Abstract
Somatic cell nuclear transfer (SCNT) is an important method of breeding quality varieties, expanding groups, and preserving endangered species. However, the viability of SCNT embryos is poor, and the cloned rate of animal production is low in pig. This study aims to investigate the gene function and establish a disease model of Banna miniature inbred pig. SCNT with donor cells derived from fetal, newborn, and adult fibroblasts was performed, and the cloning efficiencies among the donor cells were compared. The results showed that the cleavage and blastocyst formation rates did not significantly differ between the reconstructed embryos derived from the fetal (74.3% and 27.4%) and newborn (76.4% and 21.8%) fibroblasts of the Banna miniature inbred pig (P>0.05). However, both fetal and newborn fibroblast groups showed significantly higher rates than the adult fibroblast group (61.9% and 13.0%; P<0.05). The pregnancy rates of the recipients in the fetal and newborn fibroblast groups (60% and 80%, respectively) were higher than those in the adult fibroblast group. Eight, three, and one cloned piglet were obtained from reconstructed embryos of the fetal, newborn, and adult fibroblasts, respectively. Microsatellite analyses results indicated that the genotypes of all cloning piglets were identical to their donor cells and that the genetic homozygosity of the Banna miniature inbred pig was higher than those of the recipients. Therefore, the offspring was successfully cloned using the fetal, newborn, and adult fibroblasts of Banna miniature inbred pig as donor cells.
Introduction
Banna miniature inbred pigs have been bred since the 1980s from full and half siblings. As unique, highly miniature inbred pigs, Banna miniature inbred pigs can serve as large mammalian models with high homozygotic genes and clear genetic background [1], [2]. Given their similar anatomical and physiological features to humans, these animals can be used in various biomedical studies, including disease models, transgenesis, genomics, and xenotransplantation for medical research [3]. Some special traits also appear in inbreeding, such as blindness, deafness, spinal column bend, maxilla defect, and tumor. This particular phenotype provides valuable resources for studying relative human diseases. However, these individuals are hardly reproducible because of their impaired fertility or lethality. Thus, establishing a cloning system is essential to reproduce Banna miniature inbred pigs with unique traits for application to studies in various fields.
Somatic cell nuclear transfer (SCNT) is an important method of breeding quality varieties, expanding groups, and preserving endangered species [4]. This method was successfully applied in calf [5], mouse [6], goat [7], pig [8], rabbit [9], cat [10], rat [11], horse [12], mule [13], dog [14], ferret [15], buffalo [16], and camel [17] since the world’s first cloned sheep was obtained in 1996 [18]. Feasible SCNT procedures were established in pig. However, miniature pigs, such as the National Institutes of Health miniature pigs [19] and Clawn miniature pigs, have low cloning efficiency [20]. Under such circumstances, several studies focused on nuclear donor cells, which are generally believed to affect the cloning efficiency in mammals. In cattle, fetal fibroblasts are reportedly more effective than newborn fibroblasts [21]. In pig, fetal fibroblasts are more effective than adult fibroblasts as well as cumulus and oviduct cells [22]. Cell cycle synchronization through differentiation induction enables the effective production of cloned pigs [23]. In mouse, the appropriate combinations of cell type and genotype may improve the efficiency of somatic cell cloning and fetal survival after embryo transfer [24]. However, the cloning process and efficiency in Banna miniature inbred pigs remain unclear.
The present study aims to establish the nuclear transfer technology system of Banna miniature inbred pig and to investigate the effect of different donor cells, i.e., fetal, newborn, and adult fibroblasts, on the developmental competence of SCNT embryos as well as on the cloning efficiency of this pig.
Materials and Methods
All animal experiments were performed with the approval of the Animal Care Committee of Yunnan Agricultural University, China.
Chemicals
Unless otherwise stated, all chemicals were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Preparation of Donor Cells
Fetuses (47 days old) isolated from the 22nd generation in the No. 133-family of Banna miniature inbred pig were washed three times with phosphate-buffered saline. After removing the head, limbs, and viscera, the fetuses were minced and digested in Dulbecco’s modified Eagle’s medium (DMEM; Gibco) containing 20% fetal bovine serum (FBS; Hyclone), 1% penicillin-streptomycin, and 1 mg/mL Collagenase IV for 4 h at 37°C. The cells were centrifuged at 1000 rpm for 5 min, suspended in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin, and then cultured in a flask until grown to 90% confluence. The cells were then passaged and frozen in DMEM containing 20% FBS and 10% dimethylsulfoxide for future use.
Ear tissues were collected from a newborn piglet of the 18th generation in the No. 111-family and from an adult pig of the 23rd generation in the No. 133-family of Banna miniature inbred pig. The fibroblasts were isolated and cultured using the same procedure as described above.
In vitro Maturation of Oocytes
Porcine ovaries were collected from Hongteng slaughterhouse (Chenggong Ruide Food Co., Ltd, Kunming, Yunnan Province, China) with the permission to use animal parts for this study. The ovaries were transported to the laboratory at 25°C to 30°C in 0.9% (w/v) NaCl solution supplemented with 75 mg/mL potassium penicillin G and 50 mg/mL streptomycin sulfate. Cumulus-oocyte complexes were obtained from follicles 3 mm to 6 mm in diameter using an 18-gauge needle connected to a 10 mL disposable syringe. Cumulus-oocyte complexes with at least three layers of compacted cumulus cells were selected, and approximately 50 oocytes were cultured in 200 µL drops of TCM-199 medium supplemented with 0.1 mg/mL pyruvic acid, 0.1 mg/mL l-cysteine hydrochloride monohydrate, 10 ng/mL epidermal growth factor, 10% (v/v) porcine follicular fluid, 75 mg/mL potassium penicillin G, 50 mg/mL streptomycin sulfate, and 10 IU/mL eCG and hCG (Teikoku Zouki Co., Tokyo, Japan) at 38.5°C in an atmosphere with 5% CO2 (100% humidity) (APC-30D, ASTEC, Japan).
Nuclear Transfer
SCNT was performed as previously described [23], [25]. After culturing for 38 h to 42 h, oocytes with expanded cumulus cells were briefly treated with 0.1% (w/v) hyaluronidase and denuded of cumulus cells using a finely drawn glass capillary pipette. Oocytes extruding the first polar body with uniform cytoplasm were cultured in NCSU23 medium supplemented with 0.1 µg/mL demecolcine, 0.05 M sucrose, and 4 mg/mL bovine serum albumin (BSA) for 0.5 h to 1 h. The oocytes were enucleated by aspirating the first polar body and adjacent cytoplasm using a bevelled pipette (approximately 20 µm in diameter) in Tyrode’s lactate medium supplemented with 10 µM hydroxyethyl piperazineethanesulfonic acid (HEPES), 0.3% (w/v) polyvinylpyrrolidone, and 10% FBS in the presence of 0.1 µg/mL demecolcine and 5 µg/mL cytochalasin B. Any protrusion observed on the surface of an oocyte was removed along with the polar body. Fetal, newborn, and adult fibroblasts of the fourth to ninth passages were used as nuclear donors after cell cycle synchronization by 0.5% FBS serum starvation for 48 h. A single donor cell was inserted into the perivitelline space of an enucleated oocyte.
Donor cells were fused with the recipient cytoplasts with a single direct current pulse of 200 V/mm for 20 µs using an embryonic cell fusion system (ET3, Fujihira Industry Co. Ltd., Tokyo, Japan) in fusion medium [0.25 M d-sorbic alcohol, 0.05 mM Mg(C2H3O2)2, 20 mg/mL BSA, and 0.5 mM HEPES (free acid)]. The reconstructed embryos were cultured for 2 h in PZM-3 and then activated with a single pulse of 150 V/mm for 100 µs in an activation medium containing 0.25 M d-sorbic alcohol, 0.01 mM Ca(C2H3O2)2, 0.05 mM Mg(C2H3O2)2, and 0.1 mg/mL BSA. The reconstructed embryos were equilibrated in PZM-3 supplemented with 5 µg/mL cytochalasin B for 2 h at 38.5°C in humidified atmosphere of 5% CO2, 5% O2, and 90% N2 (APM-30D, ASTEC, Japan).
Culture of Embryos
Reconstructed embryos were cultured in PZM-3 medium and then placed in an incubator supplied with 5% CO2, 5% O2, and 90% N2 at 38.5°C in a humidified atmosphere. Cleavage and blastocyst formation were monitored on days 2 and 7, respectively. Differential nuclear staining of inner cell mass (ICM) and trophectoderm (TE) cell of the blastocysts was performed. Cell counts were carried out after staining with 10 µg/mL propidium iodide and 10 µg/mL Hoechst33342 under a laser scanning confocal microscope (TCS SP5II, LEICA, Germany) [26], [27].
Embryo Transfer
Crossbred (Large White/Landrace Duroc) prepubertal gilts weighing 100 kg to 120 kg were used as the surrogate mothers of the cloned embryos. They were checked for estrus at 09∶00 and 18∶00 h daily. Reconstructed embryos cultured for 6 and 30 h after activation were surgically transferred to the oviducts of the estrous surrogate mother by feeding a 14 cm Tom cat catheter (Tyco Healthcare Group LP, MA, USA) through the fimbriae at 0 and 9 h after the first standing estrus was exhibited, respectively. Pregnancy was detected at approximately 23 days after activation using an ultrasound scanner (HS-101V, Honda Electonics Co., Ltd., Yamazuka, Japan).
Microsatellite Analysis
Parentage analysis was performed in piglets produced by SCNT and the surrogate recipient to confirm the genetic identity of the SCNT piglets with the donor cells. The isolated genomic DNA samples obtained from each newborn piglet (ear tissue) and recipient (ear tissue) were used for microsatellite analysis and sent to a company that specializes in parentage verification for swine (Shanghai GeneCore BioTechnologies Co., Ltd.). Microsatellite analysis of the genomic DNA was performed using 11 porcine-specific microsatellite markers (S0026, S0070, S0155, S0226, SW122, SW24, SW72, SW830, SW840, SW857, and SW936) labeled with the fluorescent dye carboxyfluorescein (FAM).
Statistical Analysis
For proportional data, the differences between groups were analyzed for variance using the Statview® software package. The level of significance was set at P<0.05.
Results
Effect of the Donor Cell Type on the Development of Embryos Derived from SCNT
The effect of the donor cell type on the development of embryos derived from SCNT was investigated (Table 1). The cleavage rate and blastocyst formation rate of the reconstructed embryos did not significantly differ between the fetal (74.3% and 27.4%) and newborn (76.4% and 21.8%) fibroblast groups (P>0.05), but both groups exhibited significantly higher rates than the adult fibroblast group (61.9 and 13.0%; P<0.05). Our results showed that the blastocysts derived from the fetal fibroblasts had more ICM cells, TE cells, total cells and TCM/total cell than those derived from the adult fibroblasts; however, no significant difference was observed among the three groups (P>0.05; Table 2).
Table 1. Effects of different donor cells on the development of SCNT embryos of Banna miniature inbred pig.
| Donor cell type | No. of embryos (Repeats) | No. of cleavage (%) | No. of blastocyst (%) |
| Fetal fibroblast | 895(10) | 667(74.3±7.7)a | 254(27.4±5.1)a |
| Newborn fibroblast | 843(9) | 655(76.4±7.1)a | 186(21.8±3.5)a |
| Adult fibroblast | 1279(10) | 780(62.1±7.9)b | 168(13.5±4.8)b |
Values with different superscript letters within a column are significantly different (a,b P < 0.05).
Table 2. Comparison of ICM and TE cell of blastoysts derived from different donor cells.
| Doner cell type | No. of blastocyst | No. of cell | ICM/total cells(%) | ||
| ICM | TE | Total | |||
| Fetal fibroblast | 10 | 10.3±2.6a | 36.5±5.6a | 46.8±7.2a | 21.8±4.3a |
| Newborn fibroblast | 10 | 8.5±2.0a | 33.4±6.6a | 41.9±8.0a | 20.8±3.0a |
| Adult fibroblast | 10 | 6.9±1.7a | 31.0±5.3a | 37.9±6.5a | 18.2±2.6a |
Values with same superscript letters within a column are not significantly different (a P>0.05).
Effect of the Donor Cell Type on the Implantation Rate of Embryos Derived from SCNT
Reconstructed embryos derived from fetal, newborn, and adult fibroblasts were transferred to five, five, and three surrogate mothers, respectively. For the fetal, newborn, and adult fibroblasts, the number of pregnancies were three (60.0%), four (80.0%), and one (33.3%), respectively, and the number of deliveries were three (60.0%), one (20.0%), and one (33.3%), respectively (Table 3). Eight, three, and one cloned piglets were obtained from the fetal, newborn, and adult fibroblasts (Figure 1). Two of three neonatuses from the newborn fibroblasts died shortly after birth because of neonatal asphyxia caused by dystocia. In the newborn fibroblast group, the uteri of two surrogate mothers were dissected on pregnancy day 120, and 20 and 13 absorbed fetuses were collected, which took on a lump without the shape of a normal fetus. Another surrogate mother was midway aborted. The birth weight of the piglets derived from the fetal, newborn, and adult fibroblasts were 817.8, 741.3, and 925.0 g, respectively (Table 4); no significant difference in body weight was observed among the three groups (P>0.01). These weights of the fetal, newborn, and adult fibroblast groups were significantly greater than that of the control groups (P<0.01).
Table 3. Development of cloned embryos derived from different donor cells after being transferred to surrogate gilts.
| Donor cell type | No. of surrogates | No. of transferred embryos | No. of pregnancy (%) | No. of delivery (%) | Offspring (dead) |
| Fetal fibroblast | 5 | 246.0±65.7 | 3(60.0%)a | 3(60.0%)a | 8 |
| Newborn fibroblasts | 5 | 148.0±40.3 | 4(80.0%)a | 1(20.0%)a | 3(2) |
| Adult fibroblast | 3 | 304.7±20.0 | 1(33.3%)a | 1(33.3%)a | 1 |
Values with same superscript letters within a column are not significantly different (a P>0.05).
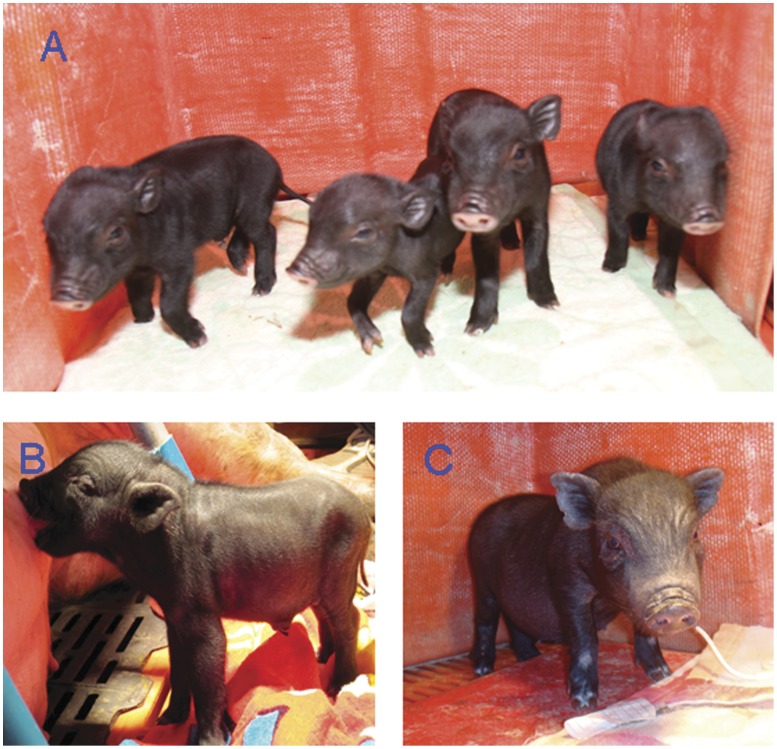
Figure 1. Banna miniature inbred pigs cloned by SCNT.
Piglets derived from (A) fetal fibroblasts, (B) newborn fibroblasts, and (C) adult fibroblasts.
Table 4. Comparison of birth weight of cloned piglets derived from different donor cells.
| Donor cell type | Fetal fibroblast (♂) | Newborn fibroblast (♂) | Adult fibroblast (♀) | Control | |
| ♂ | ♀ | ||||
| No. of piglets | 8 | 3 | 1 | 20 | 20 |
| Birth weight(g) | 817.8±157.1a | 741.3±156.0a | 925a | 518.3±114.4b | 503.6±110.4b |
Values with different superscript letters within a column are significantly different (a,b P < 0.01).
DNA Parentage Analysis
Parentage analysis was performed on the cloned piglets, donor cells, and surrogate females. The genotype of each piglet from fetal, newborn, and adult fibroblasts was identical to the donor cell but different from its surrogate mother. Only two, one, and two heterozygous loci were observed in the 11 porcine-specific microsatellite markers of the donor cell line from the fetal, newborn, and adult fibroblasts, respectively. The gene homozygosity of all donor cells was higher than that of the surrogate mothers (Tables 5, 6, 7).
Table 5. Microsatellite analysis of cloned piglets derived from fetal fibroblasts.
| Marker | Dye name | PCR annealing temp | Genotypes of Recipient | Cell line genotypes (BN133) | Genotypes of litter | |||||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||||
| S0026 | FAM | 55 | 97 | 93/101 | 91/97 | 97 | 97 | 97 | 97 | 97 | 97 | 97 | 97 | 97 |
| S0070 | FAM | 55 | 271/273 | 285/287 | 263 | 271/273 | 271/273 | 271/273 | 271/273 | 271/273 | 271/273 | 271/273 | 271/273 | 271/273 |
| S0155 | FAM | 55 | 142/152 | 142/152 | 152/156 | 142 | 142 | 142 | 142 | 142 | 142 | 142 | 142 | 142 |
| S0226 | FAM | 55 | 183/195 | 193 | 183 | 195 | 195 | 195 | 195 | 195 | 195 | 195 | 195 | 195 |
| SW122 | FAM | 55 | 108 | 108/110 | 106/110 | 108 | 108 | 108 | 108 | 108 | 108 | 108 | 108 | 108 |
| SW24 | FAM | 55 | 103 | 101/103 | 115 | 103 | 103 | 103 | 103 | 103 | 103 | 103 | 103 | 103 |
| SW72 | FAM | 55 | 97 | 99/109 | 97 | 97 | 97 | 97 | 97 | 97 | 97 | 97 | 97 | 97 |
| SW830 | FAM | 50 | 180/184 | 168 | 180 | 180/184 | 180/184 | 180/184 | 180/184 | 180/184 | 180/184 | 180/184 | 180/184 | 180/184 |
| SW840 | FAM | 55 | 135 | 117/123 | 125/129 | 135 | 135 | 135 | 135 | 135 | 135 | 135 | 135 | 135 |
| SW857 | FAM | 55 | 139/155 | 139/151 | 143/153 | 139 | 139 | 139 | 139 | 139 | 139 | 139 | 139 | 139 |
| SW936 | FAM | 55 | 97 | 95/109 | 93/119 | 97 | 97 | 97 | 97 | 97 | 97 | 97 | 97 | 97 |
For each microsatellite marker, genotype was determined by size (base pairs). Two numbers for each sample at each locus represent the PCR product size at that particular locus.
Litters 1,2,3 came from recipient 1; litters 4,5,6 came from recipient 2; and the rest came from recipient 3.
Table 6. Microsatellite analysis of cloned piglets derived from newborn fibroblasts.
| Marker | Dye name | PCR annealing temp | Genotypes of Recipient | Cell line genotypes | Genotypes of litter | ||
| 1 | 2 | 3 | |||||
| S0026 | FAM | 55 | 97 | 93 | 93 | 93 | 93 |
| S0070 | FAM | 55 | 273/283 | 273 | 273 | 273 | 273 |
| S0155 | FAM | 55 | 152/158 | 142 | 142 | 142 | 142 |
| S0226 | FAM | 55 | 181 | 195 | 195 | 195 | 195 |
| SW122 | FAM | 55 | 112 | 108 | 108 | 108 | 108 |
| SW24 | FAM | 55 | 103 | 115 | 115 | 115 | 115 |
| SW72 | FAM | 55 | 106/108 | 98/110 | 98/110 | 98/110 | 98/110 |
| SW830 | FAM | 50 | 177 | 185 | 185 | 185 | 185 |
| SW840 | FAM | 55 | 125 | 125 | 125 | 125 | 125 |
| SW857 | FAM | 55 | 144 | 154 | 154 | 154 | 154 |
| SW936 | FAM | 55 | 109 | 97 | 97 | 97 | 97 |
Table 7. Microsatellite analysis of cloned piglets derived from adult fibroblasts.
| Marker | Dye name | PCR annealing temp | Genotypes of Recipient | Cell line genotypes | Genotypes of litter |
| S0026 | FAM | 55 | 92/96 | 96 | 96 |
| S0070 | FAM | 55 | 263/271 | 271 | 271 |
| S0155 | FAM | 55 | 146/158 | 142 | 142 |
| S0226 | FAM | 55 | 180/198 | 192 | 192 |
| SW122 | FAM | 55 | 110/118 | 108 | 108 |
| SW24 | FAM | 55 | 115 | 103 | 103 |
| SW72 | FAM | 55 | 98/106 | 97 | 97 |
| SW830 | FAM | 50 | 180 | 180/185 | 180/185 |
| SW840 | FAM | 55 | 125 | 125/135 | 125/135 |
| SW857 | FAM | 55 | 152 | 152 | 152 |
| SW936 | FAM | 55 | 108 | 98 | 98 |
Discussion
This study is the first to report on successful cloning using the fetal, newborn, and adult fibroblasts of Banna miniature inbred pig. This pig can be extensively used as a large animal model in biomedical research. It can also be used a source of organs for xenotransplantation in humans because of its highly homozygotic genes and clear genetic background.
However, low cloning efficiency has hampered the production of cloned animals. Several reports indicated that the type of donor cell can affect the birth rate. In mouse, an appropriate interaction between cell type and genotype can improve cloning efficiency [24], [28]. In cattle, clones derived from adult cells aborted in the later stages of pregnancy and calves developing to term show a higher number of abnormalities than those derived from newborn or fetal cells [29]. The simultaneous coordination of the donor cell type and cell cycle stage can maximize the overall cloning efficiency [30]. However, in buffalos, cumulus cells are a more efficient nuclear donor for SCNT than skin fibroblast and granulosa cell lines [31]. Sheep also shows breed-specific variability in terms of cloned embryo development [32]. Nevertheless, neither the donor cell type nor the gender significantly affects the overall efficiency of the in vitro production of SCNT sheep embryo [33]. In rabbit, embryos reconstructed with fresh cumulus cells have a more efficient developmental potential than those reconstructed with fetal fibroblasts in vivo and in vitro [34]. In pig, the type of donor somatic cell is important for the development of cloned embryos; fetal fibroblasts are the most effective among adult and fetal fibroblasts, cumulus and oviduct cells [22]. However, comparisons show that adult cells of any type are inferior to fetal fibroblasts in terms of reconstructed embryo development.
Our results reconfirmed the fact that the type of donor somatic cell is critical for determining developmental competence. Moreover, these results further confirmed that fetal fibroblasts have the highest efficiency as donor cells in SCNT for the cloning of highly inbred Banna miniature pigs in three types of donor fibroblast, whereas adult fibroblasts have the least efficiency. The fetal fibroblasts of Banna miniature inbred pig have a cloning efficiency of 0.65%, which is higher than that (0.4%) of newborn fibroblasts. However, these cloning efficiencies were both higher than that (0.1%) of adult fibroblasts. The very low efficiency of adult fibroblasts as donor cells in SCNT could be attributed to the very low cleavage rate and blastocyst formation rate as well as to the very low numbers of ICM, TE and total cells in the blastocysts. In addition, compared with the fetal and newborn fibroblasts in the primary culture, adult fibroblasts showed slightly slower proliferation rate (data not shown). Previous reports demonstrated that the developmental rates of cloned embryos remain similar regardless of the donor age in several other different species [35]–[37]. However, significant differences in developmental rate and birth rate exist among the donor cells of different ages in pigs [22]. The possible reason for the decreased potential of fibroblasts as donor cells in producing cloned healthy live birth with increasing age may also be attributed to the differentiation status of donor cells. Fetal cells are highly undifferentiated and more amenable to reprogramming after reconstruction than differentiated cells [22], [38]. Furthermore, the somatic cells of adult animals accumulate more genetic aberrations and are more terminally differentiated than fetal cells [39], [40]. Thus, somatic cells are more likely to fail at full-term development with increasing age.
Several studies reported SCNT attempts in miniature pig. The donor cells of these pigs are all derived only from fetal fibroblasts. The cloning efficiency of the Chinese Bama miniature pig is 0.11% [1/870 (no. offspring/no. embryos transferred in the recipients; similarly hereinafter)] [41]. The efficiencies of producing cloned Potbelly miniature pigs from the lung and kidney of male newborn Meishan pigs as recipients are 8.57% and 3.57% (3/35 and 2/56), respectively [42]. Both the reconstructed embryos of Bama and Potbelly miniature inbred pigs were transferred at the two- to four-cell stage. Clawn miniature pig developed from cloned embryos was transferred to recipients after culturing for 6 h to 40 h and showed a cloning efficiency of 2.35% (2/85) [20]. Yucatan miniature pig was successfully cloned at an efficiency of 1.1% (7/631). Embryos were cultured for less than 1 h before being surgically transferred into the recipient [43]. The production efficiencies of cloned Nippon Institute for Biological Sciences strain miniature pigs using male and female fetal fibroblasts as nucleus donors range from 0.64% (2/314) to 0.9% (3/331) by transferring reconstructed embryos cultured for 1 day to 2 days into miniature and common domestic pigs [44]. In the National Institute of Health miniature inbred pig, the cloning efficiency is 1.3% (21/1610) using fetal fibroblasts as donor cells and transferring on the same day of SCNT [19]. In several inbred mice that have not been cloned (such as C57BL/6 and C3H/He), the cloning efficiencies of cloned inbred strains are extremely low, similar to the DBA/2 and 129/Sv strains [24], [37]. For the first time that Banna miniature inbred pigs were cloned using fetal fibroblasts, the cloning efficiency is 0.65% for transferring at the one- to two-cell stage. The cloning efficiency of the miniature pig is relatively low compared with others, which may be ascribed to the inbred genetic background and incorrect epigenetic modification resulting from imperfect genomic reprogramming [19], [45], [46]. Previous studies have reported that SCNT-derived clones are prone to various abnormal phenotypes, including large birth weight [47], [48]. Our result showed that the birth weight of cloned Banna miniature piglets is much larger than that of non-cloned Banna miniature inbred pigs. This finding can be attributed to the different body sizes of the surrogate mothers. In this study, all reconstructed embryos were transferred into crossbred Large White/Landrace Duroc surrogated mothers. The body size of the surrogated mother for the cloned Banna miniature piglet was approximately 200 kg, which is larger than that of Banna miniature inbred sow (approximately 50 kg). Although the uterus of the surrogate mother is larger than that of the Banna miniature inbred sow, it carries fewer cloned fetuses than the Banna miniature inbred sow. Thus, the cloned Banna miniature piglet fetuses can obtain more nutrition and large developmental space from large surrogate mothers than non-cloned Banna miniature inbred pigs from Banna miniature inbred sow. As a result, cloned Banna miniature inbred piglets have significantly larger birth weights than non-cloned Banna miniature inbred pigs. DNA parentage was performed, the genotype of each litter was identical to its donor cell but different from its surrogate mother. As an inbred line, the homozygosis of the donor cell genotype of the Banna miniature pig is higher than that of the others.
In conclusion, Banna miniature inbred pig offspring was successfully cloned using the fetal, newborn, and adult fibroblasts of this animal as donor cells. The cloning efficiency of the fetal fibroblasts was significantly higher than those of the other two fibroblasts. In addition to the establishment of a cloning system, physiological and reproductive studies on cloned Banna miniature inbred pig are required. The results will benefit animal models, transgenesis, genomics, and xenotransplantation.
Acknowledgments
The authors gratefully acknowledge Professor Hiroshi Nagashima of Meiji University, Japan, for his technical assistance in the SCNT procedures and Dr. Xin Wang of Stanford University School of medicine for critically reading the manuscript.
Funding Statement
This study was supported by the Major Research Plan from the Ministry of Science and Technology of China (Grant No. 2011CB944200), the National Natural Science Foundation of China (Grant No.31060308) and Yunnan Provincial Program for Introducing High-level Scientists (Grant No. 2011HA011). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Yu P, Zhang L, Li SF, Li YP, Cheng JQ, et al. (2004) Screening and analysis of porcine endogenous retrovirus in Chinese Banna minipig inbred line. Transplant Proc 36: 2485–2487. [DOI] [PubMed] [Google Scholar]
- 2. Zeng R, Zeng YZ (2005) Molecular cloning and characterization of SLA-DR genes in the 133-family of the Banna miniature inbred pig. Anim Genet 36: 267–269. [DOI] [PubMed] [Google Scholar]
- 3. Crabbe JC, Metten P, Cameron AJ, Wahlsten D (2005) An analysis of the genetics of alcohol intoxication in inbred mice. Neurosci Biobehav Rev 28: 785–802. [DOI] [PubMed] [Google Scholar]
- 4. Prather RS, Hawley RJ, Carter DB, Lai L, Greentein L (2003) Transgenic swine for biomedicine and agriculture. Theriogenology 59: 115–123. [DOI] [PubMed] [Google Scholar]
- 5. Kato Y, Tani T, Sotomaru Y, Kurokawa K, Kato JY, et al. (1998) Eight calves cloned from somatic cells of single adult. Science 282: 2095–2098. [DOI] [PubMed] [Google Scholar]
- 6. Wakayama T, Perry ACF, Zuccotti M, Johnsonk K R, Yanagimachi R (1998) Full term developmentof mice from enucleated oocytes injected with cumulus cell nuclei. Nature 394: 369–374. [DOI] [PubMed] [Google Scholar]
- 7. Baguisi A, Behboodi E, Melican DT, Pollock JS, Destrempes MM, et al. (1999) Production of goats by somatic cell nuclear transfer. Nat Biotechnol 17: 456–461. [DOI] [PubMed] [Google Scholar]
- 8. Polejaeva IA, Chen SH, Vaught TD, Page RL, Mullins J, et al. (2000) Cloned pigs produced by nuclear transfer from adult somatic cells. Nature 407: 86–90. [DOI] [PubMed] [Google Scholar]
- 9. Chesné P, Adenot PG, Viglietta C, Baratte M, Boulanger L, et al. (2002) Cloned rabbits produced by nuclear transfer from adult somatic cells. Nat Biotechnol 20: 366–369. [DOI] [PubMed] [Google Scholar]
- 10. Shin T, Kraemer D, Pryor J, Liu L, Rugila J, et al. (2002) A cat cloned by nuclear transplantation. Nature 415: 859. [DOI] [PubMed] [Google Scholar]
- 11. Zhou Q, Renard JP, Le Friec G, Brochard V, Beaujean N, et al. (2003) Generation of fertile cloned rats by regulating oocyte activation. Science 302: 1179. [DOI] [PubMed] [Google Scholar]
- 12. Galli C, Lagutina I, Crotti G, Colleoni S, Turini P, et al. (2003) Pregnancy: a cloned horse born to its dam twin. Nature 424: 635. [DOI] [PubMed] [Google Scholar]
- 13. Woods GL, White KL, Vanderwall DK, Li GP, Aston KI, et al. (2003) A mule cloned from fetal cells by nuclear transfer. Science 301: 1063. [DOI] [PubMed] [Google Scholar]
- 14. Lee BC, Kim MK, Jang G, Oh HJ, Yuda F, et al. (2005) Dogs cloned from adult somatic cells. Nature 436: 604. [DOI] [PubMed] [Google Scholar]
- 15. Li Z, Sun X, Chen J, Liu X, Wisely SM, et al. (2006) Cloned ferrets produced by somatic cell nuclear transfer. Dev Biol 293: 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shi D, Lu F, Wei Y, Cui K, Yang S, et al. (2007) Buffalos (Bubalus bubalis) cloned by nuclear transfer of somatic cells. Biol Reprod. Biology of Reproduction 77: 285–291. [DOI] [PubMed] [Google Scholar]
- 17. Wani NA, Wernery U, Hassan FA, Wernery R, Skidmore JA, et al. (2010) Production of the First Cloned Camel by Somatic Cell Nuclear Transfer. Biol Reprod 82: 373–379. [DOI] [PubMed] [Google Scholar]
- 18. Campbell KH, McWhir J, Ritchie WA, Wilmut I (1996) Sheep cloned by nuclear transfer from a cultured cell line. Nature 380: 64–66. [DOI] [PubMed] [Google Scholar]
- 19. Zhao J, Ross JW, Hao Y, Spate LD, Walters EM, et al. (2009) Significant Improvement in Cloning Efficiency of an Inbred Miniature Pig by Histone Deacetylase Inhibitor Treatment after Somatic Cell Nuclear Transfer. Biol Reprod 81: 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miyoshi K, Inoue S, Himaki T, Mikawa S, Yoshida M (2007) Birth of cloned miniature pigs derived from somatic cell nuclear transferred embryos activated by ultrasound treatment. Mol Reprod Dev. Molecular Reproduction and Development 74: 1568–1574. [DOI] [PubMed] [Google Scholar]
- 21. Zakhartchenko V, Mueller S, Alberio R, Schernthaner W, Stojkovic M, et al. (2001) Nuclear transfer in cattle with non-transfected and transfected fetal or cloned transgenic fetal and postnatal fibroblasts. Mol Reprod Dev 60: 362–369. [DOI] [PubMed] [Google Scholar]
- 22. Lee GS, Hyun SH, Kim HS, Kim DY, Lee SH, et al. (2003) Improvement of a porcine somatic cell nuclear transfer technique by optimizing donor cell and recipient oocyte preparations. Theriogenology 59: 1949–1957. [DOI] [PubMed] [Google Scholar]
- 23. Tomii R, Kurome M, Wako N, Ochiai T, Matsunari H, et al. (2009) Production of cloned pigs by nuclear transfer of preadipocytes following cell cycle synchronization by differentiation induction. J Reprod Dev 55: 121–127. [DOI] [PubMed] [Google Scholar]
- 24. Inoue K, Ogonuki N, Mochida K, Yamamoto Y, Takano K, et al. (2003) Effects of donor cell type and genotype on the efficiency of mouse somatic cell cloning. Biol Reprod 69: 1394–1400. [DOI] [PubMed] [Google Scholar]
- 25. Kurome M, Hisatomi H, Matsumoto S, Tomii R, Ueno S, et al. (2008) Production efficiency and telomere length of the cloned pigs following serial somatic cell nuclear transfer. J Reprod Dev 54: 254–258. [DOI] [PubMed] [Google Scholar]
- 26. Macháty Z, Day BN, Prather RS (1998) Development of early porcine embryos in vitro and in vivo. Biol Reprod 59: 451–455. [DOI] [PubMed] [Google Scholar]
- 27. Koo DB, Kim YJ, Yu I, Kim HN, Lee KK, et al. (2005) Effects of in vitro fertilization conditions on preimplantation development and quality of pig embryos. Anim Reprod Sci 90(1–2): 101–110. [DOI] [PubMed] [Google Scholar]
- 28. Thuan NV, Kishigami S, Wakayama T (2010) How to Improve the Success Rate of Mouse Cloning Technology. J Reprod Dev 56: 20–30. [DOI] [PubMed] [Google Scholar]
- 29. Kato Y, Tani T, Tsunoda Y (2000) Cloning of calves from various somatic cell types of male and female adult, newborn and fetal cows. J Reprod Fertil 120: 231–237. [PubMed] [Google Scholar]
- 30. Wells DN, Laible G, Tucker FC, Miller AL, Oliver JE, et al. (2003) Coordination between donor cell type and cell cycle stage improves nuclear cloning efficiency in cattle. Theriogenology 59: 45–59. [DOI] [PubMed] [Google Scholar]
- 31. Pandey A, Gupta SC, Gupta N (2010) Comparative Potential of Cultured Skin Fibroblast, Cumulus, and Granulosa Cell to Produce Somatic Cell Nuclear Transfer (SCNT) Preimplantation Embryos in Buffaloes (Bubalus bubalis) in Relation to Gene Expressions. Cell Reprogram.Cellular reprogramming 12: 357–368. [DOI] [PubMed] [Google Scholar]
- 32. Dinnyes A, King T, Wilmut I, De Sousa PA (2001) Sheep somatic cell nuclear transfer: effect of breed and culture system on embryonic and fetal development. Theriogenology 55: 264. [Google Scholar]
- 33. Hosseini SM, Moulavi F, Foruzanfar M, Hajian M, Abedi P, et al. (2008) Effect of donor cell type and gender on the efficiency of in vitro sheep somatic cell cloning. Small Ruminant Res 78: 162–168. [Google Scholar]
- 34. Tian J, Song J, Li H, Yang D, Li X, et al. (2012) Effect of donor cell type on nuclear remodelling in rabbit somatic cell nuclear transfer embryos. Reprod Domest Anim 47: 544–552. [DOI] [PubMed] [Google Scholar]
- 35. Hill JR, Winger QA, Long CR, Looney CR, Thompson JA, et al. (2000) Development rates of male bovine nuclear transfer embryos derived from adult and fetal cells. Biol Reprod 62: 1135–1140. [DOI] [PubMed] [Google Scholar]
- 36. Kasinathan P, Knott JG, Moreira PN, Burnside AS, Jerry DJ, et al. (2001) Effect of fibroblast donor cell age and cell cycle on development of bovine nuclear transfer embryos in vitro. Biol Reprod 64: 1487–1493. [DOI] [PubMed] [Google Scholar]
- 37. Wakayama T, Yanagimachi R (2001) Mouse cloning with nucleus donor cells of different age and type. Molecular Reproduction and Development 58: 376–383. [DOI] [PubMed] [Google Scholar]
- 38. Yao YX, Li XC, Zhang Y, Qiao LM, Guan WJ, et al. (2008) Effect of different choices and treatments with donor cells on reprogramming. Yi Chuan 30: 1392–1396. [DOI] [PubMed] [Google Scholar]
- 39. Tian XC, Kubota C, Enright B, Yang X (2003) Cloning animals by somatic cell nuclear transfer–biological factors. Reprod Biol Endocrinol 1: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Heidari B, Shirazi A, Tajic P, Ahmadi E, Nazari H, et al. (2010) Effect of donor cell age on development of ovine nuclear transfer embryos in vitro. Zygote 18: 331–338. [DOI] [PubMed] [Google Scholar]
- 41. Li Y, Liu J, Dai J, Xing F, Fang Z, et al. (2010) Production of cloned miniature pigs by enucleation using the spindle view system. Reprod Domest Anim 45: 608–613. [DOI] [PubMed] [Google Scholar]
- 42. Hoshino Y, Uchida M, Shimatsu Y, Miyake M, Nagao Y, et al. (2005) Developmental competence of somatic cell nuclear transfer embryos reconstructed from oocytes matured in vitro with follicle shells in miniature pig. Cloning Stem Cells 7: 17–26. [DOI] [PubMed] [Google Scholar]
- 43. Estrada JL, Collins B, York A, Bischoff S, Sommer J, et al. (2008) Successful cloning of the Yucatan minipig using commercial/occidental breeds as oocyte donors and embryo recipients. Cloning Stem Cells 10: 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kurome M, Ishikawa T, Tomii R, Ueno S, Shimada A, et al. (2008) Production of transgenic and non-transgenic clones in miniature pigs by somatic cell nuclear transfer. J Reprod Dev 54: 156–163. [DOI] [PubMed] [Google Scholar]
- 45. Armstrong L, Lako M, Dean W, Stojkovic M (2005) Epigenetic modification is central to genome reprogramming in somatic cell nuclear transfer. Stem Cells 24: 805–814. [DOI] [PubMed] [Google Scholar]
- 46. Niemann H, Tian XC, King WA, Lee RS (2008) Epigenetic reprogramming in embryonic and foetal development upon somatic cell nuclear transfer cloning. Reproduction 135: 151–163. [DOI] [PubMed] [Google Scholar]
- 47. Hirayama H, Sawai K, Hirayama M, Hirai T, Kageyama S, et al. (2011) Prepartum maternal plasma glucose concentrations and placental glucose transporter mRNA expression in cows carrying somatic cell clone fetuses. J Reprod Dev 57: 57–61. [DOI] [PubMed] [Google Scholar]
- 48. Martin M, Adams C, Wiseman B (2004) Pre-weaning performance and health of pigs born to cloned (fetal cell derived) swine versus non-cloned swine. Theriogenology 62: 113–122. [DOI] [PubMed] [Google Scholar]