Abstract
Background and Purpose
Retention of substances from systemic circulation in the brain and testes are limited due to high levels of P-glycoprotein (P-gp) in the luminal membranes of brain and testes capillary endothelial cells. From a clinical perspective, P-gp rapidly extrudes lipophilic therapeutic agents, which then fail to reach efficacious levels. Recent studies have demonstrated that acute administration of selective serotonin reuptake inhibitors (SSRI) can affect P-gp function, in vitro and in vivo. However, little is known concerning the time-course of these effects or the effects of different SSRI in vivo.
Experimental Approach
The P-gp substrate, tritiated digoxin ([3H] digoxin), was co-administered with fluoxetine or sertraline to determine if either compound increased drug accumulation within the brains and testes of mice due to inhibition of P-gp activity. We undertook parallel studies in endothelial cells derived from brain microvessels to determine the dose-response and time-course of effects.
Key Results
In vitro, sertraline resulted in rapid and potent inhibition of P-gp function in brain endothelial cells, as determined by cellular calcein accumulation. In vivo, a biphasic effect was demonstrated. Brain accumulation of [3H] digoxin was increased 5 minutes after treatment with sertraline, but by 60 minutes after sertraline treatment, brain accumulation of digoxin was reduced compared to control. By 240 minutes after sertraline treatment brain digoxin accumulation was elevated compared to control. A similar pattern of results was obtained in the testes. There was no significant effect of fluoxetine on P-gp function, in vitro or in vivo.
Conclusions and Implications
Acute sertraline administration can modulate P-gp activity in the blood-brain barrier and blood-testes barrier. This clearly has implications for the ability of therapeutic agents that are P-gp substrates, to enter the brain when co-administered with SSRI.
Introduction
Phospho-glycoprotein (P-gp) was first discovered in mammalian tumour cells where it confers drug resistance [1]. It is a plasma membrane ATP binding cassette (ABC)-transporter of the multidrug resistance (MDR) family. P-gp can actively transport a wide range of structurally diverse xenobiotics against a concentration gradient [2]. Specific substrates include anticancer anthracyclines (doxorubicin) and alkaloids (vincristine, vinblastine), immunosuppressives (cyclosporine A), cardiac glycosides (digoxin) and steroid hormones (aldosterone, cortisol and dexamethasone) [3], [4]. P-gp is also expressed in number of normal tissues where it plays a role in absorption, distribution and excretion. It is present at the biliary canalicular surface of hepatocytes, luminal surface of cells of the jejunum and colon, the kidney and the apical membrane of the placental syncytiotrophoblast [5], [6], [7], [8]. High levels of P-gp are also found in the luminal membranes of the endothelial cells that line the microvessels of the blood-brain barrier (BBB) and blood-testis barrier (BTB) where it functions to prevent transfer of compounds from the blood into the brain and testis [9], [10], [11], [12].
The presence of drug efflux transporters in the blood capillary cells of the BBB and BTB present a major clinical obstacle: many lipophilic therapeutic agents are rapidly extruded out, and thus fail to reach efficacious levels within the brain and/or testis. This has been demonstrated in patients treated for brain or testicular tumours, epilepsy, brain HIV, Alzheimer's disease and Parkinson's disease [13], [14], [15]. Thus, the search for P-gp reversal agents has been an area of intense research [16]. The first generation candidates were found to require effective doses that exceeded their safety limits, leading to adverse side effects and toxicity. The second and third generation candidates while less toxic, have produced highly variable results in clinical trials [17], [18], [19].
Recent studies have demonstrated that the selective serotonin reuptake inhibitors (SSRI) are substrates of P-gp [20]. In L-MDR1 cells and primary porcine brain endothelial cells it was shown that sertraline, its metabolite desmethylsertraline, and paroxetine inhibited P-gp function. These effects were comparable to the potency of the classic P-gp inhibitor, quinidine [21]. Further, the cytotoxicity of vinblastine, mitomycin C, paclitaxel and doxorubicin was increased in cancer cell lines when these drugs were administered simultaneously with fluoxetine. This was also found in vivo, with reduced tumour volumes in mice co-administered doxorubicin and fluoxetine compared to doxorubicin alone [16], [22].
A recent study in pregnant mice has shown that sertraline increased P-gp mediated substrate efflux in the placenta, but reduced P-gp mediated substrate efflux in the maternal and fetal BBB, 4 hours after administration [23]. Therefore, the aim of the current study was to determine the time course of the inhibition of P-gp at the BBB and BTB by sertraline and fluoxetine. As such, we established the most effective dose for SSRI mediated modulation of P-gp using guinea pig primary brain endothelial cells. We then determined whether these effects could be repeated in an in vivo mouse model.
Materials and Methods
Primary Endothelial Cell Culture
Primary brain microvascular endothelial cells were isolated from 6 two-week old male guinea pigs, as been described in details previously [24]. Animals were anesthetized with isoflurane (2–3%, IsoFlo®, Isoflurane, USP, Abbott Laboratories, Limited, Saint-Laurent, Quebec) prior to euthanasia by decapitation. All animal studies were performed using protocols approved by the Animal Care Committee at the University of Toronto (Protocol: 20007062) and in accordance with the Canadian Council for Animal Care.
Brains were rapidly removed and homogenized on ice in sterile Medium 199 (Invitrogen, Carlsbad, CA) supplemented with antibiotics-antimycotics (Invitrogen). Homogenates were centrifuged at 1000 g for 5 minutes at 4°C. Pellets were resuspended in 17.5% Dextran/Hank's Balanced Salt Solution, (Sigma-Aldrich, St. Louis, MO) and centrifuged at 4200 g for 15 minutes at 4°C. The vessel fraction was digested in Collagenase I (1 mg/mL in Dulbecco's Modified Eagle Medium (DMEM); Sigma-Aldrich) for 15 minutes at 37°C. Following enzymatic digestion, the vessel/collagenase mixture was centrifuged at 500 g for 10 minutes at 4°C. The collagenase supernatant was removed and cells were resuspended in culture medium. Cells were grown to confluence in (0.5%) gelatin-coated flasks (Becton Dickinson Biosciences, Franklin Lakes, NJ), trypsinized, and re-plated in gelatin-coated 96-well plates (Becton Dickinson Biosciences). At confluence, cells were utilized for calcein accumulation assay. We have previously extensively characterized the cells derived through these isolation and culture conditions and have shown that cells stain positive for von Willebrand factor, glucose transporter 1 and zonula occludens 1, confirming endothelial phenotype [24].
Calcein Accumulation Assay
Calcein-acetoxymethyl (AM) is a specific substrate of P-gp and is actively extruded from brain microvascular endothelial cells. Once in the cell, calcein-AM is rapidly and irreversibly cleaved by non-specific esterases to form fluorescent calcein. Calcein, which is not a substrate of P-gp, remains in the cell. Therefore, accumulation of fluorescent calcein is used as a reliable measure of P-gp function [24].
Media was aspirated from cells grown in 96-well plates, and washed with Tyrode's Salt solution supplemented with 1 g/L sodium bicarbonate (Sigma-Aldrich, St. Louis, MO). A 200 µL Tyrode mixture containing 1 µM calcein-AM (Sigma-Aldrich) and sertraline (Sigma-Aldrich; 10−3–10−8 M), fluoxetine (Sigma-Aldrich; 10−3–10−6 M) or verapamil (Sigma-Aldrich; 10−3, 10−4 M) was added to wells. Control wells contained calcein-AM (1 µM) with no SSRI, and background was established with Tyrode solution alone. All treatments were undertaken in octuplet in 6 independent experiments with brain endothelial cells from 6 independent guinea pigs. Sertraline and verapamil treated cells were incubated for 15, 60, 120 and 240 minutes. Fluoxetine treated cells were incubated for 15 and 60 minutes. At the end of incubation, plates were put on ice to stop transfer, and washed twice with cold Tyrode solution, lysed, and accumulation of fluorescent calcein was measured using a fluorescent plate reader (Excitation/Emission: 485/510 nm; Biotek, Winooski, VT). Relative fluorescence is presented as percent control well fluorescence with background subtracted [24]. The cytotoxic effects of sertraline, fluoxetine and verapamil were assessed using trypan blue as previously described [24].
Animal Studies
Animals
Male FVB mice (12–20 weeks of age) were purchased from Taconic (Germantown, NY). There were 6–9 control, 4–7 sertraline-treated and 4–7 fluoxetine treated mice used in each experimental group. Mice were housed (3–4/cage) with food and water available ad libitum.
Experimental Protocol
The protocol was adapted from studies previously performed in our and other laboratories [6], [25]. Mice were intravenously injected (tail vein) with either fluoxetine or sertraline (10 mg/kg) and [3H] digoxin (mixture: 0.05 mg/kg, unlabeled digoxin (Sigma-Aldrich) with [3H] digoxin 1 µCi/30 g body weight (PerkinElmer, Boston, MA)). To determine the time course of the fluoxetine effects (10 mg/kg), experiments were conducted as above, however mice were killed 5 minutes, 15 minutes, 1, 4, 12 and 24 hours after injection of fluoxetine and [3H] digoxin. For the sertraline time course experiment (10 mg/kg) an additional 1 minute time point was included. Control mice were injected with [3H] digoxin and saline to control for volume. At each time point, the number of mice in each treatment group was as follows (control, n = 6–9; sertraline, n = 4–7; fluoxetine, n = 4–7). At 1 and 5 minutes, mice were killed with isoflurane. For the remaining time points, mice were killed with an intraperitoneal injection of sodium pentobarbital (120 mg/kg, MTC Pharmaceuticals, ON). Blood was collected via cardiac puncture into tubes containing heparin, and plasma was separated by centrifugation. In addition to plasma, the brain, left and right testes, heart, a portion of the right lobe of the liver and left kidney were collected to measure accumulation of [3H] digoxin. Digoxin is not metabolized by the cytochrome P450 enzymes and is considered to be the benchmark substrate for assessing P-gp activity [6], [19], [25], [26].
Tissue Processing
Tissues were processed as described previously [25], [27]. Briefly, the brain, testes, heart, liver and kidney were homogenized in PBS (2 µL/g of tissue) and an aliquot of tissue homogenate (200 µL brain, liver and kidney; 100 µL testis and heart) and plasma (100 µL) were solubilized in SOLVABLE (PerkinElmer). Hydrogen peroxide (30%; 100 uL) was then added to decolorize samples and optimize counting efficiency prior to addition of scintillation fluid (10 mL; Ultima-Gold, PerkinElmer). Radioactivity (disintegrations per minute; DPM) was determined on a Tri-Carb Beta-Counter (PerkinElmer, address). Levels of [3H] digoxin are presented as a ratio of the tissue to plasma concentration to represent penetration into the tissue, or as raw DPM counts.
Statistical Analysis
Data is presented as mean ± standard error of the mean (SEM). Data from the left and right testis were not significantly different and were averaged. Calcein uptake was analyzed using 1-way analysis of variance (ANOVA) with Dunnett's post hoc. Time data was analyzed using unpaired t-test between control and SSRI treated mice. If the variance was found to differ significantly, data was log-transformed and reanalyzed. If variance remained significantly different, the Mann-Whitney U test was used. Significance was set at p<0.05.
Results
In Vitro: Time-dependent effects of SSRI on P-gp function
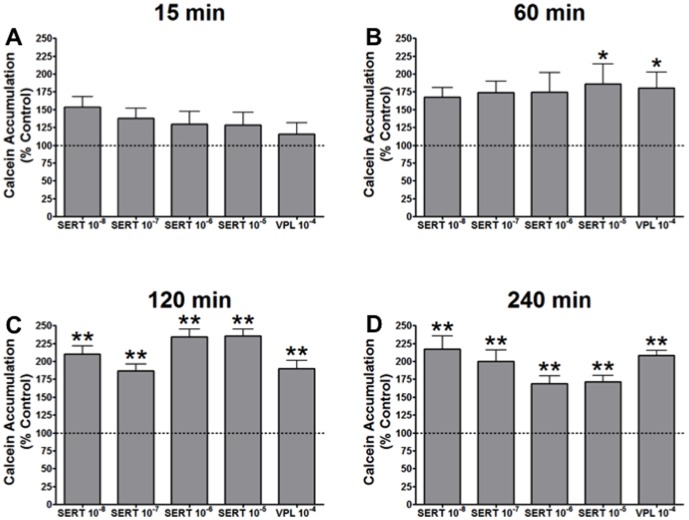
All data are presented as the percentage of the fluorescent calcein accumulation in untreated control cells. Therefore, an increase in relative calcein accumulation represents inhibition of P-gp function. There was no significant increase in calcein accumulation at 15 minutes with sertraline treatment (Fig. 1A). At 60 minutes, sertraline treatment (10−5 M) resulted in an 86% increase in calcein accumulation compared to control cells (p<0.05; Fig. 1B). By 120 minutes, all doses of sertraline resulted in significant inhibition of P-gp function – peaking at a 135% increase in calcein accumulation in cells treated with 10−5 M sertraline (p<0.01; Fig. 1C). Likewise at 240 minutes, all doses of sertraline displayed significant inhibition of P-gp function (P<0.05; Fig. 1D).
Figure 1. Calcein accumulation in guinea pig primary brain endothelial cells (n = 6) presented as % of control with increasing doses of sertraline (SERT) and 10−4 of verapamil (VPL).
Calcein accumulation was assessed at (A) 15 min, (B) 60 min, (C), 120 min and (D) 240 min. * indicates p<0.05 and ** indicates p<0.01 compared to control cells.
Levels of inhibition caused by sertraline treatment were comparable to the inhibition induced by treatment with the commonly used P-gp inhibitor, verapamil (Fig. 2). P-gp inhibition by verapamil (10−4 M) followed a similar profile to that observed with sertraline (10−5 M). Treatment of cells with fluoxetine (10−5 M) resulted in no inhibition of P-gp at any of the time points tested (Fig. 2). Higher doses of sertraline and fluoxetine (10−3 & 10−4), and verapamil (10−3) resulted in significant cell death (>90%; data not shown) as determined by Trypan blue staining.
Figure 2. Calcein accumulation in guinea pig primary brain endothelial cells (n = 6) presented as % of control with 10−5 dose of sertraline (SERT), fluoxetine (FLX) and 10−4 of verapamil (VPL) assessed at 60 min.

* indicates p<0.05 compared to control cells.
In Vivo: Time-dependent effects of SSRI on P-gp function
Analysis of the brain to plasma DPM ratio revealed that [3H] digoxin levels were significantly higher in the brains of the sertraline treated mice at 5 minutes (Fig. 3A; p<0.05). Conversely, at 1 hour, sertraline treatment resulted in a lower brain to plasma [3H] digoxin ratio (p<0.01). However, by 4 hours, sertraline treated mice again demonstrated a higher brain to plasma ratio of [3H] digoxin (p<0.05) compared to vehicle-treated controls. Analysis of the absolute brain DPM showed that [3H] digoxin levels were significantly elevated in the sertraline treated mice at 4 (p<0.05) and 12 hours (p<0.01) compared to saline treated mice (Fig. 3B). Analysis of the absolute plasma DPM levels (Fig. 3C) revealed no difference between control and sertraline groups, except at 12 hours, when there was a significant elevation of plasma [3H] digoxin levels in sertraline-treated compared to the control mice (p<0.01).
Figure 3. Accumulation of [3H] digoxin in FVB mice (n = 4–9/group) co-administered sertraline (10 mg/kg; closed bars) or saline (control; open bars).

Mice were euthanized at various time points (1 min, 5 min, 15 min, 1 h, 4 h, 12 h, 24 h) and DPM measured in the brain, testes and plasma. All data is expressed as mean±SEM. (A) brain∶plasma DPM ratio, (B) brain DPM, (C) plasma DPM. * indicates p<0.05 vs. control. ** indicates p<0.01 vs. control.
In the testes (Fig. 4A), a very similar pattern of digoxin accumulation occurred. Sertraline treatment resulted in an increase in the testes to plasma [3H] digoxin ratio at 1 minute (p<0.05), a decrease at 1 hour (p<0.01) and an increase at 4 hours (p<0.01). Analysis of the heart to plasma DPM [3H] digoxin ratio revealed that at 4 hours the ratio was significantly higher in the mice treated with sertraline compared to control (Fig. 4B; p<0.01). There was no effect of sertraline on the liver or kidney to plasma [3H] digoxin ratios at any time point (data not shown). There was no significant effect of fluoxetine on accumulation [3H] digoxin in the brain (Table 1) or testes (Table 2) at any time point.
Figure 4. Accumulation of [3H] digoxin in FVB mice (n = 4–9) co-administered sertraline (10 mg/kg; closed bars) or saline (control; open bars).

Mice were euthanized at various time points (1 m, 5 min, 15 min, 1 h, 4 h, 12 h, 24 h) and DPM measured in the testes, heart and plasma. All data is expressed as mean±SEM. (A) testes∶plasma DPM ratio, (B) heart∶plasma DPM ratio. * indicates p<0.05 vs. control. ** indicates p<0.01 vs. control.
Table 1. Brain to plasma ratio (mean ± SEM) of [3H] digoxin in mice intravenously injected with fluoxetine (10 mg/kg) or saline (control) with digoxin (0.05 mg/kg) at different times.
| Treatment | Time | |||||
| 5 minutes | 15 minutes | 1 hour | 4 hours | 12 hours | 24 hours | |
| Control | 0.0187±0.0017 | 0.0430±0.012 | 0.617±0.08 | 0.481±0.12 | 1.01±0.28 | 1.32±0.16 |
| Fluoxetine | 0.0177±0.003 | 0.0339±0.012 | 0.426±0.076 | 0.262±0.071 | 0.987±0.60 | 1.79±0.33 |
N = 4–10 mice.
Table 2. Testes to plasma ratio (mean ± SEM) of [3H] digoxin in mice intravenously injected with fluoxetine (10 mg/kg) or saline (control) with digoxin (0.05 mg/kg) at different times.
| Treatment | Time | |||||
| 5 minutes | 15 minutes | 1 hour | 4 hours | 12 hours | 24 hours | |
| Control | 0.0294+0.007 | 0.253+0.116 | 1.136+0.809 | 0.5135+0.209 | 0.923+0.223 | 1.924+1.419 |
| Fluoxetine | 0.0334+0.005 | 0.360+0.002 | 0.101+0.019 | 0.360+0.053 | 0.739+0.575 | 1.280+0.382 |
N = 4–10 mice.
Discussion
The aim of the current study was to determine if sertraline or fluoxetine could inhibit P-gp activity in the BBB and the BTB and, as such, facilitate the accumulation of therapeutic agents in the brain and testis, respectively. Over 24 hours, we investigated the time course of the effect. In vitro, sertraline treatment of primary brain endothelial cells resulted in significant inhibition of P-gp, even at the lowest dose tested (10−8 M). Indeed, sertraline was as potent an inhibitor of P-gp as the widely used inhibitor, verapamil. In contrast, P-gp function in brain endothelial cells was not altered by fluoxetine at any dose or time point, in vitro. In vivo, we demonstrated that there was a biphasic time-dependent effect of sertraline on the activity of P-gp at the BBB and BTB. Sertraline led to increased brain accumulation of digoxin at 5 minutes and 240 minutes (i.e. inhibited P-gp activity), but reduced accumulation of digoxin at 60 minutes (i.e. stimulated P-gp activity). An identical profile was identified at the BTB. Consistent with the in vitro evidence, in vivo, fluoxetine did not significantly affect P-gp function at the BBB or the BTB.
A recent study demonstrated that the effect of sertraline on P-gp activity is tissue-specific [23]. Simultaneous administration of [3H] digoxin and sertraline to pregnant mice resulted in inhibition of P-gp activity at the maternal and fetal BBB, and thus increased brain accumulation of [3H] digoxin. However, at the placental barrier, there was an increase in P-gp activity 240 minutes after administration. The findings in the brain are in line with the present study where we demonstrated increased accumulation of [3H] digoxin at 240 minutes in the brains of adult male mice. The consistency of the findings demonstrates that sertraline is an inhibitor of P-gp in endothelial cells of the brain in male, female and fetal tissues [23].
The effect of sertraline on P-gp activity at barrier sites in vivo, was biphasic. We demonstrated a substantial initial inhibition of P-gp by sertraline at 5 minutes in the brain and similar inhibition was identified at 1 minute in the testis. The initial effects of sertraline were highly transient at both of these barrier sites. Indeed, at 15 minutes (brain) and 5 minutes (testes) there was no difference in tissue accumulation of [3H] digoxin between the vehicle and sertraline treated animals. At 1 hour, sertraline-treated mice exhibited a decreased brain and testis to plasma ratio of [3H] digoxin compared to control, indicating that at that time, sertraline was increasing P-gp activity at both the BBB and BTB. In contrast, at 4 hours, there was once again increased accumulation within the brains and testes of sertraline treated animals, indicating inhibition of P-gp activity. One explanation for the biphasic relationship is the potential for the sertraline metabolite, desmethylsertraline, to inhibit P-gp, in vivo. Indeed, it has been shown in vitro in two cell types that the metabolite is a potent inhibitor of P-gp [21]. Future studies evaluating the effects of specific administration of desmethylsertraline in vivo would determine the potential of the metabolite to inhibit P-gp. Another possible explanation for the reduced digoxin accumulation in the brain at 1 h following sertraline treatment might involve changes in P-gp-independent transport of digoxin. In this connection, the organic anion transporter (Oatp1a4) has been shown to have some specificity for digoxin [28].
In the current study, there was no effect of fluoxetine on [3H] digoxin accumulation in vivo, in line with the in vitro results. In other studies, there have been mixed findings on the effect of fluoxetine on P-gp activity. In L-MDR1, a porcine kidney epithelial cell line transfected with the human MDR1 gene, and porcine primary brain endothelial cells fluoxetine and its metabolites were not found to be potent inhibitors of P-gp at 60 minutes [21]. However, in tumor cells, an inhibitory effect of fluoxetine on P-gp function has been described [16], [22]. Mice co-administered doxorubicin and fluoxetine exhibited reduced tumor volumes compared to those given doxorubicin alone [16]. In another study, cytotoxicity and intracellular accumulation of doxorubicin were increased following fluoxetine treatment of cells derived from a human colorectal adenocarcinoma [22]. One possibility for the lack of an effect of fluoxetine on P-gp in the present study is the duration of treatment. Our study and those of Weiss et al. determined transport effects of fluoxetine over 0–240 minutes. In the studies by Peer et al. and Argov et al., cells were incubated for 10 hours and 2 hours respectively, and mice were treated over a 3 week period [16], [22].
Of particular clinical interest is the finding that at 4 hours sertraline treatment resulted in a doubling of accumulation of digoxin in the heart. P-gp is expressed in the ventricular myocardium in human tissue. It is localized in the arterioles and capillaries where its function is to extrude substances back into circulation [29]. Consistent with our results, it has previously been found in abcb1a/b (−/−) mice that after 4 hours, digoxin levels were higher in the heart than wild-type controls [6]. The fact that sertraline can potently inhibit P-gp function in the heart leading to accumulation of drug substrates, may provide opportunities for improving efficacy of cardiac drugs.
Although P-gp is one of the main transporters in the brain, testis and other sites throughout the body, there are other transporters that are also functionally important. Breast cancer resistance protein (BCRP) is another ABC drug efflux protein found in various tissues such as the vascular endothelium, the epithelium of the small intestines and the proximal tubules of the kidney where it affects absorption, distribution and excretion of xenobiotics [30]. To our knowledge however, there are currently no studies that have investigated the use of fluoxetine or sertraline on BCRP activity.
In conclusion, we have shown that there is a biphasic effect of sertraline on P-gp activity at the BBB in vivo, and that this effect is mirrored in the BTB. The complexities of the effects of sertraline and fluoxetine in the regulation of P-gp that we have identified in vivo, likely underlie the inconsistencies that have emerged in the clinical use of P-gp reversal agents in clinical trials. The fact that acute treatment with sertraline can modulate P-gp function at important blood-barrier sites, in vivo, without causing major affects on plasma pharmacokinetics, may be utilized in the development of future therapeutics targeted at sites normally protected by P-gp and must be taken into consideration when studying drug-drug interactions.
Funding Statement
This study was funded by the Canadian Institutes for Health Research 452740 to WG and SGM and Doctoral Research Award to MI. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gros P, Ben Neriah YB, Croop JM, Housman DE (1986) Isolation and expression of a complementary DNA that confers multidrug resistance. Nature 323: 728–31. [DOI] [PubMed] [Google Scholar]
- 2. Uhr M, Grauer MT (2003) abcb1ab P-glycoprotein is involved in the uptake of citalopram and trimipramine into the brain of mice. J Psychiatr Res 37: 179–85. [DOI] [PubMed] [Google Scholar]
- 3. Seelig A (1998) A general pattern for substrate recognition by P-glycoprotein. Eur J Biochem 251: 252–61. [DOI] [PubMed] [Google Scholar]
- 4. Seelig A, Blatter XL, Wohnsland F (2000) Substrate recognition by P-glycoprotein and the multidrug resistance-associated protein MRP1: a comparison. Int J Clin Pharmacol Ther 38: 111–21. [DOI] [PubMed] [Google Scholar]
- 5. Bansal T, Jaggi M, Khar RK, Talegaonkar S (2009) Emerging significance of flavonoids as P-glycoprotein inhibitors in cancer chemotherapy. J Pharm Pharm Sci 12: 46–78. [DOI] [PubMed] [Google Scholar]
- 6. Schinkel AH, Wagenaar E, van Deemter L, Mol CA, Borst P (1995) Absence of the mdr1a P-Glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J Clin Invest 96: 1698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sun M, Kingdom J, Baczyk D, Lye SJ, Matthews SG, et al. (2006) Expression of the multidrug resistance P-glycoprotein, (ABCB1 glycoprotein) in the human placenta decreases with advancing gestation. Placenta 27: 602–9. [DOI] [PubMed] [Google Scholar]
- 8. van der Valk P, van Kalken CK, Ketelaars H, Broxterman HJ, Scheffer G, et al. (1990) Distribution of multi-drug resistance-associated P-glycoprotein in normal and neoplastic human tissues. Analysis with 3 monoclonal antibodies recognizing different epitopes of the P-glycoprotein molecule. Ann Oncol 1: 56–64. [PubMed] [Google Scholar]
- 9. Cordon-Cardo C, O'Brien JP, Boccia J, Casals D, Bertino JR, et al. (1990) Expression of the multidrug resistance gene product (P-glycoprotein) in human normal and tumor tissues. J Histochem Cytochem 38: 1277–87. [DOI] [PubMed] [Google Scholar]
- 10. Cordon-Cardo C, O'Brien JP, Casals D, Rittman-Grauer L, Biedler JL, et al. (1989) Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci U S A 86: 695–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, et al. (1989) Immunohistochemical localization in normal tissues of different epitopes in the multidrug transport protein P170: evidence for localization in brain capillaries and crossreactivity of one antibody with a muscle protein. J Histochem Cytochem 37: 159–64. [DOI] [PubMed] [Google Scholar]
- 12. Tsuji A, Terasaki T, Takabatake Y, Tenda Y, Tamai I, et al. (1992) P-glycoprotein as the drug efflux pump in primary cultured bovine brain capillary endothelial cells. Life Sci 51: 1427–37. [DOI] [PubMed] [Google Scholar]
- 13. Bart J, Hollema H, Groen HJ, de Vries EG, Hendrikse NH, et al. (2004) The distribution of drug-efflux pumps, P-gp, BCRP, MRP1 and MRP2, in the normal blood-testis barrier and in primary testicular tumours. Eur J Cancer 40: 2064–70. [DOI] [PubMed] [Google Scholar]
- 14. Loscher W, Potschka H (2005) Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog Neurobiol 76: 22–76. [DOI] [PubMed] [Google Scholar]
- 15. Luna-Tortos C, Fedrowitz M, Loscher W (2010) Evaluation of transport of common antiepileptic drugs by human multidrug resistance-associated proteins (MRP1, 2 and 5) that are overexpressed in pharmacoresistant epilepsy. Neuropharmacology 58: 1019–32. [DOI] [PubMed] [Google Scholar]
- 16. Peer D, Dekel Y, Melikhov D, Margalit R (2004) Fluoxetine inhibits multidrug resistance extrusion pumps and enhances responses to chemotherapy in syngeneic and in human xenograft mouse tumor models. Cancer Res 64: 7562–9. [DOI] [PubMed] [Google Scholar]
- 17. Carlson RW, O'Neill AM, Goldstein LJ, Sikic BI, Abramson N, et al. (2006) A pilot phase II trial of valspodar modulation of multidrug resistance to paclitaxel in the treatment of metastatic carcinoma of the breast (E1195): a trial of the Eastern Cooperative Oncology Group. Cancer Invest 24: 677–81. [DOI] [PubMed] [Google Scholar]
- 18. Lhomme C, Joly F, Walker JL, Lissoni AA, Nicoletto MO, et al. (2008) Phase III study of valspodar (PSC 833) combined with paclitaxel and carboplatin compared with paclitaxel and carboplatin alone in patients with stage IV or suboptimally debulked stage III epithelial ovarian cancer or primary peritoneal cancer. J Clin Oncol 26: 2674–82. [DOI] [PubMed] [Google Scholar]
- 19. Mayer U, Wagenaar E, Dorobek B, Beijnen JH, Borst P, et al. (1997) Full blockade of intestinal P-glycoprotein and extensive inhibition of blood-brain barrier P-glycoprotein by oral treatment of mice with PSC833. J Clin Invest 100: 2430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Uhr M, Grauer MT, Holsboer F (2003) Differential enhancement of antidepressant penetration into the brain in mice with abcb1ab (mdr1ab) P-glycoprotein gene disruption. Biol Psychiatry 54: 840–6. [DOI] [PubMed] [Google Scholar]
- 21. Weiss J, Dormann SM, Martin-Facklam M, Kerpen CJ, Ketabi-Kiyanvash N, et al. (2003) Inhibition of P-glycoprotein by newer antidepressants. J Pharmacol Exp Ther 305: 197–204. [DOI] [PubMed] [Google Scholar]
- 22. Argov M, Kashi R, Peer D, Margalit R (2009) Treatment of resistant human colon cancer xenografts by a fluoxetine-doxorubicin combination enhances therapeutic responses comparable to an aggressive bevacizumab regimen. Cancer Lett 274: 118–25. [DOI] [PubMed] [Google Scholar]
- 23. Bhuiyan M, Petropoulos S, Gibb W, Matthews SG (2012) Sertraline alters multidrug resistance phosphoglycoprotein activity in the mouse placenta and fetal blood-brain barrier. Repro Sci 19: 407–415. [DOI] [PubMed] [Google Scholar]
- 24. Iqbal M, Gibb W, Matthews SG (2011) Corticosteroid regulation of P-glycoprotein in the developing blood-brain barrier. Endocrinology 152: 1067–79. [DOI] [PubMed] [Google Scholar]
- 25. Petropoulos S, Kalabis GM, Gibb W, Matthews SG (2007) Functional changes of mouse placental multidrug resistance phosphoglycoprotein (ABCB1) with advancing gestation and regulation by progesterone. Reprod Sci 14: 321–8. [DOI] [PubMed] [Google Scholar]
- 26. Endres CJ, Hsiao P, Chung FS, Unadkat JD (2006) The role of transporters in drug interactions. Eur J Pharm Sci 27: 501–17. [DOI] [PubMed] [Google Scholar]
- 27. Petropoulos S, Gibb W, Matthews SG (2010) Effect of glucocorticoids on regulation of placental multidrug resistance phosphoglycoprotein (P-gp) in the mouse. Placenta 31: 803–10. [DOI] [PubMed] [Google Scholar]
- 28. Westholm DE, Rumbley JN, Salo DR, Rich TP, Anderson GW (2008) Organic anion-transporting polypeptides at the blood-brain and blood-cerebrospinal fluid barriers. Curr Top Dev Biol 80: 135–70. [DOI] [PubMed] [Google Scholar]
- 29. Solbach TF, Konig J, Fromm MF, Zolk O (2006) ATP-binding cassette transporters in the heart. Trends Cardiovasc Med 16: 7–15. [DOI] [PubMed] [Google Scholar]
- 30. Kalabis GM, Petropoulos S, Matthews SG (2007) Breast cancer resistance protein (Bcrp1/Abcg2) in mouse placenta and yolk sac: ontogeny and its regulation by progesterone. Placenta 10: 1073–81. [DOI] [PubMed] [Google Scholar]