Abstract
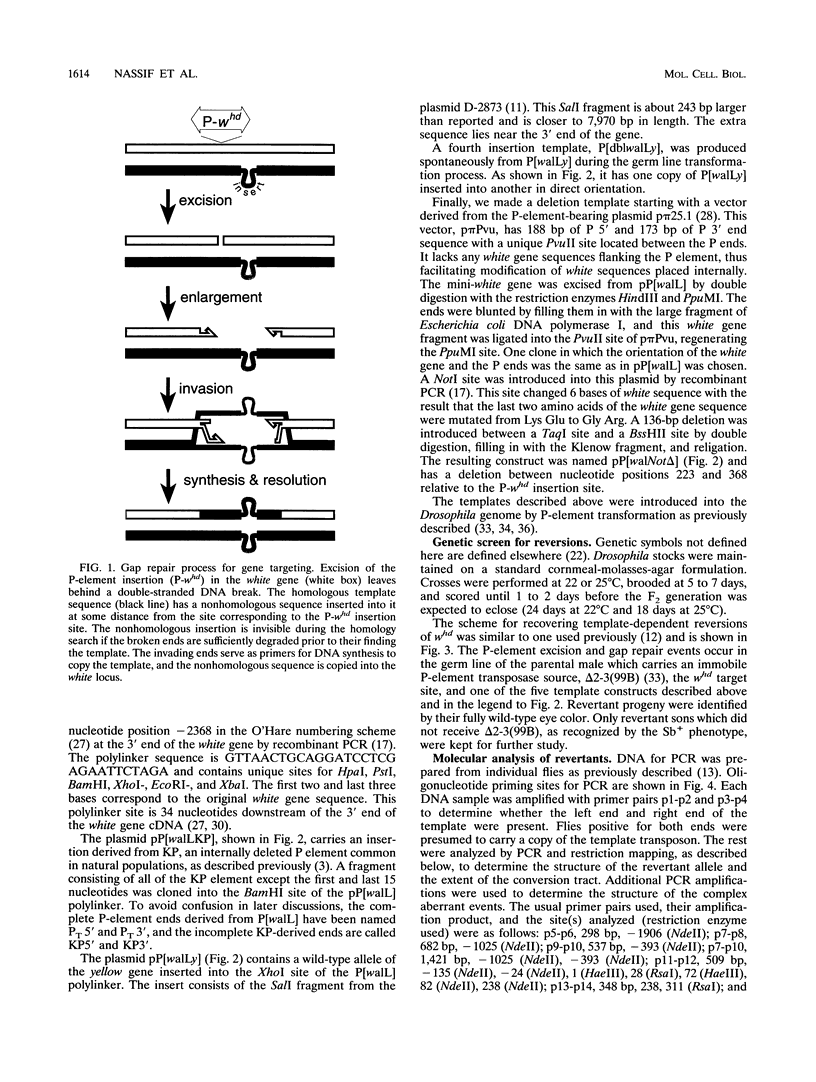
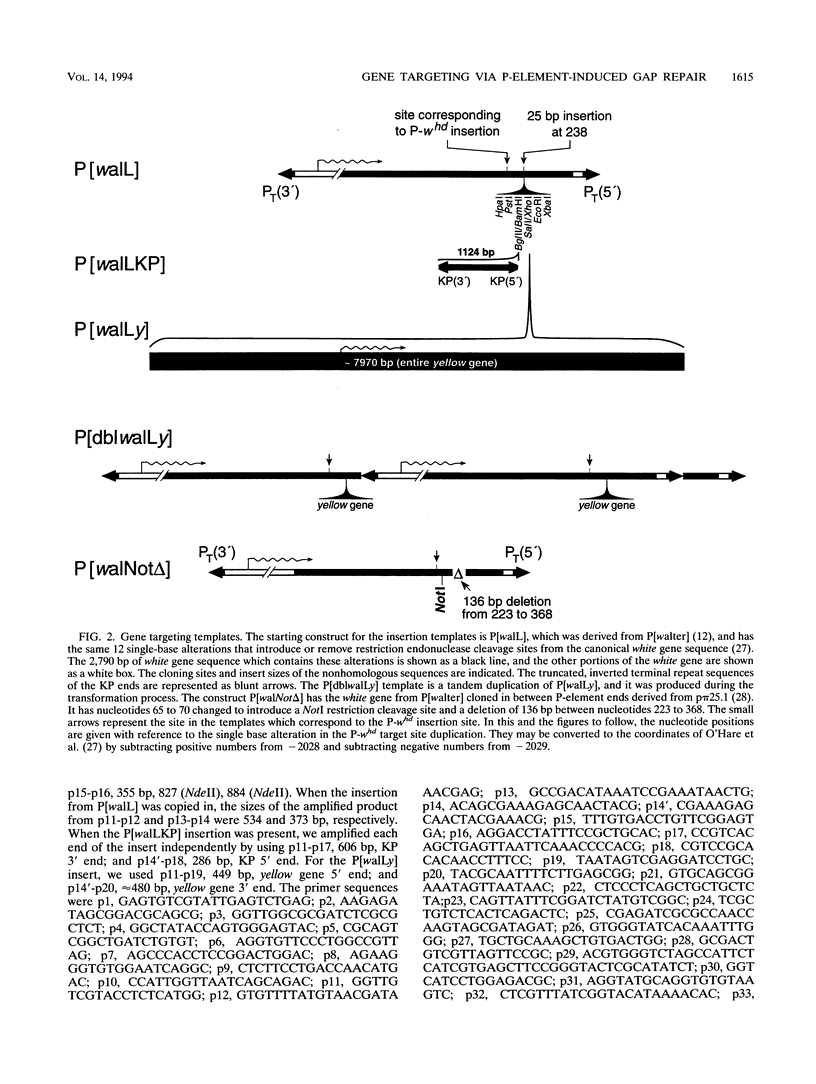
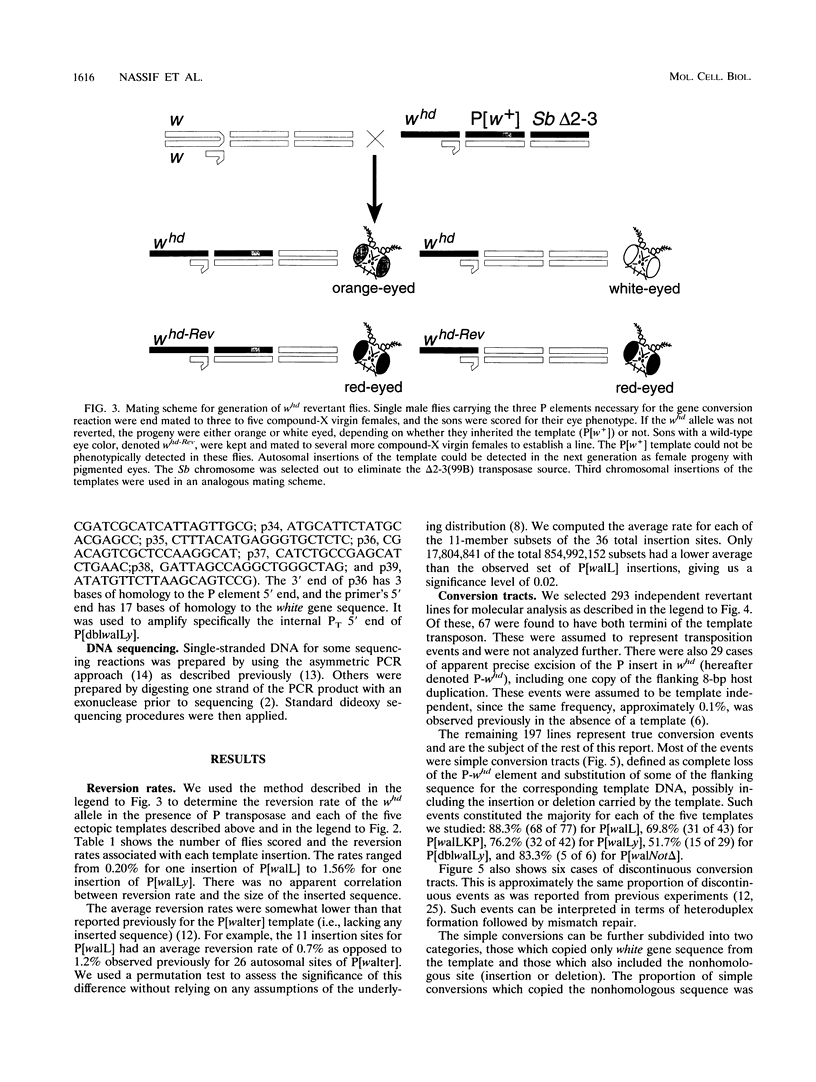
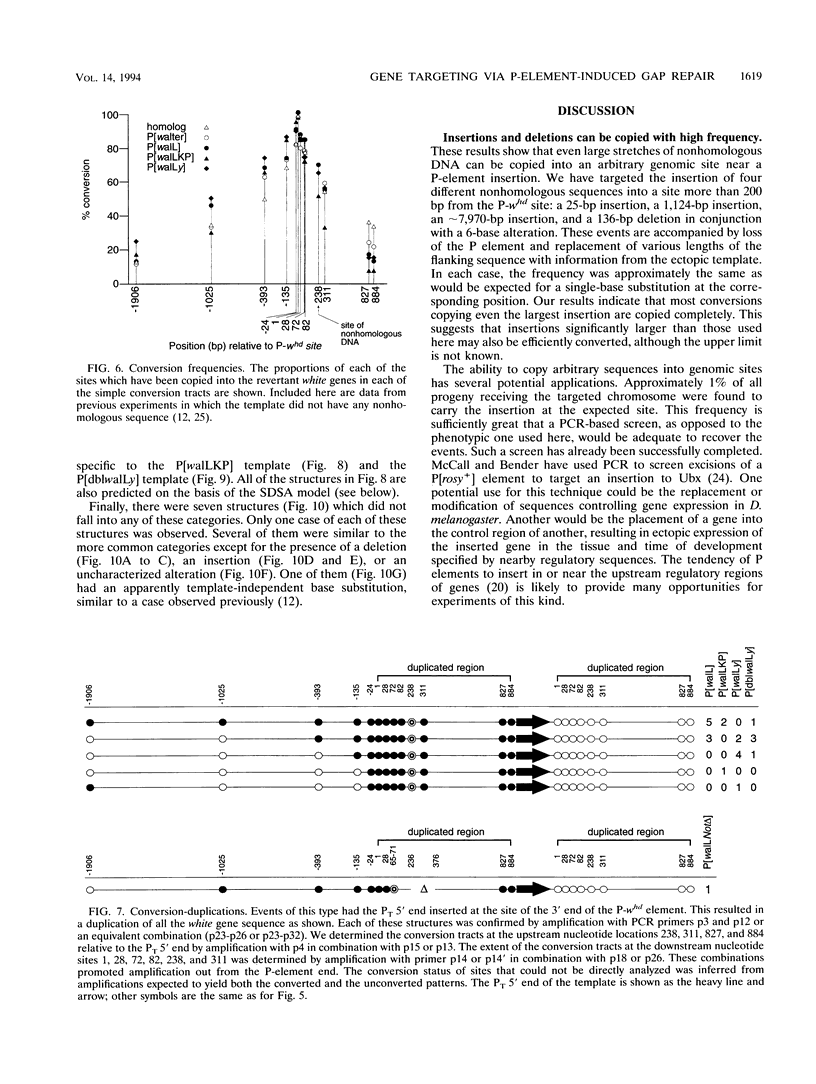
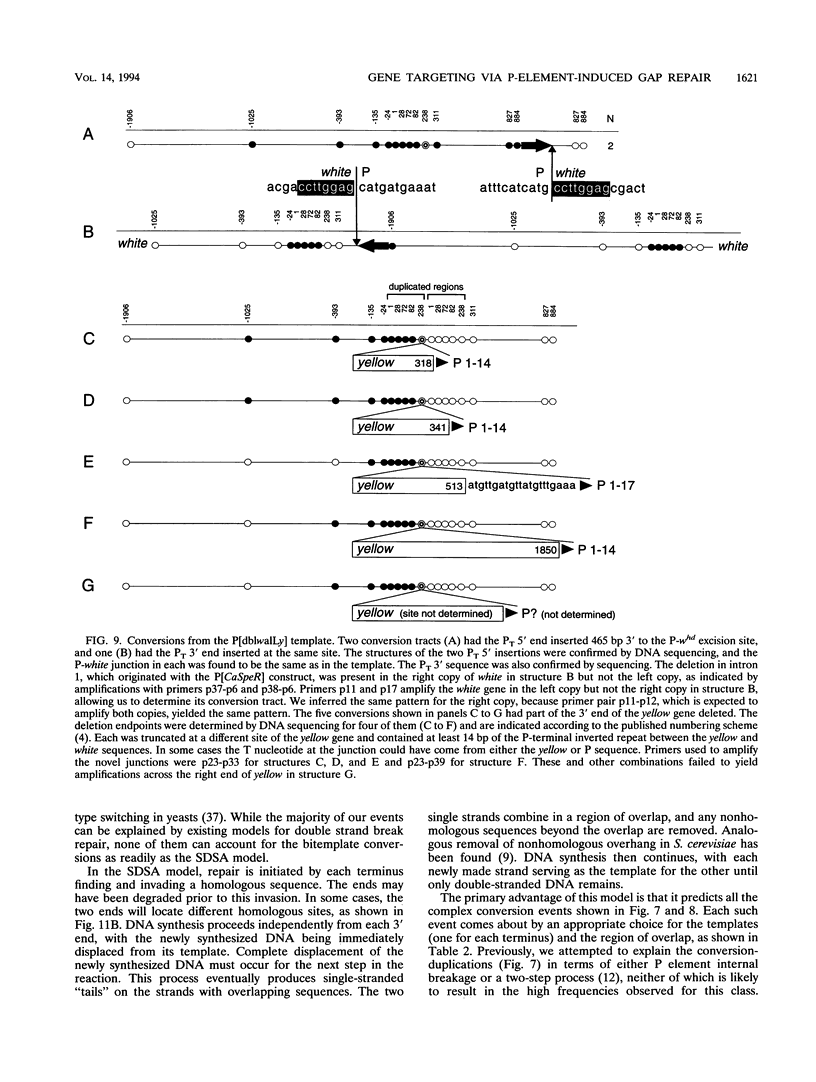
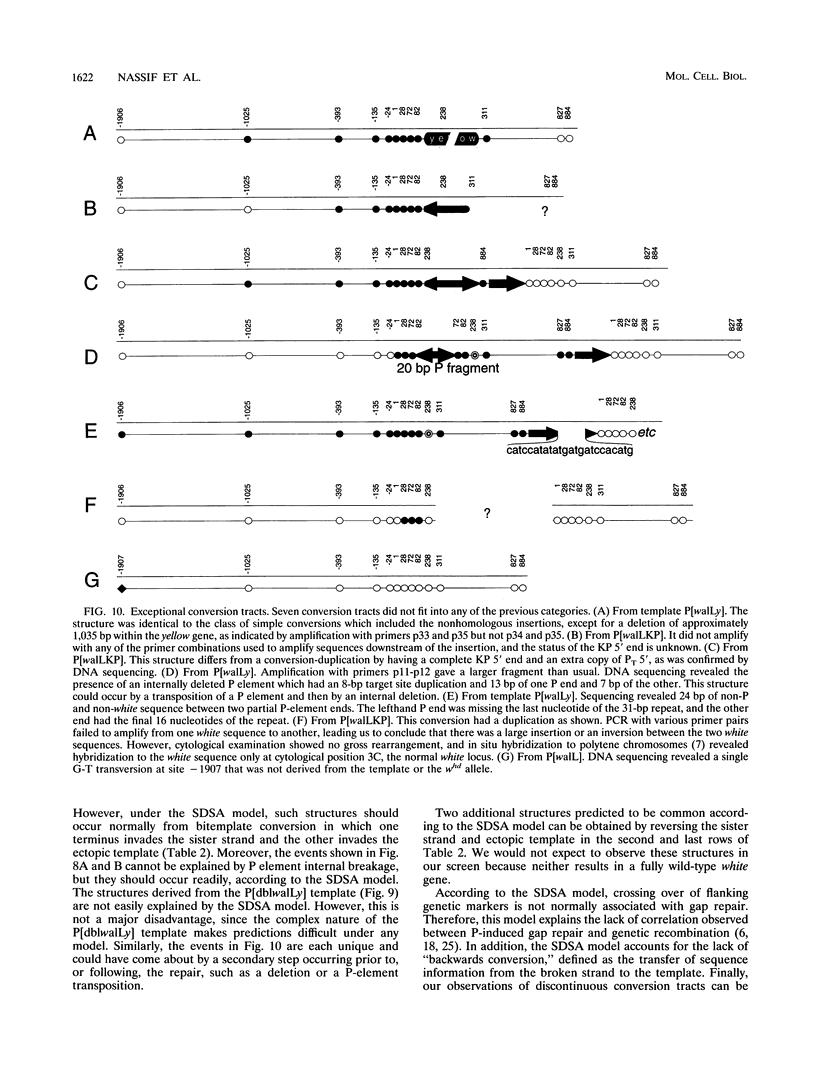
P-element-induced gap repair was used to copy nonhomologous DNA into the Drosophila white locus. We found that nearly 8,000 bp of nonhomologous sequence could be copied from an ectopic template at essentially the same rate as a single-base substitution at the same location. An in vitro-constructed deletion was also copied into white at high frequencies. This procedure can be applied to the study of gene expression in Drosophila melanogaster, especially for genes too large to be manipulated in other ways. We also observed several types of more complex events in which the copied template sequences were rearranged such that the breakpoints occurred at direct duplications. Most of these can be explained by a model of double strand break repair in which each terminus of the break invades a template independently and serves as a primer for DNA synthesis from it, yielding two overlapping single-stranded sequences. These single strands then pair, and synthesis is completed by each using the other as a template. This synthesis-dependent strand annealing (SDSA) model as a possible general mechanism in complex organisms is discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banga S. S., Boyd J. B. Oligonucleotide-directed site-specific mutagenesis in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1735–1739. doi: 10.1073/pnas.89.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastia D., Germino J., Crosa J. H., Ram J. The nucleotide sequence surrounding the replication terminus of R6K. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2095–2099. doi: 10.1073/pnas.78.4.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black D. M., Jackson M. S., Kidwell M. G., Dover G. A. KP elements repress P-induced hybrid dysgenesis in Drosophila melanogaster. EMBO J. 1987 Dec 20;6(13):4125–4135. doi: 10.1002/j.1460-2075.1987.tb02758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia W., Howes G., Martin M., Meng Y. B., Moses K., Tsubota S. Molecular analysis of the yellow locus of Drosophila. EMBO J. 1986 Dec 20;5(13):3597–3605. doi: 10.1002/j.1460-2075.1986.tb04688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels W. R., Johnson-Schlitz D. M., Eggleston W. B., Sved J. High-frequency P element loss in Drosophila is homolog dependent. Cell. 1990 Aug 10;62(3):515–525. doi: 10.1016/0092-8674(90)90016-8. [DOI] [PubMed] [Google Scholar]
- Fishman-Lobell J., Haber J. E. Removal of nonhomologous DNA ends in double-strand break recombination: the role of the yeast ultraviolet repair gene RAD1. Science. 1992 Oct 16;258(5081):480–484. doi: 10.1126/science.1411547. [DOI] [PubMed] [Google Scholar]
- Formosa T., Alberts B. M. DNA synthesis dependent on genetic recombination: characterization of a reaction catalyzed by purified bacteriophage T4 proteins. Cell. 1986 Dec 5;47(5):793–806. doi: 10.1016/0092-8674(86)90522-2. [DOI] [PubMed] [Google Scholar]
- Geyer P. K., Corces V. G. Separate regulatory elements are responsible for the complex pattern of tissue-specific and developmental transcription of the yellow locus in Drosophila melanogaster. Genes Dev. 1987 Nov;1(9):996–1004. doi: 10.1101/gad.1.9.996. [DOI] [PubMed] [Google Scholar]
- Gloor G. B., Nassif N. A., Johnson-Schlitz D. M., Preston C. R., Engels W. R. Targeted gene replacement in Drosophila via P element-induced gap repair. Science. 1991 Sep 6;253(5024):1110–1117. doi: 10.1126/science.1653452. [DOI] [PubMed] [Google Scholar]
- Gloor G. B., Preston C. R., Johnson-Schlitz D. M., Nassif N. A., Phillis R. W., Benz W. K., Robertson H. M., Engels W. R. Type I repressors of P element mobility. Genetics. 1993 Sep;135(1):81–95. doi: 10.1093/genetics/135.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings P. J. Recombination in the eukaryotic nucleus. Bioessays. 1988 Aug-Sep;9(2-3):61–64. doi: 10.1002/bies.950090206. [DOI] [PubMed] [Google Scholar]
- Heslip T. R., Williams J. A., Bell J. B., Hodgetts R. B. A P element chimera containing captured genomic sequences was recovered at the vestigial locus in Drosophila following targeted transposition. Genetics. 1992 Aug;131(4):917–927. doi: 10.1093/genetics/131.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Schlitz D. M., Engels W. R. P-element-induced interallelic gene conversion of insertions and deletions in Drosophila melanogaster. Mol Cell Biol. 1993 Nov;13(11):7006–7018. doi: 10.1128/mcb.13.11.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman P. D., Rio D. C. P element transposition in vitro proceeds by a cut-and-paste mechanism and uses GTP as a cofactor. Cell. 1992 Apr 3;69(1):27–39. doi: 10.1016/0092-8674(92)90116-t. [DOI] [PubMed] [Google Scholar]
- Kelley M. R., Kidd S., Berg R. L., Young M. W. Restriction of P-element insertions at the Notch locus of Drosophila melanogaster. Mol Cell Biol. 1987 Apr;7(4):1545–1548. doi: 10.1128/mcb.7.4.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. L., Sperle K., Sternberg N. Model for homologous recombination during transfer of DNA into mouse L cells: role for DNA ends in the recombination process. Mol Cell Biol. 1984 Jun;4(6):1020–1034. doi: 10.1128/mcb.4.6.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassif N., Engels W. DNA homology requirements for mitotic gap repair in Drosophila. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1262–1266. doi: 10.1073/pnas.90.4.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare K., Driver A., McGrath S., Johnson-Schiltz D. M. Distribution and structure of cloned P elements from the Drosophila melanogaster P strain pi 2. Genet Res. 1992 Aug;60(1):33–41. doi: 10.1017/s0016672300030640. [DOI] [PubMed] [Google Scholar]
- O'Hare K., Murphy C., Levis R., Rubin G. M. DNA sequence of the white locus of Drosophila melanogaster. J Mol Biol. 1984 Dec 15;180(3):437–455. doi: 10.1016/0022-2836(84)90021-4. [DOI] [PubMed] [Google Scholar]
- O'Hare K., Rubin G. M. Structures of P transposable elements and their sites of insertion and excision in the Drosophila melanogaster genome. Cell. 1983 Aug;34(1):25–35. doi: 10.1016/0092-8674(83)90133-2. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W. Fungal recombination. Microbiol Rev. 1985 Mar;49(1):33–58. doi: 10.1128/mr.49.1.33-58.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepling M., Mount S. M. Sequence of a cDNA from the Drosophila melanogaster white gene. Nucleic Acids Res. 1990 Mar 25;18(6):1633–1633. doi: 10.1093/nar/18.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirrotta V. Vectors for P-mediated transformation in Drosophila. Biotechnology. 1988;10:437–456. doi: 10.1016/b978-0-409-90042-2.50028-3. [DOI] [PubMed] [Google Scholar]
- Resnick M. A. The repair of double-strand breaks in DNA; a model involving recombination. J Theor Biol. 1976 Jun;59(1):97–106. doi: 10.1016/s0022-5193(76)80025-2. [DOI] [PubMed] [Google Scholar]
- Robertson H. M., Preston C. R., Phillis R. W., Johnson-Schlitz D. M., Benz W. K., Engels W. R. A stable genomic source of P element transposase in Drosophila melanogaster. Genetics. 1988 Mar;118(3):461–470. doi: 10.1093/genetics/118.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982 Oct 22;218(4570):341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Sugawara N., Haber J. E. Characterization of double-strand break-induced recombination: homology requirements and single-stranded DNA formation. Mol Cell Biol. 1992 Feb;12(2):563–575. doi: 10.1128/mcb.12.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak J. W., Orr-Weaver T. L., Rothstein R. J., Stahl F. W. The double-strand-break repair model for recombination. Cell. 1983 May;33(1):25–35. doi: 10.1016/0092-8674(83)90331-8. [DOI] [PubMed] [Google Scholar]
- Takasu-Ishikawa E., Yoshihara M., Hotta Y. Extra sequences found at P element excision sites in Drosophila melanogaster. Mol Gen Genet. 1992 Mar;232(1):17–23. doi: 10.1007/BF00299132. [DOI] [PubMed] [Google Scholar]
- Tsubota S. I., Huong D. V. Capture of flanking DNA by a P element in Drosophila melanogaster: creation of a transposable element. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):693–697. doi: 10.1073/pnas.88.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubota S., Schedl P. Hybrid dysgenesis-induced revertants of insertions at the 5' end of the rudimentary gene in Drosophila melanogaster: transposon-induced control mutations. Genetics. 1986 Sep;114(1):165–182. doi: 10.1093/genetics/114.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]



