Abstract
Cyclospora spp. have been identified as one of the most important intestinal pathogens causing protracted diarrhea in animals and human beings. To determine the Cyclospora species in the non-human primate Rhinopithecus roxellanae, a total of 71 fecal samples from 19 endangered snub-nosed monkeys in Shaanxi province were collected and examined using Sheater’s sugar flotation technique and by sequencing the fragments of 18S rDNA. Only two Cyclospora isolates from 2 golden snub-nosed monkeys (R. roxellanae) were obtained and identified between July 2011 and August of 2012. The sequences of the 18S rDNA for the two Cyclospora isolates were 477 bp, with no nucleotide variation between them. Phylogenetic analysis based on the 18S rDNA sequences revealed that the two Cyclospora isolates were posited into the clade Cyclospora spp. and sistered to C. colobi. These results first showed that Cyclospora infection occurred in R. roxellanae in hot and rainy weather, which would provide useful information for further understanding the molecular epidemiology of Cyclospora spp. and the control of Cyclospora infection in non-human primates as well as in human beings.
Introduction
Coccidian Cyclospora is an obligate intracellular apicomplexan protozoa that inhabits in the mucosal epithelium of the intestine or bile duct of various vertebrates [1], and sometimes it also is found in invertebrates, eg. the only species C. glomericola in the millipede [2]. Nineteen Cyclospora species have been described and identified in reptiles, insectivores, snakes, rodents, primates [1], [3], [4] and humans [5]. It was 1979 when Cyclospora spp. was firstly detected in three patients in Papua New Guinea [5] and eventually named as Cyclospora cayetanensis according to the classical morphology [6]. Diarrhea has been identified as the typical clinical symptom of cyclosporiasis in humans, especially in travelers [7], [8] and AIDS patients [9], but the severity is closely related to person’s immunity [10]–[12].
In China, Cyclospora infection in humans has been reported in Anhui, Zhejiang and Henan provinces [13]–[15]. These studies suggested that the prevalence of C. cayetanensis is higher in villages than that in towns, and the prevalence of Cyclospora infection is associated with age and season. However, the seasonality of Cyclospora infection in humans is likely to be influenced by the temperature, rainfall, humidity, and other environmental factors worldwide. For example, the Cyclospora prevalence in humans were markedly higher during warm and rainy seasons in Guatemala [16], Jordan [17], Nepal [18], [19], Peru [20], USA and Canada [21], but in Turkey the Cyclospora prevalence was noted at hot and dry seasons [22], while in Haiti [11] Cyclospora infection occurred during the cooler and drier months of the year.
Understanding the transmission of Cyclospora has important implications for controlling and preventing Cyclospora infection in humans and animals. Though the exact transmission routs for human infection with Cyclospora is still a question, several studies have provided valuable information. Contacting with soil has been identified as a risk factor for Cyclospora infection of humans based on studies of Cyclospora infections in Guatemala [16], Peru [23], and USA [24] where children and farmers are frequently infected through contacting with contaminated soil directly or indirectly [25].
Zoonotic transmission also seemed to be a risk factor. Studies in Guatemala [16], Jordan [17], and Peru [20] showed that people whose fecal samples were positive for C. cayetanensis usually had history of feeding livestock and poultry. Using PCR approach, C. cayetanensis oocysts had been detected from fecal samples of both domestic and street dogs, wild chickens inhabiting the forest regions near villages, and Macaca mulatta rhesus monkeys that wandering through the forest region [26]. All these results suggested the zoonotic nature of this parasite, although it is yet to know whether C. cayetanensis can infect these copraphagic animals naturally or these animals just pass oocysts through the gut. Moreover, several studies indicated the presence of Cyclospora-like oocysts in chickens [27], ducks [28], dairy cattle [29], monkeys [30], [31], mice [18], rats [18] and dogs [18], [32] and zoo animals (non-human primates, carnivores, and artiodactyla) [33]. Of them, the non-human primates have the closest genetic relationships with humans. C. cayetanensis, C. cercopitheci, C. colobi and C. papioni have been found in Macaca mulatta rhesus monkeys, Cercopithecus aethiops Linnaeus, Colobus guereza Ruppell, Papio anubis Lesson [26], [34]. Therefore, determination of Cyclospora spp. in monkeys would have important implications for controlling Cyclospora infection in humans.
The golden snub-nosed monkey (Rhinopithecus roxellanae) is one of the endangered and precious wild animals and has been listed as Category I in the List of Key Protected Wildlife in China. It mainly distributes in Sichuan, Gansu, Shaanxi and Hubei provinces. The objective of the present study was to determine the prevalence and species of Cyclospora in golden snub-nosed monkeys in Shaanxi province, northwestern China.
Materials and Methods
Ethics Statement
The performance of this study was strictly accord to the recommendations of the Guide for the Care and Use of Laboratory Animals of the Ministry of Health, China, and our protocol was reviewed and approved by the Research Ethics Committee of Northwest A&F University. All the fecal samples were collected from animals after the permission of the Shaanxi Rare Wildlife Rescue Breeding Research Center, with no specific permits being required by the authority for the feces collection.
Fecal Sample Collection
A total of 19 golden monkeys in Shaanxi Rare Wildlife Rescue Breeding Research Center in Shaanxi province of China were included in this survey. From July 2011 to August 2012, fresh stool samples from golden snub-nosed monkeys were collected, placed immediately in disposable plastic bags, and stored at 4°C for further detection.
Microscopic Examination and Oocysts Sporulation
All stool samples were suspended in water, filtered by 160 mesh sieve, and centrifuged at 3000 rpm for 3 min. The sediment was concentrated by Sheater’s sugar flotation technique. Fecal specimens were examined by microscopy for the presence of Cyclospora-like oocysts [29], [34]–[37]. Photomicrographs of parasites were taken using a camera.
The Cyclospora-like organism was mixed with a 2.5% aqueous (w/v) potassium dichromate (K2Cr2O7) solution in a 3∶1 ratio and stored at room temperature for morphological observation of sporulated oocysts after incubation for 10 days. The positive fecal samples with Cyclospora-like organism were kept in 2.5% aqueous (w/v) potassium dichromate solution for molecular characterization.
DNA Extraction and PCR Amplification of 18S rDNA
The dung samples were washed with water and the upper potessium dichromate solution was discarded. Genomic DNA was extracted from the fecal samples according to the protocols of the kit (Omega), and stored at −20°C until further use. The 18S rDNA of each DNA sample was amplified using the nested primer pairs described by Relman et al. [38] following the reaction system of Li et al. [29]. Each amplicon was examined by agarose gel electrophoresis to validate amplification efficiency.
Sequencing of the Amplicons and Genetic Analysis
The positive PCR products were sequenced directly by Sangon Biotech (Shanghai) with internal primers described by Relman et al. [38]. The obtained sequences were aligned with 18S rDNA sequences of Cyclospora spp. available in GenBank™ using BLAST program. Sequence differences (D) among Cyclospora spp. were determined by pairwise comparisons using the formula D = 1−(M/L), where M is the number of alignment positions at which the two sequences have a base in common, and L is the total number of alignment positions over which the two sequences are compared [39]–[41].
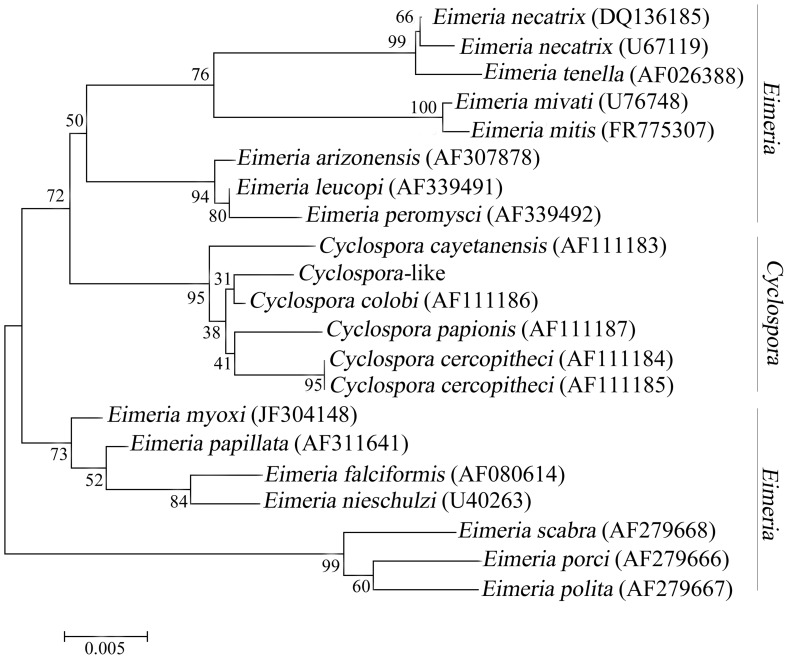
To determine the taxonomic identity of Cyclospora spp. isolated in the present study, phylogenetic relationships of parasites in the genus Cyclospora and some Eimeria species with high similarity to Cyclospora isolated herein were re-constructed using neighbor joining (NJ) method in Mega 4.0 [42] with substitution model of Kimura 2-parameter. The consensus tree was obtained after bootstrap analysis with 1000 replications, with values above 50% reported. Phylograms were drawn using the Tree View program version 1.65 [43].
Results
Of 71 fecal samples examined from July 2011 to August 2012, only 2 samples were positive for Cyclospora-like oocysts by microscopy. These two samples were then transferred into potassium dichromate solution (2.5%) and incubated at room temperature for sporulation. The sporulated oocysts were spherical, with refractile “cyst-like” structure. Two sporocysts were seen within one oocyst, and each sporocyst contained two sporozoites (Figure 1).
Figure 1. Cyclospora-like oocyst found in the feces (wet mount preparation) of the golden snub-nosed monkeys.
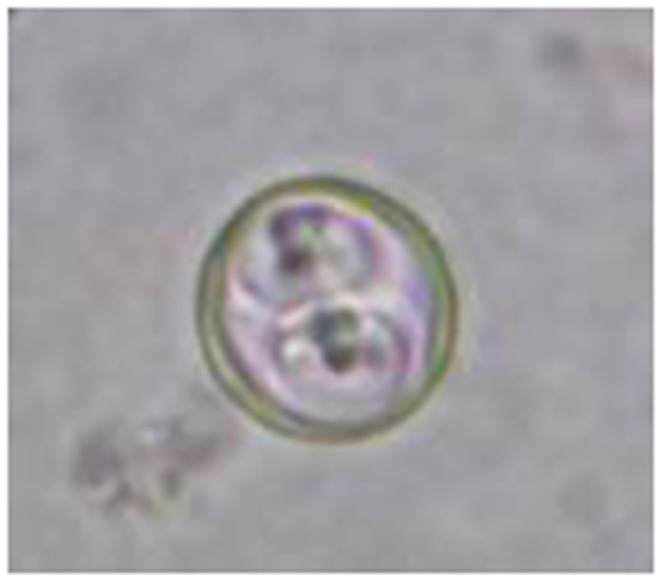
Sporulated oocyst: two sporocysts were seen within one oocyst, and each sporocyst contains two sporozoites. Magnification: 400×.
To determine the specific identity of Cyclosopora-like oocysts, genomic DNA of each isolate was extracted and used for PCR amplification of 18S rDNA. The lengths of the PCR products from both isolates were approximately 500 bp on agarose gel electrophoresis (Figure 2). Then the purified amplicons were sequenced and intra- and inter-species sequence differences in the 18S rDNA regions were determined. The two obtained sequences were identical, with 99% similarity to corresponding sequences of Cyclospora sp. available in GenBank™ (accession numbers AF111183, AF111184, AF1111185, AF111186 and AF111187). After removing the inaccurate sequences in the both ends, 477 bp of the 18S rDNA sequence were used for pairwise comparisons. Sequence differences between the Cyclospora-like isolates obtained in the present study and C. cayetanensis, C. cercopitheci, C. colobi, C. papionis were 0.8%, 1.0%, 0.4%, and 0.8%, respectively. There results suggested that the two Cyclospora-like isolates obtained in the present study may represent C. colobi.
Figure 2. Molecular identification of Cyclospora-like organism in the feces of the golden snub-nosed monkeys.
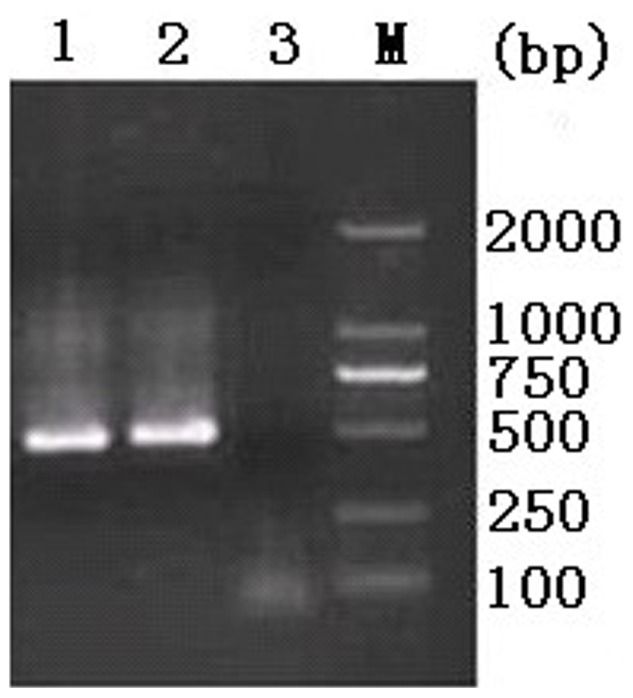
PCR products generated with nested eimeriid-range restricted primers; 500-bp amplicons were produced from the DNA of Cyclospora-like oocysts. Lane M: DL-2000 DNA Marker, lanes 1–2: oocyst specimens from golden snub-nosed monkeys 1 and 2, respectively, and lane 3: Negative control.
Then the phylogenetic relationships of some common parasites in the genera Eimeria and Cyclospora were re-constructed using NJ method based on partial 18S rDNA sequences (Figure 3). All of the Cyclospora species clustered together with high bootstrap value (95%). Within this clade, Cyclospora-like organism isolated from golden snub-nosed monkeys in the present study grouped with other monkey Cyclospora spp. and sistered to C. colobi. These results further confirmed that the Cyclospora-like organism isolated herein represents C. colobi.
Figure 3. Phylogenetic relationships of Cyclospora isolates from golden snub-nosed monkey with Cyclospora spp. and Eimeria spp.
Phylogenetic analysis was based on 18S rDNA sequences using neighbor–joining (NJ) method. The consensus tree was obtained after bootstrap analysis with 1000 replications, with values above 50% reported.
Discussion
In the present study, C. colobi was the only species found in the golden snub-nosed monkey within an observation period of one year. The common human species C. cayetanensis was not found. This finding was consistent with results of previous studies in other monkeys in Ethiopia [34] and Kenya [44]. These results may be explained by two reasons. Firstly, monkeys may be unlikely to be animal reservoirs of the human pathogen C. cayetanensis. Therefore, the transmission of Cyclosopora infection between non-human primates and human needs further study in the future. Another explanation is the limitation of the detection methods commonly used for the detection of Cyclosopora oocysts. The Sheater’s sugar flotation technique has a low sensitivity, and fecal samples with light infection intensity may not be detected.
From July 2011 to August 2012, only two Cyclosopora-positive samples were detected in July and August of 2011, respectively, which is different from study by Eberhard et al. [44] who reported that no marked seasonality was observed for Cyclosopora infection in African monkeys, despite the existence of distinct weather patterns in Kenya, East Africa. However, our results were consistent with findings of C. cayetanensis infection in humans in Henan province [15] where C. cayetanensis infection occurred in the hottest and most rainy months of the year. These results might indicate a seasonality of the infection, but may also relate to the low sensitivity of the Sheater’s sugar flotation technique used for screening the feces for detection of oocysts, or the small number of examined fecal samples (N = 71) due to the endangered nature of the golden snub-nosed monkey. In addition, the Cyclospora infection of golden monkeys could be transient due to environmental fecal contamination and immunity [13], [45], which suggested that fecal contamination might be an important mode of transmission for this parasite [46].
In conclusion, the present study isolated and characterized two Cyclospora isolates from the golden snub-nosed monkeys in Qinling Mountain in Shaanxi Province, Northwestern China. These two Cyclospora isolates represented C. colobi based on their morphological features and 18S rDNA sequences. This is the first report of C. colobi from the endangered golden snub-nosed monkeys. These results have important implications for transmission study and control of cyclosporiasis in humans and animals.
Funding Statement
This work was supported, in part, by the National Natural Science Foundation of China (Grant No. 31101805, 31101138, 30960280), the Fund for Basic scientific research (Grant No. ZD2012010 and QN2012018), the Open Funds of the State Key Laboratory of Veterinary Etiological Biology, Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences (Grant No. SKLVEB2011KFKT011), and the Special Funds for Talents in Northwest A & F University (Grant Nos. Z109021107 and 2010BSJJ015). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lainson R (2005) The genus Cyclospora (Apicomplexa: Eimeriidae), with a description of Cyclospora schneideri n. sp. in the snake Anilius scytale scytale (Aniliidae) from Amazonian Brazil-a review. Mem Inst Oswaldo Cruz 100: 103–110. [DOI] [PubMed] [Google Scholar]
- 2. Schneider A (1881) Sur les psorospermies oviformes ou coccidies, espécies nouvelles ou peu connues. Arch Zool Exp Gen 9: 387–404. [Google Scholar]
- 3. Shields JM, Olson BH (2003) Cyclospora cayetanensis: a review of an emerging parasitic coccidian. Int J Parasitol 33: 371–391. [DOI] [PubMed] [Google Scholar]
- 4. Mansfield LS, Gajadhar AA (2004) Cyclospora cayetanensis, a food- and waterborne coccidian parasite. Vet Parasitol 126: 73–90. [DOI] [PubMed] [Google Scholar]
- 5. Ashford RW (1979) Occurrence of an undescribed coccidian in man in Papua New Guinea. Ann Trop Med Parasitol 73: 497–500. [DOI] [PubMed] [Google Scholar]
- 6. Ortega YR, Gilman RH, Sterling CR (1994) A new coccidian parasite (Apicomplexa: Eimeriidae) from humans. J Parasitol 80: 625–629. [PubMed] [Google Scholar]
- 7. Long EG, Ebrahimzadeh A, White EH, Swisher B, Callaway CS (1990) Alga associated with diarrhea in patients with acquired immunodeficiency syndrome and in travelers. J Clin Microbiol 28: 1101–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoge CW, Shlim DR, Rajah R, Triplett J, Shear M, et al. (1993) Epidemiology of diarrhoeal illness associated with coccidian-like organism among travellers and foreign residents in Nepal. Lancet 341: 1175–1179. [DOI] [PubMed] [Google Scholar]
- 9. Hart AS, Ridinger MT, Soundarajan R, Peters CS, Swiatlo AL, et al. (1990) Novel organism associated with chronic diarrhoea in AIDS. Lancet 335: 169–170. [DOI] [PubMed] [Google Scholar]
- 10. Chacin-Bonilla L, Mejia de Young M, Estevez J (2003) Prevalence and pathogenic role of Cyclospora cayetanensis in a Venezuelan community. Am J Trop Med Hyg 68: 304–306. [PubMed] [Google Scholar]
- 11. Eberhard ML, Nace EK, Freeman AR, Streit TG, da Silva AJ, Lammie PJ (1999) Cyclospora cayetanensis infections in Haiti: a common occurrence in the absence of watery diarrhea. Am J Trop Med Hyg 60: 584–586. [DOI] [PubMed] [Google Scholar]
- 12. Ferreira MS (2000) Infections by protozoa in immunocompromised hosts. Mem Inst Oswaldo Cruz 95: 159–162. [DOI] [PubMed] [Google Scholar]
- 13. Wang KX, Li CP, Wang J, Tian Y (2002) Cyclospore cayetanensis in Anhui, China. World J Gastroenterol 8: 1144–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xing WL, Wu KW, Ling XY, Huang HC, Liu QZ, et al. (2002) Prevalence Survey on Cyclospora cayetanensis in Diarrhea Cases in Wenzhou. Chin J Parasit Dis 15: 320–321 (in chinese).. [PubMed] [Google Scholar]
- 15. Zhou Y, Lv B, Wang Q, Wang R, Jian F (2011) Prevalence and molecular characterization of Cyclospora cayetanensis, Henan, China. Emerg Infect Dis 17: 1887–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bern C, Hernandez B, Lopez MB, Arrowood MJ, de Mejia MA, et al. (1999) Epidemiologic studies of Cyclospora cayetanensis in Guatemala. Emerg Infect Dis 5: 766–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nimri LF (2003) Cyclospora cayetanensis and other intestinal parasites associated with diarrhea in a rural area of Jordan. Int Microbiol 6: 131–135. [DOI] [PubMed] [Google Scholar]
- 18. Sherchand JB, Cross JH (2001) Emerging pathogen Cyclospora cayetanensis infection in Nepal. Southeast Asian J Trop Med Public Health 32: 143–150. [PubMed] [Google Scholar]
- 19. Kimura K, Rai SK, Rai G, Insisiengmay S, Kawabata M, et al. (2005) Study on Cyclospora cayetanensis associated with diarrheal disease in Nepal and Loa PDR. Southeast Asian J Trop Med Public Health 36: 1371–1376. [PubMed] [Google Scholar]
- 20. Bern C, Ortega Y, Checkley W, Roberts JM, Lescano AG, et al. (2002) Epidemiologic differences between cyclosporiasis and cryptosporidiosis in Peruvian children. Emerg Infect Dis 8: 581–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Herwaldt BL (2000) Cyclospora cayetanensis: a review, focusing on the outbreaks of cyclosporiasis in the 1990s. Clin Infect Dis 31: 1040–1057. [DOI] [PubMed] [Google Scholar]
- 22. Turgay N, Yolasigmaz A, Erdogan DD, Zeyrek FY, Uner A (2007) Incidence of cyclosporiasis in patients with gastrointestinal symptoms in western Turkey. Med Sci Monit 13: CR34–CR39. [PubMed] [Google Scholar]
- 23. Madico G, McDonald J, Gilman RH, Cabrera L, Sterling CR (1997) Epidemiology and treatment of Cyclospora cayetanensis infection in Peruvian children. Clin Infect Dis 24: 977–981. [DOI] [PubMed] [Google Scholar]
- 24. Koumans EH, Katz DJ, Malecki JM, Kumar S, Wahlquist SP, et al. (1998) An outbreak of cyclosporiasis in Florida in 1995: a harbinger of multistate outbreaks in 1996 and 1997. Am J Trop Med Hyg 59: 235–242. [DOI] [PubMed] [Google Scholar]
- 25. Chacín-Bonilla L (2010) Epidemiology of Cyclospora cayetanensis: A review focusing in endemic areas. Acta Trop 115: 181–193. [DOI] [PubMed] [Google Scholar]
- 26. Chu DM, Sherchand JB, Cross JH, Orlandi PA (2004) Detection of Cyclospora cayetanensis in animal fecal isolates from Nepal using an FTA filter-base polymerase chain reaction method. Am J Trop Med Hyg 71: 373–379. [PubMed] [Google Scholar]
- 27. García-López HL, Rodríguez-Tovar LE, Medina-De la Garza CE (1996) Identification of Cyclospora in poultry. Emerg Infect Dis 2: 356–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zerpa R, Uchima N, Huicho L (1995) Cyclospora cayetanensis associated with watery diarrhoea in Peruvian patients. J Trop Med Hyg 98: 325–329. [PubMed] [Google Scholar]
- 29. Li G, Xiao S, Zhou R, Li W, Wadeh H (2007) Molecular characterization of Cyclospora-like organism from dairy cattle. Parasitol Res 100: 955–961. [DOI] [PubMed] [Google Scholar]
- 30. Gao ZY, Li GQ, Liu X, Yue CL, Cheng JL, et al. (2010) Isolation and molecular identification of Cyclospora-like organism from monkey. Chin Vet Sci 40: 30–33 (in chinese).. [Google Scholar]
- 31. Smith HV, Paton CA, Girdwood RW, Mtambo MM (1996) Cyclospora in non-human primates in Gombe, Tanzania. Vet Rec 138: 528. [PubMed] [Google Scholar]
- 32. Yai LE, Bauab AR, Hirschfeld MP, de Oliveira ML, Damaceno JT (1997) The first two cases of Cyclospora in dogs, Sao Paulo, Brazil. Rev Inst Med Trop Sao Paulo 39: 177–179. [DOI] [PubMed] [Google Scholar]
- 33. Pérez Cordón G, Hitos Prados A, Romero D, Sánchez Moreno M, Pontes A, et al. (2008) Intestinal parasitism in the animals of the zoological garden “Peña Escrita” (Almuñecar, Spain). Vet Parasitol 156: 302–309. [DOI] [PubMed] [Google Scholar]
- 34. Eberhard ML, da Silva AJ, Lilley BG, Pieniazek NJ (1999) Morphologic and molecular characterization of new Cyclospora species from Ethiopian monkeys: C. cercopitheci sp.n., C. colobi sp.n., and C. papionis sp.n. Emerg Infect Dis 5: 651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ortega YR, Sterling CR, Gilman RH, Cama VA, Díaz F (1993) Cyclospora species–a new protozoan pathogen of humans. N Engl J Med 328: 1308–1312. [DOI] [PubMed] [Google Scholar]
- 36. Long EG, White EH, Carmichael WW, Quinlisk PM, Raja R, et al. (1991) Morphologic and staining characteristics of a Cyanobacterium-like organism associated with diarrhea. J Infect Dis 64: 199–202. [DOI] [PubMed] [Google Scholar]
- 37. Wurtz R (1994) Cyclospora: a newly identified intestinal pathogen of humans. Clin Infect Dis 18: 620–623. [DOI] [PubMed] [Google Scholar]
- 38. Relman DA, Schmidt TM, Gajadhar A, Sogin M, Cross J (1996) Molecular phylogenetic analysis of Cyclospora, the human intestinal pathogen, suggests that it is closely related to Eimeria species. J Infect Dis 173: 440–445. [DOI] [PubMed] [Google Scholar]
- 39. Chilton NB, Gasser RB, Beveridge I (1995) Differences in a ribosomal DNA sequence of morphologically indistinguishable species within the Hypodontus macropi complex (Nematoda: Strongyloidea). Int J Parasitol 25: 647–651. [DOI] [PubMed] [Google Scholar]
- 40. Zhao GH, Mo XH, Zou FC, Li J, Weng YB, et al. (2009) Genetic variability among Schistosoma japonicum isolates from different endemic regions in China revealed by sequences of three mitochondrial DNA genes. Vet Parasitol 162: 67–74. [DOI] [PubMed] [Google Scholar]
- 41. Zhao GH, Blair D, Li XY, Li J, Lin RQ, et al. (2011) The ribosomal intergenic spacer (IGS) region in Schistosoma japonicum: structure and comparisons with related species. Infect Genet Evol 11: 610–617. [DOI] [PubMed] [Google Scholar]
- 42. Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) Software Version 4.0. Mol Biol Evol 24: 1596–1599. [DOI] [PubMed] [Google Scholar]
- 43. Page RD (1996) TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12: 357–358. [DOI] [PubMed] [Google Scholar]
- 44. Eberhard ML, Njenga MN, DaSilva AJ, Owino D, Nace EK, et al. (2001) A survey for Cyclospora spp. in Kenyan primates, with some notes on its biology. J Parasitol 87: 1394–1397. [DOI] [PubMed] [Google Scholar]
- 45. Hoge CW, Shlim DR, Rajah R, Triplett J, Shear M, et al. (1993) Epidemiology of diarrhoeal illness associated with coccidian-like organism among travellers and foreign residents in Nepal. Lancet 341: 1175–1179. [DOI] [PubMed] [Google Scholar]
- 46. Chacín-Bonilla L, Barrios F, Sanchez Y (2007) Epidemiology of Cyclospora cayetanensis infection in San Carlos Island, Venezuela: strong association between socio-economic status and infection. Trans R Soc Trop Med Hyg 101: 1018–1024. [DOI] [PubMed] [Google Scholar]