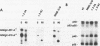Abstract
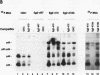
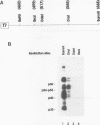
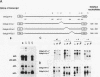
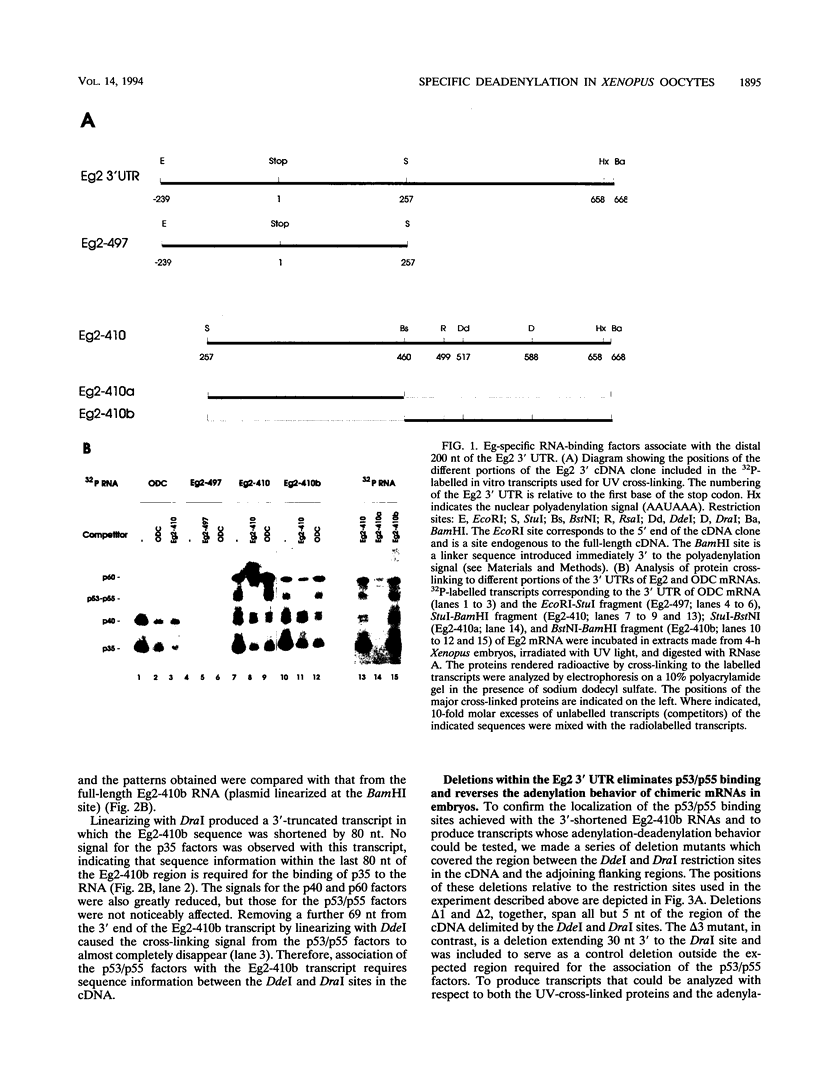
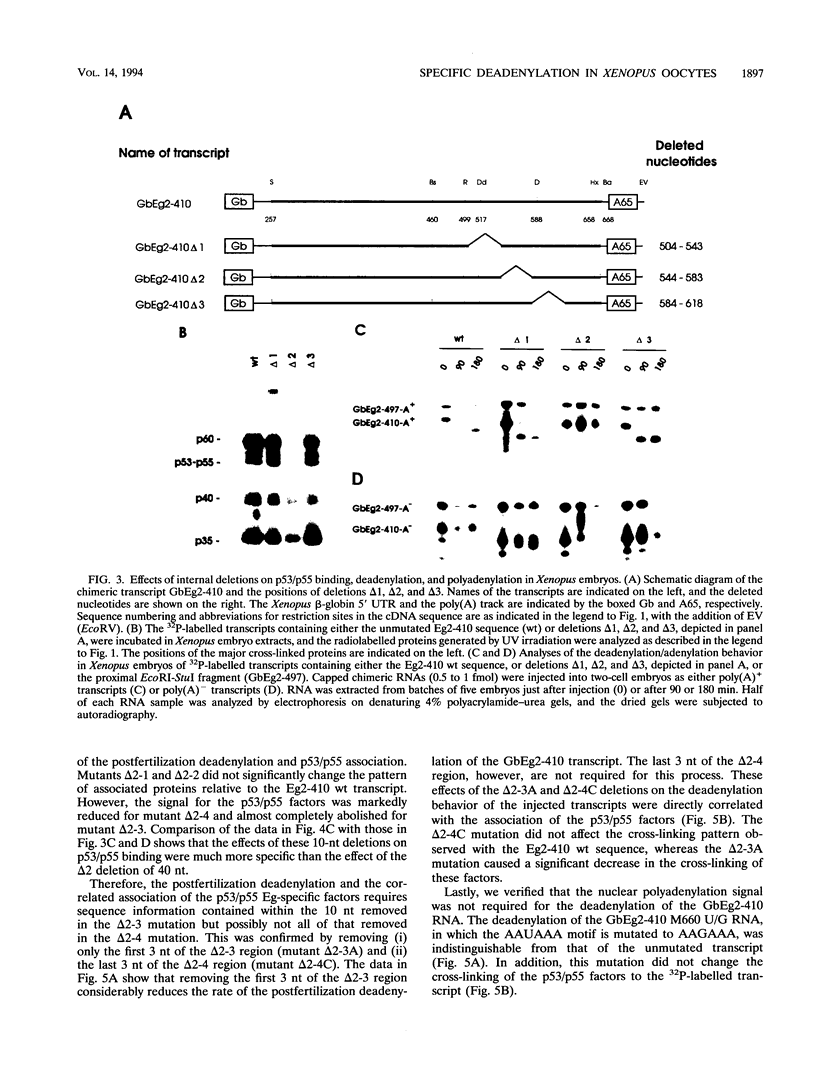
The maternal Xenopus Eg mRNAs are adenylated and translated in the mature oocyte and then, after fertilization, are deadenylated and released from polysomes. Therefore, after fertilization, a change occurs in the cellular mechanisms that control mRNA adenylation. In the study reported here, we show that the 3' untranslated region of Eg2 mRNA contains a cis-acting element that is required for the deadenylation of chimeric RNAs after fertilization. This cis-acting element is contained within a single 17-nucleotide portion of the Eg2 mRNA. Disruption of this deadenylation element allows adenylation of the chimeric transcripts in the embryo. Therefore, this cis-acting element is part of the sequence information required for the developmental switch from adenylation to deadenylation of the maternal Eg2 mRNA in Xenopus embryos.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachvarova R. F. A maternal tail of poly(A): the long and the short of it. Cell. 1992 Jun 12;69(6):895–897. doi: 10.1016/0092-8674(92)90606-d. [DOI] [PubMed] [Google Scholar]
- Brewer G., Ross J. Poly(A) shortening and degradation of the 3' A+U-rich sequences of human c-myc mRNA in a cell-free system. Mol Cell Biol. 1988 Apr;8(4):1697–1708. doi: 10.1128/mcb.8.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval C., Bouvet P., Omilli F., Roghi C., Dorel C., LeGuellec R., Paris J., Osborne H. B. Stability of maternal mRNA in Xenopus embryos: role of transcription and translation. Mol Cell Biol. 1990 Aug;10(8):4123–4129. doi: 10.1128/mcb.10.8.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. A., Sheets M. D., Wickens M. P. Poly(A) addition during maturation of frog oocytes: distinct nuclear and cytoplasmic activities and regulation by the sequence UUUUUAU. Genes Dev. 1989 Dec;3(12B):2151–2162. doi: 10.1101/gad.3.12b.2151. [DOI] [PubMed] [Google Scholar]
- Fox C. A., Wickens M. Poly(A) removal during oocyte maturation: a default reaction selectively prevented by specific sequences in the 3' UTR of certain maternal mRNAs. Genes Dev. 1990 Dec;4(12B):2287–2298. doi: 10.1101/gad.4.12b.2287. [DOI] [PubMed] [Google Scholar]
- Galili G., Kawata E. E., Smith L. D., Larkins B. A. Role of the 3'-poly(A) sequence in translational regulation of mRNAs in Xenopus laevis oocytes. J Biol Chem. 1988 Apr 25;263(12):5764–5770. [PubMed] [Google Scholar]
- Harland R., Misher L. Stability of RNA in developing Xenopus embryos and identification of a destabilizing sequence in TFIIIA messenger RNA. Development. 1988 Apr;102(4):837–852. doi: 10.1242/dev.102.4.837. [DOI] [PubMed] [Google Scholar]
- Huarte J., Stutz A., O'Connell M. L., Gubler P., Belin D., Darrow A. L., Strickland S., Vassalli J. D. Transient translational silencing by reversible mRNA deadenylation. Cell. 1992 Jun 12;69(6):1021–1030. doi: 10.1016/0092-8674(92)90620-r. [DOI] [PubMed] [Google Scholar]
- Hyman L. E., Wormington W. M. Translational inactivation of ribosomal protein mRNAs during Xenopus oocyte maturation. Genes Dev. 1988 May;2(5):598–605. doi: 10.1101/gad.2.5.598. [DOI] [PubMed] [Google Scholar]
- Jackson R. J., Standart N. Do the poly(A) tail and 3' untranslated region control mRNA translation? Cell. 1990 Jul 13;62(1):15–24. doi: 10.1016/0092-8674(90)90235-7. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuge H., Inoue A. Maturation of Xenopus laevis oocyte by progesterone requires poly(A) tail elongation of mRNA. Exp Cell Res. 1992 Sep;202(1):52–58. doi: 10.1016/0014-4827(92)90403-u. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird-Offringa I. A., de Wit C. L., Elfferich P., van der Eb A. J. Poly(A) tail shortening is the translation-dependent step in c-myc mRNA degradation. Mol Cell Biol. 1990 Dec;10(12):6132–6140. doi: 10.1128/mcb.10.12.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Guellec R., Paris J., Couturier A., Roghi C., Philippe M. Cloning by differential screening of a Xenopus cDNA that encodes a kinesin-related protein. Mol Cell Biol. 1991 Jun;11(6):3395–3398. doi: 10.1128/mcb.11.6.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legagneux V., Bouvet P., Omilli F., Chevalier S., Osborne H. B. Identification of RNA-binding proteins specific to Xenopus Eg maternal mRNAs: association with the portion of Eg2 mRNA that promotes deadenylation in embryos. Development. 1992 Dec;116(4):1193–1202. doi: 10.1242/dev.116.4.1193. [DOI] [PubMed] [Google Scholar]
- McGrew L. L., Dworkin-Rastl E., Dworkin M. B., Richter J. D. Poly(A) elongation during Xenopus oocyte maturation is required for translational recruitment and is mediated by a short sequence element. Genes Dev. 1989 Jun;3(6):803–815. doi: 10.1101/gad.3.6.803. [DOI] [PubMed] [Google Scholar]
- McGrew L. L., Richter J. D. Translational control by cytoplasmic polyadenylation during Xenopus oocyte maturation: characterization of cis and trans elements and regulation by cyclin/MPF. EMBO J. 1990 Nov;9(11):3743–3751. doi: 10.1002/j.1460-2075.1990.tb07587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munroe D., Jacobson A. Tales of poly(A): a review. Gene. 1990 Jul 16;91(2):151–158. doi: 10.1016/0378-1119(90)90082-3. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Kirschner M. W. Cyclin synthesis drives the early embryonic cell cycle. Nature. 1989 May 25;339(6222):275–280. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- Osborne H. B., Duval C., Ghoda L., Omilli F., Bassez T., Coffino P. Expression and post-transcriptional regulation of ornithine decarboxylase during early Xenopus development. Eur J Biochem. 1991 Dec 5;202(2):575–581. doi: 10.1111/j.1432-1033.1991.tb16410.x. [DOI] [PubMed] [Google Scholar]
- Paris J., Le Guellec R., Couturier A., Le Guellec K., Omilli F., Camonis J., MacNeill S., Philippe M. Cloning by differential screening of a Xenopus cDNA coding for a protein highly homologous to cdc2. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):1039–1043. doi: 10.1073/pnas.88.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris J., Osborne H. B., Couturier A., Le Guellec R., Philippe M. Changes in the polyadenylation of specific stable RNA during the early development of Xenopus laevis. Gene. 1988 Dec 10;72(1-2):169–176. doi: 10.1016/0378-1119(88)90139-4. [DOI] [PubMed] [Google Scholar]
- Paris J., Philippe M. Poly(A) metabolism and polysomal recruitment of maternal mRNAs during early Xenopus development. Dev Biol. 1990 Jul;140(1):221–224. doi: 10.1016/0012-1606(90)90070-y. [DOI] [PubMed] [Google Scholar]
- Peppel K., Baglioni C. Deadenylation and turnover of interferon-beta mRNA. J Biol Chem. 1991 Apr 15;266(11):6663–6666. [PubMed] [Google Scholar]
- Simon R., Tassan J. P., Richter J. D. Translational control by poly(A) elongation during Xenopus development: differential repression and enhancement by a novel cytoplasmic polyadenylation element. Genes Dev. 1992 Dec;6(12B):2580–2591. doi: 10.1101/gad.6.12b.2580. [DOI] [PubMed] [Google Scholar]
- Stoeckle M. Y., Guan L. High-resolution analysis of gro alpha mRNA poly(A) shortening: regulation by interleukin-1 beta. Nucleic Acids Res. 1993 Apr 11;21(7):1613–1617. doi: 10.1093/nar/21.7.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnum S. M., Wormington W. M. Deadenylation of maternal mRNAs during Xenopus oocyte maturation does not require specific cis-sequences: a default mechanism for translational control. Genes Dev. 1990 Dec;4(12B):2278–2286. doi: 10.1101/gad.4.12b.2278. [DOI] [PubMed] [Google Scholar]
- Vassalli J. D., Huarte J., Belin D., Gubler P., Vassalli A., O'Connell M. L., Parton L. A., Rickles R. J., Strickland S. Regulated polyadenylation controls mRNA translation during meiotic maturation of mouse oocytes. Genes Dev. 1989 Dec;3(12B):2163–2171. doi: 10.1101/gad.3.12b.2163. [DOI] [PubMed] [Google Scholar]
- Wahle E. The end of the message: 3'-end processing leading to polyadenylated messenger RNA. Bioessays. 1992 Feb;14(2):113–118. doi: 10.1002/bies.950140208. [DOI] [PubMed] [Google Scholar]
- Wharton R. P., Struhl G. RNA regulatory elements mediate control of Drosophila body pattern by the posterior morphogen nanos. Cell. 1991 Nov 29;67(5):955–967. doi: 10.1016/0092-8674(91)90368-9. [DOI] [PubMed] [Google Scholar]
- Wickens M. In the beginning is the end: regulation of poly(A) addition and removal during early development. Trends Biochem Sci. 1990 Aug;15(8):320–324. doi: 10.1016/0968-0004(90)90022-4. [DOI] [PubMed] [Google Scholar]
- Wilson T., Treisman R. Removal of poly(A) and consequent degradation of c-fos mRNA facilitated by 3' AU-rich sequences. Nature. 1988 Nov 24;336(6197):396–399. doi: 10.1038/336396a0. [DOI] [PubMed] [Google Scholar]