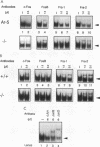
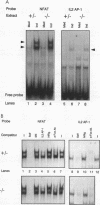
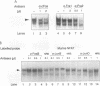
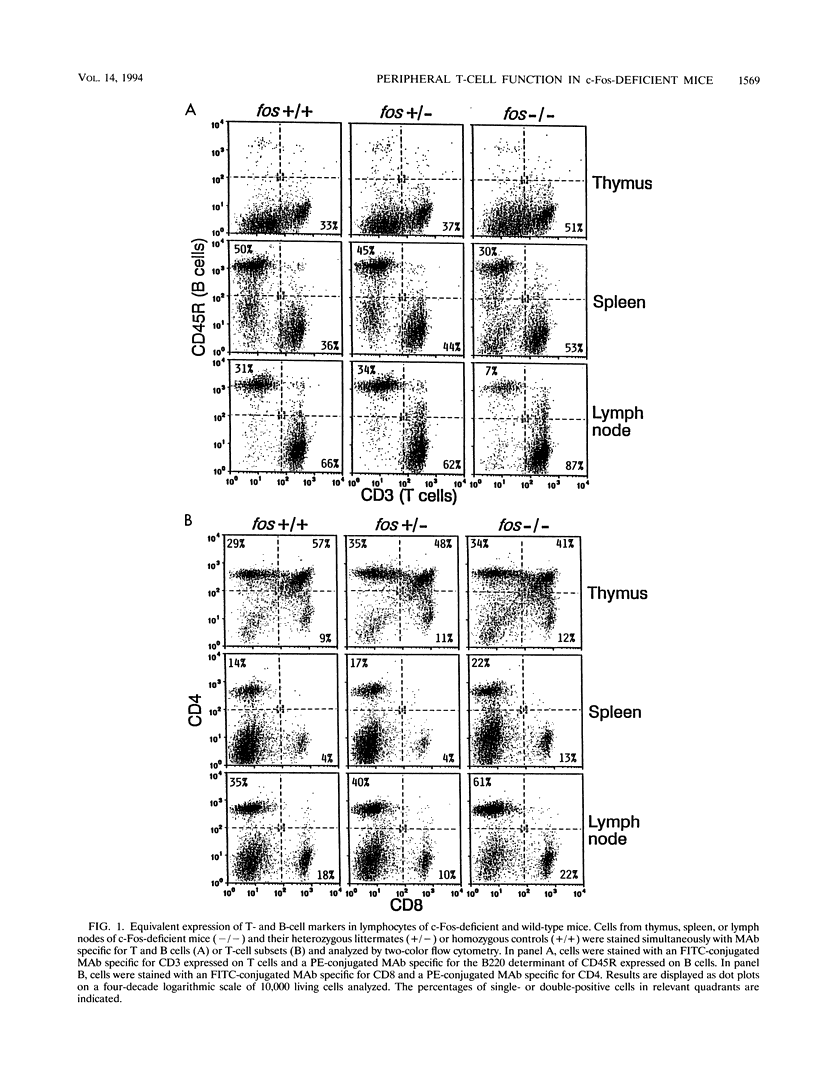
Abstract
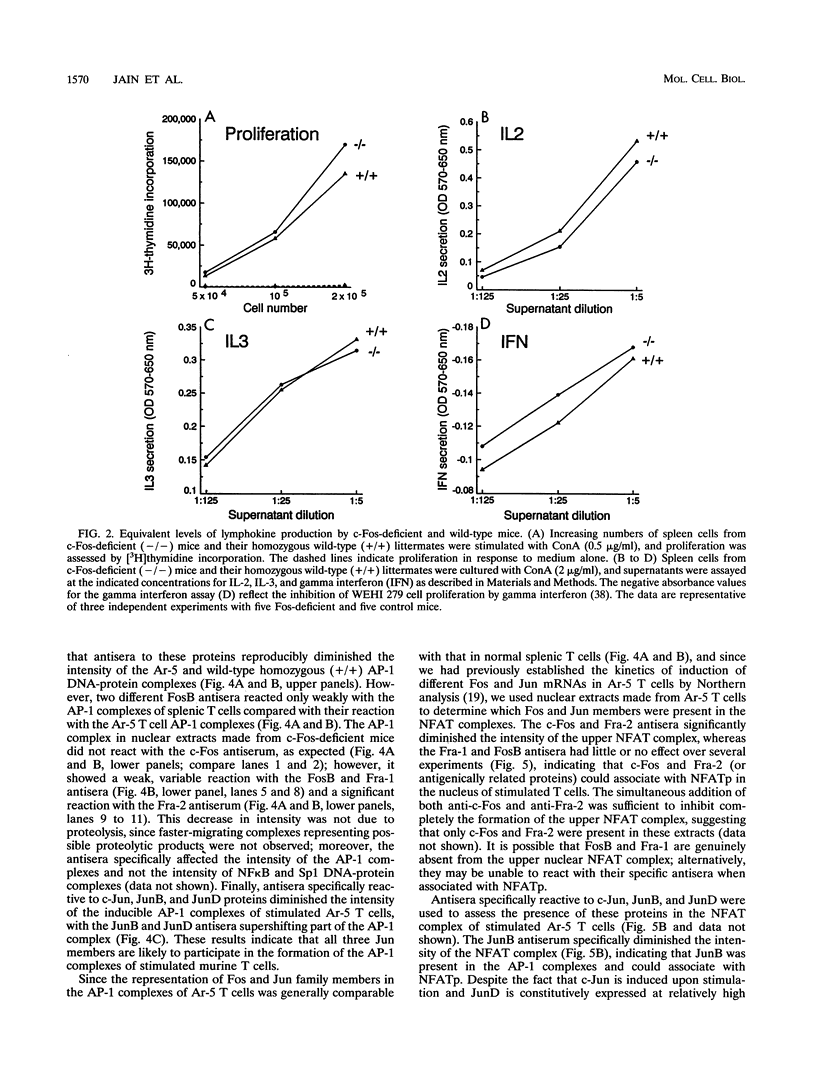
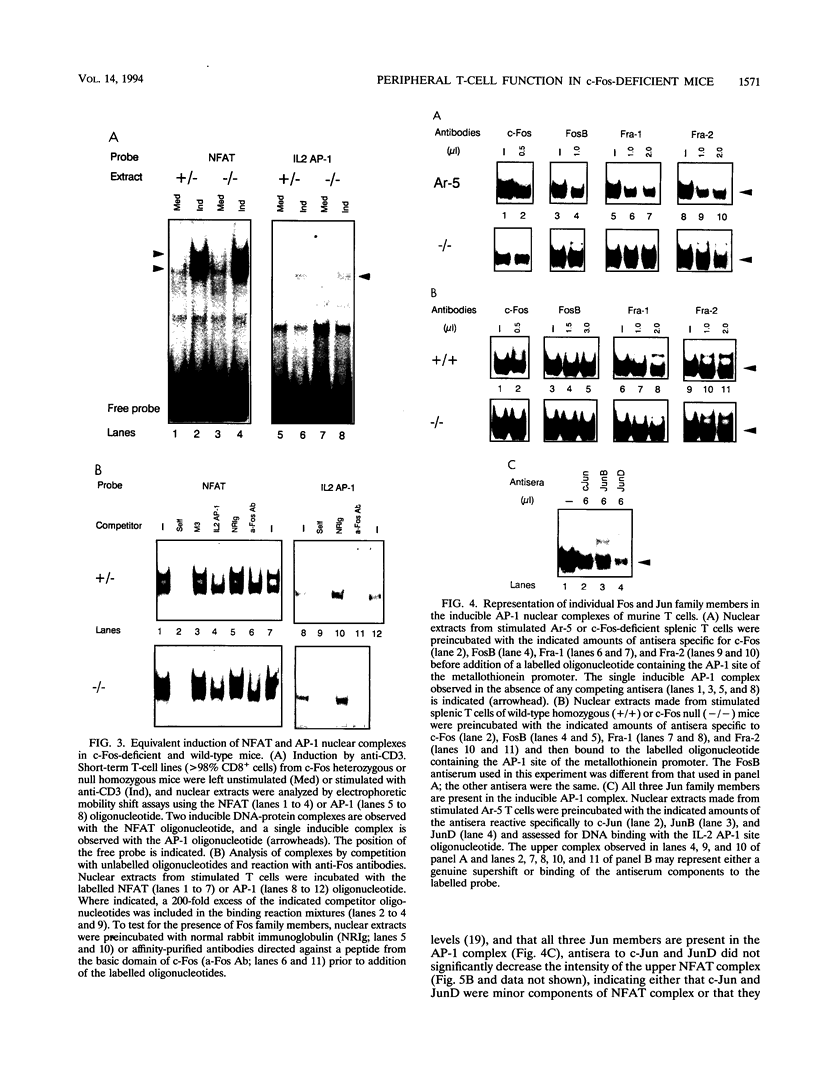
The ubiquitous transcription factors Fos and Jun are rapidly induced in T cells stimulated through the T-cell antigen receptor and regulate transcription of cytokines, including interleukin 2, in activated T cells. Since positive and negative selection of thymocytes during T-cell development also depends on activation through the T-cell receptor, Fos and Jun may play a role in thymocyte development as well. Fos and Jun act at several regulatory elements in the interleukin 2 promoter, including the AP-1 and NFAT sites. Using antisera specific to individual Fos and Jun family members, we show that c-Fos as well as other Fos family members are present in the inducible AP-1 and NFAT complexes of activated murine T cells. Nevertheless, c-Fos is not absolutely required for the development or function of peripheral T cells, as shown by using mice in which both copies of the c-fos gene were disrupted by targeted mutagenesis. c-Fos-deficient mice were comparable to wild-type mice in their patterns of thymocyte development and in the ability of their peripheral T cells to proliferate and produce several cytokines in response to T-cell receptor stimulation. Our results suggest that other Fos family members may be capable of substituting functionally for c-Fos during T-cell development and cytokine gene transcription in activated T cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birkeland M. L., Johnson P., Trowbridge I. S., Puré E. Changes in CD45 isoform expression accompany antigen-induced murine T-cell activation. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6734–6738. doi: 10.1073/pnas.86.17.6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boise L. H., Petryniak B., Mao X., June C. H., Wang C. Y., Lindsten T., Bravo R., Kovary K., Leiden J. M., Thompson C. B. The NFAT-1 DNA binding complex in activated T cells contains Fra-1 and JunB. Mol Cell Biol. 1993 Mar;13(3):1911–1919. doi: 10.1128/mcb.13.3.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabletz T., Pfeuffer I., Schorr E., Siebelt F., Wirth T., Serfling E. Transforming growth factor beta and cyclosporin A inhibit the inducible activity of the interleukin-2 gene in T cells through a noncanonical octamer-binding site. Mol Cell Biol. 1993 Feb;13(2):1155–1162. doi: 10.1128/mcb.13.2.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Lansford R., Stewart V., Young F., Alt F. W. RAG-2-deficient blastocyst complementation: an assay of gene function in lymphocyte development. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4528–4532. doi: 10.1073/pnas.90.10.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockerill P. N., Shannon M. F., Bert A. G., Ryan G. R., Vadas M. A. The granulocyte-macrophage colony-stimulating factor/interleukin 3 locus is regulated by an inducible cyclosporin A-sensitive enhancer. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2466–2470. doi: 10.1073/pnas.90.6.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D. R., Curran T. Analysis of dimerization and DNA binding functions in Fos and Jun by domain-swapping: involvement of residues outside the leucine zipper/basic region. Oncogene. 1990 Jun;5(6):929–939. [PubMed] [Google Scholar]
- Cohen D. R., Ferreira P. C., Gentz R., Franza B. R., Jr, Curran T. The product of a fos-related gene, fra-1, binds cooperatively to the AP-1 site with Jun: transcription factor AP-1 is comprised of multiple protein complexes. Genes Dev. 1989 Feb;3(2):173–184. doi: 10.1101/gad.3.2.173. [DOI] [PubMed] [Google Scholar]
- Demotz S., Grey H. M., Sette A. The minimal number of class II MHC-antigen complexes needed for T cell activation. Science. 1990 Aug 31;249(4972):1028–1030. doi: 10.1126/science.2118680. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Distel R. J., Spiegelman B. M. Protooncogene c-fos as a transcription factor. Adv Cancer Res. 1990;55:37–55. doi: 10.1016/s0065-230x(08)60467-4. [DOI] [PubMed] [Google Scholar]
- Flanagan W. M., Corthésy B., Bram R. J., Crabtree G. R. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature. 1991 Aug 29;352(6338):803–807. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- Gillis S., Smith K. A. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977 Jul 14;268(5616):154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- Goldfeld A. E., McCaffrey P. G., Strominger J. L., Rao A. Identification of a novel cyclosporin-sensitive element in the human tumor necrosis factor alpha gene promoter. J Exp Med. 1993 Oct 1;178(4):1365–1379. doi: 10.1084/jem.178.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera R., Agarwal S., Walton K., Satterberg B., Distel R. J., Goodman R., Spiegelman B. M., Roberts T. M. A direct role for c-fos in AP-1-dependent gene transcription. Cell Growth Differ. 1990 Oct;1(10):483–490. [PubMed] [Google Scholar]
- Hu-Li J., Ohara J., Watson C., Tsang W., Paul W. E. Derivation of a T cell line that is highly responsive to IL-4 and IL-2 (CT.4R) and of an IL-2 hyporesponsive mutant of that line (CT.4S). J Immunol. 1989 Feb 1;142(3):800–807. [PubMed] [Google Scholar]
- Jain J., McCaffrey P. G., Miner Z., Kerppola T. K., Lambert J. N., Verdine G. L., Curran T., Rao A. The T-cell transcription factor NFATp is a substrate for calcineurin and interacts with Fos and Jun. Nature. 1993 Sep 23;365(6444):352–355. doi: 10.1038/365352a0. [DOI] [PubMed] [Google Scholar]
- Jain J., McCaffrey P. G., Valge-Archer V. E., Rao A. Nuclear factor of activated T cells contains Fos and Jun. Nature. 1992 Apr 30;356(6372):801–804. doi: 10.1038/356801a0. [DOI] [PubMed] [Google Scholar]
- Jain J., Miner Z., Rao A. Analysis of the preexisting and nuclear forms of nuclear factor of activated T cells. J Immunol. 1993 Jul 15;151(2):837–848. [PubMed] [Google Scholar]
- Jain J., Valge-Archer V. E., Rao A. Analysis of the AP-1 sites in the IL-2 promoter. J Immunol. 1992 Feb 15;148(4):1240–1250. [PubMed] [Google Scholar]
- Jamieson C., McCaffrey P. G., Rao A., Sen R. Physiologic activation of T cells via the T cell receptor induces NF-kappa B. J Immunol. 1991 Jul 15;147(2):416–420. [PubMed] [Google Scholar]
- Johnson R. S., Spiegelman B. M., Papaioannou V. Pleiotropic effects of a null mutation in the c-fos proto-oncogene. Cell. 1992 Nov 13;71(4):577–586. doi: 10.1016/0092-8674(92)90592-z. [DOI] [PubMed] [Google Scholar]
- Johnson R. S., van Lingen B., Papaioannou V. E., Spiegelman B. M. A null mutation at the c-jun locus causes embryonic lethality and retarded cell growth in culture. Genes Dev. 1993 Jul;7(7B):1309–1317. doi: 10.1101/gad.7.7b.1309. [DOI] [PubMed] [Google Scholar]
- Karin M., Smeal T. Control of transcription factors by signal transduction pathways: the beginning of the end. Trends Biochem Sci. 1992 Oct;17(10):418–422. doi: 10.1016/0968-0004(92)90012-x. [DOI] [PubMed] [Google Scholar]
- Kerppola T. K., Luk D., Curran T. Fos is a preferential target of glucocorticoid receptor inhibition of AP-1 activity in vitro. Mol Cell Biol. 1993 Jun;13(6):3782–3791. doi: 10.1128/mcb.13.6.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovary K., Bravo R. Expression of different Jun and Fos proteins during the G0-to-G1 transition in mouse fibroblasts: in vitro and in vivo associations. Mol Cell Biol. 1991 May;11(5):2451–2459. doi: 10.1128/mcb.11.5.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovary K., Bravo R. The jun and fos protein families are both required for cell cycle progression in fibroblasts. Mol Cell Biol. 1991 Sep;11(9):4466–4472. doi: 10.1128/mcb.11.9.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Rouse R. V., Micklem H. S., Herzenberg L. A. T cell subsets defined by expression of Lyt-1,2,3 and Thy-1 antigens. Two-parameter immunofluorescence and cytotoxicity analysis with monoclonal antibodies modifies current views. J Exp Med. 1980 Aug 1;152(2):280–295. doi: 10.1084/jem.152.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner J. N., Yamamoto K. R. Regulatory crosstalk at composite response elements. Trends Biochem Sci. 1991 Nov;16(11):423–426. doi: 10.1016/0968-0004(91)90168-u. [DOI] [PubMed] [Google Scholar]
- Miner J. N., Yamamoto K. R. The basic region of AP-1 specifies glucocorticoid receptor activity at a composite response element. Genes Dev. 1992 Dec;6(12B):2491–2501. doi: 10.1101/gad.6.12b.2491. [DOI] [PubMed] [Google Scholar]
- Moore J. P., Menzel G. E., Hesketh T. R., Metcalfe J. C. C-fos gene activation in murine thymocytes by a mechanism independent of protein kinase C or a Ca2+ signal. FEBS Lett. 1988 Jun 6;233(1):64–68. doi: 10.1016/0014-5793(88)81356-5. [DOI] [PubMed] [Google Scholar]
- Nalefski E. A., Kasibhatla S., Rao A. Functional analysis of the antigen binding site on the T cell receptor alpha chain. J Exp Med. 1992 Jun 1;175(6):1553–1563. doi: 10.1084/jem.175.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northrop J. P., Ullman K. S., Crabtree G. R. Characterization of the nuclear and cytoplasmic components of the lymphoid-specific nuclear factor of activated T cells (NF-AT) complex. J Biol Chem. 1993 Feb 5;268(4):2917–2923. [PubMed] [Google Scholar]
- Nuñez G., London L., Hockenbery D., Alexander M., McKearn J. P., Korsmeyer S. J. Deregulated Bcl-2 gene expression selectively prolongs survival of growth factor-deprived hemopoietic cell lines. J Immunol. 1990 May 1;144(9):3602–3610. [PubMed] [Google Scholar]
- Rao A., Faas S. J., Cantor H. Activation specificity of arsonate-reactive T cell clones. Structural requirements for hapten recognition and comparison with monoclonal antibodies. J Exp Med. 1984 Feb 1;159(2):479–494. doi: 10.1084/jem.159.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds D. S., Boom W. H., Abbas A. K. Inhibition of B lymphocyte activation by interferon-gamma. J Immunol. 1987 Aug 1;139(3):767–773. [PubMed] [Google Scholar]
- Rüther U., Müller W., Sumida T., Tokuhisa T., Rajewsky K., Wagner E. F. c-fos expression interferes with thymus development in transgenic mice. Cell. 1988 Jun 17;53(6):847–856. doi: 10.1016/s0092-8674(88)90289-9. [DOI] [PubMed] [Google Scholar]
- Serfling E., Barthelmäs R., Pfeuffer I., Schenk B., Zarius S., Swoboda R., Mercurio F., Karin M. Ubiquitous and lymphocyte-specific factors are involved in the induction of the mouse interleukin 2 gene in T lymphocytes. EMBO J. 1989 Feb;8(2):465–473. doi: 10.1002/j.1460-2075.1989.tb03399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo S. J., Gold J. S., Murphy T. L., Murphy K. M. Identification of cis-acting regulatory elements controlling interleukin-4 gene expression in T cells: roles for NF-Y and NF-ATc. Mol Cell Biol. 1993 Aug;13(8):4793–4805. doi: 10.1128/mcb.13.8.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper R. I., Pattengale P. K., Leder P. Murine interleukin-4 displays potent anti-tumor activity in vivo. Cell. 1989 May 5;57(3):503–512. doi: 10.1016/0092-8674(89)90925-2. [DOI] [PubMed] [Google Scholar]
- Thompson C. B., Wang C. Y., Ho I. C., Bohjanen P. R., Petryniak B., June C. H., Miesfeldt S., Zhang L., Nabel G. J., Karpinski B. cis-acting sequences required for inducible interleukin-2 enhancer function bind a novel Ets-related protein, Elf-1. Mol Cell Biol. 1992 Mar;12(3):1043–1053. doi: 10.1128/mcb.12.3.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman K. S., Northrop J. P., Admon A., Crabtree G. R. Jun family members are controlled by a calcium-regulated, cyclosporin A-sensitive signaling pathway in activated T lymphocytes. Genes Dev. 1993 Feb;7(2):188–196. doi: 10.1101/gad.7.2.188. [DOI] [PubMed] [Google Scholar]
- Wang Z. Q., Ovitt C., Grigoriadis A. E., Möhle-Steinlein U., Rüther U., Wagner E. F. Bone and haematopoietic defects in mice lacking c-fos. Nature. 1992 Dec 24;360(6406):741–745. doi: 10.1038/360741a0. [DOI] [PubMed] [Google Scholar]
- Wong J. G., Rao A. Mutants in signal transduction through the T-cell antigen receptor. J Biol Chem. 1990 Mar 15;265(8):4685–4693. [PubMed] [Google Scholar]
- Ymer S., Tucker W. Q., Sanderson C. J., Hapel A. J., Campbell H. D., Young I. G. Constitutive synthesis of interleukin-3 by leukaemia cell line WEHI-3B is due to retroviral insertion near the gene. Nature. 1985 Sep 19;317(6034):255–258. doi: 10.1038/317255a0. [DOI] [PubMed] [Google Scholar]