expression profiling to routine practice. Arch. Pathol. Lab. Med. 133, 860–868 [DOI] [PubMed] [Google Scholar]
- 4. Carey L., Winer E., Viale G., Cameron D., Gianni L. (2010) Triple-negative breast cancer: disease entity or title of convenience? Nat. Rev. Clin. Oncol. 7, 683–692 [DOI] [PubMed] [Google Scholar]
- 5. Khramtsov A. I., Khramtsova G. F., Tretiakova M., Huo D., Olopade O. I., Goss K. H. (2010) Wnt/β-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am. J. Pathol. 176, 2911–2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu C. C., Prior J., Piwnica-Worms D., Bu G. (2010) LRP6 overexpression defines a class of breast cancer subtype and is a target for therapy. Proc. Natl. Acad. Sci. U.S.A. 107, 5136–5141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Geyer F. C., Lacroix-Triki M., Savage K., Arnedos M., Lambros M. B., MacKay A., Natrajan R., Reis-Filho J. S. (2011) β-catenin pathway activation in breast cancer is associated with triple-negative phenotype but not with CTNNB1 mutation. Mod. Pathol. 24, 209–231 [DOI] [PubMed] [Google Scholar]
- 8. Clevers H. (2006) Wnt/β-catenin signaling in development and disease. Cell 127, 469–480 [DOI] [PubMed] [Google Scholar]
- 9. Lin S. Y., Xia W., Wang J. C., Kwong K. Y., Spohn B., Wen Y., Pestell R. G., Hung M. C. (2000) β-catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc. Natl. Acad. Sci. U.S.A. 97, 4262–4266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ryo A., Nakamura M., Wulf G., Liou Y. C., Lu K. P. (2001) Pin1 regulates turnover and subcellular localization of β-catenin by inhibiting its interaction with APC. Nat. Cell Biol. 3, 793–801 [DOI] [PubMed] [Google Scholar]
- 11. Nakopoulou L., Mylona E., Papadaki I., Kavantzas N., Giannopoulou I., Markaki S., Keramopoulos A. (2006) Study of phospho-β-catenin subcellular distribution in invasive breast carcinomas in relation to their phenotype and the clinical outcome. Mod. Pathol. 19, 556–563 [DOI] [PubMed] [Google Scholar]
- 12. Lindvall C., Zylstra C. R., Evans N., West R. A., Dykema K., Furge K. A., Williams B. O. (2009) The Wnt co-receptor Lrp6 is required for normal mouse mammary gland development. PLoS. ONE. 4, e5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang L., Wu X., Wang Y., Zhang K., Wu J., Yuan Y. C., Deng X., Chen L., Kim C. C., Lau S., Somlo G., Yen Y. (2011) FZD7 has a critical role in cell proliferation in triple negative breast cancer. Oncogene 30, 4437–4446 [DOI] [PubMed] [Google Scholar]
- 14. Jönsson M., Borg A., Nilbert M., Andersson T. (2000) Involvement of adenomatous polyposis coli (APC)/β-catenin signalling in human breast cancer. Eur. J. Cancer 36, 242–248 [DOI] [PubMed] [Google Scholar]
- 15. Schlosshauer P. W., Brown S. A., Eisinger K., Yan Q., Guglielminetti E. R., Parsons R., Ellenson L. H., Kitajewski J. (2000) APC truncation and increased β-catenin levels in a human breast cancer cell line. Carcinogenesis 21, 1453–1456 [PubMed] [Google Scholar]
- 16. Brennan K. R., Brown A. M. (2004) Wnt proteins in mammary development and cancer. J. Mammary Gland Biol. Neoplasia 9, 119–131 [DOI] [PubMed] [Google Scholar]
- 17. Sorlie T., Tibshirani R., Parker J., Hastie T., Marron J. S., Nobel A., Deng S., Johnsen H., Pesich R., Geisler S., Demeter J., Perou C. M., Lønning P. E., Brown P. O., Børresen-Dale A. L., Botstein D. (2003) Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl. Acad. Sci. U.S.A. 100, 8418–8423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schepers G. E., Teasdale R. D., Koopman P. (2002) Twenty pairs of sox: extent, homology, and nomenclature of the mouse and human sox transcription factor gene families. Dev. Cell 3, 167–170 [DOI] [PubMed] [Google Scholar]
- 19. Foster J. W., Dominguez-Steglich M. A., Guioli S., Kwok G., Weller P. A., Stevanović M., Weissenbach J., Mansour S., Young I. D., Goodfellow P. N. (1994) Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature 372, 525–530 [DOI] [PubMed] [Google Scholar]
- 20. Wagner T., Wirth J., Meyer J., Zabel B., Held M., Zimmer J., Pasantes J., Bricarelli F. D., Keutel J., Hustert E., Wolf U., Tommerup N., Schempp W., Scherer G. (1994) Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell 79, 1111–1120 [DOI] [PubMed] [Google Scholar]
- 21. De Santa Barbara P., Bonneaud N., Boizet B., Desclozeaux M., Moniot B., Sudbeck P., Scherer G., Poulat F., Berta P. (1998) Direct interaction of SRY-related protein SOX9 and steroidogenic factor 1 regulates transcription of the human anti-Müllerian hormone gene. Mol. Cell Biol. 18, 6653–6665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Crombrugghe B., Lefebvre V., Behringer R. R., Bi W., Murakami S., Huang W. (2000) Transcriptional mechanisms of chondrocyte differentiation. Matrix Biol. 19, 389–394 [DOI] [PubMed] [Google Scholar]
- 23. Kamachi Y., Uchikawa M., Kondoh H. (2000) Pairing SOX off: with partners in the regulation of embryonic development. Trends Genet. 16, 182–187 [DOI] [PubMed] [Google Scholar]
- 24. Guo W., Keckesova Z., Donaher J. L., Shibue T., Tischler V., Reinhardt F., Itzkovitz S., Noske A., Zürrer-Härdi U., Bell G., Tam W. L., Mani S. A., van Oudenaarden A., Weinberg R. A. (2012) Slug and Sox9 cooperatively determine the mammary stem cell state. Cell 148, 1015–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang Z. C., Lin M., Wei L. J., Li C., Miron A., Lodeiro G., Harris L., Ramaswamy S., Tanenbaum D. M., Meyerson M., Iglehart J. D., Richardson A. (2004) Loss of heterozygosity and its correlation with expression profiles in subclasses of invasive breast cancers. Cancer Res. 64, 64–71 [DOI] [PubMed] [Google Scholar]
- 26. Lu X., Lu X., Wang Z. C., Iglehart J. D., Zhang X., Richardson A. L. (2008) Predicting features of breast cancer with gene expression patterns. Breast Cancer Res. Treat. 108, 191–201 [DOI] [PubMed] [Google Scholar]
- 27. Matros E., Wang Z. C., Lodeiro G., Miron A., Iglehart J. D., Richardson A. L. (2005) BRCA1 promoter methylation in sporadic breast tumors: relationship to gene expression profiles. Breast Cancer Res. Treat. 91, 179–186 [DOI] [PubMed] [Google Scholar]
- 28. Wang H., Leav I., Ibaragi S., Wegner M., Hu G. F., Lu M. L., Balk S. P., Yuan X. (2008) SOX9 is expressed in human fetal prostate epithelium and enhances prostate cancer invasion. Cancer Res. 68, 1625–1630 [DOI] [PubMed] [Google Scholar]
- 29. Li C., Wong W. H. (2001) Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc. Natl. Acad. Sci. U.S.A. 98, 31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang H., McKnight N. C., Zhang T., Lu M. L., Balk S. P., Yuan X. (2007) SOX9 is expressed in normal prostate basal cells and regulates androgen receptor expression in prostate cancer cells. Cancer Res. 67, 528–536 [DOI] [PubMed] [Google Scholar]
- 31. DasGupta R., Kaykas A., Moon R. T., Perrimon N. (2005) Functional genomic analysis of the Wnt-wingless signaling pathway. Science 308, 826–833 [DOI] [PubMed] [Google Scholar]
- 32. Tamai K., Zeng X., Liu C., Zhang X., Harada Y., Chang Z., He X. (2004) A mechanism for Wnt coreceptor activation. Mol. Cell 13, 149–156 [DOI] [PubMed] [Google Scholar]
- 33. Shang Y., Myers M., Brown M. (2002) Formation of the androgen receptor transcription complex. Mol. Cell 9, 601–610 [DOI] [PubMed] [Google Scholar]
- 34. Hennighausen L., Wall R. J., Tillmann U., Li M., Furth P. A. (1995) Conditional gene expression in secretory tissues and skin of transgenic mice using the MMTV-LTR and the tetracycline responsive system. J. Cell Biochem. 59, 463–472 [DOI] [PubMed] [Google Scholar]
- 35. Kao J., Salari K., Bocanegra M., Choi Y. L., Girard L., Gandhi J., Kwei K. A., Hernandez-Boussard T., Wang P., Gazdar A. F., Minna J. D., Pollack J. R. (2009) Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. PLoS ONE 4, e6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Neve R. M., Chin K., Fridlyand J., Yeh J., Baehner F. L., Fevr T., Clark L., Bayani N., Coppe J. P., Tong F., Speed T., Spellman P. T., DeVries S., Lapuk A., Wang N. J., Kuo W. L., Stilwell J. L., Pinkel D., Albertson D. G., Waldman F. M., McCormick F., Dickson R. B., Johnson M. D., Lippman M., Ethier S., Gazdar A., Gray J. W. (2006) A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 10, 515–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Blache P., van de Wetering M., Duluc I., Domon C., Berta P., Freund J. N., Clevers H., Jay P. (2004) SOX9 is an intestine crypt transcription factor, is regulated by the Wnt pathway, and represses the CDX2 and MUC2 genes. J. Cell Biol. 166, 37–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miller F. R., Santner S. J., Tait L., Dawson P. J. (2000) MCF10DCIS.com xenograft model of human comedo ductal carcinoma in situ. J. Natl. Cancer Inst. 92, 1185–1186 [DOI] [PubMed] [Google Scholar]
- 39. Zhou G., Lefebvre V., Zhang Z., Eberspaecher H., de Crombrugghe B. (1998) Three high mobility group-like sequences within a 48-base pair enhancer of the Col2a1 gene are required for cartilage-specific expression in vivo. J. Biol. Chem. 273, 14989–14997 [DOI] [PubMed] [Google Scholar]
- 40. Rother K., Johne C., Spiesbach K., Haugwitz U., Tschöp K., Wasner M., Klein-Hitpass L., Möröy T., Mössner J., Engeland K. (2004) Identification of Tcf-4 as a transcriptional target of p53 signalling. Oncogene 23, 3376–3384 [DOI] [PubMed] [Google Scholar]
- 41. Roarty K., Rosen J. M. (2010) Wnt and mammary stem cells: hormones cannot fly wingless. Curr. Opin. Pharmacol. 10, 643–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gavin B. J., McMahon A. P. (1992) Differential regulation of the Wnt gene family during pregnancy and lactation suggests a role in postnatal development of the mammary gland. Mol. Cell Biol. 12, 2418–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bühler T. A., Dale T. C., Kieback C., Humphreys R. C., Rosen J. M. (1993) Localization and quantification of Wnt-2 gene expression in mouse mammary development. Dev. Biol. 155, 87–96 [DOI] [PubMed] [Google Scholar]
- 44. Weber-Hall S. J., Phippard D. J., Niemeyer C. C., Dale T. C. (1994) Developmental and hormonal regulation of Wnt gene expression in the mouse mammary gland. Differentiation 57, 205–214 [DOI] [PubMed] [Google Scholar]
- 45. Kouros-Mehr H., Werb Z. (2006) Candidate regulators of mammary branching morphogenesis identified by genome-wide transcript analysis. Dev. Dyn. 235, 3404–3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brisken C., Heineman A., Chavarria T., Elenbaas B., Tan J., Dey S. K., McMahon J. A., McMahon A. P., Weinberg R. A. (2000) Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev. 14, 650–654 [PMC free article] [PubMed] [Google Scholar]
- 47. Lindvall C., Evans N. C., Zylstra C. R., Li Y., Alexander C. M., Williams B. O. (2006) The Wnt signaling receptor Lrp5 is required for mammary ductal stem cell activity and Wnt1-induced tumorigenesis. J. Biol. Chem. 281, 35081–35087 [DOI] [PubMed] [Google Scholar]
- 48. Imbert A., Eelkema R., Jordan S., Feiner H., Cowin P. (2001) δ N89 β-catenin induces precocious development, differentiation, and neoplasia in mammary gland. J. Cell Biol. 153, 555–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang J., Li Y., Liu Q., Lu W., Bu G. (2010) Wnt signaling activation and mammary gland hyperplasia in MMTV-LRP6 transgenic mice: implication for breast cancer tumorigenesis. Oncogene 29, 539–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Badders N. M., Goel S., Clark R. J., Klos K. S., Kim S., Bafico A., Lindvall C., Williams B. O., Alexander C. M. (2009) The Wnt receptor, Lrp5, is expressed by mouse mammary stem cells and is required to maintain the basal lineage. PLoS ONE 4, e6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zeng Y. A., Nusse R. (2010) Wnt proteins are self-renewal factors for mammary stem cells and promote their long-term expansion in culture. Cell Stem Cell 6, 568–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bastide P., Darido C., Pannequin J., Kist R., Robine S., Marty-Double C., Bibeau F., Scherer G., Joubert D., Hollande F., Blache P., Jay P. (2007) Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J. Cell Biol. 178, 635–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mori-Akiyama Y., van den Born M., van Es J. H., Hamilton S. R., Adams H. P., Zhang J., Clevers H., de Crombrugghe B. (2007) SOX9 is required for the differentiation of paneth cells in the intestinal epithelium. Gastroenterology 133, 539–546 [DOI] [PubMed] [Google Scholar]
- 54. Vidal V. P., Chaboissier M. C., Lützkendorf S., Cotsarelis G., Mill P., Hui C. C., Ortonne N., Ortonne J. P., Schedl A. (2005) Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr. Biol. 15, 1340–1351 [DOI] [PubMed] [Google Scholar]
- 55. Furuyama K., Kawaguchi Y., Akiyama H., Horiguchi M., Kodama S., Kuhara T., Hosokawa S., Elbahrawy A., Soeda T., Koizumi M., Masui T., Kawaguchi M., Takaori K., Doi R., Nishi E., Kakinoki R., Deng J. M., Behringer R. R., Nakamura T., Uemoto S. (2011) Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat. Genet. 43, 34–41 [DOI] [PubMed] [Google Scholar]
- 56. Topol L., Chen W., Song H., Day T. F., Yang Y. (2009) Sox9 inhibits Wnt signaling by promoting β-catenin phosphorylation in the nucleus. J. Biol. Chem. 284, 3323–3333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Akiyama H., Lyons J. P., Mori-Akiyama Y., Yang X., Zhang R., Zhang Z., Deng J. M., Taketo M. M., Nakamura T., Behringer R. R., McCrea P. D., de Crombrugghe B. (2004) Interactions between Sox9 and β-catenin control chondrocyte differentiation. Genes Dev. 18, 1072–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Afonja O., Raaka B. M., Huang A., Das S., Zhao X., Helmer E., Juste D., Samuels H. H. (2002) RAR agonists stimulate SOX9 gene expression in breast cancer cell lines: evidence for a role in retinoid-mediated growth inhibition. Oncogene 21, 7850–7860 [DOI] [PubMed] [Google Scholar]
- 59. Müller P., Crofts J. D., Newman B. S., Bridgewater L. C., Lin C. Y., Gustafsson J. A., Ström A. (2010) SOX9 mediates the retinoic acid-induced HES-1 gene expression in human breast cancer cells. Breast Cancer Res. Treat 120, 317–326 [DOI] [PubMed] [Google Scholar]
- 60. Polakis P. (2000) Wnt signaling and cancer. Genes Dev. 14, 1837–1851 [PubMed] [Google Scholar]
- 61. Hatsell S., Rowlands T., Hiremath M., Cowin P. (2003) β-catenin and Tcfs in mammary development and cancer. J. Mammary Gland Biol. Neoplasia 8, 145–158 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.