Abstract
Purpose
Several epidemiologic studies have evaluated the association between statins and lung cancer risk, whereas randomized controlled trials (RCTs) on cardiovascular outcomes provide relevant data as a secondary end point. We conducted a meta-analysis of all relevant studies to examine this association.
Methods
A systematic literature search up to March 2012 was performed in PubMed database. Study-specific risk estimates were pooled using a random-effects model.
Results
Nineteen studies (5 RCTs and 14 observational studies) involving 38,013 lung cancer cases contributed to the analysis. They were grouped on the basis of study design, and separate meta-analyses were conducted. There was no evidence of an association between statin use and risk of lung cancer either among RCTs (relative risk [RR] 0.91, 95% confidence interval [CI] 0.76–1.09), among cohort studies (RR 0.94, 95% CI 0.82–1.07), or among case-control studies (RR 0.82, 95% CI 0.57–1.16). Low evidence of publication bias was found. However, statistically significant heterogeneity was found among cohort studies and among case-control studies. After excluding the studies contributing most to the heterogeneity, summary estimates were essentially unchanged.
Conclusion
The results of our meta-analysis suggest that there is no association between statin use and the risk of lung cancer.
Introduction
Lung cancer is by far the most common cause of cancer mortality in the United States and throughout the world. According to the International Agency for Research on Cancer for 2008, about 1.6 million individuals were diagnosed with lung cancer and 1.4 million died as a result, which makes it the first-leading cause of cancer death in men and second in women globally [1]. In the United States, lung cancer is expected to account for 26% of all female cancer deaths and 29% of all male cancer deaths in 2012 [2].
Lung cancer stands out from other types of cancers because of our recognition of the major modifiable risk factor to the disease- exposure to tobacco smoke [3]. However, not all lung cancer cases are linked to cigarette smoking. Other risk factors include exposure to asbestos, haloethers, nickel, arsenic, and polycyclic aromatic hydrocarbons. Potential risk factors include genetic factors, dietary factors, and the presences of underlying benign forms of parenchymal lung diseases such as pulmonary fibrosis and chronic obstructive lung disease [4]–[5]. To date, no chemopreventive agent has been identified as an effective means to reduce the incidence of lung cancer.
Statins are inhibitors of 3-hydroxy-3-methyl glutaryl-coenzyme A reductase which is the rate-limiting enzyme in mevalonate synthesis. Statins are commonly used as cholesterol-lowering medications and have demonstrated the beneficial effects on cardiovascular morbidity and mortality [6]. As such, statins are some of the most widely prescribed drugs worldwide. Rodent studies suggested that statins may be carcinogenic [7]. In contrast, several preclinical studies indicate that these drugs may have cancer chemopreventive properties, through their interactions with essential cellular functions, such as cell proliferation and differentiation [8], [9]. Recently, meta-analysis of RCTs of statins for cardiovascular outcomes demonstrated no association between statin use and the risk of cancer [10]. However, the end-point of all cancers is not very sensitive and a negative finding does not suggest a lack of an effect at a particular site. Therefore, the effect of statins on the risk of lung cancer remains to be determined. To address this issue, we conducted a detailed meta-analysis of studies published in peer-reviewed literature.
Materials and Methods
Search Strategy
A systematic literature search up to March of 2012 was performed in PubMed database to identify relevant studies. Search terms included “HMG-CoA reductase inhibitor(s),” “statin(s)” combined with “cancer(s),” or “neoplasm(s)”. The search was limited to English language articles and those with human subjects. The title and abstract of studies identified in the search were scanned to exclude any clearly irrelevant studies. The full texts of the remaining articles were read to determine whether they contained information on the topic of interest. Furthermore, to find any additional published studies, a manual search was performed by checking all the references of retrieved articles. All searches were conducted independently by 2 authors (MT and XS). The results were compared, and any questions or discrepancies were resolved through iteration and consensus.
Study Selection
To be eligible, studies had to fulfill the following 4 inclusion criteria: 1) RCTs or observational studies (case-control or cohort); 2) report results on statin use; 3) lung cancer incidence as the outcome of interest; and 4) reported the estimate of relative risk (RR) with their corresponding 95% confidence interval (CI) (or sufficient data to calculate of these effect measure). RCTs were considered eligible if they evaluated a statin therapy compared with placebo or no treatment, had no other intervention difference between the experimental and the control group. Studies reporting different measures of RR like risk ratio, rate ratio, hazard ratio (HR), and odds ratio (OR) were included in the meta-analysis. In practice, these measures of effect yield a similar estimate of RR, since the absolute risk of lung cancer is low.
Data Extraction
Information from studies was extracted independently by 2 researchers (MT and XS), with disagreements resolved by consensus. The following data were collected: the first author’s last name, year of publication, country in which the study was performed, study design, years of follow-up or the study period, study participants age range, number of subjects and number of lung cancer cases, covariates controlled for in the analysis, and RR estimates with corresponding 95% CIs. If a study provided several risk estimates, the most completely adjusted estimate was extracted. Risk ratios and 95% CIs were calculated for each RCT by reconstructing contingency tables based on the number of participants randomly assigned and the number of participants with incident lung cancer (intention-to treat analysis). Differences in data extraction were resolved by consensus, referring back to the original article.
The quality of included RCTs was assessed based on Cochrane handbook [11], by recording seven items of bias risk: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data addressed, free of selective reporting, and free of other bias (follow-up ≥ 4 years). Each of the seven items is scored as ‘‘low risk,’’ ‘‘unclear risk,’’ or ‘‘high risk.” Meanwhile, the included cohort and case-control studies were assessed based on the 9-star Newcastle-Ottawa Scale for quality of non-randomized studies in meta-analyses [12].
Statistical analysis
Studies were grouped on the basis of study design, and two separate meta-analyses were conducted: one meta-analysis of RCTs and a second meta-analysis of observational studies. This was done to examine consistency of results across varying study designs with different potential biases.
Study-specific risk estimates were extracted from each article, and log risk estimates were weighted by the inverse of their variances to obtain a pooled risk estimate. Studies were combined by using the DerSimonian and Laird random-effects model, which considers both within- and between-study variations [13].
Q and I2 statistics were used to examine whether the results of studies were homogeneous [14]. For the Q statistic, a P value < 0.10 was considered statistically significant for heterogeneity; for I2, a value >50% is considered a measure of severe heterogeneity. When statistical heterogeneity was detected, sensitivity analyses were performed. Publication bias was evaluated with Egger’s regression test in which P value less than 0.10 was considered representative of statistically significant publication bias [15]. All statistical analyses were performed with Stata software, version 10 (Stata Corp, College Station, Texas).
Results
Literature Search
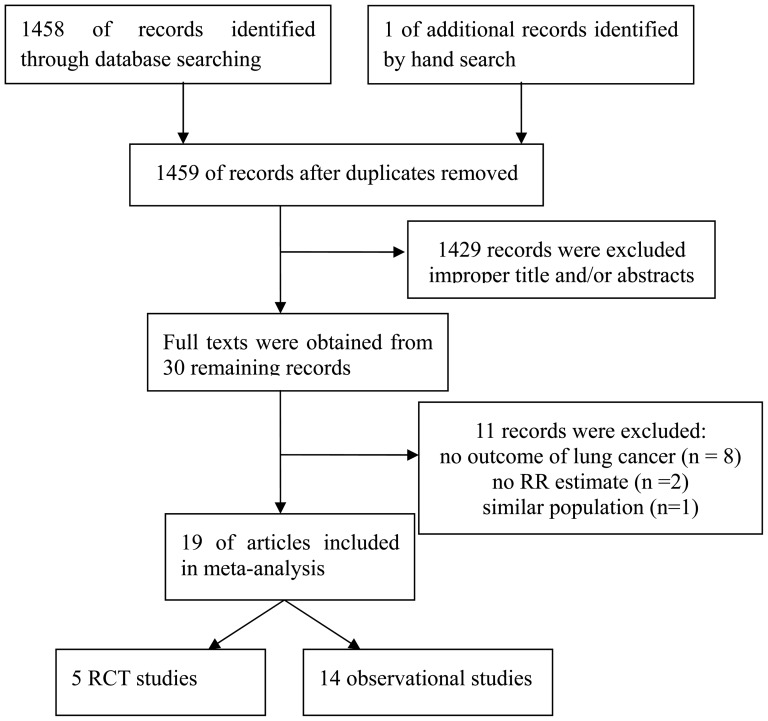
Our initial search strategy retrieved a total of 1459 citations. After the titles and abstracts were screened, 1429 articles were excluded because they were laboratory studies, review articles, or irrelevant to the current study. We identified 30 potentially relevant articles concerning statin use in relation to lung cancer risk. Eight publications were excluded because they investigated the association of statin with risk of total cancer and lung cancer was not among collected data [16]–[23]. Two articles were excluded because they did not provide RR estimate [24], [25] and one article was excluded because it reported on similar population [26]. Finally, 19 articles [27]–[45] concerning statin use and lung cancer risk (including 5 RCT studies and 14 observational studies) were included in this meta-analysis (Figure 1). We performed this meta-analysis in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement (File 1) [46].
Figure 1. Flow diagram of study identification.
Four of five RCTs were placebo controlled, whereas one RCT [28] was a non-blinded trial comparing statin treatment with a usual care control group. All RCTs were multi-center trials and reported site-specific cancer outcomes (secondary end points) including lung cancer. Therefore, we were able to conduct a post hoc analysis of these trials and calculate risk ratios for lung cancer in an intention-to-treat analysis. Study designs, along with the RR estimates and 95% CIs, are listed in Table 1 for the RCTs and in Table 2 for the observational studies. Six observational studies [36]–[38], [40], [43], [44] were reported RR estimates of the association between long-term statin use and risk of lung cancer (Table 3).
Table 1. Randomized controlled trials included in the meta-analysis.
| Study | Exposure | Duration (y) | Users | Non-users | Incident Lung Cancer | RR | 95% CI | |
| Statins | Controls | |||||||
| AFCAPS (27) | Lovastation | Mean,5.2 | 3,304 | 3,301 | 22 | 17 | 1.29 | 0.69–2.43 |
| ALLHAT-LLT (28) | Pravastatin | Mean, 4.8 | 5,170 | 5,185 | 63 | 78 | 0.81 | 0.58–1.13 |
| LIPS (29) | Fluvastatin | Median, 3.9 | 844 | 833 | 5 | 3 | 1.65 | 0.39–6.86 |
| 4S (30) | Simvastatin | Median, 10.4 | 2,221 | 2,223 | 25 | 31 | 0.81 | 0.48–1.36 |
| WOSCOPS (31) | Pravastatin | Mean, 4.9 | 3,291 | 3,286 | 102 | 109 | 0.93 | 0.76–1.09 |
Abbreviations: RR, risk ratio; AFCAPS, Air Force/Texas Coronary Atherosclerosis Prevention Study; ALLHAT-LLT, Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial; LIPS, Lesol Intervention Prevention; 4S, Scandinavian Simvastatin Survival Study; HPS, Heart Protection Study; WOSCOPS, West of Scotland Coronary Prevention Study.
Table 2. Observational studies included in the meta-analysis.
| Study | Year | Country | Design | Study period | Age, y | N. of participant | LC Cases | RR | 95% CI | Adjustments* |
| Blais (32) | 2000 | Canada | C-C | 1988–1994 | ≥65 | 5,962 | 70 | 0.94 | 0.43–2.05 | 1–6 |
| Kaye (33) | 2004 | UK | C-C | 1990–2002 | 50–89 | 18,088 | 259 | 0.9 | 0.6–1.3 | 1, 2, 7–9 |
| Graaf (34) | 2004 | Netherlands | C-C | 1985–1998 | NR | 20,105 | 445 | 0.89 | 0.56–1.42 | 1–5, 10–16 |
| Friis (35) | 2005 | Denmark | Co | 1989–2002 | 30–80 | 334,754 | 3,399 | 0.92 | 0.72–1.16 | 1, 2, 15, 16, 17 |
| Coogan (36) | 2007 | US | C-C | 1991–2005 | 40–79 | 8,813 | 464 | 0.7 | 0.4–1.1 | 1, 2, 8, 9, 15, 18, 19–22 |
| Setoguchi (37) | 2007 | US | Co | 1994–2003 | >65 | 31,723 | 216 | 1.11 | 0.77–1.60 | 1, 2, 3, 7, 10, 11, 15, 19, 23–36 |
| Khurana (38) | 2007 | US | C-C | 1998–2004 | 18–100 | 483,733 | 7,280 | 0.55 | 0.52–0.59 | 1, 2, 8, 9, 10, 18, 19 |
| Farwell (39) | 2008 | US | Co | 1997–2005 | 66.5 | 62,842 | 867 | 0.70 | 0.60–0.81 | 1, 9, 10, 15, 33, 37–49 |
| Friedman (40) | 2008 | US | Co | 1994–2003 | >20 | 361,859 | 1042 | 1.09† | 0.96–1.23 | 50 |
| Haukka (41) | 2009 | Finland | Co | 1996–2005 | 60.0 | 944,962 | 5129 | 0.81 | 0.77–0.86 | 1, 2, 5 |
| Hippisley (42) | 2010 | UK | Co | 2002–2008 | 30–84 | 2,121,786 | 6001 | 1.03† | 0.94–1.21 | 1, 8, 9, 51–54 |
| Vinogradova (43) | 2011 | UK | C-C | 1998–2008 | 30–100 | 450379 | 10,163 | 1.07 | 0.99–1.16 | 1, 2, 8, 9, 10, 15, 25, 39, 40, 49, 55 |
| Jacobs (44) | 2011 | US | Co | 1997–2007 | >60 | 133,255 | 1,926 | 1.04† | 0.95–1.14 | 1, 2, 8–10, 16, 19, 20, 39, 40, 56, 57 |
| Cheng (45) | 2012 | Taiwan | C-C | 2005–2008 | >50 | 1485 | 297 | 0.82 | 0.58–1.15 | 1, 2, 4, 10, 15, 16, 58 |
Abbreviations: LC, lung cancer; RR, relative risk; C-C, case control; Co, cohort; NR, not reported.
1, age; 2, sex; 3, comorbidity score;4, other lipid-lowering therapy; 5, duration of follow-up; 6, history of neoplasia; 7, number of physician visits; 8, body mass index; 9, smoking status; 10, diabetes; 11, prior hospitalizations, 12, use of diuretics; 13, use of angiotensin-converting enzyme inhibitor; 14, use of calcium channel blockers; 15, use of nonsteroidal anti-inflammatory drugs; 16, hormone replacement therapy; 17, use of cardiovascular drugs; 18, alcohol use; 19, race; 20, education; 21, study center; 22, interview year; 23, inflammatory bowel disease; 24, benign mammary dysplasia; 25, arthritis; 26, use of gastroprotective drugs; 27, estrogen use; 28, obesity; 29; tobacco abuse; 30, mammography; 31, gynecologic examination; 32, Papanicolaou smear; 33, colonoscopy; 34, stool occult blood; 35, distinct generic medicines taken; 36, prior nursing home stay; 37, weight; 38, thyroid disease; 39, hypertension; 40 cardiovascular disease; 41, renal failure;42, chest pain; 43, mental illness; 44, alcoholism; 45, lung disease; 46, gastrointestinal disease; 47, prostate disease; 48, total cholesterol; 49, aspirin use ; 50,calendar year; 51,Townsend score, 52, any other cancer; 53,corticosteroids; 54, asthma; 55, Cox2-inhibitors; 56, physical activity; 57, history of elevated cholesterol; 58, tuberculosis.
The risk estimate was calculated by post hoc analysis.
Table 3. Studies evaluating the association between long-term statin use and risk of lung cancer.
| Study | LC cases | RR | 95% CI | Definition of ‘‘long-term’’ statin use |
| Coogan (36) | 10 | 0.9 | 0.4 to 2.1 | >5 years |
| Setoguchi (37) | 80 | 1.02 | 0.59 to 1.74 | ≥3 years |
| Khurana (38) | 269 | 0.23 | 0.20 to 0.26 | >4 years |
| Friedman (40) | 119 (men) | 1.06 | 0.88 to 1.28 | >5 year |
| Friedman (40) | 78 (women) | 1.17 | 0.93 to 1.46 | >5 year |
| Vinogradova (43) | 558 | 1.18 | 1.05 to 1.34 | ≥49 months |
| Jacobs (44) | 340 | 1.08 | 0.93 to 1.25 | ≥5 year |
Abbreviations: LC, lung cancer; RR, relative risk. CI, confidence intervals.
Table 4 illustrates our opinion about each item of bias risk for included RCTs, most of the items were at ‘‘low risk’’ based on Cochrane handbook. Table 5 summarizes the quality scores of cohort studies and case-control studies based on the Newcastle-Ottawa Scale. Most of the observational studies score 5 or more, suggesting a reasonable good quality of the cohort and case-control studies.
Table 4. Methodological quality of included randomized controlled trials.* .
| Study | Random sequence generation | Allocation concealment | Blinding of participant and personal | Blinding of outcome assessment | Incomplete outcome data addressed | Free of selective reporting | Free of other bias† |
| AFCAPS (27) | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| ALLHAT-LLT (28) | Yes | Yes | Unclear | Yes | Yes | Yes | Yes |
| LIPS (29) | Yes | Yes | Yes | Yes | Yes | Yes | No |
| 4S (30) | Yes | Unclear | Yes | Yes | Yes | Unclear | Yes |
| WOSCOPS (31) | Yes | Unclear | Yes | Yes | Yes | Yes | Yes |
Abbreviations: AFCAPS, Air Force/Texas Coronary Atherosclerosis Prevention Study; ALLHAT-LLT, Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial; LIPS, Lesol Intervention Prevention; 4S, Scandinavian Simvastatin Survival Study; HPS, Heart Protection Study; WOSCOPS, West of Scotland Coronary Prevention Study.
Yes, low risk of bias; Unclear, unclear risk of bias; No, high risk of bias.
Follow-up ≥4 years.
Table 5. Methodological quality of included cohort studies and case–control studies based on the Newcastle–Ottawa Scale.
| Cohort studies | Selection | Comparability | Outcome | Total score | |||||
| Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Outcome of interest was not present at start of study | Control for important factor or additional factor | Assessment of outcome | Follow-up long enough for outcomes to occur † | Adequacy of follow-up of cohort | ||
| Friis (35) | * | * | * | * | * | * | 6 | ||
| Setoguchi (37) | * | * | * | * | * | * | * | 7 | |
| Farwell (39) | * | * | * | * | ** | * | * | * | 9 |
| Friedman (40) | * | * | * | * | * | * | 6 | ||
| Haukka (41) | * | * | * | * | * | * | * | 7 | |
| Hippisley (42) | * | * | * | ** | * | * | * | 8 | |
| Jacobs (44) | * | * | * | ** | * | * | * | 8 | |
Follow-up ≥4 year.
Meta-analysis of RCTs
Five RCTs contributed to the analysis [27]–[31]. A total 29,658 individuals participated in these trials: 14,830 in treatment groups and 14,828 in control groups (Table 1). The participants had a mean follow-up of approximately 5.8 years. The overall rate of lung cancer was 1.46% in the statin group (217 incident cases) and 1.61% in the control group (238 incident cases). Figure 2 graphs the RR estimates and 95% CI from the individual trials and the pooled results. Statin use was not found to be associated with the risk of lung cancer (RR 0.91, 95% CI 0.76–1.09). The Cochran’s Q test resulted in a P = 0.63 (Q = 2.57), and the corresponding quantity I2 was 0%, both indicating that study results were homogeneous. The P value for the Egger test was P = 0.30, suggesting a low probability of publication bias.
Figure 2. In RCT studies, risk estimates of lung cancer associated with statin use.
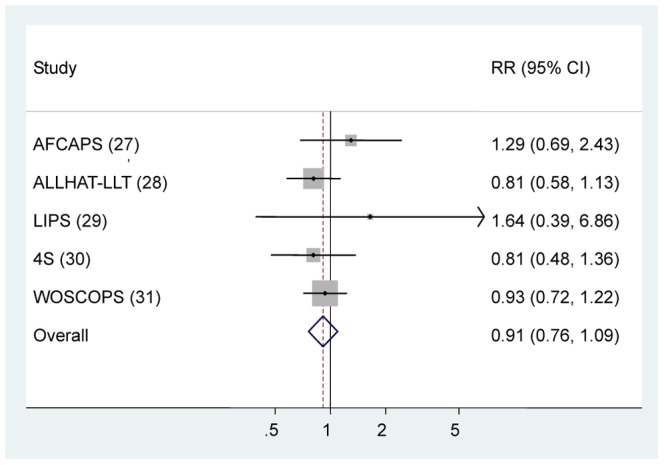
Squares indicate study-specific risk estimates (size of the square reflects the study-specific statistical weight, i.e., the inverse of the variance); horizontal lines indicate 95% confidence intervals (CIs); diamonds indicate summary risk estimate with its corresponding 95% confidence interval. Abbreviations: RR, risk ratio; AFCAPS, Air Force/Texas Coronary Atherosclerosis Prevention Study; ALLHAT-LLT, Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial; LIPS, Lesol Intervention Prevention; 4S, Scandinavian Simvastatin Survival Study; HPS, Heart Protection Study; WOSCOPS, West of Scotland Coronary Prevention Study.
When the analysis was restricted to trials that evaluated statin therapy compared with placebo [27], [29]–[31], the results did not substantially change (RR 0.96, 95% CI 0.77–1.20). Similarly, after stratifying the data in two subgroups (lipophilic v lipophobic statins), we did not find any statistically significant association between lipophilic or lipophobic statins and lung cancer risk (Table 6).
Table 6. Meta-analysis results.
| Study type | References | RR | 95% CI | Heterogeneity test | ||
| Q | P | I2 (%) † | ||||
| RCTs | 27–31 | 0.91 | 0.76 to 1.09 | 2.57 | 0.633 | 0 |
| Placebo-controlled RCTs | 27,29–31 | 0.96 | 0.77 to 1.20 | 1.86 | 0.601 | 0 |
| RCTs of lipophilic statins | 27,29,30 | 1.02 | 0.69 to 1.50 | 1.74 | 0.419 | 0 |
| RCTs of lipophobic statins | 28,31 | 0.88 | 0.72 to 1.09 | 0.44 | 0.509 | 0 |
| Observational studies | 32–45 | 0.88 | 0.75 to 1.04 | 267.72 | <0.001 | 95.1 |
| Cohort studies | 35,37,39,40–42,44 | 0.94 | 0.82 to 1.07 | 49.09 | <0.001 | 87.8 |
| Case-control studies | 32–34,36,38,43,45 | 0.82 | 0.57 to 1.16 | 169.01 | <0.001 | 96.4 |
| Long-term statin use | 36–38, 40, 43, 44 | 0.81 | 0.42 to 1.56 | 416.93 | <0.001 | 98.8 |
| Adjust for smoking | ||||||
| No | 32,34,35,37,40,41,45 | 0.93 | 0.80 to 1.08 | 20.87 | 0.002 | 71.3 |
| yes | 33,36,38,39,42–44 | 0.84 | 0.64 to 1.11 | 237.68 | <0.001 | 97.5 |
Abbreviations: RR, relative risk; CI, confidence intervals; RCT, randomized controlled trial.
I2 is interpreted as the proportion of total variation across studies that are due to heterogeneity rather than chance.
Meta-Analysis of Observational Studies
The 14 relevant studies were published between 2000 and 2012 (Table 2) including 7 cohort studies [35], [37], [39], [40]–[42] and 7 case-control studies [32]–[34], [36], [38], [43], [45]. A total of 4,979,746 participants, including 37,558 lung cancer cases were involved in these studies and followed for 4–15 years. All studies evaluated exposure to statins and the risk of lung cancer except for one study [36] that examined the use of all cholesterol-lowering drugs. Five studies reported RR [32], [33], [35], [41], [44], 5 reported OR [34], [36], [38], [43], [45], and 4 reported HR [37], [39], [40], [42]. Most studies provided risk estimates that were adjusted for age (12 studies), sex (10 studies), smoking (7 studies), use of nonsteroidal anti-inflammatory drugs (7 studies), and diabetes (7 studies); fewer were adjusted for body mass index (6 studies), and alcohol use (2 studies) (Table 2).
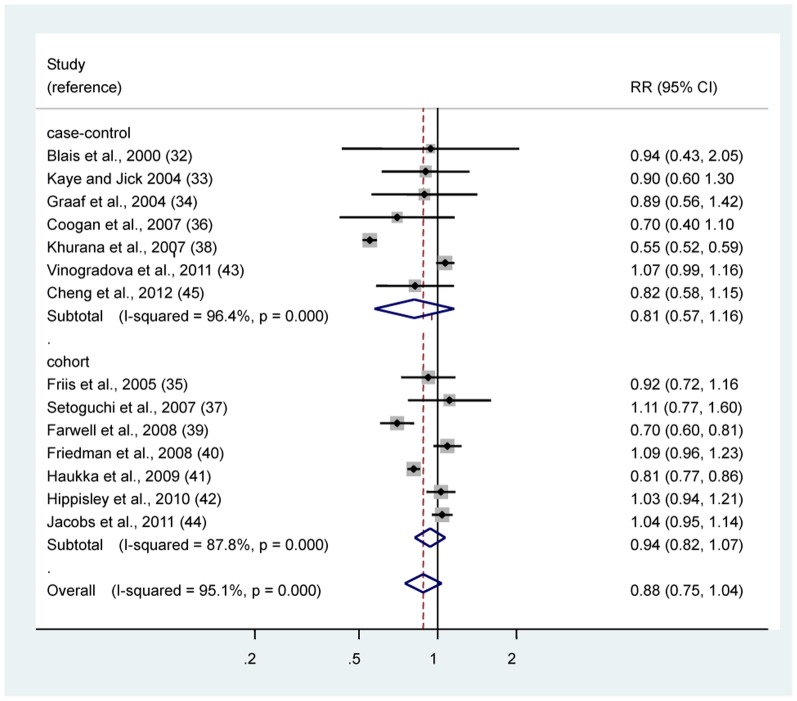
The multivariable-adjusted RRs of lung cancer for statin use in individual observational studies and summary estimates are shown in Figure 3. The overall RR of lung cancer for statin use was 0.88 (95% CI 0.75–1.04) for observational studies combined. There was statistically significant heterogeneity among studies (P < 0.001; I2 = 95.1%). The Egger test showed no evidence of publication bias (P = 0.43).
Figure 3. In observational studies, risk estimates of lung cancer associated with statin use.
Squares indicate study-specific risk estimates (size of the square reflects the study-specific statistical weight, i.e., the inverse of the variance); horizontal lines indicate 95% confidence intervals (CIs); diamonds indicate summary risk estimate with its corresponding 95% confidence interval.
To examine consistency across varying study designs with different potential biases, we stratified data into subgroups on the basis of study design. No significant association between statins and risk of lung cancer among case-control studies (RR 0.81, 95% CI 0.57–1.16) and cohort studies (RR 0.94, 95% CI 0.82–1.07). Significant heterogeneity was also observed among case-control studies (P < 0.001; I2 = 96.4%) and cohort studies (P < 0.001; I2 = 87.8%). By using a stepwise process, we determined that most of the heterogeneity was accounted for one study by Khurana et al. [38] in case-control studies. When this studies were excluded, the summary estimate was essentially unchanged (RR 0.99, 95% CI 0.87–1.11), but a concomitant shift in heterogeneity was measured by the Q-test (from P < 0.001 to P = 0.335). And we also found that most of the heterogeneity was accounted for two studies by Farwell et al. [39] and by Haukka et al. [41] in cohort studies. When these two studies were excluded, the summary estimate was essentially unchanged (RR 1.04, 95% CI 0.98–1.11), but a concomitant shift in heterogeneity was measured by the Q-test (from P < 0.001 to P = 0.790).
Further, six studies [36]–[38], [40], [43], [44] reported RR estimates of the association between long-term statin use and the risk of lung cancer (Table 3). Based on the results from these studies, the calculated combined RR for lung cancer in long-term statin use was found to be 0.81 (95 % CI 0.42–1.56) (Table 6). Stratified analysis by adjustment for smoking did not show any statistically significant difference in summary estimates between strata (Table 6).
Combined Analysis
Furthermore, we performed a combined analysis of RCTs and observational studies. Statin use was not found to be associated with the risk of lung cancer (RR 0.89, 95% CI 0.77–1.03). The Cochran’s Q test resulted in a P < 0.001 (Q = 268.59), and the corresponding quantity I2 was 93.3%. However, this particular analysis was dominated by the observational studies (14 studies). These studies accounted for the 81.2% in the random-effects model.
Discussion
This present meta-analysis included 19 clinical studies (5 RCTs and 14 observational studies), involving a total of 5,009,404 participants and 38,013 lung cancer cases. Overall, both meta-analyses of RCTs and observational studies showed no evidence for an association between statin use and the risk of lung cancer. Our results are in accord with recent meta-analyses on the association between statin use and other site-specific cancers. Likewise, they concluded that statins do not offer any substantial increase or reduction in colorectal, pancreatic, melanoma, or breast cancer risk [47]–[50].
In our subgroup analyses, the results were not substantially affected by study design, RCTs of lipophilic or lipophobic statins, and studies of long-term statin use, which reinforce our confidence in the validity of the conclusion that statin use was not associated with lung cancer risk. Although significant heterogeneity was observed among cohort and case-control studies, summary estimates were essentially unchanged after excluding the studies contributing most to the heterogeneity.
Several meta-analyses have evaluated the association between statins and lung cancer risk [51]–[53]. In the meta-analysis of twenty case-control studies [51], Taylor et al found a significant association between statin usage and any cancer, but when stratified by cancer type, only the association with colon cancer remained. However, the studies were significantly heterogeneous (P < 0.01) and these case-control studies were susceptible to various biases. A 2007 meta-analysis included RCTs and observational studies concluded that statin use was not associated with lung cancer risk. And this meta-analysis included 12 observational studies, only 3 were limited to lung cancer [52]. The recent meta-analysis by Kuoppala et al. [53] used hierarchical quality-based methods in evaluation and contained several observational studies not included in previous reports. This meta-analysis showed that statins had no effect on the incidence of lung cancer. However, the effect estimate for lung cancer had wide range (median RR 0.92, range 0.83 to 3.0) and the strength of evidence was weak.
Although we found no association between statin use and lung cancer risk in clinical studies, several preclinical studies indicate that statins may have cancer chemopreventive properties. The mechanistic data suggest that statins’ chemopreventive potential against cancer through their inhibition of the mevalonate pathway [54]. The mevalonate pathway is an important metabolic pathway that provide cell with bioactive molecules which play a key role in multiple cellular processes such as membrane integrity, cell signaling, protein synthesis, and cell cycle progression [55]. Statins’ inhibition of HMG-CoA reductase prevents the conversion of HMG-CoA to mevalonate, and thereby reduce levels of mevalonate and its downstream products, probably resulting in control of tumor initiation, growth, and metastasis [56], [57].
Increasing evidence also suggests that statins might enhance the antitumor activity of various cytokines and chemotherapeutic agents. In a phase 2 study of irinotecan, cisplatin, and simvastatin for untreated extensive-disease small cell lung cancer (ED-SCLC), the results indicated that the addition of simvastatin to irinotecan and cisplatin might improve the outcome of heavy smokers with ED-SCLC [58]. And another phase 2 study of gefitinib plus simvastatin versus gefitinib alone showed that simvastatin might improve the efficacy of gefitinib in that subgroup of gefitinib-resistant non-SCLC patients [59]. Because this field is new, only a few clinical trials have been reported so far. Therefore, the combined treatment of tumors with statins and anticancer drugs is an area of research that warrants future study.
Interestingly, the recent study by Nielsen et al [60] suggested that statin use was associated with a substantial decline in cancer-related mortality. They assessed mortality among patients from the entire Danish population who had received a diagnosis of cancer between 1995 and 2007. The study design provided substantial power to evaluate mortality from cancer with limited selection bias. However, the study does have some major limitations that may influence the interpretation of the results [61]. One limitation is that the important information on smoking and other risk factors (such as surgery) are not available. Another limitation is that there is no clear pattern of decreased mortality with increased dose [61]. Therefore, more studies are needed to verify the findings in other populations, taking into account treatment, smoking status, and other risk factors.
The present study has several strengths. First, this latest meta-analysis combined all relevant literature published up to March of 2012. Moreover, 19 clinical studies were included in our meta-analysis, reporting data of 38,013 lung cancer cases. Meta-analysis of studies with large numbers of incident cases provides high statistical power for estimating the relationship between exposure and outcome risk.
Nevertheless, our meta-analysis has several limitations. First, the included studies were different in terms of study design and definitions of drug exposure. However, our findings were stable and robust in the subgroup analyses. Second, a meta-analysis is not able to solve problems with confounding factors that could be inherent in the included studies. Inadequate control for confounders may bias the results in either direction, toward exaggeration or underestimation of risk estimates. In our meta-analysis, each observational study adjusted RR for different confounding factors which might be a source of heterogeneity. Third, heterogeneity may also be introduced because of methodologic and demographic differences among studies. We used appropriate well-motivated inclusion criteria to maximize homogeneity, and performed sensitivity and subgroup analyses to investigate potential sources of heterogeneity. Finally, inherent in any review process of published studies is the possibility of publication bias. Our search was restricted to studies published in indexed journals. In this meta-analysis, we did not search for unpublished studies or for original data. However, we found no evidence of substantial publication bias.
In summary, findings from this meta-analysis indicated that statin use was not associated with the risk of lung cancer.
Supporting Information
PRISMA Checklist for the meta-analysis.
(DOC)
Funding Statement
The authors have no support or funding to report.
References
- 1. Jemal A, Bray F (2011) Center MM, Ferlay J, Ward E, et al (2011) Global cancer statistics. CA Cancer J Clin 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 2. Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62: 10–29. [DOI] [PubMed] [Google Scholar]
- 3. Ezzati M, Henley SJ, Lopez AD, Thun MJ (2005) Role of smoking in global and regional cancer epidemiology: current patterns and data needs. Int J Cancer 116: 963–971. [DOI] [PubMed] [Google Scholar]
- 4. Wen J, Fu J, Zhang W, Guo M (2011) Genetic and epigenetic changes in lung carcinoma and their clinical implications. Mod Pathol 24: 932–943. [DOI] [PubMed] [Google Scholar]
- 5. Adcock IM, Caramori G, Barnes PJ (2011) Chronic obstructive pulmonary disease and lung cancer: new molecular insights. Respiration 81: 265–284. [DOI] [PubMed] [Google Scholar]
- 6. Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, et al. (2005) Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomized trials of statins. Lancet 366: 1267–1278. [DOI] [PubMed] [Google Scholar]
- 7. Newman TB, Hulley SB (1996) Carcinogenicity of lipid-lowering drugs. JAMA 275: 55–60. [PubMed] [Google Scholar]
- 8. Dimitroulakos J, Marhin WH, Tokunaga J, Irish J, Gullane P, et al. (2002) Microarray and biochemical analysis of lovastatin-induced apotosis of squamous cell carcinoma. Neoplasia 4: 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rubins JB, Greatens T, Kratzke RA, Tan AT, Polunovsky VA, et al. (1998) Lovastatin induces apoptosis in malignant mesothelioma cells. Am J Respir Crit Care Med 157: 1616–1622. [DOI] [PubMed] [Google Scholar]
- 10. Cholesterol Treatment Trialists' (CTT) Collaboration, Emberson JR, Kearney PM, Blackwell L, Newman C, et al (2012) Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One 7: e29849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins JP, Green S (2011) Cochrane handbook for systematic reviews of interventions. Available: http://www.cochrane-handbook.org/. Accessed 2012 Dec 26.
- 12.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, et al. (2012) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 2012 Dec 26.
- 13. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 14. Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 15. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pfeffer MA, Keech A, Sacks FM, Cobbe SM, Tonkin A, et al. (2002) Safety and tolerability of pravastatin in long-term clinical trials: prospective Pravastatin Pooling (PPP) Project. Circulation 105: 2341–2346. [DOI] [PubMed] [Google Scholar]
- 17. Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, et al. (2002) PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 360: 1623–1630. [DOI] [PubMed] [Google Scholar]
- 18. Heart Protection Study Collaborative Group, Bulbulia R, Bowman L, Wallendszus K, Parish S, et al (2011) Effects on 11-year mortality and morbidity of lowering LDL cholesterol with simvastatin for about 5 years in 20,536 high-risk individuals: a randomised controlled trial. Lancet 378: 2013–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. LIPID Study Group (Long-term Intervention with Pravastatin in Ischaemic Disease) (2002) Long-term effectiveness and safety of pravastatin in 9014 patients with coronary heart disease and average cholesterol concentrations: the LIPID trial follow-up. Lancet 359: 1379–1387. [DOI] [PubMed] [Google Scholar]
- 20. Sever PS, Chang CL, Gupta AK, Whitehouse A, Poulter NR, et al. (2011) The Anglo-Scandinavian Cardiac Outcomes Trial: 11-year mortality follow-up of the lipid-lowering arm in the U.K. . Eur Heart J 32: 2525–2532. [DOI] [PubMed] [Google Scholar]
- 21. Smeeth L, Douglas I, Hall AJ, Hubbard R, Evans S (2009) Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. Br J Clin Pharmacol 67: 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang X, So WY, Ma RC, Ko GT, Kong AP, et al. (2009) Low LDL cholesterol, albuminuria, and statins for the risk of cancer in type 2 diabetes: the Hong Kong diabetes registry. Diabetes Care 32: 1826–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marelli C, Gunnarsson C, Ross S, Haas S, Stroup DF, et al. (2011) Statins and risk of cancer: a retrospective cohort analysis of 45,857 matched pairs from an electronic medical records database of 11 million adult Americans. J Am Coll Cardiol 58: 530–537. [DOI] [PubMed] [Google Scholar]
- 24. Sato S, Ajiki W, Kobayashi T, Awata N (2006) PCS Study Group (2006) Pravastatin use and the five-year incidence of cancer in coronary heart disease patients: from the prevention of coronary sclerosis study. J Epidemiol 16: 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karp I, Behlouli H, Lelorier J, Pilote L (2008) Statins and cancer risk. Am J Med 121: 302–309. [DOI] [PubMed] [Google Scholar]
- 26. Haukka J, Niskanen L, Partonen T, Lönnqvist J, Tiihonen J (2012) Statin usage and all-cause and disease-specific mortality in a nationwide study. Pharmacoepidemiol Drug Saf 21: 61–69. [DOI] [PubMed] [Google Scholar]
- 27. Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, et al. (1998) Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 279: 1615–1622. [DOI] [PubMed] [Google Scholar]
- 28. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group (2002) The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA 288: 2998–3007. [DOI] [PubMed] [Google Scholar]
- 29. Serruys PW, de Feyter P, Macaya C, Kokott N, Puel J, et al. (2002) Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA 287: 3215–3222. [DOI] [PubMed] [Google Scholar]
- 30. Strandberg TE, Pyörälä K, Cook TJ, Wilhelmsen L, Faergeman O, et al. (2004) Mortality and incidence of cancer during 10-year follow-up of the Scandinavian Simvastatin Survival Study (4S). Lancet 364: 771–777. [DOI] [PubMed] [Google Scholar]
- 31. Ford I, Murray H, Packard CJ, Shepherd J, Macfarlane PW, et al. (2007) Long-term follow-up of the West of Scotland Coronary Prevention Study. N Engl J Med 357: 1477–1486. [DOI] [PubMed] [Google Scholar]
- 32. Blais L, Desgagné A, LeLorier J (2000) 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors and the risk of cancer: a nested case-control study. Arch Intern Med 160: 2363–2368. [DOI] [PubMed] [Google Scholar]
- 33. Kaye JA, Jick H (2004) Statin use and cancer risk in the General Practice Research Database. Br J Cancer 90: 635–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Graaf MR, Beiderbeck AB, Egberts AC, Richel DJ, Guchelaar HJ (2004) The risk of cancer in users of statins. J Clin Oncol 22: 2388–2394. [DOI] [PubMed] [Google Scholar]
- 35. Friis S, Poulsen AH, Johnsen SP, McLaughlin JK, Fryzek JP, et al. (2005) Cancer risk among statin users: a population-based cohort study. Int J Cancer 114: 643–647. [DOI] [PubMed] [Google Scholar]
- 36. Coogan PF, Rosenberg L, Strom BL (2007) Statin use and the risk of 10 cancers. Epidemiology 18: 213–219. [DOI] [PubMed] [Google Scholar]
- 37. Setoguchi S, Glynn RJ, Avorn J, Mogun H, Schneeweiss S (2007) Statins and the risk of lung, breast, and colorectal cancer in the elderly. Circulation 115: 27–33. [DOI] [PubMed] [Google Scholar]
- 38. Khurana V, Bejjanki HR, Caldito G, Owens MW (2007) Statins reduce the risk of lung cancer in humans: a large case-control study of US veterans. Chest 131: 1282–1288. [DOI] [PubMed] [Google Scholar]
- 39. Farwell WR, Scranton RE, Lawler EV, Lew RA, Brophy MT, et al. (2008) The association between statins and cancer incidence in a veterans population. J Natl Cancer Inst 100: 134–139. [DOI] [PubMed] [Google Scholar]
- 40. Friedman GD, Flick ED, Udaltsova N, Chan J, Quesenberry CP Jr, et al. (2008) Screening statins for possible carcinogenic risk: up to 9 years of follow-up of 361,859 recipients. Pharmacoepidemiol Drug Saf 17: 27–36. [DOI] [PubMed] [Google Scholar]
- 41. Haukka J, Sankila R, Klaukka T, Lonnqvist J, Niskanen L, et al. (2010) Incidence of cancer and statin usage--record linkage study. Int J Cancer 126: 279–284. [DOI] [PubMed] [Google Scholar]
- 42. Hippisley-Cox J, Coupland C (2010) Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ 340: c2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vinogradova Y, Coupland C, Hippisley-Cox J (2011) Exposure to statins and risk of common cancers: a series of nested case-control studies. BMC Cancer 11: 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jacobs EJ, Newton CC, Thun MJ, Gapstur SM (2011) Long-term use of cholesterol-lowering drugs and cancer incidence in a large United States cohort. Cancer Res 71: 1763–1771. [DOI] [PubMed] [Google Scholar]
- 45. Cheng MH, Chiu HF, Ho SC, Yang CY (2012) Statin use and the risk of female lung cancer: a population-based case-control study. Lung Cancer 75: 275–279. [DOI] [PubMed] [Google Scholar]
- 46. Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for ystematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 51: 264–269 W264. [DOI] [PubMed] [Google Scholar]
- 47. Bonovas S, Filioussi K, Flordellis CS, Sitaras NM (2007) Statins and the risk of colorectal cancer: a meta-analysis of 18 studies involving more than 1.5 million patients. J Clin Oncol 25: 3462–3468. [DOI] [PubMed] [Google Scholar]
- 48. Cui X, Xie Y, Chen M, Li J, Liao X, et al. (2012) Statin use and risk of pancreatic cancer: a meta-analysis. Cancer Causes Control 23: 1099–1111. [DOI] [PubMed] [Google Scholar]
- 49. bonovas s, nikolopoulos g, filioussi k, peponi e, bagos p, et al. (2010) can statin therapy reduce the risk of melanoma? a meta-analysis of randomized controlled trials. EUR J EPIDEMIOL 25: 29–35. [DOI] [PubMed] [Google Scholar]
- 50. undela k, srikanth v, bansal d (2012) statin use and risk of breast cancer: a meta-analysis of observational studies. breast cancer res treat 135: 261–269. [DOI] [PubMed] [Google Scholar]
- 51. Taylor ML, Wells BJ, Smolak MJ (2008) Statins and cancer: a meta-analysis of case-control studies. Eur J Cancer Prev 17: 259–268. [DOI] [PubMed] [Google Scholar]
- 52. Browning DR, Martin RM (2007) Statins and risk of cancer: a systematic review and metaanalysis. Int J Cancer 120: 833–843. [DOI] [PubMed] [Google Scholar]
- 53. Kuoppala J, Lamminpää A, Pukkala E (2008) Statins and cancer: A systematic review and meta-analysis. Eur J Cancer 44: 2122–2132. [DOI] [PubMed] [Google Scholar]
- 54. Wong WW, Dimitroulakos J, Minden MD, Penn LZ (2002) HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia 16: 508–519. [DOI] [PubMed] [Google Scholar]
- 55. buhaescu i, izzedine h (2007) mevalonate pathway: a review of clinical and therapeutical implications. clin biochem 40: 575–584. [DOI] [PubMed] [Google Scholar]
- 56. khanzada uk, pardo oe, meier c, downward j, seckl mj, et al. (2006) potent inhibition of small-cell lung cancer cell growth by simvastatin reveals selective functions of ras isoforms in growth factor signaling. oncogene 25: 877–887. [DOI] [PubMed] [Google Scholar]
- 57. maksimova e, yie ta, rom wn (2008) in vitro mechanisms of lovastatin on lung cancer cell lines as a potential chemopreventive agent. lung 186: 45–54. [DOI] [PubMed] [Google Scholar]
- 58. Han JY, Lim KY, Yu SY, Yun T, Kim HT, et al. (2011) A phase 2 study of irinotecan, cisplatin, and simvastatin for untreated extensive-disease small cell lung cancer. Cancer 117: 2178–2185. [DOI] [PubMed] [Google Scholar]
- 59. Han JY, Lee SH, Yoo NJ, Hyung LS, Moon YJ, et al. (2011) A randomized phase II study of gefitinib plus simvastatin versus gefitinib alone in previously treated patients with advanced non-small cell lung cancer. Clin Cancer Res 17: 1553–1560. [DOI] [PubMed] [Google Scholar]
- 60. Nielsen SF, Nordestgaard BG, Bojesen SE (2012) Statin use and reduced cancer-related mortality. N Engl J Med 367:1792–1802. 61. Caporaso NE (2012) Statins and cancer-related mortality--let's work together. N Engl J Med 367: 1848–1850. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist for the meta-analysis.
(DOC)