Background: Gadd45a inhibits tumor initiation and progression via multiple pathways.
Results: Gadd45a disruption stimulates tumor angiogenesis by increasing VEGFa expression and STAT3 transcriptional activity.
Conclusion: Gadd45a suppresses tumor angiogenesis.
Significance: These findings give insights into Gadd45a functions in inhibiting tumors and indicate Gadd45a to be an effective target in anticancer treatment.
Keywords: Gene Regulation, Signal Transduction, STAT Transcription Factor, Tumor Metastases, Tyrosine Protein Kinase (Tyrosine Kinase)
Abstract
Gadd45a, a p53-regulated and DNA damage-inducible gene, is implicated in protection against tumor malignancy, although the underlying mechanism remains to be defined further. Here we demonstrate that Gadd45a plays an important role in suppression of tumor angiogenesis. Gadd45a deletion significantly increases microvessel density in tumors and stimulates an angiogenic response in a chicken embryo chorioallantoic membrane assay. Disruption of endogenous Gadd45a promotes tube formation and migration of endothelial cells. We further show that Gadd45a deletion increases phosphorylation of STAT3 at Ser-727 and, in turn, elevates the STAT3 transcriptional activity. This process substantially induces both expression and secretion of VEGFa, a STAT3 responsive gene, and promotes tumor angiogenesis. Interestingly, Gadd45a is able to physically associate with mammalian target of rapamycin (mTOR), a kinase that mediates Ser-727 phosphorylation of STAT3. The interaction of Gadd45a with mTOR suppresses STAT3 phosphorylation at Ser-727 and leads to down-regulated expression of VEGFa. Further analysis reveals that Gadd45a overexpression attenuates the association between mTOR and STAT3, whereas Gadd45a disruption strengthens this interaction, indicating that Gadd45a suppression of STAT3 phosphorylation is mainly through the dissociation of mTOR with STAT3. Taken together, these findings provide the first evidence that Gadd45a inhibits tumor angiogenesis via blocking of the mTOR/STAT3 pathway.
Introduction
Gadd45a, a ubiquitously expressed and DNA damage-responsive protein, is induced by varieties of genotoxic stress agents such as UV radiation, ionizing radiation, methyl methanesulfonate, and hydroxyurea. It plays important roles in suppressing cell proliferation, mediating cell cycle arrest, promoting apoptosis, inducing DNA repair, and stabilizing genomics (1–4). Mouse embryonic fibroblasts (MEFs)2, derived from Gadd45a knockouts, exhibit centrosome amplification, incomplete cytokinesis, increase of cell proliferation, loss of normal cellular senescence, and reduction in DNA repair (5). Gadd45a is an especially dominant factor in protecting against carcinogenesis. High-frequency mutations or abnormal epigenetic modification of the Gadd45a locus have been identified in pancreatic cancer, breast cancer, and prostate cancer (6–8). It has also been found that Gadd45a suppresses tumor invasion and metastasis by decreasing MMPs and maintaining cell-to-cell adhesion in vitro (9, 10). In vivo experiments have demonstrated that Gadd45a knockout mice display an increase in ionizing radiation- and chemical carcinogen-induced tumors. Most of them are more aggressively malignant than the control mice (5, 11). To date, vast implications unveil the key roles of Gadd45a in tumor initiation and progression. However, little insight has been gained into Gadd45a regulation of tumor angiogenesis, which needs further investigation.
Angiogenesis takes place in series of pathological processes, including inflammatory reactions, ischemic diseases, and cancer. In early research it has been demonstrated that invasive tumor growth and metastasis depend on the formation of new blood vessels (12). It is expected that the growth of avascular tumors can be delayed effectively by restricting nutrient supply and waste exchange. Angiogenesis also assists tumor cells to leave the primary site and enter circulation and, therefore, facilitates tumor metastasis. On the other hand, these results imply that suppressing angiogenesis can be a potential strategy in tumor progression control.
Tumor angiogenesis is a complex process involving the interplay between environmental and genetic signals. Various mutations in cancer may abnormally activate stimulators of angiogenesis and result in switching on the neovasculature. Among all pluripotent transcription factors, STAT3 plays a critical role in tumor angiogenesis, development, and metastasis (13, 14). Its constitutive activation has been frequently observed in various cancers, which generally indicates a poor prognosis (15, 16). STAT3 has also been confirmed to activate the expression of angiogenic stimulators, including VEGFa and MMPs (14). The transcriptional activity of STAT3 is suggested to be activated by its phosphorylation at Tyr-705 and maximized by its phosphorylation at Ser-727 (17). It is noted that the second process can be mediated by mTOR (18), which is a serine/threonine protein kinase and controls cell growth, motility, survival protein synthesis, and transcription (19). The mTOR growth signals are abnormally hyperactive in cancers, rendering mTOR a potential target in cancer therapy (19). It has also been found that mTOR promotes angiogenesis by stabilizing HIF-1a and stimulating VEGFa expression (20).
In this report we show that Gadd45a inhibits tumor angiogenesis by comprehensively considering the functionalities from both STAT3 and mTOR at the same time. We demonstrate that Gadd45a disruption increases expression of VEGFa and promotes formation of tumor blood vessels. Moreover, Gadd45a expression disrupts the interaction between mTOR and STAT3 and suppresses phosphorylation of STAT3 at Ser-727. These findings provide the first demonstration that Gadd45a plays a negative role in tumor angiogenesis by blocking the mTOR/STAT3 pathway.
EXPERIMENTAL PROCEDURES
Cell Culture and Transfection
Gadd45a+/+ and Gadd45a−/− MEFs transformed by Ras/E1A were kindly provided by Professor Albert J. Fornance of Georgetown University (5). The two MEFs as well as HeLa cells were cultured with DMEM containing 10% FBS. Human umbilical vein endothelial cells (HUVECs) were maintained using endothelial cell medium (Sciencell). For transient transfections, cells were grown on 60-mm plates in ∼60–70% confluence and transfected with 8 μg of plasmid or 200 pmol of siRNA by using Lipofectamine 2000 (Invitrogen).
Reagents and Plasmids
Rapamycin was purchased from Calbiochem. siRNAs for Gadd45a and STAT3 were synthesized by Invitrogen. The antibodies, including STAT3, P-Ser STAT3, P-Tyr STAT3, and Gadd45a, were available commercially from Cell Signaling Technology. Antibodies against von Willebrand Factor, CD31, VEGFa, myc, PCNA, and BAX were ordered from Millipore, Abcam, R&D, and Santa Cruz Biotechnology, respectively. mTOR antibodies were purchased from both Cell Signaling Technology and Millipore. The Myc-Gadd45a vector was described in our previous study (1). pTA-luc and pSTAT3-TA-luc were kindly provided by Professor Yung H. Wong of Hong Kong University (21).
Microarray Analysis
Total RNA was isolated with TRIzol from cultured cells. Expression profiling arrays and statistical analysis were performed by Capitalbio Corp. (Beijing, China).
Quantitative Reverse Transcription PCR
Total RNA isolation was performed by following the procedure in the microarray analysis. cDNA was synthesized from 5 μg of total RNA by using a Superscript II first-strand synthesis kit (Invitrogen). Then, specific gene expression was detected by using SYBR Premix EX TaqII (TaKaRa) in an ABI PRISM 7300 sequence detection system.
Western Blotting Assay
A Western blotting assay was conducted as described previously in our earlier report (10). Specific proteins were detected by chemiluminescense (APPLYGEN, China) with primary antibodies and horseradish peroxidase-conjugated secondary antibodies (Promega).
Conditioned Media Concentration
Cells were grown on 100-mm plates in about 80% confluence and cultured with media free of serum for 24 h. Conditioned media were collected after centrifugation at 2000 rpm at the temperature of 4 °C for 10 min. After being centrifuged at 12,000 rpm for 20 min, the supernatants were ultrafiltered with an Amicon Ultra-10K device (Millipore) at 5000 rpm for 20 min. Concentrated media (10 μg) were analyzed by Western blot analysis.
GST Pull-down and Immunoprecipitation
GST-Gadd45a fusion protein was expressed and isolated as described in Ref. 2. It was purified by glutathione-Sepharose 4B (GE Healthcare, Buckinghamshire, UK). After glutathione-Sepharose 4B-conjugated GST fusion proteins were mixed with cellular lysates (1 mg) for 6 h at 4 °C, it was washed five to seven times with lysis buffer and boiled in loading buffer. The binding proteins were analyzed by Western blotting assay. For immunoprecipitation, cellular lysates (1 mg) were incubated with 5 μg of antibody for 6 h at 4 °C. After addition of the protein A/G Plus-agarose (Santa Cruz Biotechnology), it was incubated for another 6 h at 4 °C. The agarose with immunocomplexes was treated as a GST pull-down assay.
Luciferase Reporter Assay
pSTAT3-TA-luc and internal control plasmid pRL-SV40 were cotransfected into cells. The luciferase assay was performed by using a dual-luciferase reporter assay system (Promega). Luciferase activity was normalized against an internal control to correct for the variations in transfection efficiency.
Endothelial Migration and Matrigel Endothelial Cell Tube Formation Assays
Cell-conditioned media were collected and stored at −80 °C. HUVECs (1 × 105) were transferred into each transwell insert (24-well plate, 8.0 μm, Corning). A mixture of conditioned medium (300 μl) and DMEM (300 μl) with 2% FBS was added into the lower compartment of the transwell. After 4 h of incubation, the cells that migrated through the membrane were fixed using 70% methanol and stained with crystal violet. The average number of cells was calculated from three fields of each insert. For tube formation assay, HUVECs (1.5 × 105) were suspended in the mixture of conditioned medium (300 μl) and DMEM (300 μl) with 10% FBS and seeded on a 24-well plate coated with 30% Matrigel (300 μl/well, BD Biosciences). Tube formation was observed after incubation for 4–6 h at 37 °C. The number of the tubular structures was measured from each field.
Chicken Embryo Chorioallantoic Membrane (CAM) Assay
Seven-day-old chicken embryos (Vital River Co., Beijing, China) were windowed to expose the CAM. Autoclaved filter papers (5 mm in diameter) were placed on the CAMs. Conditioned medium (200 μl) was added to the filter papers twice per day. After 3 days, chicken embryos were fixed and photographed. The number of blood vessels was measured in each field.
Xenograft Assays
All animal experiments were performed in accordance with relevant institutional and national guidelines and regulations. Four-week-old immune-deficient mice (BALBC/C-nu/nu, Vital River Co.) were injected subcutaneously with MEFs (1 × 106) suspended in 100 μl 0.9% sodium chloride (NaCl) solution. The volume of the xenograft tumor was calculated as length × width2 × 0.5. After 16 days the mice were sacrificed. The xenograft tumors were harvested, weighed, and photographed.
Immunohistochemistry
Xenograft tumors were fixed in 4% paraformaldehyde solution overnight, embedded in paraffin, and processed into 5-μm sections. The sections were stained with antibodies and visualized by diaminobenzidine (DAB).
PCNA and BCL2-associated X protein (BAX) immunostaining were analyzed quantitatively. The staining density was scored as 0 = no staining; 1 = weak; 2 = moderate; and 3 = strong. The portion of positively stained cells was scored as 1 = 1–4%; 2 = 5–20%; 3 = 21–40%; 4 = 41–60%; 5 = 61–80%; and 6 = 81–100%.
Blood vessels were detected with von Willebrand factor and CD31 staining. Vascular density was determined by calculating the average number of microvessels from five highly vascular fields in each section.
Statistics
Statistical analysis was performed by Student's t test. p < 0.05 (*) and 0.01 (**) were considered to be significant and highly significant, respectively.
RESULTS
Deletion of Gadd45a Significantly Promotes Tumor Angiogenesis
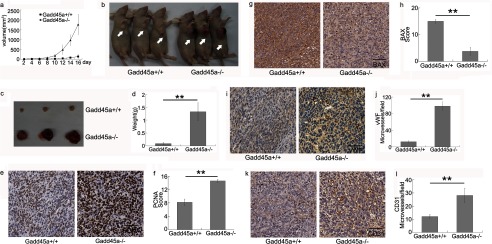
To investigate the role of Gadd45a in tumor angiogenesis, both Gadd45a+/+ and Gadd45a−/− MEFs were implanted subcutaneously into nude mice. Both MEFs were transformed by Ras/E1A. Blood vessels in xenografts were examined by immunohistochemistry. Fig. 1a summarizes the dependence of average tumor volume on time. Clearly, Gadd45a−/− MEF tumors grew more rapidly, and the tumor weights were significantly higher, than those seen in the Gadd45a+/+ counterparts after 16 days (Fig. 1, b–d). The two types of xenograft tumors were immunostained with PCNA and BAX antibodies for evaluation of proliferation and apoptosis. It is noted that PCNA expression was greatly increased in Gadd45a−/− MEF tumors compared with Gadd45a+/+ ones (Fig. 1, e and f), whereas Gadd45a+/+ MEF tumors expressed a higher level of BAX than the Gadd45a−/− ones (Fig. 1, g and h). The above observations show that cells in Gadd45a−/− MEF tumors have much higher proliferative and lower apoptotic activities. After harvesting xenograft tumors, a close inspection revealed that Gadd45a deletion tumors were redder than the control tumors (Fig. 1c), implying a substantial difference in vascularity between the two groups. The blood vessel density was analyzed by immunohistochemical staining with von Willebrand factor and CD31 antibodies, which showed an 8-fold and 2-fold increase of microvessel density, respectively, in tumors with disrupted Gadd45a as compared with the wild-type ones (Fig. 1, i–l). These results clearly show that Gadd45a is involved in suppressing tumor angiogenesis in addition to inhibiting cell growth.
FIGURE 1.
In vivo experiments show that Gadd45a deletion promotes tumor growth and angiogenesis. Gadd45a+/+ or Gadd45a−/− MEFs (1.0 × 106) were injected subcutaneously into the right armpit of mice (n = 8 for each group). a, the xenograft tumor volume (length × width2 × 0.5) was measured every other day for a total of 16 days. Average tumor volume was presented as mean ± S.E. b, after 16 days the mice were sacrificed, and tumors were removed (c). d, the weights of tumors were presented as mean ± S.E. **, p < 0.01. e and g, PCNA or BAX expressions in the two tumors were compared by immunostaining. Scale bar = 50 μm. f and h, the bar represents the scores of immunostaining analysis (staining density × score of the positively stained cell portion) as mean ± S.E. **, p < 0.01. i and k, the blood vessels in tumors were immunostained with von Willebrand factor (vWF) or CD31 antibody and counterstained with hematoxylin. Scale bar = 50 μm. j and l, microvessel density in tumors was presented as mean ± S.E. **, p < 0.01.
Gadd45a Defect Cell-conditioned Media Stimulate Angiogenesis Both in Vivo and in Vitro
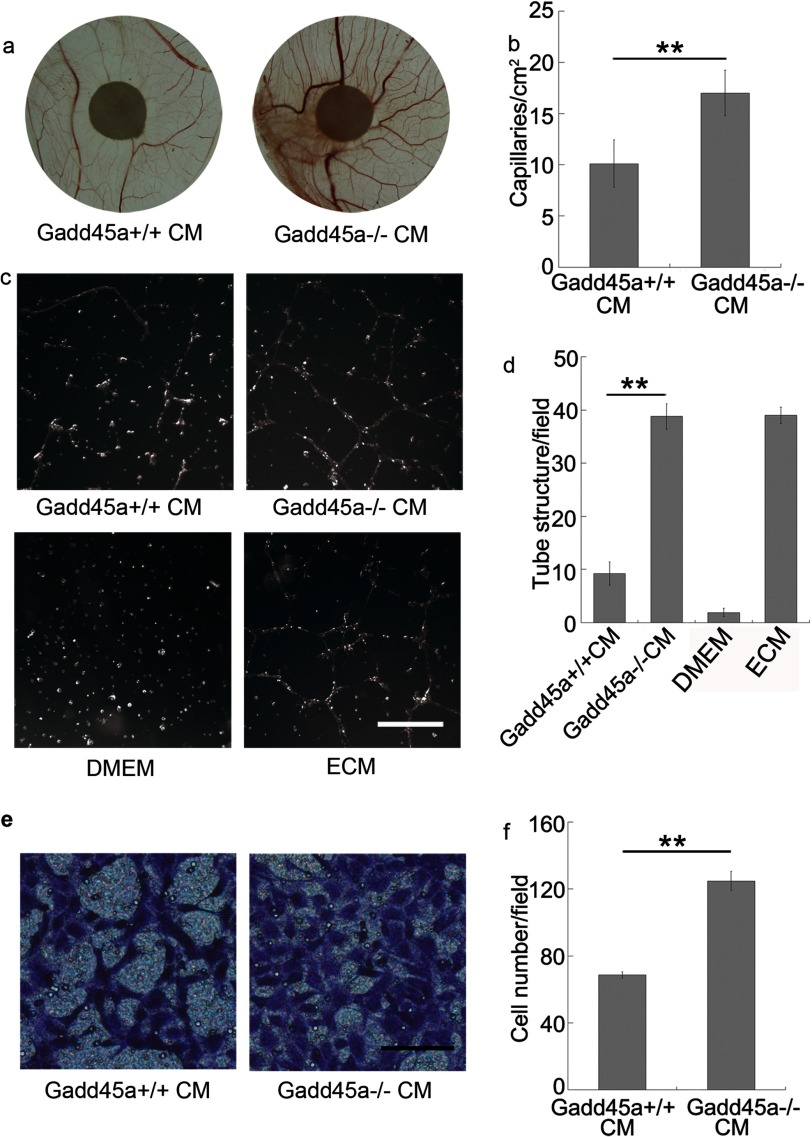
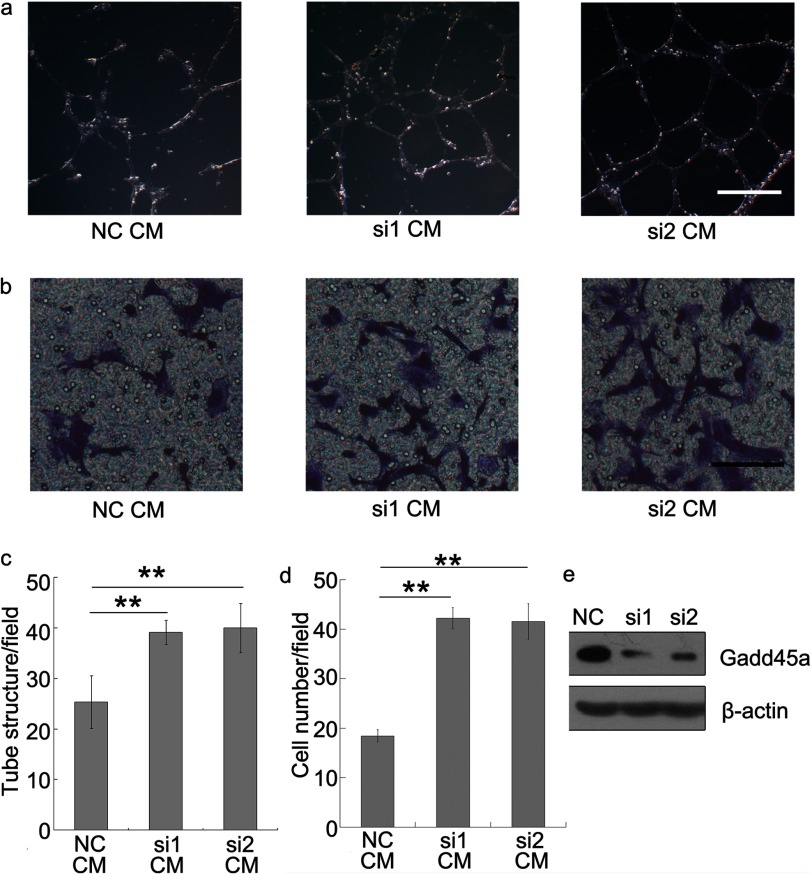
To further confirm the correlation between Gadd45a deletion and tumor angiogenesis, Gadd45a+/+ and Gadd45a−/− MEF-conditioned media (CM) were assayed for angiogenic activity, as shown in Fig. 2. In vivo experiments showed that Gadd45a−/− MEF CM induced a stronger angiogenic response in a chicken embryo CAM assay than its counterpart (Fig. 2, a and b). The difference largely reflects the distinct ability of CM from the two MEFs in modification of endothelial cell phenotype. From in vitro angiogenic assays, human umbilical vein endothelial cell (HUVEC) tube formation on Matrigel was increased by the addition of Gadd45a−/− MEF CM, which led to a much higher tube density (∼4 times) (Fig. 2, c and d). It was also noted that HUVEC migration was greatly promoted by Gadd45−/− MEF CM, compared with Gadd45+/+ MEF CM (Fig. 2, e and f). Furthermore, we performed angiogenic assays with CM from Gadd45a knockdown HeLa cells. As expected, the CM promoted both HUVEC tube formation and migration (Fig. 3) in comparison with the control CM. As a consequence, it confirms that Gadd45a functions as a suppressor in tumor angiogenesis.
FIGURE 2.
Conditioned media by Gadd45a−/− MEFs stimulates angiogenesis in CAM and promotes tube formation and migration of endothelial cells. a, angiogenic response in a chicken embryo CAM assay after 3 days. Autoclaved filter papers (5 mm in diameter) were applied to CAMs and loaded with 200 μl of medium conditioned by Gadd45a+/+ or Gadd45a−/− MEFs two times a day. b, the neovasculature number/field as mean ± S.E. n = 8. **, p < 0.01. c, tube formation assay. HUVECs (1.5 × 105) were mixed with conditioned media from Gadd45a+/+ or Gadd45a−/− MEFs and incubated for 4–6 h on Matrigel. DMEM with 10% FBS and endothelial cell medium (ECM) were used as negative and positive controls, respectively. Scale bar = 500 μm. d, the number of tube structures/field was presented as mean ± S.E. n = 3. **, p < 0.01. e, tube migration assay. HUVECs (1.0 × 105) were seeded in each insert of a transwell. The lower compartment of the transwell was supplied with conditioned medium from Gadd45a+/+ or Gadd45a−/− MEFs. After incubation for 4–6 h, the inserts were harvested, fixed using 70% methanol, and stained with crystal violet. f, the number of cells that migrated through the membrane/field was presented as mean ± S.E. n = 3. **, p < 0.01. Scale bar = 100 μm.
FIGURE 3.
Gadd45a knockdown HeLa conditioned media promote tube formation and migration of endothelial cells. The media conditioned by Gadd45a knockdown HeLa (si1 and si2) were collected. Tube formation (a) and migration assays (b) of HUVECs were performed following the procedure described in Fig. 2. NC, negative control. c, the number of tube structures/field was presented as mean ± S.E. n = 3. **, p < 0.01. d, the number of cells that migrated through the membrane/field was presented as mean ± S.E. n = 3. **, p < 0.01. Scale bar = 500 μm (a) and 100 μm (b). e, Western blot analysis evaluated the efficiency of Gadd45a knockdown.
Disruption of Gadd45a Stimulates Angiogenesis by Increasing the Expression of VEGFa
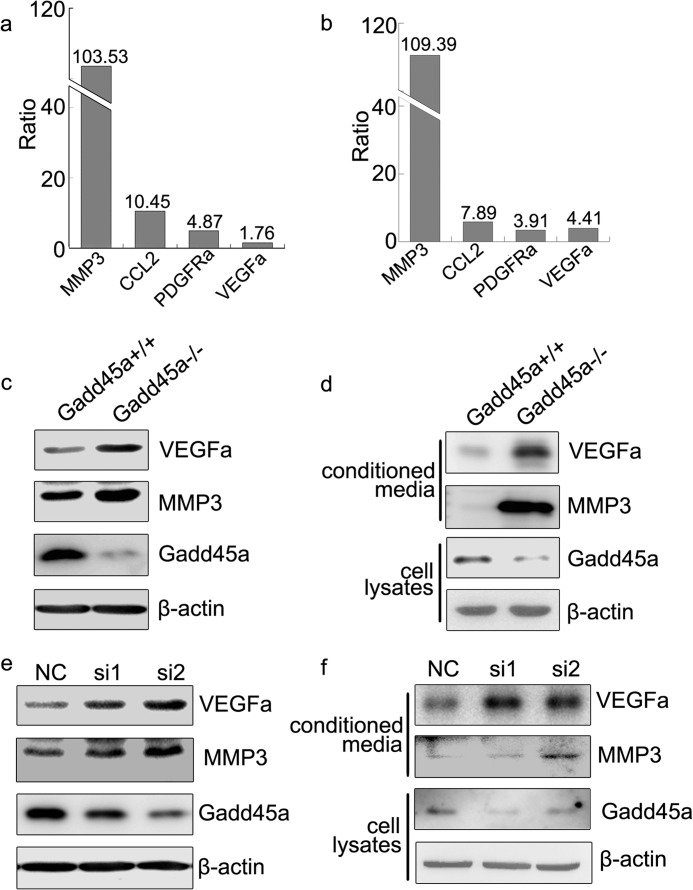
The mechanism by which Gadd45a negatively regulates tumor angiogenesis was investigated using expression profiling arrays to screen genes expressed in Gadd45a+/+ and Gadd45a−/− MEFs. It was found that angiogenic factors, including VEGFa, CCL2, platelet-derived growth factor receptor (PDGFRa), and MMP3 (22–24) were up-regulated significantly by Gadd45a deletion (Fig. 4a). The same conclusion was obtained by the quantitative RT PCR experiments (Fig. 4b). Moreover, both VEGFa and MMP3 were also confirmed at the protein level (Fig. 4c). To be more conclusive, the secreted and extracellular proteins VEGFa and MMP3 were examined further in the media conditioned by Gadd45a+/+ and Gadd45a−/− MEFs, respectively. In all circumstances, higher concentrations of VEGFa and MMP3 were detected in Gadd45a−/− MEF CM as compared with that in Gadd45a+/+ CM (Fig. 4d). The same experiments were also performed in Gadd45a knockdown HeLa cells at the protein level and gave similar results of increased expression and secretion of VEGFa and MMP3 (Fig. 4, e and f).
FIGURE 4.
Gadd45a deletion increases VEGFa and MMP3 expression. a, the ratio of MMP3, CCL2, PDGFRa, and VEGFa expression in Gadd45a−/− MEFs to that in Gadd45a+/+ MEFs from an expression profiling array assay. The ratio number is at the top of each bar. Data for Gadd45a+/+ MEFs were normalized to 1. b, quantitative RT PCR analysis shows the expression ratio of the genes in Gadd45a−/− MEFs to that in Gadd45a+/+ MEFs. The ratio numbers are at the top of the bars. Data of Gadd45a+/+ MEFs were normalized to 1. c, Western blot verification of VEGFa and MMP3 expression in the two types of MEFs. d, conditioned media by both MEFs were concentrated by ultrafiltration. A Western blot analysis was utilized to analyze secreted VEGFa and MMP3 in the media. e and f, VEGFa and MMP3 were further evaluated in HeLa cells and corresponding conditioned media after knocking down Gadd45a. NC, negative control.
Neutralizing Antibody to VEGFa Decreases Angiogenic Activity of Media Conditioned by Gadd45a+/+ and Gadd45a−/− MEFs
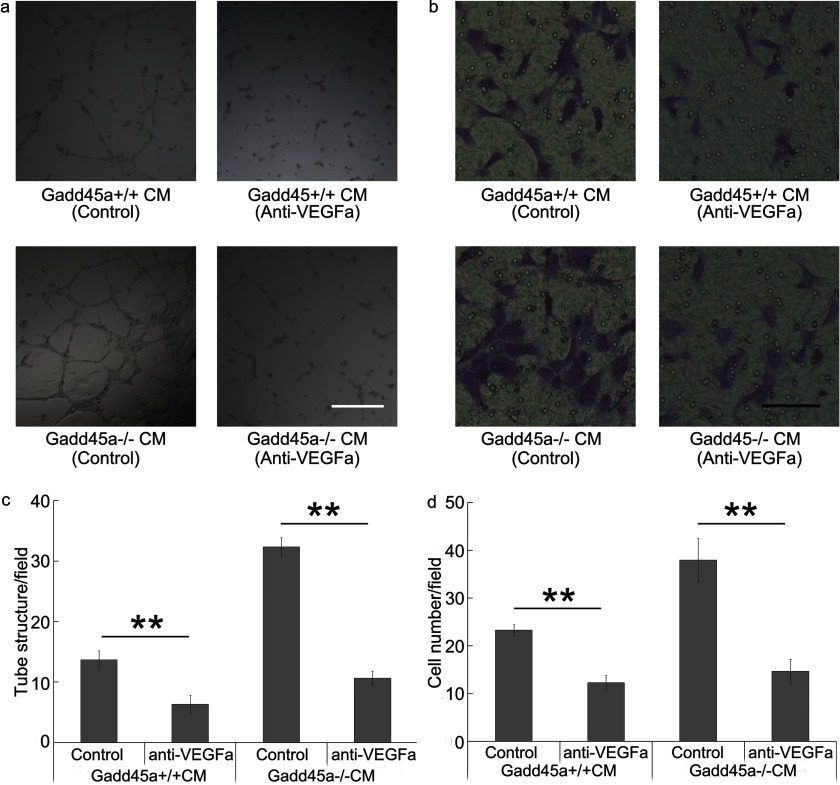
It has been confirmed that VEGFa is one of the most important angiogenic factors. To evaluate the importance of VEGFa in the angiogenic process regulated by Gadd45a, CMs from Gadd45a+/+ and Gadd45a−/− MEFs with neutralizing antibody to VEGFa were employed to analyze endothelial cell tube formation and migration (Fig. 5). Fig. 5, a and c, showed that VEGFa neutralizing antibody greatly suppressed HUVEC tube formation in CMs from both MEFs. Especially in the migration assay, the neutralizing antibody also significantly decreased the migration activity of HUVECs stimulated by media conditioned by the two MEFs (Fig. 5, b and d). Therefore, it can be concluded that disruption of endogenous Gadd45a stimulates angiogenesis by increasing VEGFa expression.
FIGURE 5.
Neutralizing antibody to VEGFa reduced angiogenic activity of CM from Gadd45a+/+ and Gadd45a−/− MEFs. a and b, after adding neutralizing antibody (0.2 μg/ml) to VEGFa into media conditioned by Gadd45a+/+ and Gadd45a−/− MEFs, tube formation and a migration assay of HUVEC were performed as described in Fig. 2. Scale bar = 500 μm (a) and 100 μm (b). c, the number of tube structures/field was presented as mean ± S.E. n = 3. **, p < 0.01. d, the number of cells that migrated through the membrane/field was presented as mean ± S.E. n = 3. **, p < 0.01.
Gadd45a Suppresses Ser-727 Phosphorylation of STAT3
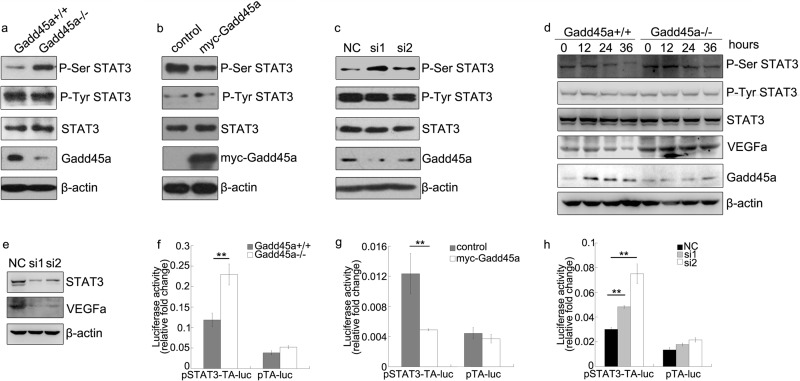
STAT3, an important multifunction transcriptional factor in tumor development and angiogenesis, has been proven to directly induce VEGFa (25). STAT3 transcriptional activity is regulated by the phosphorylation on Tyr-705 (P-Tyr STAT3) and Ser-727 (P-Ser STAT3) (17). To understand the role of Gadd45a in inhibiting tumor angiogenesis, a series of assays were conducted to examine whether Gadd45a modulates the phosphorylation of STAT3. As shown in Fig. 6a, P-Ser STAT3 was clearly up-regulated in Gadd45a−/− MEFs compared with Gadd45a+/+ MEFs. However, the similar levels of P-Tyr STAT3 were identified in the two MEFs. It was also observed that only P-Ser STAT3, but not P-Tyr STAT3, was preferentially suppressed in HeLa cells after Gadd45a overexpression (Fig. 6b). Accordingly, knocking down Gadd45a in HeLa cells significantly induced P-Ser STAT3, whereas P-Tyr STAT3 remained the same levels (Fig. 6c). The effect of Gadd45a on P-Ser STAT3 was examined further by withdrawing serum. As expected, Gadd45a was highly elevated under the condition of serum starvation, and both P-Ser STAT3 and VEGFa were rapidly decreased in Gadd45a+/+ MEFs following the serum starvation compared with Gadd45a−/− (Fig. 6d). The induction of VEGFa by STAT3 was also confirmed by knocking down STAT3 in HeLa cells (Fig. 6e). These observations indicate that Gadd45a is able to down-regulate the phosphorylation of STAT3 at Ser-727, which contributes significantly to tumor angiogenesis suppression by Gadd45a.
FIGURE 6.
Gadd45a defect leads to up-regulated P-Ser STAT3 and transcriptional activity. a, Gadd45a+/+ and Gadd45a−/− MEFs were harvested and prepared for Western blot analysis with P-Ser STAT3, P-Tyr STAT3, and Gadd45a antibodies. b and c, HeLa cells were transiently transfected with myc-Gadd45a vector or siRNAs for Gadd45a. The variation of the phosphorylation of STAT3 and total STAT3 was analyzed by immunoblots 36 h after transfection. Myc-Gadd45a was detected with myc antibody. NC was a negative control of siRNA. d, Gadd45a+/+ and Gadd45a−/− MEFs were collected for an immunoblotting assay after serum starvation for different time points, which are marked at the top of the panels. β-actin was used as the loading control. e, VEGFa expression was further evaluated after STAT3 knocked down in HeLa cells. f, for the luciferase assay, pSTAT3-TA-luc and internal control plasmid pRL-SV40 were transiently cotransfected into Gadd45a+/+ and Gadd45a−/− MEFs. The luciferase activity was normalized against the internal control. The data were presented as mean ± S.E. n = 3. g and h, the STAT3 reporter luciferase assay was performed after pSTAT3-TA-luc and pRL-SV40 were cotransfected with myc-Gadd45a vector or siRNAs for Gadd45a into HeLa cells. The relative luciferase activity was presented as mean ± S.E. n = 3. **, p < 0.01).
Because the phosphorylation of STAT3 at Ser-727 is necessary for achieving the maximal transcriptional activity of STAT3, further effort was put to understand whether Gadd45a regulates STAT3 activity via modulating its phosphorylation process. Fig. 6f showed that abrogation of Gadd45a in Gadd45a−/− MEFs increased STAT3 reporter luciferase activity in the luciferase reporter gene assay compared with wild-type MEFs. This result was further verified by the reduced STAT3 transcriptional activity following Gadd45a overexpression in HeLa cells (Fig. 6g). Similarly, STAT3 transcriptional activity was enhanced by the Gadd45a knockdown in HeLa cells (Fig. 6h). Taken together, Gadd45a defect results in increased VEGFa expression and enhanced transcriptional activity of STAT3 via increasing the STAT3 phosphorylation on Ser-727.
P-Ser STAT3 in Gadd45a+/+ and Gadd45a−/− MEFs Is Regulated by mTOR
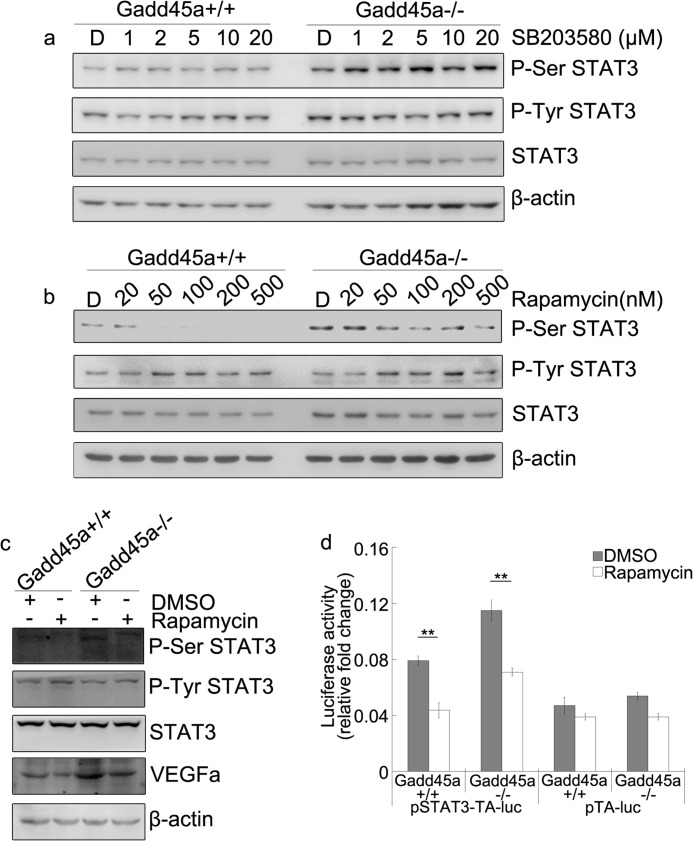
It is known that P-Ser STAT3 is induced by a series of kinases, such as p38 and mTOR (26), and that Gadd45a activates the JNK/p38 pathway by interaction with MEKK4/MTK1 (27). However, we observed that P-Ser STAT3 in Gadd45a+/+ and Gadd45a−/− MEFs has minimal response to SB203580 (p38 inhibitor) treatment (Fig. 7a). The activation of P-Ser STAT3 may be associated with mTOR (18). The effect of the mTOR inhibitor, i.e. rapamycin, on P-Ser STAT3 was examined in both MEFs. The results in Fig. 7b showed that P-Ser STAT3 was greatly attenuated in both Gadd45a+/+ and Gadd45a−/− MEFs because of the rapamycin treatment, whereas P-Tyr STAT3 was nearly unaffected. VEGFa in both MEFs was decreased substantially after rapamycin treatment (Fig. 7c). Meanwhile, the luciferase activity of STAT3 reporter in the two MEFs was found to be reduced because of the presence of rapamycin (Fig. 7d). These results demonstrate that the P-Ser STAT3 in both MEFs is directly regulated by mTOR, suggesting that Gadd45a suppression of P-Ser STAT3 might be medicated through its action on mTOR protein kinase.
FIGURE 7.
Rapamycin suppresses phosphorylation of STAT3 on Ser-727 in Gadd45a+/+ and Gadd45a−/− MEFs. Exponentially growing Gadd45a+/+ and Gadd45a−/− MEFs were treated with the indicated doses of SB203580 (a) and rapamycin (b) for 12 h. DMSO (labeled “D” on the top of panels) used as the negative control. The variation of P-Ser STAT3, P-Tyr STAT3, and total STAT3 were analyzed by immunoblot analyses. c, the variation of VEGFa was detected by immunoblot analyses in the two MEFs after rapamycin (100 nm) treatment for 36 h. DMSO, dimethyl sulfoxide. d, the STAT3 reporter luciferase assay was performed as in Fig. 4e in the two MEFs after the same treatment as in Fig. 5c. The relative luciferase activity was presented as mean ± S.E. n = 3. **, p < 0.01.
Gadd45a Disrupts the Association between mTOR and STAT3
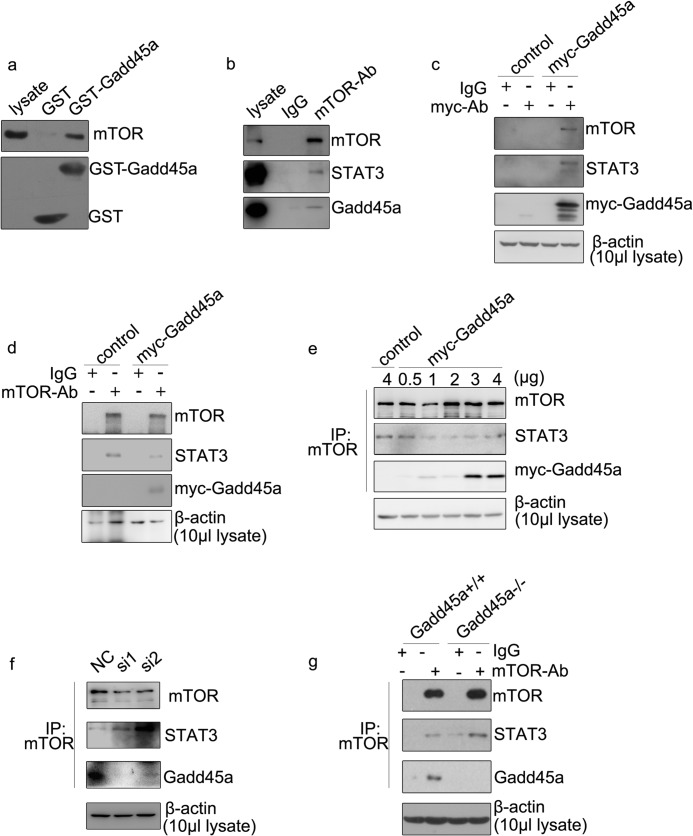
To further explore how Gadd45a regulates P-Ser STAT3, we examined the physical associations among Gadd45a, STAT3, and mTOR, as shown in Fig. 8a. This result provided the first evidence that Gadd45a interacts with mTOR in a GST pull-down assay with GST-tagged Gadd45a protein and lysates from HeLa cells. Next, cellular lysates from HeLa cells were immunoprecipitated with mTOR antibody and followed by Western blot analysis. Both mTOR and Gadd45a were detected in the immune complexes (Fig. 8b). In addition, Myc-Gadd45a or control vectors were transfected into HeLa cells. Cells were lysed for immunoprecipitation with myc or mTOR antibodies, and the immunocomplexes were subjected to Western blotting assays. In agreement with the observations in GST pull-down and immunoprecipitation assays, myc-Gadd45a fusion protein was able to associate with mTOR (Fig. 8, c and d). Meanwhile, the findings in Fig. 8, b and d, clearly identified the association of mTOR with STAT3. Interestingly, this association was reduced substantially in the presence of Gadd45a (Fig. 8d), indicating a possible regulatory effect of Gadd45a on the interaction between mTOR and STAT3. To confirm this observation, HeLa cells transfected with different doses of myc-Gadd45a vector were harvested for immunoprecipitation with mTOR antibody. An inverse correlation was shown in Fig. 8e. Increasing the myc-Gadd45a protein level led to reduction in the association between mTOR and STAT3. As expected, such an association was strengthened significantly when endogenous Gadd45a was knocked down using the siRNA approach or abrogated in Gadd45a−/− MEFs (Fig. 8, f and g). Taken together, these findings suggest that Gadd45a attenuates the association between mTOR and STAT3 by its interaction with mTOR. Therefore, a Gadd45a defect leads to enhanced association between mTOR and STAT3 which, in turn, up-regulates P-Ser STAT3 and STAT3 transcriptional activity.
FIGURE 8.
Gadd45a regulates the association between STAT3 and mTOR. a, a GST pull-down assay was performed with GST-Gadd45a and cell lysates from HeLa cells. A GST tag alone was used as an internal control. The binding proteins were analyzed with mTOR and GST antibodies. b, extracted whole proteins from HeLa cells were immunoprecipitated with mTOR antibody. The precipitates were analyzed by immunoblot analyses. HeLa cells were harvested 36 h after transient transfection with myc-Gadd45a or control vector and prepared for immunoprecipitation with myc (c) and mTOR (d) antibodies (Ab). The precipitated complexes were analyzed with immunoblot analyses. Myc-Gadd45a was detected with myc antibody. e, HeLa cells were transiently transfected with gradually increased doses of myc-Gadd45a vector. 36 h after transfection, cells were collected and prepared for immunoprecipitation (IP) with mTOR antibody. Immunocomplexes were examined by immunoblot analysis. f, HeLa cells were harvested 48 h after transient transfection of siRNA against Gadd45a. Whole protein extracts were prepared for immunoprecipitation with mTOR antibody. Immunoblot analysis was used to detected immunocomplexes. NC, negative control. g, Gadd45a+/+ and Gadd45a−/− MEFs were harvested for immunoprecipitation with mTOR antibody. The precipitated complexes were analyzed as in f. Non-specified IgG was used as an internal control. β-actin was used as the loading control.
DISCUSSION
As a multifunctional protein, Gadd45a is one of the most critical downstream targets of tumor suppressors p53 and BRCA1 and plays a key role in regulation of cell proliferation, apoptosis, cell cycle checkpoint, and genomic stability. Several lines of investigations demonstrate that Gadd45a suppresses metastasis via maintaining cell-to-cell adhesion and cell contact inhibition (10) and participates in protecting against tumor malignancy (5, 11). Gadd45a knockout mice are susceptible to DNA damage-inducible tumors (11). However, the investigations about Gadd45a suppressing tumor formation are increasing, but the mechanisms still need further understanding.
Angiogenesis symbolizes the tumor malignant transformation and contributes to tumor expansion and metastasis. Growth of new blood vessels takes place in a complex process, including the cooperation between genomic and environmental factors. A series of mutations and abnormal genomic modifications are involved to stimulate its formation in malignant tumors by promoting expression of angiogenic factors. In this study, we find that Gadd45a deletion significantly increases the microvessel formation in xenograft tumors and a CAM assay in vivo. Meanwhile, Gadd45a-defect cell CM promotes migration and tube formation of endothelial cells in vitro. Moreover, Gadd45a is involved in regulating the expression of VEGFa, which is a key stimulator of angiogenesis. By endogenously disrupting Gadd45a, both the expression and the secretion of VEGFa are enhanced. Meanwhile, neutralizing antibody against VEGFa suppresses endothelial cell tube formation and migration by CM from both Gadd45a+/+ and Gadd45a−/− MEFs. These evidences prove that Gadd45a functioned as a suppressor of tumor angiogenesis via decreasing VEGFa expression. VEGFa is regulated by various transcriptional factors including HIF1a, TWIST, and STAT3, which are commonly dysregulated in human cancers. However, Gadd45a has little effect on HIF1a and TWIST expression (data not shown). Our observation demonstrates that the regulation effect of Gadd45a on angiogenesis is closely related to the correlation between Gadd45a and STAT3. Knockdown of STAT3 obviously decreases the expression of VEGFa. The multiple pieces of evidence in this study show that the STAT3 phosphorylation at Ser-727 is largely affected by the status of cellular Gadd45a (Fig. 6, a–d). Thus, it can be concluded that Gadd45a regulation of VEGFa expression is mainly related to STAT3.
It has been well known that STAT3 activation is sufficient to induce tumor development (13) and contributes to cancer expansion and tumor angiogenesis (25). The activation of STAT3 directly promotes the expression VEGFa. Therefore, it is critical to unveil the factors in determination of STAT3 activity. The transcriptional activity depends highly on phosphorylation of two key residues. Tyr-705 phosphorylation, typically mediated by JAK, participates in STAT3 dimerization and activation, whereas Ser-727 phosphorylation accounts for achieving the maximal transcriptional activity by affecting the recruitment of cofactors (17, 28). Besides, a recent study suggests that Ser-727 phosphorylation can also activate STAT3 without the presence of P-Tyr705 (29). For the key roles of both P-Ser and P-Tyr, much effort has been made to elucidate the Gadd45 action on P-Ser and P-Tyr of STAT3. We show that Gadd45a deletion or knockdown increase the P-Ser STAT3, whereas Gadd45a overexpression reduces P-Ser STAT3. However, Gadd45a has little effect on P-Tyr STAT3. The transcriptional activity of STAT3 shows the similar trend in relation to Gadd45a in the STAT3 reporter luciferase assay experiment (Fig. 6, f–h). Furthermore, disruption of endogenous Gadd45a enhances both expression and secretion of VEGFa, which is a key target of STAT3 (Fig. 4, c–f).
Gadd45a is known to be induced by serum starvation. A crucial step is to examine how Gadd45a suppresses angiogenesis under this condition. Our study reveals that P-Ser STAT3 and VEGFa are rapidly decreased by Gadd45a induction in the Gadd45a+/+ MEFs after serum withdrawal (Fig. 6d). These findings suggest that Gadd45a suppresses VEGFa expression by decreasing P-Ser STAT3 to inhibit the transcriptional activity. Considering that the phosphorylation of STAT3 on Ser-727 is mediated by a series of kinases, such as p38 and mTOR (26), Gadd45a suppressed P-Ser STAT3 is likely mediated through its action on these upstream kinases of STAT3. Therefore, we have hypothesized that Gadd45a regulation of STAT3 might be via its interaction with mTOR/STAT3 pathway. Sequence analysis of STAT3 revealed a putative mTOR signaling (TOS) motif that indicates STAT3 as a target of raptor recruitment and mTORC1-mediated phosphorylation (30). The STAT3 transcriptional activity is strengthened by mTOR via inducing P-Ser727. This process is sensitive to rapamycin, a specific mTOR inhibitor (18). In agreement with our hypothesis, we have found that P-Ser STAT3 is obviously decreased after rapamycin treatment in Gadd45a+/+ and Gadd45a−/− MEFs, whereas it is not affected by the p38 inhibitor. Accordingly, STAT3 reporter luciferase activity and VEGFa in both MEFs are also suppressed by rapamycin treatment (Fig. 7, c and d), indicating the role of mTOR in phosphorylation of STAT3 in this cell model. Interestingly, the GST pull-down and immunoprecipitation experiments demonstrate strong interactions between Gadd45a and mTOR that lead to dissociation of mTOR/STAT3 complexes (Fig. 8, d and e). Consequently, it can be explained that the disruption of mTOR/STAT3 abrogates the STAT3 phosphorylation at Ser-727 by mTOR and down-regulates the transcriptional activity of STAT3.
In summary, we have demonstrated that Gadd45a may functions as a negative regulator in tumor angiogenesis via blocking of the mTOR/STAT3 pathway. Gadd45a disruption strengthens the association between mTOR and STAT3 and then induces the phosphorylation of STAT3 on Ser-727 to enhance its transcriptional activity. This process leads to an increase in both expression and secretion of VEGFa. These findings show a new correlation between Gadd45a and tumor angiogenesis and unveil the mechanisms of Gadd45a in protecting against carcinogenesis. As revealed by this research, it is emphasized that Gadd45a is an important target in conceiving effective anticancer treatment.
Acknowledgments
We thank Professor Albert J. Fornace and M. Christine Hollander for providing the immortalized Gadd45a−/− and matching +/+ MEF cells and Professor Yung H. Wong for providing pTA-luc and pSTAT3-TA-luc.
This work was supported by 973 National Key Fundamental Research Program of China Grant 2009CB521801 and National Natural Science Foundation of China Grant 81021061.
- MEF
- mouse embryonic fibroblast
- mTOR
- mammalian target of rapamycin
- HUVEC
- human umbilical vein endothelial cell
- CM
- conditioned medium/media
- CAM
- chorioallantoic membrane
- MMP
- matrix metalloproteinase
- PCNA
- proliferating cell nuclear antigen.
REFERENCES
- 1. Jin S., Antinore M. J., Lung F. D., Dong X., Zhao H., Fan F., Colchagie A. B., Blanck P., Roller P. P., Fornace A. J., Jr., Zhan Q. (2000) The GADD45 inhibition of Cdc2 kinase correlates with GADD45-mediated growth suppression. J. Biol. Chem. 275, 16602–16608 [DOI] [PubMed] [Google Scholar]
- 2. Tong T., Ji J., Jin S., Li X., Fan W., Song Y., Wang M., Liu Z., Wu M., Zhan Q. (2005) Gadd45a expression induces Bim dissociation from the cytoskeleton and translocation to mitochondria. Mol. Cell. Biol. 25, 4488–4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carrier F., Georgel P. T., Pourquier P., Blake M., Kontny H. U., Antinore M. J., Gariboldi M., Myers T. G., Weinstein J. N., Pommier Y., Fornace A. J., Jr. (1999) Gadd45, a p53-responsive stress protein, modifies DNA accessibility on damaged chromatin. Mol. Cell. Biol. 19, 1673–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hildesheim J., Bulavin D. V., Anver M. R., Alvord W. G., Hollander M. C., Vardanian L., Fornace A. J., Jr. (2002) Gadd45a protects against UV irradiation-induced skin tumors, and promotes apoptosis and stress signaling via MAPK and p53. Cancer Res. 62, 7305–7315 [PubMed] [Google Scholar]
- 5. Hollander M. C., Sheikh M. S., Bulavin D. V., Lundgren K., Augeri-Henmueller L., Shehee R., Molinaro T. A., Kim K. E., Tolosa E., Ashwell J. D., Rosenberg M. P., Zhan Q., Fernández-Salguero P. M., Morgan W. F., Deng C. X., Fornace A. J., Jr. (1999) Genomic instability in Gadd45a-deficient mice. Nat. Genet. 23, 176–184 [DOI] [PubMed] [Google Scholar]
- 6. Wang W., Huper G., Guo Y., Murphy S. K., Olson J. A., Jr., Marks J. R. (2005) Analysis of methylation-sensitive transcriptome identifies GADD45a as a frequently methylated gene in breast cancer. Oncogene 24, 2705–2714 [DOI] [PubMed] [Google Scholar]
- 7. Yamasawa K., Nio Y., Dong M., Yamaguchi K., Itakura M. (2002) Clinicopathological significance of abnormalities in Gadd45 expression and its relationship to p53 in human pancreatic cancer. Clin. Cancer Res. 8, 2563–2569 [PubMed] [Google Scholar]
- 8. Ramachandran K., Gopisetty G., Gordian E., Navarro L., Hader C., Reis I. M., Schulz W. A., Singal R. (2009) Methylation-mediated repression of GADD45α in prostate cancer and its role as a potential therapeutic target. Cancer Res. 69, 1527–1535 [DOI] [PubMed] [Google Scholar]
- 9. Hildesheim J., Belova G. I., Tyner S. D., Zhou X., Vardanian L., Fornace A. J., Jr. (2004) Gadd45a regulates matrix metalloproteinases by suppressing ΔNp63α and β-catenin via p38 MAP kinase and APC complex activation. Oncogene 23, 1829–1837 [DOI] [PubMed] [Google Scholar]
- 10. Ji J., Liu R., Tong T., Song Y., Jin S., Wu M., Zhan Q. (2007) Gadd45a regulates β-catenin distribution and maintains cell-cell adhesion/contact. Oncogene 26, 6396–6405 [DOI] [PubMed] [Google Scholar]
- 11. Hollander M. C., Kovalsky O., Salvador J. M., Kim K. E., Patterson A. D., Haines D. C., Fornace A. J., Jr. (2001) Dimethylbenzanthracene carcinogenesis in Gadd45a-null mice is associated with decreased DNA repair and increased mutation frequency. Cancer Res. 61, 2487–2491 [PubMed] [Google Scholar]
- 12. Folkman J. (1971) Tumor angiogenesis. Therapeutic implications. N. Engl. J. Med. 285, 1182–1186 [DOI] [PubMed] [Google Scholar]
- 13. Bromberg J. F., Wrzeszczynska M. H., Devgan G., Zhao Y., Pestell R. G., Albanese C., Darnell J. E., Jr. (1999) Stat3 as an oncogene. Cell 98, 295–303 [DOI] [PubMed] [Google Scholar]
- 14. Yu H., Kortylewski M., Pardoll D. (2007) Crosstalk between cancer and immune cells. Role of STAT3 in the tumour microenvironment. Nat. Rev. Immunol. 7, 41–51 [DOI] [PubMed] [Google Scholar]
- 15. Yan S., Zhou C., Zhang W., Zhang G., Zhao X., Yang S., Wang Y., Lu N., Zhu H., Xu N. (2008) β-Catenin/TCF pathway upregulates STAT3 expression in human esophageal squamous cell carcinoma. Cancer Lett. 271, 85–97 [DOI] [PubMed] [Google Scholar]
- 16. Chen C. L., Hsieh F. C., Lieblein J. C., Brown J., Chan C., Wallace J. A., Cheng G., Hall B. M., Lin J. (2007) Stat3 activation in human endometrial and cervical cancers. Br. J. Cancer 96, 591–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wen Z., Zhong Z., Darnell J. E., Jr. (1995) Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell 82, 241–250 [DOI] [PubMed] [Google Scholar]
- 18. Yokogami K., Wakisaka S., Avruch J., Reeves S. A. (2000) Serine phosphorylation and maximal activation of STAT3 during CNTF signaling is mediated by the rapamycin target mTOR. Curr. Biol. 10, 47–50 [DOI] [PubMed] [Google Scholar]
- 19. Guertin D. A., Sabatini D. M. (2007) Defining the role of mTOR in cancer. Cancer Cell 12, 9–22 [DOI] [PubMed] [Google Scholar]
- 20. Hudson C. C., Liu M., Chiang G. G., Otterness D. M., Loomis D. C., Kaper F., Giaccia A. J., Abraham R. T. (2002) Regulation of hypoxia-inducible factor 1α expression and function by the mammalian target of rapamycin. Mol. Cell. Biol. 22, 7004–7014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu A. M., Lo R. K., Wong C. S., Morris C., Wise H., Wong Y. H. (2006) Activation of STAT3 by G alpha(s) distinctively requires protein kinase A, JNK, and phosphatidylinositol 3-kinase. J. Biol. Chem. 281, 35812–35825 [DOI] [PubMed] [Google Scholar]
- 22. Stamatovic S. M., Keep R. F., Mostarica-Stojkovic M., Andjelkovic A. V. (2006) CCL2 regulates angiogenesis via activation of Ets-1 transcription factor. J. Immunol. 177, 2651–2661 [DOI] [PubMed] [Google Scholar]
- 23. Balasubramanian S. P., Brown N. J., Reed M. W. (2002) Role of genetic polymorphisms in tumour angiogenesis. Br. J. Cancer 87, 1057–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cao R., Bråkenhielm E., Li X., Pietras K., Widenfalk J., Ostman A., Eriksson U., Cao Y. (2002) Angiogenesis stimulated by PDGF-CC, a novel member in the PDGF family, involves activation of PDGFR-αα and -αβ receptors. FASEB J. 16, 1575–1583 [DOI] [PubMed] [Google Scholar]
- 25. Niu G., Wright K. L., Huang M., Song L., Haura E., Turkson J., Zhang S., Wang T., Sinibaldi D., Coppola D., Heller R., Ellis L. M., Karras J., Bromberg J., Pardoll D., Jove R., Yu H. (2002) Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 21, 2000–2008 [DOI] [PubMed] [Google Scholar]
- 26. Schindler C., Levy D. E., Decker T. (2007) JAK-STAT signaling. From interferons to cytokines. J. Biol. Chem. 282, 20059–20063 [DOI] [PubMed] [Google Scholar]
- 27. Takekawa M., Saito H. (1998) A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell 95, 521–530 [DOI] [PubMed] [Google Scholar]
- 28. Schuringa J. J., Schepers H., Vellenga E., Kruijer W. (2001) Ser727-dependent transcriptional activation by association of p300 with STAT3 upon IL-6 stimulation. FEBS Lett. 495, 71–76 [DOI] [PubMed] [Google Scholar]
- 29. Androutsellis-Theotokis A., Leker R. R., Soldner F., Hoeppner D. J., Ravin R., Poser S. W., Rueger M. A., Bae S. K., Kittappa R., McKay R. D. (2006) Notch signalling regulates stem cell numbers in vitro and in vivo. Nature 442, 823–826 [DOI] [PubMed] [Google Scholar]
- 30. Inoki K., Ouyang H., Li Y., Guan K. L. (2005) Signaling by target of rapamycin proteins in cell growth control. Microbiol. Mol. Biol. Rev. 69, 79–100 [DOI] [PMC free article] [PubMed] [Google Scholar]