Abstract
Diffuse large B-cell lymphoma (DLBCL) comprises 2 molecularly distinct subgroups of non-germinal center B-cell-like (non-GCB) and germinal center B-cell-like (GCB) DLBCLs, with the former showing relatively poor prognosis. In the present study, we analyzed the clinicopathological features of 39 patients with localized nasal/paranasal DLBCL. Immunohistochemistry-based subclassification revealed that 11 patients (28%) were of the GCB-type according to Hans’ algorithm and 11 (28%) were of the GCB-type according to Choi’s algorithm. According to both Hans’ and Choi’s algorithms, the non-GCB type was predominant. Nevertheless, prognosis was good. Overall survival did not differ significantly between the GCB and non-GCB subgroups (Hans’ algorithm: p = 0.57, Choi’s algorithm: p = 0.99). Furthermore, the prognosis of localized nasal/paranasal DLBCL was better than that of other localized extranodal DLBCLs. The prognosis of extranodal DLBCL is usually considered poorer than that of nodal DLBCL. However, in our study, no difference was noted between patients with localized nasal/paranasal DLBCL and patients with localized nodal DLBCL. In conclusion, although the non-GCB subtype is thought to show poor prognosis, in our study, the prognosis for localized nasal/paranasal DLBCL patients was good irrespective of subclassification.
Introduction
Although heterogeneous in nature, diffuse large B-cell lymphoma (DLBCL) can be classified into 2 distinct subtypes on the basis of genetic profiling: the germinal center B-cell-like (GCB) phenotype and the non-germinal center B-cell-like (non-GCB) phenotype [1], [2], [3]. Notably, patients belonging to the former group have a better prognosis than those belonging to the latter group. Hans et al. reported that these DLBCL subtypes can be easily distinguished on the basis of immunohistological staining for CD10, BCL6, and MUM1 proteins [2]. Later, Choi et al. added 2 new antibodies, FoxP1 and GCET1 [4], and Choi’s algorithm is reported to achieve better prognostic classification than Hans’ algorithm [5]. Extranodal non-GCB-like DLBCL is generally characterized by poor prognosis regardless of its localized disease, but localized primary non-tonsillar oral DLBCL exhibits favorable prognosis even in cases of the non-GCB subtype [6]. Nasal/paranasal DLBCL is uncommon, and the GCB and non-GCB subtypes of this disease have not yet been examined. In this study, we aimed to clarify the clinicopathological features of localized nasal/paranasal DLBCL.
Materials and Methods
Patients
We selected 39 Japanese patients diagnosed with localized nasal/paranasal DLBCL between 1995 and 2010 and reviewed our institution’s pathology department database to obtain the medical records of these patients. We only evaluated localized lymphomas, because the primary sites of advanced lymphomas are difficult to determine. All 39 cases were diagnosed as primary extranodal DLBCLs. Patients were defined as having extranodal DLBCL when the disease was confined to one or more extranodal sites and showed no (or only minor) nodal involvement after the staging procedures [7], [8]. This group of patients was then compared with 39 patients with localized nodal DLBCLs diagnosed at our institution [9]. The samples and the medical records (clinical history, treatment and survival data) used in our study was approved by the Institute Review Board (IRB) at Okayama University. Written informed consent was waived by our institutional review board, since our study was limited to the use of excess human tissue samples and medical records.
Histological Examination and Immunohistochemistry
Surgically resected or biopsied specimens of localized nasal/paranasal DLBCLs were fixed in 10% formaldehyde and embedded in paraffin. Serial sections (4 µm) were cut from each paraffin-embedded tissue block, and several of these sections were stained with hematoxylin. To subclassify the GCB- or non-GCB- type of DLBCL, immunohistochemistry was performed on formalin-fixed, paraffin-embedded sections using an automated Bond Max stainer (Leica Biosystems, Melbourne, Australia). The primary antibodies used were as follows: CD20 (L26, 1∶200; Novocastra, Newcastle-upon-Tyne, UK), CD3 epsilon (LN10, 1∶200; Novocastra), BCL6 (D8, 1∶100; SantaCruz), CD5 (4C7, 1∶100; Novocastra), GCET1 (RAM341, 1∶100; Abcam), CD10 (56C6, 1∶50; Novocastra), MUM1 (MUM1p, 1∶50; Dako), FoxP1 (JC12, 1∶500; LifeSpan Biosciences, Seattle, USA), and Ki-67 (MIB-1, 1∶5000; Novocastra). For each section, 10 high-power fields were recorded, quantitated, and averaged to calculate the estimated percentage of positively immunostained cells. Negativity for CD10, BCL6, and MUM1 was defined as <30% positively stained tumor cells, and positivity was defined as >30% positively stained tumor cells. As an exception, for Choi’s algorithm, negativity for MUM1 was defined as <80% positively stained tumor cells, and positivity, as >80% positively stained tumor cells. Negativity for GCET1 and FoxP1 staining was defined as <80% positively stained tumor cells, and positivity, as >80% positively stained tumor cells. Ki-67 immunoreactivity was evaluated semi-quantitatively by using the average estimated percentage of positive cells in the 10 recorded high-power fields [4].
Statistical Analysis
All data were analyzed using STATA software (version 9.0; Stata Corp., College Station, TX, USA). Actuarial overall survival curves were calculated using the Kaplan-Meier method, and differences were examined using the log-rank test to determine significant prognostic factors [10]. Overall survival was defined as the time from diagnosis to death from any cause or to the last follow-up visit.
Results
Characteristics of the Nasal/Paranasal DLBCL Cases
Table 1 and Table 2 summarize the characteristics of the localized nasal/paranasal DLBCL patients. The median age of the 39 patients was 76 years (range, 33–98 years). The patient population comprised 21 men and 18 women. According to Choi’s algorithm, 11 of the 39 patients (28%) were of the GCB- type and 28 (72%) were of the non-GCB- type. According to Hans’ algorithm, 11 (28%) were of the GCB- type and 28 of the 39 patients (72%) were of the non-GCB type (Table 3). According to the Ann Arbor classification, 33 patients were at clinical stage IE and 6 were at stage IIE. According to the International Prognostic Index, 4 patients were at low- intermediate risk and 13 were at low risk. Histologically, all cases were classified as DLBCL (Fig. 1). All patients were newly presenting with no prior treatment history.
Table 1. Clinical data for 39 localized nasal/paranasal DLBCLs.
| Patient no. | Age (years) | Sex | Primary site | IPI | LDH>normal values | Tumor size (maximum diameter; cm) | CS | Treatment | Relapse | Follow-up period | Follow-up |
| (months) | status | ||||||||||
| 1 | 93 | M | left nasal cavity | L or LI | No | NA | IE | RT | NA | 3 | DOAD |
| 2 | 72 | F | right nasal cavity | LI or HI | Yes | 5 | IE | R-THP-COP | NA | NA | NA |
| 3 | 57 | M | left nasal cavity/paranasal sinus | L | No | NA | IE | CHOP+RT | − | 48 | Alive,FOD |
| 4 | 60 | F | left nasal cavity | L | No | NA | IE | R-CHOP+RT | − | 34 | Alive,FOD |
| 5 | 94 | F | left nasal cavity | L or LI or HI | NA | NA | IE | NA | NA | NA | NA |
| 6 | 69 | F | left nasal cavity/paranasal sinuses | L or LI or HI | No | 3.7 | IE | CHOP+RT | − | NA | NA |
| 7 | 76 | M | left nasal cavity/paranasal sinuses | L | No | 4 | IE | Epi-COP+RT | − | 83 | Alive,FOD |
| 8 | 83 | F | right nasal cavity/paranasal sinuses | LI | Yes | 6 | IE | Epi-COP | − | 4 | DOD |
| 9 | 56 | F | right nasal cavity/paranasal sinus/lymph nodes | L | No | 3.5 | IIE | CHOP+RT | − | 101 | Alive,FOD |
| 10 | 85 | F | right nasal cavity | HI | Yes | 3.8 | IE | RT | + | 13 | DOAD |
| 11 | 73 | F | left nasal cavity/lymph nodes/paranasal sinuses | L | No | NA | IIE | CHOP+RT | NA | 7 | AWD |
| 12 | 74 | M | left nasal cavity | LI or HI | Yes | NA | IE | CHOP+RT | + | 50 | Alive,FOD |
| 13 | 89 | F | left nasal cavity/paranasal sinus | LI or HI | Yes | NA | IE | NA | NA | NA | NA |
| 14 | 81 | F | left nasal cavity | L or LI | No | NA | IE | R-THPCOP+RT | − | 17 | Alive,FOD |
| 15 | 67 | M | paranasal sinus | L or LI or HI | NA | NA | IE | CHOP | − | 125 | Alive,FOD |
| 16 | 64 | F | left nasal cavity/paranasal sinus | L | No | NA | IE | RT+chemotherapy | NA | NA | NA |
| 17 | 89 | F | left nasal cavity/paranasal sinus/lymph node | L or LI | NA | NA | IIE | NA | NA | NA | NA |
| 18 | 84 | F | right nasal cavity/pharynx | L or LI or HI | NA | NA | IE | NA | NA | NA | NA |
| 19 | 77 | M | left nasal cavity/paranasal sinus | L or LI | NA | NA | IE | NA | NA | NA | NA |
| 20 | 75 | M | right nasal cavity/paranasal sinus | L | No | NA | IE | NA | − | 19 | DOAD |
| 21 | 77 | M | right nasal cavity/paranasal sinus | LI or HI | Yes | 5.5 | IE | R-THP-COP | + | 17 | DOD |
| 22 | 79 | M | left nasal cavity | L or LI | No | 3 | IE | chemo+RT | − | 11 | Alive,FOD |
| 23 | 71 | F | left nasal cavity/lymph nodes | L or LI | No | 2.5 | IIE | chemotherapy | − | 35 | Alive,FOD |
| 24 | 98 | M | right nasal cavity | LI or HI | Yes | 2 | IE | untreatment | NA | 0 | DOAD |
| 25 | 80 | M | bilateral nasal cavities/paranasal sinus/lymph nodes | L | No | 3.7 | IIE | R-THP-COP | + | 36 | AWD |
| 26 | 65 | M | nasal cavity | L | No | NA | IE | R-CHOP+RT | − | 30 | Alive,FOD |
| 27 | 68 | M | right paranasal sinus/lymph nodes | LI | No | 2.4 | IIE | R-CHOP | − | 20 | Alive,FOD |
| 28 | 33 | M | left nasal cavity | L | No | 3 | IE | NA | NA | NA | NA |
| 29 | 77 | F | right paranasal sinuses | L or LI | NA | NA | IE | R-CHOP+MTX | + | 23 | Alive,FOD |
| 30 | 80 | M | right nasal cavity/right paranasal sinuses | LI | No | 2 | IE | untreatment | NA | 3 | AWD |
| 31 | 78 | F | right nasal cavity | L or LI or HI | NA | 2.5 | IE | NA | NA | NA | NA |
| 32 | 72 | M | left paranasal sinuses | L or LI | No | 5 | IE | NA | NA | NA | NA |
| 33 | 77 | M | right nasal cavity | L or LI or HI | NA | NA | IE | R-THP-COP+RT | + | 23 | AWD |
| 34 | 76 | M | left nasal cavity/paranasal sinuses | L | No | 4 | IE | R-THP-COP+RT | − | 9 | Alive,FOD |
| 35 | 78 | F | right nasal cavity | L or LI | No | NA | IE | untreatment | NA | 2 | AWD |
| 36 | 68 | M | left nasal cavity/paranasal sinuses | L | No | 4 | IE | R-CHOP | NA | 1 | DOD |
| 37 | 74 | F | left nasal cavity | L or LI | No | NA | IE | R-CHOP+RT | − | 12 | Alive,FOD |
| 38 | 57 | M | right paranasal sinuses | L | No | 3 | IE | NA | NA | 27 | Alive,FOD |
| 39 | 83 | M | left nasal cavity/paranasal sinus | LI | No | 3.6 | IE | R-THP-COP+RT | + | 48 | Alive,FOD |
CHOP, cyclophosphamide, adriamycin, vincristine, prednisolone; CS, clinical stage; F, female; FOD, free of disease; AWD, alive with disease; DOD, dead of disease; DOAD, dead of another disease; IPI, International Prognostic Index; L, low; LDH, lactate dehydrogenase; LI, low–intermediate;
M, male; NA, not available; MTX, methotrexate; R-, with rituximab; RT, radiation; THP-COP, pirarubicin, cyclophosphamide, vincristine, prednisolone; Epi-COP, epirubicin, cyclophosphamide, vincristine, prednisolone.
Table 2. Clinical characteristics of patients with localized nasal/paranasal DLBCL.
| Characteristic | No. of patients (%) |
| Sex | |
| Male | 21(54) |
| Female | 18(46) |
| Age (y), median (range) | 76 (33–98) |
| Ann Arbor stage | |
| I | 33(85) |
| II | 6(15) |
| LDH | |
| NormalElevated | 24(77)7(23) |
| PS | |
| 0–1 | 15(79) |
| 2 or more | 4(21) |
| IPI | |
| L-LI | 27(96) |
| HI-H | 1(4) |
| Treatment | |
| chemotherapy | 8(32) |
| chemotherapy+RT | 15(60) |
| RT alone | 2(8) |
| Complete Remission | |
| yes | 22(88) |
| no | 3(12) |
Table 3. Clinical and phenotypic characteristics of patients with GCB-type and non-GCB-type DLBCL.
| Total (n = 39) | Hans’ algorithm | P | Choi’ algorithm | p | |||
| GCB (n = 11) | non-GCB (n = 28) | GCB (n = 11) | non-GCB (n = 28) | ||||
| Sex (male/female) | 21/18 | 6/5 | 15/13 | 0.96 | 6/5 | 14/14 | 0.80 |
| Age (y), median (range) | 76 (33–98) | 75 (57–98) | 77 (33–94) | 0.61 | 77 (57–98) | 76 (33–94) | 0.98 |
| Age >60 | 34/39 (87%) | 10/11 (91%) | 24/28 (86%) | 0.66 | 10/11 (91%) | 24/28 (86%) | 0.66 |
| PS >1 | 4/19 (21%) | 0/5 (0%) | 4/14 (29%) | 0.18 | 0/5 (0%) | 4/14 (29%) | 0.18 |
| B symptoms | 1/29 (3%) | 1/8 (13%) | 0/21 (0%) | 0.099 | 1/8 (13%) | 0/21 (0%) | 0.099 |
| LDH >normal | 7/31 (23%) | 2/8 (25%) | 5/23 (22%) | 0.85 | 2/9 (22%) | 5/22 (23%) | 0.98 |
| Median survival (months) | 23 (1–125+) | 35 (11–125+) | 20 (1–101+) | 0.57 | 35 (11–48+) | 23 (1–125+) | 0.99 |
| Immunophenotype | |||||||
| CD10 | 8/39 (21%) | 8/11 (73%) | 0/28 (0%) | <0.0001 | 6/11 (55%) | 2/28 (7%) | 0.00097 |
| MUM1(Hans) | 28/39 (72%) | 5/11 (45%) | 23/28 (82%) | 0.022 | |||
| MUM1(Choi) | 24/39 (62%) | 3/11 (27%) | 21/28 (75%) | 0.0058 | |||
| BCL6 | 25/39 (64%) | 9/11 (82%) | 16/28 (57%) | 0.15 | 10/11 (91%) | 15/28 (54%) | 0.029 |
| FOXP1 | 29/39 (74%) | 6/11 (55%) | 23/28 (82%) | 0.076 | |||
| GCET1 | 12/39 (31%) | 3/11 (27%) | 9/28 (32%) | 0.77 | |||
Abbreviations: GCB, germinal center B-cell; PS, performance status; BM, bone marrow; LDH, lactate dehydrogenase; FOXP1, forehead box protein 1; GCET1, germinal center B-cell expressed transcript 1.
Figure 1. Histological and immunohistochemical features.

Diffuse infiltration and proliferation of large lymphoma cells (Hematoxylin–eosin staining).
Phenotypic Features of the Localized Nasal/Paranasal DLBCL Cases
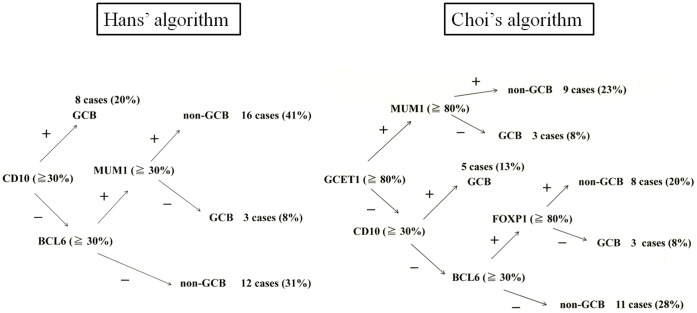
Table 4 summarizes the phenotypic features of the localized nasal/paranasal DLBCL patients. The B-cell immunophenotype of the lymphomas was confirmed by immunoreactivity with antibodies to CD20 in 39 cases. Although no cases were positive for CD5, 8 (21%) were positive for CD10 and 25 (64%) were positive for BCL6. For MUM1 staining, 28 cases (72%) were positive according to Hans’ algorithm and 24 cases (62%) were positive according to Choi’s algorithm. Furthermore, 29 cases (74%) were positive for FoxP1 and 12 (31%) were positive for GCET1. Of the 11 cases (28%) classified as GCB- type according to Hans’ algorithm, 8 were CD10-positive cases (20%), and 3 were CD10-negative, BCL6-positive, MUM1-negative cases (8%). Of the 11 (28%) classified as GCB- type according to Choi’s algorithm, 3 were GCET1-positive, MUM1-negative cases (8%); 5 were GCET1-negative, CD10-positive cases (13%); and 3 GCET1-negative, CD10-negative, BCL6-positive, FoxP1-negative cases (8%). Of the 28 cases (72%) classified as the non-GCB- type according to Hans’ algorithm, 12 were CD10-negative, BCL6-negative cases (31%) and 16 were CD10-negative, BCL6-positive, MUM1-positive cases (41%). Of the 28 cases (72%) classified as the non-GCB- type according to Choi’s algorithm, 9 were GCET1-positive, MUM1-positive cases (23%); 8 were GCET1-negative, CD10-negative, BCL6-positive, FoxP1-positive cases (20%); and 11 were GCET1-negative, CD10-negative, BCL6-negative cases (28%) (Fig. 2). The non-GCB- type was dominant according to both algorithms, but the prognosis for these cases was good. Overall survival did not differ significantly between the non-GCB type and GCB type groups (p = 0.57, Hans’ algorithm, p = 0.99, Choi’s algorithm) (Fig. 3).
Table 4. Immunohistochemical findings of localized nasal/paranasal DLBCLs.
| Patient no. | CD3 | CD5 | CD10 | CD20 | Ki-67 labeling (%) | MUM1 (Hans) | MUM1 (Choi) | BCL6 | EBER | FOXP1 | GCET1 | subtype(Hans) | subtype(Choi) |
| 1 | − | − | − | + | 21 | U.D. | − | − | − | + | − | Non-GCB | Non-GCB |
| 2 | − | − | p+ | + | 43 | − | − | + | − | + | − | GCB | GCB |
| 3 | − | − | + | + | 43 | − | − | + | − | − | − | GCB | GCB |
| 4 | − | − | − | + | 80 | + | + | − | − | + | − | Non-GCB | Non-GCB |
| 5 | − | − | − | + | 59 | + | + | − | − | + | − | Non-GCB | Non-GCB |
| 6 | − | − | − | + | 54 | + | + | + | − | + | − | Non-GCB | Non-GCB |
| 7 | − | − | − | + | 71 | + | + | + | − | + | − | Non-GCB | Non-GCB |
| 8 | − | − | − | + | 82 | + | + | − | − | + | − | Non-GCB | Non-GCB |
| 9 | − | − | − | + | 71 | − | − | − | − | + | − | Non-GCB | Non-GCB |
| 10 | − | − | − | + | 61 | + | + | + | − | − | + | Non-GCB | Non-GCB |
| 11 | − | − | − | + | 50 | + | + | + | − | + | − | Non-GCB | Non-GCB |
| 12 | − | − | − | + | 34 | + | + | + | − | − | + | Non-GCB | Non-GCB |
| 13 | − | − | − | + | 55 | + | + | + | − | + | + | Non-GCB | Non-GCB |
| 14 | − | − | − | + | 70 | + | + | + | − | + | + | Non-GCB | Non-GCB |
| 15 | − | − | + | + | 64 | + | + | − | − | − | + | GCB | Non-GCB |
| 16 | − | − | − | + | 33 | − | − | + | − | − | + | GCB | GCB |
| 17 | − | − | − | + | 61 | + | + | − | − | + | + | Non-GCB | Non-GCB |
| 18 | − | − | − | + | 72 | + | + | − | − | − | − | Non-GCB | Non-GCB |
| 19 | − | − | − | + | 63 | + | + | + | − | − | − | Non-GCB | GCB |
| 20 | − | − | − | + | 67 | − | − | + | − | − | − | GCB | GCB |
| 21 | − | − | − | + | 80 | + | + | − | − | − | − | Non-GCB | Non-GCB |
| 22 | − | − | + | + | 80 | + | + | − | − | + | − | GCB | GCB |
| 23 | − | − | + | + | 72 | − | − | + | − | + | + | GCB | GCB |
| 24 | − | − | + | + | 90 | + | + | + | − | + | − | GCB | GCB |
| 25 | − | − | − | + | 73 | − | − | + | − | + | + | GCB | GCB |
| 26 | − | − | − | + | 81 | + | + | + | − | + | − | Non-GCB | Non-GCB |
| 27 | − | − | − | + | 52 | + | + | + | − | + | − | Non-GCB | Non-GCB |
| 28 | − | − | − | + | 72 | + | − | − | − | + | − | Non-GCB | Non-GCB |
| 29 | − | − | + | + | 97 | + | + | + | − | + | + | GCB | Non-GCB |
| 30 | − | − | − | + | 90 | − | − | − | − | + | − | Non-GCB | Non-GCB |
| 31 | − | − | + | + | 77 | + | − | + | − | + | − | GCB | GCB |
| 32 | − | − | − | + | 49 | − | − | − | − | + | − | Non-GCB | Non-GCB |
| 33 | − | − | − | + | 88 | + | + | + | − | + | + | Non-GCB | Non-GCB |
| 34 | − | − | − | + | 54 | + | + | + | − | + | − | Non-GCB | Non-GCB |
| 35 | − | − | − | + | 58 | + | − | + | − | − | − | Non-GCB | GCB |
| 36 | − | − | − | + | 92 | + | − | + | − | + | − | Non-GCB | Non-GCB |
| 37 | − | − | − | + | 61 | − | + | + | − | + | − | Non-GCB | Non-GCB |
| 38 | − | − | − | + | 90 | + | − | − | − | + | − | Non-GCB | Non-GCB |
| 39 | − | − | − | + | 83 | + | + | + | − | + | + | Non-GCB | Non-GCB |
EBER, Epstein–Barr virus-encoded small RNA; GCB, germinal center B-cell; FOXP1, forehead box protein 1; GCET1; germinal center B-cell expressed transcript 1.
Figure 2. Distribution of GCB and non-GCB type according to Hans et al. and Choi et al.
Figure 3. Kaplan–Meier plot showing overall survival for patients with localized nasal/paranasal DLBCL.
Therapeutic Response and Outcome
Follow-up clinical data were available for 28 patients. The duration of follow-up ranged from 1 to 125 months (mean, 29 months). Fifteen patients were initially treated with chemotherapy plus irradiation, 9 were treated with chemotherapy alone, and 2 were treated with irradiation alone. Twenty-two patients achieved complete remission. Although 7 patients relapsed, 3 of these patients achieved complete remission following alternative chemotherapy. Salvage treatments for the 7 relapsed patients were R-MFP (methotrexate, fluorouracil, low dose cisplatin, and rituximab), R-THP-COP (cyclophosphamide, vincristine, prednisolone, pirarubicin, and rituximab), R-MTX (methotrexate and rituximab), and CHASER (cyclophosphamide, high dose cytarabine, dexamethasone, etoposide, and rituximab) plus radiation. At the time of reporting, 16 patients were disease- free and 3 patients had died of the disease.
Comparison of the Clinicopathological Characteristics of Localized Nasal/Paranasal DLBCL and Localized Nodal DLBCL
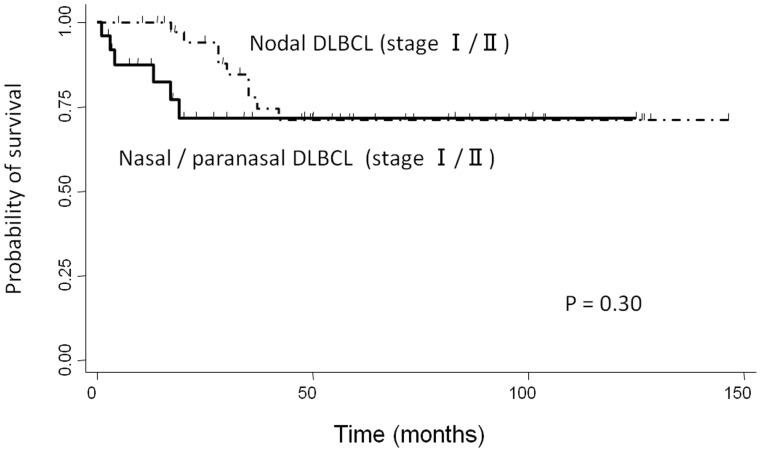
The clinicopathological characteristics of nasal/paranasal DLBCL and localized nodal DLBCL are summarized in Table 5. Nasal/paranasal DLBCL patients showed good prognosis. The slight difference in the overall survival between these patients and patients with localized nodal DLBCL was not significant (p = 0.30) (Fig. 4). Moreover, analysis using the χ2-test revealed a significant difference between the 2 groups with regard to age distribution and immunophenotype. Localized nasal/paranasal DLBCL patients were more likely to be more than 60 years old than localized nodal DLBCL patients (p = 0.018). In addition, localized nasal/paranasal DLBCL patients showed significantly higher positivity for MUM1 than localized nodal DLBCL patients according to Choi’s algorithm (p = 0.00023, χ2-test).
Table 5. Clinical and phenotypic characteristics of patients with localized nasal/paranasal DLBCL and localized nodal DLBCL.
| localized nasal/paranasal DLBCL | localized nodal DLBCL | p | |
| Total (n = 39) | Total (n = 39) | ||
| Sex (male/female) | 21/18 | 23/16 | 0.65 |
| Age (y), median (range) | 76 (33–98) | 70 (33–79) | 0.0010 |
| Age >60 | 34/39 (87%) | 25/39 (64%) | 0.018 |
| IPI : L-LI | 27/28 (96%) | 38/39 (97%) | 0.81 |
| Relapse | 7/22 (32%) | 16/39 (41%) | 0.48 |
| LDH >normal | 7/31 (23%) | 7/39 (18%) | 0.63 |
| Median survival (months) | 23 (1–125+) | 49 (4–146+) | 0.30 |
| Immunophenotype | |||
| CD10 | 8/39 (21%) | 16/39 (41%) | 0.050 |
| MUM1(Hans) | 28/39 (72%) | 28/39 (72%) | 1.0 |
| MUM1(Choi) | 24/39 (62%) | 8/39 (21%) | 0.00023 |
| BCL6 | 25/39 (64%) | 31/39 (79%) | 0.13 |
| FOXP1 | 29/39 (74%) | 32/39 (82%) | 0.41 |
| GCET1 | 12/39 (31%) | 17/39 (44%) | 0.24 |
Abbreviations: GCB, germinal center B-cell; PS, performance status; LDH, lactate dehydrogenase.
Figure 4. Comparison of overall survival between localized nasal/paranasal DLBCL and localized nodal DLBCL.
Discussion
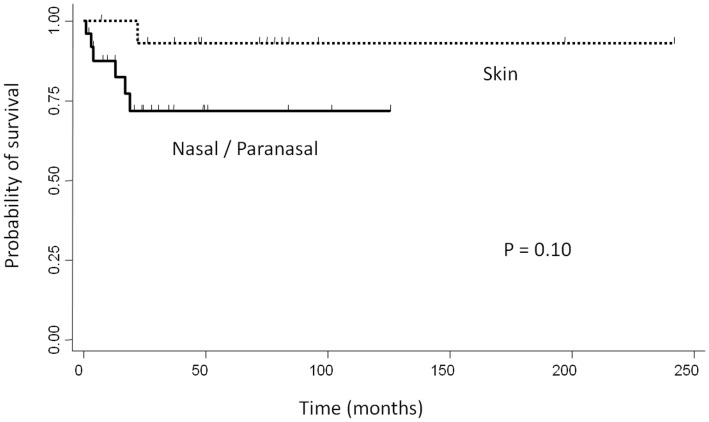
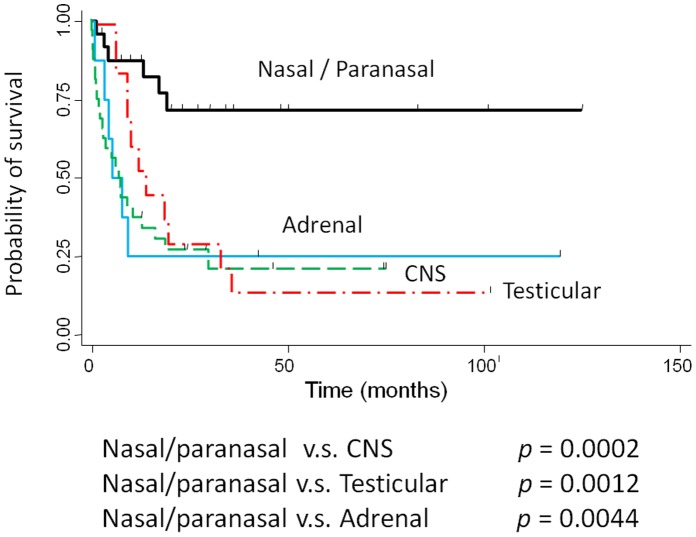
DLBCL is the most frequent and aggressive lymphoma, representing a heterogeneous group that includes de novo large B-cell lymphomas, as well as transformed lymphomas from follicular or mucosa-associated lymphoid tissue lymphomas [11]. Recent studies have demonstrated that DLBCL can be further subclassified into 2 major prognostic categories according to Hans et al.: the GCB- type and the non-GCB- type [1], [2]. However, Hans’ algorithm has been superseded by a new algorithm devised by Choi et al., and results obtained using Choi’s algorithm closely correlate with those of gene expression profiling for predicting prognosis [5]. In general, the non-GCB- type of DLBCL is associated with a significantly poorer prognosis than the GCB- type [1]; however, it has recently been established that this may not be true for extranodal DLBCL. Patients with localized primary non-tonsillar oral DLBCL presented with a favorable clinical course despite having the non-GCB- type [6]. Similarly, in our study, the non-GCB- type of localized nasal/paranasal DLBCL was the dominant type following subclassification according to both algorithms, but the prognosis of these patients was good. Moreover, the prognosis of localized nasal/paranasal DLBCL was as good as that of primary cutaneous DLBCL [12] (p = 0.10) (Fig. 5) and was statistically better than that of other localized extranodal DLBCLs (CNS [13], testis [14], and adrenal gland [15]) ( p = 0.0002, p = 0.0012, and p = 0.0044, respectively) (Fig. 6). Generally, extranodal non-GCB-like DLBCLs are characterized by poor prognosis, and the incidence of non-GCB- type DLBCLs among extranodal DLBCLs is 83–100%, although this value differs according to the organ of manifestation [16], [17], [18], [19]. According to previous reports, DLBCLs of the central nervous system [16], breast [17], stomach [20], leg type [21], testis [18], and intravascular type [19] are predominantly of the non-GCB- type, an observation consistent with the finding in our study of localized nasal/paranasal DLBCL cases. However, patients with CNS, breast, and testicular DLBCL exhibit poor prognosis, regardless of the localized disease [16], [17], [18], [22]. As shown for primary cutaneous B-cell lymphoma, findings of genes expression analysis suggest that primary non-leg-type cutaneous DLBCL and primary cutaneous DLBCL, leg type have expression profiles similar to those of GCB- type and non-GCB- type DLBCLs, respectively [23]. Therefore, primary non-leg-type cutaneous DLBCL is predominantly associated with an excellent prognosis [12], [24]. According to the recent World Health Organization (WHO) classification, subsets of DLBCLs arising in peculiar extranodal sites have been categorized as distinct disease subgroups (primary DLBCLs of the CNS, primary cutaneous DLBCLs, leg-type) or as distinct disease entities (primary mediastinal large B-cell lymphoma), on the basis of specific clinical and/or pathologic features [25], [26]. When the cases in our study are included, extranodal disease is common among DLBCL patients [27]. It is thought that there are important clinical differences between nodal and extranodal DLBCL and that the most reliable distinction can be made in patients with stage I disease. For these patients, extranodal DLBCL is independently associated with poor survival [27]. Therefore, we also compared the clinicopathological profiles of localized nasal/paranasal DLBCLs with localized nodal DLBCLs. This analysis showed that localized nasal/paranasal DLBCL was associated with good prognosis and no difference was noted in the prognosis compared with localized nodal DLBCL. In recent years, the use of rituximab has improved the prognosis of DLBCL patients, and CHOP (cyclophosphamide, adriamycin, vincristine, and prednisolone) therapy combined with rituximab (R-CHOP) is currently a standard chemotherapy for DLBCL [28]. In our study, no significant difference was noted in the number of patients treated with rituximab between localized nasal/paranasal DLBCL and localized nodal DLBCL patients (p = 0.24). Therefore, the prognosis of localized nasal/paranasal DLBCLs was favorable regardless of treatment with rituximab. In conclusion, the prognosis of localized nasal/paranasal DLBCL patients was good irrespective of the disease subclassification, although the non-GCB- type of DLBCLs are usually thought to be associated with a poor prognosis.
Figure 5. Comparison of overall survival between localized nasal/paranasal DLBCL and localized skin DLBCL.
Figure 6. Comparison of overall survival between localized nasal/paranasal DLBCL and localized adrenal, CNS, and testicular DLBCLs.
Funding Statement
The authors have no support or funding to report.
References
- 1. Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, et al. (2000) Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403: 503–511. [DOI] [PubMed] [Google Scholar]
- 2. Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, et al. (2004) Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 103: 275–282. [DOI] [PubMed] [Google Scholar]
- 3. Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, et al. (2002) The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 346: 1937–1947. [DOI] [PubMed] [Google Scholar]
- 4. Choi WW, Weisenburger DD, Greiner TC, Piris MA, Banham AH, et al. (2009) A new immunostain algorithm classifies diffuse large B-cell lymphoma into molecular subtypes with high accuracy. Clin Cancer Res 15: 5494–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meyer PN, Fu K, Greiner TC, Smith LM, Delabie J, et al. (2011) Immunohistochemical methods for predicting cell of origin and survival in patients with diffuse large B-cell lymphoma treated with rituximab. J Clin Oncol 29: 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sato Y, Onishi N, Morito T, Takata K, Mizobuchi K, et al. (2009) Patients with localized primary non-tonsillar oral diffuse large B-cell lymphoma exhibit favorable prognosis despite a non-germinal center B-cell-like phenotype. Cancer Sci 100: 42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. d’Amore F, Christensen BE, Brincker H, Pedersen NT, Thorling K, et al. (1991) Clinicopathological features and prognostic factors in extranodal non-Hodgkin lymphomas. Danish LYFO Study Group. Eur J Cancer 27: 1201–1208. [DOI] [PubMed] [Google Scholar]
- 8. Krol AD, le Cessie S, Snijder S, Kluin-Nelemans JC, Kluin PM, et al. (2003) Primary extranodal non-Hodgkin’s lymphoma (NHL): the impact of alternative definitions tested in the Comprehensive Cancer Centre West population-based NHL registry. Ann Oncol 14: 131–139. [DOI] [PubMed] [Google Scholar]
- 9. Habara T, Sato Y, Takata K, Iwaki N, Okumura H, et al. (2012) Germinal Center B-Cell-Like versus Non-Germinal Center B-Cell-Like as Important Prognostic Factor for Localized Nodal DLBCL. J Clin Exp Hematop 52: 91–99. [DOI] [PubMed] [Google Scholar]
- 10. Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, et al. (1977) Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer 35: 1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pileri SA, Dirnhofer S, Went P, Ascani S, Sabattini E, et al. (2002) Diffuse large B-cell lymphoma: one or more entities? Present controversies and possible tools for its subclassification. Histopathology 41: 482–509. [DOI] [PubMed] [Google Scholar]
- 12. Hembury TA, Lee B, Gascoyne RD, Macpherson N, Yang B, et al. (2002) Primary cutaneous diffuse large B-cell lymphoma: a clinicopathologic study of 15 cases. Am J Clin Pathol 117: 574–580. [DOI] [PubMed] [Google Scholar]
- 13. Hattab EM, Martin SE, Al-Khatib SM, Kupsky WJ, Vance GH, et al. (2010) Most primary central nervous system diffuse large B-cell lymphomas occurring in immunocompetent individuals belong to the nongerminal center subtype: a retrospective analysis of 31 cases. Mod Pathol 23: 235–243. [DOI] [PubMed] [Google Scholar]
- 14. Li D, Xie P, Mi C (2010) Primary testicular diffuse large B-cell lymphoma shows an activated B-cell-like phenotype. Pathol Res Pract 206: 611–615. [DOI] [PubMed] [Google Scholar]
- 15. Mozos A, Ye H, Chuang WY, Chu JS, Huang WT, et al. (2009) Most primary adrenal lymphomas are diffuse large B-cell lymphomas with non-germinal center B-cell phenotype, BCL6 gene rearrangement and poor prognosis. Mod Pathol 22: 1210–1217. [DOI] [PubMed] [Google Scholar]
- 16. Camilleri -Broët S, Crinière E, Broët P, Delwail V, Mokhtari K, et al. (2006) A uniform activated B-cell-like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas: analysis of 83 cases. Blood 107: 190–196. [DOI] [PubMed] [Google Scholar]
- 17. Yoshida S, Nakamura N, Sasaki Y, Yoshida S, Yasuda M, et al. (2005) Primary breast diffuse large B-cell lymphoma shows a non-germinal center B-cell phenotype. Mod Pathol 18: 398–405. [DOI] [PubMed] [Google Scholar]
- 18. Al-Abbadi MA, Hattab EM, Tarawneh MS, Amr SS, Orazi A, et al. (2006) Primary testicular diffuse large B-cell lymphoma belongs to the nongerminal center B-cell-like subgroup: A study of 18 cases. Mod Pathol 19: 1521–1527. [DOI] [PubMed] [Google Scholar]
- 19. Murase T, Yamaguchi M, Suzuki R, Okamoto M, Sato Y, et al. (2007) Intravascular large B-cell lymphoma (IVLBCL): a clinicopathologic study of 96 cases with special reference to the immunophenotypic heterogeneity of CD5. Blood 109: 478–485. [DOI] [PubMed] [Google Scholar]
- 20. Connor J, Ashton-Key M (2007) Gastric and intestinal diffuse large B-cell lymphomas are clinically and immunophenotypically different. An immunohistochemical and clinical study. Histopathology 51: 697–703. [DOI] [PubMed] [Google Scholar]
- 21. Campo E, Chott A, Kinney MC, Leoncini L, Meijer CJ, et al. (2006) Update on extranodal lymphomas. Conclusions of the Workshop held by the EAHP and the SH in Thessaloniki, Greece. Histopathology 48: 481–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zucca E, Conconi A, Mughal TI, Sarris AH, Seymour JF, et al. (2003) Patterns of outcome and prognostic factors in primary large-cell lymphoma of the testis in a survey by the International Extranodal Lymphoma Study Group. J Clin Oncol 21: 20–27. [DOI] [PubMed] [Google Scholar]
- 23. Hoefnagel JJ, Dijkman R, Basso K, Jansen PM, Hallermann C, et al. (2005) Distinct types of primary cutaneous large B-cell lymphoma identified by gene expression profiling. Blood 105: 3671–3678. [DOI] [PubMed] [Google Scholar]
- 24. Vural F, Saydam G, Cagirgan S, Ertekin B, Hekimgil M, et al. (2008) Primary cutaneous B-cell lymphoma: report of eight cases and review of the literature. Int J Dermatol 47: 675–680. [DOI] [PubMed] [Google Scholar]
- 25. Deckert M, Engert A, Brück W, Ferreri AJ, Finke J, et al. (2011) Modern concepts in the biology, diagnosis, differential diagnosis and treatment of primary central nervous system lymphoma. Leukemia 25: 1797–1807. [DOI] [PubMed] [Google Scholar]
- 26. Steidl C, Gascoyne RD (2011) The molecular pathogenesis of primary mediastinal large B-cell lymphoma. Blood 118: 2659–2669. [DOI] [PubMed] [Google Scholar]
- 27. Møller MB, Pedersen NT, Christensen BE (2004) Diffuse large B-cell lymphoma: clinical implications of extranodal versus nodal presentation–a population-based study of 1575 cases. Br J Haematol 124: 151–159. [DOI] [PubMed] [Google Scholar]
- 28. Yamaguchi H, Hirakawa T, Inokuchi K (2011) Importance of relative dose intensity in chemotherapy for diffuse large B-cell lymphoma. J Clin Exp Hematop 51: 1–5. [DOI] [PubMed] [Google Scholar]