Background: The junctional localization and RhoGEF activity of Syx mediate cell junction integrity.
Results: PKD-mediated phosphorylation and 14-3-3 binding prevent the junctional localization and suppress the GEF activity of Syx.
Conclusion: 14-3-3 proteins modulate the function of Syx in the context of cell-cell adhesion.
Significance: Understanding mechanisms that regulate junction integrity is crucial in vascular and tumor biology.
Keywords: Cadherins, Cell Junctions, Endothelial Cell, Guanine Nucleotide Exchange Factor (GEF), Protein Kinase D (PKD), RhoA, 14-3-3
Abstract
Syx is a Rho-specific guanine nucleotide exchange factor (GEF) that localizes at cell-cell junctions and promotes junction stability by activating RhoA and the downstream effector Diaphanous homolog 1 (Dia1). Previously, we identified several molecules, including 14-3-3 proteins, as Syx-interacting partners. In the present study, we show that 14-3-3 isoforms interact with Syx at both its N- and C-terminal regions in a phosphorylation-dependent manner. We identify the protein kinase D-mediated phosphorylation of serine 92 on Syx, and additional phosphorylation at serine 938, as critical sites for 14-3-3 association. Our data indicate that the binding of 14-3-3 proteins inhibits the GEF activity of Syx. Furthermore, we show that phosphorylation-deficient, 14-3-3-uncoupled Syx exhibits increased junctional targeting and increased GEF activity, resulting in the strengthening of the circumferential junctional actin ring in Madin-Darby canine kidney cells. These findings reveal a novel means of regulating junctional Syx localization and function by phosphorylation-induced 14-3-3 binding and further support the importance of Syx function in maintaining stable cell-cell contacts.
Introduction
The Rho GTPases function as molecular switches that cycle between a GTP-bound active state and a GDP-bound inactive state and are involved in a range of signaling pathways that control the actin cytoskeleton and fundamental cellular processes, like cell adhesion and cell migration (1). Guanine nucleotide exchange factors (GEFs)2 catalyze the exchange of GDP for GTP. Coordinated signaling by RhoA, Rac1, and Cdc42 GTPases regulates cell junction formation and cell polarization by modulating junctional components and the actin cytoskeleton (2). Precise spatiotemporal regulation of Rho GTPases by GEFs is thought to regulate the cross-talk between adhesion and polarity complexes (3, 4). Despite extensive characterization of the Rho GTPases, their activation by junction-associated GEFs, and especially the mechanisms by which these GEFs are regulated, remain poorly understood.
We recently identified Syx, as a junctional RhoGEF that associates with multiple members of the Crumbs polarity complex, promotes junction stability by signaling through RhoA and Diaphanous homolog 1 (Dia1), and mediates the opposing effects of VEGF and angiopoietin 1 on endothelial junction integrity (5). Depletion of Syx results in endothelial cell junction defects in vitro and in vivo. Syx belongs to the Dbl family of GEFs (6). Besides its Dbl/pleckstrin homology domains, Syx contains a PDZ binding motif (PBM) that is required for its recruitment to the junctions (5). Syx is proposed to play a role in endothelial cell migration and is important for angiogenesis and vascular barrier function in vivo (5, 7, 8). Both Syx activity and localization to junctions are critical for these effects, suggesting that misregulation of Syx function results in vascular defects. The mechanisms that regulate Syx localization and function are largely unclear.
In addition to its interaction with the myosin VI adaptor protein synectin (9), the scaffold protein multiple PDZ domain protein 1 (Mupp1) (7, 10), the protein associated with Lin7 (PALS1), and Lin7, we identified several 14-3-3 isoforms as novel Syx-binding partners (5). 14-3-3 family members associate with a diverse number of proteins, including many with oncogenic or tumor suppressor properties (11, 12). Homo- or heterodimers of 14-3-3 proteins bind to select phosphoserine/threonine residues, induce conformational change, and alter the localization, stability, and/or function of the bound protein (13). The localization and dimerization of 14-3-3 proteins are in turn regulated by post-translational modifications such as phosphorylation and acetylation (13). 14-3-3σ and 14-3-3ζ have been suggested to play a role in cell polarization by associating with Par3 (14, 15). However, the role of 14-3-3 proteins on junction stability remains unknown.
In this study, we explored the functional significance of the interaction between Syx and 14-3-3 proteins. Our data suggest that PKD phosphorylation regulates 14-3-3 binding to Syx. More importantly, a phospho-deficient, 14-3-3-uncoupled Syx mutant S92A/S938A displays elevated GEF activity and enhanced localization to areas of cell-cell contact. Altogether, these findings provide a mechanistic insight into how 14-3-3 proteins can modulate junction stability by altering the localization and GEF activity of Syx.
EXPERIMENTAL PROCEDURES
Cell Culture and Transfection
HeLa and MDCK cells were cultured in DMEM (Cellgro) with 10% fetal bovine serum (Invitrogen). HeLa and MDCK cells were transfected with TransIT-HeLaMonster (Mirus) and Lipofectamine 2000 (Invitrogen) according to the manufacturers' instructions, respectively.
Antibodies and Reagents
The following antibodies were used: mouse anti-Syx (KIAA0720, 5A9; Abnova); mouse and rabbit anti-HA (Cell Signaling); mouse anti-GFP/YFP 3E6, mouse anti-ZO1, monoclonal rabbit anti-GFP/YFP (Invitrogen); rabbit pan anti-14-3-3 (K-19), mouse anti-RhoA (26C4) (Santa Cruz Biotechnology); rabbit anti-GST, rabbit anti-actin (Sigma). Phalloidin 594 (Molecular Probes) was used to stain for actin filaments in immunofluorescence experiments.
Phorbol 12-myristate 13-acetate (PMA; Sigma) was dissolved in DMSO to a stock concentration of 100 μm. Protease and phosphatase inhibitor mixtures (Pierce) were used in all buffers (refer to immunoprecipitation section) for the generation of cell lysates.
DNA Constructs and Recombinant Protein
Full-length YFP-tagged murine Syx and HA-tagged PKD WT, kinase-active, and kinase-dead have been described previously (9, 16). Murine Syx truncation mutants were PCR-amplified from pEYFP-mSyx and then subcloned into pEYFP-C1 using HindIII and BamHI restriction sites. Point mutations were introduced in the respective Syx constructs (YFP-Syx, YFP-Syx(1–630), and YFP-Syx(791–1073)) to encode alanine substitutions at Ser92, Ser167, Ser294, Ser806, Ser936, Ser938, and Ser964 using the QuikChange Multisite-directed Mutagenesis kit (Stratagene). GST-tagged 14-3-3 epsilon (ϵ), HA-tagged 14-3-3 beta (β), epsilon (ϵ), gamma (γ), sigma (σ), and zeta (ζ) were purchased from Addgene. All DNA constructs generated were verified by DNA sequencing. pSuper-PKD1-RNAi and pSuper-PKD2-RNAi vectors were used as described previously to knock down PKD1 and PKD2 (17).
Recombinant GST-14-3-3ϵ was produced in Escherichia coli BL21 DE3 (Invitrogen). Briefly, overnight culture of BL21 cells transformed with pGEX-4T1-14-3-3ϵ was induced with 1 mm isopropyl 1-thio-β-d-galactopyranoside (Sigma) at room temperature for 3 h and harvested by centrifugation; harvested bacterial pellet was lysed with extraction buffer (0.5% Nonidet P-40 in 1× PBS, pH 7.4, plus protease inhibitor mixture), sonicated, and clarified by centrifugation. The supernatant was incubated with glutathione-agarose beads (Sigma) at 4 °C for 1 h. The beads were then washed five times with extraction buffer, and bound proteins were eluted with elution buffer (50 mm Tris, 100 mm NaCl, 1 mm DTT, 20 mm glutathione, pH 8.4). The concentration and purity of the eluted protein were evaluated by SDS-PAGE and Coomassie Blue staining (Pierce).
Immunofluorescence, Immunoprecipitation, and Immunoblotting
MDCK cells were seeded on coverslips in 35-mm 6-well tissue culture dishes and transfected with Lipofectamine 2000; cells were fixed with methanol (10 min, −20 °C) or 3% paraformaldehyde (30 min, followed by 5-min permeabilization with 0.2% Triton X-100 containing 1× PBS) the following day as reported previously (18) and probed with primary antibodies followed by incubation with Alexa Fluor secondary antibodies (Invitrogen). Images were acquired with a Zeiss LSM 510 META confocal laser-scanning microscope.
For immunoprecipitation, protein G beads (Invitrogen) were conjugated with either mouse monoclonal anti-Syx (KIAA0720; Abnova) or mouse anti-GFP 3E6 (Invitrogen) overnight at 4 °C in 1× PBS. HeLa cells transfected with the respective constructs were lysed with Triton X-100 lysis buffer (50 mm Tris, 150 mm NaCl, 1 mm EDTA, 1% Triton X-100, pH 7.4). Cell lysates were cleared by centrifugation, and the supernatant was incubated with the antibody-coated protein G beads at 4 °C for 1 h; beads were subsequently washed four times with Triton X-100 lysis buffer, and bound proteins were eluted by boiling in loading buffer.
Total and immunoprecipitated protein samples were analyzed using SDS-PAGE, transferred to nitrocellulose, and probed with the respective primary antibodies. Peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch) were detected using ECL (GE Healthcare).
Active Rho and RhoGEF Pulldown Assay
The level of activated Rho GTP was determined in HeLa cells using Rhotekin pulldown assays as described previously (19). Briefly, HeLa cells were transfected with the corresponding constructs and lysed with Rho activity lysis buffer (20 mm HEPES, 100 mm NaCl, 10% glycerol, 0.5% Nonidet P-40, 0.2% deoxycholic acid, 100 mm MgCl2, pH 7.5); cell lysates were collected and centrifuged, and the cleared supernatants were incubated with reconstituted GST-fused Rhotekin-Rho binding domain (RBD) protein beads (Cytoskeleton) at 4 °C for 1 h. Beads were washed four times with the lysis buffer, and bound proteins were eluted by boiling in loading buffer. Active RhoGEF pulldown assay was performed using Triton X-100 lysis buffer (50 mm Tris, 150 mm NaCl, 1 mm EDTA, 1% Triton X-100, pH 7.4) and RhoA G17A-conjugated glutathione beads (Cell Biolabs); the procedure was identical as described above.
RESULTS
Association of 14-3-3 with Syx Requires Ser92 and Ser938
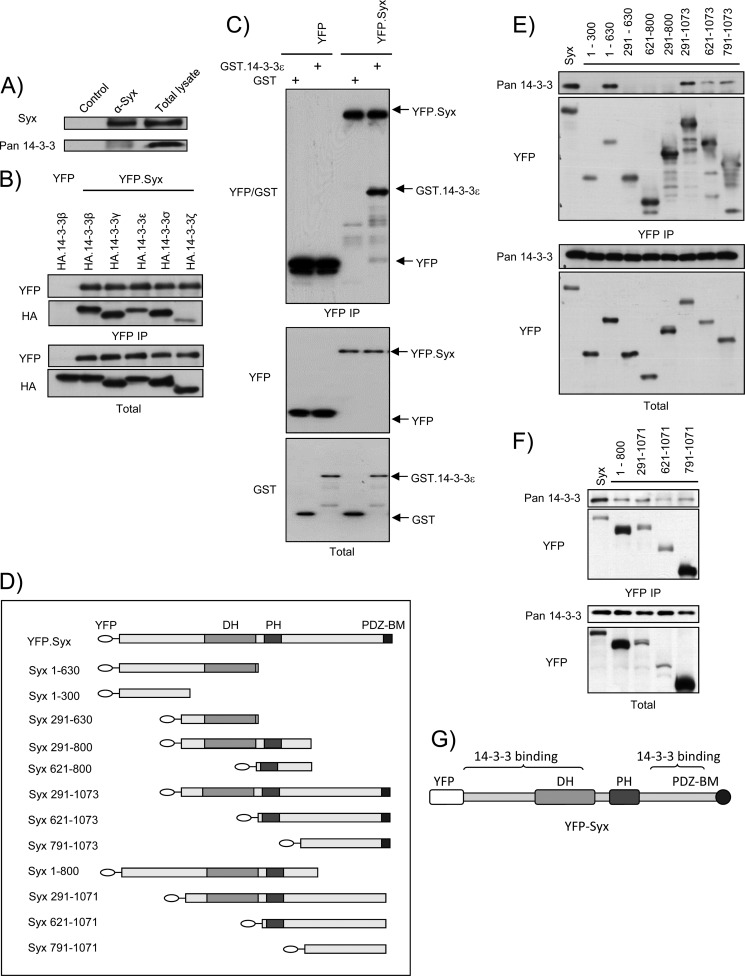
Previously, we identified 14-3-3 proteins as novel Syx-binding partners in our proteomics analysis (5). To verify the interaction, we initially used a pan 14-3-3 antibody to show the presence of endogenous 14-3-3 proteins in Syx immunoprecipitates from HeLa cells (Fig. 1A). We further demonstrated that ectopically expressed Syx associates with multiple 14-3-3 isoforms by co-immunoprecipitating HA-tagged 14-3-3β, ϵ, γ, σ, or ζ with YFP-Syx in HeLa cells (Fig. 1B). To provide biochemical evidence, we performed pulldown experiments where extracts of HeLa cells transiently expressing YFP or YFP-Syx were incubated with GST or purified recombinant GST-tagged 14-3-3ϵ (Fig. 1C). YFP-Syx pulled down GST-14-3-3ϵ proteins but not GST. In addition, YFP alone did not interact with either GST or GST-14-3-3ϵ (Fig. 1C). These data demonstrate that full-length Syx interacts with 14-3-3 proteins inside cells or in a cell-free environment.
FIGURE 1.
Syx associates with 14-3-3 proteins at its termini. A, endogenous Syx co-immunoprecipitates with 14-3-3 proteins. Confluent HeLa cells were lysed and subjected to immunoprecipitation with unconjugated (control) or anti-Syx antibody-conjugated protein G beads. Total and immunoprecipitated cell lysates were analyzed by Western blotting. Pan anti-14-3-3 (K-19) antibody was used. B, YFP-Syx interacts with HA-14-3-3β, ϵ, γ, σ, and ζ. HeLa cells were co-transfected with plasmids encoding YFP or YFP-Syx and HA-14-3-3β, ϵ, γ, σ, or ζ. Cells were lysed and subjected to immunoprecipitation with anti-YFP antibody-conjugated protein G beads. Total and immunoprecipitated cell lysates were analyzed by Western blotting using anti-YFP and anti-HA antibodies. C, YFP-Syx binds GST-14-3-3ϵ in vitro. YFP or YFP-Syx was expressed in HeLa cells and immunoprecipitated with anti-YFP antibody-conjugated protein G beads; YFP and YFP-Syx-bound beads were subsequently incubated with GST or purified recombinant GST-14-3-3ϵ (see “Experimental Procedures”). Protein complexes were eluted and analyzed with Western blotting using anti-YFP and anti-GST antibodies. D, schematic domain structure depicts YFP-tagged murine Syx and Syx truncation variants. The first and last amino acids of each murine Syx fragment are shown as numbers in the corresponding labeling. E and F, 14-3-3 proteins bind two distinct regions of Syx. HeLa cells were transfected with the indicated constructs and subjected to immunoprecipitation (IP) with anti-YFP antibody-conjugated protein G beads. Co-immunoprecipitated 14-3-3 proteins were detected by immunoblotting with pan anti-14-3-3 (K-19) antibody. G, schematic diagram denotes regions of Syx where 14-3-3 proteins bind.
14-3-3 proteins often form dimers and associate with multiple phosphorylated serines on target proteins. For example, 14-3-3β modulates the completion of cytokinesis by binding to the phosphorylated Ser346 and Ser368 on PKCϵ (20); the phosphorylation of Ser166 and Ser186 on Mdm2 regulates its association with 14-3-3σ, which stabilizes Mdm2 and modulates the p53 pathway (21). These studies indicate that more than one 14-3-3-binding residue may exist for a given 14-3-3-interacting protein. We therefore hypothesized that 14-3-3 dimers bind to two distinct phosphorylated residues of Syx.
To narrow down the region that 14-3-3 interacts with Syx, we generated and tested a panel of murine Syx truncation mutants (Fig. 1D). 14-3-3 proteins co-immunoprecipitated with several Syx fragments (Fig. 1E). Notably, 14-3-3 proteins associated with both Syx(1–630) and Syx(791–1073), two nonoverlapping Syx fragments, suggesting that there are two distinct 14-3-3 binding sites on Syx. Because the PBM of Syx associates with multiple binding partners, it is possible that these proteins may themselves interact with 14-3-3 proteins. Therefore, we further tested three additional Syx truncation mutants that lack the PBM and found all three associate with 14-3-3 proteins (Fig. 1F). Thus, our co-immunoprecipitation analysis strongly suggests that Syx contains two 14-3-3 binding sites: one toward its N terminus (amino acids 1–630) and the other close to its C terminus (amino acids 791–1071) (Fig. 1G).
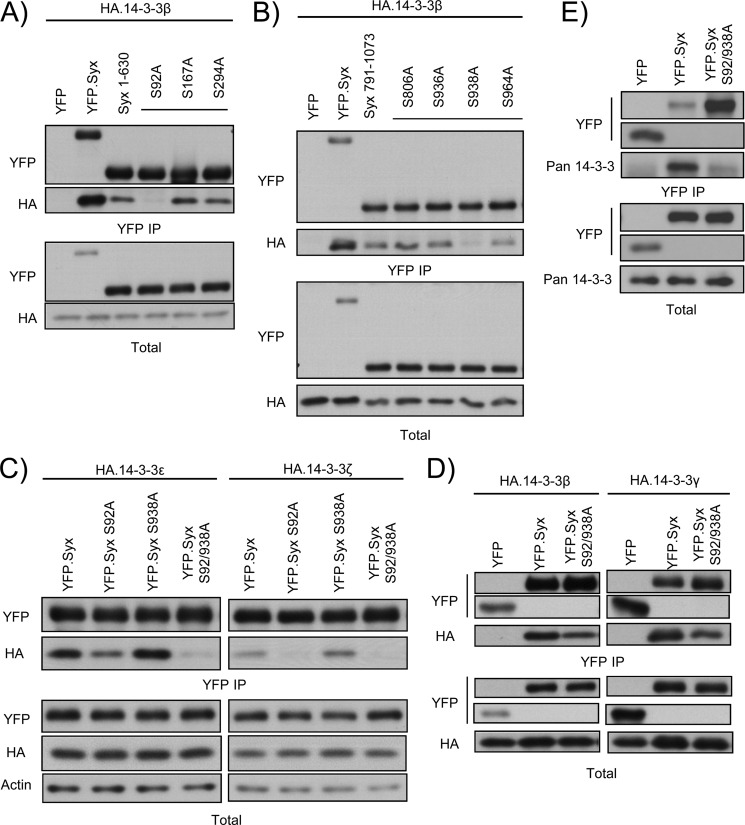
Two 14-3-3 phospho-binding consensus motifs have been identified so far: RSXpSXP (mode I) and RXY/FXpSXP (mode II) (22). However, neither consensus motif is found in the amino acid sequence of Syx. Interestingly, unconventional motifs that do not conform to the established modes of interaction have also been shown to mediate 14-3-3 binding (13). Phospho-peptide mass spectrometry analysis revealed numerous phosphorylated serine residues on Syx.3 To pinpoint the single residue responsible for 14-3-3 binding at each terminus of murine Syx, we generated single serine to alanine mutants using truncated Syx constructs (Syx(1–630) and Syx(791–1073)), to exclude 14-3-3 brought down by the other residue. Co-immunoprecipitation analysis showed a significant decrease in the association of truncated Syx (Syx(1–630) and Syx(791–1073)) with 14-3-3β compared with that of full-length Syx (Fig. 2, A and B). Importantly, mutating Ser92 (Ser100 in human Syx) and Ser938 (Ser955 in human Syx) to alanine on Syx(1–630) and Syx(791–1073), respectively, abolished the binding of 14-3-3β to the corresponding Syx fragments (Fig. 2, A and B). Mutation of other residues, including Ser167, Ser294, Ser806, Ser936, and Ser964, did not interfere with 14-3-3β association.
FIGURE 2.
Ser92 and Ser938 mediate 14-3-3 binding to Syx. A, Ser92 is a putative 14-3-3-binding residue. HeLa cells were co-transfected with plasmids encoding HA-14-3-3β and YFP, YFP-Syx, YFP-Syx(1–630), or one of three YFP-Syx(1–630) mutants in which a single serine was mutated to alanine. Cells were subjected to immunoprecipitation, and protein samples were analyzed by Western blotting. Only the replacement of Ser92 with alanine abolished the interaction between Syx(1–630) and 14-3-3β. B, Ser938 is a putative 14-3-3-binding residue. As in A, HeLa cells were co-transfected with plasmids encoding HA-14-3-3β and YFP, YFP-Syx, YFP-Syx(791–1073), or one of four YFP-Syx(791–1073) in which a single serine was mutated to alanine. Only the replacement of Ser938 with alanine abolished the interaction between Syx(791–1073) and 14-3-3β. Note that Ser806 was previously shown as a PKD-mediated phosphorylation target; mutating Ser806 to alanine, however, has no effect on the binding of 14-3-3β. C and D, Ser92 and Ser938 are essential residues for 14-3-3 interaction. HeLa cells were co-transfected with the indicated constructs and subjected to immunoprecipitation. Protein samples were analyzed by Western blot analysis. Mutating Ser92 and Ser938 on Syx either abolished or diminished its interaction with 14-3-3 isoforms. Note that mutation of Ser938 alone in C has no clear effect on 14-3-3 binding, but resulted in a decrease or absence of 14-3-3 co-immunoprecipitates when combined with S92A mutation. E, endogenous 14-3-3 proteins fail to co-immunoprecipitate with Syx S92A/S938A. HeLa cells were transfected with the indicated constructs and subjected to immunoprecipitation. Protein samples were analyzed by Western blotting. Mutating Ser92 and Ser938 on Syx significantly diminished its interaction with endogenous 14-3-3 proteins.
We subsequently generated single and double mutants of full-length Syx S92A, S938A, and S92A/S938A and assessed their ability to interact with 14-3-3 isoforms. Mutation of Ser92 on Syx resulted in complete ablation of 14-3-3ζ binding and significantly decreased interaction with 14-3-3ϵ (Fig. 2C). Single mutation of Ser938 had no discernible effect on the interaction between Syx and 14-3-3 proteins, but reduced the binding of 14-3-3ϵ when combined with the mutation of Ser92 (Fig. 2C). These data suggest that, whereas Ser938 has minor and isoform-specific effects, Ser92 is the major residue that influences 14-3-3 binding. Diminished interaction was also observed between the Syx double mutant S92A/S938A and two other 14-3-3 isoforms (β and γ) compared with that of wild type Syx (Fig. 2D). Importantly, endogenous 14-3-3 proteins failed to co-immunoprecipitate with the S92A/S938A Syx double mutant (Fig. 2E). The incomplete abrogation of exogenous 14-3-3 binding may be attributed to the interaction of overexpressed HA-14-3-3 proteins with alternative serine residues. In addition, Syx-interacting partners, which themselves can be regulated by 14-3-3 proteins, may also account for the residual association. It is clear, however, that Ser92 and Ser938 are the major sites involved in the association between Syx and 14-3-3 proteins.
PKD-mediated Phosphorylation of Syx at Ser92 Induces 14-3-3 Binding
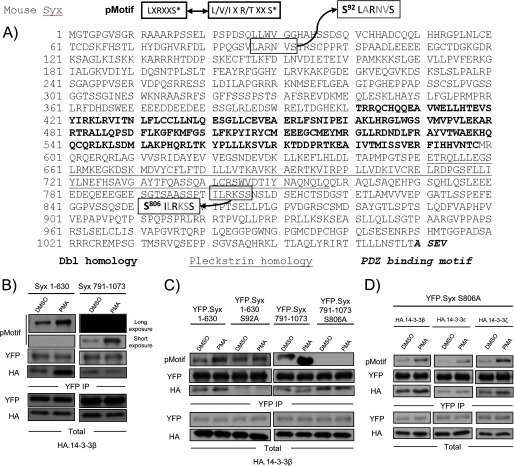
Phospho-peptide mass spectrometry analysis revealed the phosphorylation of Ser92, which conforms to a putative PKD phosphorylation motif (LXRXXS*) (Fig. 3A). To examine whether Ser92 is phosphorylated by PKD we utilized pMotif, an antibody that recognizes PKD phosphorylated motifs (23). Using pMotif, we showed previously that the major site of VEGF-induced, PKD-mediated phosphorylation on Syx is Ser806 (5). Consistent with this, the phosphorylation of Syx(791–1073) was readily detectable by the pMotif antibody (Fig. 3B, right). As expected, the PKD-mediated phosphorylation of Syx(791–1073) was due to the presence of Ser806 (Fig. 3C, right). Interestingly, basal level phosphorylation was observed on immunoprecipitated Syx(1–630) (when exposure of the immunoblot to the x-ray film was prolonged), and this phosphorylation was increased upon PMA stimulation, a condition that induces PKD activation (Fig. 3B, left). Notably, mutating Ser92 to alanine abolished the PMA-induced increase of phosphorylation on Syx(1–630), further suggesting that Ser92 is a PKD phosphorylation target. Furthermore, binding of HA-14-3-3β positively correlated with increased phosphorylation of Syx(1–630) at Ser92 (Fig. 3, B and C, left). In contrast, no change in HA-14-3-3β binding was detected for Syx(791–1073) or Syx(791–1073) S806A upon PMA treatment (Fig. 3C, right), indicating that PKD-mediated phosphorylation of Ser806 does not affect 14-3-3 binding.
FIGURE 3.
PMA/PKD mediated phosphorylation of Syx at Ser92. A, Ser92 is a putative PKD phosphorylation site. The pMotif antibody recognizes a consensus motif that contains a leucine at the −5 position and an arginine at the −3 position. Ser92 and Ser806 are the only residues within Syx that conform to the consensus motif. B, Syx(1–630) is a PKD phosphorylation target. HeLa cells were co-transfected with the indicated constructs and subjected to DMSO (control) or PMA (100 nm, 10 min) treatment prior to immunoprecipitation. Protein samples were analyzed by Western blot. X-ray film was exposed to the same blot for different lengths of time to visualize the phosphorylation (by pMotif) of Syx(1–630) and Syx(791–1073). C, both control and PMA-induced 14-3-3 associations require Syx Ser92. As in B, transfected cells were subjected to immunoprecipitation after treatment with DMSO (control) or PMA (100 nm, 10 min). Protein samples were analyzed by Western blotting. Note that the mutation of Ser92 to alanine abrogated the PMA-induced increase in phosphorylation seen with pMotif in Syx(1–630). D, Ser92 is phosphorylated by PKD in addition to Ser806. A PMA-induced increase in pMotif phosphorylation and 14-3-3 binding was observed upon transfecting cells with full-length Syx mutant YFP-Syx S806A. As in B, transfected cells were subjected to immunoprecipitation, and protein samples were analyzed by Western blotting.
To confirm this observation in full-length Syx, we repeated the same immunoprecipitation experiments using YFP-Syx S806A, which binds 14-3-3 equally well as wild type Syx (supplemental Fig. 1). An identical pattern was observed, where increased Syx phosphorylation and 14-3-3 (β, ϵ, and ζ) binding were detected upon PMA stimulation (Fig. 3D). Therefore, the data suggest that PKD is an important modulator of Syx phosphorylation and association with 14-3-3 proteins.
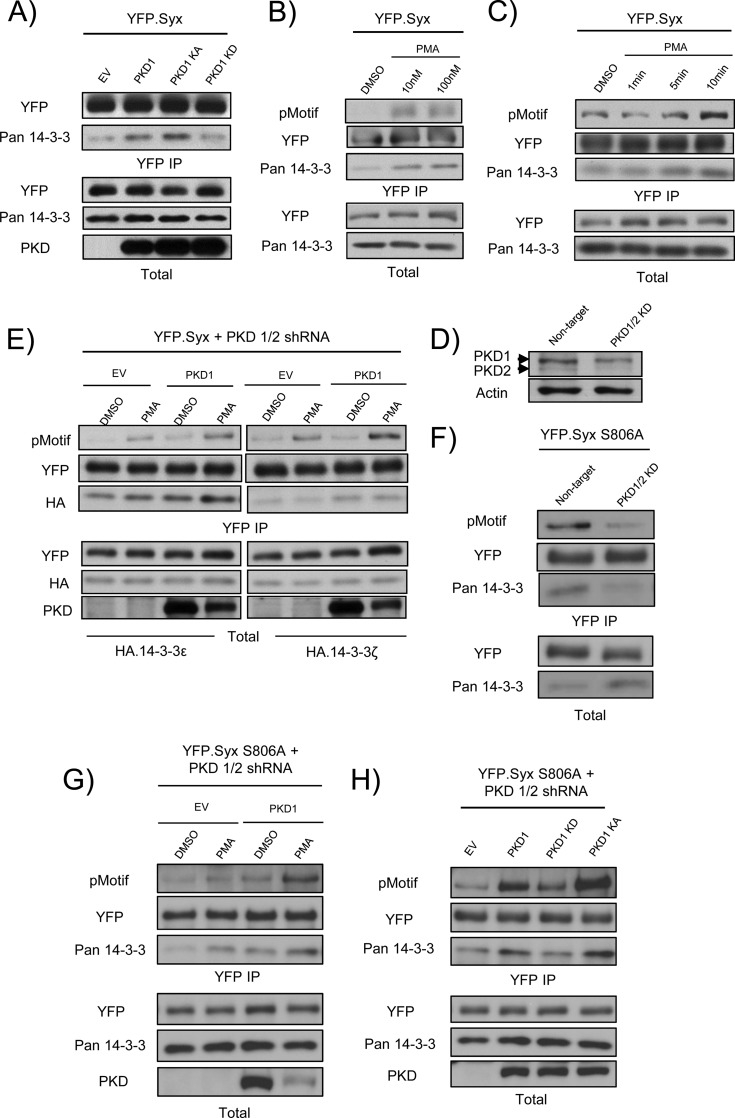
To test further this hypothesis, we co-expressed YFP-Syx with PKD1 variants and observed a higher amount of co-immunoprecipitated endogenous 14-3-3 proteins in HeLa cells that expressed either wild type or kinase-active (but not kinase-dead) PKD1 (Fig. 4A). Increased endogenous 14-3-3 binding to YFP-Syx also correlated strongly with PMA-induced PKD activation in a concentration- and time-dependent manner (Fig. 4, B and C). We validated these observations by expressing YFP-Syx, PKD1, and HA-14-3-3ϵ or ζ in PKD1/2-depleted HeLa cells (Fig. 4D) and performing co-immunoprecipitation analyses. Binding of 14-3-3ϵ and ζ to Syx positively correlated with the expression of PKD1 (Fig. 4E). To determine whether the PKD-mediated phosphorylation of Ser92 is physiological and correlates with binding of endogenous 14-3-3 proteins, we expressed and immunoprecipitated YFP-Syx S806A from PKD1/2-depleted cells. Down-regulation of endogenous PKD1/2 decreased both Ser92 phosphorylation and 14-3-3 binding (Fig. 4F). Furthermore, increased Ser92 phosphorylation and 14-3-3 binding positively correlated with the expression of wild type PKD1 and PMA stimulation (Fig. 4G) and the expression of wild type and kinase-active PKD1 (Fig. 4H) in PKD1/2-depleted cells. Combined, the data indicate that the interaction between Syx and 14-3-3 proteins is regulated by PKD-mediated phosphorylation at Ser92.
FIGURE 4.
PKD1 induces binding of 14-3-3 to Syx. A, interaction between Syx and 14-3-3 increases in the presence of PKD1. HeLa cells were co-transfected with the indicated constructs and subjected to immunoprecipitation. Expression of wild type or kinase-active (KA) PKD1, but not kinase-dead (KD) PKD1, increases the binding of endogenous 14-3-3 proteins to YFP-Syx. B and C, PMA induces 14-3-3 binding to Syx. HeLa cells were transfected with YFP-Syx and subjected to DMSO (control) or PMA treatment (10 versus 100 nm PMA in B, 1, 5, or 10 min of 100 nm PMA in C prior to lysing and immunoprecipitation. Increased 14-3-3 binding to Syx was observed in a concentration- and time-dependent fashion. D, shRNA mediated down-regulation of PKD1 and PKD2. Western blot analysis shows the protein levels of PKD1/2 in nontarget versus PKD1 and PKD2 shRNA-expressing HeLa cells. E, association of YFP-Syx with 14-3-3 isoforms is enhanced by PKD1 activation. PKD1/2-depleted HeLa cells were transfected with YFP-Syx, pcDNA (empty vector (EV)) or PKD1, and HA-14-3-3ϵ or ζ and subjected to DMSO (control) or PMA (100 nm) treatment prior to immunoprecipitation. Total and immunoprecipitated protein samples were analyzed by Western blotting. F, Down-regulation of endogenous PKD1/2 suppresses Ser92 phosphorylation and 14-3-3 binding. PKD1/2-depleted HeLa cells were transfected with YFP-Syx S806A and subjected to immunoprecipitation. A decrease in pMotif signal and 14-3-3 binding was observed in YFP-Syx S806A upon PKD1/2 depletion. G, Ser92 phosphorylation and 14-3-3 binding are increased by PMA stimulation and PKD1 expression. PKD1/2-depleted HeLa cells were transfected with YFP-Syx S806A and subjected to DMSO (control) or PMA treatment (100 nm, 10 min) prior to lysing and immunoprecipitation. Stepwise increase of pMotif staining and co-immunoprecipitated 14-3-3 was observed with PMA stimulation or PKD1 expression, or both. H, increase of Ser92 phosphorylation and 14-3-3 binding is dependent on PKD1 expression. PKD1/2-depleted HeLa cells were co-transfected with the indicated constructs and subjected to immunoprecipitation. Expression of wild type or kinase-active PKD1, but not kinase-dead PKD1, increased the pMotif staining of YFP-Syx S806A and the binding of endogenous 14-3-3 proteins to YFP-Syx S806A.
Binding of 14-3-3 Proteins Modulates the Function of Syx
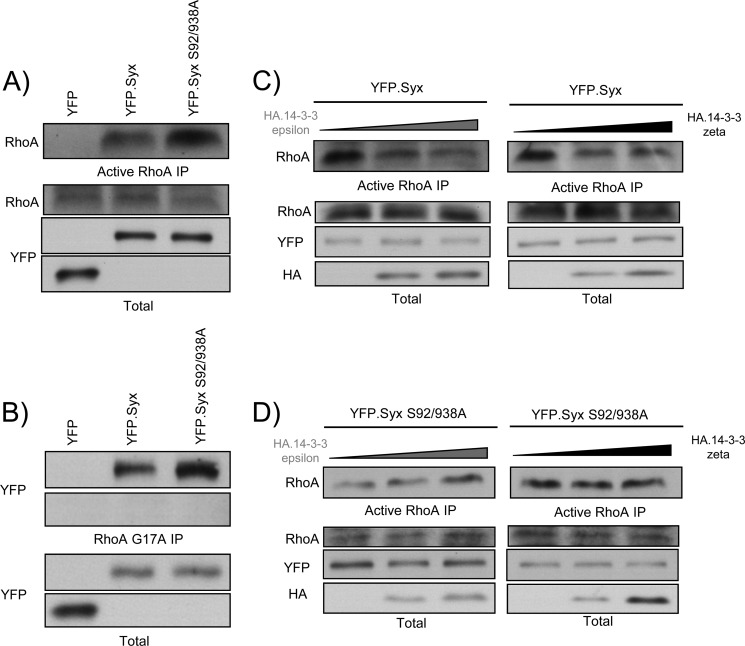
To determine the physiological significance of 14-3-3 binding, we sought to evaluate the guanine nucleotide exchange activity of 14-3-3-uncoupled Syx. Because Syx was classified as a RhoA-specific GEF (6), experiments utilizing GST-tagged Rhotekin-RBD protein beads were performed to pull down GTP-bound RhoA from lysates of HeLa cells expressing YFP, YFP-Syx, or YFP-Syx S92A/S938A. Expression of YFP-Syx increased the global RhoA activation in cells compared with YFP alone; meanwhile, Syx S92A/S938A-expressing cells exhibited significantly higher RhoA activity than Syx-expressing cells (Fig. 5A). To demonstrate that the observed increase in RhoA activation was a direct result of increased GEF activity of Syx S92A/S938A, we performed active RhoGEF pulldown assays. By coupling recombinant RhoA G17A to glutathione beads, the nucleotide-free RhoA mutant acts as a trap and binds activated RhoGEFs with high affinity (24). In agreement with our active RhoA assay, a substantially higher amount of Syx S92A/S938A was brought down in the active RhoGEF pulldown assay (Fig. 5B).
FIGURE 5.
Binding of 14-3-3 proteins inhibits the nucleotide exchange activity of Syx. A, 14-3-3-uncoupled Syx has high GEF activity. HeLa cells were transfected with plasmids encoding YFP, YFP-Syx, or YFP-Syx S92A/S938A. Cells were lysed 24 h after transfection, and the supernatants were incubated with GST-fused Rhotekin-RBD beads to bind active RhoA. Total and pulled-down active RhoA were determined by SDS-PAGE and immunoblotting. B, Syx S92A/S938A is highly active. As in A, cells were transfected with the indicated constructs and lysed 24 h after transfection. The supernatants were incubated with RhoA G17A-conjugated glutathione beads to pull down active RhoGEFs. Total and pulled-down active Syx were analyzed by SDS-PAGE and immunoblotting. C, expression of 14-3-3ϵ and ζ inhibits Syx-induced RhoA activation. HeLa cells were co-transfected with YFP-Syx and an increasing amount (0, 1, or 2 μg, respectively) of HA-14-3-3ϵ or ζ. As in A, active RhoA pulldown assay was performed, and protein samples were analyzed by Western blotting. D, Syx S92A/S938A-induced RhoA activation is unaffected by the expression of 14-3-3ϵ or ζ. As in C, active RhoA was pulled down from lysates of HeLa cells co-expressing YFP-Syx S92A/S938A and an increasing amount (0, 1, or 2 μg, respectively) of HA-14-3-3ϵ or ζ. Total and active RhoA were determined by SDS-PAGE and immunoblotting.
The observation that Syx S92A/S938A exhibits elevated nucleotide exchange activity suggested that 14-3-3 binding suppresses the GEF activity of Syx. To test this hypothesis, we performed active RhoA pulldown assays using lysates from HeLa cells that co-expressed YFP-Syx and HA-tagged 14-3-3ϵ or ζ. A high level of RhoA activation was observed when cells were transfected with Syx. The Syx-induced RhoA activation, however, was counteracted by the increased expression of 14-3-3ϵ or ζ (Fig. 5C). In contrast, this suppression of RhoA activation did not occur when 14-3-3-uncoupled Syx S92A/S938A was co-expressed with 14-3-3 proteins (Fig. 5D), indicating that this effect of 14-3-3 proteins was not due to off-target effects but specific to Syx binding. Therefore, the data strongly argue that 14-3-3 binding modulates the GEF activity of Syx.
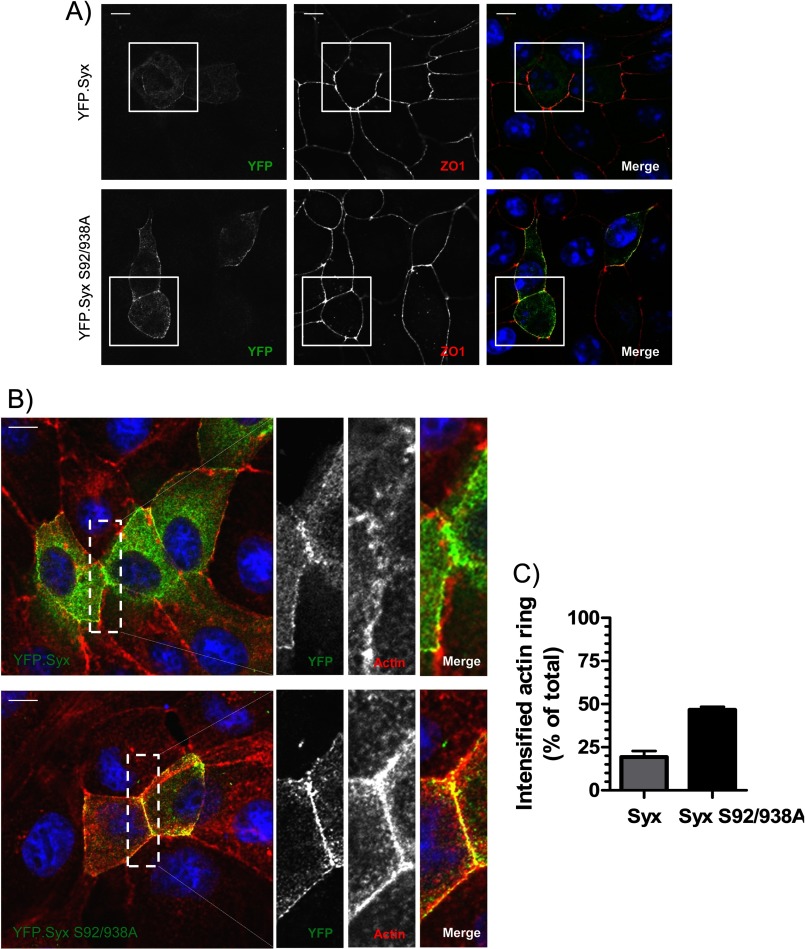
Previously, we demonstrated that the targeting of Syx to areas of cell-cell contact is critical for junction integrity (5). Therefore, in addition to the effects on RhoA activity, we sought to determine whether 14-3-3 binding also affects Syx localization. When expressed in MDCK cells, 14-3-3-uncoupled Syx showed a notably stronger targeting to the cell junctions compared with its wild type counterpart (Fig. 6A, boxes). This observation implied that the interaction with 14-3-3 proteins could modify the intracellular localization of Syx.
FIGURE 6.
14-3-3-uncoupled Syx is strongly targeted to the cell border and enhances circumferential actin accumulation. A, Syx S92A/S938A localizes strongly at areas of cell-cell contact. MDCK cells were transfected with plasmids encoding YFP-Syx or YFP-Syx S92A/S938A. Cells were fixed and immunostained for YFP, ZO1, and DAPI. B, Syx S92A/S938A induces the accumulation of circumferential actin at cell contacts. MDCK cells were transfected with the indicated constructs, fixed 24 h after transfection, and immunostained for YFP, phalloidin (F-actin), and DAPI. C, A quantitative analysis of enhanced circumferential actin staining in transfected cells from B was performed. The intensity of circumferential actin at the junctions of transfected cells was compared with that of immediate neighboring nontransfected cells using ImageJ; transfected cells that exhibited intensified circumferential actin staining are expressed as percentage of total cells counted (mean ± S.E. (error bars), n = 3, 50 cells/coverslip analyzed). Scale bars, 10 μm.
We showed previously that Syx mediates junction stability by activating RhoA and its effector Dia1 specifically at cell-cell contacts (5). To further evaluate the function of Syx S92A/S938A, we assessed its ability to induce changes to the circumferential actin cytoskeleton, which correlates with the maturity and stability of adherens junctions. Cells expressing Syx S92A/S938A, but not wild type Syx, displayed enriched circumferential actin staining compared with neighboring cells (Fig. 6B). When the intensity of cortical actin staining was quantified, we observed significantly increased junctional actin staining in cells expressing Syx S92A/S938A (Fig. 6C). The strengthening of the cortical actin ring is likely the result of elevated GEF activity of Syx S92A/S938A, which increases RhoA activation at areas of cell-cell contact. Our data support the importance of junctional Syx in regulating cell-cell adhesion and further delineate the molecular mechanisms that regulate Syx function.
DISCUSSION
The ubiquitously expressed 14-3-3 isoforms bind to a large number of proteins and mediate a remarkable range of cellular activities. However, regulation of cell-cell adhesion by 14-3-3 proteins is largely unknown. Here, our data strongly suggest that Syx-regulated junction stability is in part modulated by PKD-mediated 14-3-3 binding.
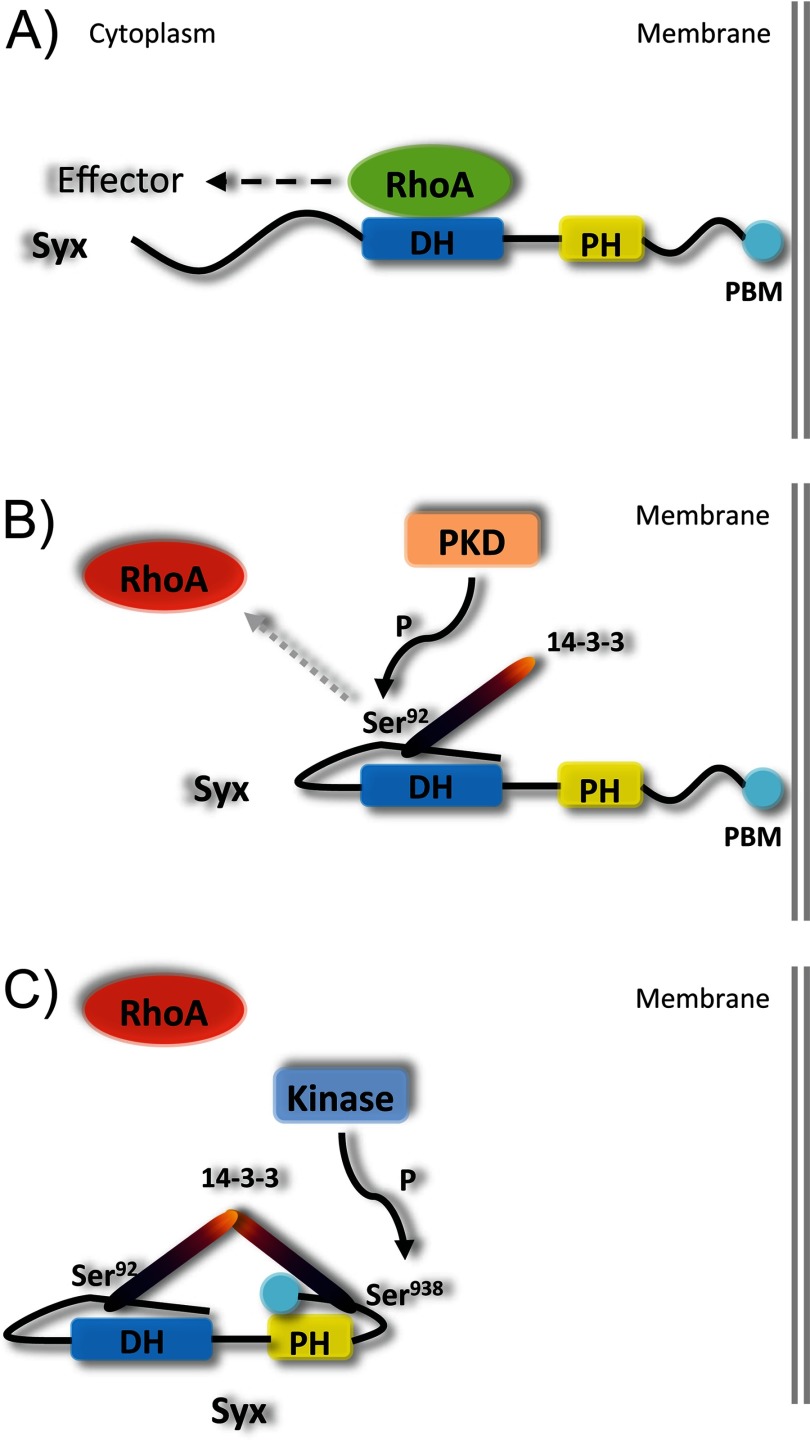
There are two aspects that define the functional relationship between Syx and 14-3-3 proteins: the effects of 14-3-3 binding on the localization and the guanine nucleotide exchange activity of Syx. The 14-3-3-uncoupled mutant Syx S92A/S938A exhibits strong junctional localization, suggesting that the binding of 14-3-3 proteins has a negative impact on Syx localization and therefore its function to maintain junction stability. Our previous study identified Syx as a target of VEGF-induced, PKD1-mediated phosphorylation at Ser806, an event that induced the mislocalization of Syx away from cell junctions. Mutating Ser806 to alanine did not interfere with the ability of Syx to bind 14-3-3 proteins, but it did induce Mupp1 association and promoted the junctional localization of Syx (5). It also allowed us to detect the unexpected phosphorylation of Ser92 by PKD1. From a structural perspective, phosphorylation of Ser806 may induce conformational changes in Syx that promote not the association per se, but the dimerization of 14-3-3 proteins associated with the N- and C-terminal regions of Syx and the subsequent dissociation from cell junctions (Fig. 7). Importantly, prior data suggest that the N-terminal domain (Syx(1–300)) can suppress Syx exchange activity (5). Indeed, several RhoGEFs are subjected to inhibitory intramolecular interactions between their N-terminal domains and their catalytic Dbl homology domains (25). Assuming a similar mode of action, 14-3-3 binding at the N and C termini and subsequent dimerization could promote an inactive Syx conformation, thus explaining the negative effect of 14-3-3 binding on Syx GEF activity (Fig. 7). An intramolecular interaction between Syx(1–300) with the Dbl homology domain may also explain the observed lack of 14-3-3 binding to Syx(1–300) (Fig. 1E), despite the presence of Ser92 within this fragment. We postulate that the intramolecular interaction results in three-dimensional folding changes that allow Ser92 phosphorylation and association with 14-3-3 proteins. Whatever the mechanism, it is clear that PKD is a key regulator of junctional Syx function. Adhesion is a dynamic process, and as such, the function of Syx is highly regulated by a multitude of events, including its autoinhibitory N terminus, the phosphorylation of Ser92, Ser806, and Ser938 (of which the upstream kinase is currently unknown), and the association of 14-3-3 proteins.
FIGURE 7.
Proposed mechanism of PKD-mediated 14-3-3 binding to Syx. A, membrane-localized Syx activates RhoA locally. B, PKD-mediated phosphorylation of Ser92 upon proper folding of Syx N terminus induces binding of 14-3-3 protein. C, phosphorylation of Ser938 promotes 14-3-3 binding at the C terminus. Additional phosphorylation of Ser806 (data not shown) by PKD induces conformational changes to the C terminus and brings the two 14-3-3 proteins into close proximity. As a consequence, dimerization of terminally bound 14-3-3 proteins locks Syx in an inactive state (suppresses GEF activity), and Syx is displaced from areas of cell-cell contact. PH, pleckstrin homology domain; DH, Dbl homology domain.
RhoA is thought to regulate signaling events that contribute to cell junction formation and preservation (4, 26). Dia1, a RhoA effector that is proposed to regulate cell junction stability (27, 28), belongs to the formin family and can remodel the actin cytoskeleton (29). We previously identified Dia1 as the key downstream effector of Syx in regulating junction integrity (5). Here, we observed increased RhoA activation and intensified actin staining at areas of cell-cell contact in cells expressing Syx S92A/S938A. We postulate that the increased junctional targeting and elevated GEF activity of Syx S92A/S938A results in the subsequent Rho-mediated activation of Dia1, the expansion of the circumferential actin bundle, and the stabilization of cell-cell contacts.
In conclusion, our findings indicate a role for 14-3-3 proteins in the regulation of cell-cell junctions by modulating the function of Syx. Based on studies conducted on Syx thus far, it is likely that Syx is regulated by the integration of signals from multiple upstream pathways. Uncovering additional mechanisms will provide further insights as to how Syx functions. Interestingly, our data suggest that the junction disruptive effects of tumor promoting phorbol esters may be mediated by Syx dysfunction. Finally, despite its function in junction integrity, the role of Syx in human cancer is currently unknown.
Supplementary Material
Acknowledgments
We thank Drs. Alan Fields, Aubrey Thompson, Nicole Murray, and Larry Karnitz (Mayo Clinic) for critical comments.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 NS069753 and R21 NS070117 (to P. Z. A.) and R01 GM086435 and 10BG11 (to P. S.). This work was also supported by the Mayo Graduate School (to S. P. N.).

This article contains supplemental Fig. 1.
S. P. Ngok, R. Geyer, and P. Z. Anastasiadis, unpublished data.
- GEF
- guanine nucleotide exchange factor
- Dia1
- Diaphanous homolog 1
- DMSO
- dimethyl sulfoxide
- MDCK
- Madin-Darby canine kidney
- PBM
- PDZ binding motif
- PMA
- phorbol 12-myristate 13-acetate
- RBD
- Rho binding domain.
REFERENCES
- 1. Etienne-Manneville S., Hall A. (2002) Rho GTPases in cell biology. Nature 420, 629–635 [DOI] [PubMed] [Google Scholar]
- 2. Jaffe A. B., Hall A. (2005) Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21, 247–269 [DOI] [PubMed] [Google Scholar]
- 3. Iden S., Collard J. G. (2008) Crosstalk between small GTPases and polarity proteins in cell polarization. Nat. Rev. Mol. Cell Biol. 9, 846–859 [DOI] [PubMed] [Google Scholar]
- 4. Nelson W. J. (2008) Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem. Soc. Trans. 36, 149–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ngok S. P., Geyer R., Liu M., Kourtidis A., Agrawal S., Wu C., Seerapu H. R., Lewis-Tuffin L. J., Moodie K. L., Huveldt D., Marx R., Baraban J. M., Storz P., Horowitz A., Anastasiadis P. Z. (2012) VEGF and angiopoietin-1 exert opposing effects on cell junctions by regulating the RhoGEF Syx. J. Cell Biol. 199, 1103–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Toledo M., Coulon V., Schmidt S., Fort P., Blangy A. (2001) The gene for a new brain specific RhoA exchange factor maps to the highly unstable chromosomal region 1p36.2–1p36.3. Oncogene 20, 7307–7317 [DOI] [PubMed] [Google Scholar]
- 7. Ernkvist M., Luna Persson N., Audebert S., Lecine P., Sinha I., Liu M., Schlueter M., Horowitz A., Aase K., Weide T., Borg J. P., Majumdar A., Holmgren L. (2009) The Amot/Patj/Syx signaling complex spatially controls RhoA GTPase activity in migrating endothelial cells. Blood 113, 244–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garnaas M. K., Moodie K. L., Liu M. L., Samant G. V., Li K., Marx R., Baraban J. M., Horowitz A., Ramchandran R. (2008) Syx, a RhoA guanine exchange factor, is essential for angiogenesis in vivo. Circ. Res. 103, 710–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu M., Horowitz A. (2006) A PDZ-binding motif as a critical determinant of Rho guanine exchange factor function and cell phenotype. Mol. Biol. Cell 17, 1880–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Estévez M. A., Henderson J. A., Ahn D., Zhu X. R., Poschmann G., Lübbert H., Marx R., Baraban J. M. (2008) The neuronal RhoA GEF, Tech, interacts with the synaptic multi-PDZ-domain-containing protein, MUPP1. J. Neurochem. 106, 1287–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morrison D. K. (2009) The 14-3-3 proteins: integrators of diverse signaling cues that impact cell fate and cancer development. Trends Cell Biol. 19, 16–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tzivion G., Gupta V. S., Kaplun L., Balan V. (2006) 14-3-3 proteins as potential oncogenes. Semin. Cancer Biol. 16, 203–213 [DOI] [PubMed] [Google Scholar]
- 13. Aitken A. (2006) 14-3-3 proteins: a historic overview. Semin. Cancer Biol. 16, 162–172 [DOI] [PubMed] [Google Scholar]
- 14. Ling C., Zuo D., Xue B., Muthuswamy S., Muller W. J. (2010) A novel role for 14-3-3σ in regulating epithelial cell polarity. Genes Dev. 24, 947–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hurd T. W., Fan S., Liu C. J., Kweon H. K., Hakansson K., Margolis B. (2003) Phosphorylation-dependent binding of 14-3-3 to the polarity protein Par3 regulates cell polarity in mammalian epithelia. Curr. Biol. 13, 2082–2090 [DOI] [PubMed] [Google Scholar]
- 16. Eiseler T., Döppler H., Yan I. K., Kitatani K., Mizuno K., Storz P. (2009) Protein kinase D1 regulates cofilin-mediated F-actin reorganization and cell motility through slingshot. Nat. Cell Biol. 11, 545–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Storz P., Döppler H., Toker A. (2004) Protein kinase Cδ selectively regulates protein kinase D-dependent activation of NF-κB in oxidative stress signaling. Mol. Cell. Biol. 24, 2614–2626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yanagisawa M., Anastasiadis P. Z. (2006) p120 catenin is essential for mesenchymal cadherin-mediated regulation of cell motility and invasiveness. J. Cell Biol. 174, 1087–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yanagisawa M., Huveldt D., Kreinest P., Lohse C. M., Cheville J. C., Parker A. S., Copland J. A., Anastasiadis P. Z. (2008) A p120 catenin isoform switch affects Rho activity, induces tumor cell invasion, and predicts metastatic disease. J. Biol. Chem. 283, 18344–18354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saurin A. T., Durgan J., Cameron A. J., Faisal A., Marber M. S., Parker P. J. (2008) The regulated assembly of a PKCϵ complex controls the completion of cytokinesis. Nat. Cell Biol. 10, 891–901 [DOI] [PubMed] [Google Scholar]
- 21. Wood N. T., Meek D. W., Mackintosh C. (2009) 14-3-3 binding to Pim-phosphorylated Ser-166 and Ser-186 of human Mdm2: potential interplay with the PKB/Akt pathway and p14(ARF). FEBS Lett. 583, 615–620 [DOI] [PubMed] [Google Scholar]
- 22. Yaffe M. B., Rittinger K., Volinia S., Caron P. R., Aitken A., Leffers H., Gamblin S. J., Smerdon S. J., Cantley L. C. (1997) The structural basis for 14-3-3:phosphopeptide binding specificity. Cell 91, 961–971 [DOI] [PubMed] [Google Scholar]
- 23. Döppler H., Storz P., Li J., Comb M. J., Toker A. (2005) A phosphorylation state-specific antibody recognizes Hsp27, a novel substrate of protein kinase D. J. Biol. Chem. 280, 15013–15019 [DOI] [PubMed] [Google Scholar]
- 24. García-Mata R., Wennerberg K., Arthur W. T., Noren N. K., Ellerbroek S. M., Burridge K. (2006) Analysis of activated GAPs and GEFs in cell lysates. Methods Enzymol. 406, 425–437 [DOI] [PubMed] [Google Scholar]
- 25. Rossman K. L., Der C. J., Sondek J. (2005) GEF means go: turning on Rho GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 6, 167–180 [DOI] [PubMed] [Google Scholar]
- 26. Braga V. M. (2002) Cell-cell adhesion and signalling. Curr. Opin. Cell Biol. 14, 546–556 [DOI] [PubMed] [Google Scholar]
- 27. Carramusa L., Ballestrem C., Zilberman Y., Bershadsky A. D. (2007) Mammalian diaphanous-related formin Dia1 controls the organization of E-cadherin-mediated cell-cell junctions. J. Cell Sci. 120, 3870–3882 [DOI] [PubMed] [Google Scholar]
- 28. Ryu J. R., Echarri A., Li R., Pendergast A. M. (2009) Regulation of cell-cell adhesion by Abi/Diaphanous complexes. Mol. Cell. Biol. 29, 1735–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chesarone M. A., DuPage A. G., Goode B. L. (2010) Unleashing formins to remodel the actin and microtubule cytoskeletons. Nat. Rev. Mol. Cell Biol. 11, 62–74 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.