Abstract
The plant-specific LBD (LATERAL ORGAN BOUNDARIES domain) genes belong to a major family of transcription factor that encode a zinc finger-like domain. It has been shown that LBD genes play crucial roles in the growth and development of Arabidopsis and other plant species. However, no detailed information concerning this family is available for apple. In the present study, we analyzed the apple (Malus domestica) genome and identified 58 LBD genes. This gene family was tested for its phylogenetic relationships with homologous genes in the Arabidopsis genome, as well as its location in the genome, structure and expression. We also transformed one MdLBD gene into Arabidopsis to evaluate its function. Like Arabidopsis, apple LBD genes also have a conserved CX2CX6CX3C zinc finger-like domain in the N terminus and can be divided into two classes. The expression profile indicated that apple LBD genes exhibited a variety of expression patterns, suggesting that they have diverse functions. At the same time, the expression analysis implied that members of this apple gene family were responsive to hormones and stress and that they may participate in hormone-mediated plant organogenesis, which was demonstrated with the overexpression of the apple LBD gene MdLBD11, resulting in an abnormal phenotype. This phenotype included upward curling leaves, delayed flowering, downward-pointing flowers, siliques and other abnormal traits. Based on these data, we concluded that the MdLBD genes may play an important role in apple growth and development as in Arabidopsis and other species.
Introduction
LBD (LATERAL ORGAN BOUNDARIES domain) gene in Arabidopsis is a newly discovered and unique transcription factor family that has been assigned to this functional group on the basis of its nuclear localization and capacity to bind to a DNA motif [1], [2], [3], [4], [5]. Until now, LBD genes were found only in plant databases, indicating that this unique gene family may only regulate plant-specific processes [6]. The LBD gene family can be divided into two classes according to the structure of the LOB (LATERAL ORGAN BOUNDARIES) domain in the N terminus [6], [7]. Class I LBD genes contain a perfectly conserved CX2CX6CX3C zinc finger-like domain and an LX6LX3LX6L leucine zipper-like coiled-coil motif, while class II LBD genes only have a conserved zinc finger-like domain [6], [8]. The zinc-finger-like domain is presumably required for DNA binding, and the C-terminal leucine zipper-like sequence is probably involved in protein dimerization [6], [7] LBD proteins have varied expression patterns ranging from temporal to tissue differences, suggesting that they may function in diverse processes [6]. Numerous LBD genes are expressed at the adaxial base of plant lateral organs, and they play critical roles in lateral organ development during a plant’s growth [4], [6], [9], [10]. When observed under normal growth conditions, no obvious phenotype could be found in some loss-of-function lbd mutants, indicating that the LBD gene is functionally redundant or required during growth under specific environmental conditions [1], [6], [11].
By now, several members of the LBD family have been functionally identified in different species. Among class I LBD genes, the LOB gene encodes a DNA-binding protein and can interact with members of the bHLH (basic helix-loop-helix) family of transcription factors, while the interaction between bHLH proteins and LOB reduced the affinity of LOB for its consensus DNA motif [2]. RA2 (Ramosa 2), a maize ortholog of LOB, has been shown to regulate reproductive growth [12], [13]. The AS2/AtLBD6 gene influence the expression of class I KNOX genes through its interaction with MYB domain transcription factor AtAS1 and ERECTA, thereby regulating the establishment of leaf polarity [8], [14], [15], [16], [17], [18], [19], [20], [21], [22]. AtASL1/AtLBD36 and AtLBD12/AtASL5 have phenotypes that are similar to those found in AtAS2/AtLBD6 overexpression plants [23].The maize ZmLBD19 gene dimerize with the maize AtAS1 ortholog RS2 (ROUGH SHEATH2) and influence female gametophyte development [20], [24]. Together with AtLBD16 and AtLBD29, AtLBD18 is regulated by AtARF7 and AtARF19 and plays a role in lateral root initiation [1], [3], [5], [25], [26], [27], [28]. Crl1/Arl1, which is a homolog of AtLBD29, has a similar function in rice [29], [30], [31]. The JAGGED LATERAL ORGANS/JLO gene regulates Arabidopsis embryo and root development [32], [33]. The expression of DDA1/LBD25 is reduced by exogenous auxin, and a dda1 mutant change the number of lateral roots [34]. AtLBD3/AtASL9 is induced by exogenous cytokinin treatment, indicating that this gene plays an important role in cytokinin-responsive biological events in plants [35]. PtaLBD1 regulates secondary growth in poplar [36]. The AtLBD20 gene takes part in jasmonate signaling in response to Fusarium wilt in Arabidopsis [37], AtLBD1 may be involved in sustaining indeterminate cell fate of SAMs in Cockscomb [38]. For class II AtLBD genes, AtLBD37, AtLBD38 and AtLBD39 are induced by nitrate and involved in anthocyanin synthesis and nitrate metabolism [11]. AtLBD41 is proposed to be involved in leaf dorsoventral determination [10]. Extensive studies of the AtLBDs in various plant species have provided a better understanding of this gene family. As of this publication, 43 Arabidopsis LBD proteins and 35 of rice homologs have been found [6], [39]. In maize and poplar, the LBD gene family consists of 43 and 57 members [4], [40], [41], respectively. Most recently, 3 LBD genes are considered as strong candidates of Co locus controlling columnar growth habit in apple [42], suggesting that LBD genes may play an important role in the control of apple tree architecture. However, it is yet unknown how many LBD genes in apple genome and how about their genomic organization in 17 apple chromosomes.
In this study, a genome-wide survey of the LBD gene family was conducted using the apple genome database [43]. The genome sequences of all apple LBD genes were identified with online software. Their genome structure, chromosome distribution and expression patterns were analyzed in silico. Subsequently, real time PCRs and semi-quantitative RT-PCRs were carried out to confirm the expression patterns in different organs and in response to hormones and various stimuli. In addition, MdLBD11 was functionally characterized in transgenic Arabidopsis. This study will serve as a foundation for future research into the functional roles of MdLBD genes.
Results
The Identification and Annotation Information of the LBD Genes in Apple
To identify the LBD proteins in apple, a local BLAST program and the Hidden Markov Model of the SMART and Pfam tools were used, and a total of 58 LBD-like genes from the entire apple genome were identified. We discovered that all of these LBD-like protein sequences possess a modular structure and conserved LOB motifs. As a result, 58 putative MdLBD proteins were found (Table 1). And we named each gene on the basis of its location on the chromosome. We noted the gene identifier, genomic position, pI (isoelectric point), length of amino acid, and protein size in Table 1. From the table, we can see that all the identified LBD genes encode proteins ranging from 130 (MdLBD47) to 431 (MdLBD26) amino acids along with a protein mass from 14.9 kD to 44.3 kD and protein pIs ranging from 4.63 (MdLBD51) to 9.69 (MdLBD47).
Table 1. Gene search and genetic nomenclature based on chromosomal position.
| Gene identifier | Gene name | Genomic position | Size(aa) | Mass(Da) | pI |
| MDP0000151784 | MdLBD1 | chr01∶14333863.14334877 | 5.83 | 24997.89 | 230 |
| MDP0000123013 | MdLBD2 | chr01∶14336168.14337182 | 5.83 | 24997.89 | 230 |
| MDP0000879799 | MdLBD3 | chr01∶14339802.14340857 | 5.93 | 25224.84 | 232 |
| MDP0000319331 | MdLBD4 | chr01∶16654621.16655986 | 5.31 | 22966.92 | 212 |
| MDP0000123797 | MdLBD5 | chr01∶18212023.18212767 | 5.05 | 24303.39 | 216 |
| MDP0000220708 | MdLBD6 | chr01∶21271862.21272392 | 8.37 | 19277.78 | 176 |
| MDP0000194766 | MdLBD7 | chr01∶21348445.21349196 | 6.36 | 18781.5 | 168 |
| MDP0000278334 | MdLBD8 | chr01∶21731069.21732173 | 9.06 | 28901.21 | 261 |
| MDP0000167580 | MdLBD9 | chr01∶21747577.21749606 | 9.21 | 21690.61 | 189 |
| MDP0000193830 | MdLBD10 | chr02∶15182073.15182800 | 8.26 | 19754.84 | 181 |
| MDP0000562305 | MdLBD11 | chr02∶15438443.15439345 | 8.22 | 32845.8 | 300 |
| MDP0000305695 | MdLBD12 | chr02∶17692925.17693725 | 9.56 | 27020.33 | 245 |
| MDP0000317227 | MdLBD13 | chr02∶17696966.17697805 | 9.19 | 30751.13 | 279 |
| MDP0000254224 | MdLBD14 | chr02∶22462044.22466145 | 7.22 | 28591.6 | 273 |
| MDP0000429067 | MdLBD15 | chr02∶29675335.29678584 | 8.18 | 24836.09 | 228 |
| MDP0000861160 | MdLBD16 | chr03∶5441634.5442565 | 6.42 | 19506.22 | 174 |
| MDP0000318244 | MdLBD17 | chr03∶9623522.9625101 | 5.51 | 23040.14 | 213 |
| MDP0000218986 | MdLBD18 | chr03∶9636639.9638218 | 5.51 | 23040.14 | 213 |
| MDP0000943252 | MdLBD19 | chr04∶2582353.2582886 | 8.03 | 19402.92 | 177 |
| MDP0000155138 | MdLBD20 | chr04∶2667092.2667817 | 5.8 | 18924.55 | 169 |
| MDP0000643326 | MdLBD21 | chr04∶22958911.22959657 | 7.25 | 27154.82 | 248 |
| MDP0000133546 | MdLBD22 | chr04∶22964675.22965421 | 6.94 | 25710.46 | 235 |
| MDP0000294210 | MdLBD23 | chr05∶4850682.4853618 | 6.08 | 43454.21 | 394 |
| MDP0000197526 | MdLBD24 | chr05∶7149837.7150580 | 6.29 | 27026.23 | 247 |
| MDP0000777060 | MdLBD25 | chr05∶7775605.7776396 | 6.04 | 28568.98 | 263 |
| MDP0000284409 | MdLBD26 | chr05∶11232036.11242489 | 8.54 | 47730.03 | 431 |
| MDP0000261146 | MdLBD27 | chr05∶25844239.25845114 | 8.64 | 25111.65 | 224 |
| MDP0000247079 | MdLBD28 | chr05∶25883261.25884136 | 8.81 | 25148.14 | 224 |
| MDP0000753623 | MdLBD29 | chr06∶11066243.11066769 | 8.28 | 15775.72 | 139 |
| MDP0000130659 | MdLBD30 | chr06∶19262057.19262656 | 8.55 | 21504.55 | 199 |
| MDP0000153290 | MdLBD31 | chr06∶20514370.20515613 | 6.14 | 41855.15 | 369 |
| MDP0000697271 | MdLBD32 | chr07∶5286028.5286741 | 8.38 | 26658.38 | 237 |
| MDP0000200379 | MdLBD33 | chr07∶5319673.5320383 | 8.58 | 26490.15 | 236 |
| MDP0000223109 | MdLBD34 | chr07∶17590374.17591071 | 5.88 | 20009.99 | 172 |
| MDP0000822986 | MdLBD35 | chr07∶20982923.20984041 | 6.19 | 25177.01 | 231 |
| MDP0000276955 | MdLBD36 | chr08∶8082319.8082765 | 8.26 | 16593.61 | 148 |
| MDP0000487015 | MdLBD37 | chr08∶12183285.12186216 | 8.42 | 31397.98 | 288 |
| MDP0000747679 | MdLBD38 | chr08∶12200557.12203488 | 8.42 | 31397.98 | 288 |
| MDP0000190306 | MdLBD39 | chr09∶2721683.2722786 | 5.92 | 34682.85 | 257 |
| MDP0000136037 | MdLBD40 | chr09∶5735343.5736376 | 8.45 | 32638.87 | 305 |
| MDP0000121821 | MdLBD41 | chr09∶12599870.12600742 | 6.36 | 17816.15 | 158 |
| MDP0000139025 | MdLBD42 | chr10∶8653279.8654145 | 7.53 | 23785.97 | 315 |
| MDP0000268890 | MdLBD43 | chr10∶21049069.21053744 | 7.64 | 19443.01 | 176 |
| MDP0000121203 | MdLBD44 | chr10∶24617746.24620393 | 9.06 | 25207.91 | 230 |
| MDP0000430686 | MdLBD45 | chr10∶27855557.27861011 | 6.55 | 44319.96 | 395 |
| MDP0000265429 | MdLBD46 | chr11∶5634618.5635555 | 6.78 | 19659.53 | 176 |
| MDP0000277239 | MdLBD47 | chr12∶24251934.24252987 | 9.69 | 14936.27 | 130 |
| MDP0000273432 | MdLBD48 | chr12∶31634852.31637028 | 9.04 | 33470.66 | 300 |
| MDP0000254288 | MdLBD49 | chr14∶24069525.24070194 | 5.08 | 23696.41 | 311 |
| MDP0000794101 | MdLBD50 | chr15∶1713236.1714021 | 7.46 | 24529.95 | 226 |
| MDP0000133936 | MdLBD51 | chr15∶4034464.4035214 | 4.63 | 24368.26 | 218 |
| MDP0000239820 | MdLBD52 | chr15∶25823210.25825670 | 8.72 | 29926.14 | 275 |
| MDP0000151330 | MdLBD53 | chr15∶25823768.25825218 | 9.03 | 36853.69 | 335 |
| MDP0000257227 | MdLBD54 | chr15∶33651689.33653060 | 6.07 | 35261.09 | 314 |
| MDP0000848210 | MdLBD55 | chr17∶2027358.2027888 | 6.89 | 19516.68 | 176 |
| MDP0000131964 | MdLBD56 | UN | 8.38 | 23121.3 | 213 |
| MDP0000145761 | MdLBD57 | UN | 8.38 | 22915.01 | 211 |
| MDP0000216231 | MdLBD58 | UN | 5.68 | 42576.48 | 375 |
Phylogenetic and Gene Structure Analysis of the LBD Genes
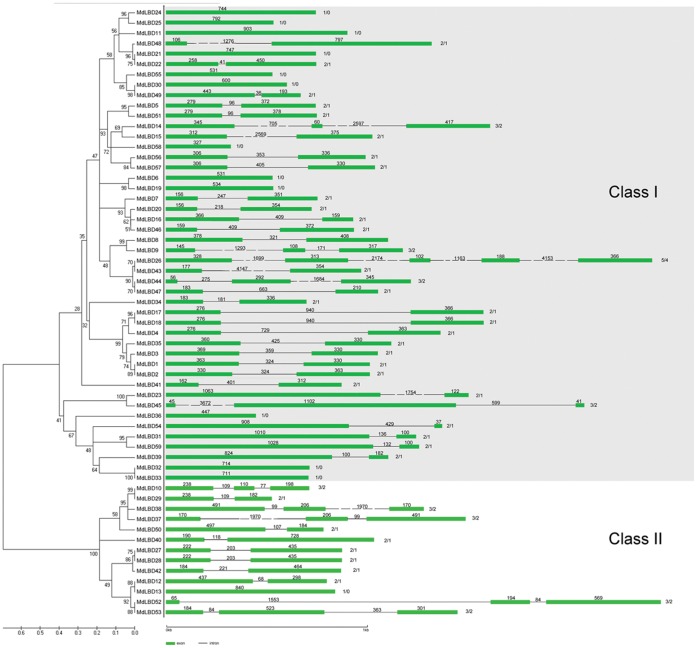
To evaluate the evolutionary relationships among the 58 apple LBD proteins, we performed a phylogenetic analysis based on their full length amino acid sequences. We identified two subfamilies (class I and class II) as being monophyletic (Fig. 1), and 11 sister pairs of paralogous LBDs (MdLBD5/51, MdLBD6/19, MdLBD8/9, MdLBD24/25, MdLBD30/49, MdLBD17/18, MdLBD23/45, MdLBD31/59, MdLBD31/59, MdLBD32/33 and MdLBD10/29) were found, 10 of which had very strong bootstrap support (>90%). Our results suggest a clear paralogous pattern of LBD gene divergence by gene duplication for the apple.
Figure 1. The phylogenetic tree and gene structure analysis of the MdLBD proteins.
The amino acid sequences of the LBD proteins were aligned with ClustalX, and the phylogenetic tree was constructed using the neighbor-joining method of MEGA 5.0 software. Each node is represented by a number that indicates the bootstrap value for 1000 replicates. The scale bar represents 0.1 substitutions per sequence position (left). The right side illustrates the exon-intron organization of the corresponding LBD genes. The exons and introns are represented by the green boxes and black lines, respectively. The numbers indicate the length of the exons or introns. The scale bar represents 1000 bp (right).
Structural analyses were intended to provide valuable information concerning duplication events when interpreting phylogenetic relationships within gene families. Thus, we analyzed the exon/intron structures of LBD family genes (right panel in Fig. 1). In apples, the exon number ranged from 1 within twelve genes to 5 in MdLBD26. Most MdLBD genes shared 1, 2 or 3 exons. Specifically, 35 genes had two exons, 12 genes had one exon, and only one gene had 5 exons. Most members within the same subgroup shared a similar intron/exon structure and gene length. The conserved intron/exon structure in each subgroup supported their close evolutionary relationship and the stated classification of subfamilies.
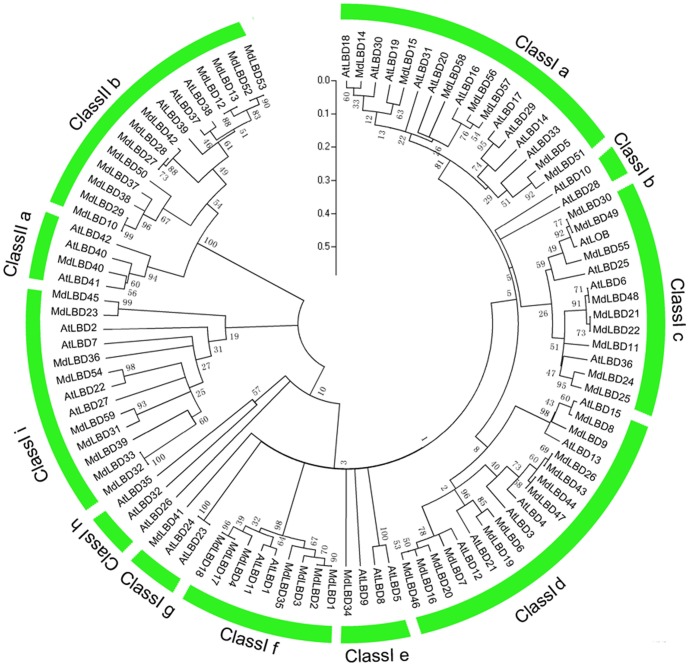
To examine the evolutionary patterns of MdLBD with those of Arabidopsis and then group them into established subfamilies, a phylogenetic tree was generated with full length protein sequences (Fig. 2). As in rice and Arabidopsis, the MdLBD clearly fell into two classes, class I and class II, which had 45 and 13 separate genes relative to 37 and 6 genes in Arabidopsis, as shown in Fig. 1. The presence of twice as many class II apple LBD genes compared with Arabidopsis indicates that this class of gene may have more functions in apple development. Class I and class II families were further divided into 8 and 2 groups. In class I, the subgroups were named from class Ia to class Ii, while class IIa and class IIb comprised the class II group. Three Arabidopsis LBD genes (AtLBD40,AtLBD41,AtLBD42) that were clustered into class IIa corresponded with only one LBD gene (MdLBD40) in apple. On the other hand, there were 12 apple LBD genes grouped in class IIb with only three genes (AtLBD37, AtLBD38, and AtLBD39) in Arabidopsis. It is reported that AtLBD37, AtLBD37, AtLBD38 and AtLBD39 function as transcript regulators in response to nitrate and regulate gene expression relative to anthocyanin biosynthesis along with nitrate uptake and transport [11]. We conclude that class IIb LBD genes may play a broader function in apple.
Figure 2. The phylogenetic analysis of LBD genes in apple and Arabidopsis.
The amino acid sequences of the LBD proteins were aligned with Clustal X, and the phylogenetic tree was constructed using the neighbor-joining method of MEGA 5.0 software. Each node is represented by a number that indicates the bootstrap value for 1000 replicates. The scale bar represents 0.1 substitutions per sequence position. Each LBD subfamily is indicated by an arc.
Chromosomal Location Analysis of MdLBDs
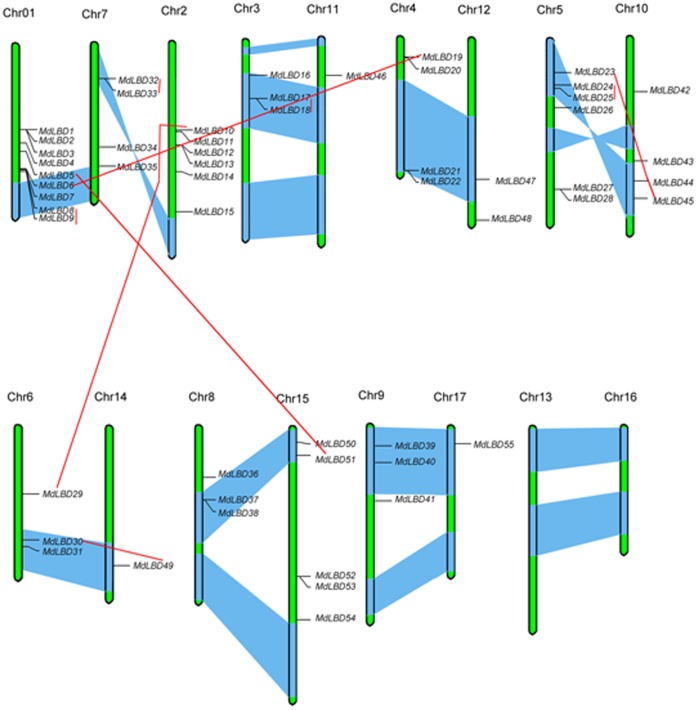
Fifty-five genes were located in 15 out of 17 chromosomes, and 3 MdLBD genes (MdLBD56, MdLBD57 and MdLBD58) were localized to unassembled genomic sequence scaffolds and thus were not mapped to any particular chromosome (Fig. 3). Chromosome 01 encompassed the largest number with 9 MdLBD genes, followed by Chr02 and Chr05 (6 genes per chromosome). Five MdLBD genes were distributed in Chr15, four genes were distributed in Chr04, 7 genes were distributed in Chr03, 10 genes were distributed in Chr06, and three genes were distributed in Chr08. Two LBD genes were identified in Chr12, while only one gene was located on Chr11, 14 and 17. In addition, further investigation showed that the distribution of each type of LBD genes was significantly irregular (Fig. 3), while no MdLBD genes were located on Chr13 or Chr16. Among them, a total of 21 genes were found in the segmental duplication blocks (Fig. 3), and five homologous pairs (MdLBD17/18, MdLBD24/25, MdLBD23/45, MdLBD32/33 and MdLBD30/49) were definitely located on the duplicated blocks.
Figure 3. The chromosomal mapping analysis of the LBD gene family in apple.
The scale bar represents a 10.0 Mb chromosomal distance. The chromosome number (Chr01-Chr17) is indicated at the top of each chromosome. To simplify the presentation, we named the putative LBD genes from MdLBD1 to MdLBD58 on the basis of the LBD family and gene order on the chromosomes from Chr01 to Chr17, respectively. Segmented duplicate homologous blocks are indicated with a blue shadow. Sister paralogous pairs are indicated by a red line.
Sequence Alignment and Conserved Motifs of MdLBD Genes
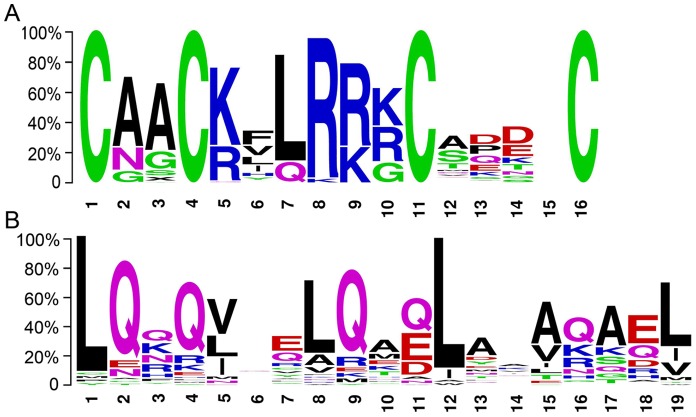
In Arabidopsis, the LBD genes had a conserved LOB domain in the N terminus of the genes, and there were two conserved blocks in the LOB domain of the class I proteins, that is, the C block and GAS block. To identify conserved domains within the MdLBD genes, we performed an alignment within all of the MdLBD genes and a separate one for the class I and class II protein sequences. As with the AtLBD genes, multiple sequence alignment showed that all 58 predicted MdLBD protein sequences had a completely conserved CX2CX6CX3C zinc finger-like domain while a LX6LX3LX6L leucine zipper-like domain existed only in 17 of the class I LBD genes (Fig. 4; Fig. S1). In contrast with the AtLBD genes, the MdLBD genes had completely conserved H/G amino acids at the GAS block. The GAS block ended with a DPVYG motif but not with a DP (V/I) YG motif.
Figure 4. Conserved domains of MdLBDs gene family.
A. The CX2CX6CX3C zinc finger-like domain sequence logos. B. The LX6LX3LX6L leucine zipper-like domain sequences Logos. Sequence alignment of two domains by ClustalX and conserved motifs Logos was performed by the WebLogo program.
Spatial and Temporal Expression and Gene Response Analysis of some of the MdLBD Genes
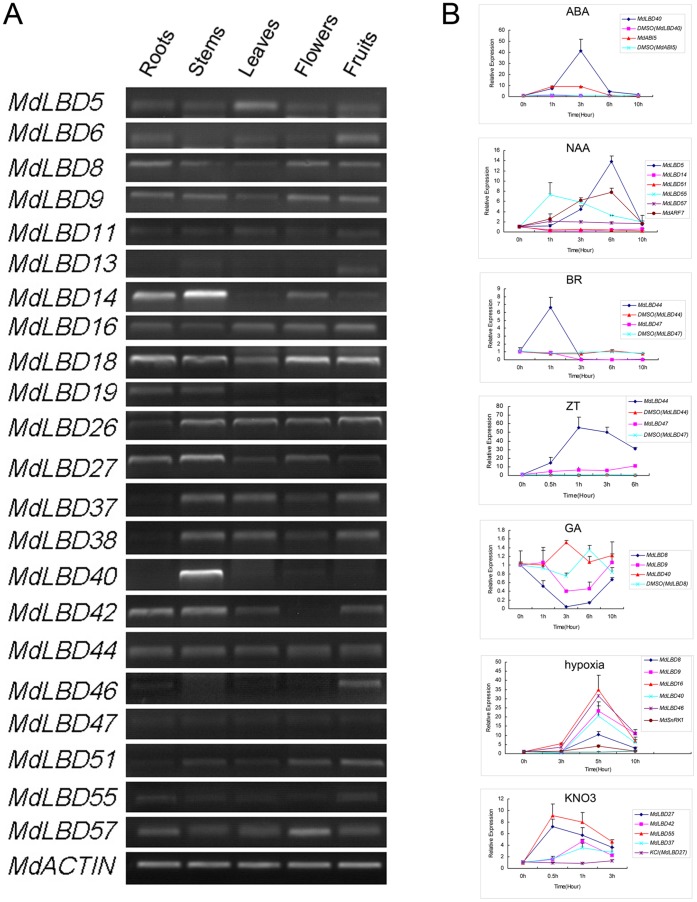
Semi-quantitative RT-PCR analysis was used to investigate the expression patterns of some of the MdLBD genes in the following organs: roots, stems, leaves, flowers and fruits. We found that transcripts of MdLBD genes could be detected in all tissues (Fig. 5A). Transcripts of MdLBD19 were detected mainly in roots and leaves, and transcripts of MdLBD40 were predominantly present in stems. Transcripts of MdLBD14 could be detected in all tissues except the leaves, and the expression of MdLBD42 could be detected in all organs except in flowers (Fig. 5A).
Figure 5. Tissue-specific expression profiles and gene response for the MdLBD genes.
A. Tissue-specific expression profiles of MdLBD genes. Expression levels of MdLBD genes were examined by semi-qRT-PCR in apple roots (R), stems (S), leaves (L), flowers (FL) and fruits (F). The MdACTIN was performed as an internal control. B. QRT-PCR analysis of MdLBD genes in response to multiple treatments. MdLBD genes expression treated by 15 mM KNO3 (15 mM KCl was treated as a control), hypoxia, and 100 µM of ABA, NAA, 6-BA, GA and BR, the MdACTIN was performed as an internal control.
It was discovered that AtLBD genes can respond to different treatments, such as auxin, cytokinin, and nitrate. Based on the existing microarray experiments that were compiled in Genevestigator, we found that they also responded to the hormones ABA (abscisic acid), GA (gibberellin) and BR (brassinosteroid); the abiotic stress of hypoxia; and biotic stress from Pst (Pseudomonas syringae) (Fig. S2). We then analyzed the gene expression of MdLBD gene homologs corresponding to AtLBD genes. Bioinformatics analysis was used to predict the homologous gene in apple in comparison to Arabidopsis, although some differences existed between the two plants. RT-PCR analysis was performed to determine mRNA expression levels. We selected 21 genes that showed high homology with the Arabidopsis gene for expression assays under hormone treatment with NAA (1-naphthlcetic acid), GA (gibberellins), 6-BA (6-Benzylaminopurine), ABA (abscisic acid), and BR (brassinolide). In the RT-PCR assay, almost all detected MdLBD genes were especially responsive to one or more treatments (Fig. 5B). For example, the relative transcript level of MdLBD40 was induced by approximately 40-fold after 3 hours of treatment with ABA when compared with the control (which was treated with DMSO), and a 9-fold increase was induced in MdABI5, a marker gene responding to ABA, which suggests that MdLBD40 was ABA-responsive. Transcripts also increased by 14-fold, 7-fold and 2-fold for MdLBD5, MdLBD55 and MdLBD57, respectively, at their peak levels when NAA was applied. Slightly down-regulated with MdLBD14 and MdLBD51, MdARF7 here was used as a marker gene. The BR induced the MdLBD44 gene by more than 6-fold but repressed MdLBD47 rapidly. MdLBD44 and MdLBD47 can also be induced by ZT by no less than 50-fold and 10-fold, respectively, at the peak. In response to GA, the expression of MdLBD8 had a rapid reduction, while no significant change took place in the transcription of MdLBD9 and MdLBD40. There were 10-fold to 35-fold changes for MdLBD8, MdLBD40, MdLBD9, MdLBD46 and MdLBD16 after about 5 h of exposure to hypoxia. MdSnRK1 was used as a control. Nitrate-induced expression showed a maximum of 3-fold to 9-fold increase in MdLBD55, MdLBD42, MdLBD27 and MdLBD37 genes at the peak. These results indicate that the function of LBD genes may be conserved between different species. Various kinds of regulating hormone treatments suggest a potential function for the MdLBD gene in hormone-mediated plant organogenesis.
MdLBD11 has a Similar Function to the Homologous Arabidopsis Gene
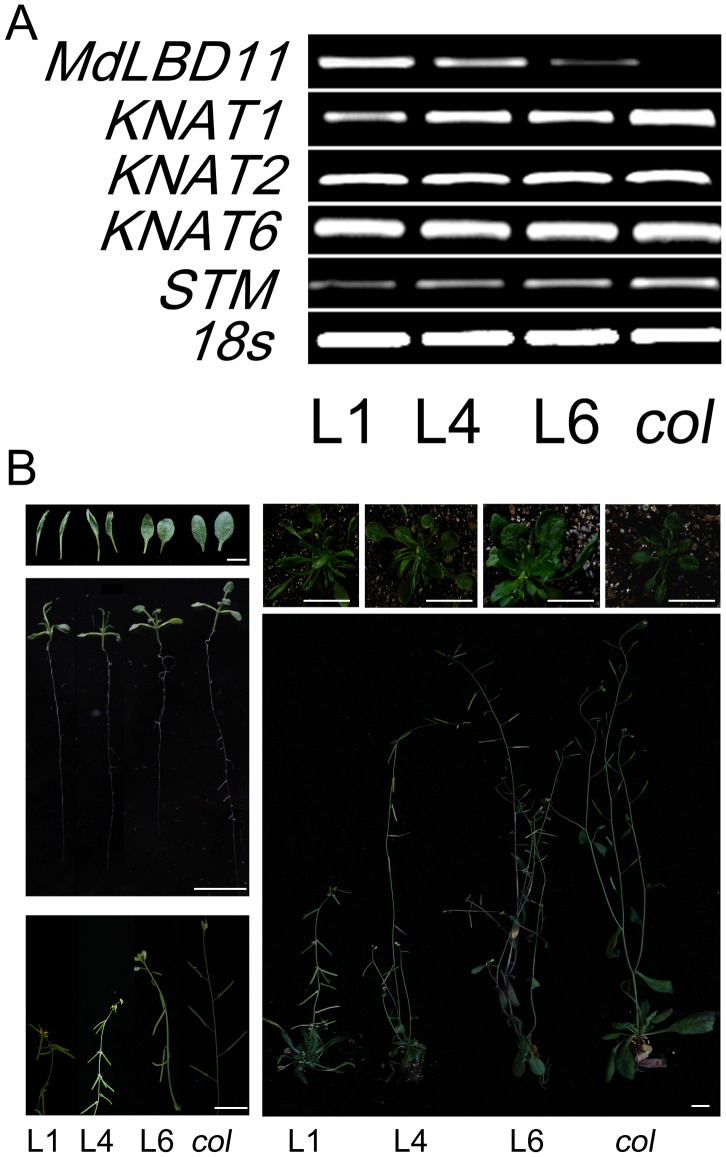
In order to know more about the biological functions of MdLBD genes, MdLBD11 was chosen for functional characterization. According to the sequence alignment and phylogenetic analysis, MdLBD11 was highly similar to Arabidopsis AtASL1 and AtAS2 (Fig. S3). First, its cDNA was introduced into Arabidopsis. Consequently, three Arabidopsis transgenic lines were obtained with different ectopic expression levels (Fig. 6A). When compared with the WT control, all transgenic lines exhibited abnormal phenotypes in relation to vegetative development and growth, including reduced plant stature, short petioles and small and upward curled leaves. Meanwhile, transgenic plants also experienced long juvenility, and their downward-pointing flowers and siliques had short pedicels (Fig. 6B). The extent of abnormal phenotypes was positively correlated with the expression level of MdLBD11 in transgenic lines, indicating that the ectopic expression of MdLBD11 resulted in the transgenic phenotypes.
Figure 6. Ectopic expression of MdLBD11 changes the phenotype in transgenic Arabidopsis.
A. Expression analysis of MdLBD11 and KNOX genes in transgenic Arabidopsis. Expression levels of MdLBD11 and KNOX genes were detected in transgenic Arabidopsis in comparison with wide type lines, and three transgenic lines were selected for expression analysis. B. Morphological characteristics of transgenic Arabidopsis. Two week-old rosettes (upper), one month-old (middle) and two month-old (bottom) transgenic Arabidopsis were compared with wide type lines. Bars = 1 cm.
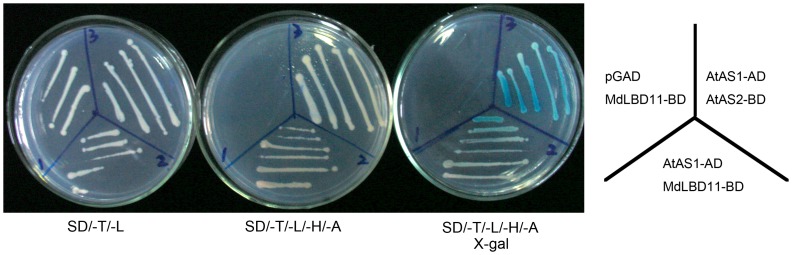
Furthermore, to examine how MdLBD11 regulates plant development and growth, yeast two-hybrid assay was conducted. The result showed that MdLBD11 interacted with Arabidopsis AtAS1 (Fig. 7). Meanwhile, the expressions of the class I KNOX genes were analyzed in MdLBD11 overexpression lines. The results showed that all transgenic lines produced less KNAT1 and STM transcripts than the WT control, with no obvious transcription difference in KNAT2 and KNAT6 (Fig. 6A), suggesting that MdLBD11 regulated plant development and growth, at least partially if not completely by modulating class I KNOX gene expression, just as AtAS2 and AtASL1 in Arabidopsis. Furthermore, the results indicated that MdLBD11 functioned with a conserved mechanism like its Arabidopsis counterparts AtAS2 and AtASL1.
Figure 7. MdLBD11 interacts with AtAS1 in a yeast two-hybrid assay.
Yeast strains containing pGAD-AtAS1, pGBD-MdLBD11, and pGBD-AtAS2 were assayed for LacZ expression, pGAD-AtAS1 in combination with pGBD-AtAS2 was used as a positive control, and pGAD with pGBD-MdLBD11 was used as a negative control. Yeast grew on SD/−T/−L medium to select for both the bait and prey proteins (left). SD/−T/−L/−A/−H media allow the growth of only positively interacting clones. SD/−T/−L/−A/−H media plus X-gal allow the growth of only the positively interacting clones.
Discussion
Characterization of the Apple LBD Gene Family
LBD genes belong to a newly found unique transcript factor family. They ubiquitously exist in higher plants and may regulate plant-specific processes [4], [6]. Based on a global survey through the released apple genome database, 58 MdLBDs were identified from 57,386 annotated genes in apple genome [43]. Each of them was featured with a conserved LOB domain which ubiquitously exists in LBD genes of other plant species [4], [6]. They were localized to 15 chromosomes, except chr13 and chr16 out of the total of 17 apple chromosomes. Meanwhile, the cDNA of MdLBD11L was isolated with a RACE approach. Its nucleotide sequence is highly similar to that of MdLBD11 cDNA. However, there is no genomic sequence information found corresponding to MdLBD11L in apple genome database. Similarly, 2 out of 3 MdLBD candidates for Co locus can not be found in apple genome database [42], suggesting that there may be more other MdLBDs existing in the unknown genomic gaps. In fact, only 81.3% genomic sequences are overlapped on the apple genome [43].
The LBD gene families contain 43, 35 and 35 members in Arabidopsis, rice, and maize, respectively [6], [39], [40]. However, apple genome has at least 58 MdLBDs. Therefore, LBD gene family evolutionarily expanded in apple, maybe due to the chromosome duplication. Many angiosperms undergo whole genome duplication events. The recent gene duplication events were the most important for the rapid expansion and evolution of gene families [44]. In apple, the chromosome homologies derived from the recent genome-wide duplication allow inference of the cytological events that have led to the number and composition of the extant apple chromosomes, from a putative nine-chromosome ancestor to the 17-chromosome [43]. In this study, we found 11 sister pairs of paralogous LBDs. Among them, 5 homologous pairs were definitely located on the duplicated blocks (Fig. 3). These data support that the chromosome duplication events occur in apple genome.
Phylogenetic Analysis and Evolution of Apple LBD Genes
Phylogenetic analysis and evolutionary relationship of the LBD proteins have been intensively studied in Arabidopsis and rice [6], [39], which divides the LBD gene family into Class I and class II [4], [6], [7]. Here phylogenetic trees combining apple and Arabidopsis LBD proteins were constructed to analyze the relationship of 58 apple LBDs with their Arabidopsis counterparts. As a result, a total of 101 apple and Arabidopsis LBDs were divided into 10 clades, among of which 2 clades, i.e. class Ib and class Ih, exist only in Arabidopsis (Fig. 2). Therefore, 58 MdLBDs were distributed in the other 8 clades. In Arabidopsis, LBD genes in Class IIb are involved in the regulation of nitrate uptaking and anthocyanin synthesis [11]. In this study, it was found that Class IIb has 12 apple and 3 Arabidopsis LBD members, respectively (Fig. 1; Fig. 2). Apple has 4-fold more LBD members in class IIb than Arabidopsis, suggesting that woody apple tree evolutionarily needs more class IIb LBD genes to adapt barren soil and to regulate complex processes of fruit coloration. This may be an evolution diversity of LBD genes between herbaceous Arabidopsis and woody apple plants, just like between herbaceous rice and maize and woody poplar [39], [40], [41].
Structural analysis is a useful tool to mine valuable information concerning duplication events and phylogenetic relationships within gene families. In apples, the exon numbers of LBD genes ranged from 1 to 5, with more than half of them containing 2 exons. This is similar in Arabidopsis and rice, suggesting that the genetic evolution of the LBD genes structure is conserved in different angiosperms. Besides, most of the MdLBD members within the same clade share a similar intron/exon structure and gene length (Fig. 1), indicating their close evolutionary relationship.
So far, all known LBD proteins that are detected in various species have a conserved zinc finger-like domain, while a leucine zipper-like coiled-coil motif is found only in the majority of class I LBD proteins [4.6]. It has been predicted that Zinc finger-like motifs in the LOB domain may function as a DNA-binding domain, and the C-terminal leucine zipper-like motif may function in a probable protein-protein interaction [4]. Moreover, the incomplete leucine zipper-like coiled-coil motif is part of the class II LBD proteins. It is believed that this domain is misfunctional for protein-protein interaction. Actually, members of MYB and bHLH proteins have been found to interact with Arabidopsis LOB, a class I LBD protein [2]. We have also demonstrated that MdLBD11 and MdLBD11L, which are two class I LBD genes, interacted with MYB transcription factor AtAS1 (Fig. 7; Fig. S4). A yeast two-hybrid assay was used to screen the potential interaction proteins with several class II MdLBD genes (MdLBD12, MdLBD27, MdLBD52), and as predicted, no proteins could be identified.
Expression Analysis Indicated LBD Genes may Play Important Roles during Plant Growth and Development
Phytohormones have long been found to play a critical role in normal plant growth and development. Auxin, cytokinin and GA are the major developmental growth regulators, whereas ABA, ETH and JA are often implicated in stress responses. Members of LBD genes in Arabidopsis, rice and other species has been found respond to different treatments, such as auxin, cytokinin, GA and nitrate and they playe a key role in plant organ boundary definition by influencing the content or transport of the nutrient and hormone [1], [4], [11], [17], [35], [45]. In Arabidopsis, AtLBD3 represss the cytokinin content and was directly activated by type-B ARR, indicating that it is a primary target of the cytokinin signal transduction pathway [35]. AtLBD16 and AtLBD28 link the auxin signal transduction cascade and are directly regulated by AtARF7 and AtARF19, which are two major auxin response genes, through the auxin responsive elements (AuxRE) and then regulate embryonic and vascular patterning [1], [3], [25], [45]. AtLBD40 is reported to be downregulated by gibberellin and upregulated by DELLA proteins [46]. In addition to transcript regulation, class I LBD proteins can also interact with some MYB and bHLH proteins to regulate plant organogenesis [2], [47]. These results confirm that LBD genes can regulate plant organ differentiation at the transcript and protein levels. LBD genes were also nutrient- and stress-responsive, revealing that they may regulate nitrogen metabolism and abiotic and biotic responses.
It was also found that the expressions of LBD genes responded to ABA and BR, hypoxia stress and Pst (Pseudomonas syringae) infection in model plants based on the online microarray database (Fig. S2), as confirmed by RT-PCR analysis in apple (Fig. 5B). Therefore, LBD genes responded to various phytohormones in apple. Furthermore, 3 apple LBD genes may be involved in the regulation of columnar growth habit [42]. As is well known, several LBD proteins interact with MYB and bHLH transcription factors which regulate organogenesis in higher plants [2], [14], [16], [17]. Therefore, it is reasonable to suppose that LBD genes may be involved in the regulation of plant organ boundary definition and shoot branching associated with various hormones by interacting with specific MYB or bHLH transcription factors. In addition, some loss-of-function lbd mutants do not exhibit abnormal development and growth [1], [6], [11], suggesting a functional redundancy of those LBD genes. Meanwhile, LBD genes clustered into the same clade show different expression patterns (Fig. 5; Fig. S5), suggesting a functional specificity for each LBD gene even in the same clade [11].
LBD Gene may have the Conserved Function to the Homologous Arabidopsis Gene
MdLBD11 transgenic Arabidopsis exhibited abnormalities both in vegetative and reproductive growth, which is highly similar to those observed in AtASL1 and AtAS2 overexpression transgenic Arabidopsis lines. This indicates that MdLBD11 has functions similar to AtASL1 and AtAS2. It is known that AtAS2 interacts with MYB transcription factor AtAS1 to regulate the expression of downstream class I KNOX genes, underlying its biological functions in Arabidopsis [8], [14], [16]. Interestingly, MdLBD11 also interacted with Arabidopsis AtAS1 and regulated the expression of KNOX genes (Fig. 6A; Fig. 7), indicating that MdLBD11 functions in a way similar to its Arabidopsis counterparts. Like AtAS1, AtLOF1 and AtLOF2 also belong to MYB transcription factor family. The phenotypes of their overexpression lines are similar to AtAS2-ovx lines [48], [49]. In this study, it was also examined whether AtLOF1 and AtLOF2 interact with specific AtLBD or MdLBD proteins. The result showed no interaction between AtLOF1/2 and AtAS2 or MdLBD11 (Fig. S4). Therefore, it is presumed that AtLOFs may interact with other LBD proteins or that they have an LBD-independent way to regulate plant development involving lateral organ formation.
Overall, our work indicates that LBD genes are conservative in structure and function in different species. By utilizing the apple genome database, it is now feasible to analyze this gene family and predict its function with bioinformatics approaches, which should be helpful for breeding and cultivation to improve agronomic traits such as shoot branching, root growth, nutrient assimilation and uptake in apple.
Materials and Methods
Ethics Statement
No specific permits were required for the described field studies. The location is not privately-owned or protected in any way, and the field studies did not involve endangered or protected species.
The Identification of MdLBD Genes in Apple
Two approaches were used to identify the members of the MdLBD gene family in apples. First, all known Arabidopsis LBD gene sequences were used in a query to perform multiple database searches against the proteome and genome files that were downloaded from the Apple GFDB database (Apple Gene Function and Gene Family Database: http://www.applegene.org/) as well as the GDR database (Genome Database for Rosaceae: http://www.rosaceae.org/) [50]. Stand-alone versions of BLAST (Basic Local Alignment Search Tool: http://blast.ncbi.nlm.nih.gov) [51], which are available from the NCBI, were used with an e-value cutoff of 1e-003. All of the protein sequences that were derived from the selected MdLBD candidate genes were examined with the domain analysis programs Pfam (Protein family: http://pfam.sanger.ac.uk/) [52] and SMART (Simple Modular Architecture Research Tool: http://smart.embl-heidelberg.de/) [53] with the default cutoff parameters. Second, we analyzed the domains of all of the apple peptide sequences using an HMM (Hidden Markov Model) [54] analysis while searching Pfam. Then, we obtained the sequences by using the PF03195 Pfam number, which contained a typical MdLBD domain, from the apple genome sequences by making use of a Perl-based script. Finally, all of the protein sequences were compared with known MdLBD sequences by applying ClustalX (http://www.clustal.org/) to verify that the sequences were candidate MdLBDs [55].
The isoelectric points and protein molecular weights were obtained with the help of the proteomics and sequence analysis tools on the ExPASy proteomics server (http://expasy.org/) [56]. The chromosomal locations were found in the GDR database by using a Perl-based program.
The Chromosomal Location and Structure of the MdLBD Genes
The chromosomal locations and gene structures were retrieved from the apple genome data that were downloaded from the GDR database. The remaining genes were mapped to the chromosomes with MapDraw [57], and the gene structures of the MdLBD genes were generated with the GSDS (http://gsds.cbi.pku.edu.cn/) [58].
Sequence Alignment and Phylogenetic Analysis
The MdLBD sequences were aligned in the ClustalX program with BLOSUM 30 as the protein weight matrix. The MUSCLE program (version 3.52) was also applied to perform multiple sequence alignments in order to confirm the ClustalX result [55], [59]. Phylogenetic trees for the MdLBD protein sequences were constructed with the NJ (neighbor-joining) method of the MEGA5 program (http://www.megasoftware.net/), as well as the p-distance for complete deletion option parameters. The reliability of the trees was tested using a bootstrapping method with 1000 replicates. The images of the phylogenetic trees were drawn in MEGA5 [60].
Expression Prediction of the MdLBD Genes Based on the Expression Profile of Arabidopsis LBD Genes
Microarray expression data from various datasets were obtained by making use of Genevestigator (https://www.genevestigator.com/gv/) with the Arabidopsis GeneChip platform (Fig. S1) [61]. We performed a local BLASTP program search in BioEdit against the Arabidopsis peptide database to find homologous genes by using the putative MdLBD genes as queries. Based on the Genevestigator selections, we obtained expression patterns that are presented as heat maps in red/green coding, which reflected the log ratio with red indicating up-regulation and green indicating down-regulation (probe sets in a 22 k Affymetrix GeneChip). A local BLASTN search against existing ESTs (which were downloaded from NCBI, 324742 records; 6 Feb 2012) was conducted to find the corresponding record for each member of these putative MdLBDs.
Plant Materials and Treatments
The apple ‘tea crabapple’ (Malus hupehensis Redh. var. pingyiensis) seedlings were used for gene isolation and expression analysis. They were kept at 25°C under long-day conditions (16 h light/8 h dark) in a sand culture. Expression levels for different tissues, leaves, flowers, fruits and roots were collected from a 5-year-old ‘Gala’ apple tree that was grown in natural conditions in the Shandong Province of China. One month-old sand culture seedlings were used for the gene expression analysis. The seedlings were treated with 15 mM KNO3 (15 mM KCl was used as a control), hypoxia and 100 µM of different kinds of hormone solutions including ABA, NAA, 6-BA, GA and BR with a 0.05% DMSO control. The treated plant materials were frozen in liquid nitrogen for RNA extraction. Arabidopsis plants were planted in the culture room under SD (8 h light/16 h dark) for a month and then moved to LD conditions (16 h light/8 h dark) at 20–22°C.
RNA Extraction, cDNA Synthesis and Gene Expression Analysis
Total RNA of apple was extracted with the hot borate method as described in our previous report [62], and total RNA of Arabidopsis was extracted with Trizol reagent (Invitrogen, USA). Two micrograms of total RNA was used to synthesize first-strand cDNA using the PrimeScript First Strand cDNA Synthesis Kit (Takara, China). RT-PCR was conducted by using cDNA templates to detect the gene expression level. Apple ACTIN and Arabidopsis ACTIN genes were employed as controls, and the analysis of each type of sample was repeated three times. The specific primers that were used for PCR analysis are listed in Table S1.
Construction of MdLBD11 Overexpression Vector
The full length cDNA of MdLBD11 was identified from the ‘Gala’ apple. The cDNA was ligated into a PMD18-T vector (Takara, China) and then digested with BamHI and cloned into a pBIN vector.
Yeast Two-hybrid (YTH) Assays
A Gal4-based two-hybrid system was used as directed by the manufacturer (Clontech, USA). The full-length encoding regions of MdLBD11, MdLBD11L and AtAS2 were ligated into the DNA binding domain vector pGBKT7 to make the bait plasmid vector. The coding sequence of AtAS1, AtLOF1 and AtLOF2 were fused to the two-hybrid activation domain vector (pGADT7) as prey. The primers that were used to create these constructs are listed in Table S1. Each pGADT7 vector was separately co-transformed with pGBKT7 vector into yeast strain Y2H for a yeast two-hybrid test. Positive colonies were selected on SD/−Trp-Leu-His-Ade medium. The positive clones of the complexes were selected and assayed for X-gal activity.
Arabidopsis Transformation
Wild type (WT) Arabidopsis ecotype Columbia (Col 0) was used. MdLBD11 was introduced into the WT (Col 0) using the floral dip method and mediated with Agrobacterium strain GV3101 [63]. The seeds of positive transgenic plants carrying the MdLBD11 constructs were individually harvested. Homozygous transgenic lines were used for further investigation.
Supporting Information
Alignment of conserved MdLBD domain sequences.
(TIF)
Expression analysis of AtLBD genes based on microarray data from Genevestigator.
(TIF)
The phylogenetic analysis and sequence alignment of MdLBD11 , MdLBD11L with the Arabidopsis corresponding genes. A. Phylogenetic analysis of MdLBD11, MdLBD11L with the Arabidopsis corresponding genes. B. Sequence alignment of MdLBD11, MdLBD11L with the Arabidopsis corresponding genes.
(TIF)
Yeast two-hybrid assay. Yeast strains containing pGAD-AtAS1, pGAD-AtMYB117 and pGBD-MdLBD11L were assayed for LacZ expression, while pGAD with pGBD-MdLBD11 were used as a negative control.
(TIF)
Tissue expression from AtLBD genes as acquired from Genevestigator.
(TIF)
Primers for gene clone, vector construction and expression analysis.
(DOC)
Funding Statement
This work was supported by the National Public Benefit (Agricultural) Research Foundation of China (201203075-3), Natural Science Foundation of China (30971969), 948 project from Ministry of Agriculture of China (2011-G21) and Program for Changjiang Scholars and lnnovative Research Team in University (IRT1155). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Reference
- 1. Okushima Y, Fukaki H, Onoda M, Theologis A, Tasaka M (2007) ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis . Plant Cell 19: 118–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Husbands A, Bell EM, Shuai B, Smith HM, Springer PS (2007) LATERAL ORGAN BOUNDARIES defines a new family of DNA-binding transcription factors and can interact with specific bHLH proteins. Nucleic Acids Res 35: 6663–6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee HW, Kim NY, Lee DJ, Kim J (2009) LBD18/ASL20 regulates lateral root formation in combination with LBD16/ASL18 downstream of ARF7 and ARF19 in Arabidopsis . Plant Physiol 151: 1377–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Majer C, Hochholdinger F (2011) Defining the boundaries: structure and function of LOB domain proteins. Trends Plant Sci 16: 47–52. [DOI] [PubMed] [Google Scholar]
- 5. Lee HW, Kim MJ, Kim NY, Lee SH, Kim J (2013) LBD18 acts as a transcriptional activator that directly binds to the EXPANSIN14 promoter in promoting lateral root emergence of Arabidopsis . Plant J 73: 212–224. [DOI] [PubMed] [Google Scholar]
- 6. Shuai B, Reynaga-Pena CG, Springer PS (2002) The lateral organ boundaries gene defines a novel, plant-specific gene family. Plant Physiol 129: 747–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Matsumura Y, Iwakawa H, Machida Y, Machida C (2009) Characterization of genes in the ASYMMETRIC LEAVES2/LATERAL ORGAN BOUNDARIES (AS2/LOB) family in Arabidopsis thaliana, and functional and molecular comparisons between AS2 and other family members. Plant J 58: 525–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Semiarti E, Ueno Y, Tsukaya H, Iwakawa H, Machida C, et al. (2001) The ASYMMETRIC LEAVES2 gene of Arabidopsis thaliana regulates formation of a symmetric lamina, establishment of venation and repression of meristem-related homeobox genes in leaves. Development 128: 1771–1783. [DOI] [PubMed] [Google Scholar]
- 9. Sun SB, Song JP, Meng LS (2012) ASYMMETRIC LEAVES2 gene, a member of LOB/AS2 family of Arabidopsis thaliana, causes an abaxializing leaves in transgenic cockscomb. Mol Biol Rep 39: 4927–4935. [DOI] [PubMed] [Google Scholar]
- 10. Chalfun-Junior A, Franken J, Mes JJ, Marsch-Martinez N, Pereira A, et al. (2005) ASYMMETRIC LEAVES2-LIKE1 gene, a member of the AS2/LOB family, controls proximal-distal patterning in Arabidopsis petals. Plant Mol Biol 57: 559–575. [DOI] [PubMed] [Google Scholar]
- 11. Rubin G, Tohge T, Matsuda F, Saito K, Scheible WR (2009) Members of the LBD family of transcription factors repress anthocyanin synthesis and affect additional nitrogen responses in Arabidopsis . Plant Cell 21: 3567–3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bortiri E, Chuck G, Vollbrecht E, Rocheford T, Martienssen R, et al. (2006) ramosa2 encodes a LATERAL ORGAN BOUNDARY domain protein that determines the fate of stem cells in branch meristems of maize. Plant Cell 18: 574–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vollbrecht E, Springer PS, Goh L, Buckler ESt, Martienssen R (2005) Architecture of floral branch systems in maize and related grasses. Nature 436: 1119–1126. [DOI] [PubMed] [Google Scholar]
- 14. Xu L, Xu Y, Dong A, Sun Y, Pi L, et al. (2003) Novel as1 and as2 defects in leaf adaxial-abaxial polarity reveal the requirement for ASYMMETRIC LEAVES1 and 2 and ERECTA functions in specifying leaf adaxial identity. Development 130: 4097–4107. [DOI] [PubMed] [Google Scholar]
- 15. Iwakawa H, Iwasaki M, Kojima S, Ueno Y, Soma T, et al. (2007) Expression of the ASYMMETRIC LEAVES2 gene in the adaxial domain of Arabidopsis leaves represses cell proliferation in this domain and is critical for the development of properly expanded leaves. Plant J 51: 173–184. [DOI] [PubMed] [Google Scholar]
- 16. Guo M, Thomas J, Collins G, Timmermans MC (2008) Direct repression of KNOX loci by the ASYMMETRIC LEAVES1 complex of Arabidopsis . Plant Cell 20: 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hay A, Barkoulas M, Tsiantis M (2006) ASYMMETRIC LEAVES1 and auxin activities converge to repress BREVIPEDICELLUS expression and promote leaf development in Arabidopsis . Development 133: 3955–3961. [DOI] [PubMed] [Google Scholar]
- 18. Byrne ME, Simorowski J, Martienssen RA (2002) ASYMMETRIC LEAVES1 reveals knox gene redundancy in Arabidopsis . Development 129: 1957–1965. [DOI] [PubMed] [Google Scholar]
- 19. Qi Y, Sun Y, Xu L, Xu Y, Huang H (2004) ERECTA is required for protection against heat-stress in the AS1/AS2 pathway to regulate adaxial-abaxial leaf polarity in Arabidopsis . Planta 219: 270–276. [DOI] [PubMed] [Google Scholar]
- 20. Phelps-Durr TL, Thomas J, Vahab P, Timmermans MC (2005) Maize rough sheath2 and its Arabidopsis orthologue ASYMMETRIC LEAVES1 interact with HIRA, a predicted histone chaperone, to maintain knox gene silencing and determinacy during organogenesis. Plant Cell 17: 2886–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Belles-Boix E, Hamant O, Witiak SM, Morin H, Traas J, et al. (2006) KNAT6: an Arabidopsis homeobox gene involved in meristem activity and organ separation. Plant Cell 18: 1900–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li H, Xu L, Wang H, Yuan Z, Cao X, et al. (2005) The Putative RNA-dependent RNA polymerase RDR6 acts synergistically with ASYMMETRIC LEAVES1 and 2 to repress BREVIPEDICELLUS and MicroRNA165/166 in Arabidopsis leaf development. Plant Cell 17: 2157–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nakazawa M, Ichikawa T, Ishikawa A, Kobayashi H, Tsuhara Y, et al. (2003) Activation tagging, a novel tool to dissect the functions of a gene family. Plant J 34: 741–750. [DOI] [PubMed] [Google Scholar]
- 24. Theodoris G, Inada N, Freeling M (2003) Conservation and molecular dissection of ROUGH SHEATH2 and ASYMMETRIC LEAVES1 function in leaf development. Proc Natl Acad Sci U S A 100: 6837–6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Okushima Y, Overvoorde PJ, Arima K, Alonso JM, Chan A, et al. (2005) Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: unique and overlapping functions of ARF7 and ARF19. Plant Cell 17: 444–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Feng Z, Zhu J, Du X, Cui X (2012) Effects of three auxin-inducible LBD members on lateral root formation in Arabidopsis thaliana . Planta 236: 1227–1237. [DOI] [PubMed] [Google Scholar]
- 27. Fan M, Xu C, Xu K, Hu Y (2012) LATERAL ORGAN BOUNDARIES DOMAIN transcription factors direct callus formation in Arabidopsis regeneration. Cell Res 22: 1169–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goh T, Joi S, Mimura T, Fukaki H (2012) The establishment of asymmetry in Arabidopsis lateral root founder cells is regulated by LBD16/ASL18 and related LBD/ASL proteins. Development 139: 883–893. [DOI] [PubMed] [Google Scholar]
- 29. Inukai Y, Sakamoto T, Ueguchi-Tanaka M, Shibata Y, Gomi K, et al. (2005) Crown rootless1, which is essential for crown root formation in rice, is a target of an AUXIN RESPONSE FACTOR in auxin signaling. Plant Cell 17: 1387–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu H, Wang S, Yu X, Yu J, He X, et al. (2005) ARL1, a LOB-domain protein required for adventitious root formation in rice. Plant J 43: 47–56. [DOI] [PubMed] [Google Scholar]
- 31. Soyano T, Thitamadee S, Machida Y, Chua NH (2008) ASYMMETRIC LEAVES2-LIKE19/LATERAL ORGAN BOUNDARIES DOMAIN30 and ASL20/LBD18 regulate tracheary element differentiation in Arabidopsis . Plant Cell 20: 3359–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bureau M, Simon R (2008) JLO regulates embryo patterning and organ initiation by controlling auxin transport. Plant Signal Behav 3: 145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xu B, Li Z, Zhu Y, Wang H, Ma H, et al. (2008) Arabidopsis genes AS1, AS2, and JAG negatively regulate boundary-specifying genes to promote sepal and petal development. Plant Physiol 146: 566–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mangeon A, Bell EM, Lin WC, Jablonska B, Springer PS (2011) Misregulation of the LOB domain gene DDA1 suggests possible functions in auxin signalling and photomorphogenesis. J Exp Bot 62: 221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Naito T, Yamashino T, Kiba T, Koizumi N, Kojima M, et al. (2007) A link between cytokinin and ASL9 (ASYMMETRIC LEAVES 2 LIKE 9) that belongs to the AS2/LOB (LATERAL ORGAN BOUNDARIES) family genes in Arabidopsis thaliana . Biosci Biotechnol Biochem 71: 1269–1278. [DOI] [PubMed] [Google Scholar]
- 36. Yordanov YS, Regan S, Busov V (2010) Members of the LATERAL ORGAN BOUNDARIES DOMAIN transcription factor family are involved in the regulation of secondary growth in Populus. Plant Cell 22: 3662–3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thatcher LF, Powell JJ, Aitken EA, Kazan K, Manners JM (2012) The lateral organ boundaries domain transcription factor LBD20 functions in Fusarium wilt Susceptibility and jasmonate signaling in Arabidopsis . Plant Physiol 160: 407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sun SB, Meng LS, Sun XD, Feng ZH (2010) Using high competent shoot apical meristems of cockscomb as explants for studying function of ASYMMETRIC LEAVES2-LIKE11 (ASL11) gene of Arabidopsis . Mol Biol Rep 37: 3973–3982. [DOI] [PubMed] [Google Scholar]
- 39. Yang Y, Yu X, Wu P (2006) Comparison and evolution analysis of two rice subspecies LATERAL ORGAN BOUNDARIES domain gene family and their evolutionary characterization from Arabidopsis . Mol Phylogenet Evol 39: 248–262. [DOI] [PubMed] [Google Scholar]
- 40. Schnable PS, Ware D, Fulton RS, Stein JC, Wei F, et al. (2009) The B73 maize genome: complexity, diversity, and dynamics. Science 326: 1112–1115. [DOI] [PubMed] [Google Scholar]
- 41. Zhu QH, Guo AY, Gao G, Zhong YF, Xu M, et al. (2007) DPTF: a database of poplar transcription factors. Bioinformatics 23: 1307–1308. [DOI] [PubMed] [Google Scholar]
- 42. Bai T, Zhu Y, Fernandez-Fernandez F, Keulemans J, Brown S, et al. (2012) Fine genetic mapping of the Co locus controlling columnar growth habit in apple. Mol Genet Genomics 287: 437–450. [DOI] [PubMed] [Google Scholar]
- 43. Velasco R, Zharkikh A, Affourtit J, Dhingra A, Cestaro A, et al. (2010) The genome of the domesticated apple (Malus×domestica Borkh.). Nat Genet 42: 833–839. [DOI] [PubMed] [Google Scholar]
- 44. Cannon SB, Mitra A, Baumgarten A, Young ND, May G (2004) The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol 4: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rast MI, Simon R (2012) Arabidopsis JAGGED LATERAL ORGANS acts with ASYMMETRIC LEAVES2 to coordinate KNOX and PIN expression in shoot and root meristems. Plant Cell 24: 2917–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zentella R, Zhang ZL, Park M, Thomas SG, Endo A, et al. (2007) Global analysis of della direct targets in early gibberellin signaling in Arabidopsis . Plant Cell 19: 3037–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kessler S, Sinha N (2004) Shaping up: the genetic control of leaf shape. Curr Opin Plant Biol 7: 65–72. [DOI] [PubMed] [Google Scholar]
- 48. Gomez MD, Urbez C, Perez-Amador MA, Carbonell J (2011) Characterization of constricted fruit (ctf) mutant uncovers a role for AtMYB117/LOF1 in ovule and fruit development in Arabidopsis thaliana . PLoS One 6: e18760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee DK, Geisler M, Springer PS (2009) LATERAL ORGAN FUSION1 and LATERAL ORGAN FUSION2 function in lateral organ separation and axillary meristem formation in Arabidopsis . Development 136: 2423–2432. [DOI] [PubMed] [Google Scholar]
- 50. Jung S, Staton M, Lee T, Blenda A, Svancara R, et al. (2008) GDR (Genome Database for Rosaceae): integrated web-database for Rosaceae genomics and genetics data. Nucleic Acids Res 36: D1034–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mount DW (2007) Using the Basic Local Alignment Search Tool (BLAST). CSH Protoc 2007: pdb top17. [DOI] [PubMed]
- 52. Punta M, Coggill PC, Eberhardt RY, Mistry J, Tate J, et al. (2012) The Pfam protein families database. Nucleic Acids Res 40: D290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Letunic I, Doerks T, Bork P (2012) SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Res 40: D302–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wu X, Song C, Wang B, Cheng J (2002) [Hidden Markov model used in protein sequence analysis]. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 19: 455–458. [PubMed] [Google Scholar]
- 55. Jeanmougin F, Thompson JD, Gouy M, Higgins DG, Gibson TJ (1998) Multiple sequence alignment with Clustal X. Trends Biochem Sci. 23: 403–405. [DOI] [PubMed] [Google Scholar]
- 56. Artimo P, Jonnalagedda M, Arnold K, Baratin D, Csardi G, et al. (2012) ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res 40: W597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu RH, Meng JL (2003) [MapDraw: a microsoft excel macro for drawing genetic linkage maps based on given genetic linkage data]. Yi Chuan 25: 317–321. [PubMed] [Google Scholar]
- 58. Guo AY, Zhu QH, Chen X, Luo JC (2007) [GSDS: a gene structure display server]. Yi Chuan 29: 1023–1026. [PubMed] [Google Scholar]
- 59. Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Grennan AK (2006) Genevestigator. Facilitating web-based gene-expression analysis. Plant Physiol 141: 1164–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yao YX, Li M, Liu Z, Hao YJ, Zhai H (2007) A novel gene, screened by cDNA-AFLP approach, contributes to lowering the acidity of fruit in apple. Plant Physiol Biochem 45: 139–145. [DOI] [PubMed] [Google Scholar]
- 63. Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana . Plant J 16: 735–743. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Alignment of conserved MdLBD domain sequences.
(TIF)
Expression analysis of AtLBD genes based on microarray data from Genevestigator.
(TIF)
The phylogenetic analysis and sequence alignment of MdLBD11 , MdLBD11L with the Arabidopsis corresponding genes. A. Phylogenetic analysis of MdLBD11, MdLBD11L with the Arabidopsis corresponding genes. B. Sequence alignment of MdLBD11, MdLBD11L with the Arabidopsis corresponding genes.
(TIF)
Yeast two-hybrid assay. Yeast strains containing pGAD-AtAS1, pGAD-AtMYB117 and pGBD-MdLBD11L were assayed for LacZ expression, while pGAD with pGBD-MdLBD11 were used as a negative control.
(TIF)
Tissue expression from AtLBD genes as acquired from Genevestigator.
(TIF)
Primers for gene clone, vector construction and expression analysis.
(DOC)