Abstract
Background
Protein kinase C (PKC) signalling is often dysregulated in gastric cancer and therefore represents a potential target in cancer therapy. The Gram-negative bacterium Helicobacter pylori, which colonises the human stomach, plays a major role in the development of gastritis, peptic ulcer and gastric adenocarcinoma.
Objective
To analyse the role of PKC isozymes as mediators of H pylori-induced pathogenesis.
Methods
PKC phosphorylation was evaluated by immunoblotting and immunohistochemistry. Gene reporter assays, RT-PCR and invasion assays were performed to assess the role of PKC in the regulation of activator protein-1 (AP-1), matrix metalloproteinase-1 (MMP-1) and the invasion of H pylori-infected epithelial cells.
Results
H pylori induced phosphorylation of PKC isozymes α, δ, θ in AGS cells, which was accompanied by the phosphorylation of PKC substrates, including PKCμ and myristoylated alanine-rich C kinase substrate (MARCKS), in a CagA-independent manner. Phospholipase C, phosphatidylinositol 3-kinase and Ca2+ were crucial for PKC activation on infection; inhibition of PKC diminished AP-1 induction and, subsequently, MMP-1 expression. Invasion assays confirmed PKC involvement in H pylori-induced MMP-1 secretion. In addition, analysis of biopsies from human gastric mucosa showed increased phosphorylation of PKC in active H pylori gastritis and gastric adenocarcinoma.
Conclusion
The targeting of certain PKC isozymes might represent a suitable strategy to interfere with the MMP-1-dependent remodelling of infected tissue and to overcome the invasive behaviour of gastric cancer cells.
Keywords: AP-1, CagA, c-Fos, MARCKS, PLC, cell signalling, adenocarcinoma, helicobacter pylori, bacterial infection, matrix metalloproteinase, helicobacter pylori—pathogenesis, inflammation, nuclear factor kappa b, signal transduction, molecular oncology, gastro-oesophageal reflux disease, barretts metaplasia, barretts carcinoma, gastro-oesphageal junction, mucosal pathology, gastritis, gastric inflammation, inflammatory bowel disease, gastrointestinal cancer, gastric neoplasia, gastric pre-cancer
Significance of this study.
What is already known on this subject?
Protein kinase C (PKC) isozymes regulate a number of cellular functions including processes related to a polarised epithelial layer formation, and exert a crucial role in carcinogenesis.
The differences in mode of activation, intracellular distribution, and expression in normal and pathological tissue suggest that there are unique and mostly not investigated roles for each particular PKC isozyme in gastrointestinal signal transduction.
H pylori-induced matrix metalloproteinase-1 (MMP-1) expression in stomach epithelium involves mitogen-activated protein kinases (MAPK).
What are the new findings?
PKCα, PKCδ, PKCθ and a number of PKC substrates are phosphorylated in H pylori-infected gastric cells independently of H pylori's virulence factor cytotoxin A associated antigen (CagA).
H pylori induces PKC through phosphatidylinositol 3-kinase (PI3K), phospholipase Cγ (PLCγ) and Ca2+.
PKCα, PKCδ and PKCθ contribute to c-Fos up-regulation and activator protein-1 (AP-1) activation in a MAPK-independent manner, leading to an increase of matrix metalloproteinase-1 expression in H pylori-infected cells.
PKC are involved in cell invasion and, therefore, could play a causative role in gastric mucosa destruction following H pylori infection.
Phosphorylated PKC is increased in gastric tissue specimens from patients with H pylori-associated gastritis and gastric adenocarcinoma.
How might it impact on clinical practice in the foreseeable future?
Post-translational modifications (eg, phosphorylation) of PKC represent a potential biomarker for diagnostics and a molecular target for treatment of H pylori-induced gastric diseases.
Introduction
Serine/threonine kinases of the protein kinase C (PKC) family are important molecules in the regulation of cellular differentiation, proliferation, apoptosis, adhesion and migration.1 PKC signalling participates in the regulation of gastric acid production2 and is often dysregulated in gastric cancer.3 4 Several PKC isoforms have been implicated in invasion and metastasis; however, the molecular mechanisms are still not well understood.
The PKC family consists of at least 10 isozymes classified into three main groups (figure 1A). Conventional PKC (cPKC) α, βI, βII and γ bind Ca2+ and phosphatidylserine and require diacylglycerol (DAG) for further activation. The novel PKC (nPKC) δ, ɛ, θ, η possess a functional C1 domain, but their C2-like domains do not contain Ca2+-binding residues. Therefore, nPKC isozymes are regulated by DAG and phosphatidylserine, but not by Ca2+. The atypical PKCs (PKCζ and PKCλ) lack both functional C1 and C2 domains and are neither Ca2+- nor DAG-dependent.5
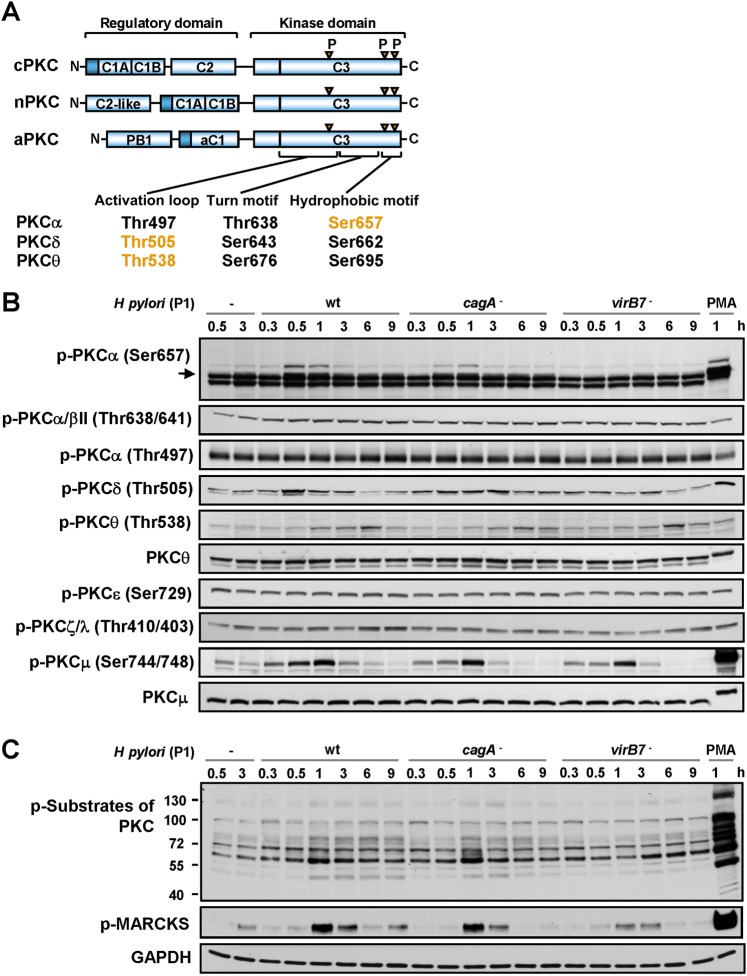
Figure 1.
H pylori activates protein kinase C (PKC). (A) The protein domains of the PKC family members, showing the pseudosubstrate (dark blue rectangle), the C1 domain that binds DAG, phosphatidylserine and phorbol esters, the C2 domain that binds Ca2+ or PIP2 (in the case of nPKC), and the C3 kinase domain. Also shown in orange are the conserved Ser/Thr residues phosphorylated during H pylori infection. (B) AGS cells were infected with H pylori P1 wt, cagA or virB7 mutants for different periods of time or were stimulated with PMA for 1 h. Cell lysates were analysed by immunoblotting using antibodies as indicated. Unphosphorylated PKCθ and PKCμ served as loading controls. (C) Analysis of phosphorylation of PKC substrates in cells treated as described in (B). GAPDH was immunodetected to show equal protein amounts in the cell samples.
In addition to their regulation by lipid second messengers, phosphorylation of conserved Ser/Thr sites within the C3 domain plays an important role in stabilisation and catalytic competence of PKC. Phosphorylation allows for the binding of the kinase domain to pseudosubstrate (within their own regulatory domain) to keep the enzyme in a latent conformation or promotes PKC binding to real substrates for full activation.6
Helicobacter pylori colonises the stomach in at least 30–50% of the world's population and increases the risk of peptic ulcers and gastric cancer. H pylori secretes effector molecules (lipopolysaccharide, VacA) into the extracellular space or injects them (CagA, muropeptides) directly into the cytoplasm of the host cell via the type IV secretion system (T4SS).7 8 Thereby H pylori controls the inflammatory, proliferative, pro- and anti-apoptotic cellular statuses.8 Other bacterial factors, including adhesins, urease, flagellae and components of the outer membrane, also contribute to the colonisation of the gastric mucosa.9 Bacteria–gastric epithelial cell interactions lead to induction of a range of matrix metalloproteinases (MMPs).10 11 MMPs participate in extracellular matrix (ECM) remodelling, the cleavage of cell adhesion molecules (eg, E-cadherin) and the processing and activation of chemoattractants and ligands for growth factor receptors,12 which leads to an increase in epithelial permeability and promotes leucocyte infiltration into the gastric mucosa.
The involvement of PKC in many cellular functions and in pathophysiology, for example, carcinogenesis, suggests that PKC may play a role in H pylori infection. However, very few studies have addressed the activation of PKC during H pylori infection. Obst et al 13 have demonstrated the translocation of PKCλ to the plasma membrane in H pylori-infected AGS cells, and Brandt et al 14 have shown the H pylori-induced phosphorylation of PKCα and PKCδ in these cells. There is only limited knowledge about the functional role of PKC in H pylori infection. By using a number of inhibitors, PKC has been demonstrated to participate in H pylori-induced alteration of the barrier properties of the epithelium15 and NF-κB-dependent cyclooxygenase-2 expression in gastric epithelial cells.16 Contradictory data exist concerning PKC involvement in IL-8 regulation in the gastric epithelium on infection.17 18
The aim of this study was to investigate the mechanisms and the functional consequences of H pylori-induced PKC activation. We show here that H pylori induces PKC in gastric epithelial cells, which involves the classical upstream PKC regulators PI3K, phospholipase Cγ (PLCγ) and Ca2+. Our data demonstrate for the first time that PKC contributes to c-Fos expression and activator protein-1 (AP-1) induction, which leads to matrix metalloproteinase-1 (MMP-1) up-regulation on H pylori infection. In addition, we show the induction of PKC phosphorylation in gastric mucosa tissue from patients with active H pylori gastritis and gastric adenocarcinoma.
Materials and methods
The antibodies and the chemicals used in this work are described in supplementary tables 1 and 2. The descriptions of the procedures for preparation of cell lysates, immunoblotting, immunofluorescence, immunohistochemistry, RNA isolation, RT-PCR, transfection, the reporter gene assay, the invasion and wound healing assays are provided in the online data supplement.
Cell culture and bacteria
AGS (ATCC) and HCA-7 (European Collection of Cell Cultures, Salisbury, UK) cells were grown in RPMI 1640 medium (PAA Laboratories, Pasching, Austria) supplemented with 10% fetal calf serum (FCS) and penicillin/streptomycin. Cells derived from human prenatal stomach tissue (HSC; Innoprot, Derio, Spain) were cultured as described previously.19 Sixteen hours before infection, the cell medium was replaced with fresh RPMI 1640 supplemented with 0.5% FCS.
The wild-type (wt) H pylori P1 strain and isogenic mutants cagA and virB7 or P12 wt and its VacA deficient mutant were cultured for 48–72 h, as described previously,20 and added to AGS cells at a multiplicity of infection of 100. In a set of experiments, the bacteria were loaded into the upper inserts of a 100 mm Transwell plate (Costar, Corning, New York, USA), and thereby separated from AGS cells cultured in the bottom chamber by a polycarbonate membrane (0.4 μM pore size).
Patients and tissue samples
Stomach biopsy specimens were obtained from 160 patients (age range 19–96 years) according to the recommendations of the updated Sydney System21 and were examined by the same experienced gastrointestinal pathologist who was blinded to the clinical and endoscopic data. Biopsies were stained with H&E, and also with Warthin–Starry–silver stain for detection of H pylori. Histological features of the gastric mucosa, including inflammation and atrophy were scored according to the updated Sydney System.21 Diagnosis of neoplasia was made according to the WHO classification 2010.
Statistical analyses
Statistical analyses of the results were performed using the Student t test. The data are expressed as the mean fold changes from at least three separate experiments ± SEM with the value of the control arbitrarily normalised to 1; p<0.05 was considered significant. The immunohistochemical data were analysed using analysis of variance (IBM SPSS 18). The statistical decisions were two-tailed with a critical probability of α=5% using a post-hoc t-test.
Results
H pylori induces phosphorylation of PKCα, PKCδ, PKCθ and PKCμ
While studying the effect of H pylori on PKC, a transient increase in phosphorylation was observed within 30 min for PKCα (Ser657), within 30–60 min for PKCδ (Thr505) and within 3–6 h for PKCθ (Thr538) following infection with P1 wt strain (figure 1A,B). Phorbol myristoyl acetate (PMA), a membrane-permeable substitute for DAG, was used as a positive control. To investigate the involvement of H pylori virulence factors in PKC phosphorylation, AGS cells were infected with H pylori mutants deficient in either CagA or VirB7 protein, which is required for the integrity of the T4SS. Both mutants adhered equally to AGS cells (data not shown). The cagA, but not the virB7, mutant induced PKCα phosphorylation. No differences between the wt, cagA or virB7 mutants were observed for PKCδ or PKCθ phosphorylation (figure 1B). Thus, H pylori induced transient phosphorylation of cPKCα in a CagA-independent, but T4SS-dependent manner; however, nPKCδ and nPKCθ were induced in a CagA- and T4SS-independent manner. No changes were detected in the Ser497, Ser729 and Thr410/Thr403 phosphorylation of PKCα, PKCɛ and PKCζ/λ, respectively (figure 1B). Further, infection of AGS cells with H pylori induced phosphorylation of PKCμ, a nPKC target,22 at the sites that correlate closely with kinase activity (figure 1B).
Intracellular localisation plays an important role in PKC function.5 6 Treatment with H pylori (or PMA) led to an accumulation of phosphorylated PKCα in the membranes and nuclei of AGS cells (supplementary figure 1A–C). In contrast to PMA, H pylori promoted no translocation of PKC isoforms δ and θ from the cytosol to membranes and nuclei (supplementary figure 1B).
To analyse the phosphorylation of PKC substrates, we used an antibody to phosphorylated Ser residues surrounded by Arg or Lys at the –2 or +2 positions and a hydrophobic residue at the +1 position.23 Figure 1C shows that wt and CagA-deficient H pylori induced a strong increase in Ser-phosphorylation of PKC substrates in AGS cells. Infection with the virB7 mutant led to a less prominent phosphorylation of PKC substrates. Actin-binding protein myristoylated alanine-rich C kinase substrate (MARCKS), a downstream target of cPKC and nPKC,24 was phosphorylated in cells infected with the wt and cagA mutant of H pylori within 1 h. Again, phosphorylation induced by the virB7 mutant was less prominent (figure 1C).
The P12 wt and vacA mutant of H pylori, as well as the P1 wt, induced the phosphorylation of PKC substrates. Heat-inactivated bacteria were not able to move, settle on the surface of the AGS cells (data not shown), or induce the phosphorylation of PKC substrates (supplementary figure 1D). Additionally, experiments using Transwell plates demonstrated that H pylori does not induce any phosphorylation of PKC substrates in the absence of direct contact with AGS cells (supplementary figure 1D). Thus, the adherence of living H pylori to host cells is required for PKC induction.
To study PKC activity in vivo, human gastric biopsies were analysed by immunohistochemistry. A pan-specific antibody, which recognises phosphorylation within the activation loop (Ser497, Ser505 and Ser538 of PKCα, PKCδ and PKCθ, respectively), was used (supplementary figure 2). PKC phosphorylation was determined in the gastric tissue of patients with H pylori-active gastritis or gastric adenocarcinoma, but not in the non-infected normal gastric mucosa (table 1).
Table 1.
Protein kinase C (PKC) phosphorylation in human gastric mucosa tissue
| Gastric mucosa biopsies | Number of specimens | Age | Gender | Mean±SEM, cells/hpf | p Value | |
| M | F | |||||
| Non-infected | 36 | 19–72 | 20 | 16 | 1.06±0.28 | |
| Hp-gastritis | 38 | 32–82 | 24 | 14 | 25.32±5.18 | 0.039* |
| Adenoma | 21 | 31–82 | 11 | 10 | 11.10±2.15 | |
| Adenocarcinoma | 65 | 34–96 | 29 | 36 | 61.37±7.56 | 0.001* |
p<0.05 versus ‘non-infected’ group, as determined by the Dunnett t-test.
In the non-infected group, 100% of specimens demonstrated an immunoreactivity less than the median of all variables (8 cells/hpf). In the ‘HP-gastritis’ and ‘adenocarcinoma’ groups, 52.6% and 81.5% of specimens, respectively, were strongly positive for phospho-PKC. In the studied biopsies, no changes in the expression of PKCθ were observed (supplementary table 3).
H pylori-induced activation of PKC involves PLCγ1, Ca2+, tyrosine kinases and PI3K
The PKC activator DAG is mainly produced from phosphatidylinositol 4,5-biphosphate (PIP2) or phosphatidylcholine (PC) through direct cleavage with phosphatidylinositol-specific PLC (PI-PLC) or PC-specific PLC (PC-PLC), respectively.25 Pretreatment of AGS cells with U73122 or D609, selective inhibitors of PI-PLC or PC-PLC, respectively, reduced the phosphorylation of PKC substrates and MARCKS following infection (figure 2A). Thus, the H pylori-induced activation of PKC involves PC-PLC and PI-PLC.
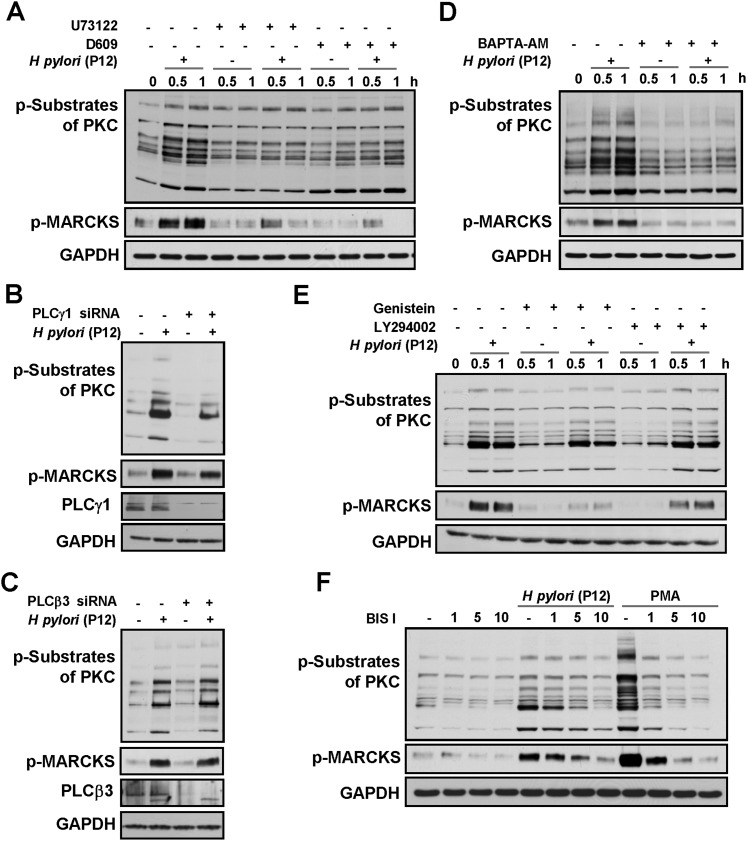
Figure 2.
Protein kinase C (PKC) activation implicates PLC, Ca2+, tyrosine kinases and PI3K. AGS cells were pre-incubated with U73122 or D609 (A), were transiently transfected with siRNAs targeting PLCγ1 (B) or PLCβ3 (C), or were pretreated with BAPTA-AM (D), genistein and LY294002 (E) or with the indicated concentrations of BIS I (in μM; (F)) and infected with H pylori P12 for 45 min or for the indicated periods of time. Cell lysates were analysed by immunoblotting using antibodies as indicated. GAPDH was immunodetected to show equal protein amounts in the cell samples.
PI-PLC comprises a group of Ca2+-dependent enzymes, including PLCβ, γ, δ, ɛ, ζ and PLCη families.26 PLCβ and PLCγ are the most studied isozymes. PLCβ (four isoforms) is induced in response to the activation of G protein-coupled transmembrane receptors. PLCβ3 is ubiquitous, whereas PLCβ1 is not expressed in the stomach; PLCβ2 and PLCβ4 are highly expressed in cells of haematopoietic origin as well as in the cerebellum and retina.27 PLCγ (two isoforms) is stimulated on activation of receptor and non-receptor tyrosine kinases.27 PLCγ1 is widely distributed, whereas PLCγ2 is expressed primarily in cells of haematopoietic origin. To determine the role of particular isozymes in infected cells, transient transfections with siRNAs targeting either PLCγ1 or PLCβ3 were performed. In contrast to PLCβ3, PLCγ1 depletion suppressed H pylori-induced phosphorylation of PKC substrates, including MARCKS (figure 2B,C). Therefore, PI-PLCγ1 contributes to PKC regulation on H pylori infection.
H pylori has been shown to provoke a CagA-independent increase of (Ca2+)i in gastric epithelial cells.28 Investigating the role of Ca2+ in PKC activation, we found that the phosphorylation of PKC substrates was dramatically reduced on treatment of the cells with the Ca2+-chelator BAPTA-AM prior to infection (figure 2D). Additionally, the tyrosine kinase inhibitor genistein and PI3K inhibitor LY294002 diminished the phosphorylation of PKC substrates, especially MARCKS (figure 2E). Taken together, these data indicate that Ca2+, tyrosine kinases and PI3K are involved in PKC regulation during infection of epithelial cells with H pylori.
To substantiate that the phosphorylation of PKC substrates reflects PKC catalytic activity, bisindolylmaleimide I (BIS I), a selective inhibitor of conventional and novel PKC, was used. The phosphorylation of PKC substrates and MARCKS in response to H pylori or PMA was completely abolished in BIS I-treated cells (figure 2F). Importantly, BIS I demonstrated no toxicity towards H pylori, in contrast to many other PKC inhibitors, including rottlerin (supplementary figure 3) and calphostin C (data not shown).
Inhibition of PKC reduces MMP-1 expression in H pylori-infected cells
While studying the role of PKC activation, we observed that BIS I significantly inhibited MMP-1 gene expression (figure 3A) and protein accumulation (figure 3B) both in H pylori-infected and in PMA-treated AGS cells. MMP-1 expression following infection with P12 wt reached a maximum at 6 h post-infection (figure 3C), and MMP-1 accumulated in the membranes and nuclei of infected cells (figure 3D). MMP-1 gene up-regulation depended on the strain used for infection, and the P1 strain was less potent in inducing MMP-1 than the P12 strain (figure 3A,E). The cagA H pylori mutant was as effective as the wt, but the virB7 mutant up-regulated MMP-1 to a lesser extent (figure 3E,F).
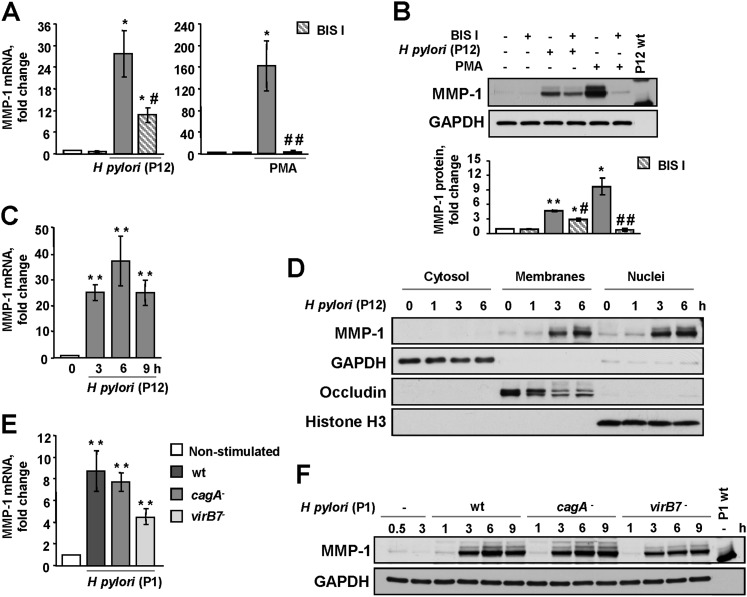
Figure 3.
H pylori up-regulates MMP-1 in a protein kinase C (PKC)-dependent manner. BIS I-treated or non-treated AGS cells were incubated with H pylori P12 wt, PMA (A–D) or H pylori P1 wt or the cagA and virB7 mutants (E, F) for 3 h or for the indicated periods of time. MMP-1 expression was analysed by qRT-PCR (A, C, E) or immunoblotting (B, D, F). The graphs in (B) summarise the densitometric analysis of three independent immunoblots (experiments). GAPDH, occludin and histone H3 were immunodetected to show the appropriate fractionation and equal protein amounts in the cell samples. Bacterial lysate was used as a negative control. *p<0.05, **p<0.01 versus non-stimulated cells; #p<0.05, ##p<0.01 versus BIS I-free stimulated cells.
Importantly, BIS I suppressed the MMP-1 expression induced by P12 wt in both the human HCA-7 colon cancer cell line and in primary stomach cells (supplementary figure 4A). Importantly, in both cell systems H pylori P12 wt induces PKC, which leads to phosphorylation of PKC substrates (supplementary figure 4B). HSC constitutively express mRNA from Muc-5ac and Muc-6 genes and stain positive for H+, K+-ATPase and pan-cytokeratins (supplementary figure 4C), which is consistent with normal gastric epithelial cells.
PKC regulates MMP-1 by activating AP-1 transcription factor
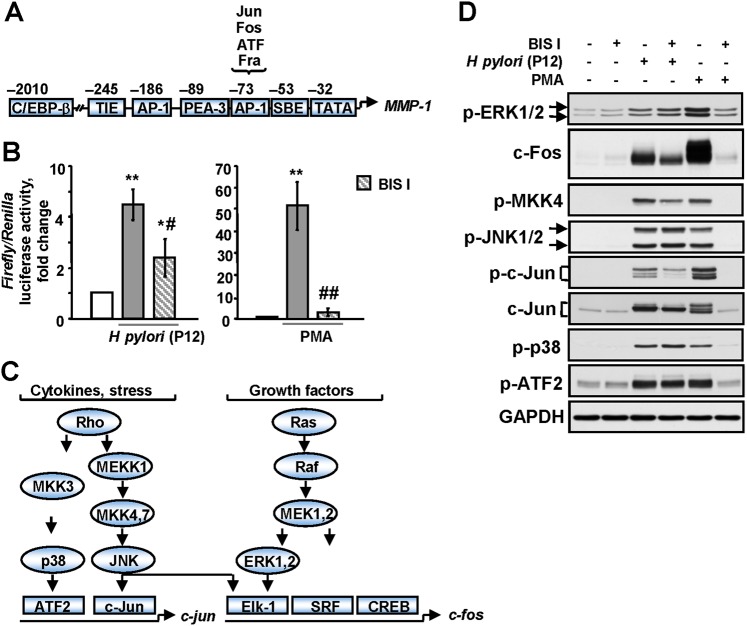
The MMP-1 promoter is predominantly regulate by AP-1 (figure 4A).29 Infection with H pylori led to a 4.4-fold increase in AP-1 activity (figure 4B). A more pronounced effect on AP-1 activity was achieved by treatment with PMA (51.5-fold induction; figure 4B). Pretreatment of the cells with BIS I completely abolished PMA-induced and diminished H pylori-induced AP-1 transactivation (figure 4B). AP-1 is a dimer that consists of Fos (c-Fos, FosB, Fra-1, Fra-2) and Jun (c-Jun, JunD, JunB) proteins and is positively regulated by mitogen-activated protein kinases (MAPK) (figure 4C).31 H pylori has been shown to activate a heterodimer composed of c-Fos and c-Jun.32 While exploring the molecular mechanism of AP-1 activation, we observed a strong phosphorylation of ERK1/2, JNK1/2, p38, c-Jun, ATF-2, JNK up-stream kinase MKK4, and accumulation of c-Jun and c-Fos in both H pylori- and PMA-treated cells (figure 4D). BIS I abolished all of the effects of PMA and reduced H pylori-induced c-Fos and c-Jun up-regulation. Surprisingly, in the infected cells, BIS I had no effect on the phosphorylation of ERK, p38 or JNK, which are considered to be up-stream regulators of c-Fos and c-Jun (figure 4C,D). The infection of AGS cells with wt H pylori and mutants showed that c-Jun was expressed following infection with the virB7 mutant, but delayed in comparison to the wt, which is in agreement with a report by Ding et al.33 However, phosphorylation of JNK1/2 and p38 was clearly T4SS-dependent (supplementary figure 5). These results indicate that in H pylori-treated cells, PKC is involved in up-regulation of the AP-1 members c-Fos and c-Jun, but the exact integrative mechanism and bacterial factors involved remain elusive.
Figure 4.
H pylori up-regulates AP-1 in a protein kinase C (PKC)-dependent manner. (A) The composition of MMP-1 promoter.30 The AP-1 element binds members of the c-Fos and c-Jun family of transcription factors. c/EBP-β, CCATT/enhancer binding protein-β; SBE, STAT binding element; TIE, TGFβ inhibitory element. (B) A reporter gene assay was performed using an inducible reporter construct encoding the firefly luciferase gene under the control of the AP-1 binding element. Firefly luciferase activity was normalised relative to Renilla's one. BIS I-treated/non-treated AGS cells were incubated with H pylori P12 wt or PMA for 3 h. *p<0.05, **p<0.01 vs non-stimulated cells; #p<0.05, ##p<0.01 vs BIS I-free stimulated cells. (C) Regulation of c-jun and c-fos expression by MAPK. (D) BIS I-treated cells were incubated with P12 wt or PMA for 1 h. The cell lysates were analysed by immunoblotting using antibodies as indicated.
Additionally, we found no accumulation of the AP-1 co-activator polyomavirus enhancer activator-3 (PEA3), which might promote MMP-1 expression in infected cells (data not shown).34
PKCα, PKCδ and PKCθ control H pylori-induced MMP-1 expression through c-Fos
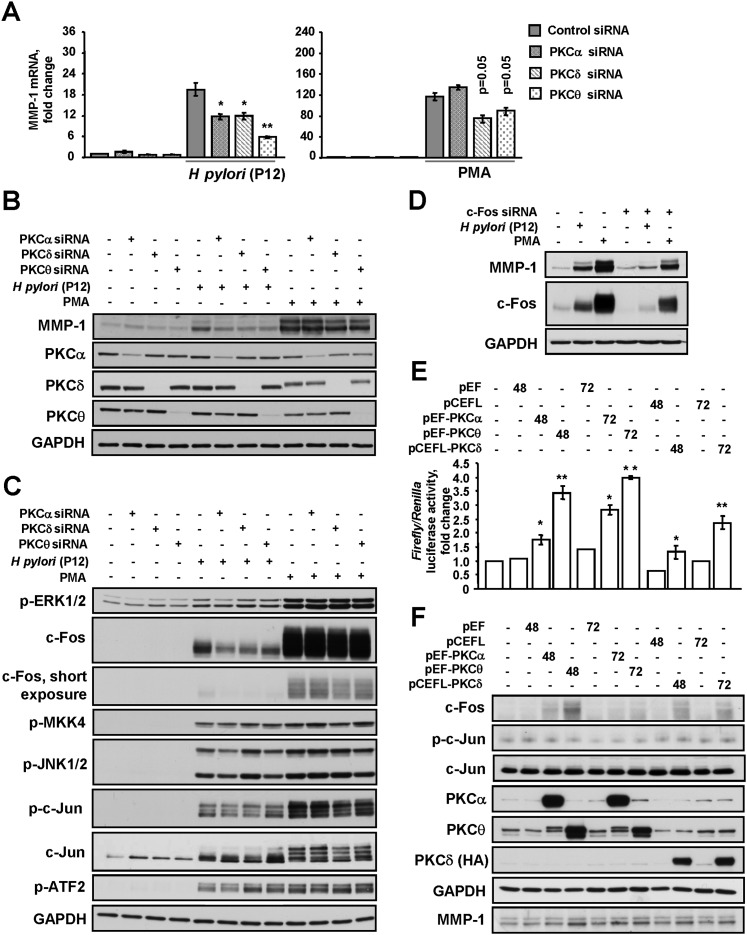
Given our results, which demonstrate that H pylori induces phosphorylation of PKCα, PKCδ and PKCθ, the involvement of these isoforms in MMP-1 regulation was subsequently tested. Specific PKC-targeting siRNAs reduced MMP-1 mRNA (figure 5A) and protein expression (figure 5B) in the infected cells. Depletion of PKCδ and PKCθ, but not PKCα, partially inhibited induction of MMP-1 by PMA (figure 5A,B).
Figure 5.
Protein kinase C (PKC) control H pylori-induced MMP-1 expression by stimulating AP-1. (A–D) AGS cells were transfected with siRNAs as indicated and then incubated with H pylori P12 wt or PMA for 3 h. MMP-1 expression was analysed by qRT-PCR (A) or by immunoblotting (B–D). (C) Cell lysates were prepared after 1-h stimulation. *p<0.05, **p<0.01 vs the respective siRNA-treated non-stimulated cells. (E, F) AGS cells were transfected with an AP-1 reporter construct and PKC-expressing or empty plasmids. After 48 or 72 h, the cells were harvested and (E) luciferase activities were estimated or (F) immunoblotting was performed using antibodies as indicated. *p<0.05, **p<0.01 versus the cells transfected with the respective empty vector.
A prominent decrease of c-Fos expression in PKCα, PKCδ or PKCθ siRNA-treated cells was observed when studying the signalling molecules involved in the activation of AP-1 in response to H pylori (figure 5C). c-Jun expression and phosphorylation of c-Jun, ERK, JNK and MKK4 were not affected (figure 5C). These results suggest that PKCα, PKCδ and PKCθ contribute to c-Fos up-regulation during infection with H pylori. In PMA-exposed cells, PKCδ depletion slightly affected the expression of c-Fos and c-Jun (figure 5C).
To confirm the crucial role of c-Fos in MMP-1 up-regulation, AGS cells were transfected with a c-Fos-targeting siRNA. On c-Fos knockdown, MMP-1 synthesis was diminished in both H pylori- and PMA-treated cells (figure 5D). Thus, c-Fos represents an important mediator in PKC-regulated MMP-1 expression.
Overexpression of PKC leads to AP-1 activation
To substantiate that PKCα, PKCδ and PKCθ regulate AP-1 in AGS cells, gene reporter assays were performed (figure 5E). Overexpression of constitutively active PKCα, δ and θ led to the transactivation of the AP-1 reporter gene 48 h post-transfection (1.8-, 1.3- and 3.5-fold, respectively) and 72 h post-transfection (2.8-, 4- and 2.1-fold, respectively). Immunoblotting revealed an accumulation of c-Fos in cells overexpressing PKC, which correlated with AP-1 activity and MMP-1 accumulation, and was most prominent in PKCθ-overexpressing cells (figure 5F). PKC overexpression did not induce the accumulation or phosphorylation of c-Jun (figure 5F). Thus, the PKC isoforms α, δ and θ regulate c-Fos leading to AP-1 activation in gastric cells.
PKC promote invasion of AGS cells in H pylori infection
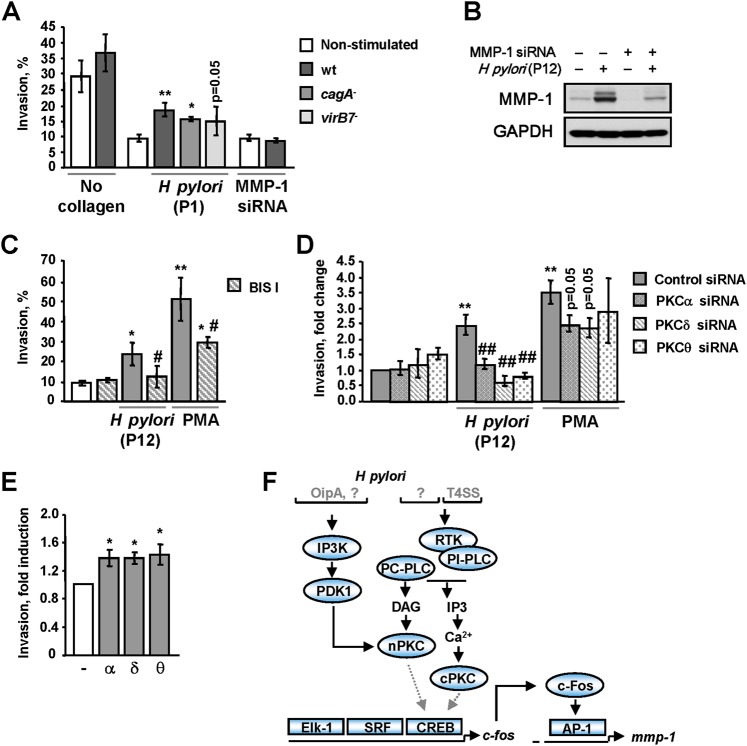
To further analyse the role of PKC in MMP-1 secretion, invasion assays using collagen I-coated filters were performed. Figure 6A shows that co-culturing of AGS cells with H pylori led to enhanced cellular invasion, which was less prominent on infection with the virB7 mutant strain in comparison to the wt and cagA strains. To assess the role of MMP-1 in H pylori-induced invasiveness, AGS cells were transfected with siRNA targeting MMP-1. Depletion of MMP-1 inhibited both basal and H pylori-induced MMP-1 expression in AGS cells (figure 6B) and suppressed invasion in response to H pylori (figure 6A). Treatment of the cells with the PKC inhibitor BIS I prior to infection markedly reduced the number of invading cells (figure 6C). Similar results were obtained for PMA.
Figure 6.
H pylori stimulates the invasive properties of AGS cells in a PKC-dependent fashion. (A) The cells were treated with control or MMP-1-targeting siRNAs, applied to the Transwell plate and further incubated with H pylori P1 wt, cagA and virB7 mutants, or PMA (4 nM) for 18 h, and the percentage invasion through collagen I-coated filters towards 5% FCS was determined. The migration rate through uncoated filters served as a methodological control. (B) The immunoblot analysis of the cells treated with scrambled or MMP-1-targeting siRNAs and infected with P12 wt for 3 h. (C–E) The invasion assay was performed using cells treated with BIS I (C) or PKC-targeting siRNAs and then stimulated with H pylori P12 wt or PMA. (E) The invasion assay was performed using cells overexpressing constitutively active PKC isozymes. (F) H pylori's T4SS and T4SS-independent factors are required for PKC activation and MMP-1 up-regulation. *p<0.05, **p<0.01 versus non-stimulated cells, #p<0.05, versus stimulated cells, ##p<0.01 versus stimulated mock-transfected cells.
Depletion of PKCα, PKCδ and PKCθ, which is crucial for MMP-1 production in response to H pylori, abolished transmigration of infected cells, indicating a functional role for these isozymes in invasion (figure 6D). Depletion of PKCα, PKCδ or PKCθ had a less prominent effect in PMA-treated cells (figure 6D). To confirm the regulatory role of PKCα, δ and θ in invasion, constitutively active isozymes were overexpressed. Figure 6E shows that PKC overexpression increased the number of invading cells. Taken together, these results indicate that MMP-1-dependent collagen I digestion involves PKC in H pylori-infected cells.
Invasion is an integrative process that depends on the adhesive and migratory behaviours of cells, in addition to their proteolytic activity towards the ECM. Therefore, involvement of PKC in regulation of cellular motility was investigated using a wound healing assay. In contrast to PMA, the P1 and P12 strains of H pylori did not stimulate wound healing (supplementary figure 6A,B). Treatment with H pylori or PMA for 24 h slightly decreased the total number of AGS cells (data not shown). Thus, H pylori-induced invasion depends mainly on the increased proteolytic activity of AGS cells. BIS I, but not siRNAs against PKCα, PKCδ or PKCθ, inhibited PMA-induced cell migration (supplementary figure 6A,C). Therefore, several PKC isozymes are engaged to stimulate both the proteolytic activity and migration of PMA-treated cells, leading to increased cell invasiveness.
In addition to the wound healing assay, the involvement of PKC in H pylori-induced scattered phenotype was studied, and no effects of BIS I or PKC-specific siRNAs on cell morphology were found (supplementary figure 7). However, PMA-induced AGS cell spreading was completely blocked by BIS I but not by siRNAs against PKCα, PKCδ or PKCθ (supplementary figure 7).
Discussion
The aim of this work was to investigate the activity and role of PKC isozymes in infected gastric epithelial cells. We show here that, on H pylori infection, cPKCα is phosphorylated within its hydrophobic motif and accumulates in both membranes and nuclei, which might represent sources of DAG35 and PKCα-interacting proteins.36 Autophosphorylation of the hydrophobic motif of PKCα has been reported to stabilise the enzyme37 and to be triggered by the mammalian target of rapamycin complex 2 and HSP90.5 Further, our results demonstrate that nPKCs δ and θ are transiently phosphorylated within their activation loops in a T4SS-independent manner. This finding is consistent with reports that both H pylori cagPAI and the outer membrane protein OipA activate phosphatidylinositol kinase 1 (PDK-1),38 which phosphorylates the activation loop of PKC, leading to enzyme maturation and activation39 (figure 6F). In contrast to the study by Brandt et al,14 we did not detect any CagA-dependent PKCδ phosphorylation at 6–9 h post-infection.
Activated PKC regulate their substrates, including MARCKS, vinculin and adducin.6 24 Our experiments demonstrate that H pylori causes phosphorylation of downstream targets of PKC, including MARCKS in a CagA- and VacA-independent manner. The phosphorylation of PKC substrates is less prominent during infection with the virB7 mutant strain. The most plausible model is that the phosphorylation of PKC substrates implicates a range of PKC isoforms that are activated independently of T4SS (eg, PKCδ and PKCθ) and via T4SS (eg, PKCα) (figure 6F).
For full activation, conventional and novel PKC require DAG, generated following PIP2 hydrolysis by PLCs. Here, we show that both PI-PLC and PC-PLC inhibitors reduce the phosphorylation of PKC substrates in H pylori-infected cells, with the PI-PLC inhibitor being more efficient. Indeed, PI-PLC-dependent hydrolysis of PIP2 yields, in addition to DAG, inositol 1,4,5-triphosphate (IP3),25 27 which provokes an increase of intracellular Ca2+. Thus, PI-PLC promotes activation of both DAG- and Ca2+-dependent PKC isozymes (figure 6F).
Within PI-PLCs, PLCγ1 plays an important role in PKC activation, as shown here using PLCγ1-targeting siRNA. Additionally, PLCγ1 activation in H pylori-infected gastric epithelial cells has been reported previously.20
Our experiments using BAPTA-AM further confirm a contributory role of intracellular Ca2+ in PKC activation on infection. As functional T4SS (but not CagA) is required for Ca2+ release during H pylori infection,28 we propose that T4SS is implicated in the regulation of Ca2+-activated PKC isozymes. Consistently, phosphorylation of Ca2+-regulated PKCα is T4SS-dependent.
It has previously been shown that PI3K signalling is activated by H pylori.38 40 PI3K, which phosphorylates PIP2 and leads to PIP3 generation, has been implicated in PDK-1 activation. Here, PI3K inhibition diminished the phosphorylation of PKC substrates and MARCKS in response to H pylori. Moreover, tyrosine kinases, which act up-stream of PLCs and PI3K, play a role in PKC activation during infection with H pylori, as demonstrated using genistein.
While studying PKC in vivo, we detected an increase of phosphorylated PKC in patients with H pylori-induced gastritis or gastric adenocarcinoma, which indicates that post-translational modifications of these enzymes may be crucial for H pylori-induced pathogenesis.
Given our results demonstrating that H pylori induces the phosphorylation of PKCα, PKCδ and PKCθ, we focused on their role in infected gastric epithelial cells. All of these PKC isoforms are involved in regulation of the cytoskeleton, adherence junctions and barrier permeability in the gastrointestinal epithelium.41 PKC may play a role in the pathogenesis of H pylori-caused diseases by affecting the integrity of the gastric epithelium.15
Gastric mucosa disturbances in response to H pylori implicate a range of MMPs, including MMP-1.10 MMP-1 not only degrades collagens I-III, VII, VIII and X, gelatin, and entactin,30 but also has functions extending beyond the degradation of the ECM components. For example, MMP-1 was found in the nucleus where it appears to confer resistance to apoptosis.42 Cytokines, growth factors and LPS induce MMP-1 synthesis via MAPK cascades in different cell types.43 44 MMP-1 is often up-regulated in gastric ulcers and cancer.10 45 Our data indicate that H pylori stimulates MMP-1 synthesis in gastric epithelial cells, which is in accordance with published data.11 19 46 Although both P1 and P12 belong to the type I cagA + vacA + katA + flaA + strains, P1 was less potent in inducing MMP-1 than the P12 strain; this finding requires further investigation. We found that MMP-1 accumulates in membranous structures and nuclei of infected cells. Further, our results show that similar to PKC activation, MMP-1 expression requires both functional T4SS and other T4SS-independent bacterial factors, for example, OipA.34 Using the PKC inhibitor BIS I or PKC-specific siRNAs, we discovered that PKCα, PKCδ and PKCθ up-regulate MMP-1, leading to enhanced invasion by infected AGS cells. Importantly, we observed no significant enhancement of migration on infection. Therefore, it is apparent that H pylori-stimulated invasion depends mainly on the proteolytic, but not the migratory, activity of AGS cells. The inhibitory effect of BIS I on MMP-1 expression was not restricted to AGS cells and was also detected in tumour HCA-7 cells and non-cancerous HSC, which suggests that this represents a common phenomenon.
It is well established that PMA, which induces a sustained activation of almost all of the PKC isoforms, up-regulates MMP-1.43 In this study, PMA stimulated MMP-1 synthesis, invasion and migration of AGS cells. Depletion of one particular PKC isoform (eg, PKCδ) had a weak effect on these processes, probably because of a contributory role of intact PKC isoforms activated by PMA.
How does PKC regulate MMP-1? PMA has been reported to activate ERK and JNK,47 leading to AP-1 assembly on the MMP-1 promoter.30 43 Consistently, PMA activates MAPK and AP-1 in AGS cells, and BIS I abolishes this effect. H pylori also induces MAPK, c-Jun and c-Fos, and activates AP-1 in AGS cells.33 48 We found that BIS I suppresses c-Fos and c-Jun expression and AP-1 activity in infected cells. Surprisingly, BIS I had no effect on the phosphorylation of ERK or JNK, which mediate MMP-1 induction by H pylori.19 46 These observations suggest that c-Jun and c-Fos regulation by PKC occurs apart from MAPK. In particular, the serum response factor (SRF) and members of the CREB/ATF family that control (together with Elk-1) c-Fos expression (figure 6F) are regulated by several Ca2+-dependent kinases, including PKC.49 Further, depletion of PKCα, PKCδ and PKCθ suppresses H pylori-induced c-Fos accumulation, and c-Fos depletion diminishes MMP-1 expression, indicating an important role of these PKC isoforms in c-Fos-dependent MMP-1 up-regulation. Indeed, in uninfected AGS cells, overexpression of active PKCα, PKCδ and PKCθ increased the amount of c-Fos, AP-1 activity and invasion through collagen I-coated filters.
With respect to the mechanistic role of H pylori virulence factors, pronounced T4SS-dependent and T4SS-independent processes exist.48 Future work on the identification of the bacterial factor(s) responsible for PKC activation will give additional insights into the mechanisms of gastric mucosa colonisation by H pylori and could provide a comprehensive picture of host–microbial interaction.
Supplementary Material
Acknowledgments
We thank G Baier and S Shaw for the PKC expression constructs, U Lendeckel and S Krueger for the MMP-1 primers, S Kahlert for the antibody to H+, K+-ATPase, and B Peters for help in ANOVA.
Footnotes
Contributors: OS: experiments, analysis, interpretation of data and manuscript preparation; MV: biopsy collection, immunohistochemistry; MN: interpretation of data, manuscript preparation, and study supervision.
Funding: The work was funded in part by the Deutsche Forschungsgemeinschaft (SFB 854) and the Bundesministerium für Bildung und Forschung (FORSYS, BMBF-0313922) by grants to MN.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Mackay HJ, Twelves CJ. Targeting the protein kinase C family: are we there yet? Nat Rev Cancer 2007;7:554–62 [DOI] [PubMed] [Google Scholar]
- 2. El-Zaatari M, Zavros Y, Tessier A, et al. Intracellular calcium release and protein kinase C activation stimulate sonic hedgehog gene expression during gastric acid secretion. Gastroenterology 2010;139:2061–71.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schwartz GK, Jiang J, Kelsen D, et al. Protein kinase C: a novel target for inhibiting gastric cancer cell invasion. J Natl Cancer Inst 1993;85:402–7 [DOI] [PubMed] [Google Scholar]
- 4. Lin KY, Fang CL, Uen YH, et al. Overexpression of protein kinase Calpha mRNA may be an independent prognostic marker for gastric carcinoma. J Surg Oncol 2008;97:538–43 [DOI] [PubMed] [Google Scholar]
- 5. Newton AC. Protein kinase C: poised to signal. Am J Physiol Endocrinol Metab 2010;298:E395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parker PJ, Murray-Rust J. PKC at a glance. J Cell Sci 2004;117:131–2 [DOI] [PubMed] [Google Scholar]
- 7. Odenbreit S, Puls J, Sedlmaier B, et al. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 2000;287:1497–500 [DOI] [PubMed] [Google Scholar]
- 8. Blaser MJ, Atherton JC. Helicobacter pylori persistence: biology and disease. J Clin Invest 2004;113:321–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andersen LP. Colonization and infection by Helicobacter pylori in humans. Helicobacter 2007;12:12–15 [DOI] [PubMed] [Google Scholar]
- 10. Menges M, Chan CC, Zeitz M, et al. Higher concentration of matrix-metalloproteinase 1 (interstitial collagenase) in Helicobacter pylori- compared to NSAID-induced gastric ulcers. Z Gastroenterol 2000;38:887–91 [DOI] [PubMed] [Google Scholar]
- 11. Göõz M, Göõz P, Smolka AJ. Epithelial and bacterial metalloproteinases and their inhibitors in Helicobacter pylori infection of human gastric cells. Am J Physiol Gastrointest Liver Physiol 2001;281:G823–32 [DOI] [PubMed] [Google Scholar]
- 12. Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol 2007;8:221–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Obst B, Schütz S, Ledig S, et al. Helicobacter pylori-induced apoptosis in gastric epithelial cells is blocked by protein kinase C activation. Microb Pathog 2002;33:167–75 [PubMed] [Google Scholar]
- 14. Brandt S, Wessler S, Hartig R, et al. Helicobacter pylori activates protein kinase C delta to control Raf in MAP kinase signalling: role in AGS epithelial cell scattering and elongation. Cell Motil Cytoskeleton 2009;66:874–92 [DOI] [PubMed] [Google Scholar]
- 15. Terrés AM, Pajares JM, Hopkins AM, et al. Helicobacter pylori disrupts epithelial barrier function in a process inhibited by protein kinase C activators. Infect Immun 1998;66:2943–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chang YJ, Wu MS, Lin JT, et al. Induction of cyclooxygenase-2 overexpression in human gastric epithelial cells by Helicobacter pylori involves TLR2/TLR9 and c-Src-dependent nuclear factor-kappaB activation. Mol Pharmacol 2004;66:1465–77 [DOI] [PubMed] [Google Scholar]
- 17. Beales IL, Calam J. Stimulation of IL-8 production in human gastric epithelial cells by Helicobacter pylori, IL-1beta and TNF-alpha requires tyrosine kinase activity, but not protein kinase C. Cytokine 1997;9:514–20 [DOI] [PubMed] [Google Scholar]
- 18. Kassai K, Yoshikawa T, Yoshida N, et al. Helicobacter pylori water extract induces interleukin-8 production by gastric epithelial cells. Dig Dis Sci 1999;44:237–42 [DOI] [PubMed] [Google Scholar]
- 19. Krueger S, Hundertmark T, Kalinski T, et al. Helicobacter pylori encoding the pathogenicity island activates matrix metalloproteinase-1 in gastric epithelial cells via JNK and ERK. J Biol Chem 2006;281:2868–75 [DOI] [PubMed] [Google Scholar]
- 20. Churin Y, Al-Ghoul L, Kepp O, et al. Helicobacter pylori CagA protein targets the c-Met receptor and enhances the motogenic response. J Cell Biol 2003;161:249–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dixon M, Genta R, Yardley J, et al. Classification of grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996;20:1161–81 [DOI] [PubMed] [Google Scholar]
- 22. Rozengurt E, Rey O, Waldron RT. Protein kinase D signalling. J Biol Chem 2005;280:13205–8 [DOI] [PubMed] [Google Scholar]
- 23. Nishikawa K, Toker A, Johannes FJ, et al. Determination of the specific substrate sequence motifs of protein kinase C isozymes. J Biol Chem 1997;272:952–60 [DOI] [PubMed] [Google Scholar]
- 24. Uberall F, Giselbrecht S, Hellbert K, et al. Conventional PKC-alpha, novel PKC-epsilon and PKC-theta, but not atypical PKC-lambda are MARCKS kinases in intact NIH 3T3 fibroblasts. J Biol Chem 1997;272:4072–8 [DOI] [PubMed] [Google Scholar]
- 25. Pollard TD, Earnshaw WC. Cell Biology. Phyladelphia, PA: Elsevier Inc, 2008:468–72 [Google Scholar]
- 26. Katan M. New insights into the families of PLC enzymes: looking back and going forward. Biochem J 2005;391:e7–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rebecchi MJ, Pentyala SN. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol Rev 2000;80:1291–335 [DOI] [PubMed] [Google Scholar]
- 28. Marlink KL, Bacon KD, Sheppard BC, et al. Effects of Helicobacter pylori on intracellular Ca2+ signalling in normal human gastric mucous epithelial cells. Am J Physiol Gastrointest Liver Physiol 2003;285:G163–76 [DOI] [PubMed] [Google Scholar]
- 29. Vincenti MP, White LA, Schroen DJ, et al. Regulating expression of the gene for matrix metalloproteinase-1 (collagenase): mechanisms that control enzyme activity, transcription, and mRNA stability. Crit Rev Eukaryot Gene Expr 1996;6:391–411 [DOI] [PubMed] [Google Scholar]
- 30. Chakraborti S, Mandal M, Das S, et al. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem 2003;253:269–85 [DOI] [PubMed] [Google Scholar]
- 31. van Dam H, Castellazzi M. Distinct roles of Jun: fos and Jun: aTF dimers in oncogenesis. Oncogene 2001;20:2453–64 [DOI] [PubMed] [Google Scholar]
- 32. Meyer-ter-Vehn T, Covacci A, Kist M, et al. Helicobacter pylori activates mitogen-activated protein kinase cascades and induces expression of the proto-oncogenes c-fos and c-jun. J Biol Chem 2000;275:16064–72 [DOI] [PubMed] [Google Scholar]
- 33. Ding SZ, Olekhnovich IN, Cover TL, et al. Helicobacter pylori and mitogen-activated protein kinases mediate AP-1 subcomponent protein expression and DNA-binding activity in gastric epithelial cells. FEMS Immunol Med Microbiol 2008;53:385–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu JY, Lu H, Sun Y, et al. Balance between PAE-3 and activator protein 1 regulates Helicobacter pylori-stimulated matrix metalloproteinase 1 expression. Cancer Res 2006;66:5111–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Neri LM, Borgatti P, Capitani S, et al. Nuclear diacylglycerol produced by phosphoinositide-specific phospholipase C is responsible for nuclear translocation of protein kinase C-alpha. J Biol Chem 1998;273:29738–44 [DOI] [PubMed] [Google Scholar]
- 36. Martelli AM, Bortul R, Tabellini G, et al. Molecular characterization of protein kinase C-alpha binding to lamin A. J Cell Biochem 2002;86:320–30 [DOI] [PubMed] [Google Scholar]
- 37. Bornancin F, Parker PJ. Phosphorylation of protein kinase C-alpha on serine 657 controls the accumulation of active enzyme and contributes to its phosphatase-resistant state. J Biol Chem 1997;272:3544–9 [DOI] [PubMed] [Google Scholar]
- 38. Tabassam FH, Graham DY, Yamaoka Y. Helicobacter pylori activate epidermal growth factor receptor- and phosphatidylinositol 3-OH kinase-dependent Akt and glycogen synthase kinase 3beta phosphorylation. Cell Microbiol 2009;11:70–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Toker A, Newton AC. Cellular signaling: pivoting around PDK-1. Cell 2000;103:185–8 [DOI] [PubMed] [Google Scholar]
- 40. Nagy TA, Frey MR, Yan F, et al. Helicobacter pylori regulates cellular migration and apoptosis by activation of phosphatidylinositol 3-kinase signaling. J Infect Dis 2009;199:641–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Farhadi A, Keshavarzian A, Ranjbaran Z, et al. The role of protein kinase C isoforms in modulating injury and repair of the intestinal barrier. J Pharmacol Exp Ther 2006;316:1–7 [DOI] [PubMed] [Google Scholar]
- 42. Limb GA, Matter K, Murphy G, et al. Matrix metalloproteinase-1 associates with intracellular organelles and confers resistance to lamin A/C degradation during apoptosis. Am J Pathol 2005;166:1555–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mackay AR, Ballin M, Pelina MD, et al. Effect of phorbol ester and cytokines on matrix metalloproteinase and tissue inhibitor of metalloproteinase expression in tumour and normal cell lines. Invasion Metastasis 1992;12:168–84 [PubMed] [Google Scholar]
- 44. Lai WC, Zhou M, Shankavaram U, et al. Differential regulation of lipopolysaccharide-induced monocyte MMP-1 and MMP-9 by p38 and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinases. J Immunol 2003;170:6244–9 [DOI] [PubMed] [Google Scholar]
- 45. Inoue T, Yashiro M, Nishimura S, et al. Matrix metalloproteinase-1 expression is a prognostic factor for patients with advanced gastric cancer. Int J Mol Med 1999;4:73–7 [DOI] [PubMed] [Google Scholar]
- 46. Pillinger MH, Marjanovic N, Kim SY, et al. Helicobacter pylori stimulates gastric epithelial cell MMP-1 secretion via CagA-dependent and -independent ERK activation. J Biol Chem 2007;282:18722–31 [DOI] [PubMed] [Google Scholar]
- 47. Schönwasser DC, Marais RM, Marshall CJ, et al. Activation of the mitogen-activated protein kinase/ERK pathway by conventional, novel, and atypical protein kinase C isotypes. Mol Cell Biol 1998;18:790–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Backert S, Naumann M. What a disorder: proinflammatory signaling pathways induced by Helicobacter pylori. Trends Microbiol 2010;18:479–86 [DOI] [PubMed] [Google Scholar]
- 49. Yamamoto KK, Gonzalez GA, Biggs WH, et al. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature 1988;334:494–8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.