Background: The GTP-binding protein Gαs facilitates myometrial quiescence during pregnancy.
Results: TNF overcomes trichostatin A (TSA)-induced myometrial relaxation. This is due to changes in levels of CBP and acetylated H4K8 within the Gαs promoter.
Conclusion: Promoter acetylation is important in governing expression of Gαs.
Significance: TSA-induced myometrial relaxation can be overcome by TNF through repression of Gαs promoter activity.
Keywords: G Protein-coupled Receptors (GPCR), G Proteins, NF-κB (NF-KB), Reproduction, Transcription Regulation, Myometrium, Parturition, Preterm Labor
Abstract
The onset of parturition is associated with a number of proinflammatory mediators that are themselves regulated by the nuclear factor κB (NF-κB) family of transcription factors. In this context, we previously reported that the RelA NF-κB subunit represses transcription and mRNA expression of the proquiescent Gαs gene in human myometrial cells following stimulation with the proinflammatory cytokine TNF. In the present study, we initially defined the functional consequence of this on myometrial contractility. Here we show that, contrary to our initial expectations, TNF did not induce myometrial contractility but did inhibit the relaxation produced by the histone deacetylase inhibitor trichostatin A, an effect that in turn was abolished by the NF-κB inhibitor N4-[2-(4-phenoxyphenyl)ethyl]-4,6-quinazolinediamine. This result suggested a role for TNF in regulating Gαs expression via activating NF-κB and modifying histone acetylation associated with the promoter region of the gene. In this context, we show that the −837 to −618 region of the endogenous Gαs promoter is occupied by cAMP-response element-binding protein (CREB), Egr-1, and Sp1 transcription factors and that CREB-binding protein (CBP) transcriptional complexes form within this region where they induce histone acetylation, resulting in increased Gαs expression. TNF, acting via NF-κB, did not change the levels of CREB, Sp1, or Egr-1 binding to the Gαs promoter, but it induced a significant reduction in the level of CBP. This was associated with increased levels of histone deacetylase-1 and surprisingly an increase in H4K8 acetylation. The latter is discussed herein.
Introduction
During pregnancy, the uterus adopts a relative state of myometrial smooth muscle inactivity termed quiescence. At the onset of labor, the quiescent state is lost, and a series of powerful myometrial contractions occur that act to expel the infant. In the developed world, premature birth complicates 6–10% of pregnancies (1). Significantly, the incidence of birth before 28 weeks of gestation (severely preterm) is increasing (3, 69) with those infants having elevated risks of major long term mental and physical handicaps (4).
There is growing evidence indicating that in the human myometrium the cessation of uterine quiescence (myometrial relaxation) and the onset of both normal and preterm labor are associated with a number of proinflammatory factors and cytokines, including TNF, IL1-β, IL-8, and COX-2, which are regulated by a family of transcription factors collectively referred to as nuclear factor κB (NF-κB)4 (Refs. 5–22; for reviews, see Refs. 23 and 24).
Although it is accepted that NF-κB is predominantly considered an activator of gene expression, a body of evidence exists that also supports the thesis that NF-κB can act as a repressor of transcription. It can do this directly by binding to DNA and recruiting inhibitory factors such as histone deacetylase proteins to the promoter (25–27) or through interaction with other transcription factors such as Egr-1, c-Myc, and Sp1 (Refs. 28–31; for a review, see Ref. 32). Of significance, however, is that NF-κB can repress transcription of certain genes via in-direct non-DNA binding mechanisms that involve competing for cellular co-activators of transcription such as CBP and p300 (33, 34). Such factors are recognized as enzymes capable of making chromatin more accessible to the transcription machinery through induction of histone acetylation indicative of epigenetic changes. Consequently, removal of these transcription factors from a given promoter by NF-κB may favor promoter histone deacetylation and subsequent transcriptional repression.
The importance of epigenetic mechanisms in the establishment, regulation, and maintenance of transcriptional activation or repression is well documented (35, 36). Chromatin is the term given to DNA when it assumes a highly ordered, structured complex within the nucleus. The fundamental unit of chromatin is the nucleosome, which consists of 150 bp of DNA wrapped around a core histone octamer (two each of H2A, H2B, H3, and H4; Ref. 37). Importantly, DNA-mediated processes, including transcription, must occur within the architectural confines of the nucleosome. In general, chromatin is highly repressive to events such as transcription because the folding of the DNA results in the steric blockade of the template, greatly reducing its accessibility by the relevant enzymes and ancillary factors. To circumvent this problem, eukaryotic cells have evolved in a manner that allows the chromatin to be carefully manipulated, thereby permitting controlled access to DNA at the appropriate temporal junction. Indeed, eukaryotes have developed multiple mechanisms to finely tune chromatin structure: a principle approach involving post-translational modification of histones, including acetylation (38), methylation (39), and phosphorylation (40), all of which are laid down in a dynamic fashion.
The full repertoire of molecular factors that perpetuate myometrial quiescence remains unknown. It is clear, however, that components of the cAMP/protein kinase A (PKA) pathway are differentially expressed in the human myometrium during pregnancy (41–47): central to this is the GTP-binding protein Gαs. Indeed, signaling through Gαs is thought to facilitate promotion of myometrial quiescence, and Gαs protein levels have been shown to be reduced at the onset of labor (41, 42). There are, however, only a limited number of reports describing the transcriptional control of the Gαs gene. The Gαs promoter itself is located on chromosome 20, is a highly GC-rich segment of DNA, and therefore contains many binding sites for transcription factors that bind GC-containing DNA sequences, including those for CREB, Egr, and Sp families (48, 50, 51, 70). Our previous studies have suggested that the promoter region of the Gαs gene is regulated by Sp-like transcription factors that require phosphorylation by PKA (44–46). Moreover, we have demonstrated that TNF-induced activation of NF-κB led to the repression of the Gαs promoter in a manner that utilized competition for the co-activator and histone acetyltransferase CBP (22). In our view, because levels of CBP are elevated in pregnant human myometrium but decline in laboring samples (52), epigenetic control of quiescence may also be important in human parturition.
In this study, we demonstrated that TNF significantly reduced trichostatin A (TSA)-induced myometrial relaxation. Moreover, the NF-κB inhibitor N4-[2-(4-phenoxyphenyl)ethyl]-4,6-quinazolinediamine (QNZ) prevented the effects of TNF. Utilizing the chromatin immunoprecipitation (ChIP) assay, we showed that the native Gαs promoter is occupied by CREB, Egr-1, Sp1, and CBP; moreover, both promoter occupancy by CBP and acetylation status of histone H4K8 were subsequently modified in the presence of TNF and the histone deacetylase inhibitor TSA. We discuss the implications of these findings.
MATERIALS AND METHODS
Selection of Patients and Tissue Collection
Women were recruited from the Department of Obstetrics and Gynaecology at the Jessop Wing, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, South Yorkshire, UK. This study received approval from the Rotherham Local Research Ethics Committee (REC Reference Number 05/Q2306/22). All patients gave informed, written consent. Lower uterine segment myometrial samples were obtained from healthy women undergoing elective caesarean section at term (n = 45; age, 16–43; gestation, 37–40 weeks) as described previously (21, 22). Myometrial smooth muscle cell cultures were then subsequently generated as detailed in Phaneuf et al. (43).
Cell Culture, Transient Transfections, Plasmids, and Luciferase Assays
Transient transfection of primary human myometrial cells was performed using the LT-1 reagent from Miras (Geneflow Ltd., Staffordshire, UK) as described by Chapman et al. (22). The Gαs-luciferase (Gαs-luc) reporter has been described previously (45). The 3x-κB-ConA-luciferase (3x-κB-ConA-luc) and enh-ConA-luciferase (ΔκB-ConA-luc) vectors were the generous gift of Prof. Ron Hay (University of Dundee, UK), and the construction of these has been reported in detail (53). The RcRSV-HA-CBP expression plasmid was supplied by Dr. Paul Hurd (Queen Mary University of London, UK) but was originally generated in the Goodman laboratory (Vollum Institute, Portland, OR), and its construction has been described elsewhere (54). The pcDNA-HDAC-1 vector was the gift of Prof. Bradley Bernstein (Department of Pathology, Massachusetts General Hospital, Harvard Medical School), and its construction has been described previously (55). For each transfection, 200 ng of luciferase reporter was used. Twenty-four hours after transfection, cells were then stimulated for a further 24 h with 10 ng/ml TNF. For experiments investigating the role of CBP, 200 ng of RcRSV-HA-CBP or 200 ng of RcRSV-NRC-MCS was used. Transfections using histone deacetylase-1 (HDAC-1) utilized 0.5, 1.0, and 1.5 μg of pcDNA-HDAC-1 or 0.5, 1.0, and 1.5 μg of pcDNA3 to normalize the amount of DNA. Luciferase assays were performed three times in triplicate as described previously (22), and data were collected using a Berthold Sirius luminometer (Geneflow Ltd.).
Immunofluorescence
Myometrial cells were cultured in a 24-well plate and upon reaching 80% confluence were stimulated with 10 ng/ml TNF for 24 h. The cells were washed in PBS and fixed in 1% (v/v) formaldehyde overnight at 4 °C. Cells were washed in PBS three times for 5 min on a rocking platform and permeabilized with PBS containing 1% (w/v) BSA and 0.1% (v/v) Triton X-100 with three 5-min washes. After washing again in PBS, RelA antiserum (Santa Cruz Biotechnology Inc., sc-372) was added to each well at a dilution of 1:100 (in PBS containing 1% (w/v) BSA) for 2 h at 37 °C. The antibody was removed with three 5-min washes in PBS, and the goat anti-rabbit IgG secondary antibody was added to the wells at a dilution of 1:200 (in PBS containing 1% (w/v) BSA) for 1 h at 37 °C. The unbound antibody was washed with three 5-min PBS washes, and the nuclear counterstain DAPI was added to the wells at a 1:20 dilution in PBS.
ChIP Assay
The ChIP assay was performed using the Magna-ChIP assay kit provided by Millipore (catalog number 17-611) following the manufacturer's guidelines. Essentially, four T-75 flasks of primary myocytes were grown to 100% confluence. Two of these flasks were then stimulated with 10 ng/ml TNF for 24 h. Cells were then fixed for 10 min using 37.5% formaldehyde added directly to the medium. This reaction was subsequently quenched by adding 2 ml of 1 m glycine made up in water. Finally, medium was removed, and cells were washed twice in ice-cold PBS (pH 7.4) supplemented with protease inhibitor mixture (Millipore). Cells were removed from each flask in the PBS:protease inhibitor mixture using a cell scraper. Appropriate samples were then pooled into control or stimulated groups, and the cell suspension was clarified by centrifugation at 4 °C at 800 × g for 5 min.
The cell pellet was resuspended in 500 μl of Magna-ChIP lysis buffer supplemented with protease inhibitor mixture. Essentially, a two-step lysis was undertaken to reduce nonspecific chromatin-IgG interactions. In the first lysis step, cells were opened, nuclei were obtained, and cells were then incubated in lysis buffer on ice for 15 min and vortexed briefly every 5 min. This suspension was clarified by centrifugation at 800 × g for 5 min at 4 °C. For the second lysis step, which opened the nuclei, the supernatant was discarded, the pellet was resuspended in 500 μl of Magna-ChIP nuclear lysis buffer, and the sample was subjected to ultrasonic cavitation on high power for three 10-s exposures to lyse the nuclei and fragment the chromatin. Chromatin fragments with an average size of ∼500 bp were obtained.
Immunoprecipitation of Chromatin-bound Complexes
The fragmented chromatin was separated into 50-μl aliquots (∼1 × 106 cells) and then diluted in 450 μl of Magna-ChIP dilution buffer. To each sample, 5 μg of immunoprecipitating antibody was added. Antisera used were anti-RelA C-terminal antibody (Santa Cruz Biotechnology Inc., sc-372), anti-Sp1 (Millipore, catalog number 07–645), anti-CREB (Cell Signaling Technology, catalog number 9192), anti-acetylated histone H3 (H3K9; Millipore, catalog number 06-599), anti-acetylated histone H4 (H4K8; Cell Signaling Technologies, catalog number 2594), anti-CBP and anti-Egr-1 (Santa Cruz Biotechnology Inc., sc-369 and sc-588, respectively), and nonspecific rabbit IgG (Abcam, catalog number ab46450). To this, a 20-μl bed volume of pre-prepared Dynabeads (Invitrogen) preadsorbed with 20 μg/ml sonicated salmon sperm DNA and 1 mg/ml BSA in Tris-EDTA buffer (pH 8.0) was added. The immunoprecipitation reactions were carried out overnight at 4 °C with constant, gentle agitation. After this period, immunoprecipitated complexes were recovered with a magnet, and the beads were washed to remove nonspecific binding using alternative high salt:low salt and LiCl washes in accordance with the manufacturer's instructions. Transcription factor-bound DNA was then eluted and purified as detailed in the Magna-ChIP protocol and stored at −20 °C.
PCR of Immunoprecipitated DNA
PCR on the immunoprecipitated DNA was carried out using primers flanking the κB sites within the IκBα promoter (sense, 5′-GACGACCCCAATTCAAATCG-3′; antisense, 5′-TCAGGCTCGGGGAATTTCC-3′) as a positive control for the ChIP assay, giving a product size of ∼300 bp (22). As a positive control for promoter occupancy by CBP, PCR on the immunoprecipitated DNA was also carried out using primers flanking the CXCL9 promoter (56). The following primer sequences were used: CXCL9 sense, 5′-TTCCACATCCAGGTAGCAACTTTG-3′; CXCL9 antisense, 5′-TGTTGGAGTGAAGTCCGAGAATGT-3′. These primers gave the expected product size of 86 bp. Reaction conditions for both controls were those originally described in Saccani et al. (57): one cycle of denaturation at 94 °C for 3 min and 36 cycles of 94 °C for 45 s, 60 °C for 1 min, and 72 °C for 1 min followed by a final elongation at 72 °C for 10 min. Samples were then resolved using 1.5% Tris acetate agarose gel electrophoresis, and a product size of ∼300 bp corresponding to the IκBα promoter was observed.
During the course of the study, it became apparent that the GC-rich nature of the Gαs promoter made its amplification by PCR difficult. Consequently, to analyze promoter occupancy in the Gαs promoter (−837 to −618; Ref. 48) (70), the following primers were used: Gαs-distal-3 sense, 5′-TTGGTTCGTGCTCGCCTCGC-3′; Gαs-distal-3 antisense, 5′-CAGTGACGACCCCTCGCACG-3′. The following protocol was also utilized to facilitate PCR from the GC-rich −837 to −618 region of the Gαs promoter. Each 25-μl reaction consisting of 5× Hercules buffer, 25 mm dNTPs, 0.25 μl of ChIP DNA, 100 pmol of Gαs-distal-3 sense, 100 pmol of Gαs-distal-3 antisense, 0.25 μl of Hercules DNA polymerase (note that the manufacturer, Agilent, did not provide a value for the number of enzyme units/μl), and 1 μl of DMSO. Reaction conditions were as follows: one cycle of denaturation at 98 °C for 3 min and 38 cycles of 98 °C for 30 s, 61 °C for 30 s, and 72 °C for 30 s followed by a final elongation at 72 °C for 5 min. Samples were then resolved using 1.5% Tris acetate agarose gel electrophoresis, and a product size of ∼219 bp (−837 to −618 bp of the Gαs promoter) was obtained.
Band Intensity Analysis of Amplified, Immunoprecipitated DNA
In this study, end point PCR was used to give semiquantitative data on enrichment levels of these respective transcription factors on each promoter. These analyses were undertaken using the SynGene (Cambridge, UK) GBox Chemi-16 gel documentation system to capture images. Subsequent band quantification was undertaken using GeneTools Version 4 quantification software (SynGene). In all cases, the value ascribed to nonspecific binding of IgG was subtracted from the specific binding value. That value was then expressed as a percentage of the input fraction with the input fraction intensity being taken as 100%. Where statistically significant changes were observed, all appropriate ChIP gels are illustrated.
Western Blot Analyses
Western blot experiments were performed as detailed in Chapman et al. (22). Briefly, RelA expression was detected using anti-RelA C-terminal antibody (Santa Cruz Biotechnology Inc., sc-372). The anti-acetyl-RelA antiserum was obtained from Cell Signaling Technology (catalog number 3045) and detects transfected levels of RelA NF-κB only when residue Lys-310 is acetylated. Gβ (Santa Cruz Biotechnology Inc., sc-378) was utilized to ensure equal well loading as detailed in Chapman et al. (22). After incubation with an appropriate secondary antiserum (125 ng of goat anti-rabbit HRP conjugates; Dako, catalog number P0448), membranes were then washed in PBS with Tween 20 and developed according to the EZ-ECL detection protocol supplied by Geneflow Ltd.
Myometrial Contractility
The effects of TNF were tested in a model of myometrial contractility at 10 and 30 ng/ml. Experiments were carried out as described in Pearson et al. (58). Briefly, strips were mounted in vitro at 37 °C in a physiological salt solution aerated with 95% O2 and 5% CO2 and allowed to contract spontaneously. Following equilibration, separate concentration-response curves for both TSA and the NF-κB inhibitor QNZ (59) were generated. The ability of QNZ to modulate the contractile response was carried out by preincubating tissue ± 10 or 30 ng/ml TNF for 1 h prior to cumulative additions of TSA. Data were analyzed by calculating activity integrals for contractions over a 30-min period, and concentration-effect curves were fitted to a four-parameter logistic equation (58).
Statistical Analysis
All experiments were performed a minimum of three times, and results are expressed as the mean ± S.E. (error bars). All data analyses were conducted using GraphPad Prism Version 5.02 (GraphPad Software, San Diego, CA). Data from transfection experiments and pharmacological studies were compared using one-way analysis of variance and analyzed further using Tukey's multiple comparison post-test. Data from two matched samples were compared using a paired, two-tailed t test; p < 0.05 was considered statistically significant.
RESULTS
TNF Inhibits TSA-induced Relaxation in Isolated Strips of Human Myometrium: a Process Inhibited by Treatment with QNZ
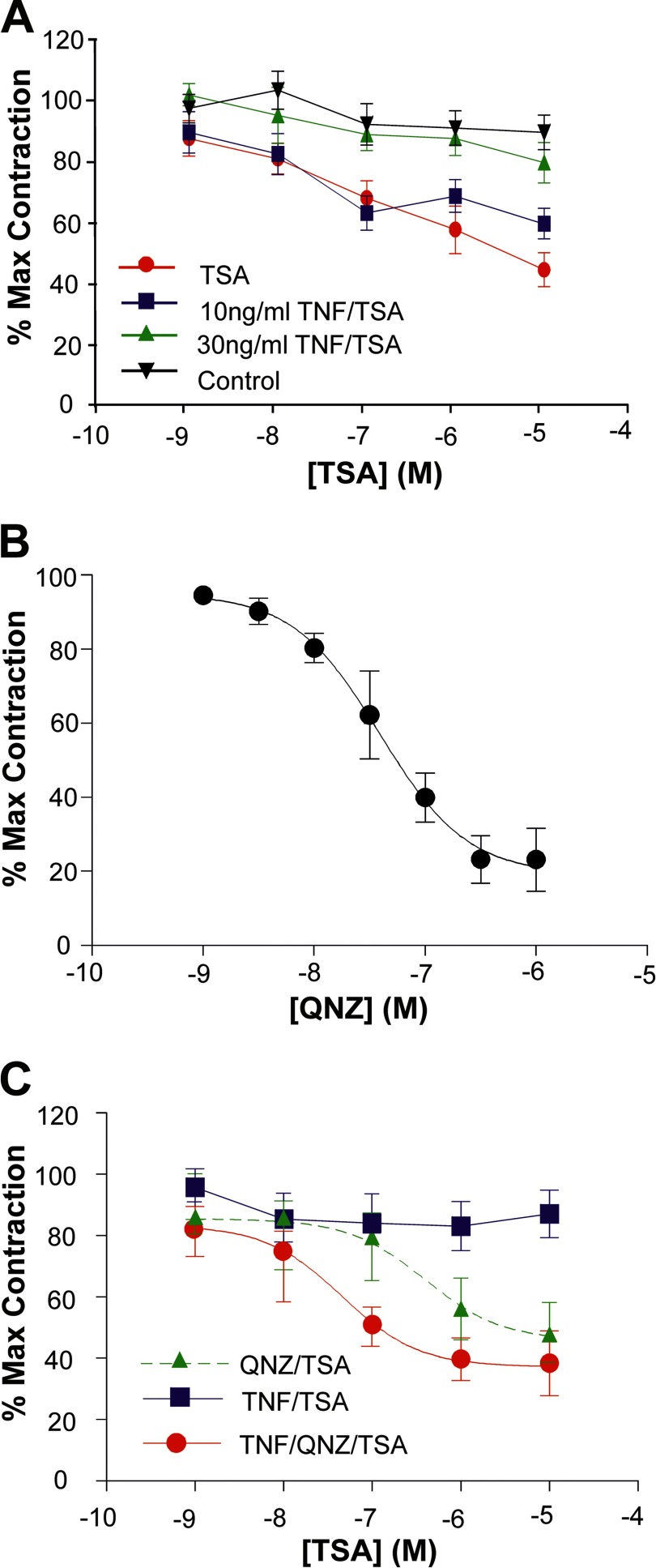
A number of proinflammatory cytokines, including TNF, are associated with the onset of both normal and preterm birth (23, 24). Taken together with data highlighting the reduction of Gαs protein levels in term and preterm laboring myometrium, we speculate that the localized inflammation within the myometrium is an important step in this transition from quiescence to contractility. Much of that work, however, has been conducted in primary cell monolayers. As such, we wanted to determine how TSA, QNZ, and TNF could influence contraction of isolated strips of human myometrium. First, cumulative addition of TSA was seen to relax spontaneously contracting, isolated myometrial strips (Fig. 1A, red line; n = 6). Significantly, whereas TNF had little effect on TSA-induced myometrial relaxation at 10 ng/ml (Fig. 1A, blue line), it did, however, greatly reduce the TSA-induced relaxation at a concentration of 30 ng/ml (Fig. 1A, green line). In support of the notion that this contraction was mediated by the NF-κB family of transcription factors, the NF-κB inhibitor QNZ, which is known to inhibit endogenous TNF synthesis (59), was also seen to inhibit myometrial contractions with an IC50 of 3.9 × 10−8 m (n = 6), suggesting a role for NF-κB in this contractile response (Fig. 1B).
FIGURE 1.
TNF, TSA, and QNZ all effect myometrial contraction. Isolated myometrial strips were prepared as described under “Materials and Methods.” A, myometrial tissue strips (n = 6 for each observation) were incubated in either 10 or 30 ng/ml TNF for 1 h followed by the cumulative addition of 10−9–10−5 m TSA to the bath. Data were analyzed using an unpaired, two-tailed t test, and results are expressed as the mean ± S.E. (error bars); p < 0.05 was considered statistically significant. Control strips were incubated with vehicle and showed no significant change in contractility with time. TSA alone caused a concentration-dependent relaxation of spontaneous myometrial contractions that was inhibited in the presence of 30 ng/ml TNF but unaffected by 10 ng/ml. B, QNZ inhibited myometrial contractility in a concentration-dependent manner with an IC50 of 3.9 × 10−8 m. C, cumulative addition of TSA to myometrial tissues preincubated with QNZ (50 nm) and TNF (30 ng/ml) produced a leftward shift in the response compared with QNZ alone.
Finally, preincubation of myometrial strips in 50 nm QNZ (a concentration close to the IC50) followed by addition of TSA also produced relaxation (Fig. 1C, green line; n = 6). In the presence of 30 ng/ml TNF and 50 nm QNZ, a leftward shift in the concentration-response curve to TSA was seen (Fig. 1C, red line; n = 6), supporting our view that myometrial relaxation induced by TSA can be modulated by TNF in a mechanism involving the NF-κB family of transcription factors.
TNF Represses Expression of the Gαs Promoter but Induces NF-κB Activity
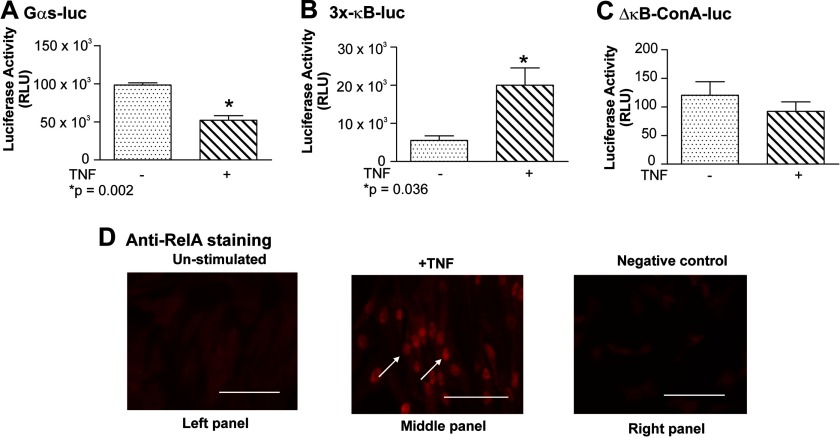
A decline in both the levels of mRNA and Gαs protein within human myometrium has been shown to accompany the transition from quiescence to labor (22, 42). Although the full details of this mechanism remain unclear, we have previously described that the TNF-induced repression of a Gαs-luc reporter vector transfected into primary myometrial myocytes is mediated by the NF-κB RelA subunit competing for limiting quantities of the key transcription cofactor CBP (22). Consequently, control experiments were undertaken to determine whether the myometrial cell cultures used in this study behaved in a similar manner. Briefly, TNF was seen to repress the Gαs-luc reporter (Fig. 2A) but to induce transcription from the NF-κB-sensitive 3x-κB-luc vector (Fig. 2B). No activity was seen when cells harboring the ConA-luc vector (which is not NF-κB-sensitive) were exposed to TNF (Fig. 2C). Because we wished to investigate the regulation of the Gαs promoter in the presence of activated NF-κB RelA, we confirmed as expected that TNF induced nuclear localization of RelA in primary myometrial myocytes (compare diffuse staining in unstimulated or nonspecific IgG-stained cells (Fig. 2D, left and right panels) with TNF-stimulated cells (Fig. 2D, middle panel)).
FIGURE 2.
TNF represses Gαs expression but induces NF-κB activity in primary myometrial myocytes. Primary myometrial cultures were transfected with 200 ng of either Gαs-luc (A), 3x-κB-ConA-luc (NF-κB-responsive) (B), or ΔκB-ConA-luc (NF-κB-unresponsive) (C). After 24 h, cells were stimulated with TNF (10 ng/ml) for 24 h. Promoter activity was quantified using a Berthold Sirius tube luminometer. All experiments were performed three times in triplicate. Data were analyzed using a paired, two-tailed t test, and results are expressed as the mean ± S.E. (error bars); p < 0.05 was considered statistically significant. As expected, TNF repressed Gαs expression (A; p = 0.002) and activated NF-κB (B; p = 0.036). No NF-κB activity was observed in a control reporter lacking κB sites (C). Immunostaining was used to demonstrate TNF-mediated induction of RelA nuclear localization in primary myometrial myocytes (D, white arrows; compare middle panel (TNF-stimulated) with left panel (un-stimulated) and right panel (negative control); scale bar, 100 μm).
The −837 to −618 Region of the Endogenous Gαs Promoter Is Occupied by CREB, Egr-1, and Sp1 and Recruits CBP
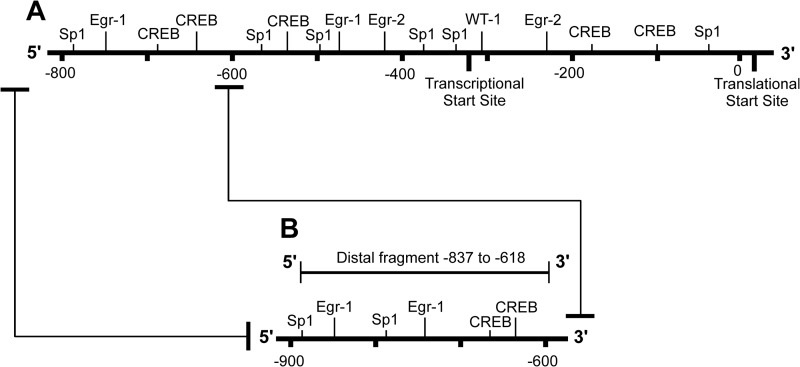
The Gαs promoter originally utilized in the Gαs-luc vector represents the region from −790 to the transcription start site (45). Previous studies have concentrated on the more proximal aspects of that promoter sequence (45). Therefore, in this study, we wished to define how the more distal aspect of the Gαs promoter, region −837 to −618, regulated expression (illustrated in Fig. 3A).
FIGURE 3.
Schematic illustration of the Gαs promoter highlighting the −837 to −618 region. The Gαs promoter originally utilized in the Gαs-luc vector represents the −790 to the transcription start site (A). In terms of experimental analyses, this study focused on the more distal aspect of this promoter (−837 to −618; B), and this was the target for use in the ChIP assays herein unless stated otherwise.
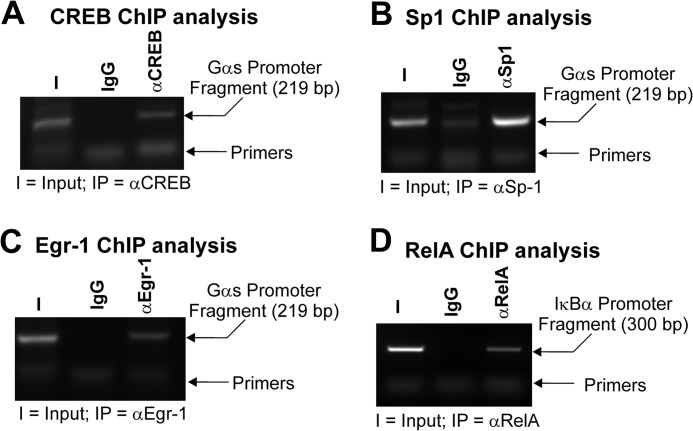
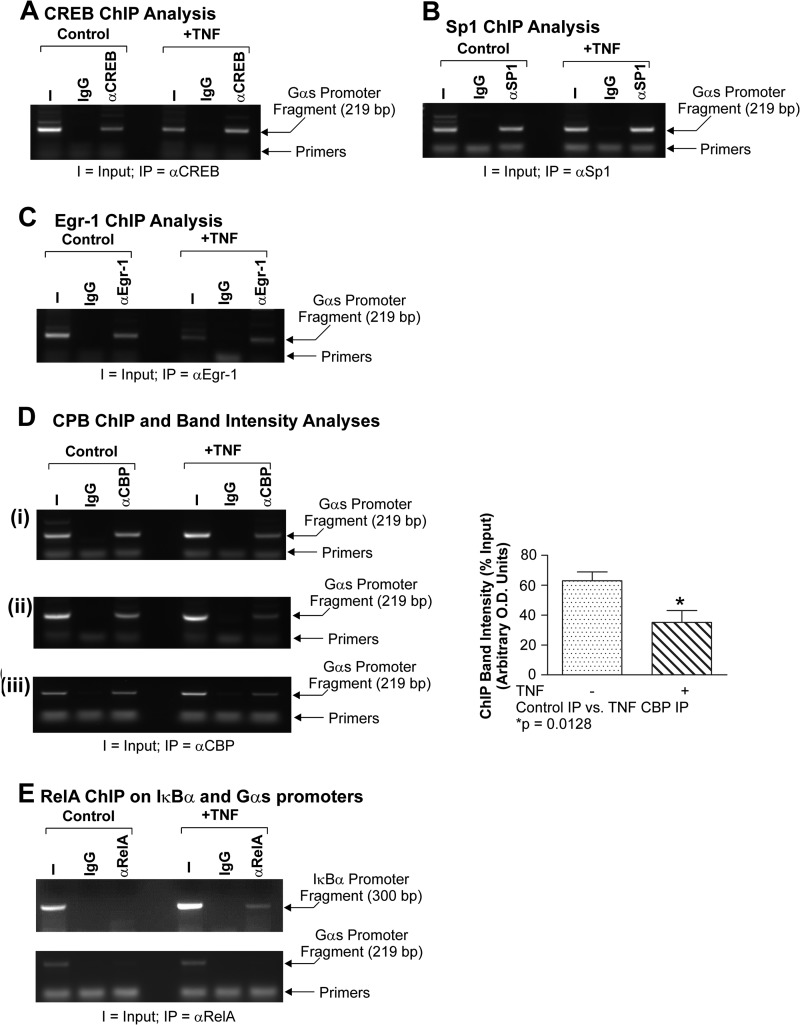
The native Gαs promoter is a GC-rich, TATA-less regulatory region (48). Using the TransFac algorithm (70), we identified many putative transcription factor binding sites. Those of particular relevance to this study included Sp, Egr, and CREB families. Sp1 has been shown to play a pivotal role in regulating the expression of TATA-less GC-rich promoters through its ability to position RNA polymerase II at the appropriate initiator site (50, 51). Moreover, Sp1 is also thought to serve as an active boundary between non-transcribed heterochromatin and transcriptionally competent euchromatin (50). As such, it was decided to examine Sp1 in this context. Egr1 was chosen because it is a potent GC-rich DNA-binding zinc finger transcription factor. Moreover, it has been shown to compete with NF-κB RelA for DNA binding, and it binds to CBP (28, 60). We decided to investigate CREB because it requires an interaction with CBP for full transactivation (61, 62), and in terms of myometrial biology, both CREB and CBP have been demonstrated to have a pivotal role in Gαs regulation (22, 45, 52). Consequently, to determine whether these factors were present on the endogenous Gαs promoter, we isolated primary myometrial smooth muscle cells as detailed above and cultured them until confluent. The ChIP assay was then utilized to determine whether these factors were present on the distal Gαs promoter fragment. Fig. 4, A–C, demonstrate that CREB, Sp1, and Egr-1 occupied the −837 to −618 aspect of the endogenous Gαs promoter, respectively. Positive controls for this experiment included occupation of the IκBα promoter by RelA NF-κB (Fig. 4D). Other endogenous Sp and Egr family members were not detected in Western analyses prior to the ChIP study (data not shown).
FIGURE 4.
The −837 to −618 Gαs promoter fragment is occupied by CREB, Sp1, and Egr-1. Primary cultures of myometrial cells were subjected to the ChIP assay. It was clear that CREB (A), Sp1 (B), and Egr-1 (C) occupied the endogenous distal Gαs promoter. TNF-induced RelA occupancy of the IκBα promoter illustrated that the ChIP assay was functioning correctly (D). I, input; IP, immunoprecipitating antibody.
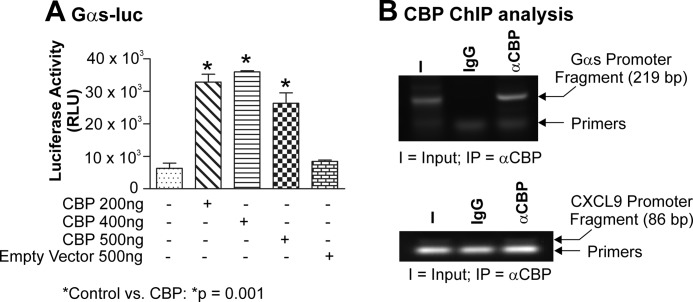
Our previous work demonstrated that when CBP and the Gαs-luc reporter vectors were co-transfected into primary myometrial cells expression of exogenous CBP was shown to enhance activity of the Gαs promoter (Fig. 4A and Ref. 22). Factors such as CBP, however, do not have an intrinsic specific DNA binding domain but can still be recruited to a promoter through direct interactions with promoter-bound heterologous factors (62). In the context of the Gαs promoter, those factors identified in Fig. 3, A–C, namely CREB, Sp1, and Egr-1, have all been documented to interact with CBP in various cells of various origins, including the myometrium (60–62). Consequently, we wished to determine whether CBP was also present within the −837 to −618 section of the native Gαs promoter. To address this notion, endogenous chromatin from primary myometrial myocytes was again subjected to the ChIP assay. Significantly, CBP was associated with this segment of the endogenous Gαs promoter in native chromatin derived from myometrial myocytes (Fig. 5B, upper panel). To support this observation, we also examined the CXCL9 promoter, which has previously also been shown to be a target of CBP (56). Again, in our studies, we were able to see CXCL9 promoter occupancy by CBP (Fig. 5B, lower panel).
FIGURE 5.
Exogenous CBP induces Gαs-luc activity, whereas endogenous CBP is recruited to the −837 to −618 region of the native Gαs promoter. Primary myometrial cultures were transfected with 200 ng of Gαs-luc and 200, 400, or 500 ng of CBP. Cells were harvested after 24 h, and luciferase activity was quantitated. All experiments were performed three times in triplicate. Data were compared using one-way analysis of variance and analyzed further using Tukey's multiple comparison test, and results are expressed as the mean ± S.E. (error bars); p < 0.05 was considered statistically significant. CBP strongly induced Gαs expression at all doses (A; p = 0.001). Using the ChIP assay on cultures of primary myometrial myocytes, it was shown that endogenous CBP also occupied the endogenous Gαs promoter (B, upper panel). CBP occupancy of the CXCL9 promoter served as the positive control (B, lower panel). I, input; IP, immunoprecipitate.
TNF Does Not Reduce Gαs Promoter Occupancy by CREB, Sp1, or Egr-1 but Does Reduce Binding by CBP
TNF has been shown to repress a Gαs promoter construct transfected into myometrial cells (22). The factors involved in regulating the natural Gαs promoter within its native context, however, have not been defined. In the present study, we used the ChIP assay on chromatin extracted from TNF-stimulated primary myometrial myocytes to determine whether there were any changes in the level of Gαs promoter occupancy by the factors identified above, namely CREB, Egr-1, and Sp1. In the presence of TNF, we did not observe any noticeable change in the levels of CREB (Fig. 6A), Egr-1 (Fig. 6B), or Sp1 (Fig. 6C) on the −837 to −618 portion of the native Gαs promoter when the myometrial cells were exposed to TNF.
FIGURE 6.
TNF does not reduce Gαs promoter occupancy by CREB, Sp1, or Egr-1 but does reduce binding by CBP. Primary cultures of myometrial cells were stimulated with TNF (10 ng/ml) for 24 h and subjected to the ChIP assay with the antisera depicted. All experiments were performed three times. Data were compared using a paired, two-tailed t test, and results are expressed as the mean ± S.E. (error bars); p < 0.05 was considered statistically significant. TNF was not seen to significantly alter the amount of bound CREB (A), Sp1 (B), and Egr-1 (C). TNF did, however, significantly reduce the amount of CBP present within the Gαs promoter (D, panels i–iii; p = 0.0128). As expected, TNF induced RelA occupancy of the IκBα promoter (E, upper panel), although as expected there was no binding of RelA to the endogenous Gαs promoter (E, lower panel). I, input; IP, immunoprecipitate.
As stated above, CBP is not thought to possess intrinsic DNA binding ability but instead relies on recruitment to the promoter through interactions with promoter-bound ancillary factors, including those identified herein. Expression of exogenous CBP alone was seen to enhance expression from the Gαs-luc reporter vector (Fig. 5A; discussed above). Moreover, we previously demonstrated that expression of exogenous CBP could relieve TNF-induced repression of the Gαs-luc promoter vector (22). That observation suggested that Gαs repression occurred because CBP was removed or lost from the transcription complex based on the Gαs promoter. To test this notion further, we undertook a series of ChIP analyses on TNF-stimulated primary myometrial myocytes to determine whether the level of CBP occupying the Gαs promoter was reduced when cells were exposed to TNF. Fig. 6D clearly illustrates that the level of CBP present within the −837 to −618 region of the endogenous Gαs promoter is reduced ∼2-fold when myometrial myocytes were exposed to TNF. Again, to confirm that this system was working efficiently, TNF-induced occupancy of the IκBα promoter by RelA served as the positive control (Fig. 6E, upper panel). Moreover, we have previously demonstrated that RelA does not bind to the Gαs promoter, and consequently, that observation was utilized in this study to confirm that the effects observed herein were specific because once again there was no binding of RelA to the endogenous Gαs promoter (Fig. 6E, lower panel).
Gαs-luc Promoter Activity Is Repressed by HDAC-1 But Induced by the HDAC Inhibitor TSA
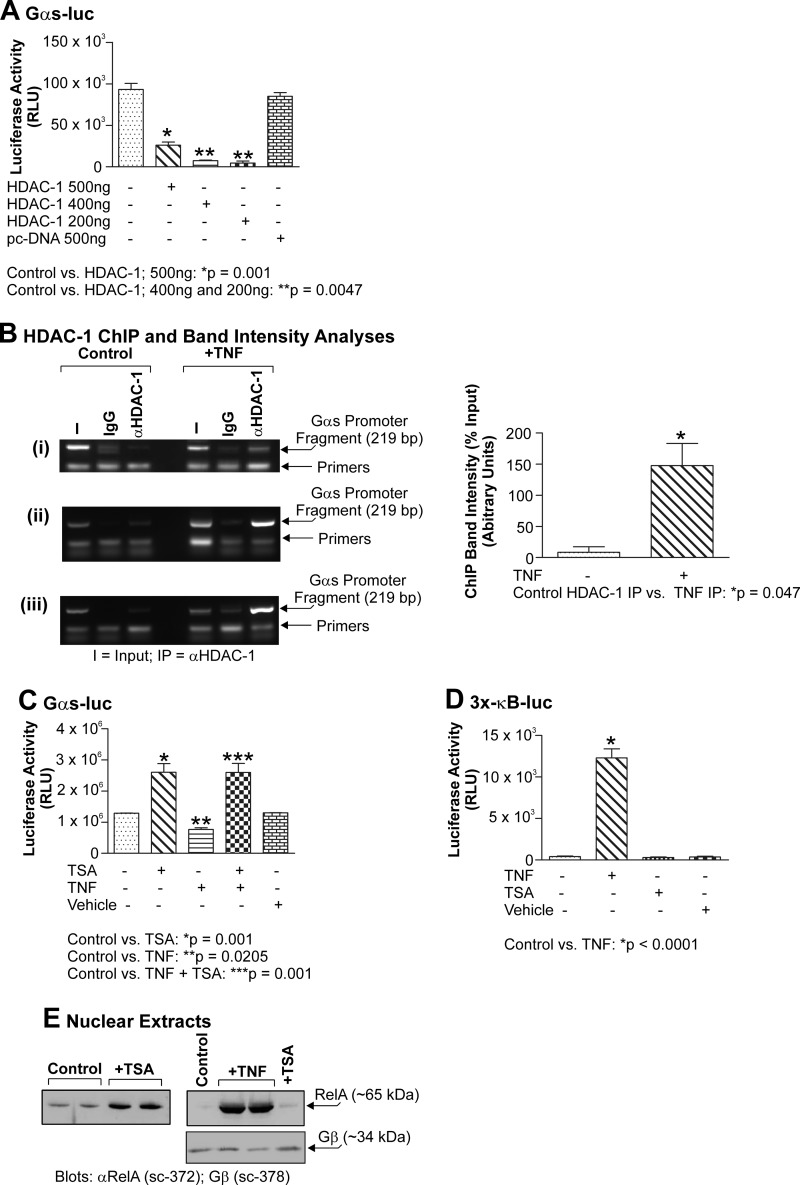
As discussed above, histone acetylation is generally associated with transcription of a given promoter, whereas reciprocal histone deacetylation generally alters chromatin structure in a manner such that it becomes refractory to transcription, thereby silencing a given gene. In terms of myometrial function, there are increasing data that suggest that deacetylation of myometrial genes may be of importance during quiescence. Indeed, both Phillips et al. (45) and Karolczak-Bayatti et al. (47) have reported the binding of HDAC-1 to two other GC-rich control regions, namely the human chorionic gonadotropin/luteinizing hormone promoter and the PKA regulatory subunit RIIα promoter. Consequently, we wished to test the notion that HDAC-1 could repress the function of the endogenous Gαs-luc promoter construct. Importantly, when monolayers of primary myometrial smooth muscle cells were co-transfected with Gαs-luc and increasing amounts of HDAC-1, HDAC-1 caused a dose-dependent repression of the Gαs-luc promoter construct (Fig. 7A). Interestingly, the effect was observed with 500 ng of HDAC-1 plasmid DNA but reached a maximal effect at the lower dose of 200 ng of HDAC-1 plasmid DNA. The reason for this is not clear but may reflect a phenomenon of promoter squelching when elevated levels of exogenous factors are introduced into the cell.
FIGURE 7.
Gαs-luc activity is repressed by HDAC-1 but induced by TSA. Primary myometrial cultures were transfected with 200 ng of Gαs-luc and 200, 400, or 500 ng of HDAC-1. Cells were harvested after 24 h, and luciferase activity was quantitated. HDAC-1 strongly repressed Gαs expression at all doses and maximally repressed Gαs expression at 200 ng (A; p = 0.0047). Primary cultures of myometrial cells were stimulated with TNF (10 ng/ml) for 24 h and subjected to the ChIP assay with anti-HDAC-1 antiserum. Data were compared using a paired, two-tailed t test, and results are expressed as the mean ± S.E. (error bars); p < 0.05 was considered statistically significant. TNF was seen to induce an increase in HDAC-1 occupancy of the endogenous Gαs promoter (B, panels i–iii; p = 0.047). Primary myometrial cultures were transfected with 200 ng of Gαs-luc and then 24 h later stimulated with TNF (10 ng/ml) for 24 h. TSA (330 nm) was then added to the culture medium for 24 h. Cells were harvested, and luciferase activity was quantitated. Data were compared using one-way analysis of variance and analyzed further using Tukey's multiple comparison test, and results are expressed as the mean ± S.E. (error bars); p < 0.05 was considered statistically significant. TSA was seen to activate Gαs-luc activity and overcome TNF-induced Gαs-luc repression (C; p < 0.001). Primary myometrial cultures were transfected with 200 ng of Gαs-luc and then 24 h later stimulated with either TNF (10 ng/ml) for 24 h, TSA (330 nm) for 24 h, or TNF and then TSA. Only TNF activated the NF-κB-sensitive 3x-κB-luc reporter (D; p = 0.0001). Cultures of primary myometrial cells were stimulated with either TNF (10 ng/ml) for 24 h, TSA (330 nm) for 24 h, or TNF and then TSA. Nuclear extracts were subsequently prepared and probed for the expression of acetyl-RelA (Lys-310) and total RelA. No acetyl-RelA was detected in response to any stimulant (not shown). TSA, however, induced significant RelA nuclear localization, but this remained less than that seen with TNF (E, upper panels). Equal loading was confirmed by blotting for Gβ (E, lower panel). All experiments were performed three times, and results are expressed as the mean ± S.E. (error bars). RLU, relative light units. I, input; IP, immunoprecipitate.
To ensure that those observations where exogenous HDAC-1 repressed expression from the Gαs-luc vector (Fig. 6A) were not merely due to promoter squelching, we also used the ChIP assay to immunoprecipitate the endogenous Gαs promoter using anti-HDAC-1 antiserum. Cultures of primary myometrial cells were treated with TNF or not stimulated at all. As seen in Fig. 7B, TNF treatment caused an association of HDAC-1 with the endogenous Gαs promoter, supporting the notion that HDAC-1 is recruited to this region in the presence of inflammatory mediators.
Because we observed that exogenous HDAC-1 could repress activity of the Gαs promoter, the obvious corollary was that the HDAC inhibitor TSA should induce activity of the Gαs-luc construct. To test this notion, primary myometrial smooth muscle cells transfected with the Gαs-luc vector were exposed to TSA (330 nm). Fig. 7C clearly demonstrates that TSA can induce expression from the Gαs-luc promoter construct. Moreover, the TNF-induced repression of Gαs could also be relieved when TSA was added to the cultures after TNF (Fig. 7C). Together, these data suggest the need for acetylation of this promoter and/or factors involved in regulating this region.
It has been reported that the NF-κB RelA subunit undergoes acetylation at key lysine residues prior to transactivation (63, 64). Moreover, HDAC inhibitors have been observed to facilitate this acetylation by reducing such deacetylase activity. As such, in terms of myometrial biology where there is a thesis that HDAC inhibitors could serve a tocolytic (labor-stopping) function, we wished to determine whether TSA could facilitate acetylation and hence activation of RelA. To do this, primary myometrial myocytes were transfected with the 3x-κB-luc reporter and stimulated with TNF, TSA, or DMSO vehicle. As can be seen in Fig. 7D, only TNF activated the NF-κB-sensitive reporter construct; neither TSA nor DMSO had any effect. Significantly, using Western analysis with an antiserum specifically recognizing acetylated RelA (acetyl-Lys- 310), we did not see any changes in RelA acetylation status in the presence of TNF, TSA, or DMSO (data not shown). We did, however, observe elevated nuclear localization of RelA when cells were exposed to TSA (Fig. 7E, left panel), but this was still markedly below those levels seen for TNF (Fig. 7E, right panel; same samples but reduced exposure time to illustrate the TNF effect). Equal loading of samples was confirmed by probing for Gβ protein (Fig. 7E).
TNF Increases Histone H4K8 Acetylation on the Gαs Promoter but Not That of Histone H3K9
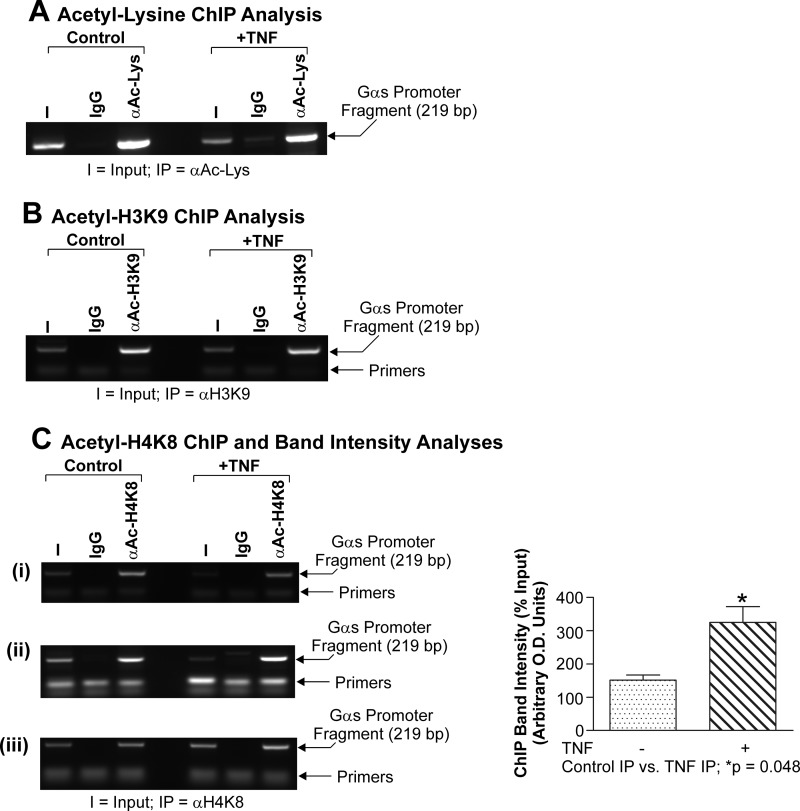
It has been well documented that CBP is a potent histone acetyltransferase targeting a variety of intracellular factors, including histones H3 and H4 (38). Acetylation of both H3 and H4 can be associated with both transcriptional activation and repression of a number of genes as they modify the inherent promoter architecture, thereby enhancing the accessibility of the promoter to RNA polymerase II (Refs. 65 and 66 and references therein). At present, however, there are no data describing whether the Gαs promoter is acetylated. Because we have identified CBP occupancy of the −837 to −618 region of the native Gαs promoter (Fig. 5B) and found that the level of CBP occupying that region of the Gαs promoter is reduced in the presence of TNF (Fig. 4D), we wanted to determine (i) whether the Gαs promoter was acetylated and (ii) whether there were changes in the acetylation status of either histone H3 or H4 within the Gαs distal promoter fragment in primary myometrial myocytes upon exposure to TNF. Once again, myocytes were stimulated with TNFα for 24 h, and further ChIP analyses were performed for pan-acetylated lysine residues, acetylated histone H3, or acetylated histone H4 within the endogenous distal Gαs fragment.
In our system, we did not observe any change in the levels of acetylated lysine within the −837 to −618 Gαs promoter region when cells were exposed to TNF (Fig. 8A). Moreover, we did not observe any change in the level of histone H3K9 acetylation as judged by those ChIP analyses between controls and TNF-stimulated cells (Fig. 8B). Significantly, however, exposure of primary myometrial smooth muscle cells to TNF was seen to induce an increase of ∼2.5-fold in the Gαs band intensity when ChIP was utilized for acetylated histone H4K8 (Fig. 8C), suggesting that this modification of the endogenous Gαs promoter is elevated in the presence of TNF.
FIGURE 8.
TNF does not alter global levels of histone acetylation or histone H3K9 acetylation but does increase histone H4K8 acetylation in the −837 to −618 Gαs promoter fragment. Primary cultures of myometrial cells were stimulated with TNF (10 ng/ml) for 24 h and subjected to the ChIP assay with the antisera depicted. Data were compared using a paired, two-tailed t test; p < 0.05 was considered statistically significant. All experiments were performed three times, and results are expressed as the mean ± S.E. (error bars). TNF did not alter the global levels of acetylated lysine-containing proteins (A) or acetylated histone H3K9 (B) within the endogenous Gαs promoter, but it induced an ∼3-fold increase in acetylated histone H4K8 levels in the endogenous −837 to −618 Gαs promoter region (C, panels i–iii). I, input; IP, immunoprecipitate.
DISCUSSION
TNF Overcomes TSA-induced Myometrial Relaxation
The cumulative addition of TSA was seen to induce relaxation in isolated myometrial strips, an effect abolished by the addition 30 ng/ml TNF, although TNF was not seen to induce myometrial contraction on its own. Whether contraction would be seen at 24 h (a similar time course utilized for the transfection and ChIP studies herein) remains unclear, although certainly at 24 h tissue viability would be brought into question. Significantly, QNZ, the NF-κB inhibitor, prevented the TNF-induced modulation of TSA-mediated relaxation. This observation suggests two things. 1) The uterotonic actions of TNF have an NF-κB-regulated component to them. 2) The uterotonic effects of TNF can only occur once other factors, not identified in this study, are acetylated (TSA is an inhibitor of deacetylation). It remains unclear which NF-κB dimers are responsible for the TNF-induced effect. Although QNZ is marketed as a specific NF-κB inhibitor, its molecular mechanism remains unclear. This is a salient point: essentially, for example, if QNZ functions through inhibition of the IκBα, then it would only serve to inhibit complexes regulated by that protein. There are certain NF-κB complexes, including p52 homodimers and p52-RelB heterodimers, that are not sensitive to IκBα inhibition (23, 32) and would therefore not be targeted by QNZ.
The Gαs Promoter Is Bound by a Number of Transcription Factors
Our current hypothesis suggests that acetylation of the Gαs promoter by CBP is a prerequisite for Gαs expression in the human myometrium, a process that would facilitate uterine quiescence seen during pregnancy (22, 52). The corollary to this, therefore, is that inflammatory mediators, including TNF, which we have previously shown to serve as a potent repressor of the Gαs promoter (25), will exert their action through repression of the Gαs promoter.
In this study, to determine how TNF may repress expression from the Gαs promoter, we have been able to demonstrate for the first time that the −837 to −618 region of the endogenous Gαs promoter is occupied by the transcription factors CREB, Egr-1, and Sp1. We were unable to determine the exact loci of these sites in this study; as such, the following may provide explanations for our observations. First, the size of the chromatin fragments we obtained after sonication was ∼500 bp (data not shown); consequently, we cannot rule out that the chromatin enrichment we observed in our ChIP assays may also include sequences located both more proximally or distally to the −837 to −618 Gαs fragment studied herein. For example, the ChIP is still specific for CREB, Egr-1, and Sp1, and the primers remain specific for the appropriate sequences flanking the −837 to −618 region, but if the specific enriched fragment contains both a more proximal or distal transcription factor binding site and the site of primer recognition, then such a result suggests direct occupancy of the region under study.
Second, to put this into the context of nucleosomes, we have investigated a fragment of 219 bp; essentially, this represents one complete nucleosomal particle with an extra 69 bp of DNA. We cannot, therefore, be certain that the effects we have observed occur throughout the entire regulatory locus of the Gαs region or whether they are restricted to this particular nucleosomal region. Such explanations, however, do not detract from our findings because they still support the thesis that these factors are binding and regulating the Gαs promoter.
We also demonstrated that exogenous histone deacetylase activity, namely HDAC-1, could repress the Gαs-luc promoter, whereas TNF stimulation induced occupancy of the Gαs −837 to −618 fragment by endogenous HDAC-1. Furthermore, the HDAC inhibitor TSA had the opposite effect and induced Gαs promoter activity. Together, these observations support the view that deacetylation of the Gαs promoter is an important regulatory mechanism. At present, however, we were unable to fully define whether the deacetylation was specific to nucleosomal histones or whether the deacetylation was more global, inducing its effects through promoter-bound ancillary factors other than histones.
H4K8 Acetylation and Gαs Promoter Repression
The obvious question posed by our data is that if deacetylation is required to reduce or silence Gαs expression why should an increased recovery of acetylated histone H4 be observed in the distal Gαs fragment in the presence of TNF. First, in terms of an explanation for this observation of increased H4K8 acetylation with TNFα, is that acetylation at H4K8 is required to stabilize the interaction between DNA and the repressor element 1-silencing transcription factor (REST) protein (for a review, see Ref. 65). This mechanism requires that the interaction between REST and DNA is stabilized by the binding of the ATP-dependent chromatin-remodeling enzyme BRG1 (which forms part of the REST complex) to H4K8 (65). In doing so, the REST complex can subsequently serve as a platform for other transcriptional repressors, including the HDAC-1- and -2-containing complex of mSin3 (65). As such, increased H4K8 acetylation (as seen in our data) induced by CBP prior to removal from the Gαs promoter region would facilitate the binding and stabilization of the REST-mSin3 complex to the distal Gαs fragment and induce repression of that promoter. Further work is needed to test this hypothesis because there is evidence that mSin3-associated HDAC-1 can deacetylate histone H3K9; in the present study, we did not see such H3K9 deacetylation in the presence of TNF.
In terms of interaction with those factors in the distal Gαs promoter, REST has been shown to bind Sp1 and Egr-1. In both studies, bidirectional regulation of the specific target genes was noted; i.e. Sp1 could activate expression of sodium channels, whereas Egr-1 stimulated expression of the Cav3.2 T-type Ca2+ channel. In the presence of REST, both these effects were repressed (66, 67). As such, we speculate that a similar mechanism may govern expression of the Gαs GTP-binding protein.
Influence of Non-genomic Protein Acetylation
Previous work has also demonstrated a key role for lysine acetylation of Hsp20 (68). This post-translational modification was seen to govern the interaction between Hsp20 and cofilin. Indeed, inhibitors of the non-nuclear lysine deacetylase KDAC8 modified the Hsp20 acetylation profile in such a manner that myometrial contractility was subsequently inhibited.
At present, the exact mechanism by which TNF influences acetylation and Gαs expression is unclear. TNF is a potent inducer of NF-κB activity; indeed, RelA NF-κB is documented to undergo CBP-mediated acetylation at Lys-218, -221, and -310 (63). Moreover, Dai et al. (64) subsequently reported that newer HDAC inhibitors, including suberoylanilide hydroxamic acid and MS-275, induced acetylation and nuclear localization of RelA. Dai et al. (64) suggested that this was necessary for NF-κB-induced antiapoptotic effects in certain leukemia cells. We did not observe TSA-induced acetylation of RelA in this study, although the antiserum used was specified by the manufacturer to only be effective against transfected RelA and not the endogenous protein; this may account for our lack of data in that experiment. We did, however, also note that TSA stimulation of myometrial cells caused an increase in RelA nuclear localization without activating the NF-κB-sensitive 3x-κB-luc reporter. The significance of that observation remains unclear at present, but it may be due to a delay in IκBα resynthesis similar to that seen for pervanadate-induced NF-κB activation (2). Moreover, it would also suggest that in the context of myometrial function a cautious approach to using HDAC inhibitors as tocolytic (labor-stopping) drugs should be used. Briefly, there is a growing body of literature interested in understanding whether HDAC inhibitors may be of use clinically as tocolytic drugs (17, 49, 68). Although such studies do present convincing evidence that HDAC inhibitors such as TSA exert robust prorelaxant effects on isolated strips of human myometrium (Refs. 17, 49, and 68 and see Fig. 1), we must, however, also consider potential unwanted effects, including acetylation and subsequent activation of non-chromatin-based factors (e.g. NF-κB RelA) that could also promote labor such as those described by Chen et al. (63). Indeed, our data demonstrate that TNF can overcome TSA-mediated myometrial relaxation. If HDAC inhibitors have a positive effect on the activity of NF-κB, then utilizing them in the clinical setting may only serve to exacerbate those premature myometrial contractions that the drug was meant to prevent.
Conclusions
In conclusion, our data demonstrate that the HDAC inhibitor TSA induces relaxation in isolated strips of human myometrium, an effect that can be overcome by stimulation of such tissues with TNF. Although TNF itself did not induce contraction, the NF-κB inhibitor QNZ did repress the TNF effect and sensitized the tissue to TSA, implying that the NF-κB family serves an important function in this modulatory role. Subsequent studies into the regulation of the proquiescent factor, the Gαs GTP-binding protein, demonstrated that the −837 to −618 region of the endogenous Gαs promoter is occupied by CREB, Egr-1, and Sp1 transcription factors. Moreover, CBP is also associated with this region: we believe CBP is required to acetylate the Gαs promoter, leading to Gαs expression. In the presence of TNF, although there was no discernible change in the level of CREB, Sp1, or Egr-1 bound to the Gαs promoter, there was significant reduction in the amount of CBP, an observation that was supported by increased levels of HDAC-1 and elevated H4K8 acetylation. We were also able to show that exogenous HDAC enzymes, namely HDAC-1, could repress the Gαs-luc construct, whereas reciprocal activation was seen with exogenous CBP or the HDAC inhibitor TSA. Taken together, our data suggest that expression from the Gαs promoter is in part governed by protein acetylation.
Acknowledgments
We thank Prof. Michael Taggart and Dr. Gaynor Miller for critical reviews of the manuscript prior to submission. We also thank Richard Goodman, Ron Hay, Bradley Berstein, Paul Hurd, and Neil Perkins for providing some of the plasmids used in this study. We are grateful to the patients of the Jessop Wing and Derby Royal Hospital for participating in this study and to the clinical staff for obtaining samples. We acknowledge Dilly Anumba for assistance in preparing the ethical review for this study.
This work was supported by United Kingdom Medical Research Council Grant G0701322 (to N. R. C.) and Jessop Wing Small Grants Scheme Grant OGN/06/03 (to N. R. C.).
- NF-κB
- nuclear factor κB
- TSA
- trichostatin A
- HDAC
- histone deacetylase
- QNZ
- N4-[2-(4-phenoxyphenyl)ethyl]-4,6-quinazolinediamine
- CREB
- cAMP-response element-binding protein
- CBP
- CREB-binding protein
- luc
- luciferase
- REST
- repressor element 1-silencing transcription factor.
REFERENCES
- 1. Lumley J. (2003) Defining the problem: the epidemiology of preterm birth. BJOG 110, Suppl. 20, 3–7 [PubMed] [Google Scholar]
- 2. Horion J., Gloire G., El Mjiyad N., Quivy V., Vermeulen L., Vanden Berghe W., Haegeman G., Van Lint C., Piette J., Habraken Y. (2007) Histone deacetylase inhibitor trichostatin A sustains sodium pervanadate-induced NF-κB activation by delaying IκBα mRNA resynthesis: comparison with tumor necrosis factor α. J. Biol. Chem. 282, 15383–15393 [DOI] [PubMed] [Google Scholar]
- 3. Government Statistical Service for the Department of Health (2005) NHS Maternity Statistics, England: 2003–04, Statistical Bulletin 2005/10, Department of Health, London [Google Scholar]
- 4. Marlow N., Wolke D., Bracewell M. A., Samara M. for the EPICure Study Group (2005) Neurologic and developmental disability at six years of age after extremely preterm birth. New. Engl. J. Med. 352, 9–19 [DOI] [PubMed] [Google Scholar]
- 5. Allport V. C., Pieber D., Slater D. M., Newton R., White J. O., Bennett P. R. (2001) Human labour is associated with nuclear factor-κB activity which mediates cyclo-oxygenase-2 expression and is involved with the “functional progesterone withdrawal”. Mol. Hum. Reprod. 7, 581–586 [DOI] [PubMed] [Google Scholar]
- 6. Elliott C. L., Allport V. C., Loudon J. A., Wu G. D., Bennett P. R. (2001) Nuclear factor κ-B is essential for up-regulation of interleukin-8 expression in human amnion and cervical epithelial cells. Mol. Hum. Reprod. 7, 787–790 [DOI] [PubMed] [Google Scholar]
- 7. Lee Y., Allport V., Sykes A., Lindstrom T., Slater D., Bennett P. (2003) The effects of labour and of interleukin-1β upon the expression of nuclear factor κB related proteins in human amnion. Mol. Hum. Reprod. 9, 213–218 [DOI] [PubMed] [Google Scholar]
- 8. Yan X., Wu Xiao C., Sun M., Tsang B. K., Gibb W. (2002) Nuclear factor κB activation and regulation of cyclooxygenase type-2 expression in human amnion mesenchymal cells by interleukin-1β. Biol. Reprod. 66, 1667–1671 [DOI] [PubMed] [Google Scholar]
- 9. Lappas M., Permezel M., Rice G. E. (2003) N-Acetyl-cysteine inhibits phospholipids metabolism, proinflammatory cytokine release, protease activity and nuclear factor-κB deoxyribonucleic acid-binding activity in human fetal membranes in vitro. J. Clin. Endocrinol. Metab. 88, 1723–1729 [DOI] [PubMed] [Google Scholar]
- 10. Yan X., Sun M., Gibb W. (2002) Localization of nuclear factor-κB (NF-κB) and inhibitory factor-κB (IκB) in human fetal membranes and deciduas at term and preterm delivery. Placenta 23, 288–293 [DOI] [PubMed] [Google Scholar]
- 11. Lappas M., Permezel M., Georgiou H. M., Rice G. E. (2004) Regulation of phospholipase isozymes by nuclear factor-κB in human gestational tissues in vitro. J. Clin. Endocrinol. Metab. 89, 2365–2372 [DOI] [PubMed] [Google Scholar]
- 12. Lappas M., Rice G. E. (2004) Phospholipase A2 isozymes in pregnancy and parturition. Prostaglandins Leukot. Essent. Fatty Acids 70, 87–100 [DOI] [PubMed] [Google Scholar]
- 13. Stjernholm-Vladic Y., Stygar D., Mansson C., Masironi B., Akerberg S., Wang H., Ekman-Ordeberg G., Sahlin L. (2004) Factors involved in the inflammatory events of cervical ripening in humans. Reprod. Biol. Endocrinol. 2, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Belt A. R., Baldassare J. J., Molnár M., Romero R., Hertelendy F. (1999) The nuclear transcription factor NF-κB mediates interleukin-1β-induced expression of cyclooxygenase-2 in human myometrial cells. Am. J. Obstet. Gynecol. 181, 359–366 [DOI] [PubMed] [Google Scholar]
- 15. Soloff M. S., Cook D. L., Jr., Jeng Y.-J., Anderson G. D. (2004) In situ analysis of interleukin-1 induced transcription of cox-2 and il-8 in cultured human myometrial cells. Endocrinology 145, 1248–1254 [DOI] [PubMed] [Google Scholar]
- 16. Lindström T. M., Bennett P. R. (2005) 15-Deoxy-Δ12,14-prostaglandin J2 inhibits interleukin-1β-induced nuclear factor-κB in human amnion and myometrial cells: mechanisms and implications. J. Clin. Endocrinol. Metab. 90, 3534–3543 [DOI] [PubMed] [Google Scholar]
- 17. Lindström T. M., Mohan A. R., Johnson M. R., Bennett P. R. (2008) Histone deacetylase inhibitors exert time-dependent effects on nuclear factor-κB but consistently suppress the expression of proinflammatory genes in human myometrial cells. Mol. Pharmacol. 74, 109–121 [DOI] [PubMed] [Google Scholar]
- 18. Soloff M. S., Izban M. G., Cook D. L., Jr., Jeng Y.-J., Mifflin R. C. (2006) Interleukin-1-induced NF-κB recruitment to the oxytocin receptor gene inhibits RNA polymerase II-promoter interactions in cultured human myometrial cells. Mol. Hum. Reprod. 12, 619–624 [DOI] [PubMed] [Google Scholar]
- 19. Mohan A. R., Sooranna S. R., Lindstrom T. M., Johnson M. R., Bennett P. R. (2007) The effect of mechanical stretch on cyclooxygenase type 2 expression and activator protein-1 and nuclear factor-κB activity in human amnion cells. Endocrinology 148, 1850–1857 [DOI] [PubMed] [Google Scholar]
- 20. Terzidou V., Lee Y., Lindström T., Johnson M., Thornton S., Bennett P. R. (2006) Regulation of the human oxytocin receptor by nuclear factor-κB and CCAAT/enhancer-binding protein-β. J. Clin. Endocrinol. Metab. 91, 2317–2326 [DOI] [PubMed] [Google Scholar]
- 21. Chapman N. R., Europe-Finner G. N., Robson S. C. (2004) Expression and DNA-binding activity of the nuclear factor κB (NF-κB) family in the human myometrium during pregnancy and labor. J. Clin. Endocrinol. Metab. 89, 5683–5693 [DOI] [PubMed] [Google Scholar]
- 22. Chapman N. R., Smyrnias I., Anumba D. O., Europe-Finner G. N., Robson S. C. (2005) Expression of the GTP-binding protein (Gαs) is repressed by the nuclear factor κB (NF-κB) RelA subunit in human myometrium. Endocrinology 146, 4994–5002 [DOI] [PubMed] [Google Scholar]
- 23. Cookson V. J., Chapman N. R. (2010) NF-κB function in the human myometrium during pregnancy and parturition. Histol. Histopathol. 25, 945–956 [DOI] [PubMed] [Google Scholar]
- 24. Golightly E., Jabbour H. N., Norman J. E. (2011) Endocrine immune interactions in human parturition. Mol. Cell. Endocrinol. 335, 52–59 [DOI] [PubMed] [Google Scholar]
- 25. Ashburner B. P., Westerheide S. D., Baldwin A. S., Jr. (2001) The p65 (RelA) subunit of NF-κB interacts with the histone deacetylase (HDAC) corepressors HDAC-1 and HDAC-2 to negatively regulate gene expression. Mol. Cell. Biol. 21, 7065–7077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rocha S., Martin A. M., Meek D. W., Perkins N. D. (2003) p53 represses cyclin D1 transcription through down regulation of Bcl-3 and inducing increased association of the p52 NF-κB subunit with histone deacetylase-1. Mol. Cell. Biol. 23, 4713–4727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Campbell K. J., Rocha S., Perkins N. D. (2004) Active repression of antiapoptotic gene expression by RelA(p65) NF-κB. Mol. Cell 13, 853–865 [DOI] [PubMed] [Google Scholar]
- 28. Chapman N. R., Perkins N. D. (2000) Inhibition of the RelA(p65) NF-κB subunit by Egr-1. J. Biol. Chem. 275, 4719–4725 [DOI] [PubMed] [Google Scholar]
- 29. Chapman N. R., Webster G. A., Gillespie P. J., Wilson B. J., Crouch D. H., Perkins N. D. (2002) A novel form of the RelA nuclear factor-κB subunit is induced by and forms a complex with the proto-oncogene c-Myc. Biochem. J. 366, 459–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Perkins N. D., Edwards N. L., Duckett C. S., Agranoff A. B., Schmid R. M., Nabel G. J. (1993) A cooperative interaction between NF-κB and Sp1 is required for HIV-1 enhancer activation. EMBO J. 12, 3551–3558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Perkins N. D., Agranoff A. B., Pascal E., Nabel G. J. (1994) An interaction between the DNA-binding domains of RelA(p65) and Sp1 mediates human immunodeficiency virus gene activation. Mol. Cell. Biol. 14, 6570–6583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Perkins N. D. (2007) Integrating cell-signalling pathways with NF-κB and IKK function. Nat. Rev. Mol. Cell Biol. 8, 49–62 [DOI] [PubMed] [Google Scholar]
- 33. Webster G. A., Perkins N. D. (1999) Transcriptional cross talk between NF-κB and p53. Mol. Cell. Biol. 19, 3485–3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wadgaonkar R., Phelps K. M., Haque Z., Williams A. J., Silverman E. S., Collins T. (1999) CREB-binding protein is a nuclear integrator of nuclear factor-κB and p53 signaling. J. Biol. Chem. 274, 1879–1882 [DOI] [PubMed] [Google Scholar]
- 35. Berger S. (2007) The complex language of chromatin regulation during transcription. Nature 447, 407–412 [DOI] [PubMed] [Google Scholar]
- 36. Suzuki M. M., Bird A. (2008) DNA methylation landscapes: provocative insights from epigenomics. Nat. Rev. Genet. 9, 465–476 [DOI] [PubMed] [Google Scholar]
- 37. Luger K., Mäder A. W., Richmond R. K., Sargent D. F., Richmond T. J. (1997) Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature 389, 251–260 [DOI] [PubMed] [Google Scholar]
- 38. Bannister A. J., Kouzarides T. (1996) The CBP co-activator is a histone acetyltransferase. Nature 384, 641–643 [DOI] [PubMed] [Google Scholar]
- 39. Rea S., Eisenhaber F., O'Carroll D., Strahl B. D., Sun Z.-W., Schmid M., Opravil S., Mechtler K., Ponting C. P., Allis C. D., Jenuwein T. (2000) Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 406, 593–599 [DOI] [PubMed] [Google Scholar]
- 40. Hurd P. J., Bannister A. J., Halls K., Dawson M. A., Vermeulen M., Olsen J. V., Ismail H., Somers J., Mann M., Owen-Hughes T., Gout I., Kouzarides T. (2009) Phosphorylation of histone H3 Thr-45 is linked to apoptosis. J. Biol. Chem. 284, 16575–16583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Europe-Finner G. N., Phaneuf S., Watson S. P., López Bernal A. (1993) Identification and expression of G-proteins in human myometrium: up-regulation of Gαs in pregnancy. Endocrinology 132, 2484–2490 [DOI] [PubMed] [Google Scholar]
- 42. Europe-Finner G. N., Phaneuf S., Tolkovsky A. M., Watson S. P., López Bernal A. (1994) Down regulation of Gαs in human myometrium in term and preterm labor: a mechanism for parturition. J. Clin. Endocrinol. Metab. 79, 1835–1839 [DOI] [PubMed] [Google Scholar]
- 43. Phaneuf S., Europe-Finner G. N., Varney M., MacKenzie I. Z., Watson S. P., López Bernal A. (1993) Oxytocin-stimulated phosphoinositide hydrolysis in human myometrial cells: involvement of pertussis toxin-sensitive and -insensitive G-proteins. J. Endocrinol. 136, 497–509 [DOI] [PubMed] [Google Scholar]
- 44. Bailey J., Sparey C., Phillips R. J., Gilmore K., Robson S. C., Dunlop W., Europe-Finner G. N. (2000) Expression of CREB, CREM and ATF2 cyclic AMP dependent transcription factors in the human myometrium during pregnancy and labour. Mol. Hum. Reprod. 6, 648–660 [DOI] [PubMed] [Google Scholar]
- 45. Phillips R. J., Bailey J., Robson S. C., Europe-Finner G. N. (2002) The differential expression of the adenylyl cyclase-stimulatory GTP-binding protein Gαs in the human myometrium during pregnancy and labor involves transcriptional regulation by cyclic AMP and binding of phosphorylated nuclear proteins to multiple GC boxes within the promoter. J. Clin. Endocrinol. Metab. 87, 5675–5685 [DOI] [PubMed] [Google Scholar]
- 46. Phillips R. J., Tyson-Capper (née Pollard) A. J., Bailey J., Robson S. C., Europe-Finner G. N. (2005) Regulation of expression of the chorionic gonadotropin/luteinizing hormone receptor gene in the human myometrium: involvement of specificity protein-1 (Sp1), Sp3, Sp4, Sp-like proteins, and histone deacetylases. J. Clin. Endocrinol. Metab. 90, 3479–3490 [DOI] [PubMed] [Google Scholar]
- 47. Karolczak-Bayatti M., Loughney A. D., Robson S. C., Europe-Finner G. N. (2011) Epigenetic modulation of the protein kinase A RIIa (PRKAR2A) gene by histone deacetylase 1 and 2 in human smooth muscle cells. J. Cell. Mol. Med. 15, 94–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kozasa T., Itoh H., Tsukamoto T., Kaziro Y. (1988) Isolation and characterization of the human Gsα gene. Proc. Natl. Acad. Sci. U.S.A. 85, 2081–2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moynihan A. T., Hehir M. P., Sharkey A. M., Robson S. C., Europe-Finner G. N., Morrison J. J. (2008) Histone deacetylase inhibitors and a functional potent inhibitory effect on human uterine contractility. Am. J. Obstet. Gynecol. 199, 167.e1–7 [DOI] [PubMed] [Google Scholar]
- 50. Smale S. T. (1997) Transcription initiation from TATA-less promoters within eukaryotic protein-coding genes. Biochim. Biophys. Acta 1351, 73–88 [DOI] [PubMed] [Google Scholar]
- 51. Wierstra I. (2008) Sp1: emerging roles—beyond constitutive activation of TATA-less housekeeping genes. Biochem. Biophys. Res. Commun. 372, 1–13 [DOI] [PubMed] [Google Scholar]
- 52. Long A. A., Chapman N. R., Innes B., Europe-Finner G. N., Robson S. C. (2005) Expression and interactions of the transcriptional co-regulators CBP/p300 in the human myometrium during pregnancy. J. Soc. Gynecol. Investig. 12, 92–97 [DOI] [PubMed] [Google Scholar]
- 53. Rodriguez M. S., Wright J., Thompson J., Thomas D., Baleux F., Virelizier J. L., Hay R. T., Arenzana-Seisdedos F. (1996) Identification of lysine residues required for signal-induced ubiquitination and degradation of IκBα in vivo. Oncogene 12, 2425–2435 [PubMed] [Google Scholar]
- 54. Kwok R. P., Lundblad J. R., Chrivia J. C., Richards J. P., Bächinger H. P., Brennan R. G., Roberts S. G., Green M. R., Goodman R. H. (1994) Nuclear protein CBP is a co-activator for the transcription factor CREB. Nature 370, 223–226 [DOI] [PubMed] [Google Scholar]
- 55. Taunton J., Hassig C. A., Schreiber S. L. (1996) A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p. Science 272, 408–411 [DOI] [PubMed] [Google Scholar]
- 56. Hiroi M., Ohmori Y. (2003) The transcriptional coactivator CREB-binding protein cooperates with STAT1 and NF-κB for synergistic transcriptional activation of the CXC ligand 9/monokine induced by interferon-γ gene. J. Biol. Chem. 278, 651–660 [DOI] [PubMed] [Google Scholar]
- 57. Saccani S., Pantano S., Natoli G. (2003) Modulation of NF-κB activity by exchange of dimers. Mol. Cell 11, 1563–1574 [DOI] [PubMed] [Google Scholar]
- 58. Pearson T., Warren A. Y., Barrett D. A., Khan R. N. (2009) Detection of EETs and HETE-generating cytochrome P-450 enzymes and the effects of their metabolites on myometrial and vascular function. Am. J. Physiol. Endocrinol. Metab. 297, E647–E656 [DOI] [PubMed] [Google Scholar]
- 59. Tobe M., Isobe Y., Tomizawa H., Nagasaki T., Takahashi H., Hayashi H. (2003) A novel structural class of potent inhibitors of NF-κB activation: structure-activity relationships and biological effects of 6-aminoquinazoline derivatives. Bioorg. Med. Chem. 11, 3869–3878 [DOI] [PubMed] [Google Scholar]
- 60. Silverman E. S., Du J., Williams A. J., Wadgaonkar R., Drazen J. M., Collins T. (1998) cAMP-response-element-binding-protein-binding-protein (CBP) and p300 are transcriptional co-activators of the early growth response factor-1 (Egr-1). Biochem. J. 336, 183–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chrivia J. C., Kwok R. P., Lamb N., Hagiwara M., Montminy M. R., Goodman R. H. (1993) Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature 365, 855–859 [DOI] [PubMed] [Google Scholar]
- 62. Goodman R. H., Smolik S. (2000) CBP/p300 in cell growth, transformation and development. Genes Dev. 14, 1553–1577 [PubMed] [Google Scholar]
- 63. Chen L. F., Mu Y., Greene W. C. (2002) Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-κB. EMBO J. 21, 6539–6548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dai Y., Rahmani M., Dent P., Grant S. (2005) Blockade of histone deacetylase inhibitor-induced RelA/p65 acetylation and NF-κB activation potentiates apoptosis in leukemia cells through a process mediated by oxidative damage, XIAP down-regulation, and c-Jun N-terminal kinase 1 activation. Mol. Cell. Biol. 25, 5429–5444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ooi L., Wood I. C. (2007) Chromatin crosstalk in development and disease: lessons from REST. Nat. Rev. Genet. 8, 544–554 [DOI] [PubMed] [Google Scholar]
- 66. van Loo K. M., Schaub C., Pernhorst K., Yaari Y., Beck H., Schoch S., Becker A. J. (2012) Transcriptional regulation of T-type calcium channel Cav3.2: bi-directionality by early growth response 1 (Egr1) and repressor element 1 (RE-1) protein-silencing transcription factor (REST). J. Biol. Chem. 287, 15489–15501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Plaisance V., Niederhauser G., al Azzouz F., Lenain V., Haefliger J.-A., Waeber G., Abderrahmani A. (2005) The repressor element silencing transcription factor (REST)-mediated transcriptional repression requires the inhibition of Sp1. J. Biol. Chem. 280, 401–407 [DOI] [PubMed] [Google Scholar]
- 68. Karolczak-Bayatti M., Sweeney M., Cheng J., Edey L., Robson S. C., Ulrich S. M., Treumann A., Taggart M. J., Europe-Finner G. N. (2011) Acetylation of heat shock protein 20 (Hsp20) regulates human myometrial activity. J. Biol. Chem. 286, 34346–34355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lawn J. E., Gravett M. G., Nunes T. M., and the GAPPS Review Group (2010) Global report on preterm birth and stillbirth (1 of 7): definitions, description of the burden and opportunities to improve data. BMC Pregnancy and Childbirth 10 (Suppl 1):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Matys V., Fricke E., Geffers R., Gößling E., Haubrock M., Hehl R., Hornischer K., Karas D., Kel A. E., Kel-Margoulis O. V., Kloos D. U., Land S., Lewicki-Potapov B., Michael H., Münch R., Reuter I., Rotert S., Saxel H., Scheer M., Thiele S., Wingender E. (2003) TRANSFAC: transcriptional regulation, from patterns to profiles. Nucleic Acids Res. 31, 374–378 [DOI] [PMC free article] [PubMed] [Google Scholar]