Abstract
The relative ineffectiveness of hematopoietic stem cells in reaching the bone marrow upon transplantation combined with the limited number of these cells available is a major reason for graft failure and delayed hematopoietic recovery. Hence, the development of strategies that could enhance homing is of high interest. Here, we provide evidence that homing is severely impaired postexposure to ionizing radiation (IR) in mice, an effect we found was time dependent and could be partially rescued using mesenchymal stromal cell (MSC) therapy. In an attempt to further increase homing, we took advantage of our observation that the granulocyte colony stimulating factor (G-CSF), a cytokine known to induce cell mobilization, is increased in the marrow of mice shortly after their exposure to IR. As such, we developed a truncated, yet functional, soluble G-CSF receptor (solG-CSFR), which we hypothesized could act as a decoy and foster homing. Using MSCs or conditioned media as delivery vehicles, we show that an engineered solG-CSFR has the potential to increase homing and hematopoietic reconstitution in mice. Altogether, our results provide novel findings at the interplay of IR and stromal cell therapy and present the regulation of endogenous G-CSF as an innovative proof-of-concept strategy to manipulate hematopoietic cell homing.
Introduction
Umbilical cord blood (UCB) cells are being increasingly used as an alternative source for hematopoietic stem cell (HSC) transplantation (review by Rocha and Broxmeyer) [1]. Despite several advantages, such as a greater availability and immune tolerance, the UCB contains a limited number of HSCs available for transplantation. Limited HSC supply, combined with the low level of these cells homing to the bone marrow (BM), can translate into an increased risk of graft failure and delayed hematopoietic engraftment [2].
Low-level homing is observed independently of the source of HSC injected and, therefore, numerous efforts have been made to enhance this important physiological process. Our comprehension of homing is limited to the role of some adhesion molecules and cytokines. In particular, injection of the granulocyte colony stimulating factor (G-CSF), a cytokine playing an essential role in granulopoiesis, induces stem cell mobilization from the BM to the peripheral blood [3,4]. Conversely, the stromal cell derived factor-1 alpha (SDF-1α), a chemoattractant present in the marrow niche, has an important role in the homing of HSC toward the BM [5,6].
Different approaches are being investigated to increase homing and engraftment of infused hematopoietic cells. The injection of a higher number of HSCs through the recent development of ex vivo expansion techniques is an attractive strategy for which safety issues remains to be determined [7]. Alternatively, some have tried increase homing by inhibiting a specific peptidase (CD26) responsible for the truncation and inactivation of SDF-1α, or increase the expression of the CXCR4 receptor by stimulating HSC with the prostaglandin E2 [8–10]. Others have looked into injecting HSCs directly into the iliac crest as a way to circumvent the need for homing [11–13]. The results from these approaches, most of which are currently being tested in phase I/II clinical trials, are thus far, either not available or disappointing.
Further studies have investigated the cotransplantation of BM-derived stromal cells (often referred to as the mesenchymal stromal cell [MSC]) together with hematopoietic cells to improve homing and engraftment [14–16]. This idea stems from the generally accepted concept that exposure to conditioning drugs (i.e., ionizing radiation (IR) and/or busulfan) damages the stromal microenvironment, preventing adequate homing and retention of the injected hematopoietic cells in the BM [17]. Work done in mouse models showed that cotransplantation of stromal cells can benefit homing and engraftment [18–20]. In contrast, the injection of stromal cells has been shown to be safe in humans, but has not yet been shown convincingly to prevent graft failure. Overall, the beneficial action of MSCs on hematopoietic recovery is still controversial, likely, in part, by the variety in the protocols currently used to isolate and inject MSCs (reviewed by Maijenburg et al.)[21].
Here, we report that homing is impaired in a time-dependent manner following exposure to IR and that stromal cell therapy can partially restore this defect. We also demonstrate, as a proof of concept, that the secretion of a G-CSF decoy receptor has the potential to modulate homing and hematopoietic reconstitution, and that therefore may represent a novel and complementary strategy to stromal cell therapy.
Materials and Methods
Mice
Eight- to 12-week-old female C57BL/6J mice were purchased from Charles River. B6.SJL-PtrcaPep3b/BoyJ mice (CD45.1) were purchased from Jackson Laboratories. All mice were allowed to acclimate at least 1 week before their use for experimentation. All in vivo manipulations were approved by the Comité Institutionnel des Bonnes Pratiques Animales en Recherche of CHU Ste-Justine (protocol No. S10–32).
Isolation, purification, and characterization of MSCs
BM was removed by flushing tibias, femurs, and iliacs with a cold phosphate buffer solution. Cleaned bones were next cut in small fragments of 1–2 mm and digested for 45 min with 0.25% collagenase type 1 (Sigma). Fresh (noncultured) MSCs were isolated from collagenase-treated bones using the EasySep® negative selection kit for mesenchymal progenitors (StemCell Technologies). The procedure was followed as recommended by the manufacturer except that 3 treatments of 0.25% collagenase solution were performed to improve cell recovery. Stromal cell purity was determined by flow cytometry using the CD45, CD31, and Ter119 antibodies (all purchased from BioLegend). Otherwise, MSCs were isolated based on their property to attach to polystyrene following culture for 7 days in the α-minimum essential medium (MEM) containing 10% fetal bovine serum and 1% penicillin/streptomycin. A stromal population was then expanded in 3% oxygen and their phenotype confirmed by flow cytometry. Where indicated, culture-expended MSCs were transduced using lentiviral particles generated as described.
Production of a soluble G-CSF decoy receptor
Full length and truncated sequences of the G-CSF receptor (G-CSFR) were amplified by PCR using the pCR Blunt II TOPO plasmid containing the full G-CSFR murine cDNA (purchased from OpenBiosystems) as a template. PCR amplicons were cloned into pENTR1A destination plasmid (Invitrogen) and used to generate lentivectors (pLentiCMV/TO/G-CSFR and pLentiCMV/TO/solG-CSFR) using the Gateway recombination technology (Invitrogen). The 3rd generation lentivector backbone was obtained from Dr. Eric Campeau (ericcampeau.com). Lentivirus production was performed using the standard method of transfection in 293T cells as previously described [22]. MSCs were transduced with lentivectors expressing the full and truncated forms of the G-CSFR. Their expression in MSCs and 293T cells was confirmed by western blot using the G-CSFR antibody (# sc-9173; Santa Cruz Biotechnology).
Western blot
Conditioned media (CM) were collected from MSC cultures in 100–150-mm dishes that were 80%–90% confluent. Briefly, cells were first rinsed with phosphate-buffered saline twice, and then transferred into serum-free media (α-MEM) for 24 h after which the CM was collected, filtered, and stored at −80°C. Where indicated, the CM was concentrated ∼75-fold using Centricon Plus-70 (Millipore) with a 30-KDa cutoff before injection. The ability of the CM containing solG-CSFR to inhibit G-CSF signaling was determined by measuring ERK1/2 phosphorylation. In brief, MSCs expressing endogenous levels of the G-CSFR were cultured at 80% confluence and starved in serum-free media for 24 h. Cells were then stimulated for 5 min with CM collected from MSCs or MSCs secreting the solG-CSFR and supplemented or not with increasing doses of recombinant G-CSF (purchased from Invitrogen). Cells were then washed with phosphate-buffered saline and resuspended in the lysis buffer containing proteinase inhibitors. After incubation on ice for 30 min, lysates were cleared by centrifugation, and supernatants were collected for western blot analysis using ERK1/2 and pERK1/2 antibodies (# ab17942 and ab50011, respectively; Abcam).
Enzyme-linked immunosorbent assay
Femurs were cleaned and marrow was flushed using a volume of 0.5 mL cold phosphate-buffered saline (PBS) containing a protease inhibitor. G-CSF-specific levels were then determined by enzyme-linked immunosorbent assay (ELISA) from BM eluates according to the manufacturer's instructions (RayBiotech).
Homing and engraftment
Where indicated, mice were exposed to a single sublethal dose of 8 Gy total body irradiation (using cobalt-60 as a source) and at various time postirradiation, mice were injected into the tail vein with 100 μL phosphate buffer saline containing 5×106 nucleated BM cells labeled with 0.5 μM carboxyfluorescein succinimidyl ester or CFSE (Invitrogen) or 4 μM PKH26 (Sigma) according to the manufacturer's instructions. Eighteen hours later, recipient mice were sacrificed and femoral BM harvested for flow cytometry analysis of the absolute number or the proportion of labeled cells per femur using CountBright™ absolute counting beads (Invitrogen). Of note, labeling of hematopoietic cells using CFSE consistently yielded a lower number of cells homing to the BM compared to when using PKH26. The reason for this difference is currently unknown. Engraftment studies were performed as described above except that 2×106 CD45.1 nucleated BM cells were injected instead of fluorescently labeled cells. Where indicated, concentrated CM was injected intraperitonealy (2 doses of 100 μL at 16 and 4 h before hematopoietic cell injection) or intravenously (one dose of 150 μL at 4 h before hematopoietic cell injection) for homing and engraftment studies, respectively. Alternatively, a fixed number of culture-expanded (gene-modified or not) MSCs were injected intraperitonealy before the assays.
Statistical analysis
Statistical analyses were performed with the Graph Pad 5.0 software using the unpaired Student t test and/or the one-way analysis of variance test. Results were considered statistically significant with p-values<0.05.
Results
Exposure to IR reduces homing to the BM of mice
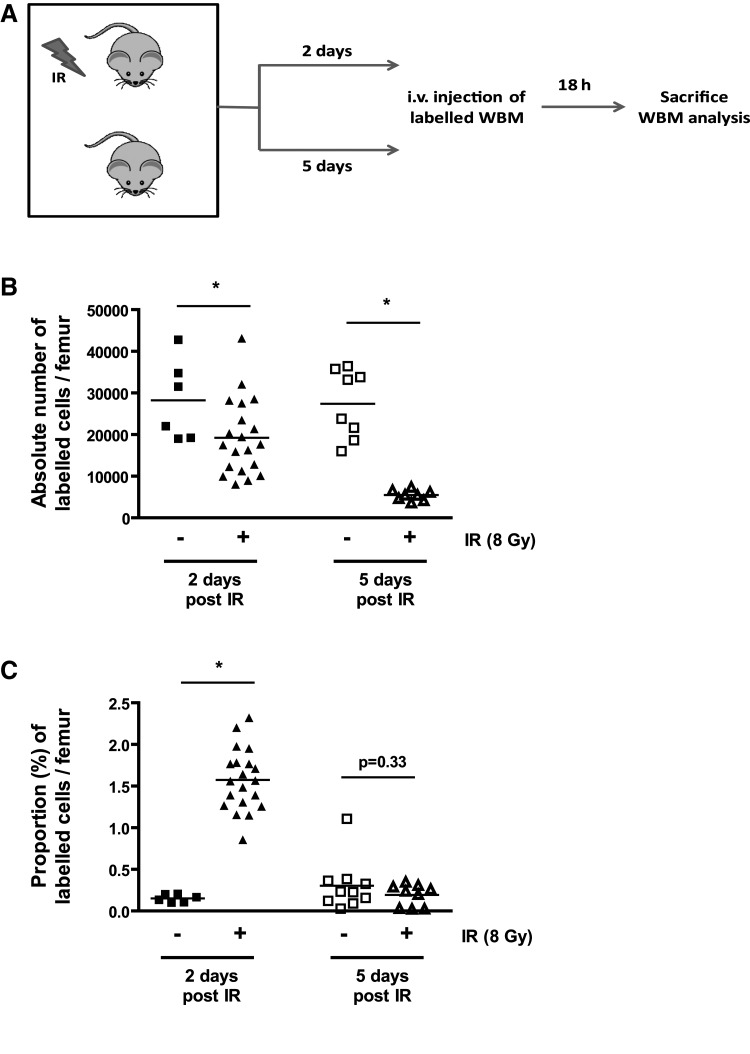
BM conditioning using IR and/or chemotherapy is a mandatory procedure to prevent the rejection of donor HSCs. Whether such conditioning benefits BM transplantation by creating a niche space and triggering the secretion of chemoattractant, such as SDF-1α, is controversial. To examine the impact of BM conditioning on cell homing in mice, we used flow cytometry and absolute cell counts of fluorescently labeled hematopoietic cells. Our results clearly demonstrate that homing to the BM is severely compromised following exposure of mice to IR (8 Gy), an effect we found was much more pronounced 5 days post-IR, when compared to 2 days post-IR (Fig. 1a, b). Noteworthy, homing was not compromised long term; as we previously showed, it is fully restored 8 weeks postexposure to IR [23]. These results (obtained using absolute cell counts) are in sharp contrast to what we observed looking at the proportion of labeled cells over the total number cells in the marrow (Fig. 1c), highlighting the importance of using the appropriate method to correctly assess homing. These data are in accordance with previous work by Collis and colleagues [24] and suggest that IR-induced damage to the marrow environment severely impairs the homing process.
FIG. 1.
Impaired homing following exposure to ionizing radiation (IR) is time dependent. (A) Schematic of the experimental procedure. Mice were irradiated or not at the dose of 8 Gy and left untreated for 2 or 5 days after which homing was determined. (B) Absolute number of PKH26+ bone marrow cells present in the femurs of mice at 2 and 5 days post their exposure (+) or not (−) to IR. (C) Same as in B except that the number of PKH26+ bone marrow cells is expressed in proportion to the number of nucleated cell present in the femur at the time of sacrifice. n=6–20, each symbol representing an individual mouse. Student t tests *P<0.05 are shown.
Injection of MSCs improves homing postexposure to IR
Alteration of the BM stroma post-IR has been proposed as a mechanism by which homing is reduced. This hypothesis is supported by data showing that injection of the MSC increases homing and/or engraftment in mice [18–20]. Yet, some reports have showed increased homing in situations where the niche had no time to regenerate, such as when stromal cells were injected simultaneously with hematopoietic cells [15,18,19]. These observations led us to wonder if the injection of stromal cells could foster homing in a situation where the microenvironment had not been damaged (in absence of exposure to IR). Moreover, we also wanted to determine the extent by which homing is restored in irradiated mice receiving stromal cell therapy compared to nonirradiated control mice.
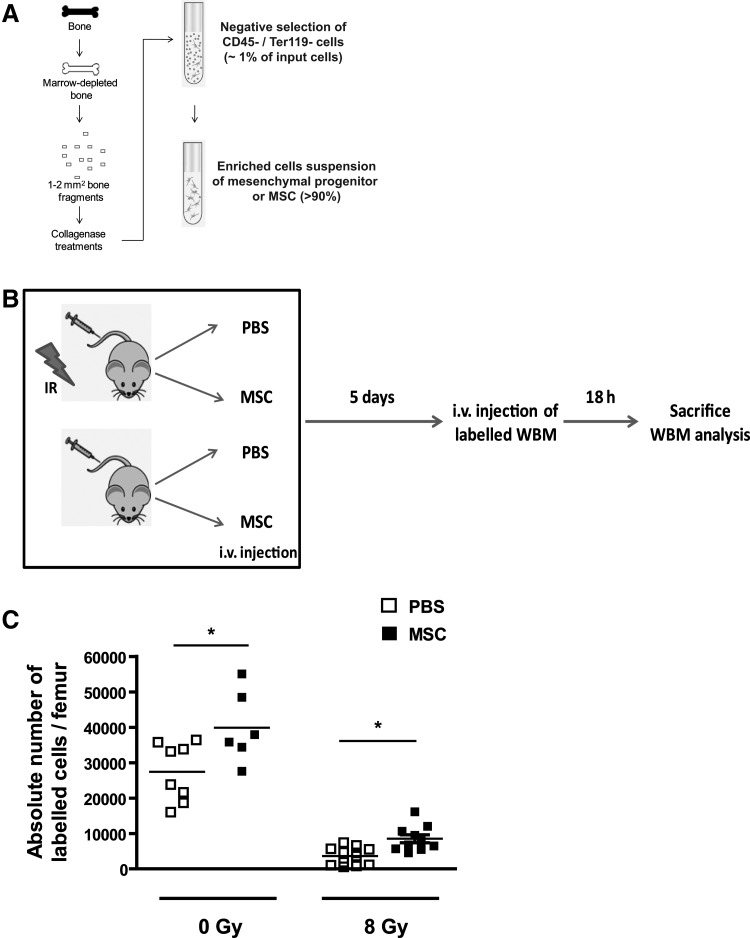
To determine the impact of cell therapy on homing, we used a population of fresh (uncultured) MSCs obtained from collagenase-treated bone and an antibody-based magnetic negative selection process (Fig. 2a). These purified cells were phenotypically defined as CD45− CD31− CD44+ CD90dim and expressed a series of osteoblast-specific genes as shown in Carbonneau et al.[23] We chose to work with this stromal population given that we had previously determined that it was damaged short term (1 week) following exposure to IR [23], and to obtain a sufficient number of cells without the need to expand them in vitro. Indeed, Rambout and Ploemacher showed that in vitro expansion of BM stromal cells compromises their functions at the niche, an observation that has subsequently been confirmed by others [25–27].
FIG. 2.
Injection of mesenchymal stromal cells (MSCs) partially restores homing post-IR. (A) Illustration of the procedure used to isolate MSCs from marrow depleted bones resulting on average in >100-fold enrichment over a nonselected population. (B) Schematic of the experimental design used to determined homing in mice that were exposed (+) or not (−) to IR (8 Gy) and immediately after injected intravenously (i.v.) with 5×104 MSCs or phosphate-buffered saline (PBS) alone. Mice were allowed a 5-day period to allow for the niche regeneration before homing was determined. (C) Homing was determined by counting by flow cytometry the absolute number of PKH26+ bone marrow cells in the femurs of mice. n=8–12, each symbol representing an individual mouse. Student t tests *P<0.05 are shown.
Thus, mice were injected intravenously with 5×104 purified stromal cells or PBS few hours following their exposure to IR. Mice were then allowed a 5-day regeneration period before homing was evaluated (Fig. 2b). Using this protocol, we observed that the injection of stromal cells could increase homing by as much as 135% in mice exposed to IR (Fig. 2c). Noteworthy, variations in the number of stromal cells injected (range 1×104 to 1×105) did not benefit homing further (data not shown). Surprisingly, injection of stromal cells in nonirradiated mice could also increase homing by 45% (Fig. 2c), suggesting that injection of stromal cells is beneficial independently of damage to the microenvironment. Still, despite a very significant benefit from the injection of stromal cells, our results also showed that homing is approximately 3-fold lower than the baseline level observed in nonirradiated mice and that alternative or complementary strategies to stromal cell therapy need to be explored to better restore homing.
Increased levels of G-CSF in the marrow of irradiated mice
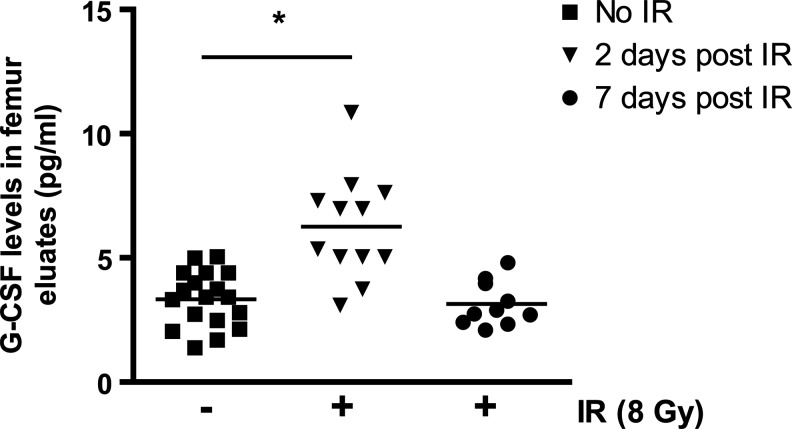
The profile of secreted cytokines within the BM microenvironment is known to be modified in response to IR; an example would be the increased expression of SDF-1α [6,28]. Among cytokines whose expression could negatively impact homing, the G-CSF is certainly a strong candidate. Whether the level of G-CSF within the niche varies following exposure to IR, however, is not known. To answer this question, we measured by ELISA the level of G-CSF in the marrow eluate of irradiated compared to nonirradiated mice femurs. Levels of G-CSF were augmented by as much as 70% forty-eight hours after exposure to IR, an increase we found was transient given that levels returned to control values 5 days later (Fig. 3). Quantitative real-time PCR analysis performed on purified marrow stromal and hematopoietic cells did not reveal any variation at the mRNA level, suggesting the absence of a G-CSF transcriptional regulation mechanism (data not shown). Based on this finding, and given the potential of G-CSF as a mobilizing agent, we hypothesized that inhibition of the G-CSF may have the potential to increase homing/retention of cells to the BM.
FIG. 3.
Increased level of granulocyte colony stimulating factor (G-CSF) in the bone marrow of irradiated mice. G-CSF levels present in the marrow eluates (see methods) collected 2 days and 1 week postexposure of mice to IR (8 Gy) and compared to levels in control (CTR) nonirradiated mice. n=10–18 femur eluates per group. One-way ANOVA test *P<0.05.
Expression of a soluble G-CSF decoy receptor increases homing and hematopoietic reconstitution
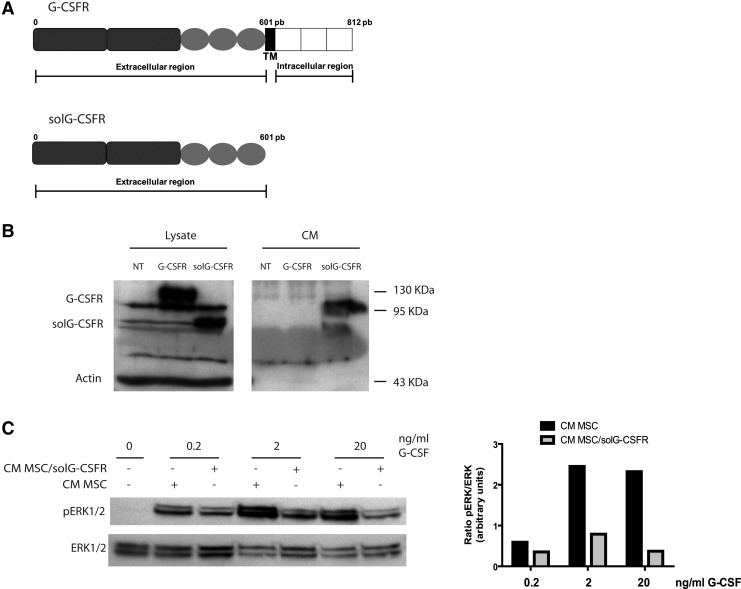
One method by which inhibition of G-CSF could be achieved is through the expression of a soluble G-CSF decoy receptor (solG-CSFR), which if expressed at a sufficiently high level, could sequester an endogenous G-CSF. Accordingly, we engineered a truncated version of the G-CSFR lacking 211 amino acids on the C-terminus corresponding to the transmembrane and intracellular domains (Fig. 4a). Cloning of the full and truncated versions of the G-CSFR in lentiviral vectors confirmed their stable expression at the expected molecular weight (Fig. 4b). Importantly, the presence of the soluble receptor was only detected in the supernatant of cells transduced with the truncated form of the G-CSFR. The capacity of the solG-CSFR to sequester G-CSF was demonstrated by showing reduced ability of recombinant G-CSF to induced ERK1/2 phosphorylation in the presence of CM containing the decoy receptor (Fig. 4c).
FIG. 4.
Generation of a soluble G-CSF decoy receptor. (A) Schematic of the G-CSF receptor and its truncated soluble version lacking the transmembrane and extracellular domains. TM=transmembrane domain. (B) Western blot analysis of G-CSF receptor expression in cell lysates and conditioned media (CM) of 293T cells that were either nontransfected (NT), transfected with the full length G-CSFR receptor (G-CSFR) or the soluble G-CSF decoy receptor (solG-CSFR). (C) Western blot analysis of pERK1/2 and total ERK1/2 expression in cell lysates of cultured murine MSCs stimulated with the indicated amount of G-CSF in presence (+) or absence (−) of CM collected from nontransduced MSCs or MSCs expressing the solG-CSFR. Also shown is the inhibition of pERK by CM collected from MSCs expressing the solG-CSFR as determined by the ratio of pERK/ERK in arbitrary units. The bar graph shown is representative of n=3 individual blots.
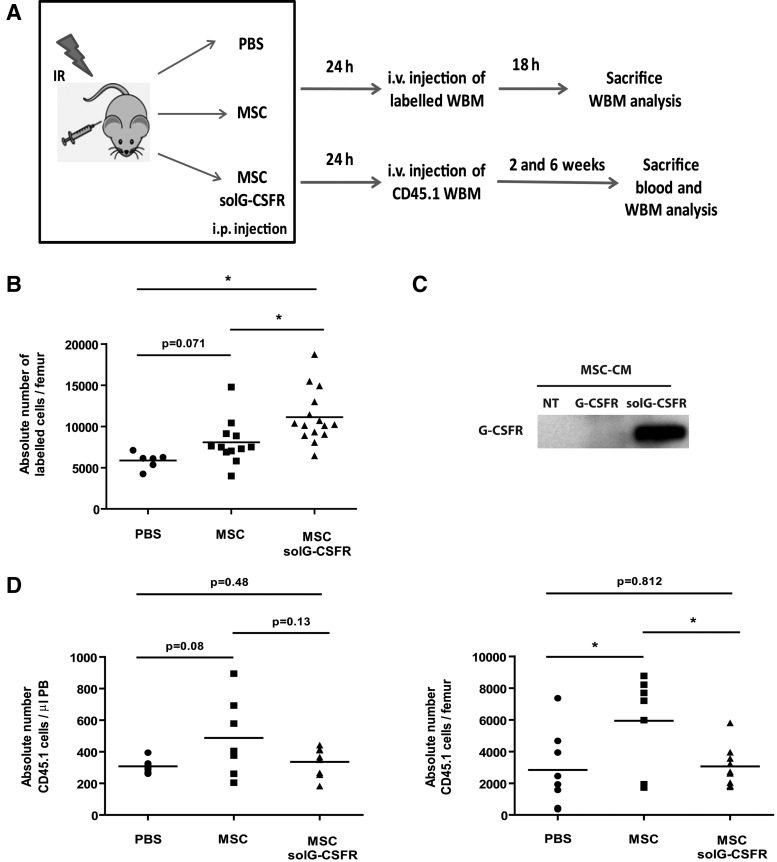
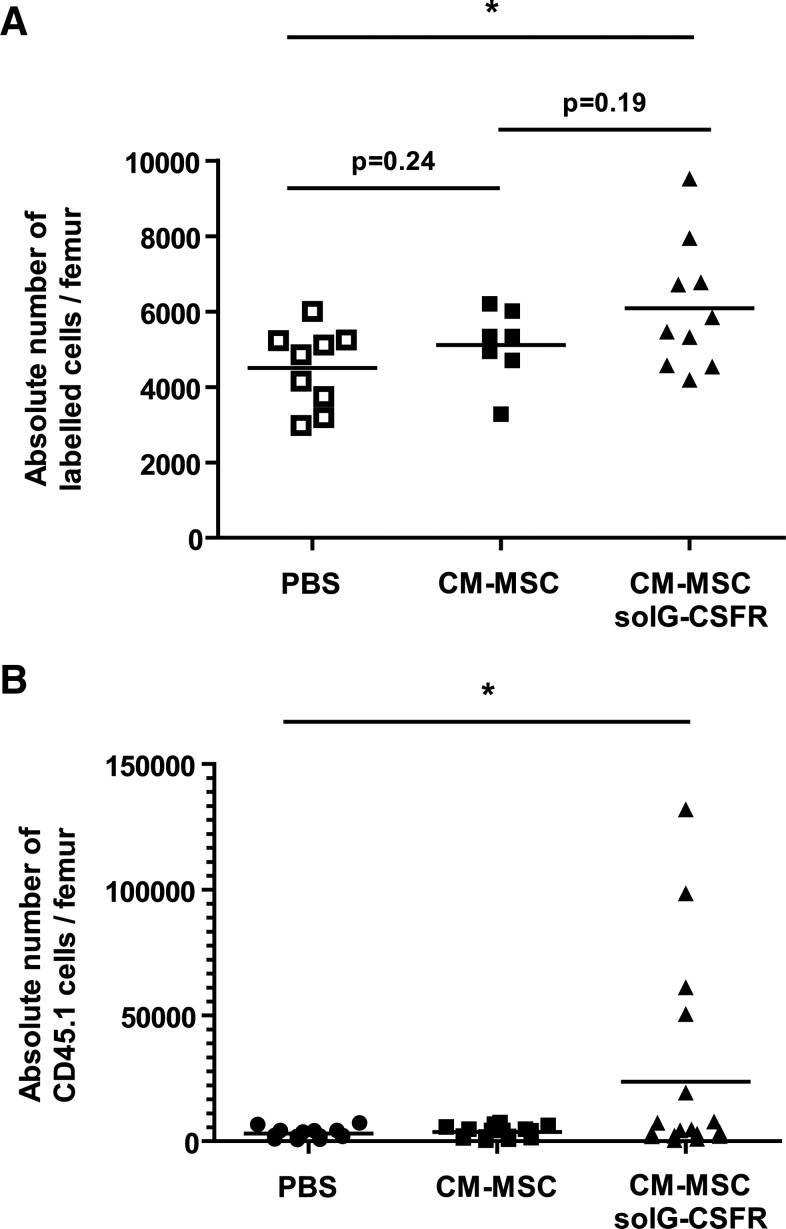
As a first attempt to sequester G-CSF systemically, we used cultured MSCs as a delivery vehicle to express the solG-CSFR in mice. We choose an experimental protocol in which 15×106 stromal cells were injected intraperitoneally a few hours after exposure of mice to IR and 1 day before homing was determined (Fig. 5a). We found the injection of stromal cells secreting the solG-CSFR could increase homing by as much as 90% when compared to mice who received PBS (Fig. 5b, c). In comparison, intraperitoneal injection of unmodified cultured stromal cells conferred only a 37% increase in homing (Fig. 5b), confirming the effectiveness of the solG-CSFR in increasing homing in this model. However, and as expected, constitutive secretion of the solG-CSFR from intraperitoneally injected stromal cells impaired hematopoietic reconstitution. Indeed, its secretion was shown to offset the beneficial action of MSCs on hematopoietic reconstitution as shown by the lower level of CD45.1 donor cells in blood and marrow of recipient mice at 13 and 45 days postinjection, respectively (Fig. 5d). In view of this, we repeated the experiments using this time-concentrated CM collected from MSCs secreting or not the solG-CSFR. Using this approach, we observed that the injection of the solG-CSFR could increase homing and accelerate short-term hematopoietic recovery (Fig. 6). Results from this proof-of-concept experiment suggest that the transient inhibition of the G-CSF by a recombinant solG-CSFR may have the potential to increase homing and could therefore represent a novel therapeutic approach to be used alone or in combination with other homing strategies.
FIG. 5.
Altered homing and engraftment by the secretion of a soluble G-CSF decoy receptor. (A) Schematic of the experiment. In brief, CD45.2 mice were sublethally irradiated at the dose of 8 Gy and immediately injected intraperitoneally (i.p.) with PBS alone or 15×106 MSCs modified or not to secrete the solG-CSFR. Twenty-four hours later, mice were injected intravenously (i.v.) with 2×106 CD45.1 or 5×106 unfractionated whole bone marrow (WBM) cells labeled with CFSE. (B) Shown is the absolute number of fluorescently labeled cells present in bone marrow (femurs) at the time of sacrifice as determined by flow cytometry. n=6–15 mice per group, each symbol representing an individual mouse. (C) The presence of the solG-CSFR in the CM of stably transduced cultured MSCs was confirmed by western blot (NT=not transduced). (D) Engraftment levels in blood and bone marrow were determined at 13 and 45 days, respectively, following the injection of WBM cells. Absolute counts of CD45.1 cells were determined by flow cytometry. n=7–9 mice per group, each symbol representing an individual mouse. Student t tests *P<0.05 are shown.
FIG. 6.
Injection of CM collected from MSCs is sufficient to alter homing and hematopoietic reconstitution. We used the same experimental design as in figure 5 except that conditioned media collected from MSCs (CM-MSCs) or MSCs secreting the solG-CSFR (CM-MSCs solG-CSRR) were injected before the injection of 2×105 CD45.1 or 5×106 unfractionated whole bone marrow (WBM) cells labeled with CFSE. (A) Shown is the absolute number of fluorescently labeled cells present in bone marrow (femurs) at the time of sacrifice as determined by flow cytometry. n=7–10 mice per group, each symbol representing an individual mouse. (B) Hematopoietic reconstitution levels in bone marrow were determined 1 week following the injection of CD45.1 cells. n=14–17 mice per group, each symbol representing an individual mouse. Student t tests *P<0.05 are shown.
Discussion
Homing of hematopoietic cells to the BM microenvironment is a complex physiological process for which the cellular and molecular determinants are not fully understood. We provide evidences that homing is severely impaired in a time-dependent manner postexposure to IR, an effect that can be partially rescued by the injection of MSCs. Intriguingly, the injection of stromal cells could increase homing in both irradiated and nonirradiated mice, suggesting that one mechanism by which stromal cells benefit homing is independent of their expected role in regenerating the niche. How exactly the injection of stromal cells positively impact homing in the absence of damage is not known, but may arise from an apparent increase in the size of the osteoblastic niche, which was showed to correlate with homing [29].
Our results also revealed that homing is still severely impaired in irradiated mice that received stromal cell therapy, an observation that may be explained in several ways. First, despite using stromal cells that had not been previously expanded in culture, only very few cells (less than 1% of the total injected) were found within the BM space 1 day after their injection (data not shown), suggesting inefficient homing of stromal cells themselves is likely a problem. Second, it is well known that exposure to IR leads not only to the apoptosis of hematopoietic cells, but also to the destruction of the BM vasculature [30], a phenotype that likely prevents the proper distribution of cells injected intravenously. With that in mind, we have tried preventing IR-induced damage by using pifithrin-α, an inhibitor of p53 who was showed to inhibit apoptosis in irradiated mice [31,32]. Unfortunately, the injection of pifithrin-α, at a dose reported to efficiently prevent apoptosis in mice, did not improve homing in our model (data not shown). Third, it is possible the amount or site of injection of stromal cells we used were inadequate to fully restore homing. These limitations originate from the fact that we worked with a stromal cell population that had not been previously expanded in vitro and, thus, for which only a very limited number of cells can be obtained. Indeed, the procedure we used only allowed the purification of 2−3×104 cells from one donor mouse following the collagenase treatment of long bones from both legs and iliac crest. Still, we believe that using noncultured stromal cell populations is highly preferable given we found the intravenous injection of an equal amount of BM stromal cells obtained following in vitro expansion had no beneficial impact on homing (data not shown). While it is difficult to compare expanded stromal cell populations with more heterogeneous freshly sorted cells, these results underline the importance of developing in vitro expansion protocols that would preserve the cellular functions and/or properties of these cells [25–27]. Noteworthy, marrow stromal cells obtained following in vitro expansion appeared to positively impact homing and hematopoietic reconstitution when injected intraperitoneally at the dose of 15×106 (Fig. 5b, d), suggesting these cells, despite the large number injected, still retained some capacity in enhancing homing.
Looking for alternative strategies to restore homing post-IR, we made the observation that the level of G-CSF is increased in marrow eluates shortly after IR. This increase was transient and did not appear to be transcriptionally regulated, suggesting that G-CSF may have been released in the marrow space from dying cells or left unconsumed because of cellular aplasia induced short-term post-IR. We believe an increase in the level of G-CSF post-IR could have a long-lasting impact on homing, possibly through the effects of G-CSF on osteoblasts homeostasis and the SDF-1α/CXCR4 axis [3,33]. It is also worth mentioning that we also observed an increase in G-CSF levels in serum of aged (20–24 months) compared to young (3–4 months) mice (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/scd). Given that aged mice have a higher number of circulating stem/progenitors cells in blood compared to young mice [34], it is tempting to speculate that small variations in the endogenous level of G-CSF may be sufficient to favor the egress of cells out of the marrow.
Our finding that a solG-CSFR can improve homing and accelerate hematopoietic reconstitution in mice is the first proof-of-concept demonstration that the inhibition of endogenous G-CSF could represent a novel therapeutic strategy. Whether this approach could be used to enhance long-term engraftment in a limiting dilution or humanized mouse model remains to be determined. Of note, the impact of our decoy receptor on homing was superior to the action of a G-CSF blocking antibody which, when injected at a dose of 10 μg per mouse (a dose reported to inhibit the action of G-CSF in mice), [35] did not significantly increase homing (Supplementary Fig. S2). Moreover, we believe the experimental conditions used in our study can be further optimized by the future development of a purified recombinant version of the solG-CSFR, which could then be injected at an optimized concentration/frequency. Also, the development of a recombinant decoy receptor with a greater affinity to G-CSF compared to the endogenous receptor may further increase its potential. As such, whether our approach could be used to enhance long-term engraftment in a limiting dilution or humanized mouse model remains to be determined.
In conclusion, our results demonstrate that homing is severely compromised in a time-dependent manner following exposure to IR, a phenotype we think is currently overlooked in the clinic. We believe it may be beneficial to develop BM transplantation protocols in which HSCs are injected more rapidly after BM conditioning. Our results also suggest that a better knowledge of cytokines released in the BM microenvironment following IR-induced damage could lead to the development of novel strategies to improve homing.
Supplementary Material
Acknowledgments
We would like to thank Vimal Krishnan and an anonymous reviewer for critical reading of the manuscript and Denise Carrier for her help with mice. This work was supported by grants from the Canadian Institute of Health Research (CIHR) #MOP-102709 and the Fonds de la recherche en santé du Québec (FRSQ) to C.M.B. B.B. have been supported by a postdoctoral fellowship from the FRSQ, C.L.C. by a studentship from the CIHR, A.F. and L.P. have been supported by a studentship from the Fondation des étoiles, and C.M.B. by a scientist award from the FRSQ.
Author Disclosure Statement
The authors declare no competing financial interests.
References
- 1.Rocha V. Broxmeyer HE. New approaches for improving engraftment after cord blood transplantation. Biol Blood Marrow Transplant. 2010;16:S126–S132. doi: 10.1016/j.bbmt.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Jetmore A. Plett PA. Tong X. Wolber FM. Breese R. Abonour R. Orschell-Traycoff CM. Srour EF. Homing efficiency, cell cycle kinetics, and survival of quiescent and cycling human CD34(+) cells transplanted into conditioned NOD/SCID recipients. Blood. 2002;99:1585–1593. doi: 10.1182/blood.v99.5.1585. [DOI] [PubMed] [Google Scholar]
- 3.Petit I. Szyper-Kravitz M. Nagler A. Lahav M. Peled A. Habler L. Ponomaryov T. Taichman RS. Arenzana-Seisdedos F, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 4.Sato N. Sawada K. Takahashi TA. Mogi Y. Asano S. Koike T. Sekiguchi S. A time course study for optimal harvest of peripheral blood progenitor cells by granulocyte colony-stimulating factor in healthy volunteers. Exp Hematol. 1994;22:973–978. [PubMed] [Google Scholar]
- 5.Aiuti A. Webb IJ. Bleul C. Springer T. Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185:111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ponomaryov T. Peled A. Petit I. Taichman RS. Habler L. Sandbank J. Arenzana-Seisdedos F. Magerus A. Caruz A, et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000;106:1331–1339. doi: 10.1172/JCI10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boitano AE. Wang J. Romeo R. Bouchez LC. Parker AE. Sutton SE. Walker JR. Flaveny CA. Perdew GH, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329:1345–1348. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell TB. Hangoc G. Liu Y. Pollok K. Broxmeyer HE. Inhibition of CD26 in human cord blood CD34+ cells enhances their engraftment of nonobese diabetic/severe combined immunodeficiency mice. Stem Cells Dev. 2007;16:347–354. doi: 10.1089/scd.2007.9995. [DOI] [PubMed] [Google Scholar]
- 9.Christopherson KW., 2nd Hangoc G. Mantel CR. Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 10.Hoggatt J. Singh P. Sampath J. Pelus LM. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113:5444–5455. doi: 10.1182/blood-2009-01-201335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castello S. Podesta M. Menditto VG. Ibatici A. Pitto A. Figari O. Scarpati D. Magrassi L. Bacigalupo A. Piaggio G. Frassoni F. Intra-bone marrow injection of bone marrow and cord blood cells: an alternative way of transplantation associated with a higher seeding efficiency. Exp Hematol. 2004;32:782–787. doi: 10.1016/j.exphem.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 12.Frassoni F. Gualandi F. Podesta M. Raiola AM. Ibatici A. Piaggio G. Sessarego M. Sessarego N. Gobbi M, et al. Direct intrabone transplant of unrelated cord-blood cells in acute leukaemia: a phase I/II study. Lancet Oncol. 2008;9:831–839. doi: 10.1016/S1470-2045(08)70180-3. [DOI] [PubMed] [Google Scholar]
- 13.Brunstein CG. Barker JN. Weisdorf DJ. Defor TE. McKenna D. Chong SY. Miller JS. McGlave PB. Wagner JE. Intra-BM injection to enhance engraftment after myeloablative umbilical cord blood transplantation with two partially HLA-matched units. Bone Marrow Transplant. 2009;43:935–940. doi: 10.1038/bmt.2008.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ball LM. Bernardo ME. Roelofs H. Lankester A. Cometa A. Egeler RM. Locatelli F. Fibbe WE. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood. 2007;110:2764–2767. doi: 10.1182/blood-2007-04-087056. [DOI] [PubMed] [Google Scholar]
- 15.Bernardo ME. Ball LM. Cometa AM. Roelofs H. Zecca M. Avanzini MA. Bertaina A. Vinti L. Lankester A, et al. Co-infusion of ex vivo-expanded, parental MSCs prevents life-threatening acute GVHD, but does not reduce the risk of graft failure in pediatric patients undergoing allogeneic umbilical cord blood transplantation. Bone Marrow Transplant. 2011;46:200–207. doi: 10.1038/bmt.2010.87. [DOI] [PubMed] [Google Scholar]
- 16.Koc ON. Gerson SL. Cooper BW. Dyhouse SM. Haynesworth SE. Caplan AI. Lazarus HM. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18:307–316. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- 17.Bernardo ME. Cometa AM. Locatelli F. Mesenchymal stromal cells: a novel and effective strategy for facilitating engraftment and accelerating hematopoietic recovery after transplantation? Bone Marrow Transplant. 2011;47:323–329. doi: 10.1038/bmt.2011.102. [DOI] [PubMed] [Google Scholar]
- 18.Chan SL. Choi M. Wnendt S. Kraus M. Teng E. Leong HF. Merchav S. Enhanced in vivo homing of uncultured and selectively amplified cord blood CD34+ cells by cotransplantation with cord blood-derived unrestricted somatic stem cells. Stem Cells. 2007;25:529–536. doi: 10.1634/stemcells.2005-0639. [DOI] [PubMed] [Google Scholar]
- 19.Nakao N. Nakayama T. Yahata T. Muguruma Y. Saito S. Miyata Y. Yamamoto K. Naoe T. Adipose tissue-derived mesenchymal stem cells facilitate hematopoiesis in vitro and in vivo: advantages over bone marrow-derived mesenchymal stem cells. Am J Pathol. 2010;177:547–554. doi: 10.2353/ajpath.2010.091042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noort WA. Kruisselbrink AB. in't Anker PS. Kruger M. van Bezooijen RL. de Paus RA. Heemskerk MH. Lowik CW. Falkenburg JH. Willemze R. Fibbe WE. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34(+) cells in NOD/SCID mice. Exp Hematol. 2002;30:870–878. doi: 10.1016/s0301-472x(02)00820-2. [DOI] [PubMed] [Google Scholar]
- 21.Maijenburg MW. van der Schoot CE. Voermans C. Mesenchymal stromal cell migration: possibilities to improve cellular therapy. Stem Cells Dev. 2012;21:19–29. doi: 10.1089/scd.2011.0270. [DOI] [PubMed] [Google Scholar]
- 22.Naldini L. Blomer U. Gallay P. Ory D. Mulligan R. Gage FH. Verma IM. Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 23.Carbonneau CL. Despars G. Rojas-Sutterlin S. Fortin A. Le O. Hoang T. Beausejour CM. Ionizing radiation-induced expression of INK4a/ARF in murine bone marrow-derived stromal cell populations interferes with bone marrow homeostasis. Blood. 2012;119:717–726. doi: 10.1182/blood-2011-06-361626. [DOI] [PubMed] [Google Scholar]
- 24.Collis SJ. Neutzel S. Thompson TL. Swartz MJ. Dillehay LE. Collector MI. Sharkis SJ. DeWeese TL. Hematopoietic progenitor stem cell homing in mice lethally irradiated with ionizing radiation at differing dose rates. Radiat Res. 2004;162:48–55. doi: 10.1667/rr3197. [DOI] [PubMed] [Google Scholar]
- 25.Morikawa S. Mabuchi Y. Kubota Y. Nagai Y. Niibe K. Hiratsu E. Suzuki S. Miyauchi-Hara C. Nagoshi N, et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009;206:2483–2496. doi: 10.1084/jem.20091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rombouts WJ. Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003;17:160–170. doi: 10.1038/sj.leu.2402763. [DOI] [PubMed] [Google Scholar]
- 27.Wang L. Liu Y. Kalajzic Z. Jiang X. Rowe DW. Heterogeneity of engrafted bone-lining cells after systemic and local transplantation. Blood. 2005;106:3650–3657. doi: 10.1182/blood-2005-02-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tabatabai G. Frank B. Mohle R. Weller M. Wick W. Irradiation and hypoxia promote homing of haematopoietic progenitor cells towards gliomas by TGF-beta-dependent HIF-1alpha-mediated induction of CXCL12. Brain. 2006;129:2426–2435. doi: 10.1093/brain/awl173. [DOI] [PubMed] [Google Scholar]
- 29.Calvi LM. Adams GB. Weibrecht KW. Weber JM. Olson DP. Knight MC. Martin RP. Schipani E. Divieti P, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 30.Kopp HG. Avecilla ST. Hooper AT. Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology. 2005;20:349–356. doi: 10.1152/physiol.00025.2005. [DOI] [PubMed] [Google Scholar]
- 31.Komarov PG. Komarova EA. Kondratov RV. Christov-Tselkov K. Coon JS. Chernov MV. Gudkov AV. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999;285:1733–1737. doi: 10.1126/science.285.5434.1733. [DOI] [PubMed] [Google Scholar]
- 32.Strom E. Sathe S. Komarov PG. Chernova OB. Pavlovska I. Shyshynova I. Bosykh DA. Burdelya LG. Macklis RM, et al. Small-molecule inhibitor of p53 binding to mitochondria protects mice from gamma radiation. Nat Chem Biol. 2006;2:474–479. doi: 10.1038/nchembio809. [DOI] [PubMed] [Google Scholar]
- 33.Christopher MJ. Rao M. Liu F. Woloszynek JR. Link DC. Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice. J Exp Med. 2011;208:251–260. doi: 10.1084/jem.20101700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrison SJ. Wandycz AM. Akashi K. Globerson A. Weissman IL. The aging of hematopoietic stem cells. Nat Med. 1996;2:1011–1016. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- 35.Shojaei F. Wu X. Zhong C. Yu L. Liang XH. Yao J. Blanchard D. Bais C. Peale FV, et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature. 2007;450:825–831. doi: 10.1038/nature06348. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.