Abstract
Background
Neurological rehabilitation after stroke lowers rates of death, dependency, and institutionalization. Little research has yet addressed the factors affecting the selection of ischemic stroke patients for rehabilitative treatment.
Method
The database for this study consisted of all cases of ischemic stroke (ICD-10 code I63) that occurred in 2010 and 2011 in the neurological inpatient care facilities participating in the “Stroke Register Northwest Germany” quality assurance project. A primary target group for rehabilitation was defined a priori (Barthel Index at discharge ≤ 65, no premorbid nursing dependency, no transfer to another acute-care hospital after initial treatment of stroke). Among these patients, factors potentially affecting the provision of rehabilitative treatment were studied with binary logistic regression and multilevel logistic regression.
Results
There were 96 955 cases of ischemic stroke in the 127 participating hospitals. 40.8% and 11.4% of these patients underwent neurological and geriatric rehabilitation, respectively. The primary target group for rehabilitation contained 14 486 patients, 14.9% of whom underwent no rehabilitation after their acute treatment. The chances of undergoing subsequent rehabilitation were higher for patients with paresis and dysarthria on admission. Female sex, older age, impaired consciousness at admission, prior history of stroke, and lack of counseling by the hospital social services were all associated with a lower probability of undergoing rehabilitation.
Conclusion
In this study, 54.4% of all ischemic stroke patients and 85.1% of all patients in a primary target group for rehabilitation that was defined a priori underwent rehabilitation after acute care for stroke. Older patients and those who had had a previous stroke were less likely to undergo rehabilitation. Counseling by hospital social services increased the probability of rehabilitation. The potential exclusion of stroke patients from rehabilitation because of old age should be critically examined in every relevant case.
Reviews of randomized controlled studies have shown that specialist neurological rehabilitation after stroke reduces subsequent risk of need for nursing care, admission to a care institution, and mortality (1– 4). Factors that have been proven to be important for successful rehabilitation include starting early, and frequent, intensive training of specific motor, cognitive, and sensory functions (1, 4). The best evidence exists for the effectiveness of inpatient rehabilitation measures (3, 5). However, systematic reviews also support the positive effects of measures provided on an outpatient or partly inpatient basis (2, 4, 6).
Treatment guidelines for neurological rehabilitation take these results into account, recommending that rehabilitation by an organized specialist, interdisciplinary team should start as soon as possible after acute cerebral insult (7, 8). Exclusion criteria for some patients that have been discussed in the literature include medical factors that could militate against intensive (early) rehabilitation care that is primarily geared to neurological symptoms. Factors that have been discussed include:
Pre-existing marked multi-morbidity (9);
Injuries or complications that prevent mobilization (7, 10, 11);
Need for technically very demanding intensive care (10, 11).
With regard to multi-morbidity, very aged patients are often referred for geriatric rehabilitation care, where there is a particular focus on the treatment of concomitant disease (12).
Neurological rehabilitation in Germany is divided into phases A to F (10); the phases relevant to the immediately post-acute period are phases B to D. In phase B, patients with severe neurological impairments are treated with intensive care interventions available. Patients who do not require intensive care and are able to cooperate with treatment to some extent are admitted to phase C, while phase D (“follow-on curative treatment”) is aimed at patients with little need for care who can manage basic activities of daily living for themselves (11, 13, 14).
Few data are available for Germany as to how in practice patients are selected for rehabilitation care. Cost bearers are guided by, among other things, function scores such as the Barthel Index (BI) or Early Rehabilitation Barthel Index (ERBI) as well as the assessment of the hospital providing acute care (9, 15). In the German sample in the CERISE studies (Collaborative Evaluation in Rehabilitation of Stroke across Europe), the following parameters reduced the probability of inpatient neurological rehabilitation care (15):
Pre-existing restrictions of function
Cognitive deficits
Low BI score
Co-morbid depression
Advanced age (15).
In addition, affiliations between the care facilities involved and sometimes the negotiation skills of the patient and his or her relatives had an influence on admission to inpatient neurological rehabilitation care. So far, however, it is unclear what proportion of patients in Germany who are in need of neurological or geriatric rehabilitation actually receive this, and what other factors play a role.
The aim of the present analysis was to investigate what clinical and sociodemographic patient characteristics contribute to stroke patients for whom rehabilitation is indicated actually receiving rehabilitation treatment when they leave acute inpatient neurological care.
Method
Standardized records in the Stroke Register Northwest Germany
The quality assurance project ”Stroke Register Northwest Germany” (http://campus.uni-muenster.de/qsnwd_projekt.html) is a collaboration between the Institute of Epidemiology and Social Medicine at the University of Münster and acute care facilities (16, 17). Data are collected on anonymized forms on paper or electronically. In addition to sociodemographic information, details of symptoms, diagnostic procedures, treatment, and complications are recorded. Subsequent rehabilitation following treatment on the acute ward is also documented, including specification of which phase, are also documented. Additional questions relate to the infrastructure at the care facility.
Patients receiving rehabilitation care
All patients who took part in neurological (phases B to D), geriatric, or other rehabilitation treatment were defined as receiving rehabilitation care.
Target rehabilitation group
The primary target group for rehabilitation in this analysis was defined by three criteria:
BI on discharge no higher than 65 points
Patient must have lived independently at home before the stroke
Patient was assigned to the primary target group for rehabilitation only if he or she had not been transferred for further acute care.
Statistical analysis
The study included all ischemic stroke patients (ICD-10 code I63) aged 18 and over who were recorded in the neurological facilities collaborating in the quality assurance project during 2010 and 2011. To describe the study population, frequencies, medians, and interquartile ranges were calculated. Differences between subgroups were analyzed using the chi-square test and U test. In addition, using multivariate regression models (binary logistic regression, multilevel logistic regression), we investigated in the target rehabilitation group (defined a priori) which patient characteristics and structural features of the acute treatment facilities (hereafter: facilities) influenced whether patients received rehabilitation care. The methods are described in detail in the Methods section.
Results
Total study group
The dataset from 2010 and 2011 was made up of 96 955 cases from 127 neurological centers. The median number of cases per facility in 2011 was 388 (interquartile range: 272 to 546). A quarter of the facilities (n = 34) had their own early rehabilitation department. Depending on the facility, between 20.6% and 100% of patients learned about the support available from social services or care services.
Overall, 40.8% (n = 39 583) of the ischemic stroke patients received phase B to D neurological rehabilitation care; 11.4% (n = 11 062) were planned to receive geriatric rehabilitation, and 2.2% (n = 2115) received other rehabilitation treatment.
Comparison of patients who did and those who did not receive rehabilitation care
Table 1 shows sociodemographic and clinical characteristics separately for patients who received rehabilitation care (54.4%, n = 52 760) and all those who received no rehabilitation in any form (45.6%, n = 44 195). The two groups differed significantly in respect of almost every sociodemographic and clinical characteristic. There was a higher proportion of men in the recipient group, and this group was also 1 year younger (median) than the patients who received no rehabilitation. Arm and leg paresis was the most frequent neurological deficit on admission. Patients who received rehabilitation care more frequently showed neurological deficits and comorbidities than patients who did not receive rehabilitation. BI and Rankin score on admission likewise showed greater impairments among those who later received rehabilitation treatment. Patients not referred for rehabilitation, on the other hand, had more often suffered a previous cerebral insult.
Table 1. Characteristics of stroke patients (age ≥ 18 years) at neurological treatment facilities for whom rehabilitation data were available (n = 96 955), stratified as recipients versus nonrecipients of rehabilitation care.
| Sociodemographic characteristics | Recipients (n = 52760) | Nonrecipients (n = 44195) | p |
| Sex | 0.00 | ||
| Male | 52.9% | 51.0% | |
| Female | 46.8% | 48.7% | |
| Age (median, interquartile) | 73.0 (64–80) | 74.0 (64–82) | 0.00 |
| Living situation before the stroke | 0.00 | ||
| Independently at home | 84.1% | 75.2% | |
| Cared for at home | 9.9% | 10.9% | |
| Cared for in an institution | 4.2% | 12.4% | |
| Symptoms on admission | |||
| State of consciousness | 0.00 | ||
| Somnolent-stuporous | 7.3% | 6.5% | |
| Comatose | 0.9% | 0.7% | |
| Pareses (arms, legs) | 78.2% | 59.4% | 0.00 |
| Impaired swallowing | 25.3% | 15.5% | 0.00 |
| Speech impairment | 50.6% | 36.9% | 0.00 |
| Language impairment | 35.1% | 28.5% | 0.00 |
| Barthel Index (median, interquartile) | 62.5 (25–87.5) | 75.0 (50–100) | 0.00 |
| Rankin scale (median, interquartile) | 3 (2–4) | 2 (1–3) | 0.00 |
| Comorbidities | |||
| Hypercholesterolemia | 56.5% | 51.9% | 0.00 |
| Hypertension | 87.3% | 83.8% | 0.00 |
| Diabetes | 32.4% | 28.6% | 0.00 |
| Atrial fibrillation | 29.6% | 26.5% | 0.00 |
| Previous myocardial infarction | 10.4% | 10.0% | 0.06 |
| Previous cerebral insult | 24.8% | 28.3% | 0.00 |
Target group for rehabilitation
In all, 14.9% (n = 14 486) of all patients fulfilled the criteria for the previously defined primary target group for rehabilitation (BI at discharge ≤ 65, lived independently at home before the stroke, not referred for further acute treatment). Around one in every two patients in this group (55.0%, n = 7971) was intended for phase B and C rehabilitation measures.
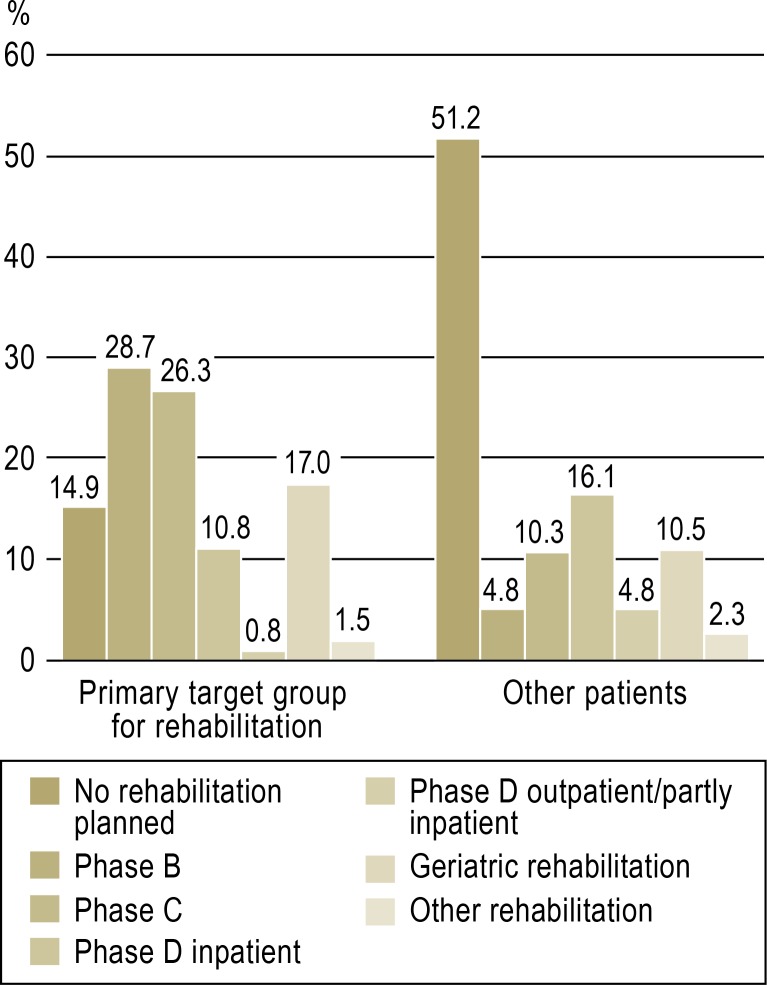
Within the target rehabilitation group, 14.9% (n = 2153) did not receive any rehabilitation. The Figure shows in detail the intended rehabilitation care in the primary target group and in the group of other patients (n = 82 469). The primary target rehabilitation group received phase B or C or geriatric rehabilitation care markedly more often than the other patients. Patients who did not belong to the primary target rehabilitation group but were still planned to receive rehabilitation care (n = 39 314) were most often assigned to phase D inpatient rehabilitation (33.0%, n = 12 972), and next most often to geriatric rehabilitation (21.6%, n = 8473).
Figure.
Planned rehabilitation care for the primary target rehabilitation group (Barthel Index at discharge ≤ 65, lived independently at home before the stroke, not referred for further acute care, n = 14 486) and for the other stroke patients (n = 82 469
Regression analysis
Tables 2 and 3 show the estimated effects from the regression analyses in the form of adjusted odds ratios (aOR) together with the related 95% confidence intervals (95% CI). The results of the simple binary logistic regression (Table 2) were very similar to those of multilevel logistic regression (Table 3), and therefore only the results of the more detailed multilevel model will be described, which contain facility infrastructure characteristics in addition to patient characteristics.
Table 2. Binary logistic regression analysis of the association between patient characteristics and receiving rehabilitation care (n = 14486) (inclusion method)*1.
| Predictor | aOR*2 (95% CI)*3 | p |
| Female sex | 0.88 (0.80–0.98) | 0.02 |
| Age (in years) | 0.94 (0.93–0.94) | 0.00 |
| Stupor | 0.60 (0.53–0.69) | 0.00 |
| Coma | 0.47 (0.33–0.66) | 0.00 |
| Pareses (arms, legs) | 2.18 (1.89–2.51) | 0.00 |
| Impaired swallowing | 1.10 (0.98–1.23) | 0.13 |
| Speech impairment | 1.19 (1.07–1.33) | 0.00 |
| Language impairment | 0.72 (0.65–0.80) | 0.00 |
| Hypercholesterolemia | 1.44 (1.30–1.59) | 0.00 |
| Hypertension | 1.32 (1.12–1.57) | 0.00 |
| Diabetes | 1.03 (0.93–1.15) | 0.56 |
| Atrial fibrillation | 1.09 (0.98–1.20) | 0.11 |
| Previous myocardial infarction | 0.87 (0.74–1.01) | 0.07 |
| Previous cerebral insult | 0.66 (0.59–0.73) | 0.00 |
| Artificial respiration*4 | 1.63 (1.29–2.06) | 0.00 |
| No information from the physician*5 | 0.63 (0.36–1.12) | 0.12 |
| No information from social or care services *6 | 0.48 (0.40–0.56) | 0.00 |
*1Analyzed the event, for patients in the primary target rehabilitation group, of receiving rehabilitation care (versus no rehabilitation care);
*2adjusted odds ratio;
*395% confidence interval;
*4while in acute care;
*5before discharge about course of illness and prevention;
*6before discharge about support available
Table 3. Multilevel logistic regression analysis of the event, for patients in the primary target rehabilitation group, of receiving rehabilitation care (versus no rehabilitation care), n = 14486 (inclusion method).
| Predictor | aOR*1 (95% CI)*2 | p |
| Female sex | 0.87 (0.79–0.97) | 0.01 |
| Age (in years) | 0.93 (0.93–0.94) | 0.00 |
| Stupor | 0.61 (0.53–0.70) | 0.00 |
| Coma | 0.47 (0.33–0.68) | 0.00 |
| Pareses (arms, legs) | 2.32 (2.00–2.70) | 0.00 |
| Impaired swallowing | 1.11 (0.98–1.25) | 0.11 |
| Speech impairment | 1.17 (1.04–1.31) | 0.01 |
| Language impairment | 0.70 (0.63–0.78) | 0.00 |
| Hypercholesterolemia | 1.54 (1.38–1.72) | 0.00 |
| Hypertension | 1.31 (1.10–1.56) | 0.00 |
| Diabetes | 1.00 (0.90–1.12) | 0.94 |
| Atrial fibrillation | 1.06 (0.96–1.18) | 0.25 |
| Previous myocardial infarction | 0.85 (0.73–1.00) | 0.05 |
| Previous cerebral insult | 0.65 (0.58–0.73) | 0.00 |
| Artificial respiration*3 | 1.57 (1.23–2.00) | 0.00 |
| No information from the physician*4 | 0.78 (0.43–1.39) | 0.4 |
| No information from social or care services*5 | 0.31 (0.25–0.37) | 0.00 |
| On-site early rehabilitation department*6 | 0.95 (0.70–1.30) | 0.74 |
| Number of cases in 2011 (in 100 cases) *6 | 1.00 (0.94–1.06) | 0.92 |
*1adjusted odds ratio;
*295% confidence interval;
*3while in acute care;
*4before discharge about course of illness and prevention;
*5before discharge about support available;
*6facility infrastructure characteristic
Speech impairments (dysarthria) on admission were associated with a higher probability of subsequent rehabilitation treatment. Also positively associated with receiving rehabilitation care were the co-morbidities high blood pressure and hypercholesterolemia, and a need for artificial respiration during the stay in acute care. One of the closest associations with subsequent rehabilitation care was found for arm and leg paresis, the presence of which was accompanied by a 2.3-fold increased probability of rehabilitation care (aOR = 2.32; 95% CI = 2.00 to 2.70).
More advanced age, female sex, and a previous cerebral insult reduced the probability of rehabilitation care. With every year that patient age exceeded the mean of about 72 years, the chance of receiving rehabilitation care reduced by about 7% (aOR = 0.93; 95% CI = 0.93 to 0.94). This means that for an 82-year-old patient, the chance of receiving rehabilitation care was 52% lower than for a 72-year-old patient.
The presence of a language impairment (aphasia) and disturbed consciousness (coma, stupor) on admission were associated with a lower probability of receiving rehabilitation care. In addition, patients who received no information from social or care services about any support on offer showed a 69% lower probability of subsequently receiving rehabilitation care (aOR = 0.31; 95% CI = 0.25 to 0.37). At the treatment facility level, none of the infrastructure characteristics showed an association with subsequent rehabilitation care.
Discussion
Main findings
The referral of ischemic stroke patients from neurological acute care to various forms of rehabilitation care was examined based on data from the Stroke Register Northwest Germany for 2010 and 2011. Out of all patients—including those with little or no impairment—four out of ten received phase B to D neurological rehabilitation care. For one in ten patients, geriatric rehabilitation was planned. For five out of ten patients, no rehabilitation was planned. The proportion of patients within the previously defined primary target group for rehabilitation (severely impaired, discharged from acute inpatient care, had been living at home independently until the stroke) who did not receive rehabilitation was much lower (14.9%). The most frequent forms of rehabilitation (at 55.0%) in the primary target rehabilitation group were phase B and C neurological rehabilitation care.
The reasons why rehabilitation care is not given need to be critically examined, not least against the background of the right, enshrined in German social legislation, to rehabilitation treatment to prevent disability and the need for care. Within the primary target group for rehabilitation, more advanced age, a previous cerebral insult, and disturbed consciousness on admission were associated with a markedly reduced chance of receiving rehabilitation. Female sex was also associated with a lower probability of rehabilitation. Although a large functional deficit and pre-existing lesions are factors that worsen the prospects of successful neurological rehabilitation (1, 12), studies have shown that age alone is not an impediment to successful rehabilitation (12, 18– 20). Despite this, other studies have also shown age to have an influence on the provision of care to stroke patients (21, 22). In relation to sex, likewise, several international studies have shown differences between men and women in stroke care, with women tending to receive poorer care (23, 24). Possible explanations include high (multi-) morbidity in female stroke patients (24), which may work against certain forms of therapy, and poorer social support (owing to the shorter life expectancy of their male partners), which can play an important part in their being accepted into rehabilitation care (15).
Neurological deficits that are treated within rehabilitation care programs, e.g., pareses or speech impairment, were associated with a higher chance of receiving rehabilitation care. Interestingly, the same was not true for the presence of aphasia, which was negatively associated with receiving rehabilitation care. One explanation of this could be that—despite the adjustment for co-morbidities and neurological deficits—patients with aphasia showed greater impairments, which could have put the success of rehabilitation in doubt. Another possible explanation could be that patients with aphasia, with their impaired language comprehension, cannot properly express their desire for rehabilitation. In a system in which negotiation skills play a part (15), such a factor could be significant.
This possible explanation is supported by the finding that lack of information from social services about the various kinds of support on offer is also associated with a markedly lower chance of rehabilitation. Information and counseling from social services could be an indicator of a facility’s commitment to after-care or a proxy variable for good quality of process. There were clear differences between centers in this regard. Other factors that were not recorded, such as rare comorbidities representing medical contraindications, or the patient’s wish not to undergo rehabilitation, might have had a strong influence on whether a patient received rehabilitation care or not. For this reason, we interpret the percentage of patients from the primary target group who did receive rehabilitation as relatively high.
In the international comparison with other Western industrialized countries, Germany also seems to come off quite positively in terms of rehabilitation care. Direct comparison between countries is possible only to a limited extent, because of the large differences that exist in health care systems and health services structures; nevertheless, the present analysis seems to suggest that more patients receive specialist neurological rehabilitation in Germany than in countries such as Canada or New Zealand (25, 26). The CERISE studies and one other European comparative study demonstrated great heterogeneity between European countries in terms of the frequency and intensity of rehabilitation care, and of target parameters such as recovery of function or mortality. According to these studies, neurological rehabilitation in Germany is characterized by a high degree of structure, time efficiency, and comparatively high therapeutic intensity. At the same time, German stroke patients showed better recovery of function, markedly better gross motor function, and relatively low mortality after a year as compared to British patients (4, 21, 27, 28).
Limitations and strengths
One strength of the present study is that the referral of stroke patients from acute inpatient neurological care to rehabilitation care was studied in a large, supraregional dataset. The analysis took into account both patient characteristics and some features of acute care facility infrastructure.
One limitation of the study is its definition of a primary target group for rehabilitation. Any definition of this kind must necessarily be arbitrary, since the limitations of routine records do not provide enough data for really appropriate selection of patients for rehabilitation care. The definition used in this study is based on transparent criteria aimed at identifying patients for whom, given the severity of impairments and their lack of need for care before the stroke event, rehabilitation care appeared to be definitely indicated. Because the criteria were deliberately narrow, they excluded patients with lesser impairments for whom rehabilitation care might also be indicated. Analyses based on the entire dataset, however, showed similar associations to those observed in the primary target group, so the main findings reported remain valid if the target group is widened.
Another limitation of this study is that participation in a quality assurance project is voluntary, and will be most of all taken up by facilities that set a high value on the treatment of stroke patients. This selection means that the associations identified cannot be straightforwardly extrapolated to facilities outside the quality assurance project.
One further limitation arises from how missing data are handled. An ad hoc procedure was employed, which could have led to distortions in the estimated associations (29). On the other hand, the proportion of missing data was low, so the extent of any distortions should be tolerable given the considerable size of the dataset.
Summary
About half of all stroke patients in the participating neurological treatment facilities received neurological or geriatric rehabilitation care. The proportion in the primary target group for rehabilitation, i.e., patients with severe impairments who had not previously required care, was considerably higher. In this group, older patients and those with previous cerebral insult had a much lower probability of receiving rehabilitation care. However, information and counseling from social services and also certain neurological symptoms markedly increased this probability. In everyday clinical practice, the question of whether advanced age really represents an exclusion criterion for rehabilitation care should be investigated individually in each case.
Supplementary Material
Methods
Standardized documentation in the Stroke Register Northwest Germany
The quality assurance project ”Stroke Register Northwest Germany” (http://campus.uni-muenster.de/qsnwd_projekt.html) has been ongoing since 1999 and is based on collaboration between the Institute of Epidemiology and Social Medicine at the University of Münster and acute care facilities (16, 17). It is the largest quality assurance register for stroke in Germany, with around 150 participating facilities with various specializations in ten German federal states. Participation is voluntary and open to all hospitals and hospital departments in any area of specialization that are involved in acute inpatient treatment of stroke patients. The register fulfills the documentation criteria of the German Stroke Association (DSG, Deutsche Schlaganfall-Gesellschaft) for the certification of stroke units.
Data collection is on anonymized paper forms or by electronic data input via module 88/1 of the current hospital information systems (KIS, Krankenhausinformationssysteme). The documentation includes the quality indicators for stroke treatment of the German Stroke Register Working Group (ADSR, Arbeitsgemeinschaft Deutscher Schlaganfall Register) (e1). In addition to sociodemographic information, stroke type, severity of impairments, and details of diagnosis, treatment, and complications are recorded. Whether rehabilitation treatment followed the acute care is also recorded, and, if so, which phase of rehabilitation. Additional questions relate to infrastructure characteristics of the treatment facility, e.g., the availability of an on-site early rehabilitation unit.
Patients receiving rehabilitation care
Recipients of rehabilitation care were defined as all those patients for whom neurological (phases B to D), geriatric, or other rehabilitation care was planned. This group includes patients who received rehabilitation immediately after leaving acute inpatient care, and those for whom rehabilitation was initiated after a short period at home or in an institution.
Target rehabilitation group
The primary target group for rehabilitation in this analysis was defined by three criteria:
Patients must have a Barthel Index (BI) of ≤ 65 at discharge. This threshold is used by cost bearers when they assign patients to phase B or C early neurological rehabilitation on the basis of the BI (e2– e4).
Patients must have lived independently at home before the stroke. This condition was intended to ensure that the need for care indicated by the BI had not already existed before the stroke.
Patients were included in the primary rehabilitation group only if they were not referred for further acute care.
Statistical analysis
The study included all patients with a stroke (ICD-10 code I63) aged ≥ 18 years from neurological departments, who were documented in 2010 and 2011 in the quality assurance project. We included only neurological departments, because the patients and infrastructure characteristics of internal medical departments are very different (e5). Patients who died during their stay in acute care were excluded.
A total of 843 cases could not be analyzed because of lack of data relating to rehabilitation. For descriptive purposes, frequencies together with medians and interquartile ranges were calculated. Differences between subgroups were analyzed using the chi-square test and the U test. In addition, the influence of patient characteristics and infrastructural features of the participating neurological departments on patients’ participation in rehabilitation was analyzed in the a priori–defined primary target rehabilitation group.
First a simple, binary logistic regression model was calculated, in which rehabilitation care represented the dependent variable. Patient characteristics were included in the model as independent variables. In the next step, a multilevel logistic regression analysis was carried out to take account of any clusters of cases within the treatment facilities. This model analyzed (infra)structural features of the facilities (“facility level”) as well as patient characteristics (“patient level”). For the regression analyses, missing values in the patient variables were assigned their own category (“missing indicator approach”). The highest percentage of missing values for any individual variable was 2.3%. For the variable “age,” mean centering was carried out to improve the interpretability of the influence of age.
Key Messages.
About half of all stroke patients documented in the register received neurological or geriatric rehabilitation care.
In the group of patients with severe impairments who were discharged from acute care, and who had been living independently at home before the stroke (= primary target group for rehabilitation), six out of seven received rehabilitation care.
Increasing age, previous cerebral insult, and disturbances of consciousness on admission were associated with a lower chance of receiving rehabilitation care.
Pareses and speech impairments on admission, and information and counseling from social services, were positively associated with receiving rehabilitation care.
In normal clinical practice, the question of whether advanced age really represents an exclusion criterion for rehabilitation should be investigated individually on a case-by-case basis.
Acknowledgments
Translated from the original German by Kersti Wagstaff, MA.
The authors are grateful to the participating hospitals and treatment centers in the quality assurance project ”Stroke Register Northwest Germany” (see list in eBox) for excellent continuous collaboration during the data collection phase.
eBox. Acknowledgments.
We are grateful to the participating centers in the quality assurance project ”Stroke Register Northwest Germany” for excellent continuous collaboration during the data collection phase.
Agnes-Karl-Krankenhaus Laatzen, Allgemeines Krankenhaus Celle, AMEOS Diakonie-Klinikum Ueckermünde, Ammerland Klinik GmbH Westerstede, Asklepios Fachklinikum Brandenburg, Asklepios Fachklinikum Lübben, Asklepios Fachklinikum Stadtroda GmbH, Asklepios Fachklinikum Teupitz, Asklepios Klinik Pasewalk, Asklepios Klinikum Uckermark, Asklepios-Kliniken Schildautal, BG-Kliniken Bergmannstrost Halle (Saale), BG Universitätsklinikum Bergmannsheil GmbH Bochum, Caritas Krankenhaus Dillingen/Saar, Carl-Thiem-Klinikum Cottbus, Christian-Albrechts-Universität Kiel, Christliches Klinikum Melle, Christliches Krankenhaus Quakenbrück, Christophorus-Kliniken GmbH Dülmen, Diakoniekrankenhaus Chemnitzer Land Hartmannsdorf, Diakoniekrankenhaus Friederikenstift GmbH Hannover, Diakoniekrankenhaus Henriettenstiftung gGmbH Hannover, Diakoniekrankenhaus Rotenburg (Wümme), Diakonissenkrankenhaus Flensburg, Dietrich-Bonhoeffer-Klinikum Neubrandenburg, DRK Krankenhaus Saarlouis, Elbe-Klinikum Stade, Elisabeth-Krankenhaus Recklinghausen, Ernst-Moritz-Arndt-Universität Greifswald, Evangelisches Bathildiskrankenhaus Bad Pyrmont gGmbH, Evangelisches Krankenhaus Bielefeld gGmbH, Evangelisches Krankenhaus Bielefeld Johannesstift, Evangelisches Krankenhaus Hattingen, Evangelisches Krankenhaus Herne, Evangelisches Krankenhaus Hamm, Evangelisches Krankenhaus Castrop Rauxel, Evangelisches Krankenhaus Oldenburg, Evangelisches Krankenhaus GmbH Gelsenkirchen, Evangelisches Krankenhaus Unna, Fachkrankenhaus Hubertusburg gGmbH Wermsdorf, Gemeinschaftskrankenhaus Herdecke, Gertrudis-Hospital Westerholt, Hanse-Klinikum Stralsund, Hanse-Klinikum Wismar GmbH, Hans-Susemihl-Krankenhaus Emden, Harz-Klinikum Wernigerode- Blankenburg, Heinrich-Braun-Krankenhaus Zwickau, Helios Kliniken Aue, Helios Klinik Borna, Helios Klinikum Erfurt, Helios Klinikum Wuppertal-Barmen, Helios-Kreiskrankenhaus Gotha-Ohrdruf, Helios Kliniken Schwerin, Helios Vogtland-Klinikum Plauen, Herz-Jesu-Krankenhaus Münster-Hiltrup, Hüttenhospital Dortmund, Immanuel Klinik Rüdersdorf, Johannes Wesling Klinikum Minden, Katholisches Krankenhaus Dortmund-West, Katholisches Krankenhaus St. Johannes-Hospital Arnsberg, Kliniken Erlabrunn gGmbH Breitenbrunn, Kliniken Maria Hilf Mönchengladbach, Klinikum Bernburg gGmbH, Klinikum Braunschweig, Klinikum Bremen-Mitte, Klinikum Bremerhaven-Reinkenheide, Klinikum Chemnitz gGmbH, Klinikum Duisburg, Klinikum Frankfurt (Oder) GmbH, Klinikum Herford, Klinikum Ibbenbüren, Klinikum Lippe-Lemgo, Klinikum Lüdenscheid, Klinikum Magdeburg gGmbH, Klinikum Meiningen GmbH, Klinikum Osnabrück, Klinikum Saarbrücken gGmbH, Klinikum St. Georg gGmbH, Klinikum Uelzen, KMG Klinikum Güstrow GmbH, Knappschaftskrankenhaus Bochum-Langendreer, Knappschaftskrankenhaus Bottrop, Knappschaftskrankenhaus Dortmund, Knappschaftskrankenhaus Püttlingen, Knappschaftskrankenhaus Recklinghausen, Knappschaftskrankenhaus Sulzbach, Krankenhaus Dresden-Friedrichstadt, Krankenhaus Plau am See, Krankenhaus St. Elisabeth-Stift Damme, Kreisklinikum Siegen, Kreiskrankenhaus Altenburg, Kreiskrankenhaus Freiberg gGmbH, Kreiskrankenhaus Greiz GmbH, Kreiskrankenhaus Gummersbach, Kreiskrankenhaus Prenzlau, Kreiskrankenhaus Prignitz gemeinnützige GmbH Perleberg, Kreiskrankenhaus Rudolf Virchow Glauchau, KRH Klinikum Nordstadt Hannover, Ludmillenstift Meppen, LWL-Klinik Lengerich, Marienhospital Letmathe Iserlohn, Marien-Hospital Marl, Martin Gropius Krankenhaus GmbH Eberswalde, Martin-Luther-Universität Halle-Wittenberg, Medizinische Hochschule Hannover, Medizinisches Zentrum Kreis Aachen Würselen, Mittelweser Kliniken GmbH Nienburg, Muldentalkliniken GmbH Krankenhaus Wurzen, Neurologische Klinik Hessisch Oldendorf, Nordwest-Krankenhaus Sanderbusch Sande, Oberhavel Kliniken GmbH – Klinik Hennigsdorf, Ökumenisches Hainich Klinikum GmbH Mühlhausen, Prosper-Hospital Recklinghausen, Ruppiner Kliniken GmbH Neuruppin, Saale-Unstrut-Klinikum Naumburg, Sächsisches Krankenhaus Rodewisch, Sächsisches Krankenhaus Altscherbitz Schkeuditz, Sächsisches Krankenhaus Arnsdorf, Sofien- und Hufeland-Klinikum GmbH Weimar, SHG Klinikum Merzig, SRH Waldklinikum Gera, St. Barbara Hospital Gladbeck, St. Bernward Krankenhaus Hildesheim, St. Elisabeth Krankenhaus Dorsten, St. Elisabeth-Hospital gem. GmbH Iserlohn, St. Elisabeth-Hospital Gütersloh, St. Elisabeth-Hospital Herten gGmbH, St. Johannes Hospital Hagen, St. Josef-Hospital Bochum, St. Josefs Hospital Cloppenburg, St. Marien-Hospital Borken, St. Marien-Hospital Hamm, St. Marien-Hospital GmbH Lünen, St. Rochus Hospital Castrop-Rauxel, St. Sixtus-Hospital Haltern am See, St. Vincenz-Krankenhaus Datteln, St. Vincenz Krankenhaus Landeshospital Paderborn, St. Vincenz Krankenhaus Menden, Städtische Kliniken Dortmund, Städtisches Klinikum Görlitz, Städtisches Klinikum Lüneburg, Städtisches Klinikum Neunkirchen/Saar, Städtisches Krankenhaus Martha-Maria Halle-Dölau, Südharz-Krankenhaus Nordhausen GmbH, Thüringen- Kliniken “Georgius Agricola” Rudolstadt, Universität Leipzig, Universitätsklinikum Münster, Universitätsklinikum Aachen, Universitätsklinikum Carl Gustav Carus Dresden, Universitätsklinikum des Saarlandes Homburg / Saar, Universitätsklinikum Göttingen, Universitätsklinikum Jena, Universitätsklinikum Rostock, Zentralklinik Bad Berka GmbH
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Dewey HM, Sherry LJ, Collier JM. Stroke rehabilitation 2007: what should it be? Int J Stroke. 2007;2:191–200. doi: 10.1111/j.1747-4949.2007.00146.x. [DOI] [PubMed] [Google Scholar]
- 2.Langhorne P, Bernhardt J, Kwakkel G. Stroke care 2 stroke rehabilitation. Lancet. 2011;14(377):1693–1702. doi: 10.1016/S0140-6736(11)60325-5. [DOI] [PubMed] [Google Scholar]
- 3.Stroke Unit Trialists’ Collaboration. Cochrane Database Syst Rev. 4. 2007. Organised inpatient (stroke unit) care for stroke. CD000197. [DOI] [PubMed] [Google Scholar]
- 4.Teasell R, Meyer MJ, McClure A, et al. Stroke rehabilitation: an international perspective. Top Stroke Rehabil. 2009;16:44–56. doi: 10.1310/tsr1601-44. [DOI] [PubMed] [Google Scholar]
- 5.Langhorne P, Duncan P. Does the organization of postacute stroke care really matter? Stroke. 2001;32:268–274. doi: 10.1161/01.str.32.1.268. [DOI] [PubMed] [Google Scholar]
- 6.Outpatient Service Trialists. Cochrane Database Syst Rev. 1. 2003. Therapy-based rehabilitation services for stroke patients at home. CD002925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duncan PW, Zorowitz R, Bates B, et al. Management of adult stroke rehabilitation care: a clinical practice guideline. Stroke. 2005;36:e100–e143. doi: 10.1161/01.STR.0000180861.54180.FF. [DOI] [PubMed] [Google Scholar]
- 8.Ackermann H, Schönle PW, Fries W. Multiprofessionelle neurologische Rehabilitation Kommission „Leitlinien der Deutschen Gesellschaft für Neurologie“. In: Diener HC, Putzki N, et al., editors. Leitlinien für Diagnostik und Therapie in der Neurologie. Stuttgart: Thieme; 2008. pp. 880–886. [Google Scholar]
- 9.Platz T, Witte OW, Liepert J, Siebler M, Audebert H, Koenig E. Neurorehabilitation nach Schlaganfall - ein Positionspapier aus dem Kompetenznetzwerk Schlaganfall. Akt Neurol. 2011;38:150–156. [Google Scholar]
- 10.Bertram M, Brandt T. Neurologisch-neurochirurgische Frührehabilitation. Eine aktuelle Bestandsaufnahme. Nervenarzt. 2007;78:1160–1174. doi: 10.1007/s00115-007-2269-1. [DOI] [PubMed] [Google Scholar]
- 11.Bundesarbeitsgemeinschaft für Rehabilitation Phaseneinteilung. BAR (ed.) Frankfurt/Main: 1995. Empfehlungen zur Neurologischen Rehabilitation von Patienten mit schweren und schwersten Hirnschädigungen in den Phasen B und C; pp. 9–15. [Google Scholar]
- 12.Knecht S, Hesse S, Oster P. Rehabilitation after stroke. Dtsch Arztebl Int. 2011;108(36):600–606. doi: 10.3238/arztebl.2011.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kompetenznetz Schlaganfall. Fachinformation Neurologische Rehabilitation. www.kompetenznetz-schlaganfall.de/reha-neuro.0.html. 2011. last accessed on 30 December.
- 14.Berliner Schlaganfallallianz. Medizinische Rehabilitation nach Schlaganfall. http://schlaganfallallianz.de/index.php?id=150. last accessed on 30 December 2011.
- 15.Putman K, De Wit L, Schupp W, et al. Inpatient stroke rehabilitation: A comparative study of admission criteria to stroke rehabilitation units in four European centres. Journal of Rehabilitation Medicine. 2007;39:21–26. doi: 10.2340/16501977-0006. [DOI] [PubMed] [Google Scholar]
- 16.Minnerup J, Wersching H, Ringelstein EB, et al. Impact of the extended thrombolysis time window on the proportion of recombinant tissue-type plasminogen activator-treated stroke patients and on door-to-needle time. Stroke. 2011;42:2838–2843. doi: 10.1161/STROKEAHA.111.616565. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt WP, Berger K, Taeger D, Lay M, Bucker-Nott HJ, Kolominsky-Rabas P. Ausstattungsmerkmale von Krankenhäusern und ihr Einfluss auf die Liegezeit von Schlaganfallpatienten. Dtsch Med Wochenschr. 2003;128:979–983. doi: 10.1055/s-2003-38956. [DOI] [PubMed] [Google Scholar]
- 18.Arsava EM, Rahman R, Rosand J, et al. Severity of leukoaraiosis correlates with clinical outcome after ischemic stroke. Neurology. 2009;72:1403–1410. doi: 10.1212/WNL.0b013e3181a18823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kugler C, Altenhoner T, Lochner P, Ferbert A. Does age influence early recovery from ischemic stroke? A study from the Hessian Stroke Data Bank. J Neurol. 2003;250:676–681. doi: 10.1007/s00415-003-1054-8. [DOI] [PubMed] [Google Scholar]
- 20.Jorgensen HS, Kammersgaard LP, Houth J, et al. Who benefits from treatment and rehabilitation in a stroke unit? A community-based study. Stroke. 2000;31:434–439. doi: 10.1161/01.str.31.2.434. [DOI] [PubMed] [Google Scholar]
- 21.Putman K, De Wit L. European comparison of stroke rehabilitation. Topics in Stroke Rehabilitation. 2009;16:20–26. doi: 10.1310/tsr1601-20. [DOI] [PubMed] [Google Scholar]
- 22.Rudd AG, Hoffman A, Down C, Pearson M, Lowe D. Access to stroke care in England, Wales and Northern Ireland: the effect of age, gender and weekend admission. Age Ageing. 2007;36:247–255. doi: 10.1093/ageing/afm007. [DOI] [PubMed] [Google Scholar]
- 23.Arrich J, Mullner M, Lalouschek W, Greisenegger S, Crevenna R, Herkner H. Influence of socioeconomic status and gender on stroke treatment and diagnostics. Stroke. 2008;39:2066–2072. doi: 10.1161/STROKEAHA.107.506147. [DOI] [PubMed] [Google Scholar]
- 24.Di Carlo A, Lamassa M, Baldereschi M, et al. Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in Europe: data from a multicenter multinational hospital-based registry. Stroke. 2003;34:1114–1119. doi: 10.1161/01.STR.0000068410.07397.D7. [DOI] [PubMed] [Google Scholar]
- 25.Gommans J, Barber A, McNaughton H, et al. Stroke rehabilitation services in New Zealand. NZMJ. 2003;116 [PubMed] [Google Scholar]
- 26.Teasell R, Meyer MJ, Foley N, Salter K, Willems D. Stroke rehabilitation in Canada: a work in progress. Top Stroke Rehabil. 2009;16:11–19. doi: 10.1310/tsr1601-11. [DOI] [PubMed] [Google Scholar]
- 27.DeWit L, Putman K, Dejaeger E, et al. Use of time by stroke patients: a comparison of four European rehabilitation centers. Stroke. 2005;36:1977–1983. doi: 10.1161/01.STR.0000177871.59003.e3. [DOI] [PubMed] [Google Scholar]
- 28.Wolfe CDA, Tilling K, Rudd A, Giroud M, Inzitari D. Variations in care and outcome in the first year after stroke: a Western and Central European perspective. J Neurol Neurosurg Psychiatry. 2004;75:1702–1706. doi: 10.1136/jnnp.2004.039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones MP. Indicator and stratification methods for missing explanatory variables in multiple linear regression. J Am Stat Assoc. 1996;91:222–230. [Google Scholar]
- e1.Heuschmann PU, Biegler MK, Busse O, et al. Development and implementation of evidence-based indicators for measuring quality of acute stroke care: the Quality Indicator Board of the German Stroke Registers Study Group (ADSR) Stroke. 2006;37:2573–2578. doi: 10.1161/01.STR.0000241086.92084.c0. [DOI] [PubMed] [Google Scholar]
- e2.Putman K, De Wit L. European comparison of stroke rehabilitation. Topics in Stroke Rehabilitation. 2009;16:20–26. doi: 10.1310/tsr1601-20. [DOI] [PubMed] [Google Scholar]
- e3.Rollnik JD, Janosch U. Current trends in the length of stay in neurological early rehabilitation. Dtsch Arztebl Int. 2010;107(16):286–292. doi: 10.3238/arztebl.2010.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e4.Stier-Jarmer M, Koenig E, Stucki G. Strukturen der neurologischen Frührehabilitation (Phase B) in Deutschland. Phys Med Rehab Kuror. 2002;12:260–271. [Google Scholar]
- e5.Unrath M, Kalic M, Berger K. Liegezeit von Patienten mit ischämischem Hirninfarkt: 10-Jahres-Trends und Analyse der Einflussfaktoren. Dtsch Med Wochenschr. 2012;137:1683–1688. doi: 10.1055/s-0032-1305213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methods
Standardized documentation in the Stroke Register Northwest Germany
The quality assurance project ”Stroke Register Northwest Germany” (http://campus.uni-muenster.de/qsnwd_projekt.html) has been ongoing since 1999 and is based on collaboration between the Institute of Epidemiology and Social Medicine at the University of Münster and acute care facilities (16, 17). It is the largest quality assurance register for stroke in Germany, with around 150 participating facilities with various specializations in ten German federal states. Participation is voluntary and open to all hospitals and hospital departments in any area of specialization that are involved in acute inpatient treatment of stroke patients. The register fulfills the documentation criteria of the German Stroke Association (DSG, Deutsche Schlaganfall-Gesellschaft) for the certification of stroke units.
Data collection is on anonymized paper forms or by electronic data input via module 88/1 of the current hospital information systems (KIS, Krankenhausinformationssysteme). The documentation includes the quality indicators for stroke treatment of the German Stroke Register Working Group (ADSR, Arbeitsgemeinschaft Deutscher Schlaganfall Register) (e1). In addition to sociodemographic information, stroke type, severity of impairments, and details of diagnosis, treatment, and complications are recorded. Whether rehabilitation treatment followed the acute care is also recorded, and, if so, which phase of rehabilitation. Additional questions relate to infrastructure characteristics of the treatment facility, e.g., the availability of an on-site early rehabilitation unit.
Patients receiving rehabilitation care
Recipients of rehabilitation care were defined as all those patients for whom neurological (phases B to D), geriatric, or other rehabilitation care was planned. This group includes patients who received rehabilitation immediately after leaving acute inpatient care, and those for whom rehabilitation was initiated after a short period at home or in an institution.
Target rehabilitation group
The primary target group for rehabilitation in this analysis was defined by three criteria:
Patients must have a Barthel Index (BI) of ≤ 65 at discharge. This threshold is used by cost bearers when they assign patients to phase B or C early neurological rehabilitation on the basis of the BI (e2– e4).
Patients must have lived independently at home before the stroke. This condition was intended to ensure that the need for care indicated by the BI had not already existed before the stroke.
Patients were included in the primary rehabilitation group only if they were not referred for further acute care.
Statistical analysis
The study included all patients with a stroke (ICD-10 code I63) aged ≥ 18 years from neurological departments, who were documented in 2010 and 2011 in the quality assurance project. We included only neurological departments, because the patients and infrastructure characteristics of internal medical departments are very different (e5). Patients who died during their stay in acute care were excluded.
A total of 843 cases could not be analyzed because of lack of data relating to rehabilitation. For descriptive purposes, frequencies together with medians and interquartile ranges were calculated. Differences between subgroups were analyzed using the chi-square test and the U test. In addition, the influence of patient characteristics and infrastructural features of the participating neurological departments on patients’ participation in rehabilitation was analyzed in the a priori–defined primary target rehabilitation group.
First a simple, binary logistic regression model was calculated, in which rehabilitation care represented the dependent variable. Patient characteristics were included in the model as independent variables. In the next step, a multilevel logistic regression analysis was carried out to take account of any clusters of cases within the treatment facilities. This model analyzed (infra)structural features of the facilities (“facility level”) as well as patient characteristics (“patient level”). For the regression analyses, missing values in the patient variables were assigned their own category (“missing indicator approach”). The highest percentage of missing values for any individual variable was 2.3%. For the variable “age,” mean centering was carried out to improve the interpretability of the influence of age.