Summary
Successful completion of mitosis requires that sister kinetochores become attached end-on to the plus ends of spindle microtubules (MTs) in prometaphase, thereby forming kinetochore microtubules (kMTs) that tether one sister to one spindle pole and the other sister to the opposite pole. Sites for kMT attachment provide at least four key functions: robust and dynamic kMT anchorage; force generation that can be coupled to kMT plus-end dynamics; correction of errors in kMT attachment; and control of the spindle assembly checkpoint (SAC). The SAC typically delays anaphase until chromosomes achieve metaphase alignment with each sister kinetochore acquiring a full complement of kMTs. Although it has been known for over 30 years that MT motor proteins reside at kinetochores, a highly conserved network of protein complexes, called the KMN network, has emerged in recent years as the primary interface between the kinetochore and kMTs. This Commentary will summarize recent advances in our understanding of the role of the KMN network for the key kinetochore functions, with a focus on human cells.
Key words: Checkpoint, Kinetochore, KNL1, MIS12, Mitosis, NDC80
Introduction
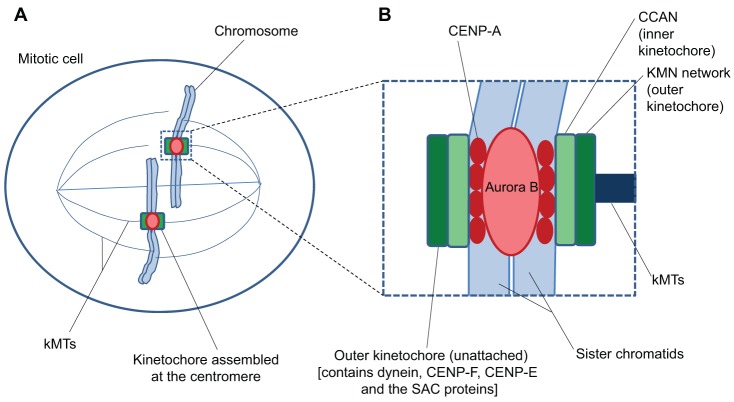
Kinetochores are mega-molecular assemblies that are formed at the centromeres of chromosomes at the onset of mitosis (Fig. 1A). Extensive research over the past two decades has identified over 80 different proteins, in ten or more major complexes, that localize to human kinetochores (Fig. 1B). The KMN (named for the Knl1 complex, the Mis12 complex and the Ndc80 complex; see below) network is part of the protein architecture within kinetochores that links centromeric DNA to the plus ends of spindle microtubules (MTs) (Cheeseman and Desai, 2008; Santaguida and Musacchio, 2009). The site where kinetochores are assembled is determined by the presence of a modified histone H3, CENP-A in humans (Cse4 in budding yeast), within nucleosomes at the periphery of each sister centromere (Fig. 1B). In addition to CENP-A-containing chromatin, the ‘inner’ domain of the kinetochore (Fig. 1B) contains a complex of peripheral centromeric proteins consisting of CENP-C, -H, -I, -K, -L, -M, -N, -O, -P, -S, -T, -U, -W and -X, called the ‘constitutive centromere-associated network’ (CCAN). A major function of CCAN is to link the MT-binding KMN network in the kinetochore ‘outer’ domain to centromeric DNA within the inner domain (Fig. 1B) (Hori et al., 2008; Hori and Fukagawa, 2012; Perpelescu and Fukagawa, 2011; Nishino et al., 2012; Saitoh et al., 1992; Takeuchi and Fukagawa, 2012). The kinase Aurora B, which localizes to centromeric chromatin before anaphase at high concentrations (Fig. 1B), has a central role in regulating the stability of kMT attachments and attachment error correction in concert with the KMN network (Cheeseman and Desai, 2008; Santaguida and Musacchio, 2009; Maresca and Salmon, 2010).
Fig. 1.
Protein composition and domain structure of the vertebrate kinetochore in mitotic cells. (A) Kinetochores are assembled at the periphery of sister centromeres on mitotic chromosomes and attach to spindle microtubules. (B) The site of kinetochore assembly is specified by the presence of the modified histone H3 CENP-A. The CCAN protein network within the inner kinetochore domain links CENP-A-containing chromatin to the KMN network within the outer kinetochore domain. The KMN network is the major interface between kMTs and their associated proteins. The peripheral region of the outer domain contains long fibrous proteins, such as CENP-F, the microtubule motors CENP-E and dynein, as well as their associated proteins, particularly in the absence of kMT attachments at prometaphase. Protein components of the SAC are also found in the outer domain. Although primarily within the peripheral region, these proteins probably also extend into the inner parts of the outer domain because many of these are connected to the KMN network. See text for details.
The highly conserved KMN network includes the Knl1 complex, which in vertebrates consists of KNL1 (also known as CASC5 or blinkin in humans, Spc105 in yeast and Spc105R in fly) and ZWINT; the Mis12 complex consisting of the four proteins MIS12 (Mtw1 or MIND in yeast), NSL1 (also called DC31 or MIS14), NNF1 (also known as PMF1) and DSN1 (also known as MIS13 in humans; KNL-3 in Caenorhabditis elegans); and the four-subunit Ndc80 complex comprising NDC80 (also known as HEC1), NUF2, SPC24 and SPC25 (Cheeseman et al., 2006; Cheeseman et al., 2004; DeLuca et al., 2006; Kops et al., 2005). The KMN network associates with kinetochores in prophase and disappears from kinetochores in telophase (Santaguida and Musacchio, 2009). The outer end of the Ndc80 complex is the primary binding site for the plus ends of spindle MTs (DeLuca and Musacchio, 2012; Gascoigne and Cheeseman, 2011; Joglekar et al., 2010; Santaguida and Musacchio, 2009; Tooley and Stukenberg, 2011), whereas the inner end is anchored either to the CCAN protein CENP-T (Hori and Fukagawa, 2012) or to the Mis12 complex (Petrovic et al., 2010). KNL1 also binds, at its outer end, to kMTs and, at its inner end, to the Mis12 complex (Fig. 1B) (Petrovic et al., 2010). The Mis12 complex is linked through CENP-C to CENP-A-containing chromatin (Fig. 1B) (Hori and Fukagawa, 2012). Both the Ndc80 complex and KNL1 have an elongated shape, extending outwards from their junctions with CENP-T or the Mis12 complex, and are oriented along the kMT axis at metaphase (DeLuca et al., 2006; Joglekar et al., 2009; Przewloka et al., 2011; Schittenhelm et al., 2009; Schittenhelm et al., 2007; Wan et al., 2009).
A number of other proteins within and at the periphery of the kinetochore outer domain depend on the presence of members of the KMN network for their kinetochore localization. These include MT-associated proteins in the proximity of kinetochore MT (kMT) plus-ends and members of the spindle assembly checkpoint (SAC) (Fig. 1B) (Musacchio and Salmon, 2007; Kops and Shah, 2012). The KMN network is also thought to associate with proteins that form the peripheral region of the outer kinetochore, including fibrous proteins, such as CENP-F, and MT motor proteins, such as the kinesin CENP-E and the cytoplasmic dynein–dynactin complex (Fig. 1B) (Cheeseman and Desai, 2008). The precise nature of the interactions between KMN network and other outer domain kinetochore proteins are only just being unraveled. In this Commentary, we will discuss recent advances in our knowledge of the structure and function of the proteins associated with KMN network and their potential roles in achieving the key functions of the kinetochore to ensure accurate chromosome segregation. We begin with the inner-most member of the KMN network, the Mis12 complex, then continue to describe the structure and function of the other two components, the Knl1 and Ndc80 complexes, and close with a discussion on other proteins and mechanisms involved in controlling the function of the KMN network.
The Mis12 complex
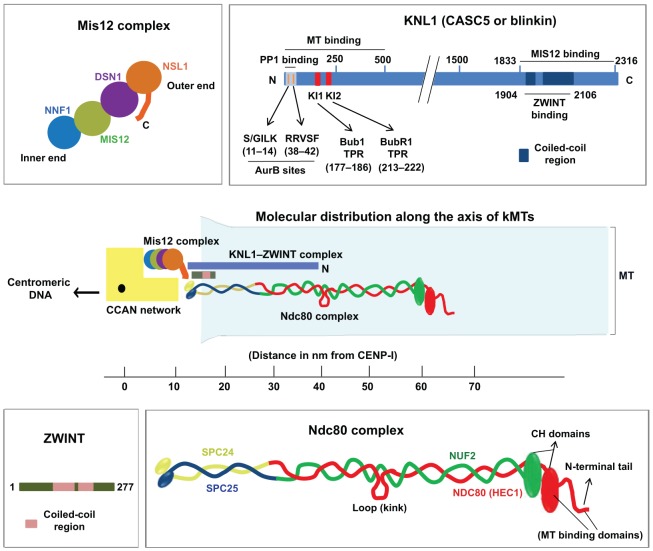
The Mis12 complex has been referred to as the kinetochore ‘keystone’ complex, as it serves as a major platform for outer kinetochore assembly (Cheeseman and Desai, 2008). The human Mis12 complex is 22-nm long and rod shaped, with the subunits linearly tethered to each other in the order NNF1, MIS12, DSN1 and NSL1, from inside to outside, within vertebrate kinetochores (Fig. 2) (Maskell et al., 2010; Petrovic et al., 2010). Electron microscopy analysis of the homologous yeast Mtw1 complex assembled in vitro essentially yields a similar elongated structure, although the yeast complex has been proposed to have a ‘comma’ or bi-lobbed shape (Hornung et al., 2011; Maskell et al., 2010). Both the yeast Dsn1 and Nsl1 subunits have been found to be elongated, with them spanning much of the length of the entire yeast complex. On the basis of in vitro protein assembly assays using purified components of the human KMN network, it has been found that the C-terminal tail of the NSL1 subunit is able to attach to both SPC24 and SPC25, which are located at the inner end of the Ndc80 complex, and to the C-terminus of KNL1 (Fig. 2) (Petrovic et al., 2010). A similar analysis of the yeast Mtw1 complex suggests that the complex has a direct link to Spc24 and Spc25 of the Ndc80 complex (Hornung et al., 2011). Hence, the current evidence suggests that even though the human and yeast Mis12 complexes might show variations in their structure, they essentially perform very similar functions.
Fig. 2.
Structure and composition of the KMN network. The molecular distribution of the components of the KMN network is shown in the centre of the figure and is projected along the axis of kMTs (shown in light blue). The CCAN (shown in yellow) forms the key connection between the KMN network at outer kinetochores and the centromeric chromatin. The human Mis12 complex (shown on the top left) consists of four subunits NNF1, MIS12, DSN1 and NSL1 that are apparently arranged linearly along the inside–outside axis of kinetochores in the sequence indicated (Petrovic et al., 2010). The C-terminal tail of the NSL1 subunit at the kinetochore-proximal outer end of the complex is important for interacting with the Ndc80 complex and KNL1, whereas the centromeric DNA-proximal inner end binds to the CCAN network. High-resolution two-color fluorescent imaging has shown that the Mis12 complex is located ∼50 nm inside of the outer end of the Ndc80 complex and ∼15 nm outside of CENP-I (part of the CCAN), and that MIS12 is probably oriented at an angle along the inner–outer kinetochore axis (Wan et al., 2009). Human KNL1 (top right) is a large multi-domain protein with the known functional domains and motifs indicated. KNL1 is elongated with its N-terminal region, which contains the proposed MT-binding site, located ∼40 nm away from CENP-I, and its C-terminal region, which is required for its kinetochore targeting (where it binds to MIS12 and its partner ZWINT), ∼15 nm away from CENP-I (Kiyomitsu et al., 2007; Petrovic et al., 2010; Wan et al., 2009). ZWINT (bottom left) is a relatively small coiled-coil rich kinetochore protein that binds the C-terminal coiled-coil domain of KNL1 and forms a tight complex with KNL1 (Petrovic et al., 2010). It has also been shown that ZWINT interacts with the Mis12 complex and the Ndc80 complex. The human Ndc80 complex (bottom right) is the major component of the core MT attachment module at kinetochores and consist of four subunits NDC80 (HEC1), NUF2, SPC24 and SPC25, which heterotetramerize through their coiled-coil regions as indicated. The N-terminal CH domain and charged unstructured tail regions of the NDC80 subunit form a bipartite MT-binding interface, whereas the globular C-terminal domains of SPC24 and SPC25 bind to CENP-T or the Mis12 complex to link the Ndc80 complex to the inner kinetochore. The CH domain of NDC80 has also been shown to be important for the retention of checkpoint proteins MAD1 and MAD2 at unattached kinetochores (Guimaraes et al., 2008; Martin-Lluesma et al., 2002; Miller et al., 2008). The conserved internal loop region of the Ndc80 complex is highly flexible, allowing the α-helical coiled-coil rod that ends in the MT-binding CH or head domains to rotate or twist at angles between 0 and 120°, with the loop region serving as a hinge. The N-terminal CH domain of NDC80 is ∼100 nm outside of CENP-A, which is bound to the centromere (not depicted), and about 65 nm outside of CENP-I within MT-bound metaphase kinetochores (Wan et al., 2009). It has also been observed that the distance between the MT-proximal NDC80 CH domain and the head domain of the centromere-proximal SPC24–SPC25 complex is only about 45 nm in human kinetochores (as compared to the 57-nm-long extended confirmation of the Ndc80 complex reported in yeast) suggesting that the MT-bound confirmation of the Ndc80 complex in human cells is slightly bent during metaphase (Wan et al., 2009).
Although it has been shown that the inner end of the Mis12 complex interacts directly with CENP-C, it is not clear which of the Mis12 complex subunits are involved in these interactions in vertebrate cells (Screpanti et al., 2011). In Drosophila melanogaster, it has been shown that the centromere-proximal Nnf1 subunit is instrumental in the interaction between the Mis12 complex and CENP-C (Przewloka et al., 2011). The yeast Mtw1 complex has also been shown to associate directly with the yeast functional homologue of CCAN, a Ctf19-containing complex called COMA (Hornung et al., 2011). As CCAN in turn directly associates with CENP-A-containing chromatin (Foltz et al., 2006; Okada et al., 2006), the Mis12 complex is an integral part of the machinery that connects the outer kinetochore to the inner centromeric DNA. Initially isolated as a key component of the core MT attachment site, Mis12 has not been shown to bind to purified MTs, even though it has been reported to enhance the MT-binding affinities of the Ndc80 complex and of KNL1 (Cheeseman et al., 2006), and recent evidence indicates that it interacts with the microtubule-associated protein (MAP) complex Ska (Chan et al., 2012).
Consistent with a role of the Mis12 complex in the recruitment of the Ndc80 and Knl1 complexes, depleting its various subunits results in defects in metaphase chromosome alignment, stable kMT formation, kinetochore bi-orientation and chromosome segregation (Kline et al., 2006). Seminal studies of the Mis12 homologue in yeast have demonstrated that the Mis12 complex performs important functions in controlling spindle structure, accurate chromosome segregation, sister centromere biorientation and separation (Goshima et al., 1999; Goshima and Yanagida, 2000). Drosophila embryos with mutations in Mis12 complex subunits also exhibit similar defects in chromosome segregation (Venkei et al., 2011). Interestingly, extensive investigations have failed to identify a Dsn1 homologue in Drosophila, and it has been proposed that this subunit has been functionally replaced in part by the fly Knl1 homologue, Spc105R, on which the other three subunits of the Mis12 complex depend for their localization to the kinetochore (Przewloka and Glover, 2009). In budding yeast, the V-shaped heterotetrameric monopolin complex, consisting of Mam1, Csm1, Lrs4 and Hrr25, has been shown to bridge the Dsn1 subunit of Mis12 with centromere-localized CENP-C and influence normal meiotic chromosome segregation (Corbett et al., 2010), but it is not known if a similar complex exists in higher vertebrates. In addition to centromere-proximal CENP-A and CCAN, Mis12 has also been shown to require the chaperone complex Hsp90–Sgt1 for its appropriate targeting to kinetochores (Davies and Kaplan, 2010). Several studies have found that the Nsl1 subunit interacts with the heterochromatic protein HP1, and that this association is important for the formation of the inner kinetochore (Kiyomitsu et al., 2010; Kiyomitsu et al., 2007; Obuse et al., 2004). However, many of the mitotic defects observed by the perturbation of HP1 could be attributed to improper kinetochore recruitment of the Ndc80 complex, as both HP1 and the Spc24 or Spc25 subunits of the Ndc80 complex have been shown to bind the same site in the C-terminal of Nsl1 and that their binding to Nsl1 is competitive in nature (Petrovic et al., 2010). A recent study has also shown that the Dsn1 subunit of the Mis12 complex and Knl1 are targets for Aurora B kinase phosphorylation that reduces the MT-binding affinity of the KMN network (Welburn et al., 2010). This study provides evidence that the phosphorylation of Dsn1 sensitizes the KMN network towards dramatic changes in MT-binding activity.
In summary, the Mis12 complex is a key component of the KMN network that is required to properly target the other two MT-binding components, the Ndc80 complex and KNL1 to kinetochores. The main function of this complex is to connect the outer kinetochore region to the inner centromeric DNA in association with the proteins of the CCAN network.
The Knl1 complex
The Knl1 complex is a heterodimer of KNL1 (also known as CASC5 or blinkin in humans, Spc105 in yeast and Spc105R in fly) with ZWINT (Petrovic et al., 2010). Human KNL1 is a large 300-kDa protein that is recruited to kinetochores by the Mis12 complex (Fig. 2).
KNL1
KNL1 has been shown to have a role in the recruitment of several outer-domain kinetochore proteins, including its binding partner ZWINT, CENP-F and the mitotic checkpoint proteins BUB1 and BUBR1 (also known as BUB1B) (Cheeseman et al., 2008; Kiyomitsu et al., 2007). X-ray crystallographic and other studies have revealed that helical motifs, called the KI motifs (∼10–12 amino acids long), in the N-terminal region of KNL1 interact with the TPR domains of BUBR1 and BUB1 and are important for maintaining normal SAC activity in human cells (Bolanos-Garcia et al., 2011; Kiyomitsu et al., 2011; Krenn et al., 2012). One of these studies has also demonstrated that the interaction between the KI motif of KNL1 and the TPR motif of BUB1 does not control the targeting of BUB1 to the kinetochore, but instead might influence the ability of BUB1 to interact with BUBR1, suggesting that KNL1 serves as a scaffold that brings together BUB1 and BUBR1 to help generate the kinetochore SAC signal (assembly of the mitotic checkpoint complex, Cdc20, Mad1, BubR1 and Bub3; Krenn et al., 2012).
Human mitotic cells that have been depleted of KNL1 are severely compromised in forming stable kMTs, and cells that enter anaphase often exhibit sister chromosome missegregation (Cheeseman et al., 2008; Kiyomitsu et al., 2007; Schittenhelm et al., 2009). In agreement with a role for KNL1 in the recruitment of BUB1 and BUBR1, both proteins are found to be displaced from kinetochores after KNL1 depletion (Kiyomitsu et al., 2007). Live imaging has revealed that these cells undergo accelerated mitosis and premature chromosome decondensation prior to the onset of anaphase (Kiyomitsu et al., 2007). Apart from recruiting BUB1 and BUBR1 to the kinetochore, KNL-1 has been shown to have a role in recruiting the Ndc80 complex and the RZZ checkpoint complex in C. elegans (Cheeseman et al., 2008; Essex et al., 2009; Gassmann et al., 2008). However, in human cells, NDC80 recruitment to kinetochores does not appear to require the function of KNL1 (Cheeseman et al., 2008; Kiyomitsu et al., 2007). KNL1 has also been shown to have MT-binding activity at its extreme N-terminus (Cheeseman et al., 2006; Espeut et al., 2012), and it has been shown in C. elegans that this is necessary for SAC activity but dispensable for the formation of proper kMT attachments and other aspects of chromosome segregation (Espeut et al., 2012). In that study, the authors expressed a mutant KNL-1 that lacked the MT-binding activity in C. elegans embryos, and found that they exhibit delays in anaphase onset and persistent checkpoint activation. Even though the amino acids at positions 1–500 are required for MT binding, the crucial sequences were narrowed down to a basic stretch of nine amino acid residues at the extreme N-terminus of the protein (Espeut et al., 2012).
The extreme N-terminus of KNL1 has also been shown to be important for the recruitment of protein phosphatase 1 (PP1) to outer kinetochores. PP1 activity counteracts the activity of the kinase Aurora B, which is involved in the destabilization of kMTs (by phosphorylating multiple sites within the KMN network), and consequently assists in accurate chromosome biorientation and alignment (Liu et al., 2010; Meadows et al., 2011; Rosenberg et al., 2011; Welburn et al., 2010). A recent study has suggested that the recruitment of PP1 to the kinetochore by KNL1 also contributes to SAC activity in an independent and possibly additive manner to that of its MT-binding activity (Espeut et al., 2012). This study further proposes that the MT-binding activity of KNL1 acts as a sensor of kMT attachment and relays this information to the checkpoint machinery for silencing of the SAC. More recently, three separate studies have shown that the yeast Knl1 homologue Spc105 is a target for the Mps1 (Mph1) checkpoint kinase and that this activity in turn is responsible for the recruitment of the Bub1–Bub3 checkpoint complex to kinetochores (London et al., 2012; Shepperd et al., 2012; Yamagishi et al., 2012). One of these studies also suggests that this phosphorylation activity of Mps1 is opposed by the phosphatase activity of Knl1-recruited PP1 (London et al., 2012).
In summary, KNL1 is a large multi-domain and multi-functional scaffold protein that is required for kinetochore targeting of several other outer-domain kinetochore proteins, including its binding partner ZWINT, the SAC proteins BUB1 and BUBR1, CENP-F and possibly, the RZZ complex. In concert with the other constituents of the KMN network, KNL1 also serves important roles in kMT attachment and silencing the SAC.
ZWINT
ZWINT (KBP-5 in C. elegans) is a 277-amino-acid kinetochore protein that initially has been implicated in the targeting of ZW10 to kinetochores (Starr et al., 2000) (Fig. 2). ZW10 is part of another outer kinetochore complex, the RZZ complex, which also contains the two proteins ROD (also known as KNTC1) and ZWILCH (Karess, 2005). The RZZ complex in turn recruits the adaptor protein Spindly that serves to attach the dynein–dynactin motor complexes to checkpoint proteins such as the MAD1–MAD2 complex (Gassmann et al., 2008; Griffis et al., 2007). A role for ZWINT in the recruitment of the RZZ complex to kinetochores has been confirmed in subsequent studies (Kops et al., 2005; Lin et al., 2006; Wang et al., 2004), Surprisingly, ZWINT was found to co-purify with the components of the KMN network, but not with the RZZ complex (Cheeseman et al., 2006; Cheeseman et al., 2004; Kops et al., 2005). Recently, ZWINT, whose structure has coiled-coil motives, has been demonstrated to form a tight complex with the C-terminal coiled-coil domain of KNL1 (Petrovic et al., 2010), and this might explain reports of interactions between ZWINT and MIS12 (Obuse et al., 2004) and the NDC80 (HEC1) subunit of the Ndc80 complex (Lin et al., 2006; Vos et al., 2011). Similar to the other constituents of the KMN network (Santaguida and Musacchio, 2009), it has also been shown that ZWINT is a stable component of the kinetochore, whereas the RZZ complex is more dynamic in its residency at metaphase kinetochores (Famulski et al., 2008). ZWINT is also known to be a substrate for phosphorylation by the Aurora B kinase. It has been proposed that this phosphorylation is required for recruitment of the RZZ complex and the dynein motor to kinetochores, which in turn is important for chromosome motility and SAC signaling (Famulski and Chan, 2007; Kasuboski et al., 2011). Very recently, a potential functional homologue of ZWINT has been identified in fission yeast as a binding partner for the yeast KNL1 homologue Spc105 (Jakopec et al., 2012).
The Ndc80 complex
A number of extensive reviews have discussed the structure and function of the Ndc80 complex (see Alushin and Nogales, 2011; DeLuca and Musacchio, 2012; Joglekar et al., 2010; Lampert and Westermann, 2011; Takeuchi and Fukagawa, 2012), and this Commentary will thus only attempt to summarize the recent advances in our understanding of this complex. The Ndc80 complex is a 57-nm-long heterotetrameric complex consisting of two dimers, NDC80 (HEC1) and NUF2, and SPC24 and SPC25, that are held together by overlapping α-helical coiled coil domains located at the C-terminus of the NDC80–NUF2 dimer and the N-terminus of the SPC24–SPC25 dimer (Cheeseman and Desai, 2008; Ciferri et al., 2008; Wei et al., 2007). The C-terminus of SPC24–SPC25 interacts with both CENP-T in humans (Gascoigne et al., 2011) and budding yeast (Bock et al., 2012; Schleiffer et al., 2012), or with the NSL1 and DSN1 components of the Mis12 complex in humans and Drosophila (see above).
Studies that have analyzed the various predicted MT-binding domains within the Ndc80 complex during mitosis suggest that both the NDC80 CH domain and its charged N-terminal tail are important for the formation of stable kMT attachments and for chromosome alignment, whereas the CH domain of NUF2, although it is non-essential for these functions, is required for producing normal MT-dependent kinetochore force and timely mitotic progression (Sundin et al., 2011; Tooley et al., 2011). Furthermore, it has been demonstrated that the N-terminal tail of NDC80 is required to interact with the negatively charged C-terminal tails (the so-called E-hooks) of tubulin monomers (Alushin et al., 2010; Ciferri et al., 2008; Tooley et al., 2011). These interactions are weakened by phosphorylation of the nine Aurora B target sites within the N-terminal tail of NDC80 (Cheeseman et al., 2006; DeLuca et al., 2006; Santaguida and Musacchio, 2009; Wei et al., 2007). It has been demonstrated in vivo that Aurora B kinase activity is important for destabilizing kMT attachment and correction of MT-attachment errors, which is highly relevant for the fidelity of mitotic chromosome segregation (Cheeseman et al., 2006; Cimini et al., 2006; DeLuca et al., 2006; Sandall et al., 2006; Welburn et al., 2010). Studies with reconstituted protein preparations in vitro show that dephosphorylated NDC80 bound to beads can track, stabilize and promote rescue of the ends of depolymerizing MTs, whereas Aurora B phosphomimetic versions of the complex are unable to do so, further emphasizing the importance of Aurora B phosphorylation for the function of the Ndc80 complex (Umbreit et al., 2012).
Structural studies have provided important details of MT binding by the Ndc80 complex (Wilson-Kubalek et al., 2008; Alushin et al., 2010). A key MT-binding region is located within the CH domain of NDC80 at a site termed ‘toe print’. The ‘toe’ serves as a sensor of the tubulin conformation that enables the Ndc80 complex to bind to straight MT protofilaments with high affinity and to curled protofilaments at the ends of depolymerizing MTs with low affinity. The Alushin et al. study and two other studies have found that the Ndc80 subunit NUF2, which also contains a CH domain, is not involved in the interaction with MTs (Sundin et al., 2011; Alushin et al., 2010; Wilson-Kubalek et al., 2008), despite a previous report showing that mutations within the NUF2 CH domain interfere with the binding of the Ndc80 complex to MTs (Ciferri et al., 2008). Additionally, Alushin et al. provide evidence that the unstructured N-terminal tail of NDC80 aids in tethering together adjacent Ndc80 complexes upon MT binding, an idea that has been supported by several other studies (Cheeseman et al., 2006; Ciferri et al., 2008; Powers et al., 2009; Tooley et al., 2011). Another recent study dissected the role of the tail domain in MT binding and Ndc80 clustering in even more detail (Alushin et al., 2012). This study shows that Aurora B sites are organized as two separate segments or zones in the tail region, one at the tubulin-binding interface and the other at the interface with an adjacent MT-bound Ndc80 complex; phosphorylation at the two different sites serves to fine tune the interaction of the Ndc80 complex with tubulin monomers and with neighboring Ndc80 complexes.
The CH domain of NDC80 is also important for recruiting spindle checkpoint proteins such as the MAD1–MAD2 complex (Guimaraes et al., 2008, Martin-Lluesma et al., 2002, Miller et al., 2008). In addition, serine 165 within the NDC80 CH domain is a target for the kinase NEK2, and this phosphorylation is important for chromosome alignment and the SAC (Wei et al., 2011). Cells expressing a non-phosphorylatable mutation of NDC80 at serine 165 are unable to recruit MAD1–MAD2 to kinetochores and exhibit premature anaphase onset with erroneous chromosome segregation. Phosphorylation of NDC80 and the Ndc80 complex by Aurora B have also been shown to be important for the recruitment of the checkpoint kinase MPS1 (also known as TTK in vertebrates) to kinetochores, leading to its activation, which is essential for the SAC to prevent anaphase (Vigneron et al., 2004; Hewitt et al., 2010; Santaguida et al., 2010; Maciejowski et al., 2010).
Taken together, the heterotetrameric Ndc80 complex emerges as the major MT-binding interface at kinetochores and uses both the N-terminal CH domain and tail domains of its NDC80 subunit to bind MTs. Phosphorylation of key amino acid residues at the N-terminal tail by Aurora B kinase negatively influences MT-binding affinity and serves as an effective mechanism to regulate the strength of kMT attachment and the process of attachment error correction. The N-terminal CH domain also plays a role in recruiting MAD1, MAD2 and Mps1 to kinetochores.
Further insights into KMN network function
Dam/Dash and Ska MAP complexes
Another active area of research has been to address the pivotal question of how the activity of the KMN network is coupled to the polymerization and depolymerization dynamics of kMT plus-ends by MAPS that potentially interact with the Ndc80 complex or the KMN network and are required for effective kMT attachments and force generation (Joglekar et al., 2010). In yeast, the Dam complex (also known as the Dash complex) is a MT-binding protein that depends on the kinetochore-bound Ndc80 complex for its localization to kinetochores. The Dam/Dash complex is composed of 10 subunits that assemble into oligomers and form either closed stable ring-like structures or open spirals around the MT lattice (Lampert and Westermann, 2011). Dam/Dash has been shown to increase the processivity of the Ndc80 complex when it is attached to depolymerizing MTs, and is also a prominent target for the yeast homologue of Aurora B kinase, Ipl1 (Alushin and Nogales, 2011, Grishchuk et al., 2008; Lampert and Westermann, 2011). Structural homologs of the Dam/Dash complex components have not been discovered in higher eukaryotes, but recent studies are beginning to shed light on other MAPs that interact with the Ndc80 complex or the KMN network. In human cells, the kinetochore-localized Ska complex has emerged as one of the major modes of these regulatory functions as discussed below.
The Ska complex was initially identified in human cells as a complex comprising two proteins SKA1 and SKA2. SKA1 and SKA2 were found to localize to spindle MTs and to concentrate at kinetochores in a manner that is dependent on kinetochore-bound NDC80 (Hanisch et al., 2006). Subsequently, a third subunit of the complex, SKA3, also called RAMA1, was discovered that localizes to kinetochores and spindle MTs, and knockdown of this protein results in identical phenotypes to those upon knockdowns of SKA1 and SKA2 (Daum et al., 2009; Gaitanos et al., 2009; Raaijmakers et al., 2009; Theis et al., 2009; Welburn et al., 2009). SKA3 is required for the proper stability and functioning of the Ska complex as a whole (Gaitanos et al., 2009). In human cells, the Ska complex is reported to be important for normal kMT formation, metaphase alignment, SAC silencing and anaphase chromosome segregation (Daum et al., 2009; Gaitanos et al., 2009; Hanisch et al., 2006; Raaijmakers et al., 2009; Theis et al., 2009; Welburn et al., 2009). The human Ska complex has been reconstituted in vitro and shown to bind MTs in a cooperative manner (Welburn et al., 2009). Moreover, that study, as well as more recent data (Schmidt et al., 2012), have shown that the Ska complex can form oligomeric assemblies that diffuse along MTs and that it binds to depolymerizing MT plus-ends along both straight and curved MT protofilaments (the Ndc80 complex binds only straight protofilaments near MT ends). The purified Ska complex exhibits processive motion at the ends of depolymerizing MTs and strengthens plus-end tracking of the purified Ndc80 complex. Moreover, the Ska complex enhances the capacity of the Ndc80 complex to bind MTs in a cooperative manner. These properties of the Ska complex, together with the requirement of MTs and the Ndc80 complex for its localization to kinetochores, suggests that the Ska complex could be a functional (but possibly not structural) homologue in vertebrates of the Dam/Dash complex in yeast (Gaitanos et al., 2009; Hanisch et al., 2006; Welburn et al., 2009; Schmidt et al., 2012).
There is also evidence that the Ska complex interacts with KNL1 and the Mis12 complex, in addition to the Ndc80 complex, and that the recruitment of Ska to kinetochores depends on all three members of the KMN network (Chan et al., 2012). That study also showed that SKA1 and SKA3 are targets for phosphorylation by Aurora B and that their phosphorylation negatively regulates the association of the Ska complex with the KMN network, which in turn inhibits the recruitment of the Ska complex to kinetochores and impairs its role in stabilizing kMT attachments.
Taken together, these studies show that the Ska complex works in close concert with the components of the KMN network, and the Ndc80 complex in particular, to provide stability to kMT attachments unless it is phosphorylated by Aurora B. Recent evidence also indicates that Ska is indeed a vertebrate functional homologue of the yeast Dam/Dash complex, but the finer details of how Ska associates with the KMN network and the MT lattice to fulfill this function are far from being resolved. Also unresolved is whether there are additional activities for the Ska complex in controlling mitotic progression, such as has been proposed by Daum et al. (Daum et al., 2009), for preventing the premature loss of sister chromatid cohesion. Further insights that can be gained from the Ska structure are presented in Box 1.
Box 1. Further insights from the structure of the Ska complex.
The crystal structure of the dimerization domains of the human Ska complex has been recently solved, which sheds light into the molecular basis of its attachment to MTs (Jeyaprakash et al., 2012). A major finding of that study is that the Ska complex is a ∼35-nm-wide W-shaped dimer, consisting of two copies of each of the SKA1, SKA2 and SKA3 subunits, and this complex can be envisaged to possess four MT-binding sites (see upper panel of the figure), one each in the SKA1 and SKA3 subunits (see lower panel of the figure).
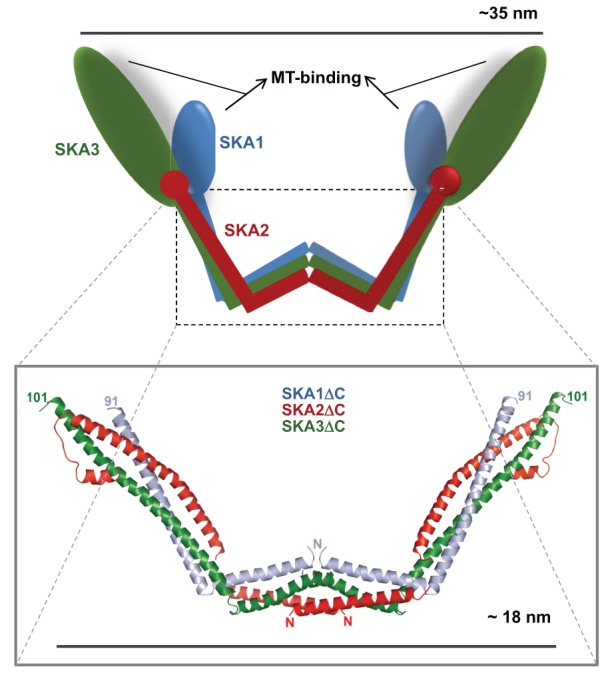
The crystal structure of the dimerization domain of the Ska complex suggests that the SKA1 subunit functions as a structural framework to bridge the interaction between SKA2 and SKA3. The structural core of the Ska monomer consists of the N-terminal helical domains of SKA1 and SKA3 and the entire SKA2, which is also completely helical. These helical structures interact with each other at two separate regions to form two intertwined helical bundles. The N-terminal ends of the short helical bundles from each of the subunit interact face-to-face to form the dimerization interphase, whereas the C-terminal ends of the longer helical bindles of each of the three subunits intertwine with each other and point outwards into the solvent to form the two arms of the W-shaped structure. The N-terminal ends of the short helices that form the central hydrophobic dimerization interface has an open confirmation that enables key molecular interactions which are utilized to accomplish effective dimerization. The globular C-terminal domains of both the SKA1 and SKA3 subunits project from the ends of the two arms of the ‘W’ after dimerization and contain the four putative MT-binding sites of the Ska complex.
The authors speculate that this proposed geometry of the Ska complex might help it to bind transversely across the curvature of MTs. This lateral linkage, in concert with the longitudinal protofilament interaction geometry of the Ndc80 complex, is proposed to enhance kinetochore attachment and processivity at depolymerizing MT ends. Cells containing Ska complexes that either lack the MT-binding domains or have mutations in key residues in the central hydrophobic dimerization core exhibit severe delays in mitotic progression or mitotic arrest (Jeyaprakash et al., 2012). Another recent study has determined that the SKA1 MT-binding site within the C-terminal domain is a winged-helix tertiary structure that is characteristic of DNA-binding proteins, and that phosphorylation of sites within this C-terminal domain inhibit MT binding. The MT-binding activity of the SKA3 C-terminal domain remains unclear (Schmidt et al., 2012). The diagram of the crystal structure was kindly provided by A. Arockia Jeyaprakash and Elena Conti (Max-Planck Institute of Biochemistry, Martinsried, Germany).
The role of the NDC80 loop domain
There is a prominent kink or bend in the structure of the Ndc80 complex ∼16 nm away from the MT-binding CH domain of NDC80. The location of the kink coincides with a break in registry of the central coiled-coil region within NDC80, which likely produces a loop in the secondary structure at this site (Wang et al., 2008). The sequence encompassing the loop was found to be evolutionarily conserved from yeast to human (Wang et al., 2008), but until very recently its function was not clear.
Two studies in yeast determined that the loop region is important for stable kMT attachment. The first study, in Saccharomyces cerevisiae, showed that the loop region is important for the recruitment of the Dam/Dash complex to kinetochores and possibly for its loading on to kMTs (Maure et al., 2011). Those authors observed that, when the loop region is deleted or mutated, there is a severe defect in the conversion of lateral to load-bearing (i.e. end-on) kMT attachments and kinetochore bi-orientation. The other study in the fission yeast Saccharomyces pombe, which also using deletion or directed mutation of key residues or sequences within the loop region, showed that this region is important for the recruitment of the MAP Dis1 (TOG or XMAP215) to kinetochores with stable end-on attachments to spindle MT ends (Hsu and Toda, 2011). Consequently, the SAC remains persistently activated in cells with mutated loop domains and they undergo prolonged mitotic arrest. Deletion of the Dis1 gene phenocopies the defects seen with loop mutations and was rescued by the artificial targeting of Dis1 to the Ndc80 complex.
These studies, along with recent studies in human cells (see Box 2), support the notion that in addition to the CH domain and N-terminal tail of NDC80, its loop domain also constitutes a MT-binding interface, thus providing a total of three different MT-binding domains within the Ndc80 complex.
Box 2. Function of the human NDC80 loop domain.
Recent studies in human cells on the role of the KMN network in kMT attachment have found that the loop domain of NDC80 (HEC1) is instrumental in stabilizing kMTs attachments (see figure) (Zhang et al., 2012; Varma et al., 2012; Matson and Stukenberg, 2012). Several lines of evidence suggest that the Ndc80 complex is slightly bent with a centrally located loop region that provides flexibility in complex confirmation. Both the CH domain and the N-terminal tail of NDC80 (see figure, shown in red) and the extreme N-terminus of KNL1 bind to MTs. In addition, the central loop region of NDC80 provides a third MT-binding site within the Ndc80 complex, albeit indirectly. Linkage from the loop domain to kMTs requires MAPs, such as Dis1 (fission yeast), the Dam/Dash complex (budding yeast), or possibly the Ska complex (human cells). In human cells, the DNA replication licensing protein CDT1 also binds to the loop region and is required for robust kMT attachment (see below). We only have limited knowledge about the exact location and orientation of these proteins relative to kMTs, and their relative positions within the kinetochore are not depicted accurately in the figure.
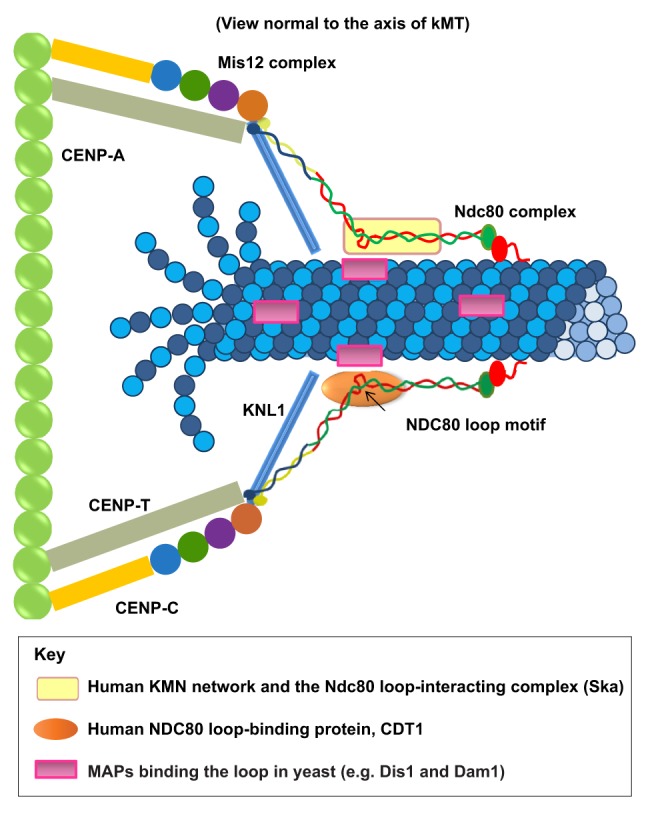
Sequence modifications in the loop region have shown that this region is important for the recruitment of other proteins that are required for normal kMT attachments. Zhang et al. found that the Ndc80 complexes assembled in the absence of the loop region are unable to associate properly with the Ska complex (Zhang et al., 2012). They proposed that the loop domain directly recruits the Ska complex to kinetochores, and that the absence of the loop-recruited Ska complex led to the observed defects in MT attachments to kinetochores in mitotic cells with loop mutations. A second study, by our group, presented a new mechanism for the stabilization of kMT attachments (Varma et al., 2012). We identified the DNA replication licensing protein CDT1 as a novel kinetochore protein that interacts with the Ndc80 complex. Inhibition of CDT1 function specifically during mitosis by either small interfering RNA-based approaches or microinjection of function-blocking antibodies induces a SAC-dependent late prometaphase arrest with severe defects in kMT attachments (Varma et al., 2012). The localization of CDT1 to kinetochores is dependent on the loop region of NDC80, and scrambling the loop region sequence induces a mitotic arrest that is similar to that observed when the loop is missing (Zhang et al., 2012) or when CDT1 is inhibited (Varma et al., 2012). Detailed analysis of the structure of the Ndc80 complex suggests that the binding of CDT1 to the loop region of kinetochore-bound NDC80 helps to maintain an extended confirmation of the Ndc80 complex in the presence of kMTs, which aids in more robust kMT attachments. Intriguingly, our study did not observe a significant decrease in the concentration of the Ska complex at kinetochores when the NDC80 loop region was mutated.
Concluding remarks and future directions
Future work will help to delineate the connections between the different components of the KMN network and the SAC (including the Mads, the Bubs and the RZZ). We already know that the CH domain of NDC80 is involved in recruiting MAD1, and that KNL1 is required for recruiting ZWINT, BUBR1 and BUB1, as well as the RZZ complex in C. elegans (Gassmann et al., 2008). Both ZWINT and the Ndc80 complex recruit the RZZ complex, which in turn appears to be a prerequisite for the recruitment of MAD1 and MAD2 (Fig. 3). However, we still lack precise knowledge of how MT attachments trigger dynein-mediated removal of SAC components (‘stripping’) and silencing of the checkpoint, or how the checkpoint feeds back into the attachment machinery of the KMN network. Part of the problem lies in the fact that the association of checkpoint proteins with the KMN network are possibly highly transient, which makes their biochemical analysis exceedingly difficult. We are slowly beginning to understand some of these mechanisms as shown by recent work that suggests that MT-binding by Knl1 is likely to be a key sensor for controlling checkpoint activity (Espeut et al., 2012), but more work is mandatory to elucidate the intricacies of the interplay between MT attachment and SAC and control mechanisms governed by Aurora B, MPS1 and PP1. As Knl1 is a large multi-domain protein, progress in our understanding of its structure and function has been relatively slow and more research in this direction is likely to be worthwhile. Although we know to some extent the functions of the N- and the C-termini of Knl1, its central region remains a ‘black box’ and analysis of this region is likely to yield valuable information of its role in the SAC and kMT attachment. An area, in which there is a high chance for immediate progress is the further study of the function of the loop in the Ndc80 complex and the proteins that interact with it. Research efforts in this direction will help us to understand if the Ska complex is indeed an orthologue of Dam/Dash and how exactly the Ska complex and the KMN network coordinate kMT attachment, force generation, attachment error correction and control of the SAC. Future studies should also shed light on the exact molecular mechanism by which the replication licensing protein CDT1 is able to influence the conformational change of Ndc80 complex in the presence of kMTs (see Box 2), and also elucidate whether different Ndc80 loop-binding proteins act in concert to accomplish stable kMT attachments.
Fig. 3.
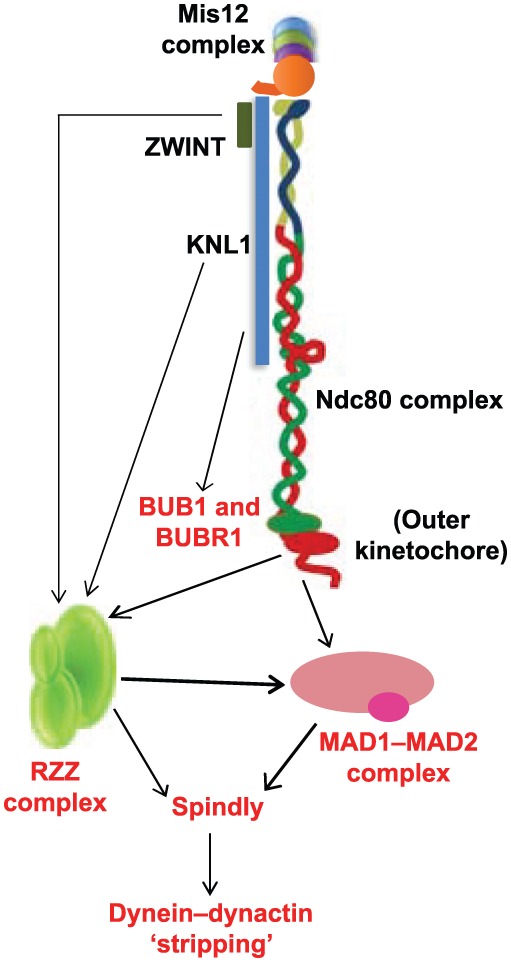
Role of the KMN network in the SAC. The schematic illustration shows the known function of KMN network components in the recruitment of SAC proteins (red) and their recruitment of each other (indicated by arrows). ZWINT (KBP-5) and KNL-1 in C. elegans are required for the recruitment of the RZZ checkpoint complex. Both the Ndc80 complex and the RZZ complex have been shown to be required for the proper localization of the MAD1–MAD2 checkpoint complex to kinetochores that are unattached in prometaphase. KNL1 is important for the kinetochore recruitment of ZWINT and the BUB1–BUBR1 checkpoint complex. Spindly (recruited by the RZZ complex) serves as an adaptor between the dynein–dynactin motor complex and the MAD1–MAD2 and RZZ complexes, and is required for the dynein motor-driven stripping of these checkpoint complexes during SAC silencing. See text for details.
Acknowledgments
We would like to acknowledge A. Arochia Jeyaprakash and Elena Conti for providing us with the high-resolution structure of the Ska complex dimerization domains. We would also like to thank Jean Cook, Jenifer Deluca, and several past and present members of the Salmon laboratory for insightful discussions.
Footnotes
Funding
The work of our laboratory is supported by the National Institutes of Health [grant number R37GM024364 and supplement to E.D.S.]. Deposited in PMC for release after 12 months.
Note added in proof
After the acceptance of this manuscript, a review written by Jakob Nilsson was published in the journal Bioessays, describing in detailed the function of the Ndc80 loop domain (Nilsson, 2012).
References
- Alushin G., Nogales E. (2011). Visualizing kinetochore architecture. Curr. Opin. Struct. Biol. 21, 661–669 10.1016/j.sbi.2011.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alushin G. M., Ramey V. H., Pasqualato S., Ball D. A., Grigorieff N., Musacchio A., Nogales E. (2010). The Ndc80 kinetochore complex forms oligomeric arrays along microtubules. Nature 467, 805–810 10.1038/nature09423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alushin G. M., Musinipally V., Matson D., Tooley J., Stukenberg P. T., Nogales E. (2012). Multimodal microtubule binding by the Ndc80 kinetochore complex. Nat. Struct. Mol. Biol. 19, 1161–1167 10.1038/nsmb.2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock L. J., Pagliuca C., Kobayashi N., Grove R. A., Oku Y., Shrestha K., Alfieri C., Golfieri C., Oldani A., Dal Maschio M.et al. (2012). Cnn1 inhibits the interactions between the KMN complexes of the yeast kinetochore. Nat. Cell Biol. 14, 614–624 10.1038/ncb2495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolanos–Garcia V. M., Lischetti T., Matak–Vinković D., Cota E., Simpson P. J., Chirgadze D. Y., Spring D. R., Robinson C. V., Nilsson J., Blundell T. L. (2011). Structure of a Blinkin-BUBR1 complex reveals an interaction crucial for kinetochore-mitotic checkpoint regulation via an unanticipated binding Site. Structure 19, 1691–1700 10.1016/j.str.2011.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Y. W., Jeyaprakash A. A., Nigg E. A., Santamaria A. (2012). Aurora B controls kinetochore-microtubule attachments by inhibiting Ska complex-KMN network interaction. J. Cell Biol. 196, 563–571 10.1083/jcb.201109001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman I. M., Desai A. (2008). Molecular architecture of the kinetochore-microtubule interface. Nat. Rev. Mol. Cell Biol. 9, 33–46 10.1038/nrm2310 [DOI] [PubMed] [Google Scholar]
- Cheeseman I. M., Niessen S., Anderson S., Hyndman F., Yates J. R., 3rd, Oegema K., Desai A. (2004). A conserved protein network controls assembly of the outer kinetochore and its ability to sustain tension. Genes Dev. 18, 2255–2268 10.1101/gad.1234104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman I. M., Chappie J. S., Wilson–Kubalek E. M., Desai A. (2006). The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell 127, 983–997 10.1016/j.cell.2006.09.039 [DOI] [PubMed] [Google Scholar]
- Cheeseman I. M., Hori T., Fukagawa T., Desai A. (2008). KNL1 and the CENP-H/I/K complex coordinately direct kinetochore assembly in vertebrates. Mol. Biol. Cell 19, 587–594 10.1091/mbc.E07-10-1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciferri C., Pasqualato S., Screpanti E., Varetti G., Santaguida S., Dos Reis G., Maiolica A., Polka J., De Luca J. G., De Wulf P.et al. (2008). Implications for kinetochore-microtubule attachment from the structure of an engineered Ndc80 complex. Cell 133, 427–439 10.1016/j.cell.2008.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimini D., Wan X., Hirel C. B., Salmon E. D. (2006). Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors. Curr. Biol. 16, 1711–1718 10.1016/j.cub.2006.07.022 [DOI] [PubMed] [Google Scholar]
- Corbett K. D., Yip C. K., Ee L. S., Walz T., Amon A., Harrison S. C. (2010). The monopolin complex crosslinks kinetochore components to regulate chromosome-microtubule attachments. Cell 142, 556–567 10.1016/j.cell.2010.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum J. R., Wren J. D., Daniel J. J., Sivakumar S., McAvoy J. N., Potapova T. A., Gorbsky G. J. (2009). Ska3 is required for spindle checkpoint silencing and the maintenance of chromosome cohesion in mitosis. Curr. Biol. 19, 1467–1472 10.1016/j.cub.2009.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A. E., Kaplan K. B. (2010). Hsp90-Sgt1 and Skp1 target human Mis12 complexes to ensure efficient formation of kinetochore-microtubule binding sites. J. Cell Biol. 189, 261–274 10.1083/jcb.200910036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca J. G., Musacchio A. (2012). Structural organization of the kinetochore-microtubule interface. Curr. Opin. Cell Biol. 24, 48–56 10.1016/j.ceb.2011.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca J. G., Gall W. E., Ciferri C., Cimini D., Musacchio A., Salmon E. D. (2006). Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell 127, 969–982 10.1016/j.cell.2006.09.047 [DOI] [PubMed] [Google Scholar]
- Espeut J., Cheerambathur D. K., Krenning L., Oegema K., Desai A. (2012). Microtubule binding by KNL-1 contributes to spindle checkpoint silencing at the kinetochore. J. Cell Biol. 196, 469–482 10.1083/jcb.201111107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex A., Dammermann A., Lewellyn L., Oegema K., Desai A. (2009). Systematic analysis in Caenorhabditis elegans reveals that the spindle checkpoint is composed of two largely independent branches. Mol. Biol. Cell 20, 1252–1267 10.1091/mbc.E08-10-1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famulski J. K., Chan G. K. (2007). Aurora B kinase-dependent recruitment of hZW10 and hROD to tensionless kinetochores. Curr. Biol. 17, 2143–2149 10.1016/j.cub.2007.11.037 [DOI] [PubMed] [Google Scholar]
- Famulski J. K., Vos L., Sun X., Chan G. (2008). Stable hZW10 kinetochore residency, mediated by hZwint-1 interaction, is essential for the mitotic checkpoint. J. Cell Biol. 180, 507–520 10.1083/jcb.200708021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltz D. R., Jansen L. E., Black B. E., Bailey A. O., Yates J. R., 3rd, Cleveland D. W. (2006). The human CENP-A centromeric nucleosome-associated complex. Nat. Cell Biol. 8, 458–469 10.1038/ncb1397 [DOI] [PubMed] [Google Scholar]
- Gaitanos T. N., Santamaria A., Jeyaprakash A. A., Wang B., Conti E., Nigg E. A. (2009). Stable kinetochore-microtubule interactions depend on the Ska complex and its new component Ska3/C13Orf3. EMBO J. 28, 1442–1452 10.1038/emboj.2009.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascoigne K. E., Cheeseman I. M. (2011). Kinetochore assembly: if you build it, they will come. Curr. Opin. Cell Biol. 23, 102–108 10.1016/j.ceb.2010.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascoigne K. E., Takeuchi K., Suzuki A., Hori T., Fukagawa T., Cheeseman I. M. (2011). Induced ectopic kinetochore assembly bypasses the requirement for CENP-A nucleosomes. Cell 145, 410–422 10.1016/j.cell.2011.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann R., Essex A., Hu J. S., Maddox P. S., Motegi F., Sugimoto A., O'Rourke S. M., Bowerman B., McLeod I., Yates J. R., 3rdet al. (2008). A new mechanism controlling kinetochore-microtubule interactions revealed by comparison of two dynein-targeting components: SPDL-1 and the Rod/Zwilch/Zw10 complex. Genes Dev. 22, 2385–2399 10.1101/gad.1687508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima G., Yanagida M. (2000). Establishing biorientation occurs with precocious separation of the sister kinetochores, but not the arms, in the early spindle of budding yeast. Cell 100, 619–633 10.1016/S0092-8674(00)80699-6 [DOI] [PubMed] [Google Scholar]
- Goshima G., Saitoh S., Yanagida M. (1999). Proper metaphase spindle length is determined by centromere proteins Mis12 and Mis6 required for faithful chromosome segregation. Genes Dev. 13, 1664–1677 10.1101/gad.13.13.1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffis E. R., Stuurman N., Vale R. D. (2007). Spindly, a novel protein essential for silencing the spindle assembly checkpoint, recruits dynein to the kinetochore. J. Cell Biol. 177, 1005–1015 10.1083/jcb.200702062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grishchuk E. L., Efremov A. K., Volkov V. A., Spiridonov I. S., Gudimchuk N., Westermann S., Drubin D., Barnes G., McIntosh J. R., Ataullakhanov F. I. (2008). The Dam1 ring binds microtubules strongly enough to be a processive as well as energy-efficient coupler for chromosome motion. Proc. Natl. Acad. Sci. USA 105, 15423–15428 10.1073/pnas.0807859105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes G. J., Dong Y., McEwen B. F., Deluca J. G. (2008). Kinetochore-microtubule attachment relies on the disordered N-terminal tail domain of Hec1. Curr. Biol. 18, 1778–1784 10.1016/j.cub.2008.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch A., Silljé H. H., Nigg E. A. (2006). Timely anaphase onset requires a novel spindle and kinetochore complex comprising Ska1 and Ska2. EMBO J. 25, 5504–5515 10.1038/sj.emboj.7601426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt L., Tighe A., Santaguida S., White A. M., Jones C. D., Musacchio A., Green S., Taylor S. S. (2010). Sustained Mps1 activity is required in mitosis to recruit O-Mad2 to the Mad1-C-Mad2 core complex. J. Cell Biol. 190, 25–34 10.1083/jcb.201002133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori T., Fukagawa T. (2012). Establishment of the vertebrate kinetochores. Chromosome Res. 20, 547–561 10.1007/s10577-012-9289-9 [DOI] [PubMed] [Google Scholar]
- Hori T., Amano M., Suzuki A., Backer C. B., Welburn J. P., Dong Y., McEwen B. F., Shang W. H., Suzuki E., Okawa K.et al. (2008). CCAN makes multiple contacts with centromeric DNA to provide distinct pathways to the outer kinetochore. Cell 135, 1039–1052 10.1016/j.cell.2008.10.019 [DOI] [PubMed] [Google Scholar]
- Hornung P., Maier M., Alushin G. M., Lander G. C., Nogales E., Westermann S. (2011). Molecular architecture and connectivity of the budding yeast Mtw1 kinetochore complex. J. Mol. Biol. 405, 548–559 10.1016/j.jmb.2010.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu K. S., Toda T. (2011). Ndc80 internal loop interacts with Dis1/TOG to ensure proper kinetochore-spindle attachment in fission yeast. Curr. Biol. 21, 214–220 10.1016/j.cub.2010.12.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakopec V., Topolski B., Fleig U. (2012). Sos7, an essential component of the conserved Schizosaccharomyces pombe Ndc80-MIND-Spc7 complex, identifies a new family of fungal kinetochore proteins. Mol. Cell. Biol. 32, 3308–3320 10.1128/MCB.00212-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaprakash A. A., Santamaria A., Jayachandran U., Chan Y. W., Benda C., Nigg E. A., Conti E. (2012). Structural and functional organization of the Ska complex, a key component of the kinetochore-microtubule interface. Mol. Cell 46, 274–286 10.1016/j.molcel.2012.03.005 [DOI] [PubMed] [Google Scholar]
- Joglekar A. P., Bloom K., Salmon E. D. (2009). In vivo protein architecture of the eukaryotic kinetochore with nanometer scale accuracy. Curr. Biol. 19, 694–699 10.1016/j.cub.2009.02.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joglekar A. P., Bloom K. S., Salmon E. D. (2010). Mechanisms of force generation by end-on kinetochore-microtubule attachments. Curr. Opin. Cell Biol. 22, 57–67 10.1016/j.ceb.2009.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karess R. (2005). Rod-Zw10-Zwilch: a key player in the spindle checkpoint. Trends Cell Biol. 15, 386–392 10.1016/j.tcb.2005.05.003 [DOI] [PubMed] [Google Scholar]
- Kasuboski J. M., Bader J. R., Vaughan P. S., Tauhata S. B., Winding M., Morrissey M. A., Joyce M. V., Boggess W., Vos L., Chan G. K.et al. (2011). Zwint-1 is a novel Aurora B substrate required for the assembly of a dynein-binding platform on kinetochores. Mol. Biol. Cell 22, 3318–3330 10.1091/mbc.E11-03-0213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyomitsu T., Obuse C., Yanagida M. (2007). Human Blinkin/AF15q14 is required for chromosome alignment and the mitotic checkpoint through direct interaction with Bub1 and BubR1. Dev. Cell 13, 663–676 10.1016/j.devcel.2007.09.005 [DOI] [PubMed] [Google Scholar]
- Kiyomitsu T., Iwasaki O., Obuse C., Yanagida M. (2010). Inner centromere formation requires hMis14, a trident kinetochore protein that specifically recruits HP1 to human chromosomes. J. Cell Biol. 188, 791–807 10.1083/jcb.200908096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyomitsu T., Murakami H., Yanagida M. (2011). Protein interaction domain mapping of human kinetochore protein Blinkin reveals a consensus motif for binding of spindle assembly checkpoint proteins Bub1 and BubR1. Mol. Cell. Biol. 31, 998–1011 10.1128/MCB.00815-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline S. L., Cheeseman I. M., Hori T., Fukagawa T., Desai A. (2006). The human Mis12 complex is required for kinetochore assembly and proper chromosome segregation. J. Cell Biol. 173, 9–17 10.1083/jcb.200509158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kops G. J., Shah J. V. (2012). Connecting up and clearing out: how kinetochore attachment silences the spindle assembly checkpoint. Chromosoma 121, 509–525 10.1007/s00412-012-0378-5 [DOI] [PubMed] [Google Scholar]
- Kops G. J., Kim Y., Weaver B. A., Mao Y., McLeod I., Yates J. R., 3rd, Tagaya M., Cleveland D. W. (2005). ZW10 links mitotic checkpoint signaling to the structural kinetochore. J. Cell Biol. 169, 49–60 10.1083/jcb.200411118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenn V., Wehenkel A., Li X., Santaguida S., Musacchio A. (2012). Structural analysis reveals features of the spindle checkpoint kinase Bub1-kinetochore subunit Knl1 interaction. J. Cell Biol. 196, 451–467 10.1083/jcb.201110013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert F., Westermann S. (2011). A blueprint for kinetochores - new insights into the molecular mechanics of cell division. Nat. Rev. Mol. Cell Biol. 12, 407–412 10.1038/nrm3133 [DOI] [PubMed] [Google Scholar]
- Lin Y. T., Chen Y., Wu G., Lee W. H. (2006). Hec1 sequentially recruits Zwint-1 and ZW10 to kinetochores for faithful chromosome segregation and spindle checkpoint control. Oncogene 25, 6901–6914 10.1038/sj.onc.1209687 [DOI] [PubMed] [Google Scholar]
- Liu D., Vleugel M., Backer C. B., Hori T., Fukagawa T., Cheeseman I. M., Lampson M. A. (2010). Regulated targeting of protein phosphatase 1 to the outer kinetochore by KNL1 opposes Aurora B kinase. J. Cell Biol. 188, 809–820 10.1083/jcb.201001006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- London N., Ceto S., Ranish J. A., Biggins S. (2012). Phosphoregulation of Spc105 by Mps1 and PP1 regulates Bub1 localization to kinetochores. Curr. Biol. 22, 900–906 10.1016/j.cub.2012.03.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejowski J., George K. A., Terret M. E., Zhang C., Shokat K. M., Jallepalli P. V. (2010). Mps1 directs the assembly of Cdc20 inhibitory complexes during interphase and mitosis to control M phase timing and spindle checkpoint signaling. J. Cell Biol. 190, 89–100 10.1083/jcb.201001050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresca T J., Salmon E D. (2010). Welcome to a new kind of tension: translating kinetochore mechanics into a wait-anaphase signal. J Cell Sci. 123, 825–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin–Lluesma S., Stucke V. M., Nigg E. A. (2002). Role of Hec1 in spindle checkpoint signaling and kinetochore recruitment of Mad1/Mad2. Science 297, 2267–2270 10.1126/science.1075596 [DOI] [PubMed] [Google Scholar]
- Maskell D. P., Hu X. W., Singleton M. R. (2010). Molecular architecture and assembly of the yeast kinetochore MIND complex. J. Cell Biol. 190, 823–834 10.1083/jcb.201002059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson D. R., Stukenberg P. T. (2012). Cdt1 throws kinetochore-microtubule attachments for a loop. Nat. Cell Biol. 14, 561–563 10.1038/ncb2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maure J. F., Komoto S., Oku Y., Mino A., Pasqualato S., Natsume K., Clayton L., Musacchio A., Tanaka T. U. (2011). The Ndc80 loop region facilitates formation of kinetochore attachment to the dynamic microtubule plus end. Curr. Biol. 21, 207–213 10.1016/j.cub.2010.12.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows J. C., Shepperd L. A., Vanoosthuyse V., Lancaster T. C., Sochaj A. M., Buttrick G. J., Hardwick K. G., Millar J. B. (2011). Spindle checkpoint silencing requires association of PP1 to both Spc7 and kinesin-8 motors. Dev. Cell 20, 739–750 10.1016/j.devcel.2011.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. A., Johnson M. L., Stukenberg P. T. (2008). Kinetochore attachments require an interaction between unstructured tails on microtubules and Ndc80(Hec1). Curr. Biol. 18, 1785–1791 10.1016/j.cub.2008.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A., Salmon E D. (2007). The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 8, 379–393 [DOI] [PubMed] [Google Scholar]
- Nilsson J. (2012). Looping in on Ndc80 - how does a protein loop at the kinetochore control chromosome segregation? Bioessays 34, 1070–1077 10.1002/bies.201200096 [DOI] [PubMed] [Google Scholar]
- Nishino T., Takeuchi K., Gascoigne K. E., Suzuki A., Hori T., Oyama T., Morikawa K., Cheeseman I. M., Fukagawa T. (2012). CENP-T-W-S-X forms a unique centromeric chromatin structure with a histone-like fold. Cell 148, 487–501 10.1016/j.cell.2011.11.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obuse C., Iwasaki O., Kiyomitsu T., Goshima G., Toyoda Y., Yanagida M. (2004). A conserved Mis12 centromere complex is linked to heterochromatic HP1 and outer kinetochore protein Zwint-1. Nat. Cell Biol. 6, 1135–1141 10.1038/ncb1187 [DOI] [PubMed] [Google Scholar]
- Okada M., Cheeseman I. M., Hori T., Okawa K., McLeod I. X., Yates J. R., 3rd, Desai A., Fukagawa T. (2006). The CENP-H-I complex is required for the efficient incorporation of newly synthesized CENP-A into centromeres. Nat. Cell Biol. 8, 446–457 10.1038/ncb1396 [DOI] [PubMed] [Google Scholar]
- Perpelescu M., Fukagawa T. (2011). The ABCs of CENPs. Chromosoma 120, 425–446 10.1007/s00412-011-0330-0 [DOI] [PubMed] [Google Scholar]
- Petrovic A., Pasqualato S., Dube P., Krenn V., Santaguida S., Cittaro D., Monzani S., Massimiliano L., Keller J., Tarricone A.et al. (2010). The MIS12 complex is a protein interaction hub for outer kinetochore assembly. J. Cell Biol. 190, 835–852 10.1083/jcb.201002070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers A. F., Franck A. D., Gestaut D. R., Cooper J., Gracyzk B., Wei R. R., Wordeman L., Davis T. N., Asbury C. L. (2009). The Ndc80 kinetochore complex forms load-bearing attachments to dynamic microtubule tips via biased diffusion. Cell 136, 865–875 10.1016/j.cell.2008.12.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przewloka M. R., Glover D. M. (2009). The kinetochore and the centromere: a working long distance relationship. Annu. Rev. Genet. 43, 439–465 10.1146/annurev-genet-102108-134310 [DOI] [PubMed] [Google Scholar]
- Przewloka M. R., Venkei Z., Bolanos–Garcia V. M., Debski J., Dadlez M., Glover D. M. (2011). CENP-C is a structural platform for kinetochore assembly. Curr. Biol. 21, 399–405 10.1016/j.cub.2011.02.005 [DOI] [PubMed] [Google Scholar]
- Raaijmakers J. A., Tanenbaum M. E., Maia A. F., Medema R. H. (2009). RAMA1 is a novel kinetochore protein involved in kinetochore-microtubule attachment. J. Cell Sci. 122, 2436–2445 10.1242/jcs.051912 [DOI] [PubMed] [Google Scholar]
- Rosenberg J. S., Cross F. R., Funabiki H. (2011). KNL1/Spc105 recruits PP1 to silence the spindle assembly checkpoint. Curr. Biol. 21, 942–947 10.1016/j.cub.2011.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh H., Tomkiel J., Cooke C. A., Ratrie H., 3rd, Maurer M., Rothfield N. F., Earnshaw W. C. (1992). CENP-C, an autoantigen in scleroderma, is a component of the human inner kinetochore plate. Cell 70, 115–125 10.1016/0092-8674(92)90538-N [DOI] [PubMed] [Google Scholar]
- Sandall S., Severin F., McLeod I. X., Yates J. R., 3rd, Oegema K., Hyman A., Desai A. (2006). A Bir1-Sli15 complex connects centromeres to microtubules and is required to sense kinetochore tension. Cell 127, 1179–1191 10.1016/j.cell.2006.09.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santaguida S., Musacchio A. (2009). The life and miracles of kinetochores. EMBO J. 28, 2511–2531 10.1038/emboj.2009.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santaguida S., Tighe A., D'Alise A. M., Taylor S. S., Musacchio A. (2010). Dissecting the role of MPS1 in chromosome biorientation and the spindle checkpoint through the small molecule inhibitor reversine. J. Cell Biol. 190, 73–87 10.1083/jcb.201001036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schittenhelm R. B., Heeger S., Althoff F., Walter A., Heidmann S., Mechtler K., Lehner C. F. (2007). Spatial organization of a ubiquitous eukaryotic kinetochore protein network in Drosophila chromosomes. Chromosoma 116, 385–402 10.1007/s00412-007-0103-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schittenhelm R. B., Chaleckis R., Lehner C. F. (2009). Intrakinetochore localization and essential functional domains of Drosophila Spc105. EMBO J. 28, 2374–2386 10.1038/emboj.2009.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleiffer A., Maier M., Litos G., Lampert F., Hornung P., Mechtler K., Westermann S. (2012). CENP-T proteins are conserved centromere receptors of the Ndc80 complex. Nat. Cell Biol. 14, 604–613 10.1038/ncb2493 [DOI] [PubMed] [Google Scholar]
- Schmidt J. C., Arthanari H., Boeszoermenyi A., Dashkevich N. M., Wilson–Kubalek E. M., Monnier N., Markus M., Oberer M., Milligan R. A., Bathe M.et al. (2012). The kinetochore-bound ska1 complex tracks depolymerizing microtubules and binds to curved protofilaments. Dev. Cell 23, 968–980 10.1016/j.devcel.2012.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Screpanti E., De Antoni A., Alushin G. M., Petrovic A., Melis T., Nogales E., Musacchio A. (2011). Direct binding of Cenp-C to the Mis12 complex joins the inner and outer kinetochore. Curr. Biol. 21, 391–398 10.1016/j.cub.2010.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepperd L. A., Meadows J. C., Sochaj A. M., Lancaster T. C., Zou J., Buttrick G. J., Rappsilber J., Hardwick K. G., Millar J. B. (2012). Phosphodependent recruitment of Bub1 and Bub3 to Spc7/KNL1 by Mph1 kinase maintains the spindle checkpoint. Curr. Biol. 22, 891–899 10.1016/j.cub.2012.03.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr D. A., Saffery R., Li Z., Simpson A. E., Choo K. H., Yen T. J., Goldberg M. L. (2000). HZwint-1, a novel human kinetochore component that interacts with HZW10. J. Cell Sci. 113, 1939–1950 [DOI] [PubMed] [Google Scholar]
- Sundin L. J., Guimaraes G. J., Deluca J. G. (2011). The NDC80 complex proteins Nuf2 and Hec1 make distinct contributions to kinetochore-microtubule attachment in mitosis. Mol. Biol. Cell 22, 759–768 10.1091/mbc.E10-08-0671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K., Fukagawa T. (2012). Molecular architecture of vertebrate kinetochores. Exp. Cell Res. 318, 1367–1374 10.1016/j.yexcr.2012.02.016 [DOI] [PubMed] [Google Scholar]
- Theis M., Slabicki M., Junqueira M., Paszkowski–Rogacz M., Sontheimer J., Kittler R., Heninger A. K., Glatter T., Kruusmaa K., Poser I.et al. (2009). Comparative profiling identifies C13orf3 as a component of the Ska complex required for mammalian cell division. EMBO J. 28, 1453–1465 10.1038/emboj.2009.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooley J., Stukenberg P. T. (2011). The Ndc80 complex: integrating the kinetochore's many movements. Chromosome Res. 19, 377–391 10.1007/s10577-010-9180-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooley J. G., Miller S. A., Stukenberg P. T. (2011). The Ndc80 complex uses a tripartite attachment point to couple microtubule depolymerization to chromosome movement. Mol. Biol. Cell 22, 1217–1226 10.1091/mbc.E10-07-0626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbreit N. T., Gestaut D. R., Tien J. F., Vollmar B. S., Gonen T., Asbury C. L., Davis T. N. (2012). The Ndc80 kinetochore complex directly modulates microtubule dynamics. Proc. Natl. Acad. Sci. USA 109, 16113–16118 10.1073/pnas.1209615109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma D., Chandrasekaran S., Sundin L. J., Reidy K. T., Wan X., Chasse D. A., Nevis K. R., DeLuca J. G., Salmon E. D., Cook J. G. (2012). Recruitment of the human Cdt1 replication licensing protein by the loop domain of Hec1 is required for stable kinetochore-microtubule attachment. Nat. Cell Biol. 14, 593–603 10.1038/ncb2489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkei Z., Przewloka M. R., Glover D. M. (2011). Drosophila Mis12 complex acts as a single functional unit essential for anaphase chromosome movement and a robust spindle assembly checkpoint. Genetics 187, 131–140 10.1534/genetics.110.119628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneron S., Prieto S., Bernis C., Labbé J. C., Castro A., Lorca T. (2004). Kinetochore localization of spindle checkpoint proteins: who controls whom? Mol. Biol. Cell 15, 4584–4596 10.1091/mbc.E04-01-0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos L. J., Famulski J. K., Chan G. K. (2011). hZwint-1 bridges the inner and outer kinetochore: identification of the kinetochore localization domain and the hZw10-interaction domain. Biochem. J. 436, 157–168 10.1042/BJ20110137 [DOI] [PubMed] [Google Scholar]
- Wan X., O'Quinn R. P., Pierce H. L., Joglekar A. P., Gall W. E., DeLuca J. G., Carroll C. W., Liu S. T., Yen T. J., McEwen B. F.et al. (2009). Protein architecture of the human kinetochore microtubule attachment site. Cell 137, 672–684 10.1016/j.cell.2009.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Hu X., Ding X., Dou Z., Yang Z., Shaw A. W., Teng M., Cleveland D. W., Goldberg M. L., Niu L.et al. (2004). Human Zwint-1 specifies localization of Zeste White 10 to kinetochores and is essential for mitotic checkpoint signaling. J. Biol. Chem. 279, 54590–54598 10.1074/jbc.M407588200 [DOI] [PubMed] [Google Scholar]
- Wang H. W., Long S., Ciferri C., Westermann S., Drubin D., Barnes G., Nogales E. (2008). Architecture and flexibility of the yeast Ndc80 kinetochore complex. J. Mol. Biol. 383, 894–903 10.1016/j.jmb.2008.08.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei R. R., Al–Bassam J., Harrison S. C. (2007). The Ndc80/HEC1 complex is a contact point for kinetochore-microtubule attachment. Nat. Struct. Mol. Biol. 14, 54–59 10.1038/nsmb1186 [DOI] [PubMed] [Google Scholar]
- Wei R., Ngo B., Wu G., Lee W. H. (2011). Phosphorylation of the Ndc80 complex protein, HEC1, by Nek2 kinase modulates chromosome alignment and signaling of the spindle assembly checkpoint. Mol. Biol. Cell 22, 3584–3594 10.1091/mbc.E11-01-0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welburn J. P., Grishchuk E. L., Backer C. B., Wilson–Kubalek E. M., Yates J. R., 3rd, Cheeseman I. M. (2009). The human kinetochore Ska1 complex facilitates microtubule depolymerization-coupled motility. Dev. Cell 16, 374–385 10.1016/j.devcel.2009.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welburn J. P., Vleugel M., Liu D., Yates J. R., 3rd, Lampson M. A., Fukagawa T., Cheeseman I. M. (2010). Aurora B phosphorylates spatially distinct targets to differentially regulate the kinetochore-microtubule interface. Mol. Cell 38, 383–392 10.1016/j.molcel.2010.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson–Kubalek E. M., Cheeseman I. M., Yoshioka C., Desai A., Milligan R. A. (2008). Orientation and structure of the Ndc80 complex on the microtubule lattice. J. Cell Biol. 182, 1055–1061 10.1083/jcb.200804170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi Y., Yang C. H., Tanno Y., Watanabe Y. (2012). MPS1/Mph1 phosphorylates the kinetochore protein KNL1/Spc7 to recruit SAC components. Nat. Cell Biol. 14, 746–752 10.1038/ncb2515 [DOI] [PubMed] [Google Scholar]
- Zhang G., Kelstrup C. D., Hu X. W., Kaas Hansen M. J., Singleton M. R., Olsen J. V., Nilsson J. (2012). The Ndc80 internal loop is required for recruitment of the Ska complex to establish end-on microtubule attachment to kinetochores. J. Cell Sci. 125, 3243–3253 10.1242/jcs.104208 [DOI] [PubMed] [Google Scholar]