Background: Inwardly rectifying potassium channels preferentially conduct currents into cells due to polyamine block.
Results: Introduction of charges in the channel pore modifies polyamine block, with a clear relationship between the position of the charge and blocker length.
Conclusion: Spermine binds deep in the channel, with longer polyamine analogs extending to shallower pore sites.
Significance: This work develops a detailed mapping approach to localize binding sites of physiologically essential channel blockers.
Keywords: Chemical Biology, Electrophysiology, Ion channels, Polyamines, Potassium Channels, Inward Rectifier
Abstract
Steeply voltage-dependent inward rectification of Kir (inwardly rectifying potassium) channels arises from blockade by cytoplasmic polyamines. These polycationic blockers traverse a long (>70 Å) pore, displacing multiple permeant ions, en route to a high affinity binding site that remains loosely defined. We have scanned the effects of cysteine modification at multiple pore-lining positions on the blocking properties of a library of polyamine analogs, demonstrating that the effects of cysteine modification are position- and blocker-dependent. Specifically, introduction of positively charged adducts results in two distinct phenotypes: either disruption of blocker binding or generation of a barrier to blocker migration, in a consistent pattern that depends on both the length of the polyamine blocker and the position of the modified cysteine. These findings reveal important details about the chemical basis and specific location of high affinity polyamine binding.
Introduction
Inwardly rectifying potassium (Kir)2 channels exhibit steeply voltage-dependent activity comparable with voltage-dependent potassium (Kv) channels, but do so without a canonical voltage-sensing domain (1–4). Rather, these channels exhibit voltage-dependent blockade by endogenous polyamines (spermine, spermidine, putrescine), polycationic organic amines that are essential for cellular growth and function (5–9). This inhibitory mechanism is essential for the sequence of events underlying electrical excitation, which would otherwise be “short-circuited” by the significant Kir channel density present in many excitable cells. Moment-to-moment changes in voltage-dependent Kir channel inhibition influence the repolarization of action potentials, and recent studies have linked genetic mutations that disrupt blockade or gating of specific Kir channels to cardiac arrhythmias (10, 11). Structural details of eukaryotic Kir channel gating are rapidly emerging (3, 4, 12–15), but structural characterization of steeply voltage-dependent blockade presents unique challenges, including the absence of a voltage gradient in protein crystals and the strong influence of permeating ion effects on blocker affinity and localization (16, 17).
Polyamine block of Kir channels involves migration of blockers through the remarkably long (>70 Å) Kir channel pore, along a trajectory that likely involves intermediate binding sites before the blocker finally reaches a stable binding site deep within the transmembrane region of the channel (18–20). At negative voltages (more negative than the K+ reversal potential), polyamines are predominantly excluded from the pore, and inward currents flow robustly. Movement of the blocker through the Kir pore is coupled to multiple permeating ions, resulting in a uniquely steep voltage dependence relative to other commonly studied channel blockers, with effective valences exceeding four elementary charges (20–22). However, current understanding of polyamine block is limited by an incomplete description of the chemical features and location of the stable polyamine binding site, its relationship to occupying permeant ions, and lack of information on how voltage alters occupancy of ion binding sites in the pore.
Studies employing site-directed mutagenesis have identified multiple residues that influence polyamine block of Kir channels. Strongly rectifying Kir channels have an acidic residue in the inner cavity region, referred to here as the “rectification controller” (Asp-172 in Kir2.1 channels), an important determinant of steeply voltage-dependent polyamine block (5, 22–24). In addition, several residues in the cytoplasmic pore of Kir channels underlie a “shallow” polyamine binding site with weak voltage dependence (20, 25–27). These shallow and deep sites are generally understood to be sequentially coupled, with blockers first interacting with the cytoplasmic domain and subsequently migrating toward the rectification controller site to generate steep voltage dependence (18). Despite these insights from mutagenesis, consensus is lacking with regard to the location of stable blocker binding in the vicinity of the rectification controller, and much of this uncertainty may arise from the complexity involved in dissecting the local and long range effects of point mutations in the long Kir channel pore (28). In this study, we present a novel approach to identify the sites occupied by polyamines in Kir channels, combining chemical modification of channels with a library of synthetic polyamine analogs (29). Our findings delineate the high affinity spermine binding site and have important implications for the molecular nature of the interaction of polyamines with strongly rectifying Kir channels.
EXPERIMENTAL PROCEDURES
Kir6.2 Channel Constructs and Expression
All point mutations in mouse Kir6.2 were prepared using the Stratagene QuikChange method. All cysteine mutations in Kir6.2 were introduced on a Kir6.2[N160D][C166S] background construct. The Kir6.2[N160D] mutation is included to confer steeply voltage-dependent, high affinity binding of spermine and other polyamines in the otherwise weakly rectifying Kir6.2 channel. Earlier studies of Kir6.2 have demonstrated that the N160D mutation in Kir6.2 (equivalent to residue Asp-172 in Kir2.1/IRK1 channels) confers a high affinity for polyamines and an effective valence of spermine block (zδ∼4–5) similar to that in Kir2.1 channels (23). The Kir6.2[C166S] mutation effectively removes any functional effects of MTS reagents or other cysteine-modifying agents. As described previously (22), Kir6.2 dimeric constructs were generated by fusing the N terminus of the “trailing subunit” and the C terminus of the “leading subunit” with a six-glycine residue linker (introduced within PCR primers).
CosM6 cells were transfected with ion channel cDNAs and GFP cDNA using the FuGENE 6 transfection reagent. Functional Kir6.2 expression also required co-transfection with SUR1. Patch clamp experiments were made at room temperature, using a perfusion chamber that allowed rapid switching of solutions.
Inside-out Patch Clamp Electrophysiology
Data were filtered at 1 kHz, and signals were digitized at 5 kHz and stored directly on a computer hard drive using Clampex software (Axon Inc.). The standard pipette (extracellular) and bath (cytoplasmic) solution used in these experiments had the following composition: 140 mm KCl, 1 mm K-EGTA, 1 mm K-EDTA, 4 mm K2HPO4, pH 7.3. Spermine was purchased from FLUKA Chemicals. PG-11179 and PG-11098 were synthesized and provided by Progen Pharmaceuticals Inc., with methods and quality control as reported previously (30, 31). MTSEA and MTSET (Toronto Research Chemicals) were dissolved in the standard recording solution on the day of experiments, to make a 10 mm stock that was stored on ice. Further dilutions to 100 μm were prepared and used immediately for channel modification.
Data Analysis
For blockade of Kir6.2[N160D]-derived channels (by spermine, ammonium, or tetramethylammonium (TMA)), conductance-voltage relationships were fit with a Boltzmann function G(V) = 1/(1 + e∧(zδF(V − V½)/kT) to yield effective valence (zδ) and V½ of blockade at a single site. This Boltzmann function is adequate to describe spermine block in Kir6.2[N160D] channels. Slightly more complex multisite models are required to describe the multiphasic features of conductance-voltage relationships of spermine block observed in Kir2.1 channels, especially at higher spermine concentrations (6, 18, 20, 32). Spermine block in the presence of ammonium derivatives (ammonium, TMA) was fitted with a sum of two Boltzmann functions: G(V) = A/(1 + e∧(zaF(V − V½a)/kT) + (1 − A)/(1 + e∧(zbF(V − V½b)/kT) (superscript characters are used to differentiate components, not as exponents) Microsoft Solver was used to fit data by a least squares algorithm.
RESULTS
MTSEA and MTSET Modification of Tandem Dimer Kir6.2 Channels
We designed a series of experiments to examine high affinity spermine binding and test two contrasting structural models of the steeply voltage-dependent polyamine binding site in Kir channels (Fig. 1A, gray clouds). Some studies have been interpreted as supporting what we refer to as the shallow binding model of polyamine block in which polyamines such as spermine are oriented with their leading amine in the vicinity of the rectification controller residue (Asp-172 in Kir2.1, N160D position in Kir6.2), and trailing amines extending toward the cytoplasmic channel entrance (12, 18). In contrast, a “deep binding” model, in which the trailing amine is oriented near the rectification controller residue, with the blocker lying predominantly between the rectification controller and the selectivity filter, has been the consistent interpretation from other studies (33, 34).
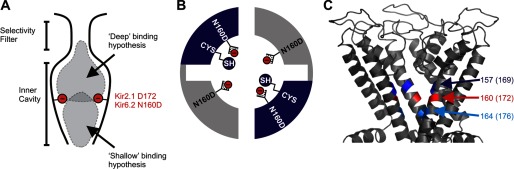
FIGURE 1.
Dimeric cysteine substituted constructs. A, graphic depiction of the Kir channel inner cavity, with shaded clouds to indicate contrasting models for regions involved in high affinity polyamine block. B, construction of tandem-linked dimers of Kir6.2[C166S][N160D]. Assembled channels have negatively charged aspartates occupying the rectification controller position (N160D) in each subunit. The second subunit (back half, dark blue) includes a substituted cysteine. C, location of cysteine substitutions at pore-lining positions in the Kir inner cavity (numbering corresponds to Kir6.2; equivalent numbering for Kir2.1 is in parentheses).
We examined the blocking properties of spermine in Kir6.2[N160D] channels with cysteines substituted at strategic pore-lining positions, before and after modification with the cationic MTS reagents MTSEA and MTSET. Kir6.2[N160D] channels comprise a functionally important inner cavity aspartate (N160D) at the equivalent position to Asp-172 in Kir2.1 channels, sufficient to confer high affinity spermine blockade similar to Kir2.1 (23). The low affinity, shallow spermine site observed in Kir2.1 is not apparent in Kir6.2[N160D] channels, making the latter a useful model channel to characterize inner cavity interactions with polyamines. Experiments were conducted in dimeric channel constructs in which two channel subunits were arranged in tandem (Fig. 1B). In all dimers, the “front half” comprised a Kir6.2[C166S][N160D] subunit (nonreactive to cysteine modification), whereas the “back half” contained a repeat of this construct, but with an additional introduced cysteine mutation (Fig. 1, B and C). Overall, this generates channels with 4 aspartates in the inner cavity and 2 introduced cysteines available for modification. MTSEA and MTSET modification of Kir pore-lining cysteines reduces currents due to a reduction of single channel conductance that depends on the number of modified subunits (35, 36). Thus, dimeric constructs limited the extent of MTS modification in the inner cavity, allowing for measurable currents both before and after modification and accurate measurement of spermine block properties in both scenarios.
Modification Effects in the Inner Cavity Can Be Modifier-dependent
We recently reported incongruous effects of MTSEA and MTSET introduced at Kir2.1[I176C] (equivalent to Kir6.2[L164C]) (32), and we have extended these observations to dimeric Kir6.2[N160D] channels. As shown in Fig. 2, B and D, MTSEA modification of Kir6.2 position 164C significantly reduces spermine affinity, shifting blockade by ∼+40 mV. In contrast, despite introducing a similar charge, MTSET modification of position 164C has virtually no effect on the potency or effective valence of spermine block (Fig. 2, A and D). Also, despite benign effects on spermine affinity, MTSET-modified channels exhibit slower kinetics of both spermine block and unblock (Fig. 2C). These findings closely recapitulate our observations in Kir2.1 (32), further confirming similar mechanisms of steeply voltage-dependent block in these two channel types.
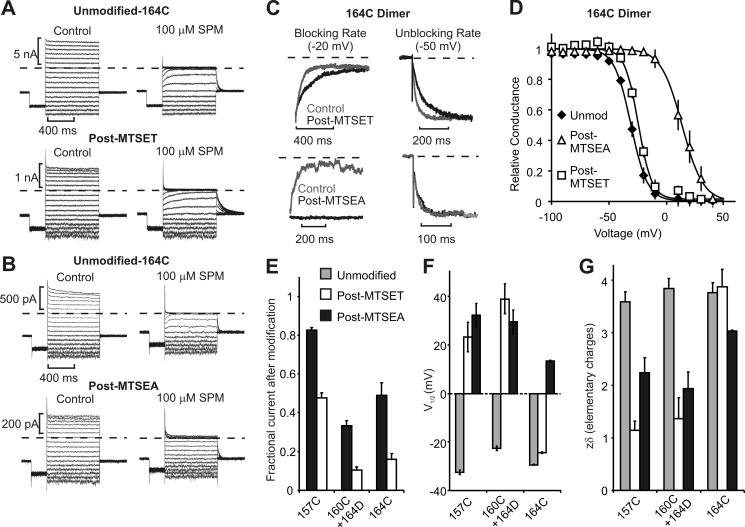
FIGURE 2.
MTSEA and MTSET modification of Kir6.2 position 164C and other inner cavity residues. A and B, spermine (SPM) block (100 μm) of the Kir6.2[164C] dimer was assessed before and after modification with MTSET or MTSEA, at voltages between −100 and +50 mV. C, expanded traces showing the kinetics of spermine block (−20 mV) and unblock (−50 mV), before and after modification by MTSET (top) or MTSEA (bottom), as indicated. D, conductance-voltage relationships illustrating block by 100 μm spermine in unmodified (Unmod) dimeric L164C channels (n = 12) and after modification with MTSEA (n = 3) or MTSET (n = 9). E–G, parameters describing the effects of modification in 157C, 160C/164D, and 164C channels. Conductance-voltage relationships were fit with Boltzmann equations to determine the V½ of 100 μm spermine block (F) and the effective valence of block (G). Error bars in panels E–G indicate S.E.
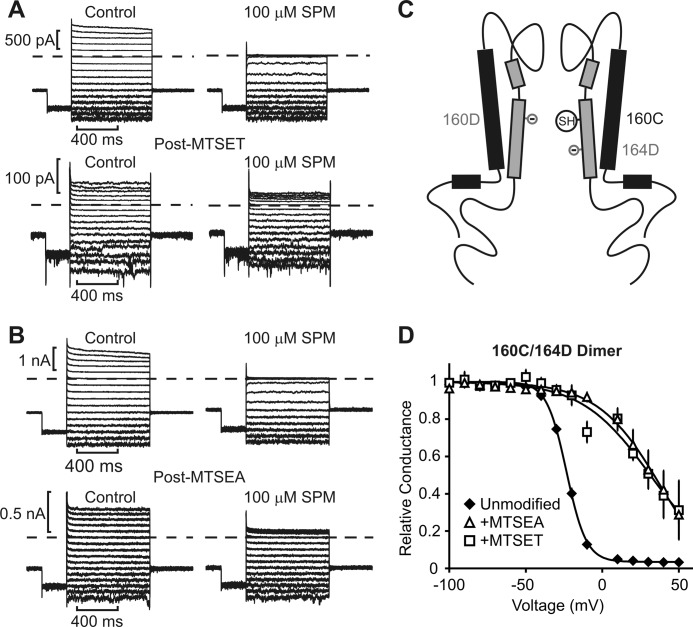
At higher positions in the inner cavity (157C and 160C), closer to the selectivity filter, the effects of MTSEA and MTSET were similar; for both modifiers, the potency and steepness (zδ) of spermine block were substantially reduced (Fig. 2, F and G), and spermine unbinding was accelerated (see Figs. 3 and 4 for detailed records of 157C and 160C/164D dimers). It is noteworthy that the 160C position was tested by constructing a 160C/164D dimer, which comprised the standard N160D/C166S mutations in the first subunit and N160C/L164D/C166S in the second subunit (Fig. 4C). This maintained a net −4 charge in the inner cavity while allowing for MTS modification of position 160C. The spermine affinity of the 160C/164D dimer was slightly weaker than channels with only N160D substitutions (Figs. 2F and 4). Another significant difference we observed for the 160C/164D dimer was some acceleration of spermine binding and unbinding kinetics (apparent in Fig. 4, A and B). For this reason, the 160C/164D channel was not used for kinetic analyses described later in this study.
FIGURE 3.
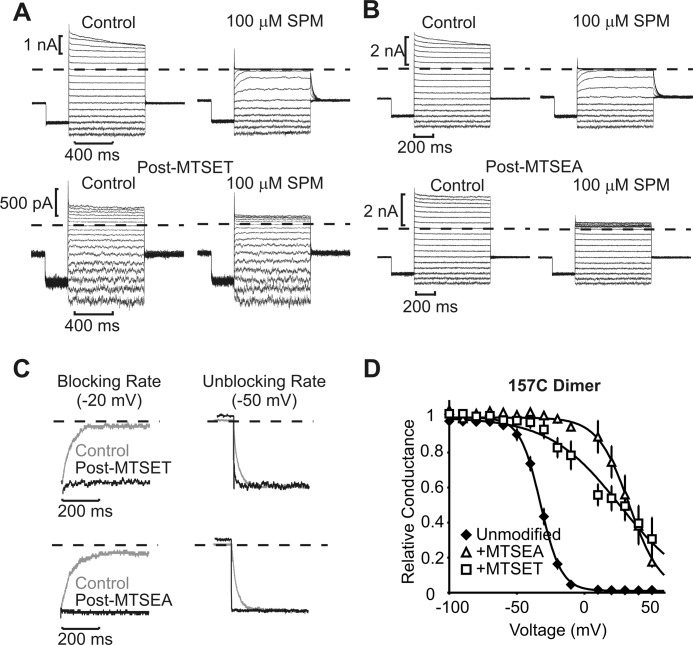
MTSEA and MTSET modification of Kir6.2 position 157C. Inside-out patches expressing the Kir6.2[157C] dimer were pulsed between −100 and +50 mV, in control or 100 μm spermine (SPM). Pulse protocols were repeated after complete modification with either MTSET (A) or MTSEA (B). C, expanded traces illustrating the blocking rate in 100 μm spermine at −20 mV and the unbinding rate at −50 mV, before and after MTSET (top) or MTSEA (bottom) modification, as indicated. D, conductance-voltage relationships illustrating the voltage dependence of block in control (n = 12) and after modification with MTSEA (n = 4) or MTSET (n = 6). Error bars indicate S.E.
FIGURE 4.
MTSEA and MTSET modification of Kir6.2 position 160C. A Kir6.2 dimer was constructed carrying N160D in the front subunit, and N160C/L164D in the back subunit. This manipulation allowed examination of modification at position 160C while maintaining four negatively charged residues in the inner cavity. Steady-state block by 100 μm spermine (SPM) was assessed before and after modification with either MTSET (A) or MTSEA (B), as described in the legend for Fig. 2. C, schematic demonstrating the overall design of the dimeric channel. SH indicates the sulfhydryl group of the cysteine (N160C) available for modification in half of the subunits. D, conductance-voltage relationships illustrating the voltage dependence of block in control (n = 11) and after modification with MTSEA (n = 5) or MTSET (n = 6). Kinetics of spermine block and unblock in the 160C/164D dimer were markedly faster than channels with four N160D residues and were not examined in detail. Error bars indicate S.E.
The effects of 157C modification also relate to a recent study characterizing loss-of-function effects of a hyperinsulinism-linked Kir6.2 mutation G156R and rescue by the N160D mutation (37). Consistent with our observation that MTSEA or MTSET modification of position 157C significantly weakens spermine block (Figs. 2F and 3), both G156K and G156R mutations abolish spermine block in Kir6.2[N160D] channels. In contrast, position 164 stands out as a unique inner cavity site where interactions between spermine and the introduced adduct depend on detailed chemical features of the modifying reagent, as reported for the equivalent residue 176 in Kir2.1 (32). Parameterized effects of MTSEA and MTSET modification on the dimeric Kir6.2 constructs are summarized in Fig. 2, E–G. Overall, the effects of modification on current magnitude (Fig. 2E) were most significant at positions 160C and 164C. However, the equilibrium properties of spermine block were clearly least disrupted by modification of position 164C. Modification of positions 157C or 160C by either MTSEA or MTSET caused dramatic rightward shifts of the V½ of block (Figs. 2F, 3D, and 4D), and smaller effective valences of block (zδ, Fig. 2G). In contrast, MTSEA modification of position 164C caused smaller changes of the V½ and effective valence, and equilibrium properties of MTSET-modified 164C dimers were very similar to unmodified channels (Fig. 2, F and G). Notably, although MTSEA modification of 164C dimers had little effect on the rate of spermine unbinding, MTSET modification slowed the blocking and unblocking kinetics (Fig. 2C, examined in more detail later).
We have previously suggested that the substantial differences between MTSEA and MTSET effects (at 164C) on spermine block reflect detailed interactions of amines with the rectification controller (32). MTSEA and MTSET are often used interchangeably for introduction of positively charged adducts to cysteine side chains, yet there are important differences in the chemical properties of these two reagents. Notably, the +1 charge in the MTSET adduct is delocalized between the quaternized nitrogen and methyl groups. In contrast, MTSEA modification introduces a primary amine, which comprises a higher charge density, and also the ability to form ionized hydrogen bonds/salt bridges with nearby carboxylates (similar interactions are not possible for the quaternary amine of MTSET). Also, the MTSEA amine is chemically identical to the terminal primary amines of spermine. When immobilized at position 164C, the MTSEA primary amine (but not the MTSET quaternary ammonium) might effectively compete for interaction with the rectification controller, without necessarily directly overlapping the spermine binding site (32). We emphasize that this differs from the effects of modification of deeper pore positions (e.g. 157C, or mutations of Gly-156), where polyamine affinity is significantly disrupted regardless of the chemical features of the substituted charge. In the context of mapping spermine binding, our findings are most consistent with a deep binding model (Fig. 1A). Because MTSET modification of 164C has little effect on spermine affinity or effective valence, a logical conclusion is that this position does not overlap with the spermine binding site (32).
Chemical Differences between MTSEA and MTSET
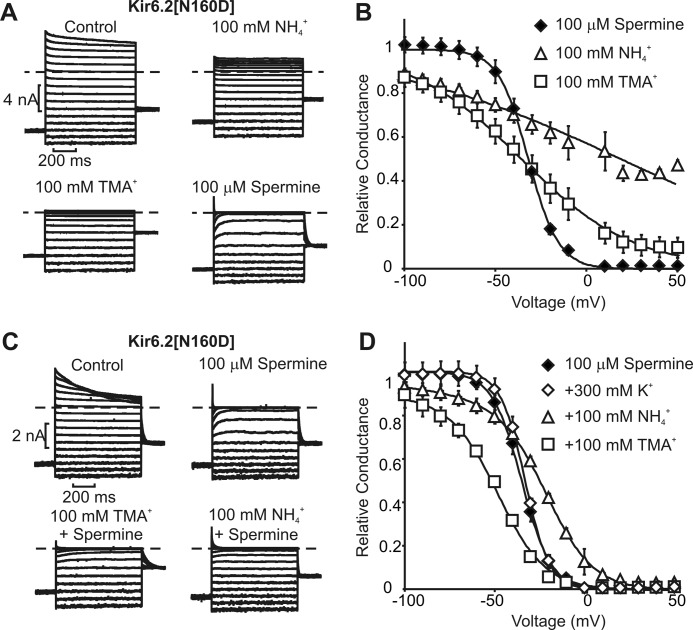
Consistent with the idea that quaternary versus lower order amine-modifying agents have different effects on spermine interactions with the rectification controller carboxylate, we observed that ammonium and TMA have significantly different effects on spermine affinity and unbinding kinetics. Although TMA (with the same quaternized ammonium structure as MTSET) is a more effective channel blocker than ammonium (Fig. 5, A and B), the latter is more effective at competing with/relieving spermine block (Fig. 5, C and D). This feature is readily apparent when examining spermine unbinding rates in the presence of ammonium versus TMA. Ammonium (100 mm) significantly accelerates the kinetics of spermine unbinding (Fig. 6, B and E, consistent with a reduction in spermine affinity), whereas 100 mm TMA exerts a “lock-in” effect and slows the rate of spermine unbinding (Fig. 6, C and E). These findings further support the notion that detailed interactions between the rectification controller carboxylate and protonatable amines are important for high affinity polyamine binding. Despite ammonium having weaker blocking potency than TMA, ammonium disrupts spermine block to a greater degree, possibly because it can generate close amine-carboxylate interactions in the inner cavity (and disrupt/compete with similar interactions of spermine). As a control, we confirmed that these effects are not related to ionic strength because the addition of comparable concentrations of KCl to the intracellular solution has little or no effect on spermine affinity or unbinding kinetics (Fig. 5D).
FIGURE 5.
Blockade of Kir6.2[N160D] channels by ammonium, tetramethylammonium, and spermine. A, currents elicited in Kir6.2[N160D] channels between −100 and +50 mV, in the presence of the indicated blockers. B, conductance-voltage relationships summarizing the experiments presented in A. Data are fit with a single Boltzmann function. C, sample currents elicited from Kir6.2[N160D] channels, illustrating spermine blockade alone or in the presence of 100 mm ammonium (NH4+) or tetramethylammonium (TMA+). D, conductance-voltage relationships summarizing experiments in B. Data are fit with a sum of two Boltzmann functions: G(V) = A/(1 + e∧(zaF(V − V½a)/kT) + (1 − A)/(1 + e∧(zaF(V − V½a)/kT) (superscript characters are used to distinguish components, not as exponents). Error bars in panels B and D indicate S.E.
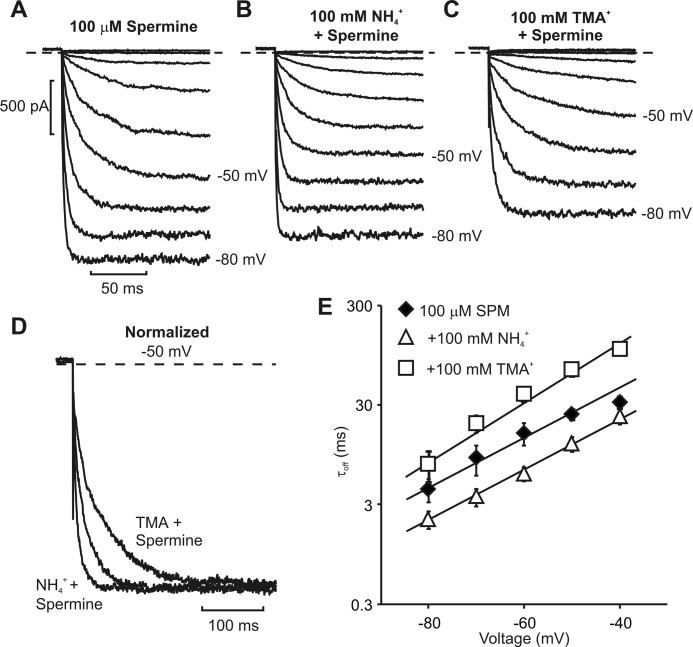
FIGURE 6.
Acceleration of spermine unbinding by internal ammonium. A–C, sample currents elicited from Kir6.2[N160D] channels, illustrating spermine unbinding at voltages between −80 and −10 mV, alone or in the presence of 100 mm ammonium (NH4+) or tetramethylammonium (TMA+). D, normalized currents depicting spermine unbinding at −50 mV, in control or in the presence of NH4+ or TMA+, as indicated. E, summarized data illustrating the rate of spermine (SPM) unbinding between −40 mV and −80 mV, in the presence of 100 mm NH4+ or TMA+. Error bars indicate S.E.
Inhibition of Blocker Potency Is Blocker-dependent
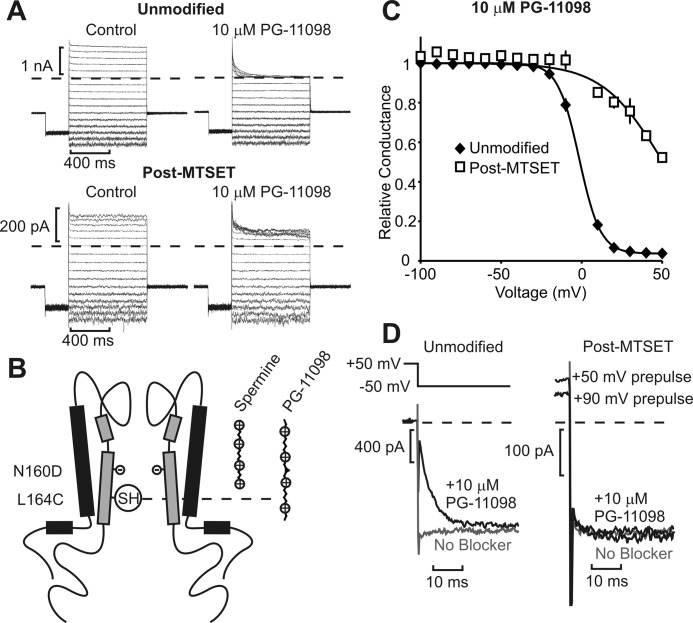
The data presented thus far (a) indicate that Kir6.2 position 164C (equivalent to Kir2.1 176C) does not overlap with the spermine binding site, (b) indicate that spermine binds similarly in the inner cavity of Kir2.1 and Kir6.2[N160D] channels, and (c) provide a rationale for the interesting position-specific differences between MTSEA and MTSET effects on spermine block. We moved on to test and develop a rigorous method for mapping polyamine binding. We first tested whether modestly extended spermine analogs such as PG-11098 (25 Å long versus 18 Å length of spermine) can extend closer to the cytoplasmic side of the inner cavity relative to spermine. We have previously demonstrated that PG-11098 inhibits (i.e. “protects” against) MTSEA modification of Kir6.2 residue 164C more effectively than does spermine (33), suggesting that 164C is at or below the lower boundary of the stable spermine binding site. We hypothesized that the slightly extended PG-11098 compound would approach 164C more closely than spermine, perhaps even overlapping with this position (shown schematically in Fig. 7B), in which case a direct overlap with substituted MTSET could significantly reduce blocker potency (in contrast to the “chemistry-dependent” effects of 164C modification on spermine block). Indeed, MTSET modification of residue 164C significantly shifted PG-11098 block to very positive voltages, indicating a significant loss of PG-11098 affinity (Fig. 7, A and C). This result contrasts starkly with the effects of 164C (MTSET) modification on spermine block (Fig. 2, A and D), where we observed a minimal change in affinity.
FIGURE 7.
Effects of MTSET modification at 164C are blocker-dependent. A, blockade of the Kir6.2[L164C] dimer was examined in the presence of 10 μm PG-11098, a synthetic spermine analog, before and after modification by MTSET. B, schematic illustrating potential interpretation, with blockers longer than spermine extending closer toward the positive charge introduced at position 164C. SH indicates the sulfhydryl group of the cysteint (L164C) available for modification in half of the subunits. C, conductance-voltage relationships before (n = 7) and after (n = 7) MTSET modification illustrate a rightward shift and smaller effective valence of PG-11098 block after MTSET. Error bars indicate S.E. D, kinetics of PG-11098 unbinding before (left panel) and after (right panel) MTSET modification. Patches were pulsed to +50 mV, in control (gray) or 10 μm PG-11098 (black), and repolarized to −50 mV to observe blocker unbinding. Prepulses to +90 mV were also used to ensure significant channel block by PG-11098 after MTSET modification, although PG-11098 unbinding remained rapid.
We also observed acceleration of PG-11098 unbinding after MTSET modification of 164C (Fig. 7D), in contrast to slower unbinding kinetics of spermine in the same conditions (Fig. 2C). To ensure that apparently rapid PG-11098 unbinding after MTSET modification was not simply due to a large fraction of unblocked channels, we pulsed to higher voltages (+90-mV prepulse in Fig. 7D) and still saw no evidence of slower blocker unbinding. We consistently observed a brief time-dependent unbinding phase in MTSET-modified channels that appears to arise from weak voltage-dependent blockade by the MTSET moiety tethered at 164C because this is observed even in the absence of a soluble blocker (Fig. 7D). In summary, these data demonstrate that MTSET modification (at 164C) can interfere with block by extended polyamines in the inner cavity, but that binding of spermine is not affected by introduction of this positive charge. The implication of this finding is that the introduced charge at position 164C does not interact with spermine, which is bound beyond this residue, whereas it effectively disrupts blockade by longer spermine analogs because their tails extend back to or beyond the 164C position (Fig. 7B).
Extended Scan of Introduced Barrier Effects on Polyamine Analogs
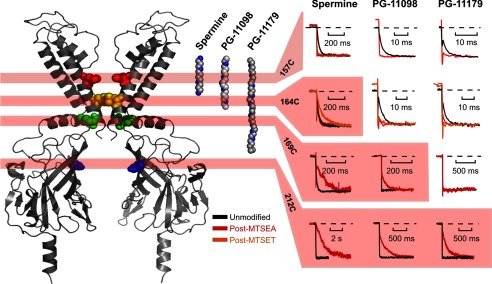
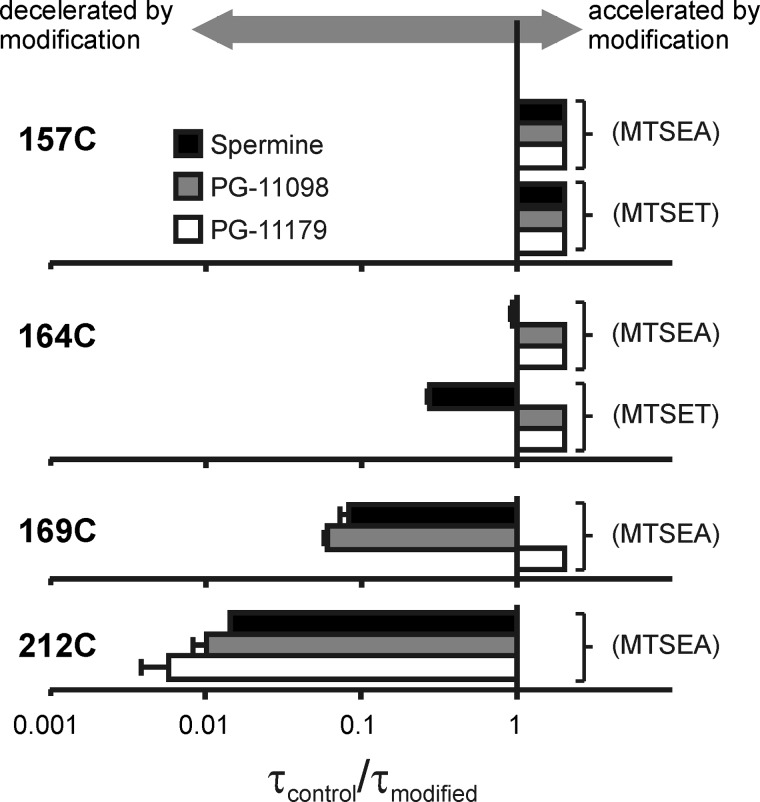
We further explored the parameter space of these experiments by modification in four different cysteine-substituted Kir6.2 channels (157C, 164C, 169C, 212C) that span a significant portion of the Kir pore (see structure in Fig. 8). We first describe blocker unbinding rates to illustrate the relationship between modification position and blocker length. Different combinations of blocker and modification position fall into two distinct categories; in some cases, blocker unbinding is accelerated beyond our ability to resolve (unblock is complete in <1 ms, consistent with a loss of blocker affinity), and in other cases blocker unbinding is slowed significantly without marked change in affinity. We will refer to the latter effect as a “barrier phenotype” because the underlying mechanism appears to be the introduction of a barrier to blocker migration (due to the incorporation of a charged adduct at a pore-lining position). In Fig. 8, we have highlighted (red shading) all the blocker/modifier combinations resulting in a barrier phenotype. Unshaded panels indicate combinations resulting in no slowing (and typically significant acceleration) of blocker unbinding. Summarized data for all modified positions, blockers, and modifying reagents are presented in Fig. 10.
FIGURE 8.
Localization of blockers in the Kir6.2 pore. Polyamine blockers are shown in fully extended conformations alongside a structural model of Kir6.2. Blocker dimensions range from “short” (i.e. spermine) to blockers that span almost the entire length of the channel pore (e.g. PG-11179). For each substituted cysteine, the kinetics of blocker unbinding at −50 mV are presented before and after modification with MTSEA (and MTSET where determined). At deep sites (L157C, top row), modification accelerates blocker unbinding. At shallower sites (164C, 169C, 212C), a barrier phenotype emerges in which modification slows the kinetics of blocker unbinding. Blocker/position combinations that exhibit a barrier phenotype are highlighted in red. The barrier phenotype emerges with modification at progressively shallower positions for progressively longer blockers, and the pattern suggests that the trailing end of PG-11179 extends beyond position 169C, but not position 212C.
FIGURE 10.
Blocker- and position-dependent emergence of the barrier phenotype by modification of pore-lining cysteines. For each substituted cysteine, the kinetics of blocker unbinding at −50 mV after modification have been normalized to the unbinding kinetics in unmodified channels. In cases where the unbinding rate after modification cannot be resolved, the τcontrol/τmodified ratio has been arbitrarily assigned a value >1 to indicate the observed acceleration of unbinding. Error bars indicate S.E.
A very clear dependence of blocker kinetics on the position of positively charged adducts emerges. At the deepest site (Kir6.2[L157C], Fig. 8, top row), unbinding of each blocker is markedly accelerated after modification (for either MTSEA or MTSET). Furthermore, progressively longer blockers demonstrate a clear progression to more shallow pore-lining sites at which a barrier phenotype appears. At position 164C (Fig. 8, second row), spermine unbinding is slowed after MTSET modification, whereas unbinding of PG-11098 and PG-11179 is clearly accelerated after 164C modification (by MTSEA or MTSET, Figs. 7 and 8). From these data, we conclude that spermine predominantly occupies space above residue 164C, whereas the longer blockers PG-11098 and PG-11179 overlap more prominently or frequently with 164C and are disrupted by modification of this position.
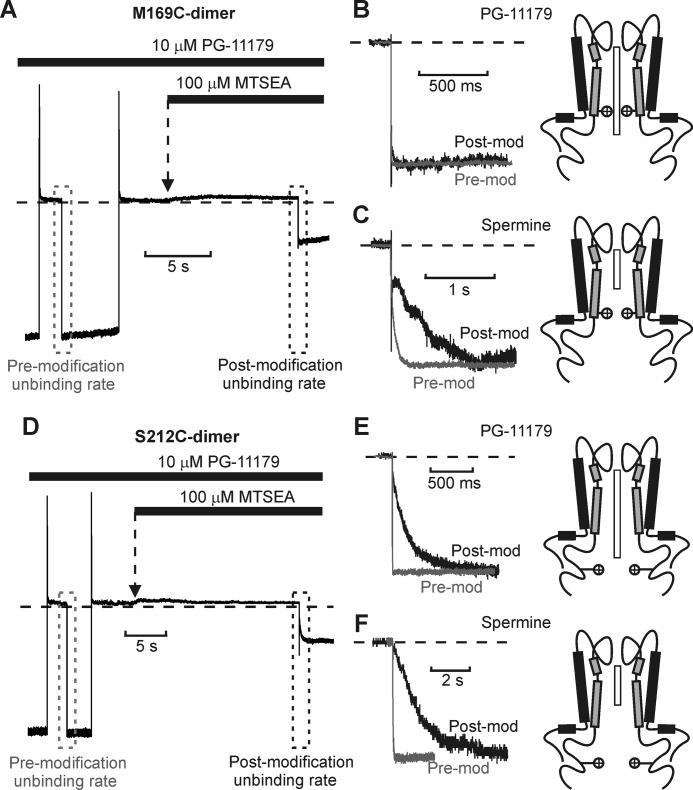
This pattern continues with positions progressively closer to the cytoplasmic channel entrance. At positions 169C and 212C, we only examined the effects of MTSEA because MTSET modification caused substantial current rundown that precluded reliable electrophysiological recordings. At these positions (Fig. 8, third and fourth rows), the barrier phenotype is apparent for spermine and PG-11098). For the longer PG-11179 compound, blockade is abolished by MTSEA modification of 169C or 212C, making unbinding rates difficult to measure. However, we observed that both 169C and 212C channels could be modified while the PG-11179 blocker was occupying the pore (with channels held at +50 mV) and that unbinding rates could then be clearly distinguished upon repolarization (Fig. 9, A and D). Using this alternative protocol, modification of 169C did not slow PG-11179 unbinding (Fig. 9B), whereas modification of 212C dramatically decelerated PG-11179 unbinding (Fig. 9E). Using similar protocols, spermine unbinding is dramatically slowed by MTSEA modification of either 169C or 212C (Fig. 9, C and F).
FIGURE 9.
Kinetic effects of MTSEA modification at Kir6.2 positions 169C and 212C. A, sample data of MTSEA modification of Kir6.2[N160D][C166S][M169C] dimeric channels blocked with PG-11179. Channels were blocked with a +50-mV pulse and then repolarized to −50 mV to observe the PG-11179 unbinding rate from unmodified channels (gray box). In the second step, channels were blocked at +50 mV and then exposed to a solution containing PG-11179 + 100 μm MTSEA and repolarized to −50 mV to observe the blocker unbinding rate from partially modified channels. B and C, expanded data illustrating relevant unbinding kinetics observed in similar experiments using PG-11179 (B) or spermine (C). MTSEA modification of residue 169 slows spermine unbinding, but has no discernible effect on PG-11179 unbinding. Graphics depict spermine and PG-11179 orientations relative to position 169C (indicated by positive charges). Post-mod, after modification; Pre-mod, before modification. D–F, identical experiments were performed with spermine and PG-11179 and modification of position 212C. MTSEA modification of 212C slows unbinding of both spermine and PG-11179.
Adjacent to the channel structure in Fig. 8 is an extended structure representation of the three blockers tested, oriented in a fashion most consistent with these data, with the “head” end of each blocker occupying a deep site and the trailing ends of PG-11098 and PG-11179 extending beyond position 164 and progressively closer to the cytoplasmic channel entrance. Viewed as a whole, our findings imply that a barrier phenotype (slower unbinding with little or no effect on affinity) arises from the modification of cysteines substituted below the trailing end of a blocker (i.e. closer to the cytoplasmic entrance).
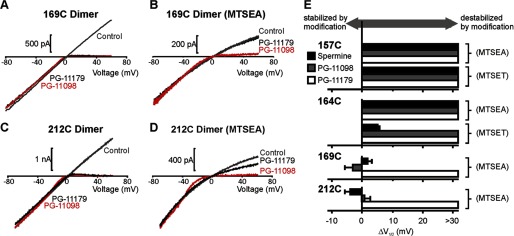
These effects of position and blocker length on unbinding rates (Fig. 10) are generally consistent with the effects on steady-state properties of polyamine block (Fig. 11). We have summarized the effects of modification on the V½ of block (parameterized as ΔV½) in Fig. 11E. These values were derived from either voltage step protocols or very slow voltage ramps (Fig. 11, A–D, used because of the extremely slow kinetics of block and unblock after modification of 169C or 212C). We again observed a well defined spatial pattern for the effects of channel modification on V½ of blockade; modification of the deepest pore-lining position (157C) attenuated block by all polyamine analogs, and modification of progressively shallower positions (164C, 169C) attenuated block only for progressively longer polyamine analogs.
FIGURE 11.
Blocker- and position-dependent effects of modification on blocker potency. A–D, for positions 169C and 212C; extremely slow (60 s) voltage ramps between −80 mV and +60 mV were used to assess the voltage dependence of block for various blockers (100 μm). E, ΔV½ was determined as the difference in V½ of block for modified versus unmodified channels (n = 3–7 per condition). V½ values of block were derived from either voltage step protocols (e.g. Fig. 2) or slow voltage ramps, as depicted in panels A–D. Error bars indicate S.E.
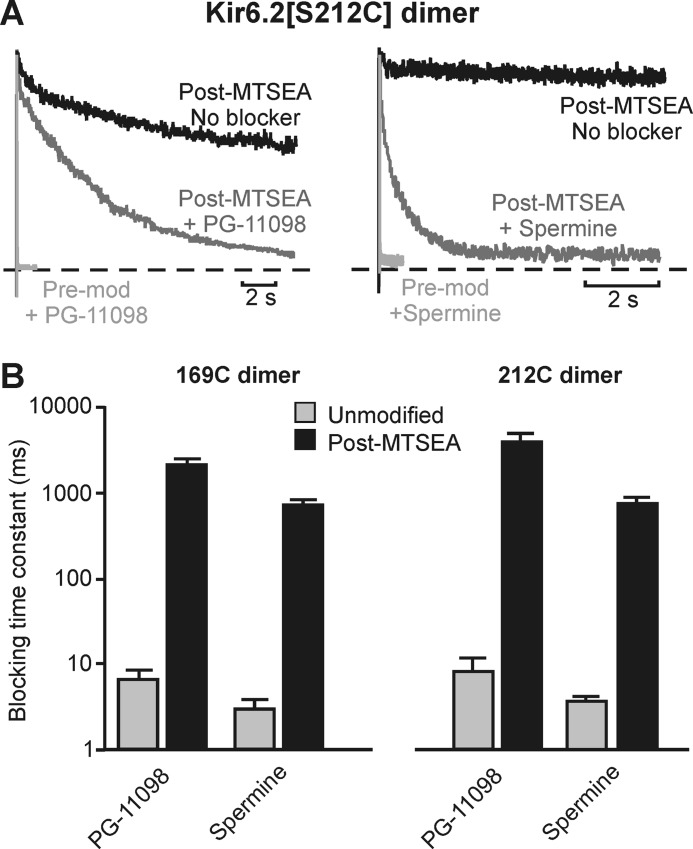
Lastly, given that MTS modification of many pore-lining positions decelerates blocker unbinding with minimal effects on blocker affinity, many barrier phenotype positions might also be expected to exhibit a significant slowing of blocker binding. This is indeed the case, highlighted here with data illustrating the effects of MTSEA modification of 212C dimeric channels on the blocking kinetics of both spermine and PG-11098 (Fig. 12A). Similar behavior is also observed after modification of 169C (Fig. 12B) and for spermine after MTSET modification of 164C (Fig. 2C). As mentioned, we were unable to block modified channels with any practical dose of PG-11179 (Fig. 11, B and D), and therefore we have not determined PG-11179 blocking rates.
FIGURE 12.
Slow blocker binding in MTSEA-modified 169C and 212C channels. A, exemplar data illustrating the effects of MTSEA modification of Kir6.2[S212C] dimeric channels on blocking kinetics of PG-11098 or spermine (both at 100 μm). Patches were pulsed from −80 mV to +50 mV after the indicated manipulations. Pre-mod, before modification. B, summarized data describing spermine and PG-11098 blocking kinetics in unmodified and MTSEA-modified 169C and 212C channels (n = 3–5 per recording condition). MTSEA modification of these positions profoundly slows blocker binding. Error bars indicate S.E.
DISCUSSION
Emerging Details of the Deep Spermine Binding Site in Kir Channels
In previous studies, we have presented multiple lines of evidence that spermine binds deep in the Kir channel inner cavity (19, 32, 33). Expansion of the chemical features of modifying reagents provides a useful additional approach to explore spermine interactions with the rectification controller. The distinct effects of MTSEA and MTSET at position 164C most likely arise from their different propensities to interact with the rectification controller carboxylate (38). At deeper pore sites (e.g. 157C), this mechanism cannot be discerned because modification disrupts spermine binding with no dependence on the chemical properties of the modifying reagent (Fig. 2). However, at position 164C, we interpret our findings to suggest that MTSEA (at 164C) disrupts spermine block indirectly because MTSET modification of the same site has no effect on spermine affinity. Rather than direct overlap with the spermine binding site, we suggest that the MTSEA amine (at 164C) engages the rectification controller and effectively competes for strong amine-carboxylate interactions. The small size of MTSEA and capacity to form ionized hydrogen bonds/salt bridges enable close engagement with the rectification controller carboxylate, whereas MTSET cannot form similar interactions. A similar effect is observed when spermine and ammonium are co-applied as ammonium weakens spermine block and accelerates spermine unbinding (Fig. 6). Tetramethyl ammonium, despite being a more potent blocker than ammonium, does not have this weakening effect on spermine block.
Evidence for a Deep Spermine Binding Site
The present study supports the hypothesis that the stable spermine binding site lies deep in the inner cavity (deep binding hypothesis, Fig. 1A). We demonstrate dichotomous effects of charge incorporation in the Kir pore. At deep sites (e.g. 157C), modification considerably accelerates spermine unbinding and disrupts the binding site for all analogs (Figs. 3 and 8). In contrast, modification of residues closer to the cytoplasmic channel entrance causes dramatic slowing of blocker unbinding without reducing affinity (Figs. 2, 8, and 9), consistent with introduction of an energetic barrier for blocker migration, rather than disruption of the binding site itself. Spermine (the shortest blocker examined) is the most tolerant of modification of pore-lining positions deep in the pore, whereas longer blockers (PG-11098, PG-11179) only tolerate modification at progressively shallower positions in the pore. In addition, blocker entry into the deep binding site is significantly slowed by modification of numerous pore-lining positions (Fig. 12).
Insights from Extended Polyamine Analogs, a Consistent Pattern of Length-dependent Effects
The extension of results to include multiple cysteine-substituted sites and progressively longer blockers solidifies a structural interpretation of a deep spermine binding site (Figs. 1 and 8). Our findings reveal a close relationship between the location of charge incorporation and the necessary length of polyamine blocker to exhibit a barrier phenotype. Considered within the spatial dimensions of the inner cavity, a rational explanation for these observations is that the leading ends of spermine and PG analogs reach a similar site deep in the inner cavity (also indicated by their similar effective valences of blockade (29, 33)), and the trailing ends of the PG analogs extend back toward 164C and beyond, such that progressively longer blockers, trailing closer to the channel cytoplasmic entrance, require charge substitution at progressively shallower sites to observe the slow unbinding barrier phenotype.
Our findings are difficult to rationalize using alternative proposed spermine binding sites. A shallow binding model has been invoked to suggest that the blocker is oriented between Kir2.1 residues 183 and 172 (Kir6.2 residues 175 and 160) (12, 18). However, our study demonstrates that positive charge substitutions in this region (at positions 164 and 169) do not change affinity and therefore cannot directly overlap with the spermine binding site, as we also reported in Kir2.1 channels (32). Furthermore, models of shallow binding cannot account for the position dependence of modification required to generate slower blocker unbinding with progressively longer blockers.
It is noteworthy that previous findings demonstrating slower blocker unbinding in MTSET-modified Kir2.1[176C] channels (and Kir6.2[160D][164C] in the current study) provoked questions as to whether slow unbinding kinetics might be an indirect result of current inhibition (32). That is, if spermine were to bind at a peripheral shallow site, smaller potassium flux after modification might cause slower relief of spermine block. The present data set seems to rule this out because no slowing of polyamine unbinding was observed in some constructs with substantial MTS-dependent current inhibition (e.g. the 160C/164D dimer). Furthermore, an indirect effect of slow permeation would be predicted to slow blocker unbinding irrespective of blocker length. Instead, we observe (Figs. 2 and 7–11) that blocker affinity and unbinding rates after pore-lining modification depend predictably on blocker length.
Conclusions
The introduction of positively charged adducts at specific sites in the Kir6.2[N160D] pore dramatically affects the equilibrium and kinetic properties of spermine block. Most remarkably, at a position between the rectification controller and helix bundle crossing, MTSET modification decelerates the kinetics of spermine unbinding, but has little effect on valence or potency of block. The data illustrate two important features of spermine block. Firstly, high affinity block involves spermine migration to a deep position in the inner cavity, between the rectification controller and the selectivity filter. Secondly, the stability of spermine in this deep binding site involves detailed chemical interactions of a hydrogen bond nature with the rectification controller carboxylate functional group. These functional experiments reveal critical structural and chemical details of voltage-dependent polyamine block that may be difficult to discern from crystallographic studies of polyamine binding in the absence of a membrane electric field.
This work was supported, in whole or in part, by National Institutes of Health Grants HL54171 (to C. G. N.) and HL53268 (to H. T. K.). This work was also supported by Canadian Institutes for Health Research (CIHR) Operating Grant MOP-97998 (to H. T. K.) and a Natural Sciences and Engineering Research Council (NSERC) Discovery grant (to H. T. K.).
- Kir
- inwardly rectifying potassium
- MTS
- methanethiosulfonate
- MTSEA
- (2-aminoethyl) methanethiosulfonate
- MTSET
- (2-(trimethylammonium)ethyl) methanethiosulfonate
- TMA
- tetramethylammonium.
REFERENCES
- 1. Lu Z. (2004) Mechanism of rectification in inward-rectifier K+ channels. Annu. Rev. Physiol. 66, 103–129 [DOI] [PubMed] [Google Scholar]
- 2. Nichols C. G., Lopatin A. N. (1997) Inward rectifier potassium channels. Annu. Rev. Physiol. 59, 171–191 [DOI] [PubMed] [Google Scholar]
- 3. Hansen S. B., Tao X., MacKinnon R. (2011) Structural basis of PIP2 activation of the classical inward rectifier K+ channel Kir2.2. Nature 477, 495–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuo A., Gulbis J. M., Antcliff J. F., Rahman T., Lowe E. D., Zimmer J., Cuthbertson J., Ashcroft F. M., Ezaki T., Doyle D. A. (2003) Crystal structure of the potassium channel KirBac1.1 in the closed state. Science 300, 1922–1926 [DOI] [PubMed] [Google Scholar]
- 5. Lopatin A. N., Makhina E. N., Nichols C. G. (1994) Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature 372, 366–369 [DOI] [PubMed] [Google Scholar]
- 6. Lopatin A. N., Makhina E. N., Nichols C. G. (1995) The mechanism of inward rectification of potassium channels: “long-pore plugging” by cytoplasmic polyamines. J. Gen. Physiol. 106, 923–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ficker E., Taglialatela M., Wible B. A., Henley C. M., Brown A. M. (1994) Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science 266, 1068–1072 [DOI] [PubMed] [Google Scholar]
- 8. Fakler B., Brändle U., Glowatzki E., Weidemann S., Zenner H. P., Ruppersberg J. P. (1995) Strong voltage-dependent inward rectification of inward rectifier K+ channels is caused by intracellular spermine. Cell 80, 149–154 [DOI] [PubMed] [Google Scholar]
- 9. Gerner E. W., Meyskens F. L., Jr. (2004) Polyamines and cancer: old molecules, new understanding. Nat. Rev. Cancer 4, 781–792 [DOI] [PubMed] [Google Scholar]
- 10. Cerrone M., Noujaim S., Jalife J. (2006) The short QT syndrome as a paradigm to understand the role of potassium channels in ventricular fibrillation. J. Intern. Med. 259, 24–38 [DOI] [PubMed] [Google Scholar]
- 11. Priori S. G., Pandit S. V., Rivolta I., Berenfeld O., Ronchetti E., Dhamoon A., Napolitano C., Anumonwo J., di Barletta M. R., Gudapakkam S., Bosi G., Stramba-Badiale M., Jalife J. (2005) A novel form of short QT syndrome (SQT3) is caused by a mutation in the KCNJ2 gene. Circ. Res. 96, 800–807 [DOI] [PubMed] [Google Scholar]
- 12. Xu Y., Shin H. G., Szép S., Lu Z. (2009) Physical determinants of strong voltage sensitivity of K+ channel block. Nat. Struct. Mol. Biol. 16, 1252–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tao X., Avalos J. L., Chen J., MacKinnon R. (2009) Crystal structure of the eukaryotic strong inward-rectifier K+ channel Kir2.2 at 3.1 Ä resolution. Science 326, 1668–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nishida M., Cadene M., Chait B. T., MacKinnon R. (2007) Crystal structure of a Kir3.1-prokaryotic Kir channel chimera. EMBO J. 26, 4005–4015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pegan S., Arrabit C., Zhou W., Kwiatkowski W., Collins A., Slesinger P. A., Choe S. (2005) Cytoplasmic domain structures of Kir2.1 and Kir3.1 show sites for modulating gating and rectification. Nat. Neurosci. 8, 279–287 [DOI] [PubMed] [Google Scholar]
- 16. Lopatin A. N., Nichols C. G. (1996) [K+] dependence of polyamine-induced rectification in inward rectifier potassium channels (IRK1, Kir2.1). J. Gen. Physiol. 108, 105–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hagiwara S., Miyazaki S., Rosenthal N. P. (1976) Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J. Gen. Physiol. 67, 621–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shin H. G., Lu Z. (2005) Mechanism of the voltage sensitivity of IRK1 inward-rectifier K+ channel block by the polyamine spermine. J. Gen. Physiol. 125, 413–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kurata H. T., Marton L. J., Nichols C. G. (2006) The polyamine binding site in inward rectifier K+ channels. J. Gen. Physiol. 127, 467–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kurata H. T., Cheng W. W., Arrabit C., Slesinger P. A., Nichols C. G. (2007) The role of the cytoplasmic pore in inward rectification of Kir2.1 channels. J. Gen. Physiol. 130, 145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guo D., Ramu Y., Klem A. M., Lu Z. (2003) Mechanism of rectification in inward-rectifier K+ channels. J. Gen. Physiol. 121, 261–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kurata H. T., Phillips L. R., Rose T., Loussouarn G., Herlitze S., Fritzenschaft H., Enkvetchakul D., Nichols C. G., Baukrowitz T. (2004) Molecular basis of inward rectification: polyamine interaction sites located by combined channel and ligand mutagenesis. J. Gen. Physiol. 124, 541–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shyng S., Ferrigni T., Nichols C. G. (1997) Control of rectification and gating of cloned KATP channels by the Kir6.2 subunit. J. Gen. Physiol. 110, 141–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wible B. A., Taglialatela M., Ficker E., Brown A. M. (1994) Gating of inwardly rectifying K+ channels localized to a single negatively charged residue. Nature 371, 246–249 [DOI] [PubMed] [Google Scholar]
- 25. Yang J., Jan Y. N., Jan L. Y. (1995) Control of rectification and permeation by residues in two distinct domains in an inward rectifier K+ channel. Neuron 14, 1047–1054 [DOI] [PubMed] [Google Scholar]
- 26. Kubo Y., Murata Y. (2001) Control of rectification and permeation by two distinct sites after the second transmembrane region in Kir2.1 K+ channel. J. Physiol. 531, 645–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fujiwara Y., Kubo Y. (2006) Functional roles of charged amino acid residues on the wall of the cytoplasmic pore of Kir2.1. J. Gen. Physiol. 127, 401–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Robertson J. L., Palmer L. G., Roux B. (2008) Long-pore electrostatics in inward-rectifier potassium channels. J. Gen. Physiol. 132, 613–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Loussouarn G., Marton L. J., Nichols C. G. (2005) Molecular basis of inward rectification: structural features of the blocker defined by extended polyamine analogs. Mol. Pharmacol. 68, 298–304 [DOI] [PubMed] [Google Scholar]
- 30. Mitchell J. L., Leyser A., Holtorff M. S., Bates J. S., Frydman B., Valasinas A. L., Reddy V. K., Marton L. J. (2002) Antizyme induction by polyamine analogues as a factor of cell growth inhibition. Biochem. J. 366, 663–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Valasinas A., Sarkar A., Reddy V. K., Marton L. J., Basu H. S., Frydman B. (2001) Conformationally restricted analogues of 1N,14N-bisethylhomospermine (BE-4-4-4): synthesis and growth inhibitory effects on human prostate cancer cells. J. Med. Chem. 44, 390–403 [DOI] [PubMed] [Google Scholar]
- 32. Kurata H. T., Zhu E. A., Nichols C. G. (2010) Locale and chemistry of spermine binding in the archetypal inward rectifier Kir2.1. J. Gen. Physiol. 135, 495–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kurata H. T., Diraviyam K., Marton L. J., Nichols C. G. (2008) Blocker protection by short spermine analogs: refined mapping of the spermine binding site in a Kir channel. Biophysical Journal 95, 3827–3839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chang H. K., Yeh S. H., Shieh R. C. (2003) The effects of spermine on the accessibility of residues in the M2 segment of Kir2.1 channels expressed in Xenopus oocytes. J. Physiol. 553, 101–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Loussouarn G., Phillips L. R., Masia R., Rose T., Nichols C. G. (2001) Flexibility of the Kir6.2 inward rectifier K+ channel pore. Proc. Natl. Acad. Sci. U.S.A. 98, 4227–4232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lu T., Nguyen B., Zhang X., Yang J. (1999) Architecture of a K+ channel inner pore revealed by stoichiometric covalent modification. Neuron 22, 571–580 [DOI] [PubMed] [Google Scholar]
- 37. Bushman J. D., Gay J. W., Tewson P., Stanley C. A., Shyng S. L. (2010) Characterization and functional restoration of a potassium channel Kir6.2 pore mutation identified in congenital hyperinsulinism. J. Biol. Chem. 285, 6012–6023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mavri J., Vogel H. J. (1994) Ion pair formation involving methylated lysine side chains: a theoretical study. Proteins 18, 381–389 [DOI] [PubMed] [Google Scholar]