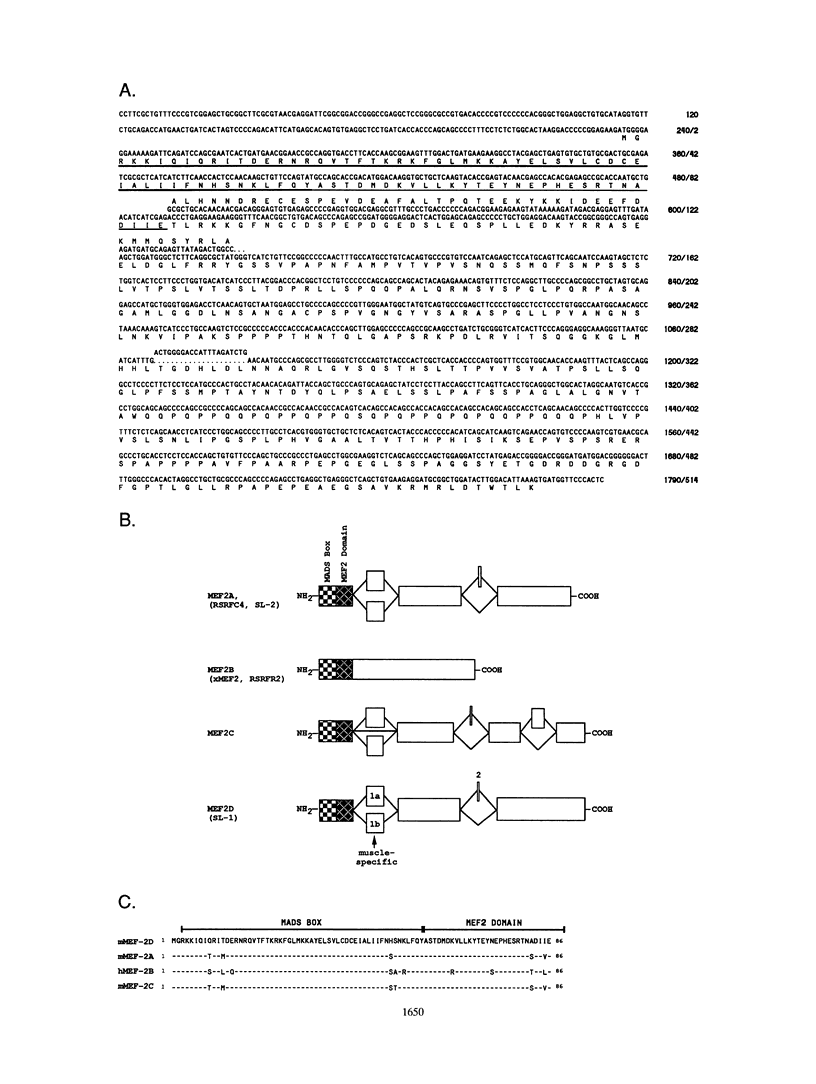
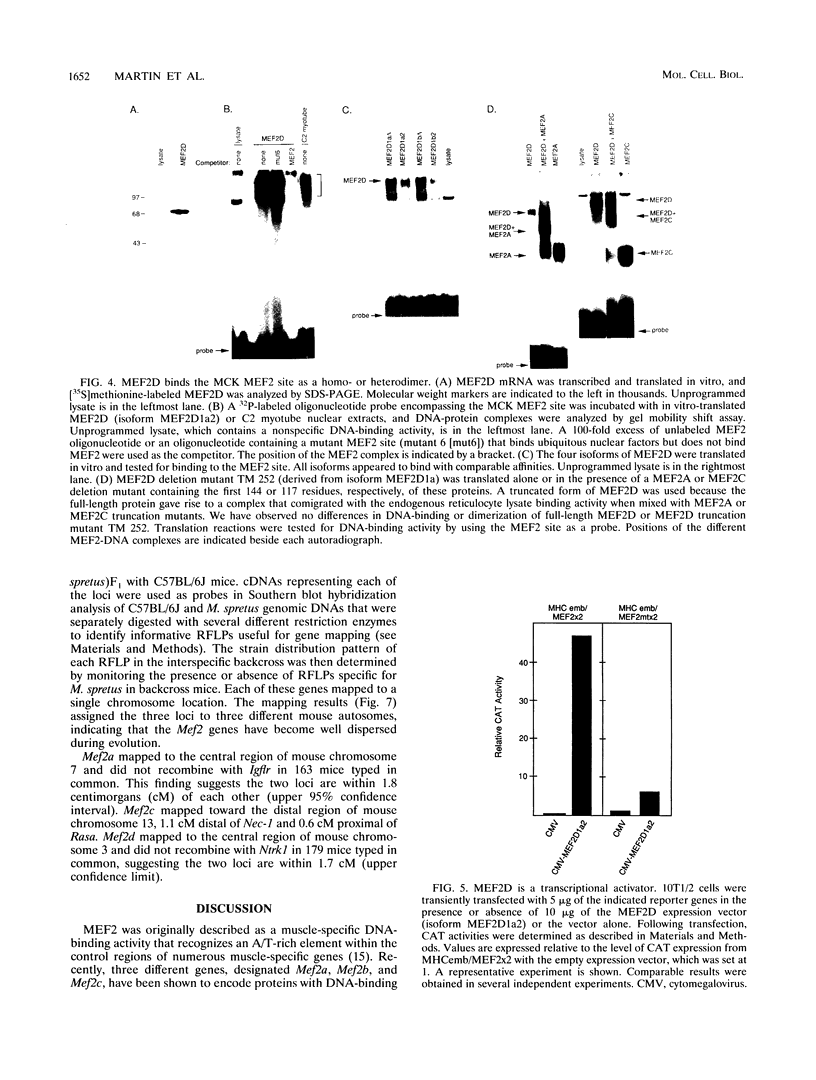
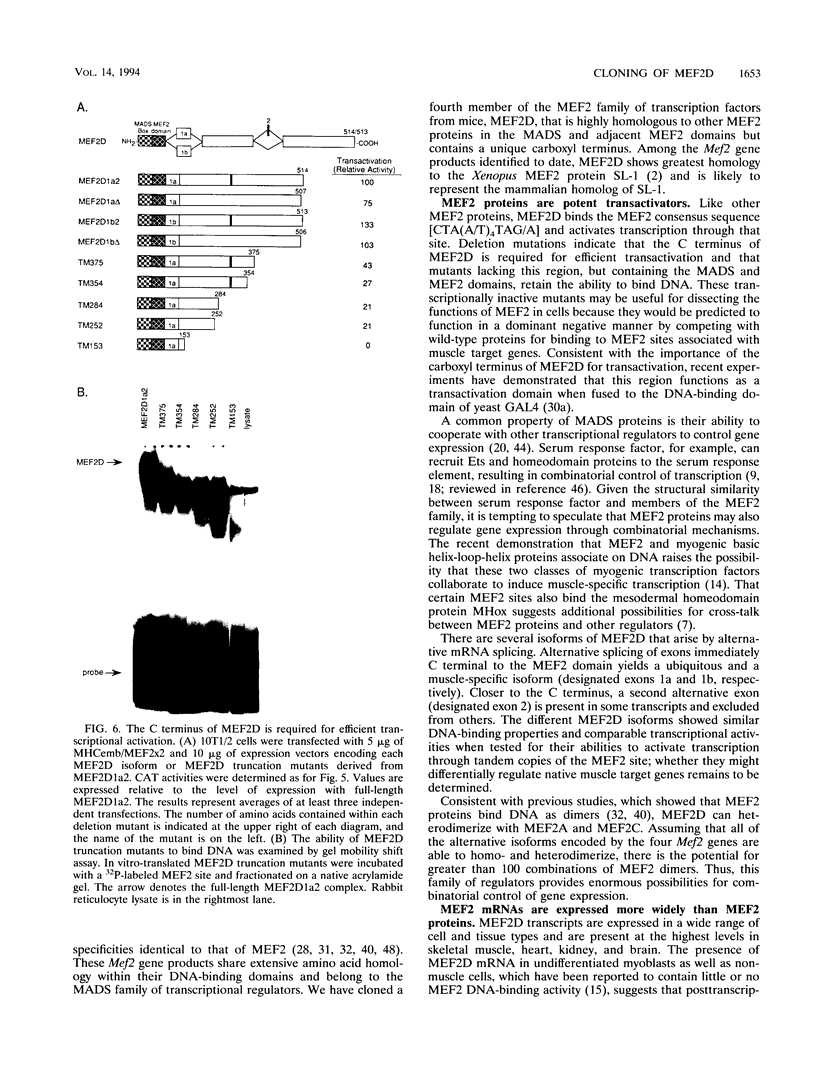
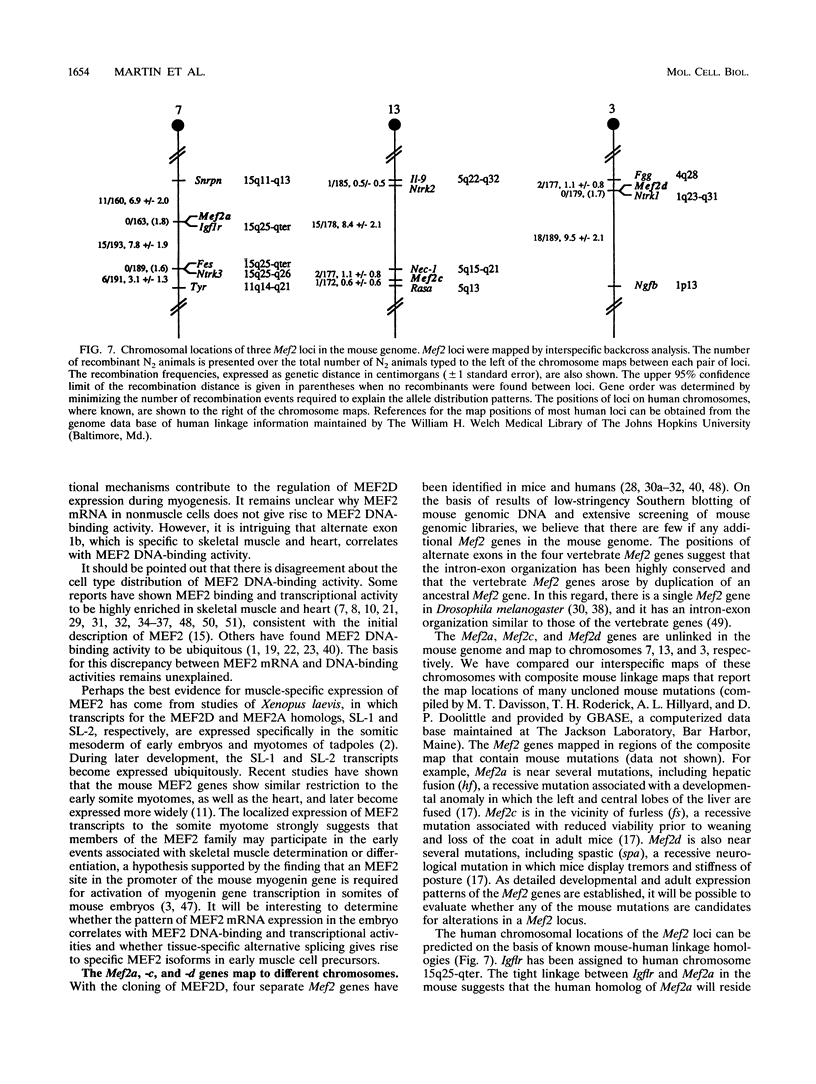
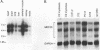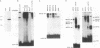Abstract
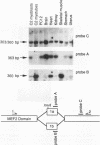
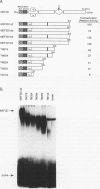
Members of the myocyte-specific enhancer-binding factor 2 (MEF2) family of transcription factors bind a conserved A/T-rich sequence in the control regions of numerous muscle-specific genes. Mammalian MEF2 proteins have been shown previously to be encoded by three genes, Mef2, xMef2, and Mef2c, each of which gives rise to multiple alternatively spliced transcripts. We describe the cloning of a new member of the MEF2 family from mice, termed MEF2D, which shares extensive homology with other MEF2 proteins but is the product of a separate gene. MEF2D binds to and activates transcription through the MEF2 site and forms heterodimers with other members of the MEF2 family. Deletion mutations show that the carboxyl terminus of MEF2D is required for efficient transactivation. MEF2D transcripts are widely expressed, but alternative splicing of MEF2D transcripts gives rise to a muscle-specific isoform which is induced during myoblast differentiation. The mouse Mef2, Mef2c, and Mef2d genes map to chromosomes 7, 13, and 3, respectively. The complexity of the MEF2 family of regulatory proteins provides the potential for fine-tuning of transcriptional responses as a consequence of combinatorial interactions among multiple MEF2 isoforms encoded by the four Mef2 genes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun T., Tannich E., Buschhausen-Denker G., Arnold H. H. Promoter upstream elements of the chicken cardiac myosin light-chain 2-A gene interact with trans-acting regulatory factors for muscle-specific transcription. Mol Cell Biol. 1989 Jun;9(6):2513–2525. doi: 10.1128/mcb.9.6.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbart R. E., Liang C. S., Smoot L. B., Laheru D. A., Mahdavi V., Nadal-Ginard B. A fourth human MEF2 transcription factor, hMEF2D, is an early marker of the myogenic lineage. Development. 1993 Aug;118(4):1095–1106. doi: 10.1242/dev.118.4.1095. [DOI] [PubMed] [Google Scholar]
- Chambers A. E., Kotecha S., Towers N., Mohun T. J. Muscle-specific expression of SRF-related genes in the early embryo of Xenopus laevis. EMBO J. 1992 Dec;11(13):4981–4991. doi: 10.1002/j.1460-2075.1992.tb05605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T. C., Wallace M. C., Merlie J. P., Olson E. N. Separable regulatory elements governing myogenin transcription in mouse embryogenesis. Science. 1993 Jul 9;261(5118):215–218. doi: 10.1126/science.8392225. [DOI] [PubMed] [Google Scholar]
- Copeland N. G., Gilbert D. J., Chrétien M., Seidah N. G., Jenkins N. A. Regional localization of three convertases, PC1 (Nec-1), PC2 (Nec-2), and furin (Fur), on mouse chromosomes. Genomics. 1992 Aug;13(4):1356–1358. doi: 10.1016/0888-7543(92)90069-5. [DOI] [PubMed] [Google Scholar]
- Copeland N. G., Jenkins N. A. Development and applications of a molecular genetic linkage map of the mouse genome. Trends Genet. 1991 Apr;7(4):113–118. doi: 10.1016/0168-9525(91)90455-y. [DOI] [PubMed] [Google Scholar]
- Courey A. J., Tjian R. Analysis of Sp1 in vivo reveals multiple transcriptional domains, including a novel glutamine-rich activation motif. Cell. 1988 Dec 2;55(5):887–898. doi: 10.1016/0092-8674(88)90144-4. [DOI] [PubMed] [Google Scholar]
- Cserjesi P., Lilly B., Bryson L., Wang Y., Sassoon D. A., Olson E. N. MHox: a mesodermally restricted homeodomain protein that binds an essential site in the muscle creatine kinase enhancer. Development. 1992 Aug;115(4):1087–1101. doi: 10.1242/dev.115.4.1087. [DOI] [PubMed] [Google Scholar]
- Cserjesi P., Olson E. N. Myogenin induces the myocyte-specific enhancer binding factor MEF-2 independently of other muscle-specific gene products. Mol Cell Biol. 1991 Oct;11(10):4854–4862. doi: 10.1128/mcb.11.10.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton S., Treisman R. Characterization of SAP-1, a protein recruited by serum response factor to the c-fos serum response element. Cell. 1992 Feb 7;68(3):597–612. doi: 10.1016/0092-8674(92)90194-h. [DOI] [PubMed] [Google Scholar]
- Edmondson D. G., Cheng T. C., Cserjesi P., Chakraborty T., Olson E. N. Analysis of the myogenin promoter reveals an indirect pathway for positive autoregulation mediated by the muscle-specific enhancer factor MEF-2. Mol Cell Biol. 1992 Sep;12(9):3665–3677. doi: 10.1128/mcb.12.9.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D. G., Olson E. N. A gene with homology to the myc similarity region of MyoD1 is expressed during myogenesis and is sufficient to activate the muscle differentiation program. Genes Dev. 1989 May;3(5):628–640. doi: 10.1101/gad.3.5.628. [DOI] [PubMed] [Google Scholar]
- Friedman K. D., Rosen N. L., Newman P. J., Montgomery R. R. Enzymatic amplification of specific cDNA inserts from lambda gt11 libraries. Nucleic Acids Res. 1988 Sep 12;16(17):8718–8718. doi: 10.1093/nar/16.17.8718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk W. D., Wright W. E. Cyclic amplification and selection of targets for multicomponent complexes: myogenin interacts with factors recognizing binding sites for basic helix-loop-helix, nuclear factor 1, myocyte-specific enhancer-binding factor 2, and COMP1 factor. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9484–9488. doi: 10.1073/pnas.89.20.9484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossett L. A., Kelvin D. J., Sternberg E. A., Olson E. N. A new myocyte-specific enhancer-binding factor that recognizes a conserved element associated with multiple muscle-specific genes. Mol Cell Biol. 1989 Nov;9(11):5022–5033. doi: 10.1128/mcb.9.11.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueneberg D. A., Natesan S., Alexandre C., Gilman M. Z. Human and Drosophila homeodomain proteins that enhance the DNA-binding activity of serum response factor. Science. 1992 Aug 21;257(5073):1089–1095. doi: 10.1126/science.257.5073.1089. [DOI] [PubMed] [Google Scholar]
- Han T. H., Lamph W. W., Prywes R. Mapping of epidermal growth factor-, serum-, and phorbol ester-responsive sequence elements in the c-jun promoter. Mol Cell Biol. 1992 Oct;12(10):4472–4477. doi: 10.1128/mcb.12.10.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz I. A regulatory hierarchy for cell specialization in yeast. Nature. 1989 Dec 14;342(6251):749–757. doi: 10.1038/342749a0. [DOI] [PubMed] [Google Scholar]
- Hidaka K., Yamamoto I., Arai Y., Mukai T. The MEF-3 motif is required for MEF-2-mediated skeletal muscle-specific induction of the rat aldolase A gene. Mol Cell Biol. 1993 Oct;13(10):6469–6478. doi: 10.1128/mcb.13.10.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson G. M., Mitchell M. T., Molloy G. R., Pearson M. L., Benfield P. A. Identification of a novel TA-rich DNA binding protein that recognizes a TATA sequence within the brain creatine kinase promoter. Nucleic Acids Res. 1988 Sep 26;16(18):8925–8944. doi: 10.1093/nar/16.18.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horlick R. A., Hobson G. M., Patterson J. H., Mitchell M. T., Benfield P. A. Brain and muscle creatine kinase genes contain common TA-rich recognition protein-binding regulatory elements. Mol Cell Biol. 1990 Sep;10(9):4826–4836. doi: 10.1128/mcb.10.9.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannello R. C., Mar J. H., Ordahl C. P. Characterization of a promoter element required for transcription in myocardial cells. J Biol Chem. 1991 Feb 15;266(5):3309–3316. [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Organization, distribution, and stability of endogenous ecotropic murine leukemia virus DNA sequences in chromosomes of Mus musculus. J Virol. 1982 Jul;43(1):26–36. doi: 10.1128/jvi.43.1.26-36.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp B. E., Pearson R. B. Protein kinase recognition sequence motifs. Trends Biochem Sci. 1990 Sep;15(9):342–346. doi: 10.1016/0968-0004(90)90073-k. [DOI] [PubMed] [Google Scholar]
- Leff S. E., Brannan C. I., Reed M. L., Ozçelik T., Francke U., Copeland N. G., Jenkins N. A. Maternal imprinting of the mouse Snrpn gene and conserved linkage homology with the human Prader-Willi syndrome region. Nat Genet. 1992 Dec;2(4):259–264. doi: 10.1038/ng1292-259. [DOI] [PubMed] [Google Scholar]
- Li Z., Paulin D. Different factors interact with myoblast-specific and myotube-specific enhancer regions of the human desmin gene. J Biol Chem. 1993 May 15;268(14):10403–10415. [PubMed] [Google Scholar]
- Martin J. F., Schwarz J. J., Olson E. N. Myocyte enhancer factor (MEF) 2C: a tissue-restricted member of the MEF-2 family of transcription factors. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5282–5286. doi: 10.1073/pnas.90.11.5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott J. C., Cardoso M. C., Yu Y. T., Andres V., Leifer D., Krainc D., Lipton S. A., Nadal-Ginard B. hMEF2C gene encodes skeletal muscle- and brain-specific transcription factors. Mol Cell Biol. 1993 Apr;13(4):2564–2577. doi: 10.1128/mcb.13.4.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermod N., O'Neill E. A., Kelly T. J., Tjian R. The proline-rich transcriptional activator of CTF/NF-I is distinct from the replication and DNA binding domain. Cell. 1989 Aug 25;58(4):741–753. doi: 10.1016/0092-8674(89)90108-6. [DOI] [PubMed] [Google Scholar]
- Molkentin J. D., Markham B. E. Myocyte-specific enhancer-binding factor (MEF-2) regulates alpha-cardiac myosin heavy chain gene expression in vitro and in vivo. J Biol Chem. 1993 Sep 15;268(26):19512–19520. [PubMed] [Google Scholar]
- Muscat G. E., Perry S., Prentice H., Kedes L. The human skeletal alpha-actin gene is regulated by a muscle-specific enhancer that binds three nuclear factors. Gene Expr. 1992;2(2):111–126. [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji Y., Hidaka K., Tsujino S., Yamamoto Y., Mukai T., Yanagihara T., Kishimoto T., Sakoda S. A single MEF-2 site is a major positive regulatory element required for transcription of the muscle-specific subunit of the human phosphoglycerate mutase gene in skeletal and cardiac muscle cells. Mol Cell Biol. 1992 Oct;12(10):4384–4390. doi: 10.1128/mcb.12.10.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navankasattusas S., Zhu H., Garcia A. V., Evans S. M., Chien K. R. A ubiquitous factor (HF-1a) and a distinct muscle factor (HF-1b/MEF-2) form an E-box-independent pathway for cardiac muscle gene expression. Mol Cell Biol. 1992 Apr;12(4):1469–1479. doi: 10.1128/mcb.12.4.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmacek M. S., Bengur A. R., Vora A. J., Leiden J. M. The structure and regulation of expression of the murine fast skeletal troponin C gene. Identification of a developmentally regulated, muscle-specific transcriptional enhancer. J Biol Chem. 1990 Sep 15;265(26):15970–15976. [PubMed] [Google Scholar]
- Pollock R., Treisman R. Human SRF-related proteins: DNA-binding properties and potential regulatory targets. Genes Dev. 1991 Dec;5(12A):2327–2341. doi: 10.1101/gad.5.12a.2327. [DOI] [PubMed] [Google Scholar]
- Schwarz-Sommer Z., Huijser P., Nacken W., Saedler H., Sommer H. Genetic Control of Flower Development by Homeotic Genes in Antirrhinum majus. Science. 1990 Nov 16;250(4983):931–936. doi: 10.1126/science.250.4983.931. [DOI] [PubMed] [Google Scholar]
- Schwarz J. J., Chakraborty T., Martin J., Zhou J. M., Olson E. N. The basic region of myogenin cooperates with two transcription activation domains to induce muscle-specific transcription. Mol Cell Biol. 1992 Jan;12(1):266–275. doi: 10.1128/mcb.12.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. L., Johnson A. D. A molecular mechanism for combinatorial control in yeast: MCM1 protein sets the spacing and orientation of the homeodomains of an alpha 2 dimer. Cell. 1992 Jan 10;68(1):133–142. doi: 10.1016/0092-8674(92)90212-u. [DOI] [PubMed] [Google Scholar]
- Tessarollo L., Tsoulfas P., Martin-Zanca D., Gilbert D. J., Jenkins N. A., Copeland N. G., Parada L. F. trkC, a receptor for neurotrophin-3, is widely expressed in the developing nervous system and in non-neuronal tissues. Development. 1993 Jun;118(2):463–475. doi: 10.1242/dev.118.2.463. [DOI] [PubMed] [Google Scholar]
- Treisman R. The serum response element. Trends Biochem Sci. 1992 Oct;17(10):423–426. doi: 10.1016/0968-0004(92)90013-y. [DOI] [PubMed] [Google Scholar]
- Yee S. P., Rigby P. W. The regulation of myogenin gene expression during the embryonic development of the mouse. Genes Dev. 1993 Jul;7(7A):1277–1289. doi: 10.1101/gad.7.7a.1277. [DOI] [PubMed] [Google Scholar]
- Yu Y. T., Breitbart R. E., Smoot L. B., Lee Y., Mahdavi V., Nadal-Ginard B. Human myocyte-specific enhancer factor 2 comprises a group of tissue-restricted MADS box transcription factors. Genes Dev. 1992 Sep;6(9):1783–1798. doi: 10.1101/gad.6.9.1783. [DOI] [PubMed] [Google Scholar]
- Zhou M. D., Goswami S. K., Martin M. E., Siddiqui M. A. A new serum-responsive, cardiac tissue-specific transcription factor that recognizes the MEF-2 site in the myosin light chain-2 promoter. Mol Cell Biol. 1993 Feb;13(2):1222–1231. doi: 10.1128/mcb.13.2.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Garcia A. V., Ross R. S., Evans S. M., Chien K. R. A conserved 28-base-pair element (HF-1) in the rat cardiac myosin light-chain-2 gene confers cardiac-specific and alpha-adrenergic-inducible expression in cultured neonatal rat myocardial cells. Mol Cell Biol. 1991 Apr;11(4):2273–2281. doi: 10.1128/mcb.11.4.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]