Abstract
Evidence supporting that deleted in azoospermia-like (DAZL) plays a key role during gametogenesis and meiosis continues to emerge. Our study aimed to determine whether overexpression of DAZL using a lentiviral approach in a somatic stem cell to germ cell in vitro differentiation culture could enhance the formation of primordial germ cell-like cells (PLCs) and oocyte-like cells (OLCs). Introduction of DAZL at the beginning of induced differentiation significantly increased the formation of Fragilis-positive PLCs, which was independent of mitotic proliferation. In addition, mRNA levels of the germ cell markers Oct4, Stella, and Vasa were also higher in the DAZL-transduced group and suppressed when DAZL was knocked down using small interference RNA. At later stages of differentiation, the expression of several genes associated with meiosis, including Scp3, Dmc1, Rec8, and Stra8, was determined to be significantly higher when DAZL was overexpressed, which was abrogated by its knockdown. Exogenous introduction of DAZL also increased the protein levels of SCP3 and VASA, which again was reversed by its knockdown. Although not a common phenomenon in the in vitro differentiation system, the percentage of SCP3-positive cells displaying meiotic chromosome patterns in the DAZL-transduced group was higher than in the control, as was the overall percentage of OLCs that were generated. The introduction of factors such as DAZL into a stem cell-to-germ cell differentiation culture may provide an opportunity to better understand the key genes and their interactions during gametogenesis, also providing a means to enhance the generation of germ cells in vitro.
Introduction
Research into the development of germ cells and primordial germ cells (PGCs), in particular, has met with considerable technical challenges due to their low numbers (<5,000 in the mouse), their location deep within the developing embryo, and their mobile nature during migration [1,2]. Although the temporal order in vivo of gametogenic events may not be precisely captured in vitro, an in vitro model is nevertheless a viable alternative to study germ cell development, offering insights into factors and mechanisms related to germ cell formation, migration, proliferation, and further differentiation into functional germ cells. The ability to generate germ cells from somatic stem cells may provide an alternative in vitro model to study germ cell biology. We have previously reported that stem cells isolated from porcine fetal skin give rise to a subpopulation of morphologically distinct PGC-like cells (PLCs) in vitro upon induction in a differentiation medium [3]. These cells are alkaline phosphatase-positive and express Oct4, Fragilis, Stella, deleted in azoospermia-like (DAZL), and VASA, which are markers indicative of germ cell formation. The PGC-like cells also undergo imprint erasure, and are capable of further differentiating into oocyte-like cells (OLCs) [3–5]. Using this model, we previously revealed that the heparin-binding growth factor midkine is a mitogen of PLCs [6], and its effect is partially elicited due to suppression of the Dazl gene. This finding was further confirmed in PGCs isolated from fetal gonads [6], validating the use of this in vitro derived model for research into the biology of PGCs.
The Dazl gene is a member of the DAZ family. This family comprises DAZ, DAZL, and BOULE, which encode a group of RNA-binding proteins [7]. The common features of the DAZ family include a conserved ribonucleoprotein-type RNA-recognition motif (RRM), and varying numbers of a unique 24-amino-acid DAZ repeat [7]. DAZL is known to be expressed during gametogenesis in both PGCs and oocytes at all stages of maturation in humans, mice, pigs, and Xenopus laevis [8–11]. The expression of DAZ family genes is specific to germ cells [12]. In addition, the role of the DAZ family on germline development appears to be conserved across species from invertebrates to mammals, which is evident by the finding that human Dazl can partially rescue the Drosophila boule mutant [13]. Disruption of the Dazl gene in mice results in a loss of germ cells and complete absence of gametes in the female, and a failure to differentiate past meiotic prophase I during spermatogenesis [8,14]. The current study sought to apply our established in vitro derived model to study the influence of DAZL on germ cell formation and differentiation.
Materials and Methods
Isolation and culture of skin-derived stem cells
All experiments related to animal material were conducted according to the Care and Use of Experimental Animals of the Canadian Council on Animal Care Guidelines, and have been approved by the University of Guelph Animal Care and Use Committee. Porcine skin-derived stem cells (SSCs) were isolated and cultured as previously described [15] from the skin of fetuses collected at day 40–45 of gestation (E40–45). Cells were passaged twice before use. SSCs from 4–6 fetuses were combined and used in each of the experiments.
Induced differentiation
Porcine PLCs and OLCs were differentiated from SSCs as previously described [3,16]. Briefly, skin sphere cells were dissociated mechanically by pipetting and plated at 1×105 cells per 60-mm tissue culture dish (Fisher Scientific Corning) treated with 0.05 mg/μL of poly-d-lysine (Sigma-Aldrich) and 0.005 mg/mL of Laminin (Sigma-Aldrich). Cells were cultured in a 0.22 μm-filtered differentiation medium [Dulbecco's modified Eagle's medium (DMEM-high glucose; Gibco), 5% heat-inactivated fetal bovine serum (FBS; Invitrogen; lot No. 586696), 0.1 mM nonessential amino acids (NEAA; Invitrogen), 0.1 mM β-mercaptoethanol (Sigma-Aldrich), and 5% porcine follicular fluid]. The porcine follicular fluid was harvested from both small (1–3 mm) and large (4–6 mm) follicles, adhering to a collection ratio of 3 small for every one large follicle. It was then centrifuged at 1,500 g for 30 min, and the supernatant was passed through a 0.22-μm filter, and stored at −80°C. Half of the medium was removed and replaced with a fresh medium every 4 days. The cultures were maintained for 30–50 days. All culturing was carried out at 38.5°C in 5% CO2.
Lentiviral transduction of the differentiating cells
The pL-SIN-Lenti-EF1α-GFP control (GFP-Con) lentiviral gene-transfer plasmid, a kind gift from Dr. Ellis, was previously used in our laboratory to successfully transduce PLCs, which gave rise to OLCs that robustly expressed GFP after further induced differentiation in vitro [3]. The pL-SIN-Lenti-EF1α-DAZL-IRES-GFP (DAZL-GFP) plasmid was constructed by inserting the complete coding sequence of the porcine Dazl gene, previously cloned from porcine oocytes [10], upstream of an internal ribosome entry site (IRES). This fragment was then transferred upstream of the GFP-coding sequence into the existing pL-SIN-Lenti-EF1α-GFP lentiviral plasmid in a manner allowing the elongation factor 1α (EF1α) promoter to drive expression of both Dazl and Gfp. DAZL- or GFP-only (GFP-Con) recombinant lentiviral particles were produced as described [3], with the following modifications. Transfected 293FT cells were cultured in a 293FT culture medium (DMEM high-glucose supplemented with 10% FBS and 1% NEAA) to produce recombinant lentivirus. The lentiviral medium was collected, passed through 0.45-μm filters, and frozen at −80°C. At day 2 (D2) of induced differentiation, the medium was replaced with 2 mL of lentivirus-containing medium supplemented with 8 μg/mL of polybrene (Sigma-Aldrich), followed by incubation for 24 h at 38.5°C, after which the medium was replaced with 3 mL of fresh differentiation medium. After 3 to 5 days, cells were evaluated for GFP expression.
RNA isolation and real-time polymerase chain reaction
At D24 and D36 of differentiation, differentiating cells were harvested with 0.02% EDTA treatment for 10 min at room temperature, and total RNA was isolated using the Total RNA Kit (Norgen Biotek Corporation, Thorold, Canada) according to the manufacturer's protocol. Reverse transcription was performed as previously described [15]. Samples were DNase treated by adding 1 μL of 10× DNase buffer and 1 U of amplification-grade DNase (Invitrogen) and then incubated for 15 min at room temperature. One microliter of EDTA (25 mM) was then added, and the samples were incubated for 10 min at 65°C. Reverse transcription (RT) was then performed by adding 0.5 μL of H2O, 5 μL of 5× first-strand buffer, 1.25 μL of random hexamer primers, 6.25 μL of 2 mM dNTPs, and 1 μL of MMLV reverse transcriptase. The samples were then incubated at 25°C for 10 min, 37°C for 50 min, and 70°C for 15 min.
Real-time polymerase chain reaction (PCR) was carried out on a Smart Cycler (Cepheid) using the Quantitect SYBR green PCR kit (Takara). A total of 500 ng of DNase-treated cDNA was added to 6.25 μL of SYBR green mix, 0.25 μL of ROX, and 0.25 μL of each forward and reverse primer at 10 pmol (final reaction volume equal to 12.5 μL). Primers, expected product sizes, and GenBank accession numbers are presented in Table 1.
Table 1.
Primers in Real-Time Polymerase Chain Reaction
| Genes | Primer sequences | Accession no., reference | Product size (bp) |
|---|---|---|---|
| RPII | F: 5′-caggagtggatcctggagac-3′ | Linher et al. [3] | 181 |
| R: 5′-ggagccatcaaaggagatga-3′ | |||
| DAZL | F: 5′-cctccaaccatgatgaatcc-3′ | Gi:31542548 | 222 |
| R: 5′-gggcaaaatatcagctcctg-3′ | |||
| OCT4 | F: 5′-cacctcaggtcggagtgg-3′ | Aj251914 | 160 |
| R: 5′-agcttggcaaattgttcgag-3′ | |||
| VASA | F: 5′-ttgcaggacgagatttgatg-3′ | AY626785 | 165 |
| R: 5′-ccaattctcgagttggtgt-3′ | |||
| FRAGILIS | F: 5′-catgtcgtctggtccctgt-3′ | Gi:47682386 | 137 |
| R: 5′-gtggaggcataggcctgg-3′ | |||
| STELLA | F: 5′-ttaatccaacccggactcag-3′ | Gi:181341661 | 173 |
| R: 5′-tggttgaggtggatattctgg-3′ | |||
| SCP3 | F: 5′-agccgtctgtggaagatcag-3′ | NM_153694 | 197 |
| R: 5′-aactccaactccttccagca-3′ | |||
| DMC1 | F: 5′-gggatacaaatgacaacaag-3′ | D64107, CV876801 | 239 |
| R: 5′-cgaaattctccaaaagcttc-3′ | |||
| REC8 | F: 5′-agcctgcttcttcctaacca-3′ | XM_001928117 | 155 |
| R: 5′-actttctctggggtcacagc-3′ | |||
| STRA8 | F: 5′-tcccgttgatgatgagatga-3′ | XM_003134656 | 179 |
| R: 5′-acagcccactccaaaacatc-3′ | |||
| GDF9b | F: 5′-ggatccagaaaagcacaacc-3′ | AF458070 | 227 |
| R: 5′-agtgtccagggatgaaatgc-3′ | |||
| CCND3 | F: 5′-tcacaggcactgagtggac-3′ | Liu et al. [10] | 176 |
| R: 5′-gatggctgtgacatctgtgg-3′ |
5-Bromo-2-deoxyuridine incorporation assay
At D24 of differentiation, nonadherent PLCs were collected from 60-mm dishes containing differentiating cells, and equal cell numbers in both the DAZL- and GFP-Con-transduced groups were seeded into wells of a 24-well plate (SARSTEDT, 83.1836), and cells were cultured in 30 μg/mL of 5-bromo-2-deoxyuridine (BrdU) for 48 h, followed by immunostaining. The BrdU incorporation assay was performed as described previously [15]. Individual fluorescent cells were counted based on both the BrdU signal and propidium iodide nuclear staining. At least, 300 cells were counted on each slide. Three independent cultures were analyzed for BrdU uptake.
Immunocytochemistry
At D24, nonadherent and loosely adherent PLCs were collected by incubating in 0.02% EDTA for 10 min at room temperature. Cells were washed twice with phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde (PFA; Fisher Scientific) in PBS for 20 min. Cells were then washed 3 times in PBS with 0.1% Tween 20 (Fisher Scientific) and incubated for 10 min, and then for 20 min in PBS with 0.1% Triton X-100 (Fisher Scientific). Cells were blocked for 2 h in PBS with 2% BSA (PBS-B) and 0.05% Triton X-100 (blocking solution), followed by an incubation with 1:400 rabbit polyclonal anti-Fragilis (Abcam) primary antibody overnight at 4°C. Cells were then washed in a blocking solution and incubated with 1:300 goat anti-rabbit IgG-R-phycoerythrin (PE; Sigma) for 2 h at room temperature. This was followed with a blocking solution wash (PBS-B) and incubation with Hoechst333258 (Sigma-Aldrich) for 5 min, followed by 3 washes with PBS-B. Cells were mounted using a fluorescent mount medium (DakoCytomation) and viewed using an Olympus BX-UCB microscope and MetaMorph image analysis software (Universal Imaging Corporation).
At D36, OLCs (≥70 μm of diameter) were collected, and the same procedure was performed using 1:400 rabbit polyclonal anti-DAZL (Abcam) and 1:400 rabbit polyclonal anti-DDX/MVH (Abcam) primary antibodies. The cross-reactivity and specificity of DAZL and VASA antibodies to porcine cell types have been verified previously [3,11].
Chromosome spreads and SCP3 staining
At D24–D28 of differentiation, loosely plate-adherent and nonadherent PLCs were collected with 0.02% EDTA 10-min incubation, and washed with a fresh medium. Cells were resuspended in wells of 6-well plates (Costar™; Cornig Incorp.) with differentiation medium plus 1 μg/mL of Brefeldin A (BFA; Sigma-Aldrich) and incubated for 6 h at 38.5°C. Cells were washed with the medium to remove the BFA and then cultured in a differentiation medium for 8 days. Cells were then collected with trypsin (Invitrogen) by incubating for 3 min at 38.5°C in an FBS-containing medium. Cells were treated with 1% sodium citrate for 20 min at room temperature, placed into 1% PFA solution, and spread onto slides. The slides were kept in humid chambers and incubated at room temperature overnight. Slides were dried at room temperature, incubated in Tris-buffered saline (TBS) with 1% BSA and 0.5% Triton X-100 (ADB) for 2 h at room temperature, treated with 1:200 rabbit polyclonal anti-SCP3 (NB300-232; NOVUS Biologicals) primary antibody, and incubated at 4°C overnight. Slides were then washed for 15 min 3 times with TBS, and incubated with ADB for 1 h at room temperature. Goat anti-rabbit PE (1:250; Sigma) was applied and incubated as secondary antibody for 2 h at 37°C. Slides were again washed with TBS, 3 times for 15 min, and stained with Hoechst (Sigma-Aldrich) for 10 min. Slides were washed once with TBS, and mounted using a mounting medium (Dako). Slides were analyzed using a fluorescence microscope (Olympus).
Small-interference RNA transfection
At D32 of differentiation of DAZL-transduced cells, morphologically distinct, nonadherent PLCs were collected and counted to determine the cell number. Cells were seeded into wells of a 24-well plate (Sarstedt) at 2×105 cells/well and cultured in 400 μL of differentiation medium without antibiotics overnight. These cells were transfected with a combination of 100 μL of medium and 20 pmol of DAZL small-interference RNA (siRNA) or control siRNA using Lipofectamine 2000™ (Invitrogen) following the manufacturer's recommendations. After incubation for 6 h at 38.5°C, the medium was replaced with 500 μL of fresh medium. After a further 48 h, these cells were collected, and the cell number was determined by counting live cells with a hemocytometer. In the control group, cells were cultured under the same conditions without transfection with siRNA or Lipofectamine 2000. RNA was isolated from both the control and treatment groups to determine the expression of meiosis- and oocyte-related genes by RT-PCR. Protein levels of DAZL, SCP3, and VASA were analyzed by western blot. Sequences for DAZL siRNA were as follows: target sequence, 5′-cac aaa taa att tcc atg gta-3′; sense strand, 5′-caa aua aau uuc cau ggu att-3′; antisense strand, 5′-uac cau gga aau uua uuu gtg-3′ (Qiagen).
Western blotting
Non- and loosely adherent PLCs collected at D36 of GFP-Con- and DAZL-GFP-transduced cells, and the cells from 3 siRNA transfection groups (nontransfection, control siRNA, and DAZL siRNA) were collected using 0.02% EDTA. For immunoblotting, protein was isolated using a radioimmunoprecipitation assay lysis buffer with complete miniprotease inhibitors (Roche) added fresh before use. Thirty micrograms of protein [as determined using a BSA protein assay kit (Pierce Chemical Co.)] was mixed with 5× reducing sample buffer, boiled for 5 min, and electrophoresed under reducing conditions on 10% polyacrylamide gels. Protein was transferred using an iBlot (Invitrogen) onto nitrocellulose membranes (Millipore). Membranes were incubated for 2 h in 5% nonfat dry-milk blocking buffer at room temperature, followed by an overnight incubation at 4°C in 1:1,000 rabbit anti-DAZL (Abcam), 1:1,000 rabbit anti-SCP3 (NOVUS Biologicals), and 1:500 rabbit anti-VASA (Abcam) primary antibodies. After washing with PBS, membranes were incubated with 1:1,000 anti-rabbit IgG HRP-linked secondary antibody (Cell Signaling Technology, Inc.) at room temperature for 1 h. All proteins were detected using the LI-COR Odyssey fluorescent scanner (LI-COR Bioscience). Blots were stripped, blocked, and reprobed between each set of antibodies. For all blots, GAPDH protein was detected as an internal loading control, through an overnight incubation at 4°C in mouse anti-GAPDH (1:10,000; Abcam), followed by a 1-h incubation with anti-mouse IgG HRP-linked secondary antibody (1:5,000; Cell Signaling Technology, Inc.) at room temperature.
Statistical analysis
For each set of data, independent experiments were repeated at least 3 times, with results representing the mean±SEM of all repeats. The statistical differences between experimental groups were determined by one-way analysis of variance, followed by the Tukey test for multiple comparisons. For single-treatment comparison to the relevant control, column statistics followed by unpaired t-tests were performed using GraphPad Prism analysis software. Results were considered significant at P<0.05, and these differences were denoted by an asterisk or different letters.
Results
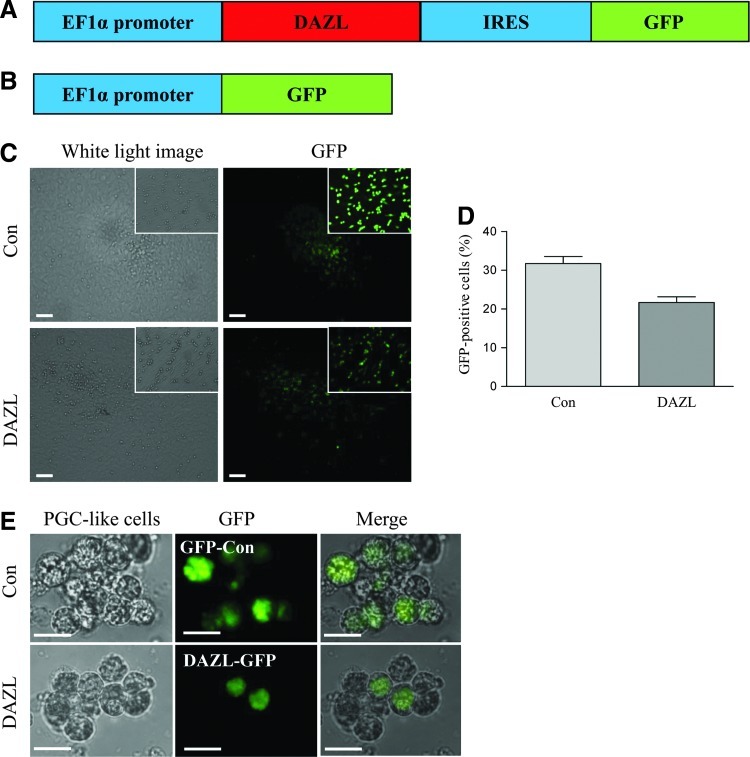
To study the potential role of DAZL on PGC formation, we constructed a recombinant lentivirus to drive Dazl expression using the Efl1α promoter, a strong constitutive mammalian promoter. The GFP-coding sequence was placed 3′ to the Dazl cDNA sequence in the expression construct (Fig. 1A), allowing a means to visually identify transduced cells. As a control, a lentivirus expressing GFP (Fig. 1B) was used. SSCs isolated from fetal pigs were transduced with the EF1α-DAZL-IRES-GFP (DAZL) or EF1α-GFP (Con) lentivirus at D2 of induced differentiation. Figure 1C and D show representative images of GFP expression at D16 of differentiation, and the average transduction ratios. At D24, GFP expression was also confirmed in the differentiated PLCs in both groups (Fig. 1E).
FIG. 1.
Overexpression of DAZL in PLCs. Diagrams showing the lentiviral expression constructs of DAZL-IRES-GFP (DAZL; A), and GFP-only control (Con; B). (C) Representative images showing the transduction efficiency. (D) Ratio of GFP-expressing cells (reflecting transduced cells) at D16 of differentiation. (E) Higher magnification of PLCs and their GFP expression in both transduced groups at D24 of differentiation. Data represent the mean±SEM of 3 independent experiments. Scale bar in (C)=100 μm; in (E)=25 μm. DAZL, deleted in azoospermia-like; PLCs, primordial germ cell-like cells; IRES, internal ribosome entry site. Color images available online at www.liebertpub.com/scd
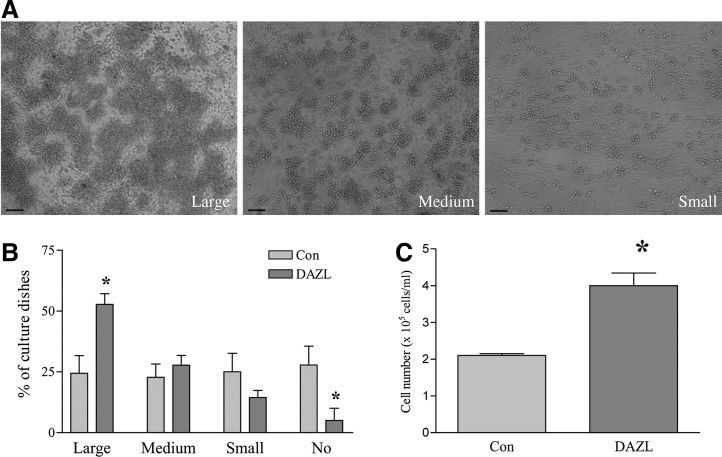
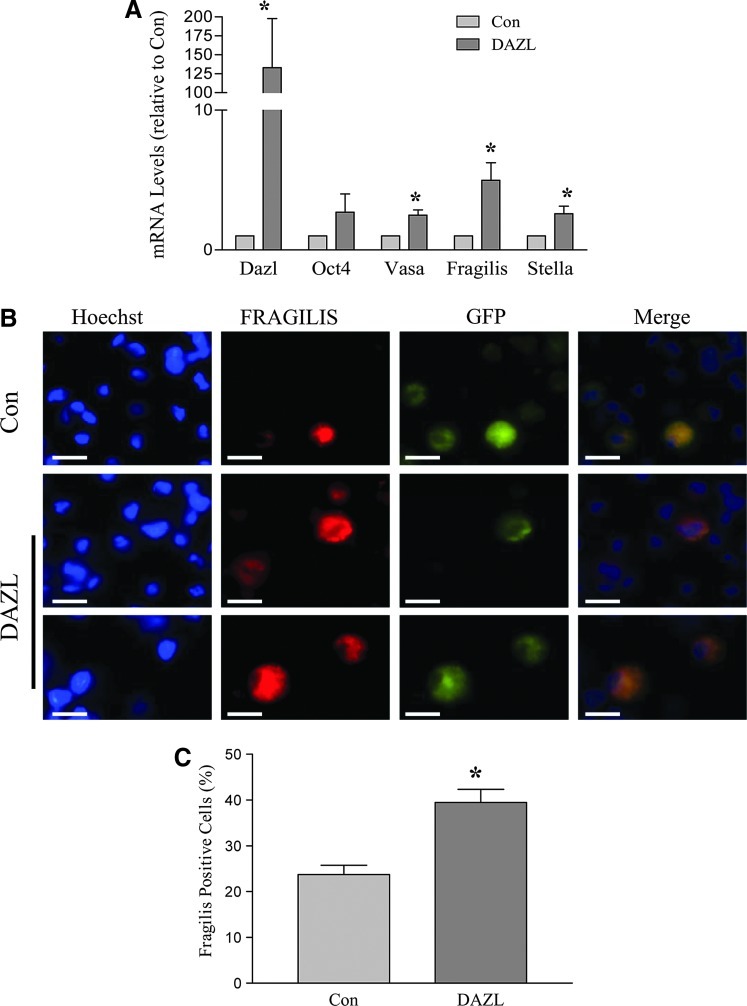
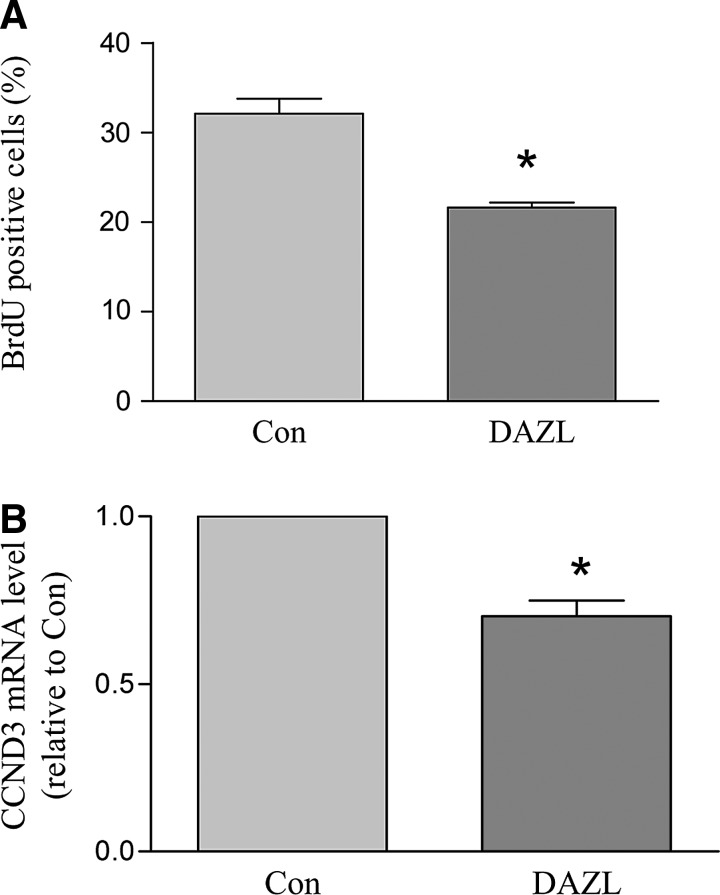
Cells that morphologically resembled PGCs usually started to form by ∼D14 of differentiation. Large, medium, and small clusters of PLCs were observed, reflecting the overall number of early germ cells that were being generated. The culture dishes were sorted as large, medium, small, and non-PLC (No), reflecting the overall size of the PLC clusters formed in the respective dishes (Fig. 2A). At D24 of differentiation, the DAZL-transduced group showed increased numbers of dishes containing large PLC clusters and a decreased number of dishes in which PLCs did not form (non-PLC dishes) compared to the GFP-transduced control group (Fig. 2B). The PLC clusters were very loosely attached onto the bottom layer of supporting cells, and could therefore be collected via incubation with a very low concentration of EDTA (0.02%). The increase in PLC formation was confirmed when the cells were collected and counted (Fig. 2C). FRAGILIS is a marker protein associated with early germ cell development. Immunocytochemistry revealed that FRAGILIS is expressed in PLCs (Fig. 3A), and that the FRAGILIS-positive cell ratio was significantly increased in the DAZL-transduced group (Fig. 3B). RT-PCR analysis of the Dazl transcript confirmed its overexpression conferred by lentiviral transduction (Fig. 3C). In addition, mRNA levels of 2 premigratory PGC markers (Fragilis and Stella) and a postmigratory PGC marker (Vasa) were also significantly higher in the DAZL-transduced group compared to the control group (P<0.05; Fig. 3C). We next sought to investigate whether the increase in the PLC number in the DAZL-transduced group was the consequence of PGC formation (stem cells differentiating into PGCs) or PGC proliferation. To accomplish this objective, PGCs were collected, and a BrdU incorporation assay was performed. We found that the percentage of BrdU-positive cells decreased in the DAZL-transduced group (Fig. 4A). Moreover, the expression of Ccnd3, a gene encoding G1/S-specific cyclin-D3, which is critical for the G-to-S phase transition during mitosis, decreased compared to that of the control group (Fig. 4B; P<0.05). This finding suggested that the increase in the PLC number due to DAZL was due to its enhancement of PGC formation, but not proliferation.
FIG. 2.
DAZL enhances PLC formation. SSCs were transduced with DAZL- or GFP (Con)-lentivirus and induced to differentiate. PLCs started to appear in the cultures from ∼D14 of differentiation onward. (A) Representative images of large, medium, and small PLC clusters. (B) Percentages of differentiating cell dishes showing large-, medium-, and small-PLC clusters in DAZL- and control (GFP)-transduced groups, respectively, at D24 of differentiation. (C) PLCs were harvested and counted, and cell numbers are shown. The total number of cells, including PLCs and supporting cells, is 12×105 cells/mL. Data represent the mean±SEM of 3 independent experiments, and an asterisk (*) indicates a significant difference from the control (B, C: t-test comparing the DAZL and control groups; P<0.05). Scale bar=100 μm. SSC, skin-derived stem cells.
FIG. 3.
DAZL enhances early germ cell marker expression. At D24 of differentiation, PLCs were collected, and RT-PCR was performed. (A) Relative mRNA levels of germ cell markers in DAZL- and GFP (Con)-transduced groups. (B) Representative images of Fragilis- and/or GFP-positive cells. (C) Fragilis-positive cell ratio in the DAZL- and GFP (con)-transduced groups. Data represent the mean±SEM of 4 independent experiments, and an asterisk (*) indicates a significant difference from the control (t-test comparing the DAZL groups and their respective controls; P<0.05). Scale bar=20 μm. PCR, polymerase chain reaction; RT, reverse transcription. Color images available online at www.liebertpub.com/scd
FIG. 4.
DAZL suppresses BrdU incorporation and decreases mitosis-related gene expression in PLCs. SSCs were transduced with DAZL- or GFP (con)-lentivirus and induced to differentiate. (A) Percentage of BrdU-positive cells in the DAZL and control (Con) groups. (B) CCND3 expression was suppressed in the DAZL-transduced group as determined by real-time RT-PCR. Data represent the mean±SEM of 3 (A) and 4 (B) independent experiments. An asterisk (*) indicates a significant difference from the control (t-test comparing the DAZL groups and their respective controls; P<0.05). BrdU, 5-bromo-2-deoxyuridine.
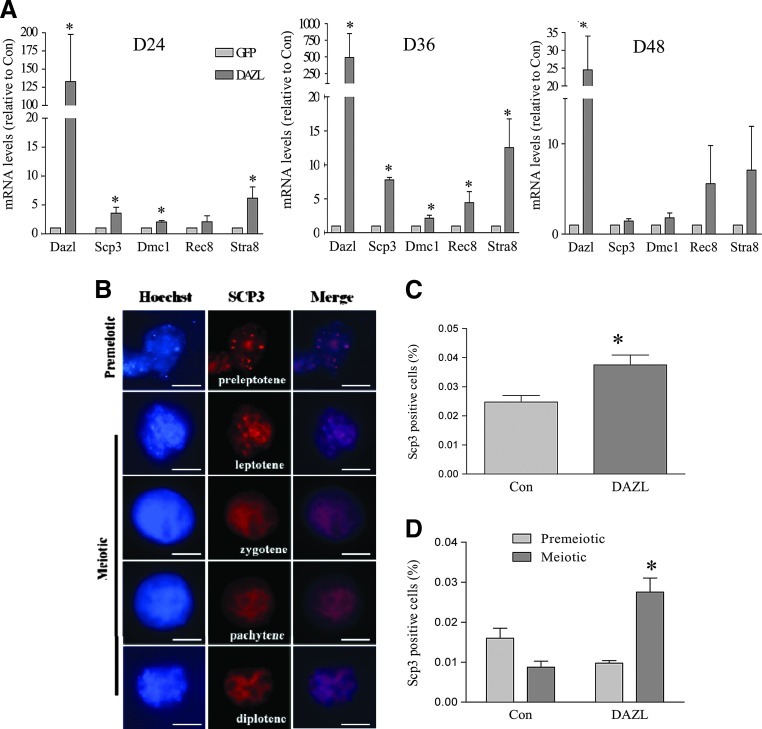
To study whether DAZL plays a role in the progression of meiosis in our in vitro generated PLCs, RT-PCR was performed on PLCs collected from different periods of differentiation. The increased expression of meiosis markers Scp3, Dmc1, and Stra8 was revealed at D24 of differentiation in the DAZL-transduced group, although no significant change in Rec8 was observed (Fig. 5A). At D36, a higher increase in meiosis marker expression in the DAZL-transduced group was shown. By D48, the appearance of germ cell-like cells in the differentiation culture declined on a morphological basis, and differences in germ cell marker expression between groups diminished (Fig. 5A). We further performed chromosome spreads and immunocytochemistry for SCP3 at around D32-D36. While ∼5% of the PLCs stained positive for SCP3, in the majority of the cells that were examined, SCP3 either localized to the cytoplasm or displayed irregular staining patterns that did not resemble any typical prophase stage. Nevertheless, a small subpopulation of PLCs did display SCP3 localization representative of different stages of prophase I. Figure 5B depicts an example of images that resemble both pre- and early-meiotic chromosomal SCP3-staining patterns. The percentages of SCP3-positive cells that did exhibit premeiotic or meiotic chromosome patterns in the DAZL- and GFP-transduced control groups are shown in Fig. 5C. A higher ratio of such cells was observed in the DAZL group (Fig. 5C, DAZL: 3.8/104 cells; Con: 2.5/104 cells). Moreover, a significantly higher percentage of the SCP3-positive cells were shown to be at the meiotic stage in the DAZL-transduced group compared to the GFP control (Fig. 5D).
FIG. 5.
DAZL promotes meiosis in PLCs. SSCs were transduced with DAZL- or GFP (con)-lentivirus and induced to differentiate. At D24, 36, and 48 of differentiation, PLCs were collected, and RT-PCR was performed. (A) mRNA levels of meiosis-specific genes in DAZL- and GFP (con)-transduced groups. (B) Representative images of premeiotic and meiotic PLCs staining positive for SCP3 as determined by chromosome spread and immunocytochemistry at around D36 of differentiation. (C) Percentage of SCP3-positive cells. (D) Percentage of SCP3-positive premeiotic and meiotic cells in the DAZL- and GFP (con)-transduced groups. Data represent the mean±SEM of 4 independent experiments. An asterisk (*) indicates a significant difference from the respective control (t-test comparing the DAZL groups and their respective controls; P<0.05). Scale bar=5 μm. Color images available online at www.liebertpub.com/scd
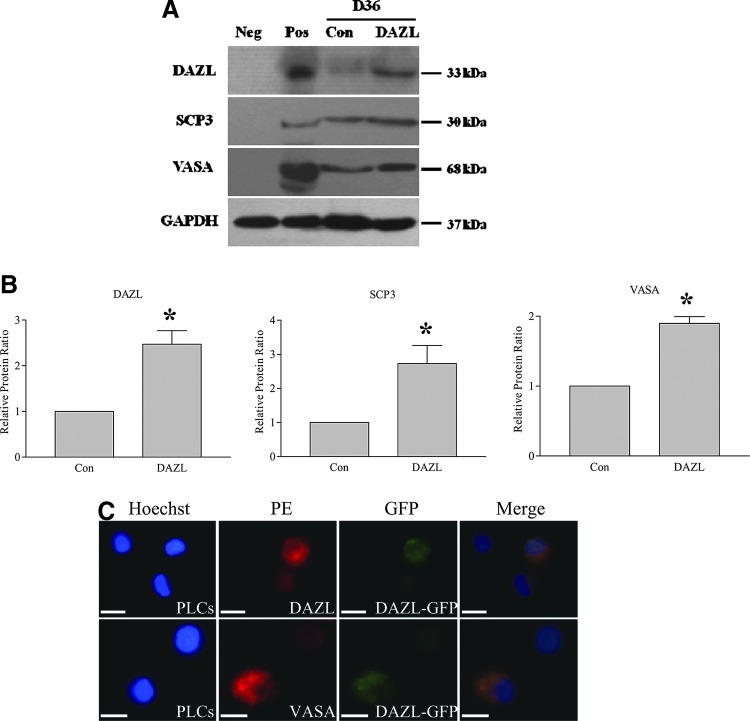
As DAZL has been reported to enhance translation of SCP3 and VASA [17], we next sought to examine whether the levels of SCP3 and VASA were influenced by DAZL in our PLCs. Western blot analysis for DAZL, SCP3, and VASA was performed on total protein from PLCs collected at D36 of differentiation. As shown in Fig. 6A and B, DAZL protein was 2.5-fold higher in DAZL-transduced PLCs than in the control, confirming that DAZL was being successfully overexpressed. SCP3 protein was 2.7-fold, and VASA protein was 1.9-fold higher, respectively, in the DAZL-traduced group compared to the GFP-transduced group. The cytoplasmic colocalization of DAZL-GFP and VASA is depicted in Fig. 6C, with no staining observed in the nuclei.
FIG. 6.
DAZL enhances meiosis marker and germ cell-specific protein expression in PLCs. SSCs were transduced with DAZL- or GFP (con)-lentivirus and induced to differentiate. At D36 of differentiation, PLCs were collected, and western blots were performed. (A) Western blot images representing the expression of DAZL, SCP3, and VASA at the protein level; GAPDH was used as a loading control. Pos: positive control=ovarian tissue; Neg: negative control=muscle tissue. (B) Densitometric quantitation depicting expression of DAZL, SCP3, and VASA in both of the DAZL- and control (Con)-transduced groups. (C) Immunocytochemistry for DAZL and VASA in DAZL-transduced PLCs. Data represent the mean±SEM of 4 independent experiments. An asterisk (*) indicates a significant difference from the control (t-test comparing the DAZL groups and their respective controls; P<0.05). Scale bar=25 μm. Color images available online at www.liebertpub.com/scd
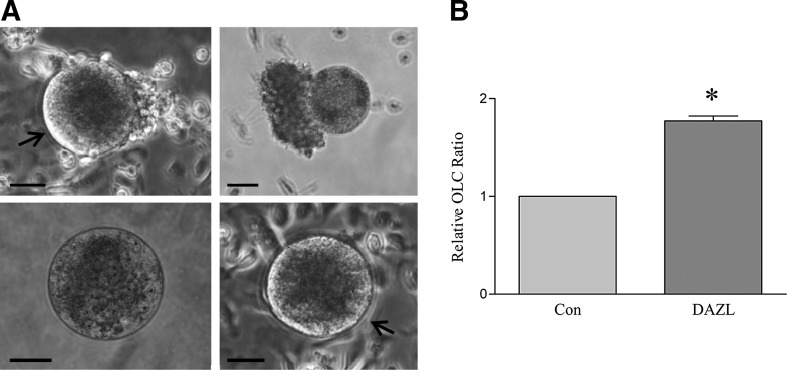
We next sought to validate the role of DAZL on germ cell marker expression using a loss-of-function approach. siRNAs against either DAZL (siDAZL) or a negative control (siCon) were transfected into DAZL-transduced PLCs collected at D32 of differentiation, and after 48 h, RT-PCR was performed on RNA isolated from these cells. Figure 7A shows that Dazl mRNA levels were significantly suppressed in the siDAZL-transfected group, confirming successful Dazl knockdown. As expected, the transcript levels of meiosis-related genes, including Scp3, Dmc1, Stra8, and Rec8, and oocyte-related genes such as Vasa and Gdf9 were decreased in siDAZL-transfected cells, whereas expression of the mitosis-related gene Ccnd3 was increased in the siDAZL-treated group (Fig. 7B). Moreover, western blot analysis revealed that SCP3 and VASA protein levels were also decreased in the DAZL siRNA knockdown group (Fig. 7C, D). Consistent with what we reported previously, OLCs formed at later stages of differentiation (Fig. 8A). To investigate whether enhanced PLC formation promoted by DAZL overexpression could also lead to an increase in the formation of late-stage germ cells, the number of OLCs≥70 μm in diameter was determined, revealing a 1.8-fold increase in their number in the DAZL-transduced group compared to the GFP-transduced control group (Fig. 8).
FIG. 7.
DAZL siRNA reverses the DAZL-mediated effect on germ cell marker expression in PLCs. SSCs were transduced with DAZL- or GFP (con)-lentivirus and induced to differentiate. At D32 of differentiation, cells were transfected with DAZL siRNA. Real-time RT-PCR and western blots were performed 48 h post-siRNA transfection. (A) Analysis of mRNA levels confirmed DAZL knockdown. (B) The influence of DAZL on germ cell marker and mitosis-related gene expression was reversed by DAZL siRNA. (C) Representative western blot images depicting the expression of DAZL, SCP3, and VASA in the different treatment groups; GAPDH was used as a loading control. (D) Densitometric quantitation depicting relative protein levels of DAZL, SCP3, and VASA in DAZL-transduced without DAZL siRNA (non-siR), DAZL-transduced +DAZL siRNA (siDazl), and DAZL-transduced + con siRNA (siCon) groups. Data represent the mean±SEM of 4 independent experiments. Different letters or an asterisk (*) indicates significant differences from the non-siR control group (analysis of variance followed by Tukey post-test; P<0.05). siRNA, small-interference RNA.
FIG. 8.
DAZL increases OLC formation. SSCs were transduced with DAZL- or GFP (con)-lentivirus and induced to differentiate. At ∼D30 and onward of differentiation, large OLCs were detected. (A) Representative images of OLCs. Arrows point to zona pellucida-like structures. (B) The relative ratio for the presence of OLCs during a differentiation experiment was increased in the DAZL-transduced group compared to the control (GFP-transduced) group. Data represent the mean±SEM of 4 independent experiments. An asterisk (*) indicates a significant difference from the control (t-test comparing the DAZL groups and their respective controls; P<0.05). Scale bar=50 μm. OLCs, oocyte-like cells.
Discussion
Evidence continues to emerge supporting the importance of DAZL in germ cell development. Indeed, Dazl is required for gametogenesis, as its knockout results in the complete lack of a PGC population [8,18]. In addition, the expression of DAZL in 12.5-dpc PGCs (a time point that coincides with the mitotic to meiotic transition) is 28-fold higher than in 10.5-dpc (mitotic) PGCs in mice [19], suggesting that it may also play a role during meiosis. It has been reported that DAZL is necessary for the expression of Stra8 and meiotic initiation in embryonic ovaries, indicating that it is an intrinsic meiotic competence factor having an obligatory function upstream of meiotic initiation [20]. Using Dazl-null mice, Haston et al. demonstrated that Dazl is required for the differentiation of murine PGCs, a process involving the transition from mitosis to meiosis, as well as imprint erasure through epigenetic programs [18]. Previous studies indicate that DAZL is a key regulator of several aspects underlying gametogenesis, and our finding that its overexpression in germ cells differentiated in vitro increases the expression of meiosis-related genes, as well as the percentage of cells that enter meiosis, supports this notion.
DAZL localizes to the nucleus and cytoplasm of fetal germ cells and to the cytoplasm of developing oocytes [21]. The cytoplasmic localization of DAZ proteins, coupled with their ability to bind to RNA homopolymers through their RRM [22,23], infers a role for DAZL in post-transcriptional regulation. DAZL protein has been associated with actively translating polyribosomes, suggesting that it may mediate aspects of protein synthesis, including mRNA stability, transportation, localization, or translation [24,25]. DAZL is known to interact with poly(A)-binding proteins (PABPs) in vivo [26]. It has been proposed that DAZL binds to a 3′UTR and thereby recruits PABP, which in turn interacts with 5′ end-associated factors to enhance translation [27]. In our study, the levels of SCP3 and VASA (Mvh) were both significantly upregulated in our PLCs. In contrast, when the level of DAZL was knocked down using a specific siRNA, SCP3 and VASA protein levels were decreased. These findings confirm the previously established regulatory role of DAZL on the expression of SCP3 and VASA in our in vitro derived germ cell system. DAZL has been shown to interact with Mvh mRNA, stimulating its translation in germ cells by binding to a site within the 3′ UTR of the Mvh transcript [28]. Germ cells derived from Dazl knockout mice contain lower levels of MVH (VASA) protein, suggesting that the RNA-binding activity of the DAZL protein mediates regulation of Mvh translation in vivo [28]. A similar regulatory mechanism was also reported to control Scp3 expression, in which DAZL facilitates Scp3 translation by interacting with the 3′UTR of the Scp3 transcript [17]. In our study, the expression of early- and late-stage germ cell markers is also increased at the mRNA level in the DAZL-overexpressing group. Although the mechanism whereby DAZL regulates the level of these transcripts is currently unclear, it is possible that DAZL enhances the translation of some yet-to-be-identified factors or pathway components, which in turn activate the transcription of these germ cell markers. Alternatively, via its RRM, DAZL may bind to the 3′UTR of the mRNAs of these germ cell markers to enhance their stability.
We have recently demonstrated that midkine stimulates PGC proliferation by downregulating Dazl [6], suggesting that DAZL plays an inhibitory role on PGC mitosis. Our finding that DAZL increased PLC numbers in our differentiation culture was therefore somewhat surprising at first glance. However, subsequent experiments suggested that the increase in the PLC number was not the consequence of enhanced proliferation, but rather an increase in the differentiation of stem cells into early germ cells. This notion was supported by the finding that BrdU incorporation and Ccnd3 expression were suppressed in PLCs in the DAZL-transduced group. CCND3 plays a key role in regulating growth factor-dependent G1-to-S phase transition, and has been shown to be highly expressed in PGCs [29]. Its downregulation may represent one of the means by which the active mitosis of PGCs is inhibited, acting as a switch to ramp-up cells entering meiosis. It is therefore possible that DAZL plays a dual role during stem-cell-to-germ-cell development: to initially facilitate the differentiation of stem cells into PLCs (PGC formation), and at later stages to promote the transition from mitosis to meiosis. It has been shown that in Dazl knockout mice, germ cell-specific genes such as Oct4, Stella, and Mvh and germ cell nuclear antigen are expressed at lower levels in the embryo [14], suggesting the involvement of DAZL in germ cell formation. Interestingly, in the current study, the expression of germ cell markers such as Fragilis, Stella, Vasa, and Oct4 was upregulated by DAZL overexpression in PLCs, and suppressed when DAZL was knocked down via siRNA. A recent report revealed that human DAZL plays a role in PGC formation [30], which supports our results obtained in a porcine somatic stem cell to germ cell differentiation model.
While a comparison between the results reported here on germ cell development in vitro with events occurring in vivo would be ideal, in vivo studies of DAZL in a porcine model are lacking. Nevertheless, relevant information can be gained from in vivo data derived from parallel in vitro studies conducted in the mouse. Recently, it was shown that by E14.5, murine germ cell numbers are significantly decreased in both male and female Dazl−/− embryos, concomitant with reduced marker expression of Vasa and SCP3 [18]. Of note, 2 other studies conducted using a murine model reported that either loss of PGCs occurred at E14.5 only in Dazl−/− males [20] or was initiated at a considerably later stage (E15.5 to E17.5) of development [8]. Irrespective of the timing, Haston et al. showed that in Dazl−/− males, imprint erasure was delayed or incomplete, and while PGCs derived from female mutant animals did enter meiosis, there appeared to be a block in meiotic progression. Notably, while 99% of the PGCs isolated from wild-type gonads demonstrated SCP3 staining in a manner representative of the pachytene stage of meiotic prophase I, only 87% and 59% of Dazl−/+ and −/− PGCs, respectively, reflected this pattern. Furthermore, in the Dazl-null group, the remaining 31% of PGCs failed to display an appropriate alignment of SCP3 [18]. These results were supported by events observed in vitro using Oct4ΔPE:GFP-positive PGC-like cells derived from embryonic stem cells during differentiation culture. Interestingly, in ESCs isolated from Dazl wild-type and −/+ animals, in vitro generation of PGCs progressed in a manner consistent with what has been previously reported in the mouse and human ESC-to-germ cell differentiation literature [31–35], with appropriate expression of germ cell markers as well as erasure and re-established imprinting [18]. This was not the case in Dazl−/− ESCs, which failed to produce a population of PGC-like cells, demonstrating that DAZL is required for directing pluripotent cells along the germ path to give rise to germ cells [18]. Our study, in combination with data obtained in mouse, clearly demonstrates that DAZL plays a central role in germ cell development and the progression from mitosis to meiosis.
Substantial studies have reported the generation of germ cell-like cells from somatic tissue-derived stem cells such as skin [3–5,16,36–38], bone marrow [39–42], and pancreas [43]. It has been demonstrated that epigenetic imprints are established during gametogenesis [44–46], and that the DNA methylation status of imprinted genes is erased and reset during PGC development [47,48]. Using the porcine skin stem cell to the PLC differentiation model utilized in the current study, it was previously revealed that 19% of the 29 individual CpG sites in the differentially methylated region 1 of the 5′ flanking region of an imprinted gene, H19, were methylated in undifferentiated skin stem cells, while 99% of CpG sites were unmethylated in this region at D25 of differentiation. These results suggest that imprint erasure occurred in the PGC-like cells undergoing induced differentiation [3]. The potential for somatic stem cells to differentiate into germ cells in vivo has also been in other previous studies [49,50]. When somatic stem cells derived from eviscerated fetuses were injected into murine blastocysts and transferred into pseudopregnant recipients, chimeric fetuses were obtained. Chimerism was detected in mesenchymal organs as well as in the genital ridges [50]. More recently, using GFP as a reporter, it was demonstrated that stem cells derived from porcine skin could incorporate into the early embryos and contribute to not only various somatic tissues of the 3 germ layers, but also the gonad of postnatal chimeras [49]. These studies suggest that somatic stem cells may provide a novel and accessible model to investigate how the germ cell path is activated, and how germ cell development is regulated in vitro. In our current study, the finding that the overall OLC number is significantly increased in the DAZL-transduced group further confirms the importance of DAZL during germ cell formation and development, supporting that the introduction of key factors such as DAZL may be a viable approach to improve in vitro germ cell induction from somatic stem cells. Nevertheless, although we show that somatic cell-derived PGC formation and meiosis are enhanced by the overexpression of DAZL, the efficiency of generating meiotic cells remains very low. Moreover, compared to a study that generated germ cells from human embryonic stem cells overexpressing DAZL [51], the percentage of meiotic cells in our somatic cell-derived differentiation system continues to be over one magnitude lower. We speculate that this may be attributed to the possibility that compared to embryonic stem cells, a considerably lower percentage of cells in our somatic stem cell population that have the required potency to enter the germ path, or alternatively, more meiotic key players are missing in our model. Future studies aimed at simultaneously introducing multiple factors into our stem cell-to-germ cell differentiation system may not only offer an opportunity to understand the key genes and their interactions during germ cell formation and differentiation, but also help to build a more efficient system of generating germ cells in vitro. Interestingly, it was recently reported that overexpression of both VASA and DAZL synergistically increased the percentage of cells positive for the late male germ cell marker ACROSIN that were differentiated from human embryonic stem cells, although their progression to meiosis was not synergistic [51].
Acknowledgments
Acknowledgement of Funding: Canada Institutes for Health Research (CIHR), National Science and Research Engineering Council (NSERC), the Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA), and National Research Foundation of Korea Grant (NRF-2011-013-E00053). The authors wish to thank staff at the University of Guelph Arkell Swine Research Station for overseeing the sow-breeding program.
Author Disclosure Statement
The authors have nothing to declare.
References
- 1.Tam PP. Snow MH. Proliferation and migration of primordial germ cells during compensatory growth in mouse embryos. J Embryol Exp Morphol. 1981;64:133–147. [PubMed] [Google Scholar]
- 2.Ginsburg M. Snow MH. McLaren A. Primordial germ cells in the mouse embryo during gastrulation. Development. 1990;110:521–528. doi: 10.1242/dev.110.2.521. [DOI] [PubMed] [Google Scholar]
- 3.Linher K. Dyce P. Li J. Primordial germ cell-like cells differentiated in vitro from skin-derived stem cells. PLoS One. 2009;4:e8263. doi: 10.1371/journal.pone.0008263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dyce PW. Shen W. Huynh E. Shao H. Villagomez DA. Kidder GM. King WA. Li J. Analysis of oocyte-like cells differentiated from porcine fetal skin-derived stem cells. Stem Cells Dev. 2011;20:809–819. doi: 10.1089/scd.2010.0395. [DOI] [PubMed] [Google Scholar]
- 5.Dyce PW. Li J. From skin cells to ovarian follicles? Cell Cycle. 2006;5:1371–1375. doi: 10.4161/cc.5.13.2898. [DOI] [PubMed] [Google Scholar]
- 6.Shen W. Park BW. Toms D. Li J. Midkine promotes proliferation of primordial germ cells by inhibiting the expression of the deleted in azoospermia-like gene. Endocrinology. 2012;153:3482–3492. doi: 10.1210/en.2011-1456. [DOI] [PubMed] [Google Scholar]
- 7.Yen PH. Putative biological functions of the DAZ family. Int J Androl. 2004;27:125–129. doi: 10.1111/j.1365-2605.2004.00469.x. [DOI] [PubMed] [Google Scholar]
- 8.Ruggiu M. Speed R. Taggart M. McKay SJ. Kilanowski F. Saunders P. Dorin J. Cooke HJ. The mouse Dazla gene encodes a cytoplasmic protein essential for gametogenesis. Nature. 1997;389:73–77. doi: 10.1038/37987. [DOI] [PubMed] [Google Scholar]
- 9.Dorfman DM. Genest DR. Reijo Pera RA. Human DAZL1 encodes a candidate fertility factor in women that localizes to the prenatal and postnatal germ cells. Hum Reprod. 1999;14:2531–2536. doi: 10.1093/humrep/14.10.2531. [DOI] [PubMed] [Google Scholar]
- 10.Liu J. Linher K. Li J. Porcine DAZL messenger RNA: its expression and regulation during oocyte maturation. Mol Cell Endocrinol. 2009;311:101–108. doi: 10.1016/j.mce.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Linher K. Cheung Q. Baker P. Bedecarrats G. Shiota K. Li J. An epigenetic mechanism regulates germ cell-specific expression of the porcine deleted in azoospermia-like (DAZL) gene. Differentiation. 2009;77:335–349. doi: 10.1016/j.diff.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Xu EY. Moore FL. Pera RA. A gene family required for human germ cell development evolved from an ancient meiotic gene conserved in metazoans. Proc Natl Acad Sci U S A. 2001;98:7414–7419. doi: 10.1073/pnas.131090498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu EY. Lee DF. Klebes A. Turek PJ. Kornberg TB. Reijo Pera RA. Human BOULE gene rescues meiotic defects in infertile flies. Hum Mol Genet. 2003;12:169–175. doi: 10.1093/hmg/ddg017. [DOI] [PubMed] [Google Scholar]
- 14.Lin Y. Page DC. Dazl deficiency leads to embryonic arrest of germ cell development in XY C57BL/6 mice. Dev Biol. 2005;288:309–316. doi: 10.1016/j.ydbio.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 15.Dyce PW. Zhu H. Craig J. Li J. Stem cells with multilineage potential derived from porcine skin. Biochem Biophys Res Commun. 2004;316:651–658. doi: 10.1016/j.bbrc.2004.02.093. [DOI] [PubMed] [Google Scholar]
- 16.Dyce PW. Wen L. Li J. In vitro germline potential of stem cells derived from fetal porcine skin. Nat Cell Biol. 2006;8:384–390. doi: 10.1038/ncb1388. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds N. Collier B. Bingham V. Gray NK. Cooke HJ. Translation of the synaptonemal complex component Sycp3 is enhanced in vivo by the germ cell specific regulator Dazl. RNA. 2007;13:974–981. doi: 10.1261/rna.465507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haston KM. Tung JY. Reijo Pera RA. Dazl functions in maintenance of pluripotency and genetic and epigenetic programs of differentiation in mouse primordial germ cells in vivo and in vitro. PLoS One. 2009;4:e5654. doi: 10.1371/journal.pone.0005654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molyneaux KA. Stallock J. Schaible K. Wylie C. Time-lapse analysis of living mouse germ cell migration. Dev Biol. 2001;240:488–498. doi: 10.1006/dbio.2001.0436. [DOI] [PubMed] [Google Scholar]
- 20.Lin Y. Gill ME. Koubova J. Page DC. Germ cell-intrinsic and -extrinsic factors govern meiotic initiation in mouse embryos. Science. 2008;322:1685–1687. doi: 10.1126/science.1166340. [DOI] [PubMed] [Google Scholar]
- 21.Nishi S. Hoshi N. Kasahara M. Ishibashi T. Fujimoto S. Existence of human DAZLA protein in the cytoplasm of human oocytes. Mol Hum Reprod. 1999;5:495–497. doi: 10.1093/molehr/5.6.495. [DOI] [PubMed] [Google Scholar]
- 22.Houston DW. Zhang J. Maines JZ. Wasserman SA. King ML. A Xenopus DAZ-like gene encodes an RNA component of germ plasm and is a functional homologue of Drosophila boule. Development. 1998;125:171–180. doi: 10.1242/dev.125.2.171. [DOI] [PubMed] [Google Scholar]
- 23.Tsui S. Dai T. Roettger S. Schempp W. Salido EC. Yen PH. Identification of two novel proteins that interact with germ-cell-specific RNA-binding proteins DAZ and DAZL1. Genomics. 2000;65:266–273. doi: 10.1006/geno.2000.6169. [DOI] [PubMed] [Google Scholar]
- 24.Tsui S. Dai T. Warren ST. Salido EC. Yen PH. Association of the mouse infertility factor DAZL1 with actively translating polyribosomes. Biol Reprod. 2000;62:1655–1660. doi: 10.1095/biolreprod62.6.1655. [DOI] [PubMed] [Google Scholar]
- 25.Maegawa S. Yamashita M. Yasuda K. Inoue K. Zebrafish DAZ-like protein controls translation via the sequence ‘GUUC’. Genes Cells. 2002;7:971–984. doi: 10.1046/j.1365-2443.2002.00576.x. [DOI] [PubMed] [Google Scholar]
- 26.Collier B. Gorgoni B. Loveridge C. Cooke HJ. Gray NK. The DAZL family proteins are PABP-binding proteins that regulate translation in germ cells. EMBO J. 2005;24:2656–2666. doi: 10.1038/sj.emboj.7600738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brook M. Smith JW. Gray NK. The DAZL and PABP families: RNA-binding proteins with interrelated roles in translational control in oocytes. Reproduction. 2009;137:595–617. doi: 10.1530/REP-08-0524. [DOI] [PubMed] [Google Scholar]
- 28.Reynolds N. Collier B. Maratou K. Bingham V. Speed RM. Taggart M. Semple CA. Gray NK. Cooke HJ. Dazl binds in vivo to specific transcripts and can regulate the pre-meiotic translation of Mvh in germ cells. Hum Mol Genet. 2005;14:3899–3909. doi: 10.1093/hmg/ddi414. [DOI] [PubMed] [Google Scholar]
- 29.Sorrentino E. Nazzicone V. Farini D. Campagnolo L. De Felici M. Comparative transcript profiles of cell cycle-related genes in mouse primordial germ cells, embryonic stem cells and embryonic germ cells. Gene Expr Patterns. 2007;7:714–721. doi: 10.1016/j.modgep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Kee K. Angeles VT. Flores M. Nguyen HN. Reijo Pera RA. Human DAZL, DAZ and BOULE genes modulate primordial germ-cell and haploid gamete formation. Nature. 2009;462:222–225. doi: 10.1038/nature08562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lacham-Kaplan O. Chy H. Trounson A. Testicular cell conditioned medium supports differentiation of embryonic stem cells into ovarian structures containing oocytes. Stem Cells. 2006;24:266–273. doi: 10.1634/stemcells.2005-0204. [DOI] [PubMed] [Google Scholar]
- 32.Geijsen N. Horoschak M. Kim K. Gribnau J. Eggan K. Daley GQ. Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature. 2004;427:148–154. doi: 10.1038/nature02247. [DOI] [PubMed] [Google Scholar]
- 33.Hubner K. Fuhrmann G. Christenson LK. Kehler J. Reinbold R. De La Fuente R. Wood J. Strauss JF., 3rd Boiani M. Scholer HR. Derivation of oocytes from mouse embryonic stem cells. Science. 2003;300:1251–1256. doi: 10.1126/science.1083452. [DOI] [PubMed] [Google Scholar]
- 34.Toyooka Y. Tsunekawa N. Akasu R. Noce T. Embryonic stem cells can form germ cells in vitro. Proc Natl Acad Sci U S A. 2003;100:11457–11462. doi: 10.1073/pnas.1932826100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clark AT. Bodnar MS. Fox M. Rodriquez RT. Abeyta MJ. Firpo MT. Pera RA. Spontaneous differentiation of germ cells from human embryonic stem cells in vitro. Hum Mol Genet. 2004;13:727–739. doi: 10.1093/hmg/ddh088. [DOI] [PubMed] [Google Scholar]
- 36.Song SH. Kumar BM. Kang EJ. Lee YM. Kim TH. Ock SA. Lee SL. Jeon BG. Rho GJ. Characterization of porcine multipotent stem/stromal cells derived from skin, adipose, and ovarian tissues and their differentiation in vitro into putative oocyte-like cells. Stem Cells Dev. 2011;20:1359–1370. doi: 10.1089/scd.2010.0203. [DOI] [PubMed] [Google Scholar]
- 37.Yang X. Qu L. Wang X. Zhao M. Li W. Hua J. Shi M. Moldovan N. Wang H. Dou Z. Plasticity of epidermal adult stem cells derived from adult goat ear skin. Mol Reprod Dev. 2007;74:386–396. doi: 10.1002/mrd.20598. [DOI] [PubMed] [Google Scholar]
- 38.Dyce PW. Liu J. Tayade C. Kidder GM. Betts DH. Li J. In vitro and in vivo germ line potential of stem cells derived from newborn mouse skin. PLoS One. 2011;6:e20339. doi: 10.1371/journal.pone.0020339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee HJ. Selesniemi K. Niikura Y. Niikura T. Klein R. Dombkowski DM. Tilly JL. Bone marrow transplantation generates immature oocytes and rescues long-term fertility in a preclinical mouse model of chemotherapy-induced premature ovarian failure. J Clin Oncol. 2007;25:3198–3204. doi: 10.1200/JCO.2006.10.3028. [DOI] [PubMed] [Google Scholar]
- 40.Johnson J. Bagley J. Skaznik-Wikiel M. Lee HJ. Adams GB. Niikura Y. Tschudy KS. Tilly JC. Cortes ML, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122:303–315. doi: 10.1016/j.cell.2005.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nayernia K. Lee JH. Drusenheimer N. Nolte J. Wulf G. Dressel R. Gromoll J. Engel W. Derivation of male germ cells from bone marrow stem cells. Lab Invest. 2006;86:654–663. doi: 10.1038/labinvest.3700429. [DOI] [PubMed] [Google Scholar]
- 42.Liu C. Xu S. Ma Z. Zeng Y. Chen Z. Lu Y. Generation of pluripotent cancer-initiating cells from transformed bone marrow-derived cells. Cancer Lett. 2011;303:140–149. doi: 10.1016/j.canlet.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 43.Danner S. Kajahn J. Geismann C. Klink E. Kruse C. Derivation of oocyte-like cells from a clonal pancreatic stem cell line. Mol Hum Reprod. 2007;13:11–20. doi: 10.1093/molehr/gal096. [DOI] [PubMed] [Google Scholar]
- 44.Borghol N. Lornage J. Blachere T. Sophie Garret A. Lefevre A. Epigenetic status of the H19 locus in human oocytes following in vitro maturation. Genomics. 2006;87:417–426. doi: 10.1016/j.ygeno.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 45.Olek A. Walter J. The pre-implantation ontogeny of the H19 methylation imprint. Nat Genet. 1997;17:275–276. doi: 10.1038/ng1197-275. [DOI] [PubMed] [Google Scholar]
- 46.Warnecke PM. Mann JR. Frommer M. Clark SJ. Bisulfite sequencing in preimplantation embryos: DNA methylation profile of the upstream region of the mouse imprinted H19 gene. Genomics. 1998;51:182–190. doi: 10.1006/geno.1998.5371. [DOI] [PubMed] [Google Scholar]
- 47.Sato S. Yoshimizu T. Sato E. Matsui Y. Erasure of methylation imprinting of Igf2r during mouse primordial germ-cell development. Mol Reprod Dev. 2003;65:41–50. doi: 10.1002/mrd.10264. [DOI] [PubMed] [Google Scholar]
- 48.Petkov SG. Reh WA. Anderson GB. Methylation changes in porcine primordial germ cells. Mol Reprod Dev. 2009;76:22–30. doi: 10.1002/mrd.20926. [DOI] [PubMed] [Google Scholar]
- 49.Zhao MT. Yang X. Lee K. Mao J. Teson JM. Whitworth KM. Samuel MS. Spate LD. Murphy CN. Prather RS. The in vivo developmental potential of porcine skin-derived progenitors and neural stem cells. Stem Cells Dev. 2012;21:2682–2688. doi: 10.1089/scd.2012.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kues WA. Petersen B. Mysegades W. Carnwath JW. Niemann H. Isolation of murine and porcine fetal stem cells from somatic tissue. Biol Reprod. 2005;72:1020–1028. doi: 10.1095/biolreprod.104.031229. [DOI] [PubMed] [Google Scholar]
- 51.Medrano JV. Ramathal C. Nguyen HN. Simon C. Reijo Pera RA. Divergent RNA-binding proteins, DAZL and VASA, induce meiotic progression in human germ cells derived in vitro. Stem Cells. 2012;30:441–451. doi: 10.1002/stem.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]