Abstract
Background
Clostridium difficile infections are becoming more common, more severe, and more likely to recur. Conventional treatment with antibiotics often fails to eradicate the infection; even when it succeeds, recurrent infection is common. Complementary treatment with probiotic agents to reconstitute the physiological intestinal flora does not yield any consistent benefit. In recent years, fecal transplantation has been used in the English-speaking countries with cure rates of about 87%, but the available evidence is limited to large case series. No randomized controlled trials have been performed. We present the case of a 73-year-old woman with intractable, recurrent enterocolitis due to Clostridium difficile who was successfully treated with fecal transplantation via colonoscopy.
Case description
Upon the completion of antibiotic treatment for a second recurrence of enterocolitis, stool in liquid suspension was introduced into the patient’s colon through a colonoscope. Prior testing had shown the stool donor to be free of acute infection or stool pathogens. The patient was given loperamide to prolong contact of the stool transplant with the colonic mucosa. She was also treated with Saccharomyces cerevisiae for four weeks.
Course
There was no clinical or microbiological evidence of a further recurrence of enterocolitis for 6 months after transplantation. Stool transplantation had no adverse effects.
Conclusion
This patient had a lasting remission of enterocolitis due to Clostridium difficile after the treatment described above. Fecal transplantation seems to be a safe and highly effective treatment for recurrent Clostridium difficile infection. It is unclear whether the administration of Saccharomyces cerevisiae confers any additional benefit.
Clostridium difficile causes approximately 10% to 20% of cases of antibiotics-associated diarrhea and is the main cause of antibiotics-associated colitis (50% to 75%) and pseudomembranous colitis (over 90%) (1– 3). Three possible situations must be distinguished when Clostridium difficile is detected in stool:
Asymptomatic colonization: up to 50% of neonates (e1) and 3% to 8% of adults (e2)
Symptomatic diarrhea with fever (30 to 50%), leukocytosis (50 to 60%), and abdominal pain or cramps (20% to 35%) (4, e3)
Severe to fulminant forms with pseudomembranous colitis and/or toxic megacolon (3, 5).
The incidence of Clostridium difficile infections has increased over the last 20 years (3). Between 2002 and 2006, incidence in Germany rose from between 1.7 and 3.8 cases to 14.8 cases per 100 000 inpatients (6). Some serious cases are caused by new, highly virulent strains (e.g. ribotype 027) (7). First-line treatment for Clostridium difficile colitis includes halting administration of the antibiotic that has triggered colitis (where possible) and antimicrobial treatment with oral metronidazole or oral vancomycin.
The greatest problems are primary treatment failure and recurrences during or after standard treatment. A meta-analysis of 39 studies (11 prospective, 21 retrospective, and seven randomized clinical trials [RCTs]) and 7005 patients reports treatment failure in 22% of cases for metronidazole, versus 14% for vancomycin. Recurrence rates were 27% for metronidazole and 24% for vancomycin (e4). Recurrences are treated either with further metronidazole or vancomycin therapy or with decreasing doses of vancomycin over a longer period (a tapering schedule). In smaller case series, newer antibiotics such as tigecycline (e5), rifaximin (e6), and nitazoxanide (e7– e9) show response rates of 86%, 79%, and 74% to 89% respectively for refractory Clostridium difficile infections. The new macrocyclic antibiotic fidaxomicin has been shown to be noninferior to vancomycin with regard to cure rate but was associated with a significantly lower recurrence rate, possibly due to a lesser impact on natural intestinal flora (8, e10, e11).
A major factor in the pathogenesis of Clostridium difficile infections is the destruction of natural intestinal flora by antibiotics, leading to a selective advantage and colonization by Clostridium difficile (3). Clindamycin has now been overtaken by cephalosporins and quinolones as the main trigger of Clostridium difficile infection (e12). Restoring intestinal flora by fecal transplant may therefore be an alternative to conventional antibiotic treatment for Clostridium difficile (9). Transplantation is performed via stool suspension enema, nasogastric tube, or colonoscopy (9– 12, e13).
A meta-analysis including a total of 17 studies (case reports and case series) and 166 patients reports cure rates of approximately 87% for recurrent Clostridium difficile colitis (10). More recent works confirm these figures, with cure rates of around 89% (Table 1). This means that fecal transplantation outcomes are significantly superior to those of antimicrobial therapy in the event of recurrence.
Table 1. Larger case series in the treatment of Clostridium difficile enterocolitis using fecal transplantation.
| No. of patients receiving fecal transplantation | No. of patients responding to treatment | Treatment response rate (%) | Transplantation method | Reference |
| 4 | 4 | 100 | Rectal enema | Eiseman B et al., 1958 (12) |
| 16 | 14 | 87 | Rectal enema/jejunal tube | Bowden TA et al., 1981 (25) |
| 55 | 46 | 84 | Rectal enema | Borody TJ et al., 1989 (24) |
| 7 | 7 | 100 | Rectal enema | Paterson DL et al., 1994 (e20) |
| 9 | 9 | 100 | Rectal enema | Gustafsson A et al., 1998 (e19) |
| 18 | 15 | 83 | Nasogastric tube | Aas et al., 2003 (e18) |
| 15 | 11 | 73 | Nasogastric tube | MacConnachie AA et al., 2009 (29) |
| 12 | 10 | 83 | Nasogastric tube | Rubin TA et al., 2009 (32) |
| 19 | 19 | 100 | Colonoscopy | Rohlke F et al., 2010 (31) |
| 12 | 12 | 100 | Colonoscopy | Yoon SS et al., 2010 (33) |
| 40 | 33 | 82.5 | Duodenal tube/colonoscopy | Garborg K et al., 2010 (26) |
| 7 | 7 | 100 | Rectal enema | Silverman MS et al., 2010 (15) |
| 77 | 70 | 91 | Colonoscopy | Brandt LJ et al., 2012 (14) |
| 43 | 37 | 86 | Colonoscopy | Hamilton MJ et al., 2012 (27) |
| 26 | 24 | 92 | Colonoscopy | Kelly CR et al., 2012 (28) |
| 70 | 66 | 94 | Colonoscopy | Mattila E et al., 2012 (30) |
| 19 | 13 | 69 | Nasojejunal tube | Polak P et al., 2011 (e21) |
| 7 | 7 | 100 | Colonoscopy | Nieuwdorp M et al., 2008 (e22) |
Individual case reports and review articles are not included. A Medline literature search was performed, using the following search terms: “clostridium difficile [and] fecal bacteriotherapy,” “clostridium difficile “[and] fecal transplantation,” “clostridium difficile [and] stool transplantation”
Surprisingly, the guidelines of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) (13) and the American guidelines (those of the Infectious Diseases Society of America, IDSA) do not mention fecal transplantation (e14). The German Society for Infectious Diseases (DGI, Deutsche Gesellschaft für Infektiologie) is currently preparing guidelines on Clostridium difficile infections. A Cochrane review on fecal transplantation is still being prepared (e15). This case report describes successful fecal transplantation in a 73-year-old female patient with recurrent Clostridium difficile infection.
Case report
Medical history and findings
The 73-year-old patient was admitted with progressive abdominal pain and diarrhea and more than 10 bowel movements per day. Her medical history was known to include numerous previous illnesses, including absolute arrhythmia with atrial fibrillation, three-vessel coronary disease with a risk profile (arterial hypertension, type 2 diabetes, and hyperlipoproteinemia), and erosive gastritis in 2011 with bleeding complications. The latter was being treated with a proton pump inhibitor (pantoprazole). At the end of June 2011 the patient had been admitted with chest pain and dyspnea. During her inpatient stay she developed pneumonia and was treated with amoxicillin/clavulanic acid until the beginning of July. Gastrointestinal symptoms began in mid-August. Viral and bacterial intestinal infections were ruled out. Clostridium difficile toxin A/B was detected in her stool. CRP (C-reactive protein) levels were significantly elevated (111.4 mg/L). Ultrasound imaging of the intestinal wall showed generalized thickening of the intestinal wall, maximum 7 mm (Figure 1). This indicated antibiotic-associated Clostridium difficile pancolitis.
Figure 1.
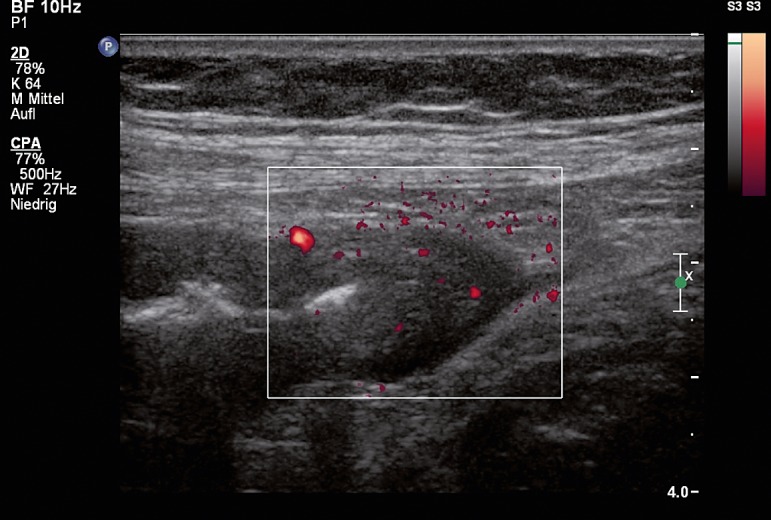
Intestinal ultrasound image of patient described in case report, showing a significant thickening of the intestinal wall and increased vascularization of the transverse colon
Progression
Initial treatment involved 3 × 400 mg oral metronidazole per day. This led to an increase in bowel movement frequency, so on the fourth day of treatment the patient was switched to 4 × 250 mg oral vancomycin per day (infusion solution as oral dosage form). Over six days of vancomycin treatment bowel movement frequency increased further, with progressive cramp-like abdominal pain. Ultrasound examinations of the intestinal wall showed persistent thickening of the intestinal wall. A switch to vancomycin enteric capsules failed to produce any clinical improvement; in fact, nausea and vomiting began, indicating possible vancomycin intolerance. Treatment was switched to 3 × 500 mg oral nitazoxanide per day, leading to rapid improvement in clinical symptoms. After a total of 16 days’ treatment, the patient was discharged with no remaining complaints.
Three weeks later the patient experienced an initial recurrence with corresponding clinical symptoms and microbiological evidence of toxin presence. In view of her failure to respond to metronidazole and vancomycin and her possible intolerance of vancomycin, nitazoxanide treatment was administered again. Evidence of Clostridium difficile in resistogram cell culture did not indicate resistance to metronidazole or vancomycin. However, symptoms did not fully resolve. We added treatment with 2 × 400 mg oral rifaximin per day, achieving normal stool consistency and bowel movement frequency. On discharge, rifaximin treatment was continued for a further 14 days in view of the protracted response to treatment.
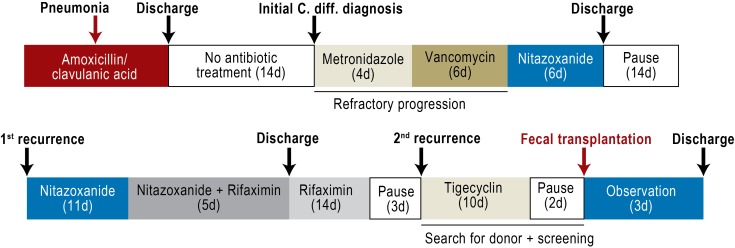
Three days after the end of rifaximin treatment diarrhea and abdominal pain recurred. A second recurrence was diagnosed (Figure 2). In light of a current meta-analysis and numerous case reports and review articles (Table 1), the option of fecal transplantation as part of a personalized attempt to cure the patient’s complaints was discussed with the patient. After informed consent had been given preparations were made for fecal transplantation. The patient’s 25-year-old granddaughter was investigated as a donor relative. Blood serum tests showed no evidence of hepatitis A, B, or C; HIV; or syphilis. Acute infection was ruled out clinically and using laboratory tests. The patient’s granddaughter had received no antibiotic treatment in the previous 12 months, according to her medical history. Three separate stool samples were negative for bacterial stool pathogens, Clostridium difficile, worm eggs, parasites, and viruses (Table 2).
Figure 2.
Diagram showing course of patient’s illness and treatment;
C. diff.: Clostridium difficile; d: day
Table 2. Tests recommended and performed for donors (a total of three stools per donor were tested, on different days).
| Material tested | Tests performed |
| Blood | Differential blood count, electrolytes, kidney and liver function tests |
| Hepatitis serum tests (anti-HAV, anti-HBc, HBs-Ag, anti-HCV) | |
| HIV serum test | |
| CMV and EBV serum tests | |
| Syphilis serum test | |
| Stool | Clostridium difficile toxin A and B (× 3) |
| Stool cultures (× 3) for Campylobacter spp., Shigella, Salmonella, Yersinia, and intestinal E. coli | |
| Stool (× 3) for adenovirus, rotavirus, and norovirus | |
| Stool microscopy (× 3) for parasites/worm eggs and Cryptosporidium/Microsporidium |
HAV: hepatitis A virus; HBc: hepatitis B core; HBs: hepatitis B surface; Ag: antigen; HCV: hepatitis C virus; HIV: human immunodeficiency virus; CMV: cytomegalovirus; EBV: Epstein–Barr virus
The authors began to administer 2 × 50 mg intravenous tigecycline per day, and the patient was symptom-free within seven days. The pretransplant antibiotic treatment was administered according to the procedure described in a retrospective, long-term observation study, in order to reduce the risk of colitis-associated perforation and microbial translocation (14). Two days after the end of tigecycline treatment, fecal transplantation was performed after intestinal lavage. Pantoprazole treatment was halted before transplantation. In addition, adjuvant Saccharomyces cerevisiae probiotic treatment was begun, as in published protocols (Box) (15). On the day of fecal transplantation 177 g of fresh stool from the donor was added to sterile saline solution and filtered through gauze several times. Next, the suspension (250 mL in total) was aliquoted and aliquots were administered through the colonoscope tube during the retraction phase from the terminal ileum (Figures 3a to (3c). Loperamide was then administered to prolong the contact between the stool suspension and the colonic mucosa (for the first six hours after fecal transplantation only).
Box. Fecal Transplant: Aims and Procedure (adapted in line with [9, 27, 28, e18]).
Aims:
To restore natural intestinal flora by administering a suspension of feces from a healthy donor
To prevent recurrence of Clostridium difficile infection
Requirements:
Identify suitable donor (see below and Table 1)
Fewer than three bowel movements per day at time of transplantation if possible
Halt antibiotic treatment two days before fecal transplantation if possible
Written consent
Procedure:
Donor selection
Rule out infectious diseases (see Table 2)
Rule out gastrointestinal disorders, particularly chronic inflammatory bowel diseases and irritable bowel syndrome (IBS)
Rule out antibiotic treatment in the previous three months
Administration of osmotic laxative on the evening before scheduled transplantation if appropriate
Preparation of materials
Weigh fresh (less than six hours old) donor stool
For application via colonoscopy, the whole stool can be used
For application via nasogastric tube, use approximately 30 to 50 g stool
Add donor stool to 250 to 500 mL (application via colonoscopy) or 25 to 100 mL (application via nasogastric tube) sterile water or saline solution
Homogenize suspension by stirring or shaking
Filter suspension through gauze, coffee filters, or 0.25 mm laboratory filters to remove solid components (filter 2 to 3 times)
Place suspension in 50 mL syringes and store at room temperature until needed
Preparation of patient
Treatment with an antibiotic effective against Clostridium difficile until 48 hours before scheduled fecal transplantation
For application via nasogastric tube, administer proton pump inhibitor on the evening before transplantation and the morning of the day of transplantation
For application via colonoscopy, perform intestinal lavage using polyethylene glycol (PEG) solutions or according to the local standard operating procedure (SOP)
Application via nasogastric tube
Fit nasogastric tube on the morning of transplantation, check positioning
Apply stool suspension through tube
Rinse with 25 mL saline solution
Remove tube
Food can be ingested immediately
Application via colonoscopy
Insert colonoscope according to local SOP
Advance as far as the terminal ileum
Working backwards, administer stool; if possible, administer most in the terminal ileum and ascending colon
Optionally, administer loperamide immediately after transplantation and six hours later
Aftercare:
Regular clinical checkups and testing of stool for Clostridium difficile at 2 weeks, 4 weeks, 3 months, and 6 months
Risks:
Usual risks of method of application: perforation, hemorrhage, etc.
Microbial translocation and sepsis, particularly in cases of severe colitis
Figure 3.


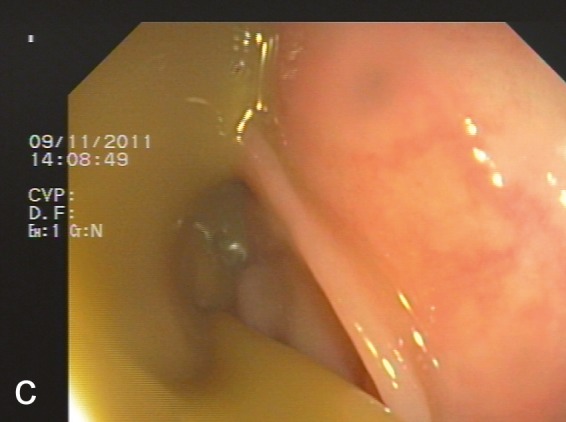
Preparing and performing fecal transplantation
a) Preparation of feces for transplantation by adding 177 g of fresh stool from the donor to sterile saline solution and filtering the suspension through gauze several times
b) Application of the filtered stool suspension through the colonoscope tube
c) Stool suspension entering the colon
The patient was discharged two days after transplantation, symptom-free with normal bowel movement frequency and stool consistency. None of the clinical and microbiological checkups at 14 days, four weeks, three months, and six months after fecal transplantation showed any indication of a recurrence of Clostridium difficile enterocolitis. Two months after fecal transplantation there was clinical evidence of herpes zoster of the thigh.
Discussion
This case report describes the successful use of fecal transplantation in a 73-year-old female patient in Germany. The course of the patient’s illness illustrates a typical situation in clinical practice, with antimicrobial treatment for a nosocomial infection leading to antibiotic-associated Clostridium difficile colitis.
In the USA the annual cost to the healthcare system of Clostridium difficile infections is estimated at $3.2 billion (14, 16). In a German case-control study, it was calculated that hospital costs were quadrupled for patients who developed nosocomial Clostridium difficile enterocolitis (17). Refractory and recurrent cases, as in the patient described here, account for a large part of this increase in costs (Figure 2). It is currently estimated that 50% to 75% of patients are readmitted to the hospital when they experience an initial recurrence (18). The recurrence rates of conventional antibiotic treatment are between 18% and 30% (16, 19). In addition, the probability of a further recurrence increases with the number of recurrences, rising to 45% to 65% after the third recurrence (20, 21). Patients with recurrent Clostridium difficile infections, who are often female, experience fever, abdominal pain, and cramps significantly more frequently (e16). This makes innovative, cost-effective treatments that promise long-lasting success particularly important.
A further risk factor for Clostridium difficile infection is the use of proton pump inhibitors. In a meta-analysis, the incidence of Clostridium difficile colitis rose by 65% (22). The pathophysiology of this phenomenon is the subject of heated discussion. Experimental data suggest that reduced stomach acid production leads to a change in intestinal flora (e17).
Worldwide, approximately 400 patients have undergone fecal transplantation to treat Clostridium difficile enterocolitis (Table 1). To date no published case reports from Germany are yet available. Cure rates are approximately 90% (12, 14, 15, 23– 33, e18– e22). A multicenter study published long-term data on fecal transplantation in 77 patients infected with Clostridium difficile (14). All the patients were treated with antibiotics for Clostridium difficile infections, receiving an average of five different drugs, with no lasting success. Patients were followed up for an average of 17 months. Primary cure rates (patient symptom-free with no evidence of recurrence after 90 days) are 91%; secondary cure rates (patient symptom-free following vancomycin treatment, with or without a second fecal transplant) are 98%. Most of the few recurrences that occurred after transplantation occurred in the context of further antibiotic treatment (14).
Interestingly, this patient’s response following single fecal transplantation was very rapid, although antibiotic treatment regimens have usually yielded success only after long periods, and combination therapy has sometimes been necessary (see also Figure 2). This observation is backed up by cases described in the literature. On average, a significant improvement in symptoms was achieved within three days (14, 23). This seems to be due, in particular, to swift repopulation by balanced intestinal flora. Because the donor had received no antibiotic treatment in the 12 months preceding stool “donation,” it can be assumed that there was no significant imbalance in her natural flora.
Molecular analyses showed that two weeks after transplantation the recipient’s intestinal bacterial flora was the same as that of the donor (34, 35). The composition and diversity of the enteric microbiome seems to play an important role in this. For example, Bacteroides spp. numbers are particularly reduced in patients with Clostridium difficile infection, but after transplantation they again become the dominant species (34, 35, e23). One alternative to fecal transplantation might be to boost intestinal flora using live bacteria or fungi (probiotics). However, administration of these does not lead to lasting colonization of the intestine because these microorganisms have not adapted to the environment of the intestines (e24, e25). Current recommendations on the use of probiotics to prevent recurrence of Clostridium difficile infection are therefore cautious (grade of recommendation B/C) (36, e26). With fecal transplantation, the bacteria used are already adapted to the gastrointestinal tract. This achieves longer-term restoration of fecal flora, for up to 24 weeks (e22).
The patient described here experienced no adverse effects, which is in line with information stated in the current literature (14, 23, 30). However, eight weeks after transplantation she did develop a herpes zoster infection. In view of the patient’s many comorbidities and her age, in our opinion this reactivation of an infection in the patient, who was surely immunocompromized, is not surprising. No varicella zoster infections have been described in patients who have received fecal transplants to date (14, 23, 30).
Limitations
Although fecal transplantation is well tolerated, it does have some limitations: For example, the preparation phase is relatively long (at least a week), as a result of donor screening. In the future one solution to this problem might be to establish a “stool bank” containing samples from suitable donors. It might also be possible to take stool samples from patients before antimicrobial treatment, so that any subsequent antibiotic-associated diarrhea could be treated with an “autologous” fecal transplant. Cryopreserved stool might be used (27).
A further problem is that as yet there are few randomized clinical trials comparing fecal transplantation to a standard treatment. An ongoing randomized trial has, for the first time, shown a significant benefit for fecal transplantation (treatment response rate 81%) over standard vancomycin treatment (31%) or vancomycin with intestinal lavage (23%) in recurrent Clostridium difficile infection (37). Interestingly, it has shown no additional benefit for intestinal lavage. A second trial, which is randomized, controlled, and blinded, compares transplantation of donor stool and transplantation of the patient’s own stool (38).
The expressions “fecal transplantation” and “stool transplantation” are likely to cause patients to reject such treatment because of a “yuck factor.” It would therefore be more advisable to use phrases such as “bacterial treatment to restore natural intestinal flora.” A recent study investigated the willingness of volunteers to undergo fecal transplantation (39). Interestingly, a majority would opt for fecal transplantation if it were recommended by their treating physician. In the case described in this paper, transplantation with feces from a relative of the patient was selected. This is also reflected in the fact that for transplantation involving donors from patients’ families (relatives or partners) the response rate is somewhat higher (93%) than for transplants from nonfamily donors (84%) (23).
In the case described here, fecal suspension was applied via colonoscopy. To date, 75% of transplantations have been performed in this way. Alternatively, transplantation may be performed through a nasogastric tube. When study participants were asked, they disliked this method of application (39), and administration via colonoscopy seems to be better accepted by patients. There are no significant differences in efficacy between application via colonoscopy and via nasogastric tube (40). However, a larger quantity of stool suspension can be administered via colonoscopy, eliminating the need for repeat administration. In the literature, the largest quantity of stool applied via the upper digestive tract is estimated at 200 mL (9, 23). With a suspension of more than 500 mL, however, the response rate was higher (97%) than with smaller volumes (80% for quantities less than 200 mL) (23). In both large case series that have been published, a single transplantation via colonoscopy successfully achieved lasting cure. In addition, patients with early recurrence despite fecal transplant were successfully cured using a second transplantation (14, 30).
Summary
Fecal transplantation is a safe, highly effective alternative to conventional antibiotic treatment for Clostridium difficile enterocolitis and takes effect rapidly. Current data, which include mainly patients treated for relapsing or recurrent Clostridium difficile infection (9– 11, 14, 23, 30, e13), indicate that the procedure should be a treatment option for this patient population in particular.
Key Messages.
The incidence of Clostridium difficile infection is increasing.
Conventional antibiotics (oral metronidazole or vancomycin) are the first-line treatment.
Recurrences are a significant clinical problem and often difficult to treat.
Imbalance in the intestinal microbial flora is a major pathogenetic sign of Clostridium difficile infection.
Fecal transplantation is a safe, effective treatment for the restoration of intestinal flora and may be a treatment option, particularly for recurrence of Clostridium difficile infection.
Footnotes
Conflict of interest statement
Dr. Härter received a one-off lecture fee from MSD Sharp & Dohme GmbH.
The remaining authors declare that no conflict of interest exists.
References
- 1.Bartlett JG. Clostridium difficile: history of its role as an enteric pathogen and the current state of knowledge about the organism. Clin Infect Dis. 1994;18(Suppl 4):265–272. doi: 10.1093/clinids/18.supplement_4.s265. [DOI] [PubMed] [Google Scholar]
- 2.Kelly CP, Pothoulakis C, LaMont JT. Clostridium difficile colitis. N Engl J Med. 1994;330:257–262. doi: 10.1056/NEJM199401273300406. [DOI] [PubMed] [Google Scholar]
- 3.Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526–536. doi: 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- 4.Gerding DN, Olson MM, Peterson LR, et al. Clostridium difficile-associated diarrhea and colitis in adults. A prospective case-controlled epidemiologic study. Arch Intern Med. 1986;146:95–100. [PubMed] [Google Scholar]
- 5.Dallal RM, Harbrecht BG, Boujoukas AJ, et al. Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications. Ann Surg. 2002;235:363–372. doi: 10.1097/00000658-200203000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burckhardt F, Friedrich A, Beier D, Eckmanns T. Clostridium difficile surveillance trends, Saxony, Germany. Emerg Infect Dis. 2008;14:691–692. doi: 10.3201/eid1404.071023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman J, Bauer MP, Baines SD, et al. The changing epidemiology of Clostridium difficile infections. Clin Microbiol Rev. 2010;23:529–549. doi: 10.1128/CMR.00082-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louie TJ, Cannon K, Byrne B, et al. Fidaxomicin preserves the intestinal microbiome during and after treatment of Clostridium difficile infection (CDI) and reduces both toxin reexpression and recurrence of CDI. Clin Infect Dis. 2012;55(Suppl 2):132–142. doi: 10.1093/cid/cis338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landy J, Al-Hassi HO, McLaughlin SD, et al. Review article: faecal transplantation therapy for gastrointestinal disease. Aliment Pharmacol Ther. 2011;34:409–415. doi: 10.1111/j.1365-2036.2011.04737.x. [DOI] [PubMed] [Google Scholar]
- 10.Bakken JS, Borody T, Brandt LJ, et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9:1044–1049. doi: 10.1016/j.cgh.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borody TJ, Campbell J. Fecal microbiota transplantation: current status and future directions. Expert Rev Gastroenterol Hepatol. 2011;5:653–655. doi: 10.1586/egh.11.71. [DOI] [PubMed] [Google Scholar]
- 12.Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958;44:854–859. [PubMed] [Google Scholar]
- 13.Bauer MP, Kuijper EJ, van Dissel JT. European Society of Clinical Microbiology and Infectious Diseases (ESCMID): treatment guidance document for Clostridium difficile infection (CDI) Clin Microbiol Infect. 2009;15:1067–1079. doi: 10.1111/j.1469-0691.2009.03099.x. [DOI] [PubMed] [Google Scholar]
- 14.Brandt LJ, Aroniadis OC, Mellow M, et al. Long-Term Follow-Up of Colonoscopic Fecal Microbiota Transplant for Recurrent Clostridium difficile Infection. Am J Gastroenterol. 2012;107:1079–1087. doi: 10.1038/ajg.2012.60. [DOI] [PubMed] [Google Scholar]
- 15.Silverman MS, Davis I, Pillai DR. Success of self-administered home fecal transplantation for chronic Clostridium difficile infection. Clin Gastroenterol Hepatol. 2010;8:471–473. doi: 10.1016/j.cgh.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien JA, Lahue BJ, Caro JJ, Davidson DM. The emerging infectious challenge of Clostridium difficile-associated disease in Massachusetts hospitals: clinical and economic consequences. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 2007;28:1219–1227. doi: 10.1086/522676. [DOI] [PubMed] [Google Scholar]
- 17.Vonberg RP, Reichardt C, Behnke M, et al. Costs of nosocomial Clostridium difficile-associated diarrhoea. J Hosp Infect. 2008;70:15–20. doi: 10.1016/j.jhin.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(Suppl 2):S88–S92. doi: 10.1093/cid/cis335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauer MP, Notermans DW, van Benthem BH, et al. Clostridium difficile infection in Europe: a hospital-based survey. Lancet. 2011;377:63–73. doi: 10.1016/S0140-6736(10)61266-4. [DOI] [PubMed] [Google Scholar]
- 20.Stepan C, Surawicz CM. Treatment strategies for C difficile associated diarrhea. Acta Gastroenterol Latinoam. 2007;37:183–191. [PubMed] [Google Scholar]
- 21.Surawicz CM, Alexander J. Treatment of refractory and recurrent Clostridium difficile infection. Nat Rev Gastroenterol Hepatol. 2011;8:330–339. doi: 10.1038/nrgastro.2011.59. [DOI] [PubMed] [Google Scholar]
- 22.Janarthanan S, Ditah I, Adler DG, Ehrinpreis MN. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol. 2012;107:1001–1010. doi: 10.1038/ajg.2012.179. [DOI] [PubMed] [Google Scholar]
- 23.Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011;53:994–1002. doi: 10.1093/cid/cir632. [DOI] [PubMed] [Google Scholar]
- 24.Borody TJ, George L, Andrews P, et al. Bowel-flora alteration: a potential cure for inflammatory bowel disease and irritable bowel syndrome? Med J Aust. 1989;150 doi: 10.5694/j.1326-5377.1989.tb136704.x. [DOI] [PubMed] [Google Scholar]
- 25.Bowden TA, Jr., Mansberger AR, Jr., Lykins LE. Pseudomembraneous enterocolitis: mechanism for restoring floral homeostasis. Am Surg. 1981;47:178–183. [PubMed] [Google Scholar]
- 26.Garborg K, Waagsbo B, Stallemo A, Matre J, Sundoy A. Results of faecal donor instillation therapy for recurrent Clostridium difficile-associated diarrhoea. Scand J Infect Dis. 2010;42:857–861. doi: 10.3109/00365548.2010.499541. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton MJ, Weingarden AR, Sadowsky MJ, Khoruts A. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107:761–767. doi: 10.1038/ajg.2011.482. [DOI] [PubMed] [Google Scholar]
- 28.Kelly CR, de Leon L, Jasutkar N. Fecal microbiota transplantation for relapsing Clostridium difficile infection in 26 patients: methodology and results. J Clin Gastroenterol. 2012;46:145–149. doi: 10.1097/MCG.0b013e318234570b. [DOI] [PubMed] [Google Scholar]
- 29.MacConnachie AA, Fox R, Kennedy DR, Seaton RA. Faecal transplant for recurrent Clostridium difficile-associated diarrhoea: a UK case series. QJM. 2009;102:781–784. doi: 10.1093/qjmed/hcp118. [DOI] [PubMed] [Google Scholar]
- 30.Mattila E, Uusitalo-Seppala R, Wuorela M, et al. Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology. 2012;142:490–496. doi: 10.1053/j.gastro.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 31.Rohlke F, Surawicz CM, Stollman N. Fecal flora reconstitution for recurrent Clostridium difficile infection: results and methodology. J Clin Gastroenterol. 2010;44:567–570. doi: 10.1097/MCG.0b013e3181dadb10. [DOI] [PubMed] [Google Scholar]
- 32.Rubin TA, Gessert CE, Aas J. Stool transplantation for older patients with Clostridium difficile infection. J Am Geriatr Soc. 2009;57 doi: 10.1111/j.1532-5415.2009.02600.x. [DOI] [PubMed] [Google Scholar]
- 33.Yoon SS, Brandt LJ. Treatment of refractory/recurrent C difficile-associated disease by donated stool transplanted via colonoscopy: a case series of 12 patients. J Clin Gastroenterol. 2010;44:562–566. doi: 10.1097/MCG.0b013e3181dac035. [DOI] [PubMed] [Google Scholar]
- 34.Grehan MJ, Borody TJ, Leis SM, et al. Durable alteration of the colonic microbiota by the administration of donor fecal flora. J Clin Gastroenterol. 2010;44:551–561. doi: 10.1097/MCG.0b013e3181e5d06b. [DOI] [PubMed] [Google Scholar]
- 35.Khoruts A, Dicksved J, Jansson JK, Sadowsky MJ. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol. 2010;44:354–360. doi: 10.1097/MCG.0b013e3181c87e02. [DOI] [PubMed] [Google Scholar]
- 36.Shanahan F. Probiotics in perspective. Gastroenterology. 2010;139:1808–1812. doi: 10.1053/j.gastro.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 37.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 2013. 2013 Jan 16; doi: 10.1056/NEJMoa1205037. Online first. [DOI] [PubMed] [Google Scholar]
- 38.Brandt LJ. Fecal Microbiota Transplantation: Patient and physician attitudes. Clin Infect Dis. 2012;55:1659–1660. doi: 10.1093/cid/cis812. [DOI] [PubMed] [Google Scholar]
- 39.Zipursky JS, Sidorsky TI, Freedman CA, Sidorsky MN, Kirkland KB. Patient attitudes towards the use of fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection. Clin Infect Dis. 2012;55:1652–1658. doi: 10.1093/cid/cis809. [DOI] [PubMed] [Google Scholar]
- 40.Postigo R, Kim JH. Colonoscopic versus nasogastric fecal transplantation for the treatment of Clostridium difficile infection: a review and pooled analysis. Infection. 2012;40:643–648. doi: 10.1007/s15010-012-0307-9. [DOI] [PubMed] [Google Scholar]
- e1.Larson HE, Barclay FE, Honour P, Hill ID. Epidemiology of Clostridium difficile in infants. J Infect Dis. 1982;146:727–733. doi: 10.1093/infdis/146.6.727. [DOI] [PubMed] [Google Scholar]
- e2.Nakamura S, Mikawa M, Nakashio S, et al. Isolation of Clostridium difficile from the feces and the antibody in sera of young and elderly adults. Microbiol Immunol. 1981;25:345–351. doi: 10.1111/j.1348-0421.1981.tb00036.x. [DOI] [PubMed] [Google Scholar]
- e3.Manabe YC, Vinetz JM, Moore RD, et al. Clostridium difficile colitis: an efficient clinical approach to diagnosis. Ann Intern Med. 1995;123:835–840. doi: 10.7326/0003-4819-123-11-199512010-00004. [DOI] [PubMed] [Google Scholar]
- e4.Vardakas KZ, Polyzos KA, Patouni K, et al. Treatment failure and recurrence of Clostridium difficile infection following treatment with vancomycin or metronidazole: a systematic review of the evidence. Int J Antimicrob Agents. 2012;40:1–8. doi: 10.1016/j.ijantimicag.2012.01.004. [DOI] [PubMed] [Google Scholar]
- e5.Larson KC, Belliveau PP, Spooner LM. Tigecycline for the treatment of severe Clostridium difficile infection. The Annals of pharmacotherapy. 2011;45:1005–1010. doi: 10.1345/aph.1Q080. [DOI] [PubMed] [Google Scholar]
- e6.Rivkin A, Gim S. Rifaximin: new therapeutic indication and future directions. Clin Ther. 2011;33:812–827. doi: 10.1016/j.clinthera.2011.06.007. [DOI] [PubMed] [Google Scholar]
- e7.Musher DM, Logan N, Bressler AM, Johnson DP, Rossignol JF. Nitazoxanide versus vancomycin in Clostridium difficile infection: a randomized, double-blind study. Clin Infect Dis. 2009;48:e41–e46. doi: 10.1086/596552. [DOI] [PubMed] [Google Scholar]
- e8.Musher DM, Logan N, Hamill RJ, et al. Nitazoxanide for the treatment of Clostridium difficile colitis. Clin Infect Dis. 2006;43:421–427. doi: 10.1086/506351. [DOI] [PubMed] [Google Scholar]
- e9.Musher DM, Logan N, Mehendiratta V, et al. Clostridium difficile colitis that fails conventional metronidazole therapy: response to nitazoxanide. J Antimicrob Chemother. 2007;59:705–710. doi: 10.1093/jac/dkl553. [DOI] [PubMed] [Google Scholar]
- e10.Crook DW, Walker AS, Kean Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis. 2012;55(Suppl 2):93–103. doi: 10.1093/cid/cis499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e11.Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422–431. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- e12.Bartlett JG, Perl TM. The new Clostridium difficile-what does it mean? N Engl J Med. 2005;353:2503–2505. doi: 10.1056/NEJMe058221. [DOI] [PubMed] [Google Scholar]
- e13.Brandt LJ, Borody TJ, Campbell J. Endoscopic fecal microbiota transplantation: “first-line” treatment for severe clostridium difficile infection? J Clin Gastroenterol. 2011;45:655–657. doi: 10.1097/MCG.0b013e3182257d4f. [DOI] [PubMed] [Google Scholar]
- e14.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA) Infection control and hospital epidemiology: the Official Journal of the Society of Hospital Epidemiologists of America. 2010;31:431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- e15.Hundal RKZ, Johnstone J, Lee C, Marshall JK. Fecal transplantation for recurrent or refractory Clostridium difficile diarrhea. Cochrane Database Syst Rev. 2011 [Google Scholar]
- e16.Fekety R, McFarland LV, Surawicz CM, et al. Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blinded trial. Clin Infect Dis. 1997;24:324–333. doi: 10.1093/clinids/24.3.324. [DOI] [PubMed] [Google Scholar]
- e17.Kanno T, Matsuki T, Oka M, et al. Gastric acid reduction leads to an alteration in lower intestinal microflora. Biochem Biophys Res Commun. 2009;381:666–670. doi: 10.1016/j.bbrc.2009.02.109. [DOI] [PubMed] [Google Scholar]
- e18.Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis. 2003;36:580–585. doi: 10.1086/367657. [DOI] [PubMed] [Google Scholar]
- e19.Gustafsson A, Lund-Tonnesen S, Berstad A, Midtvedt T, Norin E. Faecal short-chain fatty acids in patients with antibiotic-associated diarrhoea, before and after faecal enema treatment. Scand J Gastroenterol. 1998;33:721–727. doi: 10.1080/00365529850171666. [DOI] [PubMed] [Google Scholar]
- e20.Paterson DL, Iredell J, Whitby M. Putting back the bugs: bacterial treatment relieves chronic diarrhoea. Med J Aust. 1994;160:232–233. [PubMed] [Google Scholar]
- e21.Polak P, Freibergerova M, Jurankova J, et al. [First experiences with faecal bacteriotherapy in the treatment of relapsing pseudomembranous colitis due to Clostridium difficile] Klin Mikrobiol Infekc Lek. 2011;17:214–217. [PubMed] [Google Scholar]
- e22.Nieuwdorp M, van Nood E, Speelman P, et al. [Treatment of recurrent Clostridium difficile-associated diarrhoea with a suspension of donor faeces] Ned Tijdschr Geneeskd. 2008;152:1927–1932. [PubMed] [Google Scholar]
- e23.Borody TJ, Warren EF, Leis SM, et al. Bacteriotherapy using fecal flora: toying with human motions. J Clin Gastroenterol. 2004;38:475–483. doi: 10.1097/01.mcg.0000128988.13808.dc. [DOI] [PubMed] [Google Scholar]
- e24.Vollaard EJ, Clasener HA. Colonization resistance. Antimicrob Agents Chemother. 1994;38:409–414. doi: 10.1128/aac.38.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e25.Johansson ML, Molin G, Jeppsson B, et al. Administration of different Lactobacillus strains in fermented oatmeal soup: in vivo colonization of human intestinal mucosa and effect on the indigenous flora. Appl Environ Microbiol. 1993;59:15–20. doi: 10.1128/aem.59.1.15-20.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e26.Floch MH, Walker WA, Madsen K, et al. Recommendations for probiotic use-2011 update. J Clin Gastroenterol. 2011;(45 Suppl):168–171. doi: 10.1097/MCG.0b013e318230928b. [DOI] [PubMed] [Google Scholar]