Abstract
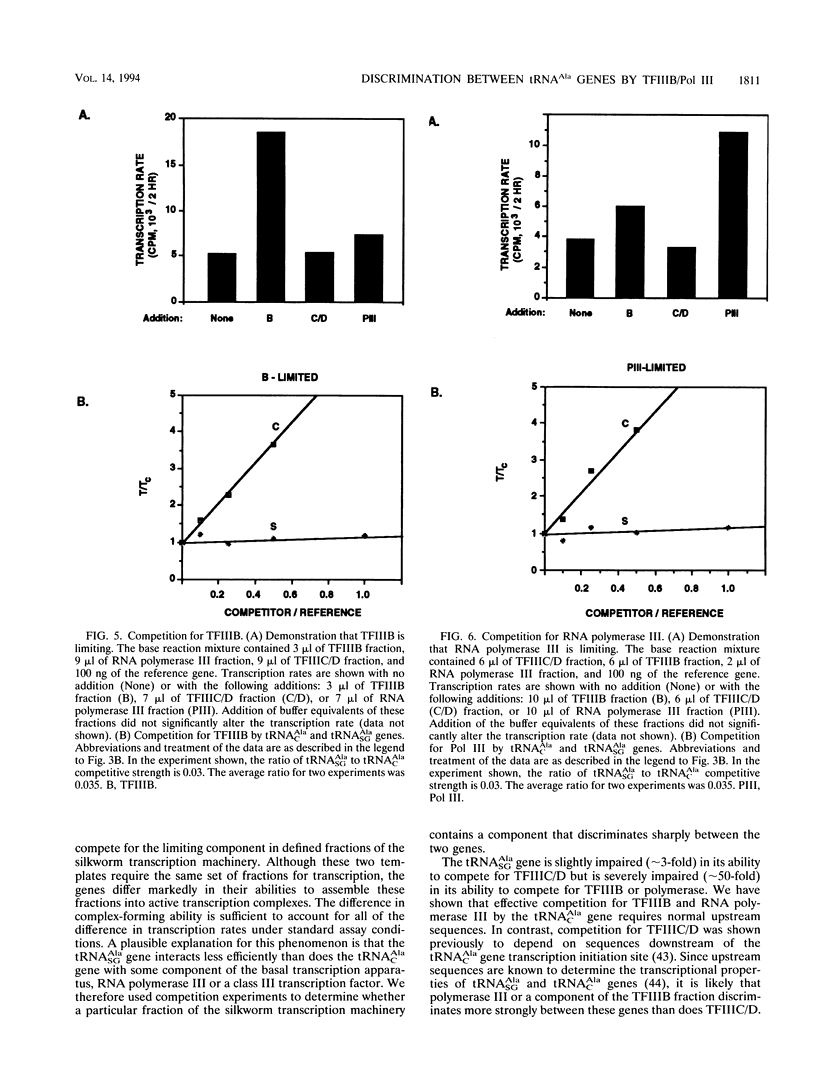
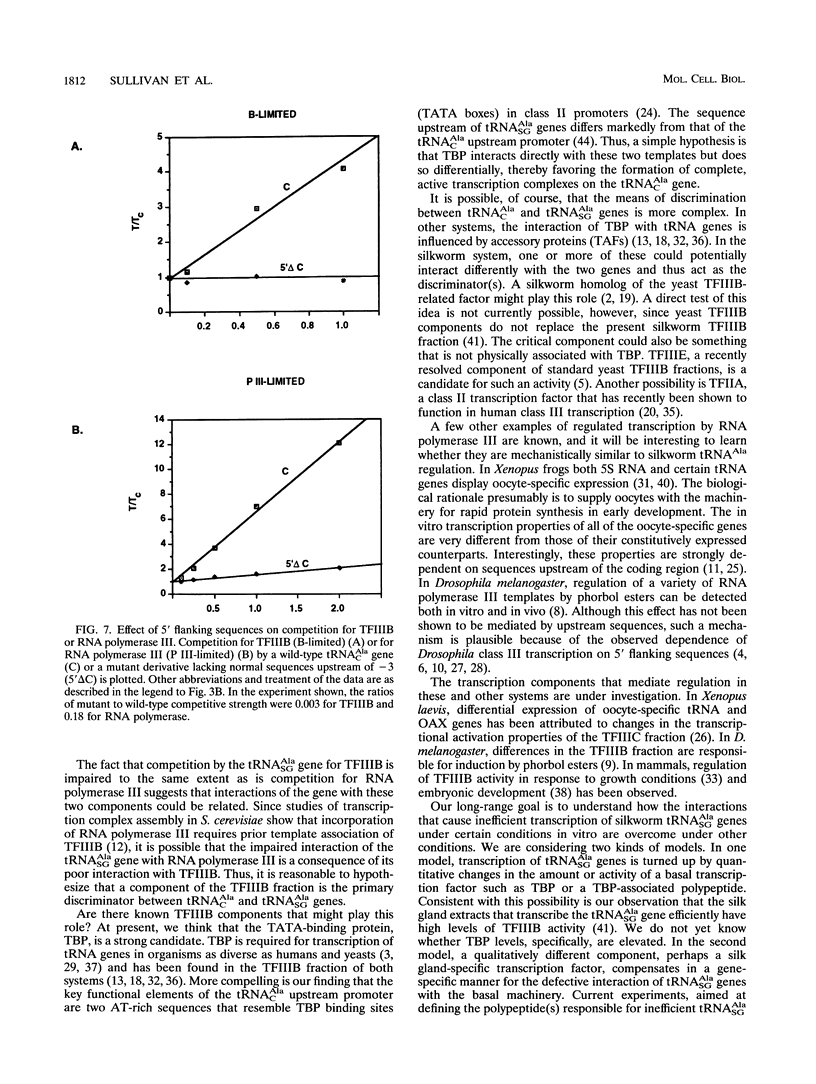
Constitutive and silk gland-specific tRNA(Ala) genes from silkworms have very different transcriptional properties in vitro. Typically, the constitutive type, which encodes tRNA(AlaC), directs transcription much more efficiently than does the silk gland-specific type, which encodes tRNA(AlaSG). We think that the inefficiency of the tRNA(AlaCG) gene underlies its capacity to be turned off in non-silk gland cells. An economical model is that the tRNA(AlaSG) promoter interacts poorly, relative to the tRNA(AlaC) promoter, with one or more components of the basal transcription machinery. As a consequence, the tRNA(AlaSG) gene directs the formation of fewer transcription complexes or of complexes with reduced cycling ability. Here we show that the difference in the number of active transcription complexes accounts for the difference in tRNA(AlaC) and tRNA(AlaSG) transcription rates. To determine whether a particular component of the silkworm transcription machinery is responsible for reduced complex formation on the tRNA(AlaSG) gene, we measured competition by templates for defined fractions of this machinery. We find that the tRNA(AlaSG) gene is greatly impaired, in comparison with the tRNA(AlaC) gene, in competition for either TFIIIB or RNA polymerase III. Competition for each of these fractions is also strongly influenced by the nature of the 5' flanking sequence, the promoter element responsible for the distinctive transcriptional properties of tRNA(AlaSG) and tRNA(AlaC) genes. These results suggest that differential interaction with TFIIIB or RNA polymerase III is a critical functional distinction between these genes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Buratowski S., Zhou H. A suppressor of TBP mutations encodes an RNA polymerase III transcription factor with homology to TFIIB. Cell. 1992 Oct 16;71(2):221–230. doi: 10.1016/0092-8674(92)90351-c. [DOI] [PubMed] [Google Scholar]
- Cormack B. P., Struhl K. The TATA-binding protein is required for transcription by all three nuclear RNA polymerases in yeast cells. Cell. 1992 May 15;69(4):685–696. doi: 10.1016/0092-8674(92)90232-2. [DOI] [PubMed] [Google Scholar]
- DeFranco D., Sharp S., Söll D. Identification of regulatory sequences contained in the 5'-flanking region of Drosophila lysine tRNA2 genes. J Biol Chem. 1981 Dec 10;256(23):12424–12429. [PubMed] [Google Scholar]
- Dieci G., Duimio L., Coda-Zabetta F., Sprague K. U., Ottonello S. A novel RNA polymerase III transcription factor fraction that is not required for template commitment. J Biol Chem. 1993 May 25;268(15):11199–11207. [PubMed] [Google Scholar]
- Dingermann T., Burke D. J., Sharp S., Schaack J., Söll D. The 5- flanking sequences of Drosophila tRNAArg genes control their in vitro transcription in a Drosophila cell extract. J Biol Chem. 1982 Dec 25;257(24):14738–14744. [PubMed] [Google Scholar]
- Garber M. E., Vilalta A., Johnson D. L. Induction of Drosophila RNA polymerase III gene expression by the phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA) is mediated by transcription factor IIIB. Mol Cell Biol. 1994 Jan;14(1):339–347. doi: 10.1128/mcb.14.1.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber M., Panchanathan S., Fan R. S., Johnson D. L. The phorbol ester, 12-O-tetradecanoylphorbol-13-acetate, induces specific transcription by RNA polymerase III in Drosophila Schneider cells. J Biol Chem. 1991 Nov 5;266(31):20598–20601. [PubMed] [Google Scholar]
- Garbers D. L. Guanylyl cyclase receptors and their endocrine, paracrine, and autocrine ligands. Cell. 1992 Oct 2;71(1):1–4. doi: 10.1016/0092-8674(92)90258-e. [DOI] [PubMed] [Google Scholar]
- Garcia A. D., O'Connell A. M., Sharp S. J. Formation of an active transcription complex in the Drosophila melanogaster 5S RNA gene is dependent on an upstream region. Mol Cell Biol. 1987 Jun;7(6):2046–2051. doi: 10.1128/mcb.7.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouilloud E., Clarkson S. G. A dispersed tyrosine tRNA gene from Xenopus laevis with high transcriptional activity in vitro. J Biol Chem. 1986 Jan 5;261(1):486–494. [PubMed] [Google Scholar]
- Kassavetis G. A., Braun B. R., Nguyen L. H., Geiduschek E. P. S. cerevisiae TFIIIB is the transcription initiation factor proper of RNA polymerase III, while TFIIIA and TFIIIC are assembly factors. Cell. 1990 Jan 26;60(2):235–245. doi: 10.1016/0092-8674(90)90739-2. [DOI] [PubMed] [Google Scholar]
- Kassavetis G. A., Joazeiro C. A., Pisano M., Geiduschek E. P., Colbert T., Hahn S., Blanco J. A. The role of the TATA-binding protein in the assembly and function of the multisubunit yeast RNA polymerase III transcription factor, TFIIIB. Cell. 1992 Dec 11;71(6):1055–1064. doi: 10.1016/0092-8674(92)90399-w. [DOI] [PubMed] [Google Scholar]
- Kassavetis G. A., Riggs D. L., Negri R., Nguyen L. H., Geiduschek E. P. Transcription factor IIIB generates extended DNA interactions in RNA polymerase III transcription complexes on tRNA genes. Mol Cell Biol. 1989 Jun;9(6):2551–2566. doi: 10.1128/mcb.9.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klekamp M. S., Weil P. A. Yeast class III gene transcription factors and homologous RNA polymerase III form ternary transcription complexes stable to disruption by N-lauroyl-sarcosine (sarcosyl). Arch Biochem Biophys. 1986 May 1;246(2):783–800. doi: 10.1016/0003-9861(86)90335-8. [DOI] [PubMed] [Google Scholar]
- Kovelman R., Roeder R. G. Sarkosyl defines three intermediate steps in transcription initiation by RNA polymerase III: application to stimulation of transcription by E1A. Genes Dev. 1990 Apr;4(4):646–658. doi: 10.1101/gad.4.4.646. [DOI] [PubMed] [Google Scholar]
- Larson D., Bradford-Wilcox J., Young L. S., Sprague K. U. A short 5' flanking region containing conserved sequences is required for silkworm alanine tRNA gene activity. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3416–3420. doi: 10.1073/pnas.80.11.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo S. M., Tanaka M., Sullivan M. L., Hernandez N. A TBP complex essential for transcription from TATA-less but not TATA-containing RNA polymerase III promoters is part of the TFIIIB fraction. Cell. 1992 Dec 11;71(6):1029–1040. doi: 10.1016/0092-8674(92)90397-u. [DOI] [PubMed] [Google Scholar]
- Meissner W., Holland R., Waldschmidt R., Seifart K. H. Transcription factor IIA stimulates the expression of classical polIII-genes. Nucleic Acids Res. 1993 Feb 25;21(4):1013–1018. doi: 10.1093/nar/21.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meza L., Araya A., Leon G., Krauskopf M. Specific alanine-tRNA species associated with fibroin biosynthesis in the posterior sild-gland of Bombyx mori L. FEBS Lett. 1977 May 15;77(2):255–260. doi: 10.1016/0014-5793(77)80246-9. [DOI] [PubMed] [Google Scholar]
- Morton D. G., Sprague K. U. In vitro transcription of a silkworm 5S RNA gene requires an upstream signal. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5519–5522. doi: 10.1073/pnas.81.17.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottonello S., Rivier D. H., Doolittle G. M., Young L. S., Sprague K. U. The properties of a new polymerase III transcription factor reveal that transcription complexes can assemble by more than one pathway. EMBO J. 1987 Jul;6(7):1921–1927. doi: 10.1002/j.1460-2075.1987.tb02452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palida F. A., Hale C., Sprague K. U. Transcription of a silkworm tRNA(cAla) gene is directed by two AT-rich upstream sequence elements. Nucleic Acids Res. 1993 Dec 25;21(25):5875–5881. doi: 10.1093/nar/21.25.5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds W. F., Johnson D. L. Differential expression of oocyte-type class III genes with fraction TFIIIC from immature or mature oocytes. Mol Cell Biol. 1992 Mar;12(3):946–953. doi: 10.1128/mcb.12.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney R. J., Harding J. D. Transcriptional activity and factor binding are stimulated by separate and distinct sequences in the 5' flanking region of a mouse tRNAAsp gene. Nucleic Acids Res. 1988 Mar 25;16(6):2509–2521. doi: 10.1093/nar/16.6.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjadi F. G., Spiegelman G. B. Modulation of a Drosophila melanogaster tRNA gene transcription in vitro by a sequence TNNCT in its 5' flank. Gene. 1987;60(1):13–19. doi: 10.1016/0378-1119(87)90208-3. [DOI] [PubMed] [Google Scholar]
- Schaack J., Sharp S., Dingermann T., Burke D. J., Cooley L., Söll D. The extent of a eukaryotic tRNA gene. 5'- and 3'-flanking sequence dependence for transcription and stable complex formation. J Biol Chem. 1984 Feb 10;259(3):1461–1467. [PubMed] [Google Scholar]
- Schultz M. C., Reeder R. H., Hahn S. Variants of the TATA-binding protein can distinguish subsets of RNA polymerase I, II, and III promoters. Cell. 1992 May 15;69(4):697–702. doi: 10.1016/0092-8674(92)90233-3. [DOI] [PubMed] [Google Scholar]
- Sprague K. U., Hagenbüchle O., Zuniga M. C. The nucleotide sequence of two silk gland alanine tRNAs: implications for fibroin synthesis and for initiator tRNA structure. Cell. 1977 Jul;11(3):561–570. doi: 10.1016/0092-8674(77)90074-5. [DOI] [PubMed] [Google Scholar]
- Stutz F., Gouilloud E., Clarkson S. G. Oocyte and somatic tyrosine tRNA genes in Xenopus laevis. Genes Dev. 1989 Aug;3(8):1190–1198. doi: 10.1101/gad.3.8.1190. [DOI] [PubMed] [Google Scholar]
- Taggart A. K., Fisher T. S., Pugh B. F. The TATA-binding protein and associated factors are components of pol III transcription factor TFIIIB. Cell. 1992 Dec 11;71(6):1015–1028. doi: 10.1016/0092-8674(92)90396-t. [DOI] [PubMed] [Google Scholar]
- Tower J., Sollner-Webb B. Polymerase III transcription factor B activity is reduced in extracts of growth-restricted cells. Mol Cell Biol. 1988 Feb;8(2):1001–1005. doi: 10.1128/mcb.8.2.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood D. C., Knickerbocker H., Gardner G., Condliffe D. P., Sprague K. U. Silk gland-specific tRNA(Ala) genes are tightly clustered in the silkworm genome. Mol Cell Biol. 1988 Dec;8(12):5504–5512. doi: 10.1128/mcb.8.12.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldschmidt R., Seifart K. H. TFIIA is required for in vitro transcription of mammalian U6 genes by RNA polymerase III. J Biol Chem. 1992 Aug 15;267(23):16359–16364. [PubMed] [Google Scholar]
- White R. J., Jackson S. P., Rigby P. W. A role for the TATA-box-binding protein component of the transcription factor IID complex as a general RNA polymerase III transcription factor. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1949–1953. doi: 10.1073/pnas.89.5.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. J., Stott D., Rigby P. W. Regulation of RNA polymerase III transcription in response to F9 embryonal carcinoma stem cell differentiation. Cell. 1989 Dec 22;59(6):1081–1092. doi: 10.1016/0092-8674(89)90764-2. [DOI] [PubMed] [Google Scholar]
- Wolffe A. P., Brown D. D. Developmental regulation of two 5S ribosomal RNA genes. Science. 1988 Sep 23;241(4873):1626–1632. doi: 10.1126/science.241.4873.1626. [DOI] [PubMed] [Google Scholar]
- Young L. S., Dunstan H. M., Witte P. R., Smith T. P., Ottonello S., Sprague K. U. A class III transcription factor composed of RNA. Science. 1991 Apr 26;252(5005):542–546. doi: 10.1126/science.1708526. [DOI] [PubMed] [Google Scholar]
- Young L. S., Rivier D. H., Sprague K. U. Sequences far downstream from the classical tRNA promoter elements bind RNA polymerase III transcription factors. Mol Cell Biol. 1991 Mar;11(3):1382–1392. doi: 10.1128/mcb.11.3.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. S., Takahashi N., Sprague K. U. Upstream sequences confer distinctive transcriptional properties on genes encoding silkgland-specific tRNAAla. Proc Natl Acad Sci U S A. 1986 Jan;83(2):374–378. doi: 10.1073/pnas.83.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. S., Takahashi N., Sprague K. U. Upstream sequences confer distinctive transcriptional properties on genes encoding silkgland-specific tRNAAla. Proc Natl Acad Sci U S A. 1986 Jan;83(2):374–378. doi: 10.1073/pnas.83.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]