Abstract
Acute alcohol intoxication decreases muscle protein synthesis, but there is a paucity of data on the ability of alcohol to regulate muscle protein degradation. Furthermore, various types of atrophic stimuli appear to regulate ubiquitin-proteasome-dependent proteolysis by increasing the muscle-specific E3 ligases atrogin-1 and MuRF1 (i.e., “atrogenes”). Therefore, the present study was designed to test the hypothesis that acute alcohol intoxication increases atrogene expression leading to an elevated rate of muscle protein breakdown. In male rats, the intraperitoneal injection of alcohol dose- and time-dependently increased atrogin-1 and MuRF1 mRNA in gastrocnemius, the latter of which was most pronounced. A comparable change was absent in the soleus and heart. The ability of in vivo-administered ethanol to increase atrogene expression was independent of the route of alcohol administration (intraperitoneal vs. oral), as well as of nutritional status (fed vs. fasted) and gender (male vs. female). The increase in atrogin-1 and MuRF1 was independent of alcohol metabolism, and the overproduction of endogenous glucocorticoids and could not be prevented by maintaining the circulating concentration of insulin-like growth factor-I. Despite marked changes in atrogene expression, acute alcohol in vivo did not alter the release of either 3-methylhistidine (MH) or tyrosine from the isolated perfused hindlimb, suggesting that the rate of muscle proteolysis remains unchanged. Moreover, alcohol did not increase the directly determined rate of protein degradation in isolated epitrochlearis muscles or cultured myocytes. Finally, no increase in atrogene expression or 3-MH release was detected in muscle from rats fed an alcohol-containing diet. Our results indicate that although acute alcohol intoxication increases atrogin-1 and MuRF1 mRNA preferentially in fast-twitch skeletal muscle, this change was not associated with increased rates of muscle proteolysis. Therefore, the loss of muscle mass/protein in response to chronic alcohol abuse appears to result primarily from a decrement in muscle protein synthesis, not an increase in degradation.
Keywords: protein degradation, protein breakdown, ubiquitin-proteasome, 3-methylhistidine, glucocorticoid, IGF-I
proximal myopathy, preferentially affecting skeletal muscle with a predominance of fast-twitch fibers, is a common metabolic phenotype of those individuals who chronically abuse alcohol (12, 48). The loss of muscle mass in this condition represents an imbalance in the rates of skeletal muscle protein synthesis vs. protein degradation. Studies from independent laboratories have provided consistent evidence that chronic alcohol consumption over many weeks decreases muscle protein synthesis (33, 49). However, an extended period of alcohol consumption is not a prerequisite for the development of this metabolic disturbance as a single intoxicating dose of ethanol also decreases muscle protein synthesis in a dose- and time-dependent manner (31, 51, 58). Importantly, the ability of alcohol to inhibit muscle protein synthesis has not only been observed under in vivo conditions but also in the isolated perfused hindlimb and in cultured myocytes (19, 31).
In contrast, there is a paucity of data related to the ability of either chronic alcohol consumption or acute intoxication to alter the other determinant of muscle protein balance—protein degradation. The plasma concentration and/or urinary excretion of 3-methylhistidine (MH) provides an estimate of whole body muscle proteolysis and has been reported to be either increased or unchanged by alcohol (30, 34, 42, 53). Finally, another study has reported that breakdown, assessed indirectly as the calculated difference between the change in the rate of protein synthesis and protein content in skeletal muscle, was actually decreased by chronic alcohol consumption (40, 50). Hence, indirect estimates of in vivo muscle protein degradation in response to alcohol are divergent, and the direct measurement of protein degradation in muscle appears lacking.
Proteolysis can proceed via several distinct pathways [e.g., lysosomal, calcium-dependent, and ubiquitin (Ub)-proteasome-dependent], although the latter mechanism appears primarily responsible for the increased protein degradation in skeletal muscle observed in various catabolic conditions (17, 35). The Ub-proteasome system contains two functional facets: the targeting of specific protein substrates for degradation and their ultimate destruction by the proteasome (54). The targeting requirement is fulfilled by the activity of a hierarchical cascade consisting of E1 (Ub-activating), E2 (Ub-conjugating), and E3 (Ub-ligating) enzymes. Proteins degraded by this pathway are first sequentially linked to monomeric Ub, forming a polyubiquitin chain. Subsequently, this chain is recognized by the 19S proteasome (i.e., regulatory cap) and leads to protein unfolding, insertion into the proteasome, and degradation of the protein into peptide fragments. Although the rate-limiting step in muscle proteolysis has not been identified, the coordinated upregulation of specific E2s and E3s and ubiquitin conjugation appears central to the atrophic response during catabolic injury.
Historically, protein ubiquitination in the N end-rule pathway by the Ub-conjugating enzyme E214k and the Ub ligase E3α was considered the primary regulatory pathway for muscle loss in wasting states (17, 35). However, a host of diverse atrophic stimuli, including immobilization, denervation, diabetes, sepsis, burn injury, excess glucocorticoids, and nutrient deprivation strongly increase the gene expression of two additional muscle-specific E3 ligases—muscle RING finger, MuRF1, and muscle atrophy F-box, MAFbx; aka atrogin-1 (2, 14, 15, 28, 36, 55). Furthermore, although we have previously reported a modest increase in both atrogin-1 and MuRF1 in rats orally gavaged twice daily for 3 days, the time- and dose-dependency, as well as the mechanism and physiological relevance, for this response was not elucidated (59). Likewise, overexpression of atrogin-1 protein reduces the size of cultured myocytes (2) and blunts cardiac hypertrophy (37). Conversely, there is an attenuation in the loss of muscle mass after muscle denervation in atrogin-1 and MuRF1 null mice (2) and after coexpression of a constitutively active form of the NF-κB activator IKK in MuRF1 null mice (4). As a consequence of their importance in upregulating muscle proteolysis, atrogin-1 and MuRF1 have collectively been referred to as atrophy-related genes or “atrogenes.” On the basis of this background, the current study was conducted to address the hypothesis that acute alcohol intoxication and chronic alcohol consumption upregulates atrogene expression, and this change will be associated with an increased rate of proteolysis in skeletal muscle composed of fast-twitch fibers.
MATERIALS AND METHODS
Experimental protocols.
All experimental protocols involving animals were approved by the Institutional Animal Care and Use Committee of The Pennsylvania State University College of Medicine and adhered to the National Institutes of Health guidelines for the use of experimental animals. Unless otherwise indicated, pathogen-free male Sprague-Dawley rats (225–250 g; Charles River Breeding Laboratories, Cambridge, MA) were used in all of the experiments. Rats were housed in a controlled environment and provided commercial laboratory food (Harlan #2018; 18% protein rodent diet; Madison, WI) and water ad libitum for at least 1 wk before the start of the study.
On the day of the study, the general experimental protocol for the first study involved the injection of ethanol (75 mmol/kg ip) into freely fed rats with subsequent removal of tissues at 1, 4, 8, or 24 h thereafter. Time-matched control animals were injected with an equal volume of 0.9% (wt/vol) sterile saline and tissues collected at either 0, 1, 4, 8, or 24 h. Rats were fasted after alcohol administration. Rats were excluded a priori from analysis if gross inspection at the time of death showed any indication that the intraperitoneal injection of alcohol or saline had been delivered directly into the liver or intestine and not into the peritoneal cavity per se. The experimental protocol was selected because this model of acute alcohol intoxication reproducibly decreases muscle protein synthesis in a dose- and time-dependent manner (24, 31, 51). We acknowledge the intraperitoneal injection of alcohol is not the most physiological route of alcohol administration, but the large majority of data related to the effect of acute alcohol on muscle protein metabolism has been generated using this well-characterized model (25). We addressed this concern related to the route of alcohol administration by comparing data from rats injected with alcohol intraperitoneally to those obtained from animals administered the same dose of ethanol (or saline) via oral gavage. Immediately prior to excision of tissues, rats were anesthetized with an injection of pentobarbital sodium (100 mg/kg ip). A portion of the gastrocnemius, soleus, and heart was immediately homogenized, and the remaining muscle was rapidly frozen between liquid nitrogen-cooled clamps. In this and all subsequent studies, frozen tissues were powdered and stored at −70°C until analyzed. Blood was collected in a heparinized syringe from the abdominal aorta and, after centrifugation, the plasma was stored at −20°C. The plasma alcohol concentration at the time of death was determined using an Analox Instruments (Lunenburg, MA) rapid analyzer.
In subsequent studies, rats were injected with one of three doses of ethanol (20, 50, or 75 mmol/kg ip body wt), while the control group was injected intraperitoneally with sterile saline. As indicated above, this highest dose of ethanol was selected because it decreases muscle protein synthesis. The two lower ethanol doses were selected on the basis of studies indicating the blood alcohol concentration would be ∼50% and 10%, respectively, of that present in the high-dose ethanol group (31). Rats were anesthetized with pentobarbital sodium 4 h after injection of alcohol or saline, and tissues and blood were sampled as described above.
Additional in vivo studies were performed in 1) overnight fasted male and female rats administered ethanol intraperitoneally and 2) fasted male rats pretreated with the alcohol dehydrogenase inhibitor 4-methylpyrazole (4-MP; 8 mg/kg, Sigma, St. Louis, MO) 30 min prior to intraperitoneal ethanol administration. This dose of 4-MP has been previously demonstrated to be effective (38, 43, 47).
To assess the importance of endogenously produced glucocorticoids, control and alcohol-treated rats were injected subcutaneously with the glucocorticoid receptor antagonist RU486 (Mifepristone; 20 mg/kg body wt; Sigma) or an equal volume (200 μl) of vehicle (DMSO) 2 h before alcohol administration. RU486 is an antiprogestin with antiglucocorticoid properties. RU486 has a high affinity for cytosolic type II glucocorticoid receptors in various target tissues and exhibits little agonist activity (39). This dose of RU486 attenuates the glucocorticoid-induced increase in catabolism and ameliorates endotoxin- or cytokine-induced changes in muscle protein balance in adult rats (10, 32). To confirm the efficacy of RU486 blockade, rats were pretreated with RU486 prior to intraperitoneal injection of dexamethasone (Dex)-sodium phosphate (100 μg/100 mg body wt) in sterile saline (0.9%). This dose of Dex was selected because it inhibits protein synthesis and stimulates protein degradation leading to muscle wasting (6, 55, 56). For all in vivo studies, data from alcohol-treated rats were compared with data from appropriate time-matched control animals.
Because alcohol decreases IGF-I and this peptide hormone can downregulate atrogene expression (28, 55), we determined whether preventing the alcohol-induced decrease in IGF-I would also prevent the concomitant increase in atrogin-1 and MuRF1. Immediately before the intraperitoneal injection of alcohol, rats were injected subcutaneously with recombinant human binary complex (5 μg/g body wt; Insmed, San Jose, CA), which consists of a 1:1 molar ratio of IGF-I and IGF binding protein (IGFBP)-3 or an equal volume (1 ml/rat) of sterile saline. The association of IGFBP-3 with IGF-I, which occurs naturally, delays the clearance of IGF-I from the circulation and thereby increases the bioavailability and bioactivity of IGF-I. This dose of the binary complex was selected based on its ability to reverse the defects in muscle protein synthesis induced by sepsis and food restriction (26, 28). Moreover, unlike the administration of native IGF-I, the binary complex does not complicate data interpretation by hormone-induced hypoglycemia. Time-matched control rats were also injected with the binary complex or saline.
Finally, to determine whether chronic alcohol feeding produced the same changes in atrogene expression as acute intoxication, rats were maintained for 12–14 wk or 28 wk on an ethanol-containing diet, in which alcohol was provided both in the drinking water and agar blocks, exactly as previously described (26, 33, 34). The duration of the alcohol feeding was selected based on previous results, indicating the presence of alcoholic skeletal and cardiac myopathy characterized by a reduction in protein synthesis and muscle mass (26, 33, 62). Time-matched control animals consumed an isonitrogenous isocaloric diet. On the day of the experiment, fed rats were anesthetized (7 AM), and the gastrocnemius and heart were sampled for subsequent analysis.
Isolated perfused hindlimb preparation.
The isolated perfused hindlimb preparation was used to directly determine whether in vivo alcohol administration alters the rate of protein degradation as assessed by 3-MH release. 3-MH, which originates by posttranslational modification of certain histidine residues in actin and myosin, is not reincorporated into protein after its release and is excreted in the urine as 3-MH or N-acetyl-3-MH. In the whole animal, the use of 3-MH as a marker of muscle proteolysis is limited because of its substantial release from the intestine (18). However, this caveat is circumvented by determining the release of 3-MH in the isolated perfused hindlimb where the gut is excluded from the perfusion. On the morning of the experiment, freely fed male rats were divided into the following groups: 1) rats injected intraperitoneally with 75 mmol/kg of alcohol and the perfusion starting 4 h after alcohol administration; 2) rats injected with alcohol and the perfusion starting 8 h after alcohol administration; 3) rats injected with endotoxin (LPS; 1 mg/kg, Escherichia coli, 026:B6; Sigma), used as a positive control, and the perfusion starting at 4 h after LPS injection; 4) rats in which cecal ligation and puncture were performed to induce a clinically relevant model of peritonitis (65) with perfusion starting 24 h later; 5) appropriate time-matched control rats; and 6) control and 4-h alcohol-treated rats perfused with buffer containing IGF-I (10 nM). At the appropriate time point, rats were anesthetized with pentobarbital sodium and the hindlimb was perfused. Briefly, a midline incision was made, and both the inferior vena cava and abdominal aorta were exposed. The abdominal aorta was cannulated, and perfusate was delivered immediately via the abdominal aorta to the hindlimb musculature. The inferior vena cava was subsequently cannulated. The perfusate consisted of a modified Krebs-Henseleit bicarbonate buffer containing 30% (vol/vol) washed bovine erythrocytes, 4.5% (wt/vol) BSA (fraction V; Sigma), 10 mM glucose, and all other amino acids at concentrations normally found in rat plasma (21). The medium was maintained at 37°C and gassed with humidified 95% O2-5% CO2. After perfusion for 30 min, the inferior vena cava cannula was then connected to the perfusion system, and recirculation of the perfusate was begun. For rats treated with alcohol in vivo, ethanol was added to the perfusate, so the final alcohol concentration was ∼250 mg/dl. The hindlimb musculature of all rats was perfused for a total length of 2 h. Samples of perfusate were collected after a 30-min washout period (time 0) and at 120 min for the determination of 3-MH and other amino acids by HPLC, exactly as previously described by our laboratory (30, 41).
Epitrochlearis muscle preparation.
The epitrochlearis muscles were dissected intact for the in vitro measurement of skeletal muscle protein metabolism, exactly as previously described (60). Briefly, epitrochlearis muscles were immediately preincubated for 30 min at 37°C in 2 ml of Krebs/Henseleit buffer (in mM): 120 NaCl, 4.8 KCl, 25 NaHCO3, 2.5 CaCl2, 1.2 KH2PO4, and 1.2 MgSO4, pH 7.4, supplemented with 5 mM HEPES, 0.1% (wt/vol) BSA (99% fatty acid free), 0.17 mM leucine, 0.20 mM valine, 0.10 mM isoleucine, 5 mM glucose, saturated with 95% O2-5% CO2 gas mixture, as previously described (60). After preincubation, muscles were transferred to fresh media (2 ml) and incubated for an additional 120 min with a change of buffer every 60 min. During the final 60 min of the incubation period, the buffer was supplemented with 1 mM l-[3H]phenylalanine (0.15 μCi/ml). At the end of the incubation, muscles were removed from the incubation buffer, trimmed of connective tissue, immersed into 2 ml of ice-cold 10% (wt/vol) TCA, and weighed. Muscles were homogenized and centrifuged at 10,000 g for 10 min at 4°C. The TCA-insoluble material was washed 3 times with 10% TCA, and the resultant pellet was solubilized in 1 M NaOH at 37°C for determination of protein, and radioactivity was incorporated into muscle protein. Tissue protein content was determined using the bicinchoninic acid procedure (BCA; Pierce Chemical, Rockford, IL) with crystalline BSA as a standard. Protein-bound radioactivity was measured using scintillation counting. Protein synthesis was calculated by dividing the protein-bound radioactivity by the specific activity of the phenylalanine in the incubation medium. Values are expressed as nanomoles of phenylalanine incorporated per milligram protein per hour. Because tyrosine is neither synthesized nor degraded in muscle, release of the amino acid from muscle into the incubation medium reflects net protein breakdown. Tyrosine in the incubation medium was assayed fluorometrically (63). Protein degradation was then estimated as the sum of the net tyrosine release into the incubation medium and protein synthesis after conversion of the rate of phenylalanine incorporation into muscle proteins into tyrosine equivalents (60). The amount of tyrosine incorporated into muscle was determined by multiplying the amount of phenylalanine incorporated by 0.77, which is the molar ratio of tyrosine to phenylalanine in mixed skeletal muscle proteins. Values for proteolysis are expressed as nanomoles of tyrosine released per milligram of protein per hour.
Cell culture.
C2C12 mouse myoblasts were purchased from American Type Culture Collection (Manassas, VA). Cells were cultured in Dulbecco's modified Eagle's medium containing 10% FBS, penicillin (100 units/ml), streptomycin (100 μg/ml), and amphotericin (25 μg/ml). Experiments were conducted using cells at the myotube stage in which 80 mM ethanol was added to cells, exactly as previously described (19, 20). Cells were pulse-labeled with [35S]-methionine/cysteine in the presence or absence of alcohol for various times to determine the rate of degradation. Some cells were collected at this time (pulse), whereas for others, the radiolabeled media were removed and replaced with fresh media lacking radioactive methionine/cysteine (chase). Myocytes were chased for 4 h in the absence (control) or presence of alcohol. Cells were collected and precipitated in 10% TCA, and the TCA-precipitable counts were determined using liquid scintillation counting. The results were then compared with those of the appropriate time-matched control group, and data were expressed as a percentage of the pulse-control value. In additional studies performed at the same time, comparably treated cells were isolated, and the content of atrogin-1 and MuRF1 mRNA was determined at 4, 8, and 24 h.
Analytical methods.
Total RNA was extracted from powdered muscle using TRI Reagent (Molecular Research Center, Cincinnati, OH). To determine atrogin-1 and MuRF1 mRNA content, an RNAse protection assay (RPA) was used. Methods and primer sequences for these mRNAs along with L32 have been previously published by our laboratory (14, 22, 28). Liver and muscle IGF-I mRNA content was also determined by RPA, exactly as previously described by our laboratory (26, 30). Polyubiquitin mRNA was determined by Northern blot analysis, and normalization of RNA loading was assessed by 18S mRNA, exactly as previously described (28). Gels were exposed to a PhosphorImager screen (Molecular Dynamics, Sunnyvale, CA), and the resultant data were quantified using Molecular Dynamics’ ImageQuant software (ImageQuant ver. 5.2). All data were normalized to L32 or 18S mRNA.
Each gastrocnemius was homogenized in four volumes of ice-cold buffer containing (in mM) 20 HEPES, pH 7.4, 2 EGTA, 50 NaF, 100 KCl, 0.2 EDTA, 50 β-glycerophosphate, 10 N-ethylmaleimide, 0.1 PMSF, 1 benzamidine, and 0.5 sodium vanadate. Homogenates were centrifuged and Western blot analysis was performed exactly as previously described (22) to determine ubiquitin conjugates in muscle from control and alcohol-treated rats.
The concentration of total IGF-I in plasma was determined using a modified acid-ethanol (0.25 N HCl:87.5% ethanol) procedure with cryoprecipitation. Samples for total IGF-I were analyzed by RIA, as previously described (26, 30).
Statistical analysis.
Experimental data for each condition are summarized as means ± SE, and the number of animals in each treatment group is provided in the figure legends. For all of the studies in which the experimental protocol involved three or more groups, data were analyzed by InStat, ver. 3.05, statistical software package (GraphPad Software, San Diego, CA) using one-way ANOVA. When ANOVA indicated a significant overall effect, differences among individual means were assessed using the Student-Neuman-Keuls test. In some instances, the experimental protocol only involved two experimental groups, and then a Student's t-test was performed. Differences between the groups were considered significant when P < 0.05.
RESULTS
Alcohol-induced increases in atrogene expression.
Initial studies were performed to examine the time- and dose-response changes in atrogene mRNA expression in various striated muscles. Alcohol was injected at a dose (75 mmol/kg ip) previously demonstrated to decrease both cardiac and skeletal muscle (gastrocnemius, not soleus) protein synthesis (29, 47, 61). Time-matched controls were performed at each time point examined, and because there was no difference in the values for either atrogene between 0 and 8 h, these values have been combined and are represented in Fig. 1 as the “Ctr group.” In addition, a control value is also included for the 24-h time point and represents the effect of fasting for this period of time. The blood alcohol concentration (in mg/dl) was 411 ± 13, 261 ± 24, 63 ± 12, and nondetectable at the 1, 4, 8, and 24-h time points, respectively.
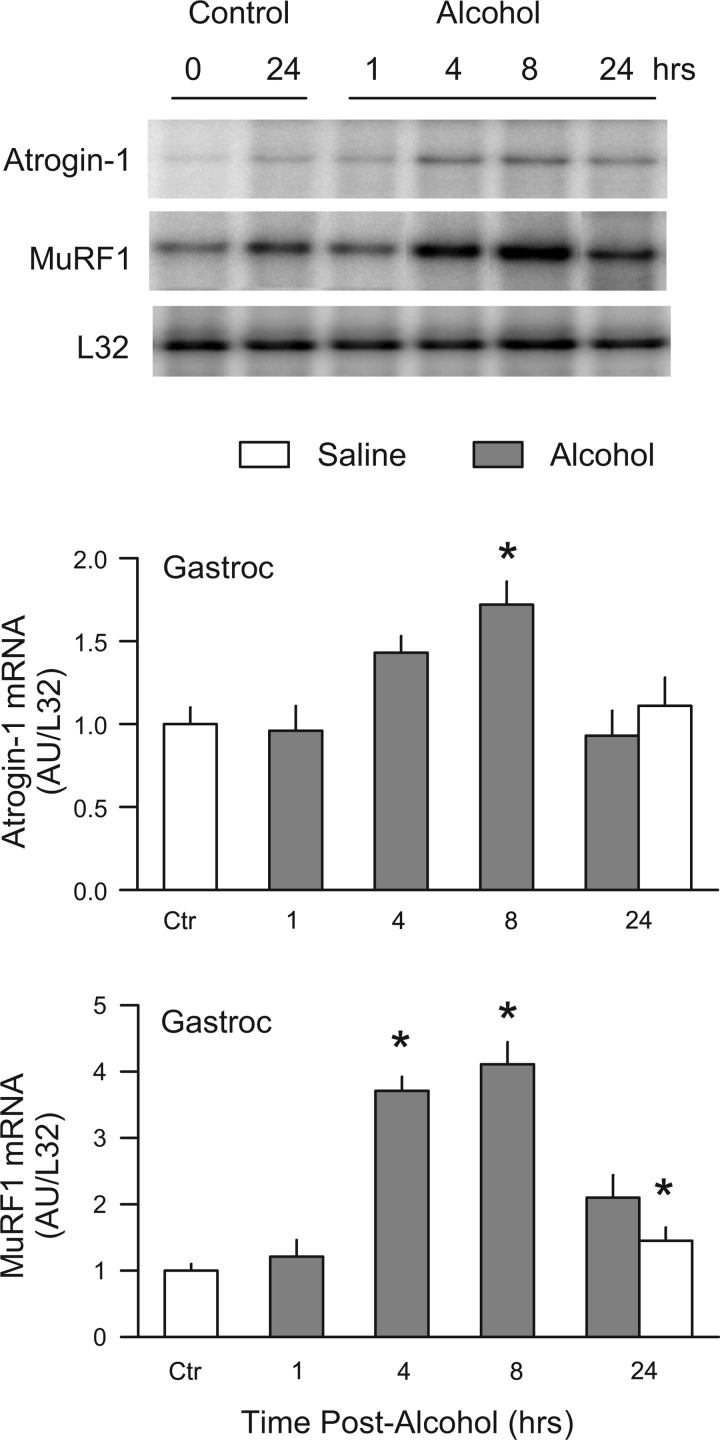
Fig. 1.
Time-dependent increase in atrogin-1 and MuRF1 mRNA in response to alcohol. Male, fed rats were injected IP with 75 mmol/kg of ethanol, and gastrocnemius sampled at 1, 4, 8, or 24 h thereafter, and either atrogin-1 or MuRF1 quantitated by RNAse protection assay (RPA). Time-matched control animals were included for each time point. The subsequent analysis revealed no differences in control muscle between 0 and 8 h, and therefore, these control values were pooled and are presented as the “Ctr” value. Inset: representative RPA for atrogin-1, MuRF1, and the loading control L32 mRNA. Values are expressed as means ± SE; n = 6 or 7 rats per group, except for the Ctr group, in which the sample size was 12. *P < 0.05 compared with Ctr group.
Acute alcohol intoxication increased the mRNA content of both atrogin-1 and MuRF1 in gastrocnemius, compared with control values (Fig. 1). The alcohol-induced increase in MuRF1 was observed at an earlier time point (i.e., 4 h) and was quantitatively greater than the increase observed for atrogin-1. An ethanol-induced increase in both atrogin-1 (70%) and MuRF1 (4-fold) was detected at 8 h, and this increase had returned to fasted control levels by 24 h. The alcohol-induced increase in atrogin-1 and MuRF1 also appeared to be dose dependent when mRNA content was determined at the 8-h time point (Fig. 2).
Fig. 2.
Dose-dependent increase in atrogin-1 and MuRF1 in response to alcohol. Male, fed rats were injected IP with either 0, 20, 50, or 75 mmol/kg body wt of ethanol and gastrocnemius (Gastroc) collected 8 h thereafter. Inset: representative RPA for atrogin-1, MuRF1, and the loading control L32 mRNA. Values are expressed as means ± SE; n = 6 or 7 rats per group. *P < 0.05 compared with “Ctr” group.
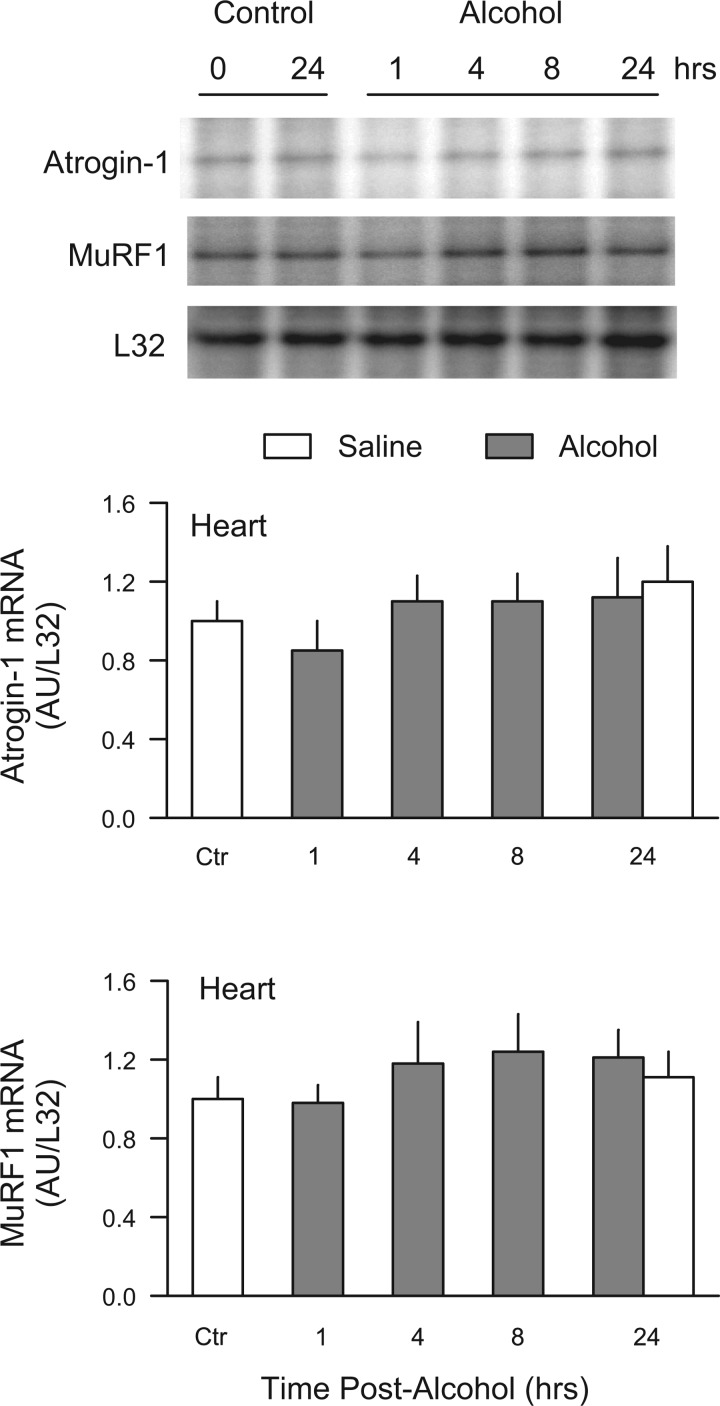
Heart and soleus muscle from the time course study described above were also analyzed. As illustrated in Fig. 3, we were unable to detect any significant change in the mRNA abundance for either atrogin-1 or MuRF1 for cardiac muscle by RPA. Moreover, no change in atrogene expression was observed in the predominantly slow-twitch soleus muscle (data not shown).
Fig. 3.
Time-dependent change in atrogin-1 and MuRF1 mRNA in heart in response to alcohol. Male, fed rats were injected IP with 75 mmol/kg of ethanol and ventricular muscle sampled at 1, 4, 8, or 24 h thereafter, and either atrogin-1 or MuRF1 quantitated by RPA. Time-matched control animals were included for each time point. The subsequent analysis revealed no differences in control muscle between 0 and 8 h, and therefore, these control values were pooled (i.e., “Ctr”). Inset: representative RPA for atrogin-1, MuRF1, and the loading control L32 mRNA. Values are expressed as means ± SE for n = 6 or 7 rats per group, except for the Ctr group where the sample size was 12. There were no statistically significant changes for either atrogene at the time points assessed.
Variables such as gender and nutritional status may influence the response of animals to alcohol and, therefore, these variables were assessed. Eight hours after administration, the blood alcohol concentrations were not significantly different between male and female rats (55 ± 9 vs. 42 ± 7 mg/dl) or between fed and fasted male rats (43 ± 8 vs. 49 ± 10 mg/dl). The ability of acute alcohol intoxication to increase MuRF1 mRNA in gastrocnemius was comparable in fasted male and female rats, as well as in fed and overnight fasted male rats, compared with appropriate control values (Table 1). Likewise, the alcohol-induced increases in atrogin-1 mRNA were also not different between male and female or in animals in the fed and fasted condition (data not shown).
Table 1.
Effect of gender, nutritional state, and alcohol metabolites on MuRF1 mRNA content in gastrocnemius in response to intraperitoneally administered alcohol
| Control | Alcohol | |
|---|---|---|
| Gender Study | ||
| Males | 1.00±0.12a | 3.89±0.35b |
| Females | 0.97±0.11a | 4.55±0.71b |
| Nutrition Study | ||
| Fasted | 1.00±0.07a | 5.21±0.63c |
| Fed | 0.73±0.05b | 3.87±0.53c |
| EtOH Metabolism Study | ||
| Control | 1.00±0.10a | 4.76±0.36b |
| 4-MP | 1.49±0.21a | 7.04±1.12b |
Values are expressed as means ± SE; n = 7 or 8 rats per treatment. Values represent arbitrary densitometric units from analysis of RNAse protection assay (RPA) for MuRF1 normalized for L32 (AU/L32). Similar alcohol (EtOH)-induced changes were detected for atrogin-1 (data not shown). 4-MP, 4-methylpyrazole.
Means with different letters are statistically different from one another within the same study (P < 0.05).
To assess whether the oxidation of alcohol was necessary for the previously mentioned effects, a separate study was performed in which fasted male rats were pretreated with the alcohol dehydrogenase inhibitor 4-MP. Administration of 4-MP markedly slowed the rate of ethanol elimination so the blood alcohol concentration determined after 8 h was significantly higher than in vehicle-treated rats given alcohol (261 ± 34 vs. 37 ± 13 mg/dl, respectively; P < 0.05). Injection of 4-MP alone into control animals appeared to increase MuRF1 mRNA of muscle from control rats, although this trend failed to achieve statistical significance (Table 1). Moreover, pretreatment of rats with 4-MP did not alter the alcohol-induced increase in MuRF1. Comparable increases in response to acute alcohol intoxication were also detected for atrogin-1 mRNA content within gastrocnemius (data not shown). These data suggest that the ability of ethanol to increase atrogene expression is mediated independently of its oxidative metabolism.
Oral ingestion of alcohol: acute and chronic.
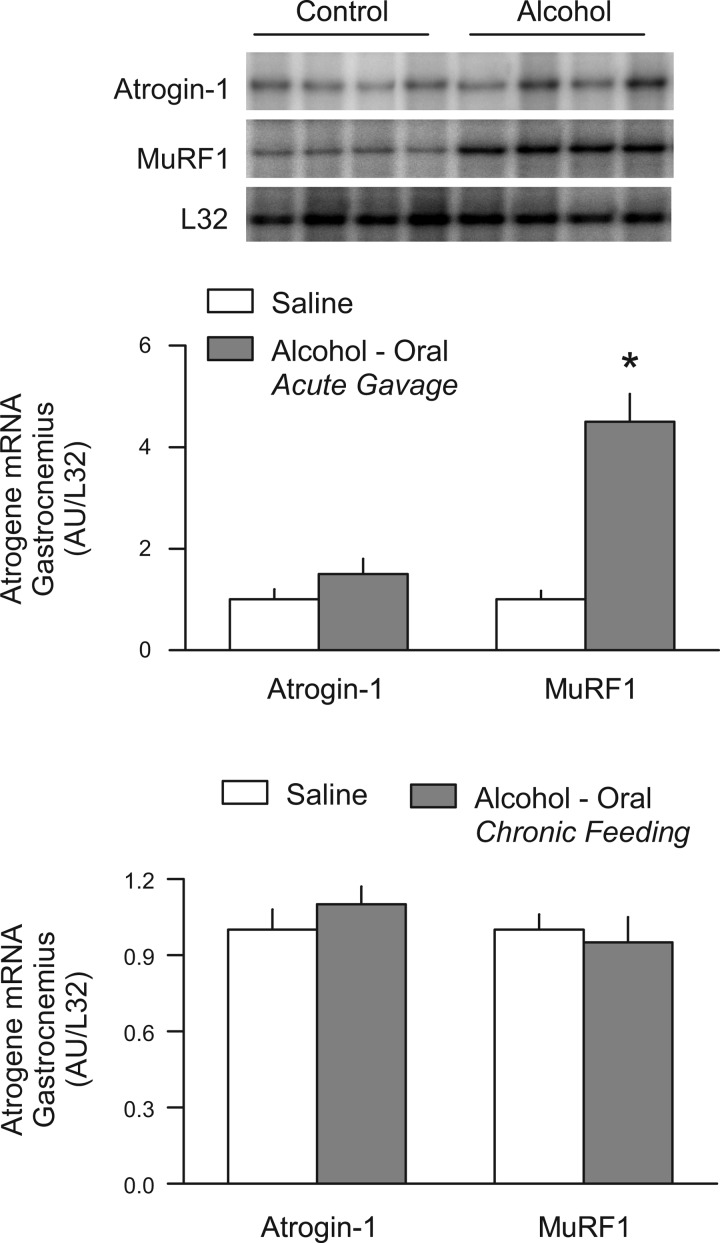
To determine whether the route of alcohol administration influences the increase in atrogene mRNA, animals were administered the same dose of alcohol (75 mmol/kg) by oral gavage. At the 8-h time point, the blood alcohol concentration averaged 33 ± 10 mg/dl in these animals. Acute oral ingestion of alcohol also increased MuRF1 (4.5-fold) in gastrocnemius, and this change was comparable to that seen in rats administered alcohol by intraperitoneal injection (Fig. 4, top and middle). In contrast, although orally administered alcohol tended to increase atrogin-1 mRNA, this change failed to achieve statistical significance because of the relatively large variability. However, the percentage increase above control values was quite similar (55%) to that observed in rats after intraperitoneal alcohol administration.
Fig. 4.
Increase in muscle atrogin-1 and MuRF1 mRNA after oral ingestion of alcohol. Top: representative RPA of atrogin-1, MuRF1, and L32 in gastrocnemius from male, fed rats administered 75 mmol/kg of alcohol via oral gavage. Middle: quantitation of all RPA data from rats administered alcohol by oral gavage where values are expressed as means ± SE for n = 6 or 7 rats per group. *P < 0.05 compared with time-matched control value. Bottom: quantitation of all RPA data determined on gastrocnemius of chronic (12–14 wk) alcohol-fed rats in which values are expressed as means ± SE for n = 10–12 per group.
In contradistinction, we failed to detect any change in either atrogin-1 or MuRF1 mRNA content in the gastrocnemius of rats consuming a nutritionally complete diet that contains ∼36% alcohol for a period of 12–14 wk, compared with control values from rats provided an isocaloric isonitrogenous diet (Fig. 4, bottom). Moreover, analysis of gastrocnemius from rats fed an alcohol-containing diet for 28 wk also did not reveal a difference in atrogene expression between alcohol-fed and pair-fed control animals (data not shown). As previously reported, animals fed the alcohol-containing diet for either 12–14 wk or 28 wk demonstrated a marked reduction in gastrocnemius mass and protein synthesis (26, 27, 33). Furthermore, no change in the mRNA expression of either atrogene was detected in hearts from rats chronically fed alcohol (data not shown).
Role of glucocorticoids and IGF-I in regulating muscle atrogenes.
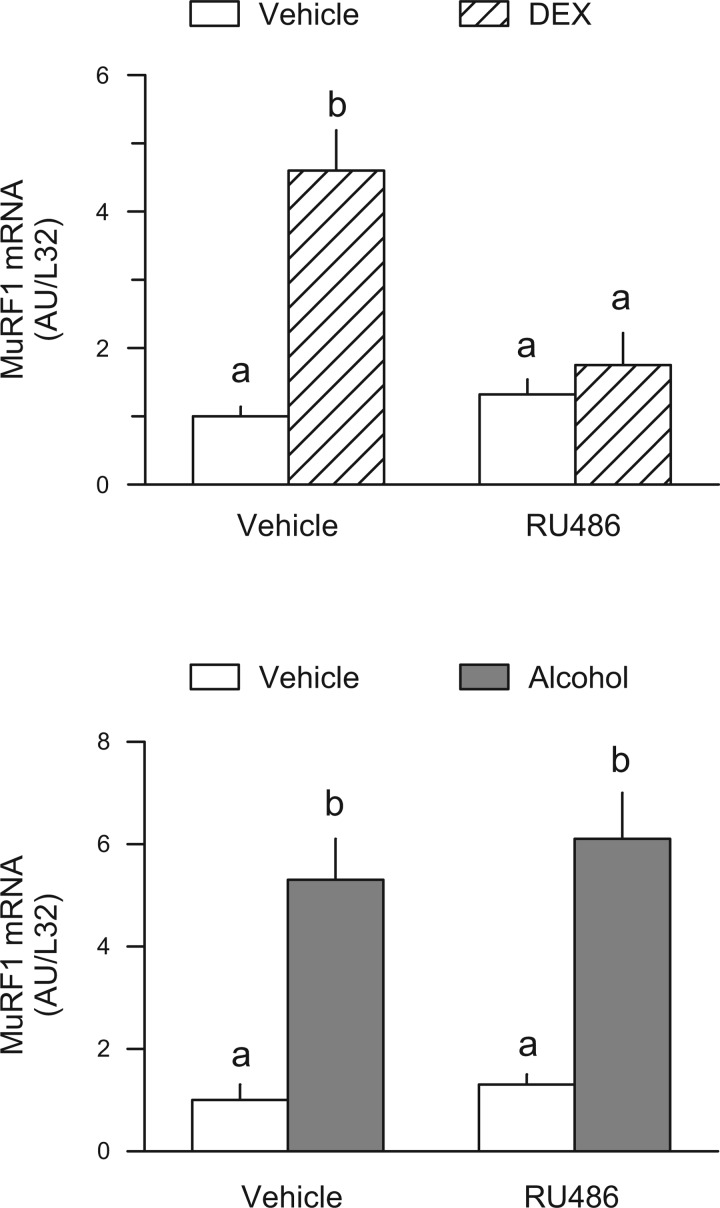
Acute alcohol intoxication has been reported to increase the plasma concentration of corticosterone (30). Moreover, the synthetic glucocorticoid dexamethasone upregulates the expression of atrogin-1 and MuRF1 both under in vivo and in vitro conditions (14, 23, 55), as well as stimulates muscle proteolysis (6, 35, 64). Therefore, we determined whether an elevation in endogenous glucocorticoids in response to alcohol was a physiological mediator for the increase in atrogin-1 and MuRF1. In vivo administration of dexamethasone increased the mRNA content for MuRF1, and this increase was essentially completely prevented in rats pretreated with the glucocorticoid receptor antagonist RU486 (Fig. 5, top). Despite the demonstrated efficacy of RU486, this antagonist was unable to ameliorate the alcohol-induced increase in MuRF1 (Fig. 5, bottom) suggesting the glucocorticoid-independent nature of the response. Similarly, although dexamethasone increased atrogin-1 in control animals, pretreatment with RU486 did not block the ability of alcohol to increase atrogin-1 mRNA (data not shown).
Fig. 5.
Role of exogenous and endogenous glucocorticoids in regulating muscle MuRF1 mRNA. Top: quantitation of RPAs for MuRF1 from gastrocnemius of rats injected with dexamethasone (DEX) alone or pretreated with the glucocorticoid receptor antagonist RU486. Bottom: quantitation of RPAs for MuRF1 mRNA in muscle of alcohol-treated (75 mmol/kg ip) rats pretreated with vehicle or RU486. Values are expressed as means ± SE for n = 5 or 6 (top) or 7 or 8 (bottom) rats per group. Values from the saline-injected control group were set at 1.0 AU. a,bMeans with different letters are statistically different from one another (P < 0.05).
A decrease in the circulating and tissue (e.g., liver and muscle) concentration of IGF-I is also seen in alcohol-treated rats (30, 34). Moreover, changes in IGF-I are inversely related to changes in both atrogene expression and rates of muscle proteolysis (25). Hence, changes in IGF-I may regulate the content of atrogin-1, MuRF1, and protein breakdown in a manner opposite to that reported for IGF-I and protein synthesis. Acute alcohol intoxication reduced the circulating concentration of total IGF-I by almost 30% (Fig. 6, top). This decline was prevented by the administration of the binary complex consisting of IGF-I and IGFBP-3. Alcohol also decreased the IGF-I mRNA content in gastrocnemius (Fig. 6, middle) and liver (data not shown). Injection of IGF-I into control animals reduced muscle IGF-I mRNA to the level seen in both groups of alcohol-treated animals. In control rats, IGF-I decreased the MuRF1 mRNA content by 45% (Fig. 6, bottom). In contrast, IGF-I failed to prevent the alcohol-induced increase in MuRF1 mRNA in skeletal muscle.
Fig. 6.
Role of IGF-I in the regulation of MuRF1. Immediately prior to the IP injection of alcohol (75 mmol/kg) or saline, rats were injected subcutaneously with a binary complex consisting of IGF-I and its natural binding partner, IGF binding protein-3, or an equal volume of saline. Blood and tissues were sampled 8 h after administration of alcohol. Values are expressed as means ± SE for n = 7 or 8 rats per group. For mRNA data, values from the saline-injected control group were set at 1.0 AU. a,b,cMeans with different letters are statistically different from one another (P < 0.05).
Alcohol-induced changes in muscle proteolysis.
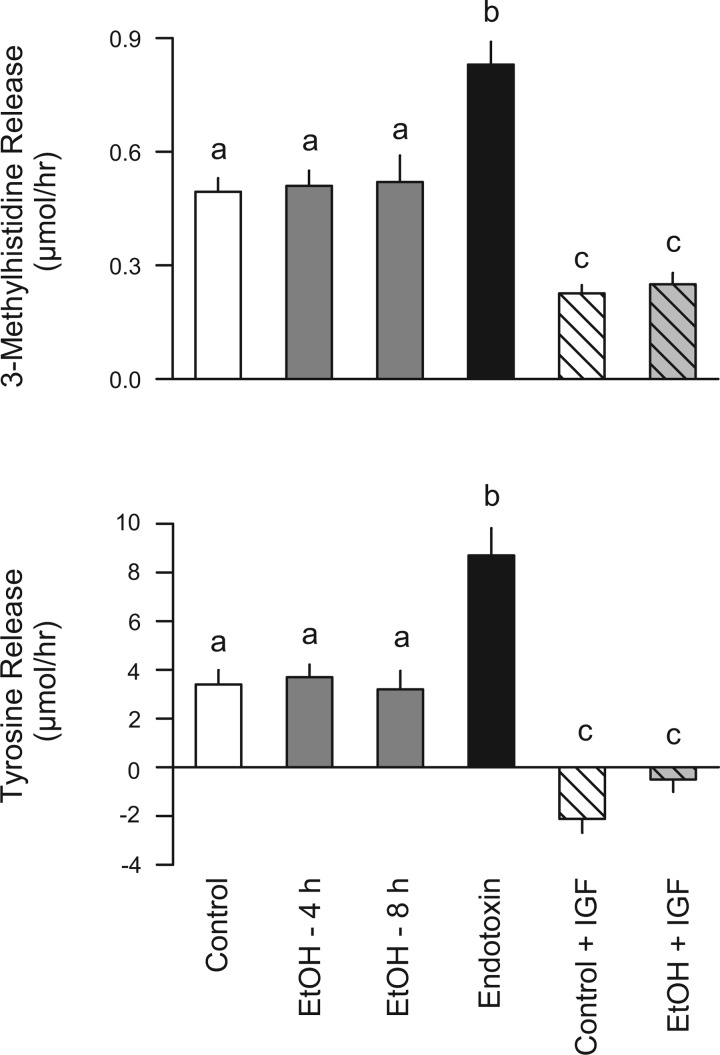
The above-mentioned data implied that acute alcohol intoxication increased the production of factors enhancing muscle protein breakdown. However, direct determinations of proteolysis were still lacking. To address this gap in our understanding, several complementary methods were used. In the first study, male rats that were fasted overnight were injected intraperitoneally with alcohol and the 3-MH release from the hindlimb determined either 4 h or 8 h later. To simulate in vivo conditions, hindlimbs from alcohol-treated rats were perfused with buffer containing alcohol at a concentration of ∼250 mg/dl to maintain the potential effect of alcohol during the course of the perfusion. As illustrated in Fig. 7 (top), in vivo ethanol exposure did not significantly enhance myofibrillar breakdown as measured by the net release of 3-MH in the perfused hindlimb. This lack of an effect cannot be attributed to methodological limitations because 3-MH release was increased 70% when assessed 4 h after in vivo injection of LPS. Furthermore, as an additional positive control, 3-MH release was also determined 24 h after induction of bacterial infection using a clinically relevant model of peritonitis, which has been previously shown to increase atrogene expression (14). Sepsis increased 3-MH release by 55%, compared with time-matched pair-fed control values (control = 0.61 ± 0.05 vs. septic = 0.94 ± 0.07 μmol/h; n = 5 per group; P < 0.05). Moreover, the inclusion of IGF-I in the perfusate for 2 h was capable of reducing 3-MH release by more than 50% in both control and alcohol-treated rats. In addition, total muscle protein breakdown, as measured by the net release of tyrosine from the perfused hindlimb, did not differ between control and alcohol-treated rats (Fig. 7, bottom). In contrast, when rats were treated in vivo with endotoxin as a positive control, we detected an ∼3-fold increase in tyrosine release. Conversely, when the hindlimb was perfused with buffer containing IGF-I for 2 h (as a negative control), the release of tyrosine was reduced in both control and alcohol-treated rats.
Fig. 7.
Effect of in vivo alcohol administration on 3-methylhistidine and tyrosine release by the isolated perfused hindlimb. Male overnight fasted rats were treated in vivo with alcohol (75 mmol/kg ip). Rats were anesthetized, and the hindlimb was isolated and perfused at either 4 h or 8 h thereafter. The perfusate was continuously oxygenated and recycled for the 2-h perfusion period, and the 3-methylhistidine concentration of the perfusate was determined at time 0 and 120 min. In alcohol-treated rats, the perfusate also contained ∼250 mg/dl of alcohol, the concentration of which remained relatively constant during the perfusion period (31). Time-matched control animals received an intraperitoneal injection of saline, and because there were no statistical differences among the various control groups, their values were combined. Positive control, rats injected in vivo with endotoxin. Negative control, control and alcohol-treated (4 h) rats perfused in situ with IGF-I (10 nM) for 2 h. Values are expressed as means ± SE for n = 8 or 9 rats per group. a,b,cMeans with different letters are statistically different from one another (P < 0.05).
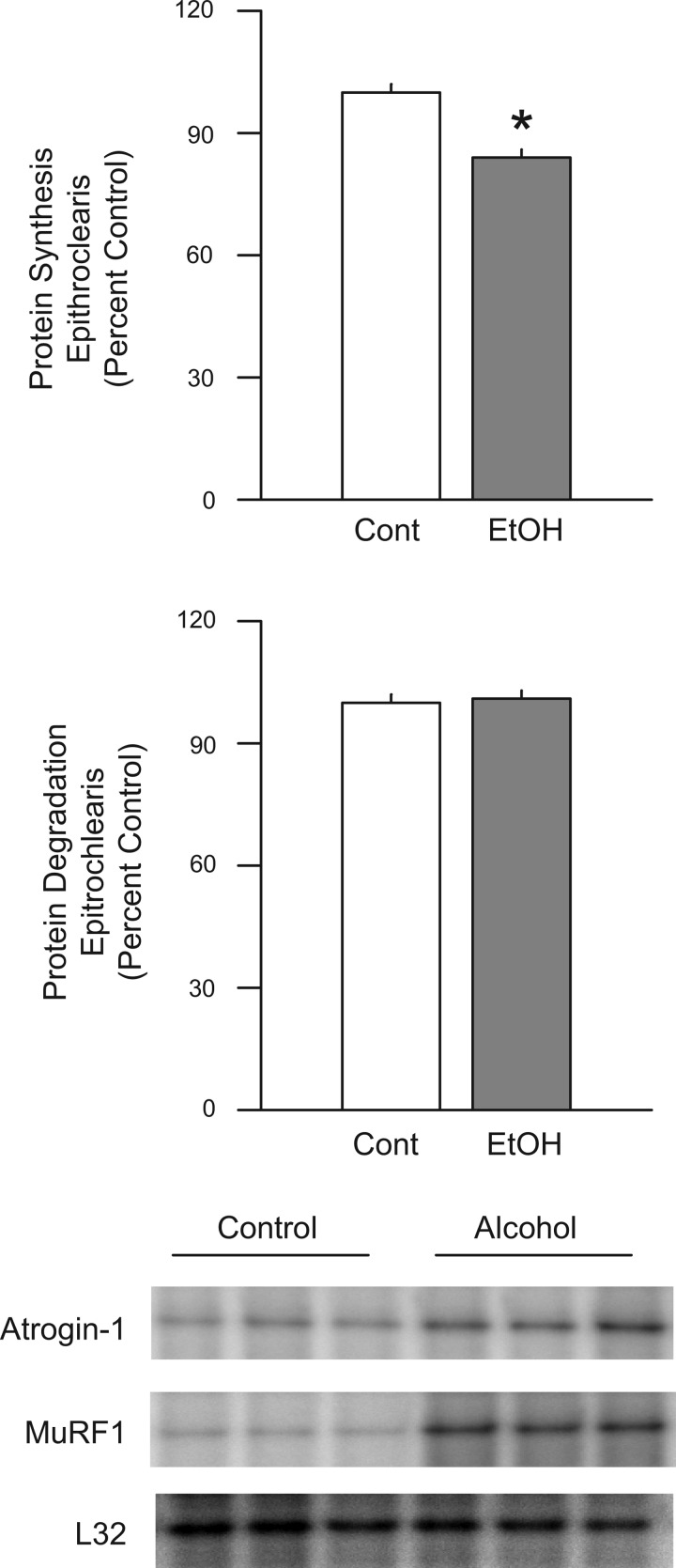
Additionally, we were unable to detect any significant change on the rate of proteolysis in the incubated epitrochlearis muscle from in vivo alcohol-treated rats, compared with control animals (Fig. 8). However, this experimental condition did result in a significant 16% reduction in protein synthesis. In a separate study, the in vivo administration of alcohol was shown to increase atrogin-1 (control = 1.00 ± 0.08 AU/L32 vs. alcohol = 1.73 ± 0.21 AU/L32; n = 7 per group; P < 0.05) and MuRF1 (1.00 ± 0.11 AU/L32 vs. 2.41 ± 0.37 AU/L32; n = 7 per group; P < 0.05) mRNA content in the epitrochlearis.
Fig. 8.
Effect of in vivo alcohol on protein balance in the incubated epitrochlearis muscle. Rats were administered alcohol in vivo (EtOH; 75 mmol/kg ip), and the epitrochlearis was isolated and incubated in vitro for the determination of protein synthesis (top) and protein degradation (middle). Values are expressed as means ± SE for n = 12 muscles per group. *P < 0.05 compared with time-matched control value. Bottom: representative RPA for atrogin-1, MuRF1, and the loading control L32 mRNA.
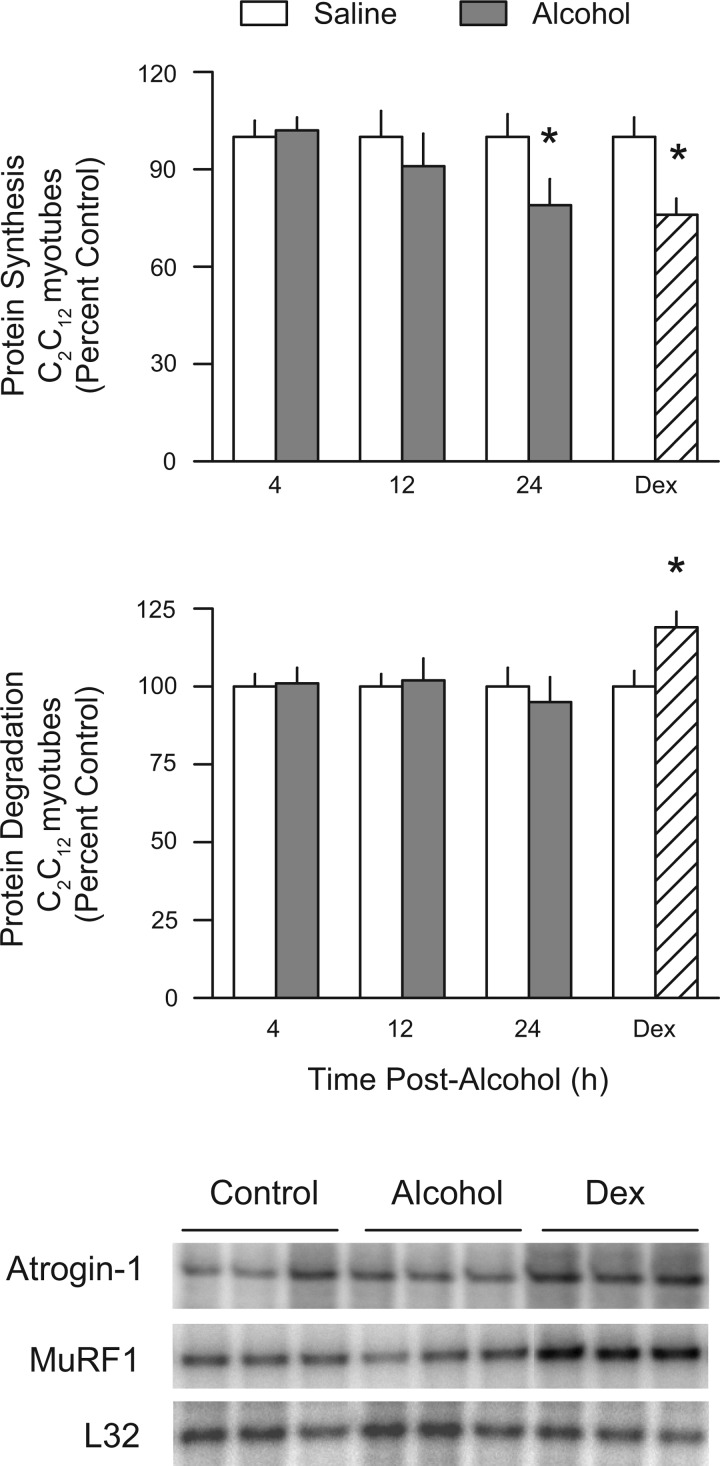
The direct effect of alcohol on proteolysis and atrogene expression was also assessed in vitro by incubating C2C12 myotubes with 80 mM ethanol for either 4, 12, or 24 h. This dose of alcohol was effective at reducing the rate of protein synthesis by 21% at the 24-h time point, compared with the time-matched control value (Fig. 9, top). In contrast, alcohol did not significantly decrease the rate of proteolysis (Fig. 9, middle). Furthermore, we detected no change in the mRNA content for either atrogin-1 or MuRF1 in myotubes incubated with alcohol for 4, 12, or 24 h (Fig. 9, bottom). As a positive control, cells were incubated with Dex for 24 h. Such a treatment decreased protein synthesis by 20%, increased protein degradation by 16%, and increased the mRNA content of both atrogin-1 and MuRF1 (Fig. 9).
Fig. 9.
Effect of alcohol on protein balance and atrogene expression in cultured C2C12 myotubes. Cells were incubated in the presence of ethanol (80 mM) for 4, 12, and 24 h, and values were compared with time-matched controls. Rates of protein synthesis (top) and protein degradation (middle) were determined in separate wells of cells. Additional myotubes were processed at the same time and showed no alcohol-induced change in atrogin-1 or MuRF1 mRNA content, compared with time-matched control values (bottom). Dexamethasone (Dex; 10 μM) was added to myotubes for 24 h and was used as a positive control. P < 0.05 compared to time-matched control value.
Finally, despite the lack of a change in muscle atrogene expression in chronically alcohol-fed rats, we also determined 3-MH release from the hindlimb of a small cohort of these animals. The release of 3-MH was not different in rats fed a nutritionally complete diet containing alcohol for 12–14 wk vs. time-matched control animals (0.29 ± 0.03 μmol/h vs. 0.31 ± 0.03 μmol/h; n = 5 each; P = not significant).
Polyubiquitin mRNA and protein.
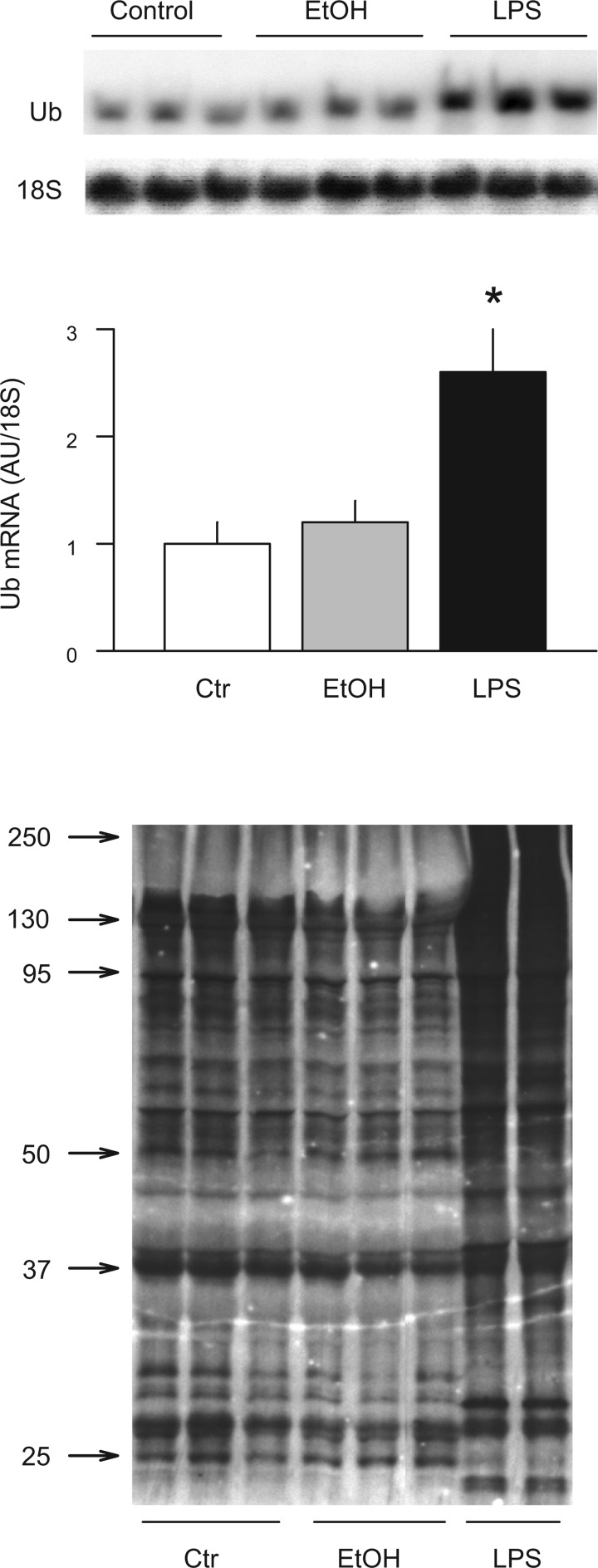
Finally, we also assessed the expression of Ub mRNA and protein in gastrocnemius because these surrogate markers appear to reliably and selectively reflect activation of Ub-proteasome-dependent proteolysis in atrophying skeletal muscle (55). As a positive control, we included gastrocnemius from endotoxin-treated rats, which showed a 2.7-fold increase in Ub mRNA in muscle by Northern blot analysis. In addition, Ub antibody immunoreactivity assessed by Western blot analysis was increased in response to endotoxin (Fig. 10). In contrast, no change in Ub mRNA or protein content was detected in gastrocnemius from rats administered alcohol intraperitoneally.
Fig. 10.
Effect of acute alcohol intoxication on mRNA content of ubiquitin (Ub) and the content of Ub conjugates in gastrocnemius. Muscle was collected 8 h after in vivo intraperitoneal administration of alcohol (EtOH; 75 mmol/kg) or endotoxin (LPS; 1 mg/kg). Top: representative Northern blot for polyubiquitin mRNA in rats injected with saline, EtOH, and LPS, respectively. Middle: Ub mRNA content was quantitated, and the data were expressed in AUs determined by densitometry and normalized to 18S. Values are means ± SE; n = 5 or 6 per group. Bottom: representative Western blot for Ub conjugates in gastrocnemius muscle. Approximate molecular weights are indicated in left margin. P < 0.05 compared to time-matched control value.
DISCUSSION
Protein balance within tissue and organs results from an orchestrated interaction of synthetic and degradative processes. The majority of research over the past decade has focused on understanding the mechanisms underlying alcohol-induced inhibition of protein synthesis. However, changes in the rate of proteolysis are clearly capable of altering protein balance. Because of its central role in the metabolism of ethanol, much of the previous research in this area relates to alcohol-induced changes in protein degradation in the liver. In this regard, chronic alcohol feeding has been reported to impair hepatic proteolysis when directly assessed by independent methods (8, 45). Moreover, a direct effect of alcohol on liver proteolysis has been demonstrated using the isolated perfused liver (46). In part, this alcohol-induced decrease in hepatic protein breakdown appears to result from an inhibition in the Ub-proteasome proteolytic pathway (1, 3, 9, 16). As the proteasome system serves to degrade oxidatively damaged proteins and abnormal proteins, this impairment has been speculated to be participatory in the development of alcoholic liver disease (44). In addition, acute alcohol intoxication also decreases the activity of various hepatic lysosomal enzymes (52).
In contrast to the relatively definitive data pertaining to hepatic proteolysis discussed above, the effect of alcohol on protein degradation in skeletal muscle is unclear and has been primarily defined using surrogate markers. For example, urinary excretion of 3-MH, which is a marker of myofibrillar degradation, has been reported to be both increased (46, 53, 53) and decreased (40) in response to chronic alcohol consumption. Moreover, the fractional rate of breakdown, estimated as the difference between muscle protein accretion and the fractional rate of protein synthesis, was calculated to be decreased in chronically alcohol-fed rats (50). However, all of these previous studies have only provided indirect estimates of muscle protein degradation. In contrast, our current data demonstrate that muscle proteolysis is not altered by either acute alcohol intoxication or chronic alcohol feeding. To assess proteolysis, we determined 3-MH release and tyrosine release using the isolated perfused hindlimb and thereby circumvented the major limitation of this technique in rats, which is the release of 3-MH from nonmuscle tissues (18). Our current data clearly indicate that there was no increase in the hindlimb release of 3-MH or tyrosine at 4–8 h after the acute administration of alcohol or after 12–14 wk of chronic alcohol consumption, compared with time-matched and nutritionally equivalent control animals. The use of positive (e.g., endotoxin and sepsis) and negative (e.g., IGF-I) controls suggests the difference between our current data and previous studies is not related to our ability to detect a change in 3-MH release and hence proteolysis. Furthermore, no effect of alcohol on proteolysis was detected in the isolated epitrochlearis muscle or in cultured C2C12 myotubes. Finally, acute alcohol intoxication also failed to alter either polyUb mRNA content or the extent of Ub conjugation in skeletal muscle, compared with control animals. Collectively, these data strongly suggest that alcohol does not modulate protein degradation and that its ability to negatively impact muscle protein balance results primarily from its inhibition of protein synthesis.
Despite the inability of ethanol to modulate muscle proteolysis, acute alcohol intoxication dramatically upregulated the expression of MuRF1 and, to a lesser extent, atrogin-1. These E3 ligases are known to target substrates for proteolysis by the ATP-dependent proteasome. Although orally administered alcohol has been shown to increase atrogin-1 and MuRF1 mRNA ∼12 h after gavage (59), the current study characterized the temporal progression for that change. The alcohol-induced increase in atrogene expression appeared maximal between 4 and 8 h after alcohol administration; however, values had returned to basal levels by 24 h. Moreover, food restriction after alcohol administration was not responsible for this increase. Of note, this increase in atrogin-1 and MuRF1 appeared restricted to predominantly fast-twitch muscles such as gastrocnemius and epitrochlearis, with no change detected in either the slow-twitch soleus or heart. On the one hand, it is clear that alcohol, as opposed to one of its oxidative metabolites, is responsible for the increase in muscle atrogene expression. However, on the other hand, we have been unable to determine whether alcohol acts directly on muscle or via the production of some yet unidentified secondary mediator. Because of the limited viability of the experimental models (∼2 h), we were unable to determine whether alcohol directly increases either atrogin-1 or MuRF1 in the isolated perfused hindlimb or in the incubated epitrochlearis muscles. However, incubation of myotubes for various periods of time up to 24 h failed to increase atrogene mRNA, suggesting the effect of alcohol is mediated indirectly. In contrast, chronic alcohol consumption as part of a nutritionally complete diet failed to increase either atrogin-1 or MuRF1. The reason for this difference is unknown but may be related to the lower blood alcohol levels achieved in the chronic, alcohol-fed model. Alternatively, chronically alcohol-fed rats may become desensitized to the effect of alcohol as a result of repeated ethanol exposure, leading to the development of tachyphylaxis.
In initial studies, we examined the increase in glucocorticoids and the decrease in IGF-I as putative secondary mediators. Several independent groups have reported that the synthetic glucocorticoid, dexamethasone, increases atrogin-1 and MuRF1 (2, 5, 15, 23). In the current study, we were able to prevent such an increase by pretreating rats with RU486. Despite the proven efficacy of the drug, RU486 failed to prevent the alcohol-induced increase in atrogene expression. These data are in contrast to the sepsis-induced increase in atrogene mRNA, which is glucocorticoid dependent (66). Moreover, alcohol decreases the circulating concentration of IGF-I via inhibition of its synthesis in both liver and skeletal muscle. Both in vivo and in vitro studies indicate that atrogene mRNA content is inversely proportional to the prevailing IGF-I concentration (7, 28, 55). However, although we could prevent the alcohol-induced reduction in blood IGF-I by the injection of a binary complex consisting of IGF-I and IGFBP-3, the increase in atrogin-1 and MuRF1 mRNA was not ameliorated. These results differ from those of other investigators, which showed that in vivo repletion of IGF-I could largely prevent the increase in atrogin-1 produced by diabetes (7) and that the localized overexpression of IGF-I in muscle blocked the increase in atrogene mRNA and muscle wasting produced by angiotensin (57). Therefore, although the identity of the secondary mediator is unknown, there is little evidence to support the causative role of either glucocorticoids or IGF-I for the alcohol-induced increase in atrogene expression.
Increased atrogene expression has been observed in a number of catabolic conditions and is closely associated with the loss of muscle protein (17, 35). Moreover, inhibition of proteasomal activity, in general, or the genetic deletion of these specific genes prevents or attenuates the atrophic response produced by various catabolic insults (2, 4, 13, 22), thereby confirming their relative importance. In contradistinction to these results, our data strongly suggest that the alcohol-induced increase in atrogin-1 and MuRF1 mRNA is causally “unrelated” to a change in protein degradation. We can only speculate as to a possible explanation for this discordant regulation between atrogene mRNA expression and muscle proteolysis after acute alcohol exposure. Alternatives include, but are not limited to, the possibility that 1) atrogin-1 and MuRF1 mRNAs are not efficiently translated into protein in this model, in which peptide-chain initiation is impaired (24, 31); 2) atrogene mRNA and protein are both upregulated, but other components of the proteolytic pathway, such as calpains (11), are actually rate-limiting; and/or 3) muscle proteolysis only results when the stress-induced increase in one or both atrogenes is of a magnitude and duration greater than that seen after alcohol. Although direct evidence is lacking, we favor the latter alternative because the upregulation of atrogin-1 and MuRF1 mRNA produced by denervation-induced muscle atrophy was markedly greater and certainly more sustained than that seen after acute alcohol intoxication (2). Furthermore, in the denervation model, the protection from muscle loss was greatest in mice lacking both atrogin-1 and MuRF1, as opposed to mice lacking only one of these atrogenes. Hence, we postulate that the alcohol-induced increase in MuRF1 and atrogin-1, the latter of which was especially modest, failed to reach some critical threshold, either in terms of magnitude or duration, to produce a sustained and detectable increase in muscle proteolysis. Finally, we cannot exclude the possibility that the alcohol-induced increase in atrogene expression is causally related to increased degradation of a specific protein or set of proteins and that more global estimates of proteolysis failed to detect such a change. In this regard, MuRF1 has been recently demonstrated to be the E3 ligase responsible for the degradation of myosin heavy chain protein in dexamethasone-treated myotubes (5).
One potential caveat regarding the interpretation of the data from the current study is that the effect of alcohol on muscle atrogene expression and proteolysis was determined under basal or nonstressed conditions. This may be of importance because alcohol has been reported to interact with other atrophic stimuli, such as immobilization, to produce synergistic increases in atrogin-1 and MuRF1 (59). Under such conditions, pretreatment of animals with the proteasome inhibitor Velcade appears to largely prevent the disuse atrophy produced by the combination of alcohol and immobilization. Therefore, while alcohol does not appear to stimulate muscle proteolysis under basal conditions, it remains possible that an increase in protein degradation plays a more prominent role under selective conditions, in which alcohol sensitizes muscle to catabolic stimuli. However, a systematic investigation of this synergistic effect is beyond the scope of the current investigation.
Perspectives and Significance
Catabolic insults produce muscle atrophy by altering either protein synthesis and/or degradation. Although the relative importance of these processes has been defined in other catabolic conditions, there is a paucity of information related to whether alcohol stimulates muscle proteolysis per se. Our previous work indicated that acute alcohol intoxication increased atrogin-1 and MuRF1 mRNA expression in gastrocnemius, thereby implying an enhanced rate of proteolysis. However, collectively, our current data do not support such an alcohol-induced change in protein breakdown. That is, while acute alcohol intoxication upregulates atrogen expression, no change in the rate of muscle proteolysis was detected in the perfused hindlimb or in the isolated epitrochlearis muscle in response to alcohol. In addition, the skeletal muscle myopathy produced by chronic alcohol consumption was associated with neither a change in atrogene expression nor a change in muscle proteolysis. Therefore, are data clearly indicate that muscle proteolysis is not increased by either acute alcohol intoxication or chronic alcohol consumption in rats. Hence, the atrophic response produced by long-term alcohol abuse appears to be primarily, if not exclusively, due to a decrease in the synthetic side of the protein balance equation. Finally, our data suggest that an increase in atrogin-1 and MuRF1 mRNA is not necessarily predictive of a coordinate change in proteolysis and that the studies using atrogene expression as the sole surrogate marker of degradation should be interpreted with caution.
Acknowledgments
The authors thank Danuta Huber and Jay Nystrom for their assistance with this project. This work was supported in part by grants from the National Institutes of Health AA-11290 (C. H. Lang) and AA-12814 (T. C. Vary).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bardag-Gorce F, Venkatesh R, Li J, French BA, French SW. Hyperphosphorylation of rat liver proteasome subunits: the effects of ethanol and okadaic acid are compared. Life Sci 75: 585–597, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Born LJ, Kharbanda KK, McVicker DL, Zetterman RK, Donohue TM Jr. Effects of ethanol administration on components of the ubiquitin proteolytic pathway in rat liver. Hepatology 23: 1556–1563, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Cai D, Frantz JD, Tawa NE Jr, Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ and Shoelson SE. IKKβ/NF-κB activation causes severe muscle wasting in mice. Cell 119: 285–298, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Clarke BA, Drujan D, Willis MS, Murphy LO, Corpina RA, Burova E, Rakhilin SV, Stitt TN, Patterson C, Latres E, Glass DJ. The E3 Ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab 6: 376–385, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Dardevet D, Sornet C, Savary I, Debras E, Patureau-Mirand P, Grizard J. Glucocorticoid effects on insulin- and IGF-I-regulated muscle protein metabolism during aging. J Endocrinol 156: 83–89, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Dehoux M, Van BR, Pasko N, Lause P, Verniers J, Underwood L, Ketelslegers JM, Thissen JP. Role of the insulin-like growth factor I decline in the induction of atrogin-1/MAFbx during fasting and diabetes. Endocrinology 145: 4806–4812, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Donohue TM, Zetterman RK and Tuma DJ. Effect of chronic ethanol administration on protein catabolism in rat liver. Alcohol Clin Exp Res 13: 49–57, 1989. [DOI] [PubMed] [Google Scholar]
- 9.Donohue TM, Zetterman RK, Zhang-Gouillon ZQ, French SW. Peptidase activities of the multicatalytic protease in rat liver after voluntary and intragastric ethanol administration. Hepatology 28: 486–491, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Fan J, Wojnar MM, Theodorakis M, Lang CH. Regulation of insulin-like growth factor (IGF)-I mRNA and peptide and IGF-binding proteins by interleukin-1. Am J Physiol Regul Integr Comp Physiol 270: R621–R629, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Fareed MU, Evenson AR, Wei W, Menconi M, Poylin V, Petkova V, Pignol B, Hasselgren PO. Treatment of rats with calpain inhibitors prevents sepsis-induced muscle proteolysis independent of atrogin-1/MAFbx and MuRF1 expression. Am J Physiol Regul Integr Comp Physiol 290: R1589–R1597, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Sola J, Estruch R, Grau JM, Pare JC, Rubin E, Urbano-Marquez A. The relation of alcoholic myopathy to cardiomyopathy. Ann Intern Med 120: 529–536, 1994. [DOI] [PubMed] [Google Scholar]
- 13.Fischer D, Gang G, Pritts T, Hasselgren PO. Sepsis-induced muscle proteolysis is prevented by a proteasome inhibitor in vivo. Biochem Biophys Res Commun 270: 215–221, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Frost RA, Nystrom GJ, Jefferson LS, Lang CH. Hormone, cytokine, and nutritional regulation of sepsis-induced increases in atrogin-1 and MuRF1 in skeletal muscle. Am J Physiol Endocrinol Metab 292: E501–E512, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA 98: 14440–14445, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutala R, Wang J, Kadapakkam S, Hwang Y, Ticku M, Li MD. Microarray analysis of ethanol-treated cortical neurons reveals disruption of genes related to the ubiquitin-proteasome pathway and protein synthesis. Alcohol Clin Exp Res 28: 1779–1788, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Hasselgren PO, Wray C, Mammen J. Molecular regulation of muscle cachexia: it may be more than the proteasome. Biochem Biophys Res Commun 290: 1–10, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Haverberg LN, Omstedt PT, Munro HN, Young VR. Ntau-methylhistidine content of mixed proteins in various rat tissues. Biochim Biophys Acta 405: 67–71, 1975. [DOI] [PubMed] [Google Scholar]
- 19.Hong-Brown LQ, Brown CR, Huber DS, Lang CH. Alcohol regulates eukaryotic elongation factor 2 phosphorylation via an AMP-activated protein kinase-dependent mechanism in C2C12 skeletal myocytes. J Biol Chem 282: 3702–3712, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Hong-Brown LQ, Pruznak AM, Frost RA, Vary TC, Lang CH. Indinavir alters regulators of protein anabolism and catabolism in skeletal muscle. Am J Physiol Endocrinol Metab 289: E382–E390, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Jefferson LS, Li JB, Rannels SR. Regulation by insulin of amino acid release and protein turnover in the perfused rat hemicorpus. J Biol Chem 252: 1476–1483, 1977. [PubMed] [Google Scholar]
- 22.Krawiec BJ, Frost RA, Vary TC, Jefferson LS, Lang CH. Hindlimb casting decreases muscle mass in part by proteasome-dependent proteolysis but independent of protein synthesis. Am J Physiol Endocrinol Metab 289: E969–E980, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Krawiec BJ, Nystrom GJ, Frost RA, Jefferson LS, Lang CH. AMP-activated protein kinase agonists increase mRNA content of the muscle-specific ubiquitin ligases MAFbx and MuRF1 in C2C12 cells. Am J Physiol Endocrinol Metab 292: E1555–E1567, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Lang CH, Frost RA, Deshpande N, Kumar V, Vary TC, Jefferson LS, Kimball SR. Alcohol impairs leucine-mediated phosphorylation of 4E-BP1, S6K1, eIF4G, and mTOR in skeletal muscle. Am J Physiol Endocrinol Metab 285: E1205–E1215, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Lang CH, Frost RA, Summer AD, Vary TC. Molecular mechanisms responsible for alcohol-induced myopathy in skeletal muscle and heart. Int J Biochem Cell Biol 37: 2180–2195, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Lang CH, Frost RA, Svanberg E, Vary TC. IGF-I/IGFBP-3 ameliorates alterations in protein synthesis, eIF4E availability, and myostatin in alcohol-fed rats. Am J Physiol Endocrinol Metab 286: E916–E926, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Lang CH, Frost RA, Vary TC. Skeletal muscle protein synthesis and degradation exhibit sexual dimorphism after chronic alcohol consumption but not acute intoxication. Am J Physiol Endocrinol Metab 292: E1497–E1506, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Lang CH, Huber D, Frost RA. Burn-induced increase in atrogin-1 and MuRF-1 in skeletal muscle is glucocorticoid independent but downregulated by IGF-I. Am J Physiol Regul Integr Comp Physiol 292: R328–R336, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Lang CH, Kumar V, Liu X, Frost RA, Vary TC. IGF-I induced phosphorylation of S6K1 and 4E-BP1 in heart is impaired by acute alcohol intoxication. Alcohol Clin Exp Res 27: 485–494, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Lang CH, Liu X, Nystrom G, Wu D, Cooney RN, Frost RA. Acute effects of growth hormone in alcohol-fed rats. Alcohol Alcohol 35: 148–158, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Lang CH, Pruznak AM, Deshpande N, Palopoli MM, Frost RA, Vary TC. Alcohol intoxication impairs phosphorylation of S6K1 and S6 in skeletal muscle independently of ethanol metabolism. Alcohol Clin Exp Res 28: 1758–1767, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Lang CH, Silvis C, Nystrom G, Frost RA. Regulation of myostatin by glucocorticoids after thermal injury. FASEB J 15: 1807–1809, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Lang CH, Wu D, Frost RA, Jefferson LS, Kimball SR, Vary TC. Inhibition of muscle protein synthesis by alcohol is associated with modulation of eIF2B and eIF4E. Am J Physiol Endocrinol Metab 277: E268–E276, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Lang CH, Wu D, Frost RA, Jefferson LS, Vary TC, Kimball SR. Chronic alcohol feeding impairs hepatic translation initiation by modulating eIF2 and eIF4E. Am J Physiol Endocrinol Metab 277: E805–E814, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Lecker SH, Goldberg AL, Mitch WE. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J Am Soc Nephrol 17: 1807–1819, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J 18: 39–51, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Li HH, Kedar V, Zhang C, McDonough H, Arya R, Wang DZ, Patterson C. Atrogin-1/muscle atrophy F-box inhibits calcineurin-dependent cardiac hypertrophy by participating in an SCF ubiquitin ligase complex. J Clin Invest 114: 1058–1071, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li TK, Theorell H. Human liver alcohol dehydrogenase: inhibition by pyrazole and pyrazole analogs. Acta Chem Scand 23: 892–902, 1969. [DOI] [PubMed] [Google Scholar]
- 39.Mao J, Regelson W, Kalimi M. Molecular mechanism of RU 486 action: a review. Mol Cell Biochem 109: 1–8, 1992. [DOI] [PubMed] [Google Scholar]
- 40.Martin FC, Peters TJ. Assessment in vitro and in vivo of muscle degradation in chronic skeletal muscle myopathy of alcoholism. Clin Sci (Lond) 68: 693–700, 1985. [DOI] [PubMed] [Google Scholar]
- 41.McNurlan MA, Garlick PJ, Steigbigel RT, Decristofaro KA, Frost RA, Lang CH, Johnson RW, Santasier AM, Cabahug CJ, Fuhrer J, Gelato MC. Responsiveness of muscle protein synthesis to growth hormone administration in HIV-infected individuals declines with severity of disease. J Clin Invest 100: 2125–2132, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Milakofsky L, Miller JM, Vogel WH. Effects of acute ethanol administration on rat plasma amino acids and related compounds. Biochem Pharmacol 35: 3885–3888, 1986. [DOI] [PubMed] [Google Scholar]
- 43.Molina PE, Lang CH, Bagby GJ, Spitzer JJ. Ethanol oxidation is not required to attenuate endotoxin-enhanced glucose metabolism. Am J Physiol Regul Integr Comp Physiol 260: R1058–R1065, 1991. [DOI] [PubMed] [Google Scholar]
- 44.Osna NA, Donohue TM Jr. Implication of altered proteasome function in alcoholic liver injury. World J Gastroenterol 13: 4931–4937, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poso AR, Hirsimaki P. Inhibition of proteolysis in the liver by chronic ethanol feeding. Biochem J 273: 149–152, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Preedy VR, Adachi J, Peters TJ, Worrall S, Parkkila S, Niemela O, Asamo M, Ueno Y, Takeda K, Yamauchi M, Sakamoto K, Takagi M, Nakajima H, Toda G. Recent advances in the pathology of alcoholic myopathy. Alcohol Clin Exp Res 25: 54S–59S, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Preedy VR, Keating JW, Peters TJ. The acute effects of ethanol and acetaldehyde on rates of protein synthesis in type I and type II fibre-rich skeletal muscles of the rat. Alcohol Alcohol 27: 241–251, 1992. [PubMed] [Google Scholar]
- 48.Preedy VR, Ohlendieck K, Adachi J, Koll M, Sneddon A, Hunter R, Rajendram R, Mantle D, Peters TJ. The importance of alcohol-induced muscle disease. J Muscle Res Cell Motil 24: 55–63, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Preedy VR, Peters TJ. The effect of chronic ethanol feeding on body and plasma composition and rates of skeletal muscle protein turnover in the rat. Alcohol Alcohol 23: 217–224, 1988. [PubMed] [Google Scholar]
- 50.Preedy VR, Peters TJ. The effect of chronic ethanol ingestion on synthesis and degradation of soluble, contractile and stromal protein fractions of skeletal muscles from immature and mature rats. Biochem J 259: 261–266, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reilly ME, Mantle D, Richardson PJ, Salisbury J, Jones J, Peters TJ, Preedy VR. Studies on the time-course of ethanol's acute effects on skeletal muscle protein synthesis: comparison with acute changes in proteolytic activity. Alcohol Clin Exp Res 21: 792–798, 1997. [PubMed] [Google Scholar]
- 52.Reilly ME, Mantle D, Salisbury J, Peters TJ, Preedy VR. Comparative effects of acute ethanol dosage on liver and muscle protein metabolism. Biochem Pharmacol 60: 1773–1785, 2000. [DOI] [PubMed] [Google Scholar]
- 53.Reinus JF, Heymsfield SB, Wiskind R, Casper K, Galambos JT. Ethanol: relative fuel value and metabolic effects in vivo. Metabolism 38: 125–135, 1989. [DOI] [PubMed] [Google Scholar]
- 54.Roos-Mattjus P, Sistonen L. The ubiquitin-proteasome pathway. Ann Med 36: 285–295, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Sacheck JM, Ohtsuka A, McLary SC, Goldberg AL. IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am J Physiol Endocrinol Metab 287: E591–E601, 2004. [DOI] [PubMed] [Google Scholar]
- 56.Shah OJ, Anthony JC, Kimball SR, Jefferson LS. Glucocorticoids oppose translational control by leucine in skeletal muscle. Am J Physiol Endocrinol Metab 279: E1185–E1190, 2000. [DOI] [PubMed] [Google Scholar]
- 57.Song YH, Li Y, Du J, Mitch WE, Rosenthal N, Delafontaine P. Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. J Clin Invest 115: 451–458, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tiernan JM, Ward LC. Acute effects of ethanol on protein synthesis in the rat. Alcohol Alcohol 21: 171–179, 1986. [PubMed] [Google Scholar]
- 59.Vargas R, Lang CH. Alcohol accelerates loss of muscle and impairs recovery of muscle mass resulting from disuse atrophy. Alcohol Clin Exp Res 32: 128–137, 2008. [DOI] [PubMed] [Google Scholar]
- 60.Vary TC, Dardevet D, Grizard J, Voisin L, Buffiere C, Denis P, Breuille D, Obled C. Differential regulation of skeletal muscle protein turnover by insulin and IGF-I after bacteremia. Am J Physiol Endocrinol Metab 275: E584–E593, 1998. [DOI] [PubMed] [Google Scholar]
- 61.Vary TC, Deiter G, Goodman SA. Acute alcohol intoxication enhances myocardial eIF4G phosphorylation despite reducing mTOR signaling. Am J Physiol Heart Circ Physiol 288: H121–H128, 2005. [DOI] [PubMed] [Google Scholar]
- 62.Vary TC, Nairn AC, Lang CH. Restoration of protein synthesis in heart and skeletal muscle after withdrawal of alcohol. Alcohol Clin Exp Res 28: 517–525, 2004. [DOI] [PubMed] [Google Scholar]
- 63.Waalkes TP, Udenfriend S. A fluorometric method for the estimation of tyrosine in plasma and tissues. J Lab Clin Med 50: 733–736, 1957. [PubMed] [Google Scholar]
- 64.Wang L, Luo GJ, Wang JJ, Hasselgren PO. Dexamethasone stimulates proteasome- and calcium-dependent proteolysis in cultured L6 myotubes. Shock 10: 298–306, 1998. [DOI] [PubMed] [Google Scholar]
- 65.Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock–a review of laboratory models and a proposal. J Surg Res 29: 189–201, 1980. [DOI] [PubMed] [Google Scholar]
- 66.Wray CJ, Mammen JM, Hershko DD, Hasselgren PO. Sepsis upregulates the gene expression of multiple ubiquitin ligases in skeletal muscle. Int J Biochem Cell Biol 35: 698–705, 2003. [DOI] [PubMed] [Google Scholar]