Abstract
Plant ascorbate peroxidases (APXs), enzymes catalyzing the dismutation of H2O2 into H2O and O2, play an important role in reactive oxygen species homeostasis in plants. The rice genome has eight OsAPXs, but their physiological functions remain to be determined. In this report, we studied the function of OsAPX2 gene using a T-DNA knockout mutant under the treatment of drought, salt and cold stresses. The Osapx2 knockout mutant was isolated by a genetic screening of a rice T-DNA insertion library under 20% PEG-2000 treatment. Loss of function in OsAPX2 affected the growth and development of rice seedlings, resulting in semi-dwarf seedlings, yellow-green leaves, leaf lesion mimic and seed sterility. OsAPX2 expression was developmental- and spatial-regulated, and was induced by drought, salt, and cold stresses. Osapx2 mutants had lower APX activity and were sensitive to abiotic stresses; overexpression of OsAPX2 increased APX activity and enhanced stress tolerance. H2O2 and MDA levels were high in Osapx2 mutants but low in OsAPX2-OX transgenic lines relative to wild-type plants after stress treatments. Taken together, the cytosolic ascorbate peroxidase OsAPX2 plays an important role in rice growth and development by protecting the seedlings from abiotic stresses through scavenging reactive oxygen species.
Introduction
Reactive oxygen species (ROS) control various signaling pathways in plants involved in stress and pathogen responses, photosynthesis, programmed cell death, hormonal action, growth and development [1], [2]. ROS might cause cellular injury through reacting with biological compounds [3]. ROS damage is one of the major mechanisms underlying the biotic and abiotic stresses including drought, high light, wounding, salt, or pathogen infection [4], [5], [6].
Cells have evolved highly regulated mechanisms to maintain a balance between ROS production and destruction [7]. Ascorbate peroxidases (APX) are antioxidant enzymes functioning in converting H2O2 into H2O and O2 [8]. Studies have shown that APXs play an important role in removing ROS in plants [1]. In Arabidopsis, cytosolic APXs (cAPXs) are critical for cellular H2O2 homeostasis and play an important role in oxidative protection of chloroplasts under abiotic stresses, including high light, heat, methyl viologen and drought stress [9], [10]. Arabidopsis APX1 is important for plant growth and development since mutation of APX1 leads to the accumulation of H2O2, inhibition of plant growth and photosynthesis, delay of flowering, and enhanced protein oxidation under high light [10]. APX2 expression is induced under high light, heat stresses and wounding conditions [11], [12]. APX2 is also critical for drought tolerance in Arabidopsis [13]. Thylakoid-bound APXs (tAPXs) are essential for photosynthetic activity and photoprotection under photooxidative stress in Arabidopsis [14].
Rice genome contains eight APX genes. OsAPX1 and OsAPX2 are in the cytosol, OsAPX3 and OsAPX4 in peroxisomes, OsAPX6 in mitochondria, and OsAPX5, OsAPX7 and OsAPX8 in chloroplasts [15]. The expressions of OsAPX1 and OsAPX2 are developmentally regulated [16]. The silencing of OsAPX1 and OsAPX2 genes individually resulted in strong effect on plant development, producing semi-dwarf phenotype [17]. The expressions of OsAPXs are also modulated by environmental stimuli, such as drought, high light, high temperature, salt stress, pathogen attacks and exogenous ABA [15], . Overexpression of OsAPX1 enhances tolerance to chilling at the booting stage in rice [18]; however, overexpression of OsAPX2 improves salt tolerance in transgenic Arabidopsis and Medicago sativa [19], [20].
Currently, the studies on rice APXs are using either overexpression or RNAi techniques, no knockout mutants have been used to explore the function of APXs in rice. The major concern regarding RNAi is that it generates knockdown mutants which can have different phenotypes compared with the knockout mutants. Another drawback of RNAi is its “off target” silencing, in which the genes with similar sequences are non-specifically silenced [21]. Therefore, it is necessary to apply insertion mutagenesis, which generates site specific null mutants, to confirm the observations obtained from the RNAi analysis [22]. On the other hand, there is only single abiotic stress (ie. either drought, or salt or cold stress) applied to elucidating the APX function in the previous studies [18], [19], [20]. In this study, we isolated a loss-of-function rice mutant Osapx2 through genetic screening of a T-DNA insertion population. The function of OsAPX2 in drought, salt and cold stresses tolerance is extensively investigated at seedling and reproductive stages.
Materials and Methods
Mutant Screening
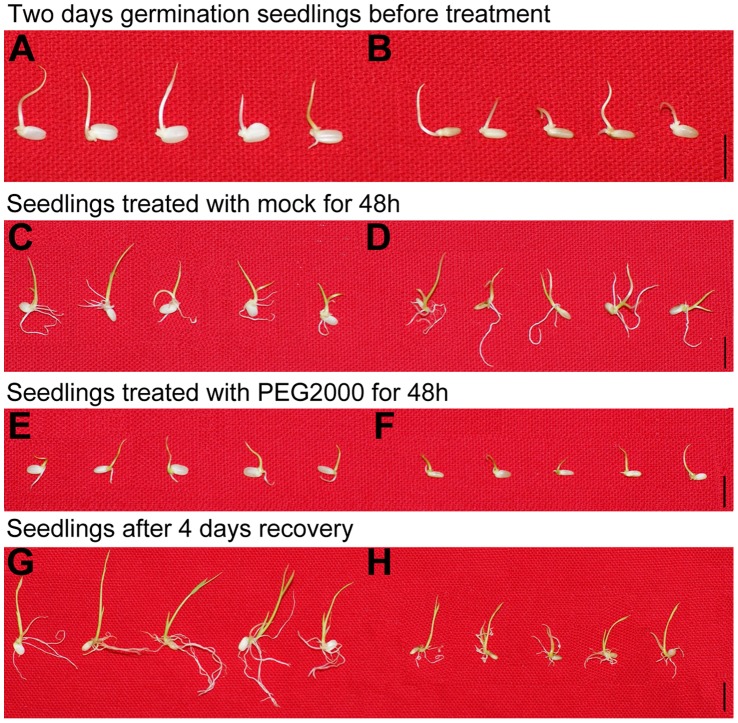
We have screened more than 100,000 T-DNA insertion rice lines generated in our laboratory with 20% PEG2000 (simulation of drought stress) [23]. Thirty seeds from each line were sown in petri dish (10 cm in diameter) with 20 ml sterile distilled water. After two days of germination, seedlings were treated with 20% PEG2000 for 48 h and recovered in water for another 48 h. One T-DNA insertion line was found to produce small seeds and exhibited severe inhibition in root elongation and seedling growth compared with the wild-type control (Nipponbare).
Anatomical Observations
Floral structures of the mutant, complementation and wild-type plants were observed under an optical microscope before flowering. Pollen viability was determined according to staining gradation under a microscope with 1% I2-KI solution and nipping into pieces with forceps to spill out pollen grains [24].
Identification of the Osapx2 Mutant
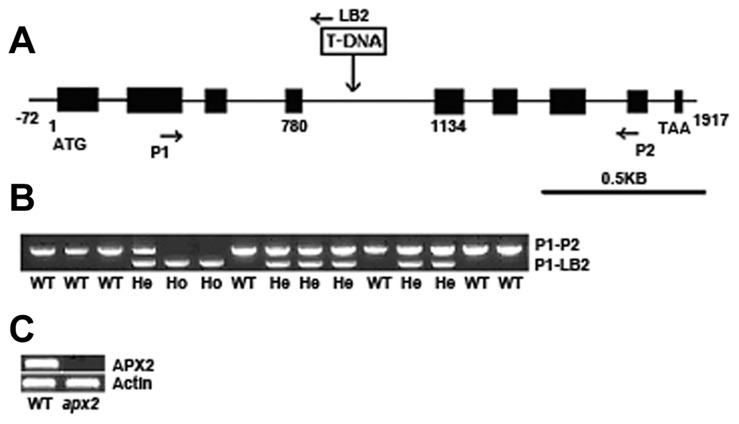
Genomic DNA flanking the T-DNA left border was amplified using polymerase chain reaction (PCR) walking with nesting-specific primer pairs according to the method described by Peng et al. [25]. The specific primers for the T-DNA left border were LB1 (5′-CGATGGCTGTGTAGAAGTACTCGC-3′) and LB2 (5′-GTTCCTATAGGGTTTCGCTCATGTGTTG-3′). The specific primers for the adaptor were APR1 (5′-GGATCCTAATACGAGTCACTATAGCGC-3′) and APR2 (5′-CTATAGCGCTCGAGCGGC-3′). PCR products were directly sequenced. The T-DNA insertion site in the mutant was defined by NCBI BLAST of the rice genome database (http://www.ncbi.nlm.nih.gov/Blast/) using the rescued flanking sequences, and BLAST results showed that the T-DNA was inserted in the fourth intron of OsAPX2.
Genotyping of Osapx2 with PCR
Genotyping of Osapx2 in T1 segregating population was performed by PCR using the following primers: P1 (5′-AACTTCCCATCCTCTCCTAC-3′), P2 (5′- CAAAGAAGGCGTCCTCATC-3′) and LB2 (5′-GTTCCTATAGGGTTTCGCTCATGTGTTG-3′) (Figure 3-A). More than 100 individual plants were analyzed. One µg of DNA was used as template with primers mixture (1 µM of P1, P2 and LB2, respectively) in a 20 ml reaction system. PCR was conducted with an initial step of 94°C incubation for 3 min and 30 cycles of 94°C for 30 s, 54°C for 30 s, and 72°C for 1 min.
Figure 3. Molecular analysis of Osapx2 mutants.
A: Structure of OsAPX2 and the location of T-DNA insertion. Black boxes indicate protein-encoding exons. Arrows indicate the locations of PCR primers used. B: Confirmation of co-segregation of the T-DNA insertion with the PEG-sensitive and small seed phenotype in leaves of T1 heterozygous progeny. Two PEG-sensitive seedlings (Ho; Homo type), PCR-amplified product only from the P1-LB2 primer set (Osapx2/Osapx2); six normal seedlings (He; Hetero type), amplified with both the P1-LB2 and P1–P2 primer sets (Osapx2/OsAPX2); and seven normal seedlings (WT), which amplified only with the P1–P2 primer set (OsAPX2/OsAPX2). C: RT-PCR expression analysis in leaves of wild-type phenotype and PEG-sensitive mutant seedlings. Actin gene is used as control.
Complementation and Over Expression of OsAPX2
OsAPX2 full-length cDNA (905 bps) was amplified by PCR with the primers OP1 containing a SalI digestion site (5′- cgGTCGACGTGAGTTGAGTTGGGGATTG-3′) and OP2 containing a PstI digestion site (5′-gctCTGCAGCCACGACAGTCTTGATCGTAC-3′). The PCR product was cloned into the pEASY-Blunt simple cloning vector (Transgen Biotech) and sequenced. For complementation construct, full-length cDNA of OsAPX2 was cloned into the vector carrying 2.1-kb OsAPX2-promoter (from -2111 bp to -1 bp) to generate pOsAPX2::OsAPX2. For the over expression construct, cDNA were excised from the vector by SalI and PstI digestion and subcloned into pCAMBIA-23A. In pCAMBIA-23A, OsAPX2 was downstream of the Actin promoter. The constructs were introduced into Agrobacterium tumefaciens (stain EHA105) by electroporation. Agrobacterium-mediated transformation was performed using vigorously growing calli derived from mature embryos of Osapx2 mutant and wild-type Nipponbare following an improved protocol described by Yang et al. [26].
RT-PCR and Quantitative RT-PCR
For molecular analysis of Osapx2 mutants, total RNA was isolated using TRIzol solution (Invitrogen) from leaves of wild-type and Osapx2 plants. For expression analysis of OsAPX2, total RNA was extracted from different tissues, including roots, leaves, blade sheath, blade ears, flowers, apical meristems and internodes. Total RNA from each sample was reversely transcribed with oligo (dT) primer and PrimeScript RT Enzyme (TaKaRa) according to the manufacturer’s instructions. For real time PCR (RT-PCR), the PCR primers for amplifying OsAPX2 were RT-1 (5′-AACTTCCCATCCTCTCCTAC-3′) and RT-2 (5′-CTCTCCTTGTGGCATCTTCC-3′). PCR conditions were as follows: preincubation at 94°C for 2.5 min, then 30 cycles at 94°C for 30 s, 54°C for 30 s, and 72°C for 1 min. The rice Actin gene, amplified with primers Actin1 (5′-TGCTATGTACGTCGCCATCCAG-3′) and Actin2 (5′-AATGAGTAACCACGCTCCGTC-3′), was selected as an internal standard to normalize the expression of OsAPX2. Quantitative real-time PCR (RT-qPCR) was performed on the iQ5 Multicolor Real-Time PCR Detection System (Bio-Rad) with real-time PCR Master Mixture (SYBR Green Mix). The primers for OsAPX2 were OsAPX2F (5′-CATCCTCTCCTACGCCGAC-3′) and OsAPX2R (5′-CCTTCAGGAGGAGGCTCAG-3′). The primers for OsAPX1 were OsAPX1F (5′- GCCGATTTCTACCAGCTTCTTG-3′) and OsAPX1R (5′- AGGGTGTGACCGCCAGAGA-3′). The rice Actin gene was amplified with primers ActinF (5′-TGCTATGTACGTCGCCATCCAG-3′) and ActinR (5′-AATGAGTAACCACGCTCCGTCA-3′). RT-qPCR was performed in triplicate for each individual line and threshold cycle values were quantified by RT-qPCR by calculating means of normalized expression using the relative quantification method [27].
Expression Profile of OsAPX2 under Stress Conditions
Rice seeds were surface sterilized for 5 min with ethanol (75% v/v) and 10 min with commercially diluted (1∶3 v/v) NaOCl twice, followed by 3 to 5 rinses with sterile distilled water. Germination was carried out for 72 h on sterile Murashige and Skoog (MS) medium in the dark at 28°C day/25°C night temperatures, 12 h light/12 h dark cycle, and 50% humidity. Two-week-old seedlings were used for abiotic stress treatments. For the drought treatment, seedlings were transferred to Whatman 3 MM paper in a sterile petri dish for 0, 0.5, 1, 2 and 4 h. For the cold treatment, 4°C for 0, 0.5, 1, 2 and 4 h; control plants were harvested at the same time. For the salt treatment, 0 mM, 50 mM, 100 mM, 150 mM and 250 mM NaCl for 1 h. OsAPX1 expression in Osapx2 mutant was analyzed under normal and stress conditions according to the method described as above, except that seedlings of wild-type and Osapx2 were harvested after 1 h treatment for drought, cold and salt (150 mM NaCl) treatments.
Histochemical GUS Assay
Plant material was prefixed in 90% acetone for 1 hour at –20°C, washed twice with staining buffer without X-Gluc and infiltrated with staining solution (100 mM sodium phosphate buffer pH 7.0, 10 mM sodium EDTA, 5 mM potassium-ferrocyanide, 5 mM potassium-ferricyanide, 0.1% Triton X-100, 0.1 mg/ml chloramphenicol, 2 mM X-Gluc) under vacuum for 20 min and incubated at 37°C for 24 h. Chlorophyll was extracted by passing through increasing concentrations of ethanol. The samples were cleared as described by Malamy & Benfey [28].
Evaluation of Abiotic Stresses Tolerance at the Seedling Stage
Seeds of Osapx2 mutant and its segregated wild-type plants were germinated and grown in pots filled with vermiculite in a greenhouse at 30°C (light) and 22°C (dark) under 16-h-light/8-h-dark conditions. Then seedlings with four leaves were treated with different stresses. Salinity treatment was performed by immersed the pots into 200 mM NaCl for 10 days. For low temperature treatment, plants were transferred to a 16°C incubator for 14 days. For drought treatment, plants were withheld water for 6 days. For stresses tolerance evaluation of OsAPX2-overexpressing plants, seedlings of OsAPX2-OX and wild-type with four leaves were treated and time courses were extended to 18 days for salinity treatment, 18 days for low temperature treatment and 10 days for drought treatment. Phenotype changes of treated plants were carefully investigated and photographed. Contents of relative water, chlorophyll, H2O2 and malondialdehyde (MDA) were measured immediately after stress treatments. Each treatment was repeated three times.
APX Activity Assays
Five-leaf seedlings grown in the half-strengthened MS liquid medium were used. For salt treatment, wild-type, Osapx2 and OsAPX2-OX plants were treated with 150 mM NaCl for 24 h; for cold treatment, plants were transferred to a 10°C incubator for 24 h; for drought treatment, plants were transferred to dry petri dishes for 2 h.
APX activity was determined according to the method of Chen and Asada (1989) with minor modifications [29]. The 200 mg tissues were homogenized in 50 mM sodium phosphate buffer (pH 7.0) containing 1 mM EDTA and 5 mM ascorbate. The 1-ml reaction mixture was composed of 50 mM sodium phosphate buffer (pH 7.0) containing 0.1 mM EDTA and 0.5 mM ascorbate, 1.54 mM H2O2, and 0.1 ml of enzyme extracts. The oxidation of ascorbate was determined by the decrease in the absorbance at 290 nm. APX activity was expressed as micromole of ascorbate oxidized per minute.
Protein content was determined using bovine serum albumin as a standard, according to the method of Bradford (1976) [30]. All enzyme activities were calculated per milligram of protein per minute.
Relative Water Content Assay
Relative water content of leaves was measured on treated and control plants. The fresh weight (FW) was measured immediately after these materials were taken. All leaves were macerated in deionized water overnight at 4°C and the leaves turgid weight (TW) was recorded. Finally these leaves dehydrated in drying cabinet at 70°C until the constant weight for 8 h and obtained the dry weight (DW). Relative water content was calculated using the equation: RWC (%) = (FW−DW)/(TW−DW) ×100% [31].
Chlorophyll Content Assay
Total chlorophyll content was determined spectrophotometrically according to the method described by Arnon (1949) [32]. The 300 mg leaves were grinded into powder with liquid nitrogen and then were transferred to a 15 ml Falcon tube. Add 5 ml of 80% acetone to the tube mix thoroughly and stand in dark overnight. Centrifugation was performed at 4°C for 15 min (3,000 rpm). Supernatant was transferred to a new centrifuge tube and measure the absorbance of chlorophyll (hereafter termed A) using spectrophotometry. The chlorophyll concentrations are calculated as follows (use 80% acetone as a blank control). Ca+b (mg/g) = [8.02×A663+20.20×A645]×V/1000×W. Where V = volume of the extract (ml); W = Weight of fresh leaves (g).
Hydrogen Peroxide Determination
Determination of H2O2 levels were carried out according to the method of Dagmar (2001) [33]. Briefly, 500 mg of leaves were homogenized in a cold mortar with a pestle and 0.2 g silicon dioxide in pre-cooled acetone (5 ml) and the homogenate was centrifuged at 12,000 g for 5 min. One milliliter of the supernatant was mixed with 0.1 ml of 5% Ti(SO4)2 and 0.2 ml of 19% ammonia. After precipitate was formed the reaction mixture was centrifuged at 12,000 g for 5 min. The resulting pellet was dissolved in 3 ml of 2 M H2SO4 and the absorbance of the solution was recorded at 415 nm. The H2O2 content was calculated according to a standard curve of H2O2 ranging from 0 to 10 µM.
Malondialdehyde Assay
Leaves were taken for determination of malondiaklehyde (MDA) content. Briefly, 200 mg of leaves were homogenized under liquid nitrogen, hydrated in 5 ml of 0.1% (w/v) trichloroacetic acid solution (TCA) and centrifuged at 12,000 g at 4°C for 10 min. One ml of the supernatants was reacted with 4 ml of 0.5% (w/v) thiobarbituric acid (TBA) solution containing 20% (w/v) TCA. The mixture was incubated at 100°C for 30 min and centrifuged at 12,000 g at 4°C for 10 min; the absorbance of the supernatant was read at 532 and 600 nm. MDA content was estimated by using extinction coefficient of 155 (mmol/L/com) [34].
Evaluation of Abiotic Stresses Tolerance at the Booting Stage
For different abiotic stress tolerance evaluation at the booting stage, wild-type plants and four T2 OsAPX2-OX transgenic lines with stable OsAPX2 over expression were grown in pots (25 cm diameter ) with soil in greenhouse at 25/19°C (day/night). When the flag leaf was just visible (about 10 days before heading), different treatments were performed. For drought treatment, water was withheld for 20 days. Then all the pots were rewatered simultaneously and left there until the plants reached maturity. For salt treatment, the pots were immersed in 200 mM NaCl for 7 days, and then were watered every day with 1 liter tap water. For cold treatment, the pots were transferred to a phytotron maintained at 14°C for 7 days, then moved to greenhouse at 25/19°C (day/night) and left there until the plants reached maturity. Stress tolerance was evaluated on the basis of mean spikelet fertility.
Statistical Analysis
All the experiments were carried out in triplicate. The values shown in the figures are mean values±SD. For multiple comparisons, means were compared by one-way analysis of variance and Duncan’s multiple range test with a 5% level of significance.
Results
Isolation of a PEG-sensitive T-DNA Insertion Mutant
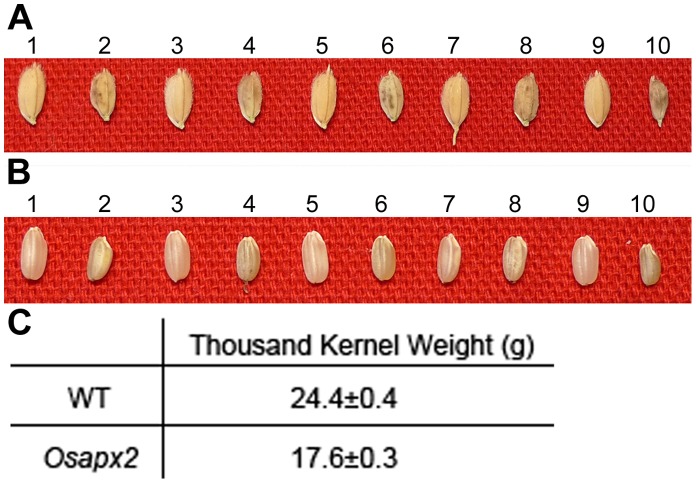
In an attempt to obtain drought sensitive rice mutants, we conducted genetic screening of a T-DNA insertion population containing more than 100,000 individual lines using 20% PEG2000. A PEG-sensitive mutant was identified with the phenotype of severe inhibition in the elongation of both shoots and roots (Figure 1). Additionally, the mutant seeds were smaller than the wild-type (Figure 2-A,B). As measured by the thousand kernel weight (TKW) of seeds, the mutant TKW was 17.6±0.3 g while that of wild-type was 24.4±0.4 g (Figure 2-C).
Figure 1. The phenotype of the PEG-sensitive T-DNA insertional rice line.
After two days germination, wild-type (WT) (A) and mutant lines (B) were treated with 20% PEG2000. WT and mutant lines were treated with mock (C and D) and 20% PEG2000 (E and F) respectively, for 48 h. Seedlings of treated WT (G) and mutant seedlings (H) were recovered for 4 days. Bar = 1 cm.
Figure 2. The seed phenotype of WT and Osapx2.
A,B: the seed phenotype of WT (odd numbers) and Osapx2 (even numbers), before dehulling (A) and after dehulling (B). C: The thousand kernel weight of WT and Osapx2 seeds.
T-DNA insertion of the mutant was confirmed by amplification of genomic fragment flanking the T-DNA insertion site using PCR walking assay [25]. BLAST search of the T-DNA flanking sequence against the rice genome database (http://blast.ncbi.nlm.nih. gov/Blast.cgi) identified that T-DNA was inserted into the fourth intron of ascorbate peroxidase 2 gene (OsAPX2, Os07 g0694700) (Figure 3-A). The full genome sequence of the OsAPX2 spans approximately 2,553-bp on chromosome 7, contains nine exons and eight introns.
In order to test the co-segregation of PEG sensitivity and T-DNA insertion in T1 segregating population, we analyzed more than 100 individual plants using gene-specific PCR primers (P1 and P2) flanking the T-DNA insertion in OsAPX2 and primer LB2 to the T-DNA left border (Figure 3-A). Results showed that the PEG- sensitive phenotype and small seed size phenotype was co-segregated with the homozygous T-DNA insertion (Figure 3-B). RT-PCR analysis showed that OsAPX2 gene expression was completely abolished in the mutant (Figure 3-C).
OsAPX2 is Important for Growth and Development in Rice
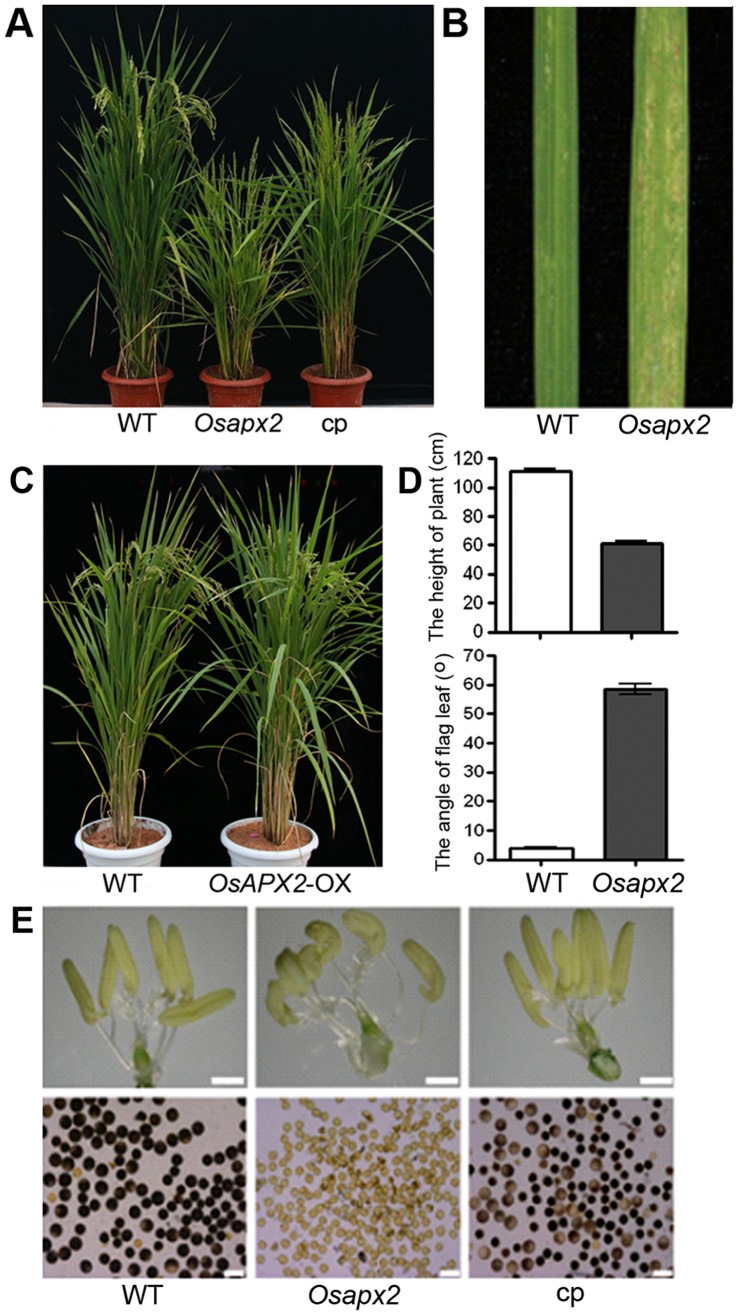
Not only were the seeds of the Osapx2 mutant smaller than the wild-type plants, but also the mutant plants were shorter than the wild-type plants. At the heading stage, the height of the mutant seedlings was about two-third of the wild-type Nipponbare (63 cm compared with 110 cm of wild-type) (Figure 4-A,D). The angle of flag leaf was increased in the mutant plants (Figure 4-D). Seedlings of the Osapx2 mutants grown both in greenhouse and paddy field showed a lesion mimic phenotype in the middle of the leaf blade during the tillering stage. After the tillering stage, the lesion spots spreaded to the whole leaves in the mutant plants (Figure 4-B). The mutant plants were completely sterile which was supported by abnormal anther morphology and defective pollen viabiligy (Figure 4-E). Genetic analyses of T1 heterozygous progeny revealed that a segregation ration of wild-type (145):sterility (55) phenotype was 3∶1 (χ2 = 0.67< χ2 0.01,1), suggesting that the mutated phenotype was controlled by a single recessive nuclear locus.
Figure 4. Characterization of wild-type, Osapx2, complementation and OsAPX2-OX plants.
A,C: The phenotype of wild-type (WT), Osapx2, complementation (cp) plants and OsAPX2-OX plants at mature stage. B: The leaves of wild-type (WT), Osapx2 (m) and complementation plants (cp) during the vegetative development stage. D: The comparison of the height and the flag leaf angle between wild-type and Osapx2 mutant plants at the mature stage. E: Comparison of anther morphology and pollen viability stained with KI among wild-type (WT), Osapx2 and complementation plants (cp). Bar = 20.0 µm.
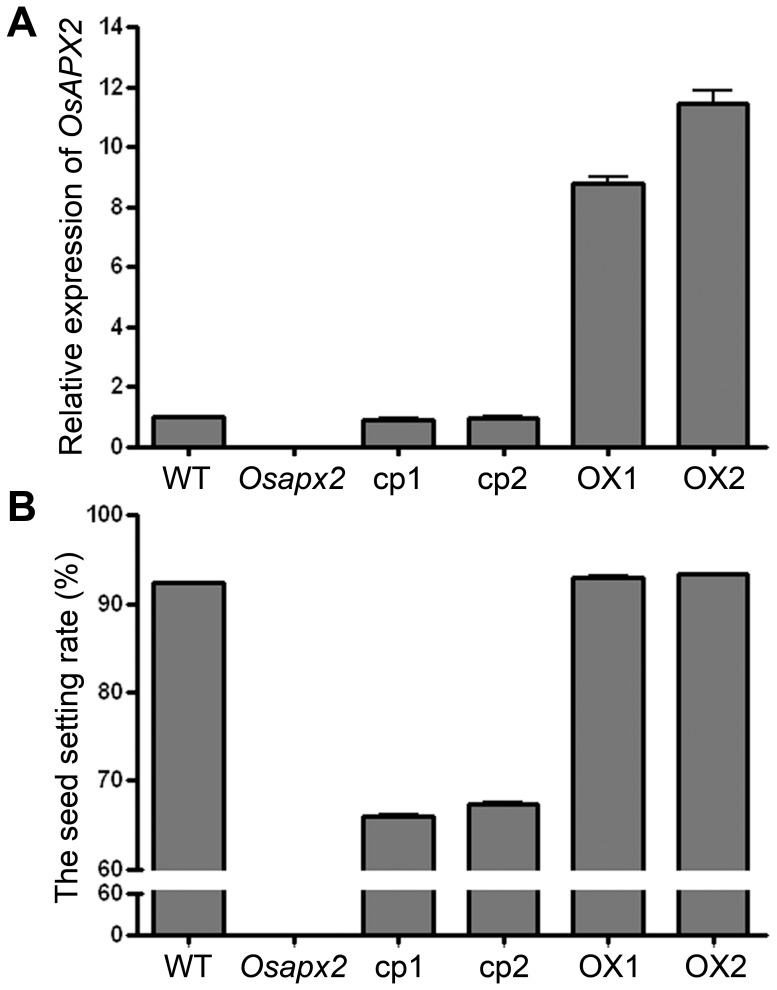
Since the homozygous mutants were male sterile, the small seeds segregated from the heterozygous lines were used for complementary test. Calli derived from homozygous Osapx2 mutant seeds were confirmed by PCR and transformed with pOsAPX2::OsAPX2, a construction of the full-length cDNA of OsAPX2 driven by its own promoter. Among all the fifty-one transgenic plants obtained, thirty five plants showed the wild-type expression level of OsAPX2 gene as determined by RT-qPCR (Figure 5-A) and recovered the wild-type phenotype in terms of seedling height, leaf appearance, anther shape, pollen viability and seed setting rate (Figure 4-A,E; Figure 5-B).
Figure 5. The expression of OsAPX2 and seed setting rate in wild-type, Osapx2, complementation and OsAPX2-OX plants.
A: RT-qPCR analysis of OsAPX2 expression in wild-type (WT), Osapx2, complementation plants (cp1 and cp2) and OsAPX2-OX plants. B: Seed setting rates of wild-type (WT), Osapx2, complementation plants (cp1 and cp2) and OsAPX2-OX plants. Values represent means ± SD of three replicates.
OsAPX2 over expression construct driven by actin promoter was introduced into wild-type rice cultivar Nipponbare via A. tumefaciens mediated transformation. Totally more than 200 individual transgenic lines were generated and confirmed by PCR detection of the transgene. OsAPX2 expression was examined by RT-qPCR of total RNA extracted from leaves of each transgenic line. OsAPX2 expression was much higher in transgenic lines relative to wild-type plants (Figure 5-A). The OsAPX2 transgenic plants have the same phenotype as the wild-type seedlings in which they had a similar seed setting rate (Figure 4-C, Figure 5-B).
OsAPX2 Expression is Spatially and Developmentally Regulated
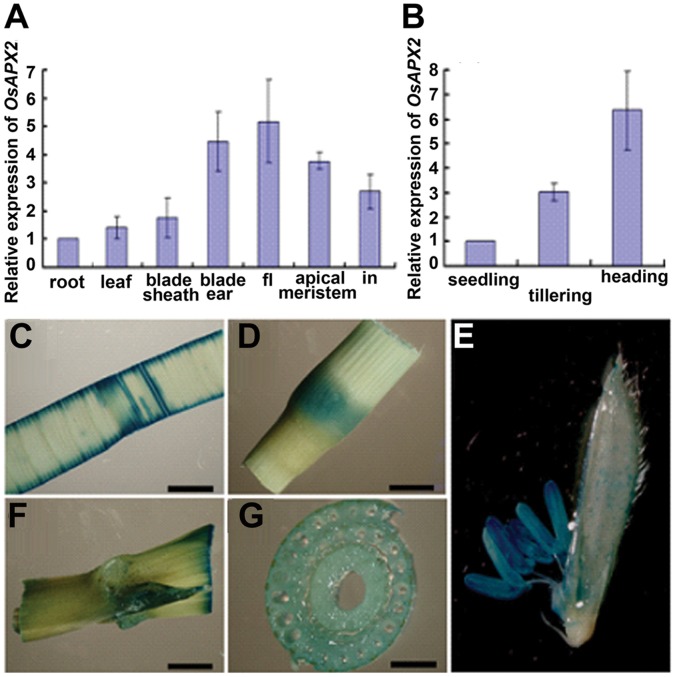
RT-qPCR was performed to analyze the expression pattern of OsAPX2 in different tissues and in leaves at different developmental stages. As show in Figure 6-A, the expression of OsAPX2 was low in root, leaf and blade sheath but high in blade ear, flower, apical meristem and internode. To further examine the expression pattern of OsAPX2 comprehensively, the pCAMBIA1391Z vector with the fusion of the OsAPX2 pormoter (from −2111 bp to −1 bp) and the GUS coding sequence was transformed into calli in the wild-type background by Agrobacterium tumefaciens-mediated transformation. Strong GUS activity was detected in organs including young leaf (Figure 6-C), internode (Figure 6-D) and blade ear (Figure 6-F), stem (Figure 6-G) and anther (Figure 6-E), which are consistent with above RT-qPCR results. Then we compared the gene expression at different developmental stages using leaves at seedling and tillering stages, and flag leaves at heading stage. Results showed that the expression of OsAPX2 was higher in mature leaves than young leaves; OsAPX2 was most strongly expressed in flag leaves at heading stage (Figure 6-B).
Figure 6. Developmentally regulated OsAPX2 expression in rice tissues.
A: RT-qPCR was performed to analysis the expression of OsAPX2 in root, leaf, blade sheath, blade ear, flower (fl), apical meristem and internode (in). B: The expression of OsAPX2 in leaves at different stages. C,D,E,F,G Expression patterns of OsAPX2 revealed by GUS staining. Leaf (D), internode (D), blade ear (F) and the transversely side of internode (G), flower (E). Bar = 200 µm.
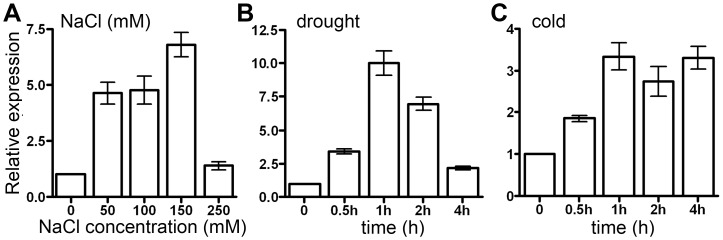
OsAPX2 Expression is Induced by Drought, Cold and Salt Stresses
According to previous reports, the expression of APX genes is modulated by different environmental stimuli. To analyze the expression pattern of OsAPX2 in response to abiotic stresses, 2-week-old rice plants were subjected to drought, cold (4°C) and salt stresses, respectively. The accumulation of the transcripts was determined by RT-qPCR. As shown in Figure 7-A, OsAPX2 expression was induced by salt stress. After salt treatment for 1 h, transcript accumulation of OsAPX2 was increased by 4.5-fold under the treatment of 50 mM and 100 mM NaCl compared with the water mock control. Gene expression was increased by 7-fold after 150 mM NaCl treatment but was reduced to control level when treated with 250 mM NaCl. Also, OsAPX2 expression was induced by drought treatment. As shown in Figure 7-B, the expression of OsAPX2 reached peak at 1 h treatment but gradually reduced after 2 h treatment. Similarly, OsAPX2 expression was induced within 0.5 h after cold treatment and was highest after 1 h treatment (Figure 7-C).
Figure 7. OsAPX2 expression response to drought, cold and salt stresses.
A: OsAPX2 expression response to salt stress. B,C: Time course of OsAPX2 expression during drought and cold treatments. Actin was used as an internal control. Data represent means ± SD of three replicates.
Since there are two cAPX genes in rice genome and OsAPX1/OsAPX2 proteins were 83% identity to each other. The expression profile of OsAPX1 was analyzed in both wild-type and indentified Osapx2 mutant seedlings under various treatments. As shown in Figure 8, the expression pattern of OsAPX1 induced by different stresses is similar in wild-type and Osapx2, while the expression of OsAPX1 induced more notably in Osapx2 mutant than wild-type by different stress treatments.
Figure 8. The expression of OsAPX1 in Osapx2.
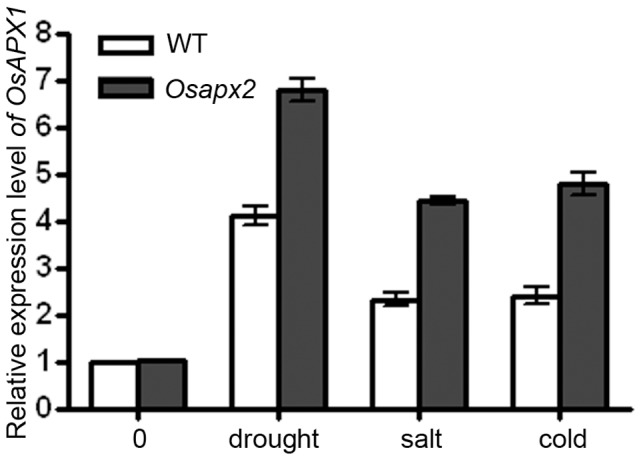
The expression of OsAPX1 was detected in leaves of wild-type and Osapx2 mutant seedlings under normal condition and after stress treatments. Data represent means ± SD of three replicates.
Expression of OsAPX2 is Critical to Abiotic Stress Tolerance
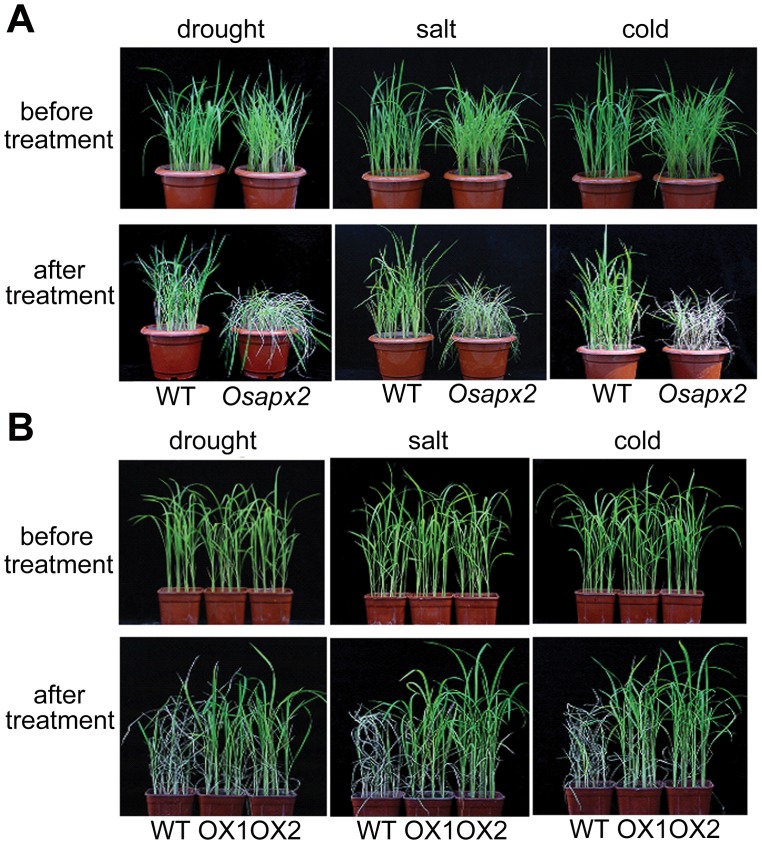
The induction of OsAPX2 expression by abiotic stresses suggests OsAPX2 might function in stress tolerance in rice. In order to demonstrate the role of OsAPX2 in abiotic stresses tolerance, we compared the growth of Osapx2 mutant, T1 OsAPX2-OX lines and wild-type plants under drought, salt and cold stresses. As shown in Figure 9, Osapx2 mutant plants were more sensitive to drought stress than its segregated wild-type plants when water withheld for 6 days (Figure 9-A). By contrast, OsAPX2-OX plants were more tolerant to drought stress than the wild-type plants; they were growing well even after water was withheld for 10 days (Figure 9-B).
Figure 9. Effect of OsAPX2 expression on drought, salt and cold tolerance in rice.
A: Without OsAPX2, Osapx2 mutant plants showed sensitive to drought, salt and cold treatments. B: OsAPX2-OX transgenic plants showed tolerance to drought, salt and cold treatments.
Similarly, Osapx2 mutant plants were more sensitive to salinity stress than the wild-type plants (Figure 9-A), while OsAPX2-OX lines were more tolerant to salt stress than the wild-type plants (Figure 8-B). When treated with 200 mM NaCl for 10 days, Osapx2 mutant plants were almost dead, and the growth of the wild-type plants was inhibited. Wild-type plants were beginning bleaching after salt stress for 18 days, while OsAPX2-OX seedlings were still green and growing well (Figure 9-B). Moreover, Osapx2 plants were more sensitive to cold stress than the wild-type plants (Figure 9-A), whereas OsAPX2-OX plants were more tolerant to cold stress than wild-type plants. When exposed to 16°C in a chamber for 14 days, Osapx2 mutant plants were becoming wilting, and the growth of the wild-type was inhibited. After cold treatment for 18 days, wild-type seedlings were becoming wilting, while OsAPX2-OX seedlings were still growing well (Figure 9-B).
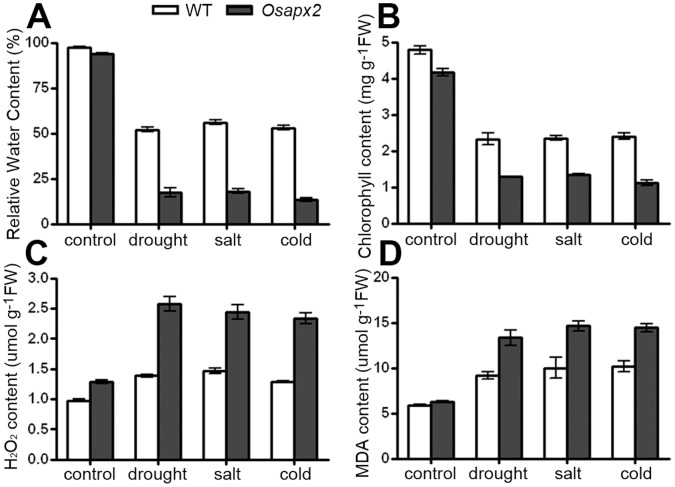
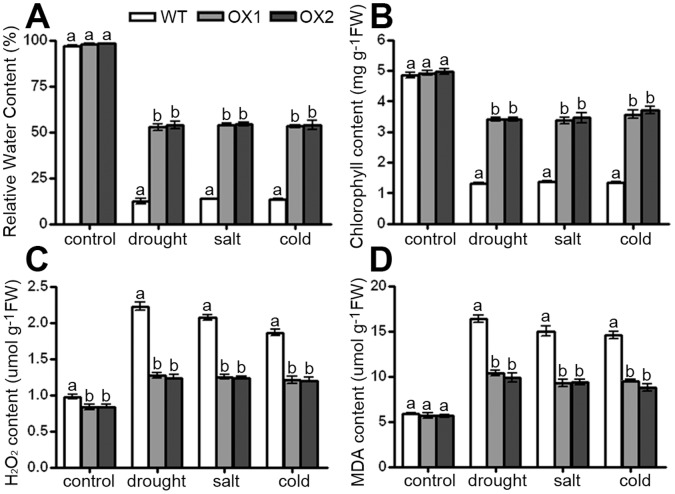
The responses of Osapx2 mutant, wild-type and OsAPX2-OX plants to abiotic stresses are consistence to their relative water content, chlorophyll content, H2O2 and MDA contents. The abiotic stress sensitive phenotype of Osapx2 was consistent with more water loss, lower chlorophyll content, higher H2O2 and MDA contents after stress treatments (Figure 10). By contrast, the abiotic stress tolerance phenotype of OsAPX2-OX was consistent with less water loss, higher chlorophyll content, and lower H2O2 and MDA contents (Figure 11).
Figure 10. The effects of stress treatments on wild-type and Osapx2 plants.
A: Relative water content. B: Chlorophyll content. C: H2O2 content. D: MDA content. Values represent the mean ± SD of three replicates.
Figure 11. The effects of stress treatments on wild-type and OsAPX2-OX plants.
A: Relative water content. B: Chlorophyll content. C: H2O2 content. D: MDA content. Data are mean of three replicates and were compared by one-way analysis of variance and Duncan’s multiple range test. Different letters (a–b) indicate significant differences (P<0.05) between lines.
Overexpression of OsAPX2 Improved Spikelet Fertility in Rice under Abiotic Stresses
Studies have shown that rice yields are affected by the abiotic stress at the booting stage [35]. In order to understand whether or not the OsAPX2 functions in improving rice yields through enhancing tolerance to abiotic stresses, we compared the spikelet fertility of both OsAPX2-OX and wild-type plants at the booting stage. The T2 transgenic OsAPX2-OX plants and wild-type plants were stressed under drought, salt and low temperature treatments at the booting stage, respectively. Four independent OsAPX2-OX lines (OX1, OX2, OX3 and OX4) were used for each treatment in our assays. As shown in Figure 12, OsAPX2-OX and wild-type plants had similar spikelet fertilities under normal condition, while significant differences were found between OsAPX2-OX transgenic lines and wild-type plants under stress treatments. For drought treatment, the spikelet fertilities were decreased by 75% in wild-type plants, but were decreased by 56%, 52%, 60% and 57% in OsAPX2-OX lines (OX1, OX2, OX3 and OX4, respectively; Figure 12). For salt treatment, the spikelet fertilities were decreased by 54% in wild-type plants, but only reduced by 38%, 33%, 39% and 37% in OsAPX2-OX lines (OX1, OX2, OX3 and OX4, respectively; Figure 12). For cold treatment, the spikelet fertilities were decreased by 76% in wild-type plants, but only were reduced by 66%, 59%, 64% and 67% in OsAPX2-OX lines (OX1, OX2, OX3 and OX4, respectively; Figure 12). Moreover, our experiments in drought shed obtained the same results, showing that OsAPX2-OX plants were more tolerant to drought stress than wild-type plants at the booting stage (Figure S1).
Figure 12. Spikelet fertility after stress treatments in T2 OsAPX2-OX transgenic plants and WT plants.
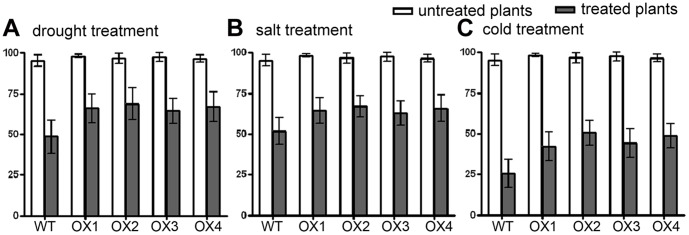
OX1, OX2, OX3 and OX4 are independent OsAPX2-OX lines. A: drought treatment, B: salt treatment, C: cold treatment. Values represent the mean ± SD (N = 10).
OsAPX2 is Essential for Scavenging H2O2 in Rice
In order to understand whether or not APX2 is important in scavenging H2O2 in rice, we analyzed the APX activity and H2O2 contents in wild-type, Osapx2 and OsAPX2-OX plants. As shown in Figure 13, APX activity in Osapx2 was lower than that of wild-type plants under both normal and stress conditions; by contrast, OsAPX2-OX transgenic lines showed higher levels of APX activity under both normal and stress conditions compared to the wild-type plants.
Figure 13. APX activity assay of wild-type, Osapx2 and OsAPX2-OX plants.
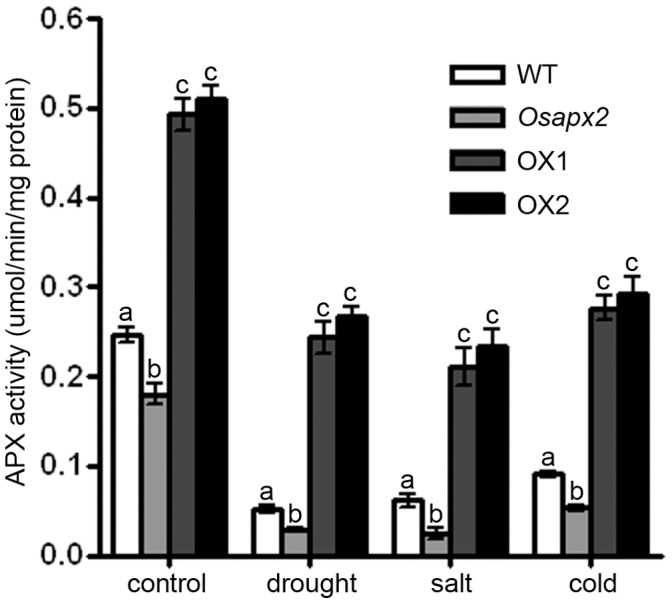
Five leaf seedlings grown in the half-strengthened MS liquid medium were treated with different treatments. For salt treatment, wild-type, Osapx2 and OsAPX2-OX plants were treated with 150 mM NaCl for 24 h; for cold treatment, plants were transferred to a 10°C incubator for 24 h; for drought treatment, plants were transferred to dry sterile petri dishes or 2 h. Different letters (a–c) indicate significant differences (P<0.05) between lines.
H2O2 content was analyzed in wild-type, Osapx2, complementation lines and OsAPX2-OX lines at different developmental stages. As shown in Figure 14, for all of the lines, the H2O2 level was lower in seedlings, but increased gradually with the growth and reached a high level in spikelets. However, H2O2 level of Osapx2 mutant plants was significantly higher than that of wild-type in all growing stage. H2O2 content of complementation lines was similar to that of wild-type plants at seedling stage, while it is still higher than that of wild-type plants at tillering stage and in spikelet. By contrast, the accumulation of H2O2 in OsAPX2-OX transgenic lines was lower than the wild-type plants.
Figure 14. H2O2 content assay of wild-type, Osapx2, complementation and OsAPX2-OX plants at different developmental stages.
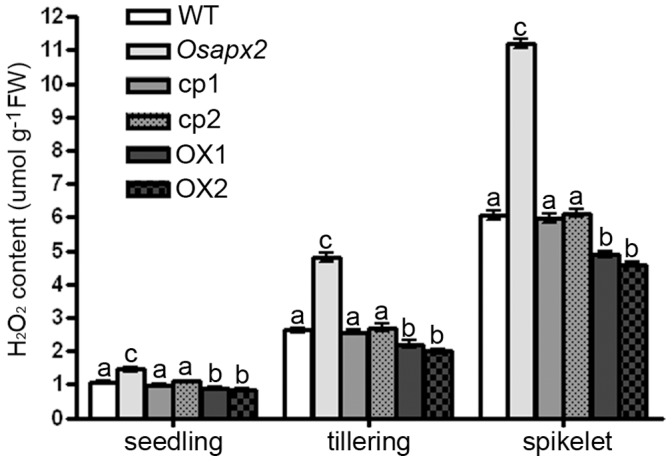
H2O2 content assay was performed in wild-type, Osapx2, independent complementation lines (cp1 and cp2) and independent OsAPX2-OX lines (OX1 and OX2) at different developmental stages. Different letters (a–c) indicate significant differences (P<0.05) between lines.
Discussion
OsAPX2 is a Central Player in Rice Growth and Abiotic Stress Tolerance
In this study, using both the Osapx2 knockout mutants and the Osapx2 overexpression lines, we demonstrated that OsAPX2 plays an important role in tolerance to abiotic stresses in rice. In addition, we found that the Osapx2 knockout mutants exhibited a pleiotropic phenotype including semi-dwarf, severe leaf minic lesion and male-sterility (Figure 4-A,E). Our results indicate that OsAPX2 not only functions in protecting rice seedlings from abiotic stresses but also is a central player in the growth and development in rice.
The fact that knocking out the Osapx2 gene causes severe growth defects and abiotic stress responses indicates that Osapx2 gene is a central component of the reactive oxygen gene network in rice. On the other hand, this also shows that the function of Osapx2 gene is not compensated by other members of the APX gene family in rice [15]. This might be true since Rosa et al. (2010) found that silencing of OsApx2 reduced OsApx1 and OsApx7 expression [17]. Similarly, knocking out the Atapx1 gene does not lead to an increase in the expression of the other APX isozymes in the APX gene family in Arabidopsis [10]. These may suggest that a more complicated compensation system exists in plants. The APX reactive oxygen scavenging network might be compensated and cooperated by other system(s) outside the APX gene family.
OsAPX2 Plays a Critical Role in Protecting the Functions of Chloroplasts in Rice
Studies from Arabidopsis have shown that the cytosolic ascorbate peroxidase APX1 plays a key role in ROS homeostasis in cells under abiotic stresses. Davletova et al. (2005) reported that in the absence of APX1, the entire chloroplastic H2O2-scavenging system collapsed, H2O2 levels increased, and protein oxidation occurred, indicating that APX1 plays a critical role in cross-compartment protection of chloroplast functions [36]. This study implies that the cytosolic ascorbate peroxidase APX1 functions as a defense barrier between the three major ROS-producing organelles of plant cells (i.e., the chloroplast, mitochondria, and peroxisomes). OsAPX2, a cytosolic ascorbate peroxidase in rice, shares the highest degree of similarity with the Arabidopsis APX1 in all 8 members of the rice APX gene family (Figure S2). Our results have shown that Osapx2 mutants were sensitive to abiotic stresses (Figure 9-A). Interestingly, Osapx2 mutants had a significant low level of chlorophylls under abiotic stresses relative to the wild-type plants (Figure 10-B). By contrast, over expression of OsAPX2 increased the chlorophyll contents to a level higher than the wild-type plants (Figure 11-B). These results suggest that the rice cytosolic ascorbate peroxidase OsAPX2 might also function as a defense barrier in cellular oxidative stress and plays a key role in cross-compartment protection of chloroplast functions in rice.
APXs Might Control Plant Growth and Development through the H2O2 Signal Transduction Pathway
Our results showed that OsAPX2 disruption inhibited seedling growth and anther development (Figure 4-A,E). Previous studies found that silencing of either OsAPX1 or OsAPX2 gene produced a semi-dwarf phenotype [17], and low temperatures caused male sterility in rice [18]. Arabidopsis APX1 knockout mutant also had a suppressed growth and development phenotype [10]. However, regulation mechanism of plant growth and development by APX genes remains unclear. Since APX function to remove cellular H2O2 and disturbing AXP activity enhances H2O2 production in plants, therefore, it is reasonable to hypothesize that APXs might control plant growth and development through the H2O2 signal transduction pathway. H2O2 is a signaling molecule and plays versatile roles in plants [37]. Studies have shown that H2O2 regulates the expression of many transcription factors, including ZAT zinc finger transcription factor, WRKY, heat shock transcription factor, and ethylene response factor [10], [36], [38]. It is possible that H2O2 might affect plant growth and development through regulating the expression of these transcription factors. Indeed, knocking out AtAPX1 gene elevated the levels of ZAT zinc finger transcription factor, WRKY, heat shock transcription factor, and ethylene response factor [10]. Moreover, the expression of a flowering related gene GIGANTEA in Arabidopsis was elevated in AtAPX1 knockout mutants. But there is no direct link between the GIGANTEA expression and the rise in H2O2 levels in AtAPX1 mutants [10]. Therefore, more studies are needed to identify the transcription factor(s) and/or gene(s) involved in APX regulation of growth and development in plants.
Supporting Information
Drought stress experiments preformed in drought shed. Black lines indicated OsAPX2-OX plants. Red lines indicated wild-type plants.
(TIF)
Phylogenetic tree of rice and Arabidopsis APX proteins. The tree was constructed with the DNAMAN tree program with amino acid sequences of Arabidospis APXs (APX1∼APX6, tAPX and sAPX) and OsAPXs (OsAPX1∼OsAPX8).
(TIF)
Acknowledgments
We thank Qilin Wang and Yi Zhang for their assistance in abiotic stresses tolerance assay. We would like to express our great thanks to the anonymous reviewers for their helpful comments which improved this paper greatly.
Funding Statement
This work was supported by grants from The National Natural Science Foundation of China (NSFC) (30771089 to Tiegang Lu) and (31070222 to Quansheng Qiu). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Scandalios JG (2002) The rise of ROS. Trends Biochem Sci 27: 483–486. [DOI] [PubMed] [Google Scholar]
- 2. Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55: 373–399. [DOI] [PubMed] [Google Scholar]
- 3. Fridovich I (1998) The trail to superoxide dismutase. Protein Science 7: 2688–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Karpinski S, Escobar C, Karpinska B, Creissen G, Mullineaux PM (1997) Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. Plant Cell 9: 627–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49: 249–279. [DOI] [PubMed] [Google Scholar]
- 6. Mittler R, Vanderauwera S, Gallery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9: 490–498. [DOI] [PubMed] [Google Scholar]
- 7. Miller G, Suzuki N, Rizhsky L, Hegie A, Koussevitzky S, et al. (2007) Double mutants deficient in cytosolic and thylakoid ascorbate peroxidase reveal a complex mode of interaction between reactive oxygen species, plant development, and response to abiotic stresses. Plant Physiol 144: 1777–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Asada K (1999) The water–water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50: 601–639. [DOI] [PubMed] [Google Scholar]
- 9. Koussevitzky S, Suzuki N, Huntington S, Armijo L, Sha W, et al. (2008) Ascorbate peroxidase 1 plays a key role in the response of Arabidopsis thaliana to stress combination. J Biol Chem 283: 34197–34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pnueli L, Liang H, Rozenberg M, Mittler R (2003) Growth suppression, altered stomatal responses, and augmented induction of heat shock proteins in cytosolic ascorbate peroxidase (Apx1)-deficient Arabidopsis plants. Plant J 34: 187–203. [DOI] [PubMed] [Google Scholar]
- 11. Panchuk II, Zentgraf U, Volkov RA (2005) Expression of the Apx gene family during leaf senescence of Arabidopsis thaliana . Planta 222: 926–932. [DOI] [PubMed] [Google Scholar]
- 12. Mullineaux PM, Karpinski S, Baker NR (2006) Spatial dependence for hydrogen peroxide-directed signaling in light-stressed plants. Plant Physiol 141: 346–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rossel JB, Walter PB, Hendrickson L, Chow WS, Poole A, et al. (2006) A mutation affecting ASCORBATE PEROXIDASE 2 gene expression reveals a link between responses to high light and drought tolerance. Plant Cell Environ 29: 269–281. [DOI] [PubMed] [Google Scholar]
- 14. Kangasjärvi S, Lepistö A, Hännikäinen K, Piippo M, Luomala EM, et al. (2008) Diverse roles for chloroplast stromal and thylakoid-bound ascorbate peroxidases in plant stress responses. Biochem J 412: 275–285. [DOI] [PubMed] [Google Scholar]
- 15. Teixeira FK, Menezes-Benavente L, Galvão VC, Margis R, Margis-Pinheiro M (2006) Rice ascorbate peroxidase gene family encodes functionally diverse isoforms localized in different subcellular compartments. Planta 224: 300–314. [DOI] [PubMed] [Google Scholar]
- 16. Agrawal GK, Jwa NS, Iwahashi H, Rakwal R (2003) Importance of ascorbate peroxidases OsAPX1 and OsAPX2 in the rice pathogen response pathways and growth and reproduction revealed by their transcriptional profiling. Gene 322: 93–103. [DOI] [PubMed] [Google Scholar]
- 17. Rosa SB, Caverzan A, Teixeira FK, Lazzarotto F, Silveira JA, et al. (2010) Cytosolic APx knockdown indicates an ambiguous redox responses in rice. Phytochemistry 71: 548–558. [DOI] [PubMed] [Google Scholar]
- 18. Sato Y, Masuta Y, Saito K, Murayama S, Ozawa K (2011) Enhanced chilling tolerance at the booting stage in rice by transgenic overexpression of the ascorbate peroxidase gene, OsAPXa. Plant Cell Rep 30: 399–406. [DOI] [PubMed] [Google Scholar]
- 19. Lu Z, Liu D, Liu S (2007) Two rice cytosolic ascorbate peroxidases differentially improve salt tolerance in transgenic Arabidopsis . Plant Cell Rep 26: 1909–1917. [DOI] [PubMed] [Google Scholar]
- 20. Guan Q, Takano T, Liu S (2012) Genetic Transformation and Analysis of Rice OsAPx2 Gene in Medicago sativa. PLoS One 7: e41233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niwa R, Slack FJ (2008) Ins and outs of RNA interference analysis. Cell Press 34–36.
- 22. Gilchrist E, Haughn G (2010) Reverse genetics techniques: engineering loss and gain of gene function in plant. Briefings in functional genomics 9(2): 103–110. [DOI] [PubMed] [Google Scholar]
- 23. Zou LP, Sun XH, Zhang ZG, Liu P, Wu JX, et al. (2011) Leaf rolling controlled by the homeodomain leucine zipper class IV gene Roc5 in rice. Plant Physiol 156: 1589–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li SC, Yang L, Deng QM, Wang SQ, Wu FQ, et al. (2006) Phenotypic characterization of a female sterile mutant in rice. J Intergr Plant Biol 48: 307–314. [Google Scholar]
- 25. Peng H, Huang H, Yang Y, Zhai Y, Wu J, et al. (2005) Functional analysis of GUS expression patterns and T-DNA integration characteristics in rice enhancer trap lines. Plant Sci 168: 1571–1579. [Google Scholar]
- 26. Yang Y, Peng H, Huang H, Wu J, Jia S, et al. (2004) Large-scale production of enhancer trapping lines for rice functional genomics. Plant Sci 167: 281–288. [Google Scholar]
- 27. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔ C T method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 28. Malamy JE, Benfey PN (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124: 33–44. [DOI] [PubMed] [Google Scholar]
- 29. Chen GX, Asada K (1989) Ascorbate peroxidase in tea leaves:occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol 30: 987–998. [Google Scholar]
- 30. Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of proteins utilizing the principal of protein–dye binding. Anal Biochem 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 31. Boyer JS (1968) Measurement of the water status of plants. Annu Rev Plant Physiol 9: 351–363. [Google Scholar]
- 32. Arnon DI (1949) Copper enzymes in isolated chloroplasts in Beta vulgaris. Plant Physiol 24: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dagmar P, Sairam RK, Srivastava GC, Singh DV (2001) Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Sci 161: 765–771. [Google Scholar]
- 34. Carmak I, Horst WJ (1991) Effects of aluminum on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant 83: 463–468. [Google Scholar]
- 35. Guan YS, Serraj R, Liu SH, Xu JL, Ali J, et al. (2010) Simultaneously improving yield under drought stress and non-stress conditions: a case study of rice (Oryza sativa L.). J Exp Bot 61: 4145–4156. [DOI] [PubMed] [Google Scholar]
- 36. Davletova S, Rizhsky L, Liang HZ (2005) Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 17: 268–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Quan LJ, Zhang B, Shi WW, Li HY (2008) Hydrogen peroxide in plants: a versatile molecule of the reactive oxygen species network. J Intergr Plant Biol 50(1): 2–18. [DOI] [PubMed] [Google Scholar]
- 38. Miller G, Shulaev V, Mittler R (2008) Reactive oxygen signaling and abiotic stress. Physiol Plantarum 133: 481–489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Drought stress experiments preformed in drought shed. Black lines indicated OsAPX2-OX plants. Red lines indicated wild-type plants.
(TIF)
Phylogenetic tree of rice and Arabidopsis APX proteins. The tree was constructed with the DNAMAN tree program with amino acid sequences of Arabidospis APXs (APX1∼APX6, tAPX and sAPX) and OsAPXs (OsAPX1∼OsAPX8).
(TIF)