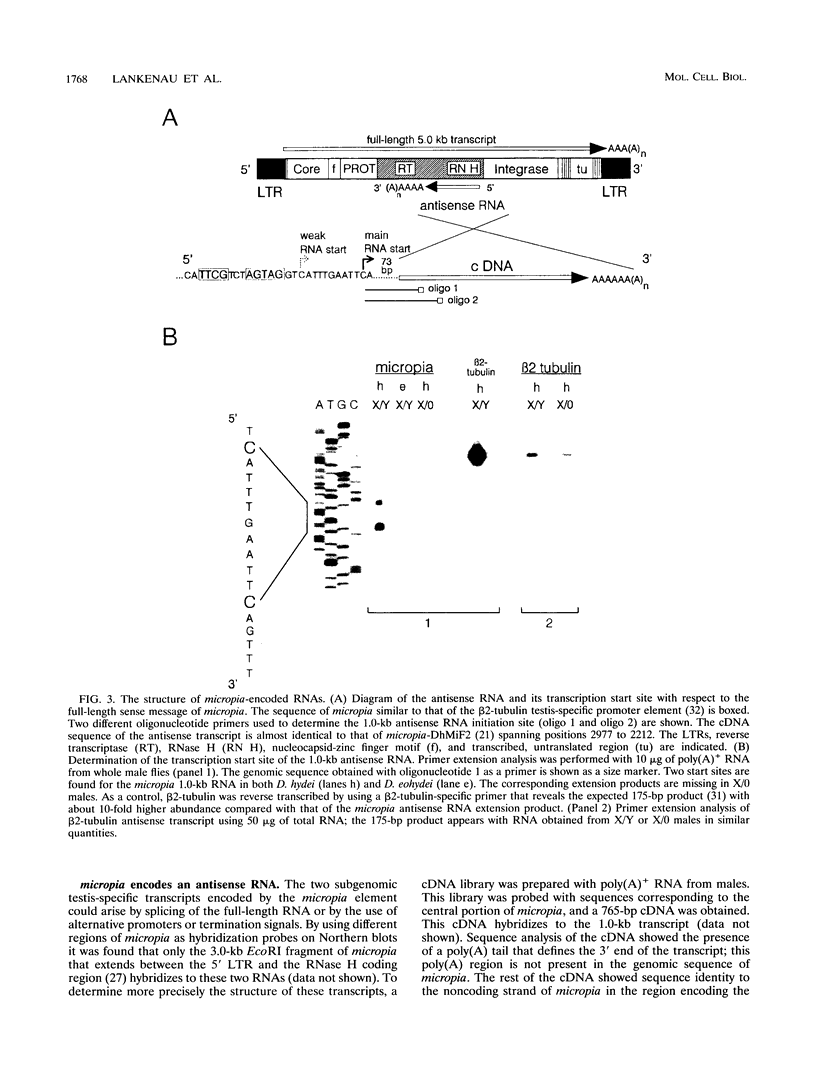
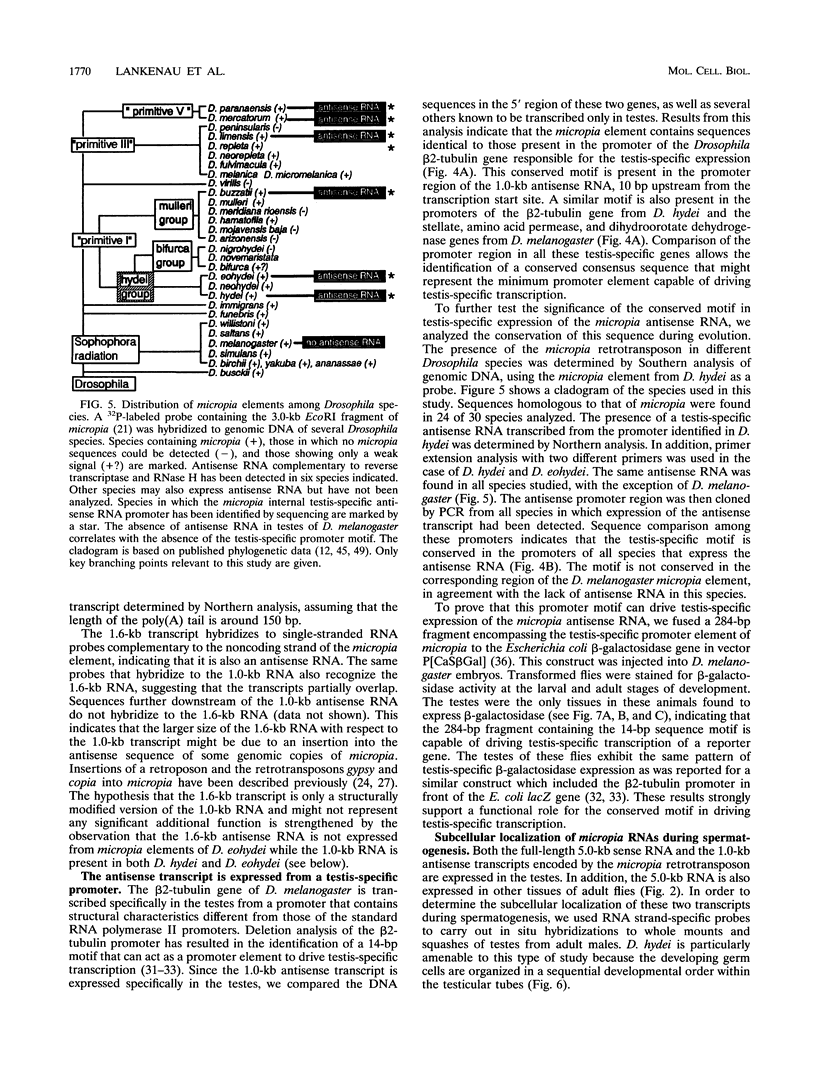
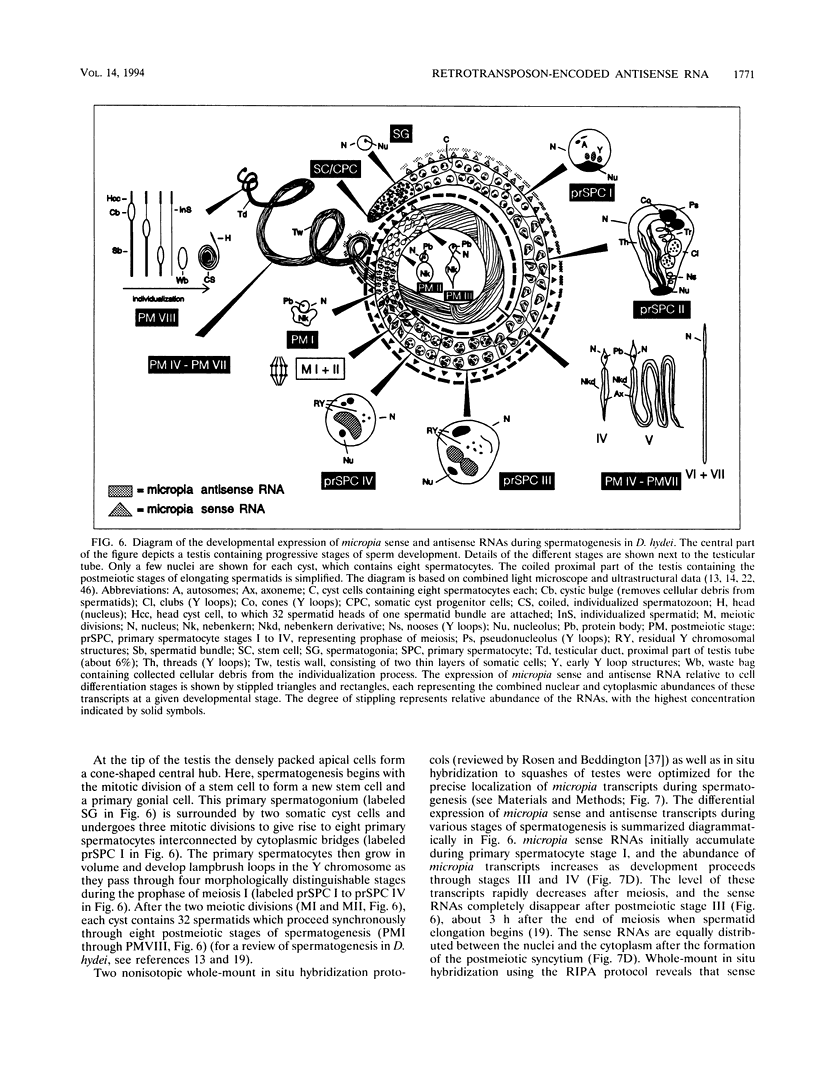
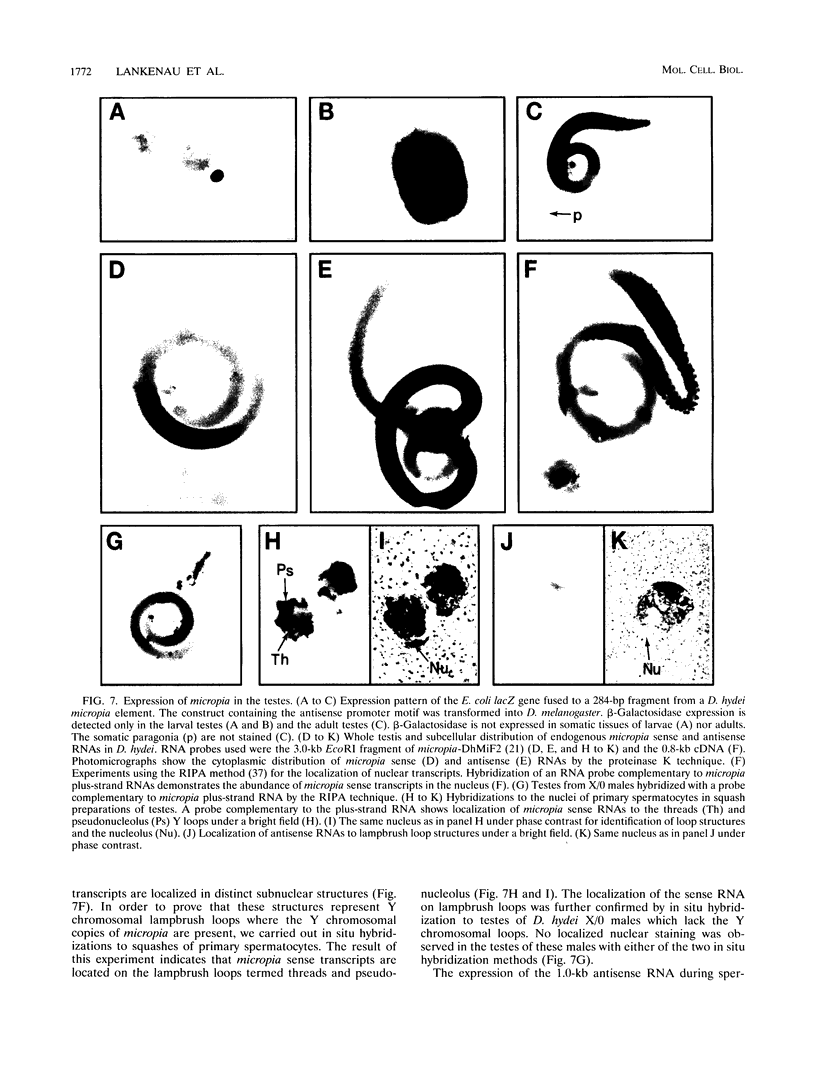
Abstract
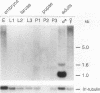
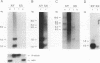
The micropia transposable element of Drosophila hydei is a long terminal repeat-containing retrotransposon present in both the autosomes and the Y chromosome. micropia expression gives rise to a complex set of sense and antisense RNAs transcribed primarily during spermatogenesis. The most abundant sense RNAs constitute an assortment of heterogeneous high-molecular-weight transcripts expressed as constituents of the Y-chromosomal lampbrush loops of primary spermatocytes. In addition, micropia encodes a full-length RNA that extends between the two long terminal repeats of the element. The major 1.0-kb antisense RNA characterized is complementary to the reverse transcriptase and RNase H coding regions of micropia. It is expressed from a testis-specific promoter during the primary spermatocyte stages and is detectable until spermatid elongation stages. Sequence comparison of this promoter with the 5' region of other testis-specific genes allows the conception of a conserved sequence that is responsible for this pattern of expression. A 284-bp fragment containing this sequence is able to drive testis-specific expression of the Escherichia coli lacZ gene in Drosophila melanogaster. This sequence is conserved in the micropia elements present in other Drosophila species that also encode an antisense RNA. The evolutionary conservation of micropia antisense RNA expression and the sequences responsible for its testis-specific transcription suggests a role for this antisense RNA in the control of germ line expression of the full-length transcript or transposon-encoded proteins.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck H. New compound (1) chromosomes and the production of large quantities of X/O males in Drosophila hydei. Genet Res. 1975 Dec;26(3):313–317. doi: 10.1017/s0016672300016116. [DOI] [PubMed] [Google Scholar]
- Boeke J. D., Corces V. G. Transcription and reverse transcription of retrotransposons. Annu Rev Microbiol. 1989;43:403–434. doi: 10.1146/annurev.mi.43.100189.002155. [DOI] [PubMed] [Google Scholar]
- Boorstein W. R., Craig E. A. Primer extension analysis of RNA. Methods Enzymol. 1989;180:347–369. doi: 10.1016/0076-6879(89)80111-9. [DOI] [PubMed] [Google Scholar]
- Finnegan D. J., Fawcett D. H. Transposable elements in Drosophila melanogaster. Oxf Surv Eukaryot Genes. 1986;3:1–62. [PubMed] [Google Scholar]
- Gerasimova T. I., Matjunina L. V., Mizrokhi L. J., Georgiev G. P. Successive transposition explosions in Drosophila melanogaster and reverse transpositions of mobile dispersed genetic elements. EMBO J. 1985 Dec 30;4(13B):3773–3779. doi: 10.1002/j.1460-2075.1985.tb04147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A., Frank D., Bolce M. E., Brown B. D., Sive H. L., Harland R. M. Localization of specific mRNAs in Xenopus embryos by whole-mount in situ hybridization. Development. 1990 Oct;110(2):325–330. doi: 10.1242/dev.110.2.325. [DOI] [PubMed] [Google Scholar]
- Hennig W., Huijser P., Vogt P., Jäckle H., Edström J. E. Molecular cloning of microdissected lampbrush loop DNA sequences of Drosophila hydei. EMBO J. 1983;2(10):1741–1746. doi: 10.1002/j.1460-2075.1983.tb01651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig W., Kremer H. Spermatogenesis of Drosophila hydei. Int Rev Cytol. 1990;123:129–175. doi: 10.1016/s0074-7696(08)60673-7. [DOI] [PubMed] [Google Scholar]
- Huijser P., Kirchhoff C., Lankenau D. H., Hennig W. Retrotransposon-like sequences are expressed in Y chromosomal lampbrush loops of Drosophila hydei. J Mol Biol. 1988 Oct 5;203(3):689–697. doi: 10.1016/0022-2836(88)90202-1. [DOI] [PubMed] [Google Scholar]
- Lankenau D. H., Hennig W. Micropia-Dm2, the nucleotide sequence of a rearranged retrotransposon from Drosophila melanogaster. Nucleic Acids Res. 1990 Jul 25;18(14):4265–4266. doi: 10.1093/nar/18.14.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankenau D. H., Huijser P., Hennig W. Characterization of the long terminal repeats of micropia elements microdissected from the Y-chromosomal lampbrush loops "threads" of Drosophila hydei. J Mol Biol. 1989 Oct 5;209(3):493–497. doi: 10.1016/0022-2836(89)90013-2. [DOI] [PubMed] [Google Scholar]
- Lankenau D. H., Huijser P., Jansen E., Miedema K., Hennig W. DNA sequence comparison of micropia transposable elements from Drosophila hydei and Drosophila melanogaster. Chromosoma. 1990 Apr;99(2):111–117. doi: 10.1007/BF01735326. [DOI] [PubMed] [Google Scholar]
- Lankenau D. H., Huijser P., Jansen E., Miedema K., Hennig W. Micropia: a retrotransposon of Drosophila combining structural features of DNA viruses, retroviruses and non-viral transposable elements. J Mol Biol. 1988 Nov 20;204(2):233–246. doi: 10.1016/0022-2836(88)90572-4. [DOI] [PubMed] [Google Scholar]
- Laverty T. R., Lim J. K. Site-specific instability in Drosophila melanogaster: evidence for transposition of destabilizing element. Genetics. 1982 Jul-Aug;101(3-4):461–476. doi: 10.1093/genetics/101.3-4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J. Detailed structure of the Drosophila melanogaster stellate genes and their transcripts. Genetics. 1990 Feb;124(2):303–316. doi: 10.1093/genetics/124.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels F., Falkenburg D., Müller A. M., Hinz U., Otto U., Bellmann R., Glätzer K. H., Brand R., Bialojan S., Renkawitz-Pohl R. Testis-specific beta 2 tubulins are identical in Drosophila melanogaster and D. hydei but differ from the ubiquitous beta 1 tubulin. Chromosoma. 1987;95(6):387–395. doi: 10.1007/BF00333989. [DOI] [PubMed] [Google Scholar]
- Michiels F., Gasch A., Kaltschmidt B., Renkawitz-Pohl R. A 14 bp promoter element directs the testis specificity of the Drosophila beta 2 tubulin gene. EMBO J. 1989 May;8(5):1559–1565. doi: 10.1002/j.1460-2075.1989.tb03540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels F., Wolk A., Renkawitz-Pohl R. Further sequence requirements for male germ cell-specific expression under the control of the 14 bp promoter element (beta 2UE1) of the Drosophila beta 2 tubulin gene. Nucleic Acids Res. 1991 Aug 25;19(16):4515–4521. doi: 10.1093/nar/19.16.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K., Rosenbaum J., Zbrzezna V., Pogo A. O. The nucleotide sequence of Drosophila melanogaster copia-specific 2.1-kb mRNA. Nucleic Acids Res. 1989 Mar 11;17(5):2134–2134. doi: 10.1093/nar/17.5.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mével-Ninio M., Mariol M. C., Gans M. Mobilization of the gypsy and copia retrotransposons in Drosophila melanogaster induces reversion of the ovo dominant female-sterile mutations: molecular analysis of revertant alleles. EMBO J. 1989 May;8(5):1549–1558. doi: 10.1002/j.1460-2075.1989.tb03539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst S. M., Corces V. G. Developmental expression of Drosophila melanogaster retrovirus-like transposable elements. EMBO J. 1987 Feb;6(2):419–424. doi: 10.1002/j.1460-2075.1987.tb04771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirrotta V., Steller H., Bozzetti M. P. Multiple upstream regulatory elements control the expression of the Drosophila white gene. EMBO J. 1985 Dec 16;4(13A):3501–3508. doi: 10.1002/j.1460-2075.1985.tb04109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen B., Beddington R. S. Whole-mount in situ hybridization in the mouse embryo: gene expression in three dimensions. Trends Genet. 1993 May;9(5):162–167. doi: 10.1016/0168-9525(93)90162-b. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A. C., Mahowald A. P. Identification and genetic localization of mRNAs from ovarian follicle cells of Drosophila melanogaster. Cell. 1979 Mar;16(3):589–598. doi: 10.1016/0092-8674(79)90032-1. [DOI] [PubMed] [Google Scholar]
- Tautz D., Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989 Aug;98(2):81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Retrovirus variation and reverse transcription: abnormal strand transfers result in retrovirus genetic variation. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):6900–6903. doi: 10.1073/pnas.90.15.6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K. T., Peacock W. J., Hardy R. W. Dynamics of spermiogenesis in Drosophila melanogaster. I. Individualization process. Z Zellforsch Mikrosk Anat. 1972;124(4):479–506. doi: 10.1007/BF00335253. [DOI] [PubMed] [Google Scholar]
- Vogt P., Hennig W., Siegmund I. Identification of cloned Y chromosomal DNA sequences from a lampbrush loop of Drosophila hydei. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5132–5136. doi: 10.1073/pnas.79.17.5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Loos F., Dijkhof R., Grond C. J., Hennig W. Lampbrush chromosome loop-specificity of transcript morphology in spermatocyte nuclei of Drosophila hydei. EMBO J. 1984 Dec 1;3(12):2845–2849. doi: 10.1002/j.1460-2075.1984.tb02218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]