Abstract
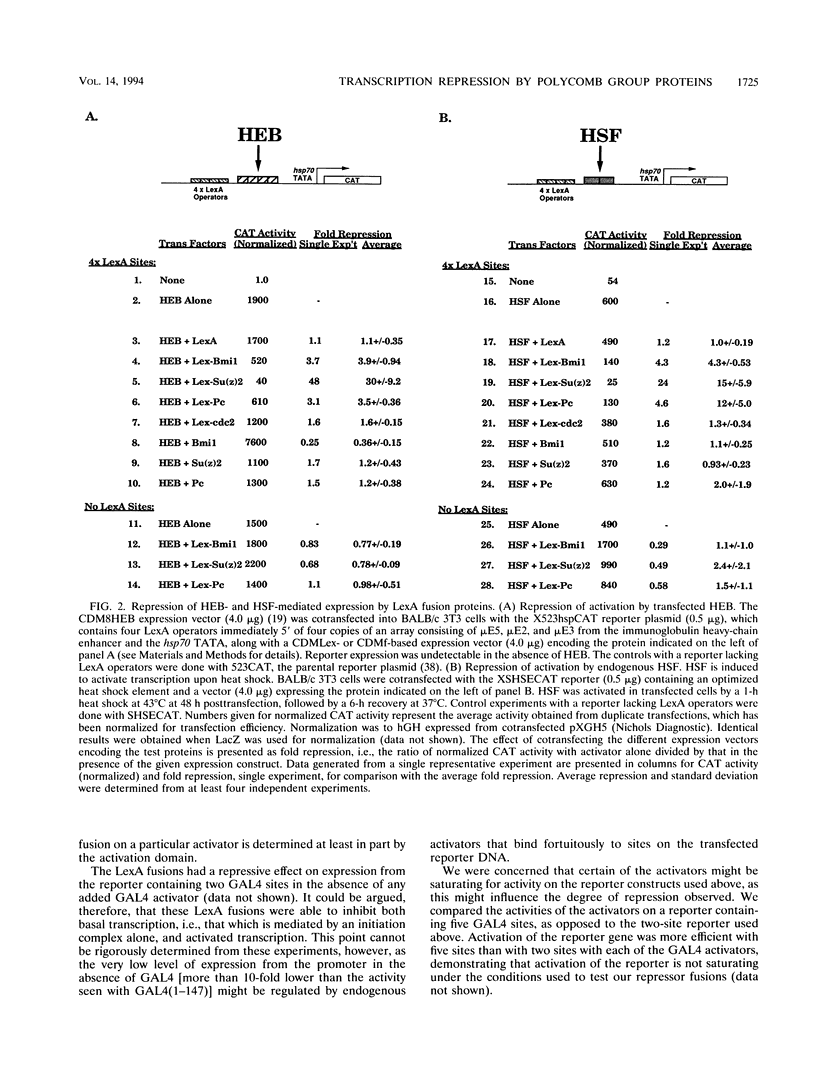
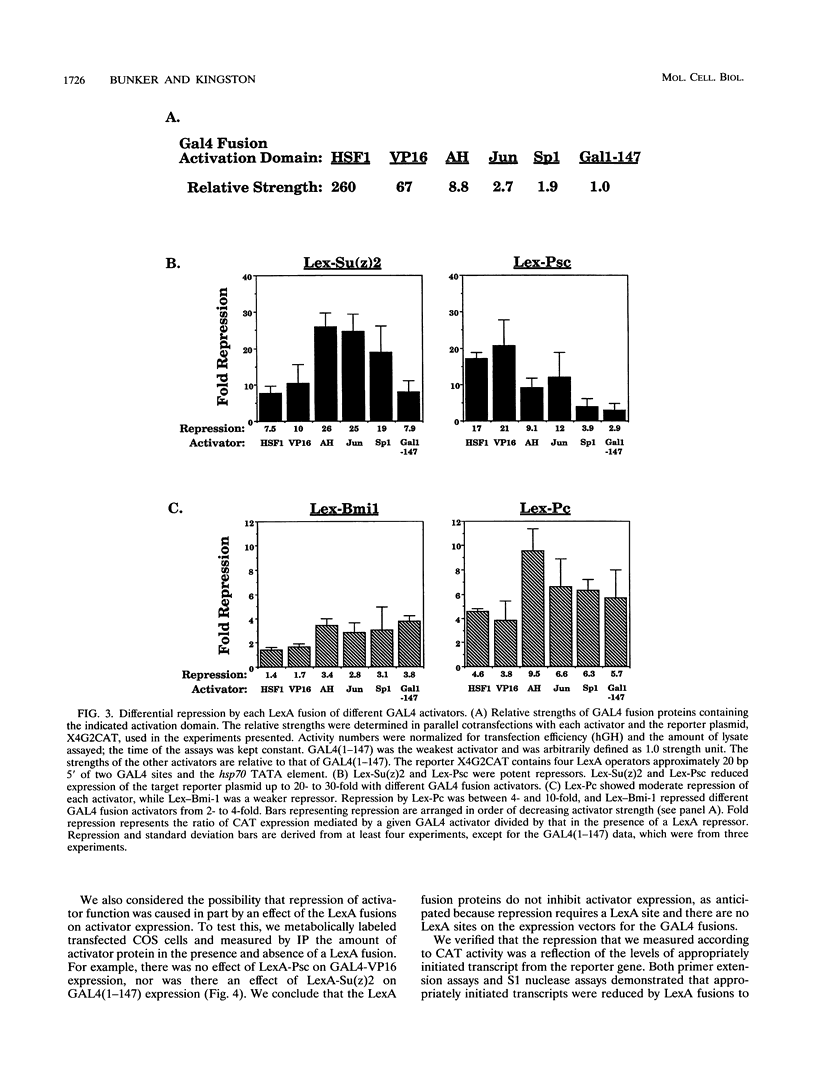
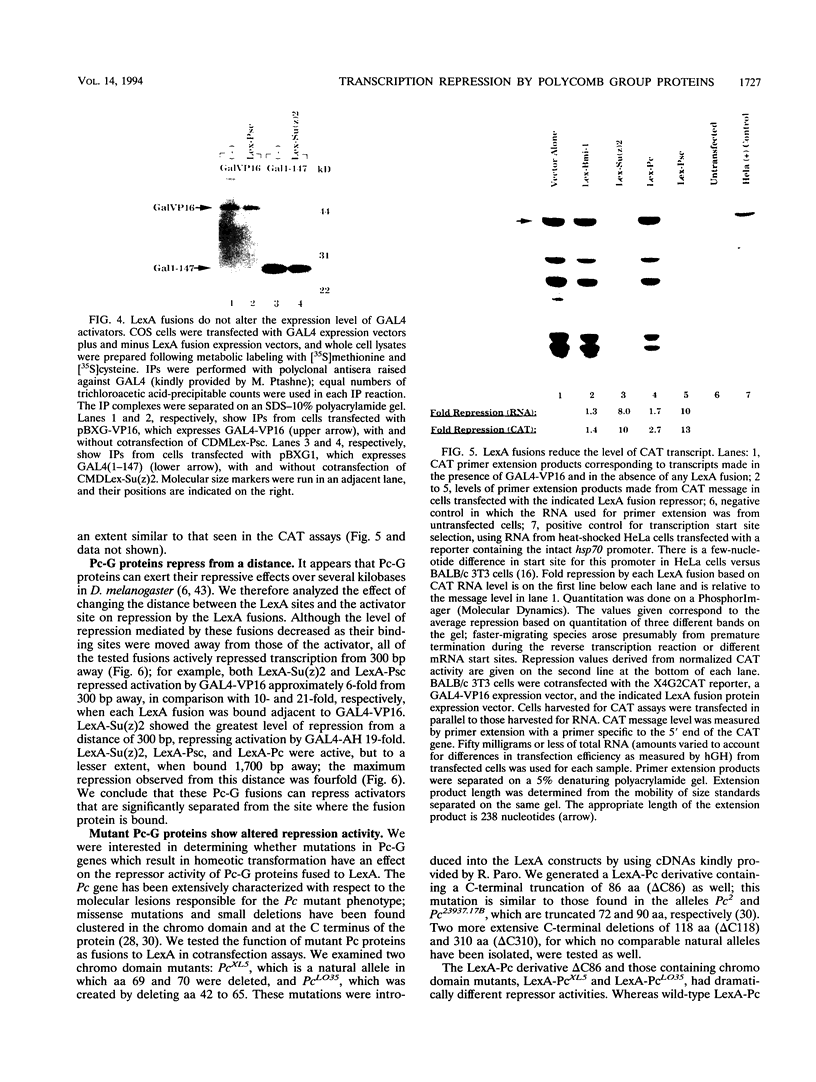
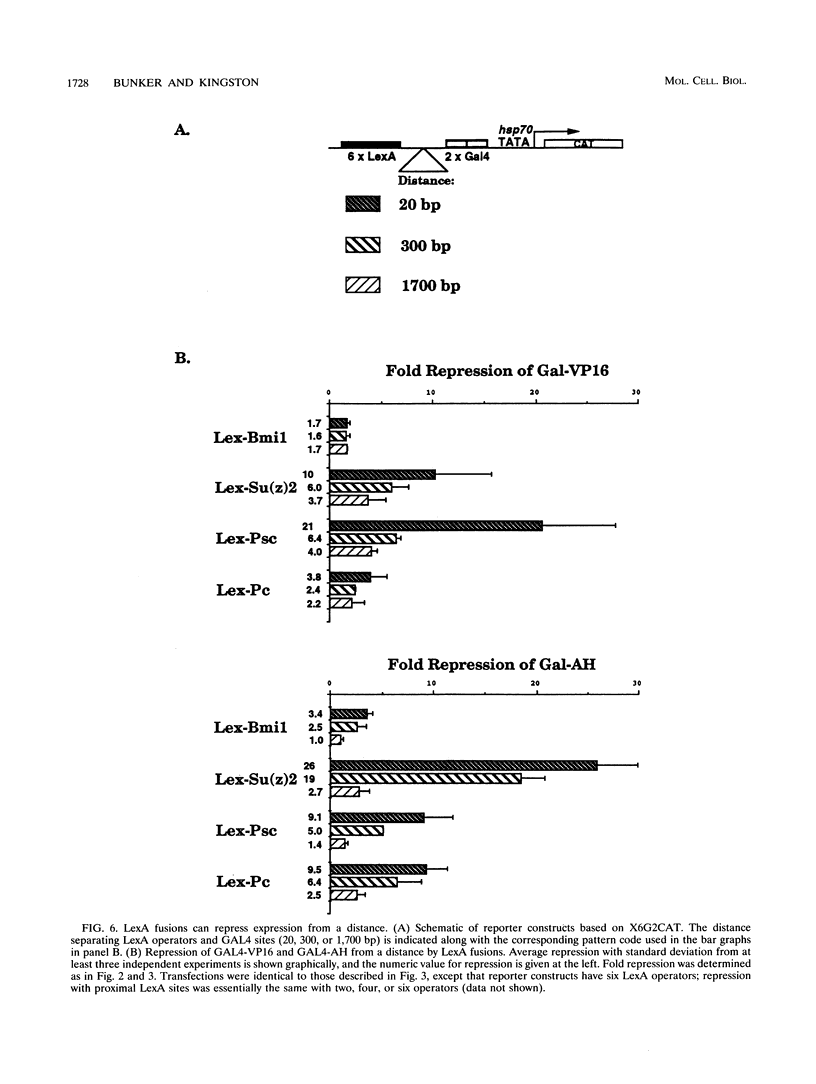
The Polycomb group (Pc-G) genes are essential for maintaining the proper spatially restricted expression pattern of the homeotic loci during Drosophila development. The Pc-G proteins appear to function at target loci to maintain a state of transcriptional repression. The murine oncogene bmi-1 has significant homology to the Pc-G gene Posterior sex combs (Psc) and a highly related gene, Suppressor two of zeste [Su(z)2]. We show here that the proteins encoded by bmi-1 and the Pc-G genes Polycomb (Pc) and Psc as well as Su(z)2 mediate repression in mammalian cells when targeted to a promoter by LexA in a cotransfection system. These fusion proteins repress activator function by as much as 30-fold, and the effect on different activation domains is distinct for each Pc-G protein. Repression is observed when the LexA fusion proteins are bound directly adjacent to activator binding sites and also when bound 1,700 bases from the promoter. These data demonstrate that the products of the Pc-G genes can significantly repress activator function on transiently introduced DNA. We suggest that this function contributes to the stable repression of targeted loci during development.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler P. N., Martin E. C., Charlton J., Jones K. Phenotypic consequences and genetic interactions of a null mutation in the Drosophila Posterior Sex Combs gene. Dev Genet. 1991;12(5):349–361. doi: 10.1002/dvg.1020120504. [DOI] [PubMed] [Google Scholar]
- Baichwal V. R., Park A., Tjian R. The cell-type-specific activator region of c-Jun juxtaposes constitutive and negatively regulated domains. Genes Dev. 1992 Aug;6(8):1493–1502. doi: 10.1101/gad.6.8.1493. [DOI] [PubMed] [Google Scholar]
- Bienz M. Molecular mechanisms of determination in Drosophila. Curr Opin Cell Biol. 1992 Dec;4(6):955–961. doi: 10.1016/0955-0674(92)90124-u. [DOI] [PubMed] [Google Scholar]
- Brunk B. P., Martin E. C., Adler P. N. Drosophila genes Posterior Sex Combs and Suppressor two of zeste encode proteins with homology to the murine bmi-1 oncogene. Nature. 1991 Sep 26;353(6342):351–353. doi: 10.1038/353351a0. [DOI] [PubMed] [Google Scholar]
- Castelli-Gair J. E., García-Bellido A. Interactions of Polycomb and trithorax with cis regulatory regions of Ultrabithorax during the development of Drosophila melanogaster. EMBO J. 1990 Dec;9(13):4267–4275. doi: 10.1002/j.1460-2075.1990.tb07875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celniker S. E., Keelan D. J., Lewis E. B. The molecular genetics of the bithorax complex of Drosophila: characterization of the products of the Abdominal-B domain. Genes Dev. 1989 Sep;3(9):1424–1436. doi: 10.1101/gad.3.9.1424. [DOI] [PubMed] [Google Scholar]
- Courey A. J., Tjian R. Analysis of Sp1 in vivo reveals multiple transcriptional domains, including a novel glutamine-rich activation motif. Cell. 1988 Dec 2;55(5):887–898. doi: 10.1016/0092-8674(88)90144-4. [DOI] [PubMed] [Google Scholar]
- Duncan I. M. Polycomblike: a gene that appears to be required for the normal expression of the bithorax and antennapedia gene complexes of Drosophila melanogaster. Genetics. 1982 Sep;102(1):49–70. doi: 10.1093/genetics/102.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dura J. M., Brock H. W., Santamaria P. Polyhomeotic: a gene of Drosophila melanogaster required for correct expression of segmental identity. Mol Gen Genet. 1985;198(2):213–220. doi: 10.1007/BF00382998. [DOI] [PubMed] [Google Scholar]
- Eissenberg J. C., James T. C., Foster-Hartnett D. M., Hartnett T., Ngan V., Elgin S. C. Mutation in a heterochromatin-specific chromosomal protein is associated with suppression of position-effect variegation in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9923–9927. doi: 10.1073/pnas.87.24.9923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field J., Nikawa J., Broek D., MacDonald B., Rodgers L., Wilson I. A., Lerner R. A., Wigler M. Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Mol Cell Biol. 1988 May;8(5):2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke A., DeCamillis M., Zink D., Cheng N., Brock H. W., Paro R. Polycomb and polyhomeotic are constituents of a multimeric protein complex in chromatin of Drosophila melanogaster. EMBO J. 1992 Aug;11(8):2941–2950. doi: 10.1002/j.1460-2075.1992.tb05364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene J. M., Kingston R. E. TATA-dependent and TATA-independent function of the basal and heat shock elements of a human hsp70 promoter. Mol Cell Biol. 1990 Apr;10(4):1319–1328. doi: 10.1128/mcb.10.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene J. M., Larin Z., Taylor I. C., Prentice H., Gwinn K. A., Kingston R. E. Multiple basal elements of a human hsp70 promoter function differently in human and rodent cell lines. Mol Cell Biol. 1987 Oct;7(10):3646–3655. doi: 10.1128/mcb.7.10.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt Y., Alexander W. S., Barri G., Klinken S. P., Adams J. M. Novel zinc finger gene implicated as myc collaborator by retrovirally accelerated lymphomagenesis in E mu-myc transgenic mice. Cell. 1991 May 31;65(5):753–763. doi: 10.1016/0092-8674(91)90383-a. [DOI] [PubMed] [Google Scholar]
- Hirschhorn J. N., Brown S. A., Clark C. D., Winston F. Evidence that SNF2/SWI2 and SNF5 activate transcription in yeast by altering chromatin structure. Genes Dev. 1992 Dec;6(12A):2288–2298. doi: 10.1101/gad.6.12a.2288. [DOI] [PubMed] [Google Scholar]
- Hu J. S., Olson E. N., Kingston R. E. HEB, a helix-loop-helix protein related to E2A and ITF2 that can modulate the DNA-binding ability of myogenic regulatory factors. Mol Cell Biol. 1992 Mar;12(3):1031–1042. doi: 10.1128/mcb.12.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. S., Gelbart W. M. Genetic analysis of the enhancer of zeste locus and its role in gene regulation in Drosophila melanogaster. Genetics. 1990 Sep;126(1):185–199. doi: 10.1093/genetics/126.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. S., Gelbart W. M. The Drosophila Polycomb-group gene Enhancer of zeste contains a region with sequence similarity to trithorax. Mol Cell Biol. 1993 Oct;13(10):6357–6366. doi: 10.1128/mcb.13.10.6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennison J. A., Tamkun J. W. Dosage-dependent modifiers of polycomb and antennapedia mutations in Drosophila. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8136–8140. doi: 10.1073/pnas.85.21.8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuziora M. A., McGinnis W. Different transcripts of the Drosophila Abd-B gene correlate with distinct genetic sub-functions. EMBO J. 1988 Oct;7(10):3233–3244. doi: 10.1002/j.1460-2075.1988.tb03190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis E. B. A gene complex controlling segmentation in Drosophila. Nature. 1978 Dec 7;276(5688):565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- Martin E. C., Adler P. N. The Polycomb group gene Posterior Sex Combs encodes a chromosomal protein. Development. 1993 Feb;117(2):641–655. doi: 10.1242/dev.117.2.641. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Gavis E. R., Kingsbury R., Axel R. Analysis of transcriptional regulatory signals of the HSV thymidine kinase gene: identification of an upstream control region. Cell. 1981 Aug;25(2):385–398. doi: 10.1016/0092-8674(81)90057-x. [DOI] [PubMed] [Google Scholar]
- Messmer S., Franke A., Paro R. Analysis of the functional role of the Polycomb chromo domain in Drosophila melanogaster. Genes Dev. 1992 Jul;6(7):1241–1254. doi: 10.1101/gad.6.7.1241. [DOI] [PubMed] [Google Scholar]
- Moazed D., O'Farrell P. H. Maintenance of the engrailed expression pattern by Polycomb group genes in Drosophila. Development. 1992 Nov;116(3):805–810. doi: 10.1242/dev.116.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paro R., Hogness D. S. The Polycomb protein shares a homologous domain with a heterochromatin-associated protein of Drosophila. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):263–267. doi: 10.1073/pnas.88.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paro R. Imprinting a determined state into the chromatin of Drosophila. Trends Genet. 1990 Dec;6(12):416–421. doi: 10.1016/0168-9525(90)90303-n. [DOI] [PubMed] [Google Scholar]
- Pearce J. J., Singh P. B., Gaunt S. J. The mouse has a Polycomb-like chromobox gene. Development. 1992 Apr;114(4):921–929. doi: 10.1242/dev.114.4.921. [DOI] [PubMed] [Google Scholar]
- Pillus L. An acquired state: epigenetic mechanisms in transcription. Curr Opin Cell Biol. 1992 Jun;4(3):453–458. doi: 10.1016/0955-0674(92)90011-z. [DOI] [PubMed] [Google Scholar]
- Rastelli L., Chan C. S., Pirrotta V. Related chromosome binding sites for zeste, suppressors of zeste and Polycomb group proteins in Drosophila and their dependence on Enhancer of zeste function. EMBO J. 1993 Apr;12(4):1513–1522. doi: 10.1002/j.1460-2075.1993.tb05795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter G., Spierer P. Position effect variegation and chromatin proteins. Bioessays. 1992 Sep;14(9):605–612. doi: 10.1002/bies.950140907. [DOI] [PubMed] [Google Scholar]
- Ruden D. M., Ma J., Li Y., Wood K., Ptashne M. Generating yeast transcriptional activators containing no yeast protein sequences. Nature. 1991 Mar 21;350(6315):250–252. doi: 10.1038/350250a0. [DOI] [PubMed] [Google Scholar]
- Ruezinsky D., Beckmann H., Kadesch T. Modulation of the IgH enhancer's cell type specificity through a genetic switch. Genes Dev. 1991 Jan;5(1):29–37. doi: 10.1101/gad.5.1.29. [DOI] [PubMed] [Google Scholar]
- Sato T., Denell R. E. Homoeosis in Drosophila: anterior and posterior transformations of Polycomb lethal embryos. Dev Biol. 1985 Jul;110(1):53–64. doi: 10.1016/0012-1606(85)90063-6. [DOI] [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987 May;84(10):3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer C. D., Wallrath L. L., Elgin S. C. Regulating genes by packaging domains: bits of heterochromatin in euchromatin? Trends Genet. 1993 Feb;9(2):35–37. doi: 10.1016/0168-9525(93)90171-D. [DOI] [PubMed] [Google Scholar]
- Simon J., Chiang A., Bender W. Ten different Polycomb group genes are required for spatial control of the abdA and AbdB homeotic products. Development. 1992 Feb;114(2):493–505. doi: 10.1242/dev.114.2.493. [DOI] [PubMed] [Google Scholar]
- Struhl G., Akam M. Altered distributions of Ultrabithorax transcripts in extra sex combs mutant embryos of Drosophila. EMBO J. 1985 Dec 1;4(12):3259–3264. doi: 10.1002/j.1460-2075.1985.tb04075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamkun J. W., Deuring R., Scott M. P., Kissinger M., Pattatucci A. M., Kaufman T. C., Kennison J. A. brahma: a regulator of Drosophila homeotic genes structurally related to the yeast transcriptional activator SNF2/SWI2. Cell. 1992 Feb 7;68(3):561–572. doi: 10.1016/0092-8674(92)90191-e. [DOI] [PubMed] [Google Scholar]
- Webster N., Jin J. R., Green S., Hollis M., Chambon P. The yeast UASG is a transcriptional enhancer in human HeLa cells in the presence of the GAL4 trans-activator. Cell. 1988 Jan 29;52(2):169–178. doi: 10.1016/0092-8674(88)90505-3. [DOI] [PubMed] [Google Scholar]
- Wedeen C., Harding K., Levine M. Spatial regulation of Antennapedia and bithorax gene expression by the Polycomb locus in Drosophila. Cell. 1986 Mar 14;44(5):739–748. doi: 10.1016/0092-8674(86)90840-8. [DOI] [PubMed] [Google Scholar]
- Winston F., Carlson M. Yeast SNF/SWI transcriptional activators and the SPT/SIN chromatin connection. Trends Genet. 1992 Nov;8(11):387–391. doi: 10.1016/0168-9525(92)90300-s. [DOI] [PubMed] [Google Scholar]
- Zink B., Engström Y., Gehring W. J., Paro R. Direct interaction of the Polycomb protein with Antennapedia regulatory sequences in polytene chromosomes of Drosophila melanogaster. EMBO J. 1991 Jan;10(1):153–162. doi: 10.1002/j.1460-2075.1991.tb07931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lohuizen M., Frasch M., Wientjens E., Berns A. Sequence similarity between the mammalian bmi-1 proto-oncogene and the Drosophila regulatory genes Psc and Su(z)2. Nature. 1991 Sep 26;353(6342):353–355. doi: 10.1038/353353a0. [DOI] [PubMed] [Google Scholar]
- van Lohuizen M., Verbeek S., Scheijen B., Wientjens E., van der Gulden H., Berns A. Identification of cooperating oncogenes in E mu-myc transgenic mice by provirus tagging. Cell. 1991 May 31;65(5):737–752. doi: 10.1016/0092-8674(91)90382-9. [DOI] [PubMed] [Google Scholar]