Summary
Fusion of lysosomes with the plasma membrane is a calcium-dependent process that is crucial for membrane repair, limiting pathogen entry and clearing cellular debris. In non-polarized cells, lysosome exocytosis facilitates rapid resealing of torn membranes. Here, we investigate the mechanism of lysosome exocytosis in polarized epithelia, the main barrier between the organism and the external environment and the first line of defense against pathogens. We find that in polarized Madin-Darby canine kidney (MDCK) cells, calcium ionophores or pore-forming toxins cause lysosomes to fuse predominantly with the basolateral membrane. This polarized exocytosis is regulated by the actin cytoskeleton, membrane cholesterol and the clathrin adaptor AP-1. Depolymerization of actin, but not microtubules, causes apical lysosome fusion, supporting the hypothesis that cortical actin is a barrier to exocytosis. Overloading lysosomes with cholesterol inhibits exocytosis, suggesting that excess cholesterol paralyzes lysosomal traffic. The clathrin adaptor AP-1 is responsible for accurately targeting syntaxin 4 to the basolateral domain. In cells lacking either the ubiquitous AP-1A or the epithelial-specific AP-1B, syntaxin 4 is non-polar. This causes lysosomes to fuse with both the apical and basolateral membranes. Consistent with these findings, RNAi-mediated depletion of syntaxin 4 inhibits basolateral exocytosis in wild-type MDCK, and both apical and basolateral exocytosis in cells lacking AP-1A or AP-1B. Our results provide fundamental insight into the molecular machinery involved in membrane repair in polarized epithelia and suggest that AP-1 is a crucial regulator of this process.
Key words: Lysosome, Polarity, Exocytosis, Membrane repair, AP-1 clathrin adaptor, SNARE protein, Fusion
Introduction
Lysosomes, long thought to be little more than terminal degradative compartments of the cell, are now recognized as central players in maintaining cellular homeostasis (Luzio et al., 2007). Melanocytes, platelets and cytotoxic T lymphocytes (CTLs) have specialized secretory lysosomes or lysosome-related organelles that store defined repertoires of proteins. In CTLs, lysosome secretion at the immunological synapse delivers lytic granules to effect target cell apoptosis (Stinchcombe et al., 2004). Exocytosis of ‘conventional’ lysosomes in response to a transient increase in intracellular calcium ([Ca2+]i) was first observed during cell invasion of Trypanosoma cruzi: binding of the parasite to the cell membrane triggers calcium influx and subsequent fusion of lysosomes with the region of the plasma membrane that surrounds the invading parasite (Andrews, 2002). Lysosome exocytosis has since emerged as an important mechanism in diverse paradigms such as membrane repair after exposure to calcium ionophores and pore-forming toxins (Divangahi et al., 2009; Jaiswal et al., 2002; Rodríguez et al., 1997) and for propagating the Ca2+ wave in astrocytes to modulate synaptic transmission (Li et al., 2008; Zhang et al., 2007). Molecular machinery implicated in calcium-induced lysosome exocytosis include the lysosomal calcium sensor synaptotagmin VII, the v-SNARE VAMP7 and t-SNAREs SNAP23, syntaxin 2 and syntaxin 4 (Rao et al., 2004).
Although lysosomes have been identified as key organelles in membrane repair, studies show that other compartments distinct from lysosomes may also participate in wound healing (Divangahi et al., 2009; McNeil and Kirchhausen, 2005; Meldolesi, 2003). Rapid resealing of torn membranes is a ubiquitous, highly conserved response crucial for cell survival (McNeil and Kirchhausen, 2005). This is especially important for barrier epithelia that directly interact with pathogens and toxins, which can cause membrane lesions, necessitating rapid wound healing. However, whether lysosomes participate in membrane repair in polarized epithelia has not yet been examined. Here, we investigated lysosome exocytosis in polarized Madin-Darby canine kidney (MDCK) cells in response to pore-forming toxins and calcium ionophores. Our results demonstrate that (i) localized increase in [Ca2+]i induces lysosomes to fuse exclusively with the basolateral membrane in MDCK cells; (ii) this polarized exocytosis is predicated on the actin cytoskeleton, membrane cholesterol and the basolateral localization of syntaxin 4; and (iii) the AP-1 clathrin adaptor specifies polarity of fusion by accurately targeting syntaxin 4 to the basolateral domain. These studies have implications for understanding lysosome function in epithelial physiology and pathology, wound healing and immunity.
Results and Discussion
Calcium-induced lysosome exocytosis occurs preferentially at the basolateral membrane in polarized MDCK cells
Because calcium-induced lysosome exocytosis has never been documented in differentiated epithelial monolayers, we first established the polarity and extent of this process. Cells on Transwell filters were exposed to the calcium ionophore ionomycin (5 or 10 µM) either in the apical, basolateral or both media. At these concentrations, ionomycin is not cytotoxic (supplementary material Fig. S1) and does not alter the trans-epithelial electrical resistance of the monolayer (supplementary material Table S1). In all cases, ∼4-6% of total cellular β-hexosaminidase (β-hex) activity was present in the basolateral medium after ionomycin exposure (Fig. 1A). Enzyme activity in the apical medium was comparable to untreated cells. Ionomycin induced the appearance of lysosome-associated membrane protein LAMP2 exclusively on the basolateral surface (Fig. 1B), suggesting that lysosomes fuse predominantly with the basolateral membrane in polarized MDCK cells. Thrombin and bombesin, which mobilize intracellular calcium stores, and the pore-forming toxin streptolysin O (SLO) also induced lysosome exocytosis at the basolateral membrane (Fig. 1C; supplementary material Fig. S2). This exclusive basolateral fusion in response to increased [Ca2+]i by various stimuli is supported by data showing that Trypanozoma cruzi enters MDCK monolayers only from the basolateral surface (Schenkman et al., 1988). Polarized exocytosis could reflect the asymmetric distribution of lysosomes and/or exocytic machinery at the basolateral surface or the existence of mechanisms that prevent apical exocytosis. Using apical and basolateral markers and ZO-1 to demarcate the tight junction, we observed that LAMP2-containing lysosomes in MDCK cells were distributed uniformly throughout the cell volume (supplementary material Fig. S3), suggesting that polarized exocytosis is not due to preferential basolateral docking of lysosomes. We then investigated mechanisms that could restrict lysosome fusion to the basolateral surface.
Fig. 1.
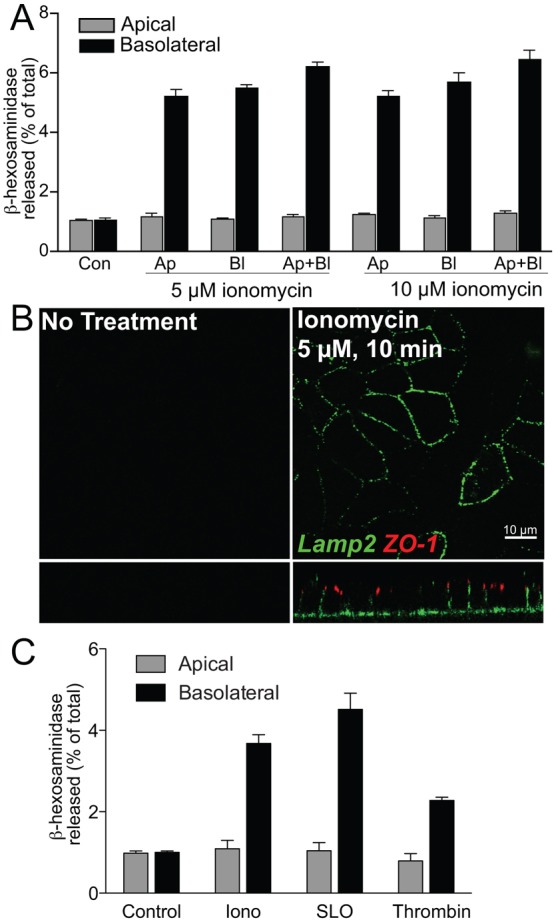
Lysosomes fuse with the basolateral membrane in response to increased [Ca2+]i. (A) β-hex activity in apical (Ap) or basolateral (Bl) media of polarized MDCK cells. Ionomycin (5 µM or 10 µM) was added to either or both compartments for 10 minutes and β-hex activity measured. Con, control untreated cells. (B) Appearance of LAMP2 (green) on the cell surface after ionomycin treatment. Confocal xy and xz sections are shown. The tight junction marker ZO-1 is in red. (C) Comparison of β-hex release after 10 minutes of incubation with 5 µM ionomycin (Iono), 150 ng/ml streptolysin O (SLO) or 1 U/ml thrombin.
Polarity of lysosome exocytosis is lost upon actin depolymerization
Exocytic fusion with the plasma membrane requires remodeling of the actin cytoskeleton and actin depolymerization increases calcium-induced lysosome exocytosis in non-polarized cells (Rodríguez et al., 1999). In polarized epithelia, actin spatially restricts exocytosis by two mechanisms: one, cortical actin acts as a fusion barrier at the apical membrane (Ehre et al., 2005; Muallem et al., 1995); and two, an intact actin cytoskeleton is required to maintain syntaxin 4 clusters at the basolateral membrane (Low et al., 2006). In MDCK cells, the actin depolymerizing drug cytochalasin D caused lysosomes to fuse apically in response to ionomycin (Fig. 2A,B), whereas cytochalasin D alone had no effect on exocytosis (supplementary material Fig. S4). Immunofluorescence analysis confirmed that cytochalasin D disrupted actin filaments and dispersed syntaxin 4, but did not alter LAMP2 distribution (supplementary material Fig. S5). On the other hand, depolymerization of microtubules by treating the cells with nocodazole also disrupted syntaxin 4 clusters (supplementary material Fig. S5), but did not alter the polarity of lysosome exocytosis (Fig. 2A,B). These data are in agreement with studies in non-polarized cells, where actin depolymerization increased and microtubule disruption had no effect on lysosome exocytosis (Jaiswal et al., 2002; Laulagnier et al., 2011). This is because microtubule-based motors are responsible for long-range movements that transport newly synthesized material and organelles such as lysosomes to specific locations within the cell, but do not participate in exocytosis (Caviston et al., 2011). Moreover, since ∼5% of total cellular β-hex is released upon exocytosis in MDCK cells, only lysosomes already pre-docked near the plasma membrane likely exocytose in response to ionomycin, as has been demonstrated by Jaiswal and colleagues (Jaiswal et al., 2002). Therefore, microtubules are critical for positioning lysosomes near the plasma membrane, whereas cortical actin is a barrier to exocytosis: although both cytochalasin D and nocodazole dispersed syntaxin 4 to the apical membrane, only cytochalasin D induced apical lysosome fusion, presumably by increasing accessibility to the plasma membrane.
Fig. 2.
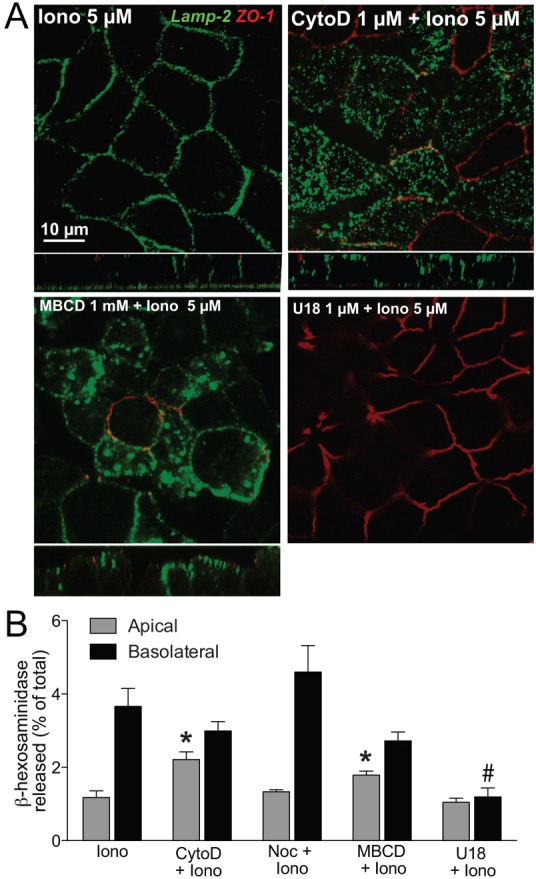
Actin depolymerization and cholesterol extraction induce apical lysosome fusion. (A,B) MDCK cells were treated with nocodazole (Noc) to destabilize microtubules, cytochalasin D (CytoD) to depolymerize actin filaments, methyl-β-cyclodextrin (MBCD) to extract membrane cholesterol or U18666A (U18) to trap cholesterol in lysosomes prior to ionomycin (Iono) treatment. (A) Surface labeling for LAMP2 (green) and ZO-1 (red). (B) β-hex activity in apical and basolateral media. *P<0.01 compared with apical β-hex release from cells treated with ionomycin alone; #P<0.01, compared with basolateral release from cells treated with ionomycin alone.
Lysosome exocytosis is sensitive to membrane cholesterol levels
Cholesterol, an essential lipid in mammalian cells, participates in membrane biogenesis, modulates protein function and drives organelle traffic. In MDCK cells, extraction of membrane cholesterol by methyl-β-cyclodextrin (MBCD) before ionomycin exposure caused apical lysosome exocytosis (Fig. 2A,B), whereas MBCD alone had no effect on exocytosis (supplementary material Fig. S4). In contrast, U18666A, a hydrophobic amine that causes cholesterol accumulation in late endosomes and lysosomes (Huynh et al., 2008), completely inhibited ionomycin-induced lysosome exocytosis (Fig. 2A,B). Neither MBCD nor U18666A altered LAMP2 or actin distribution; however, MBCD dispersed syntaxin 4 clusters apically (supplementary material Fig. S6), in agreement with data from endothelial cells (Predescu et al., 2005). Recent reports show that in fibroblasts and cardiomyocytes, cholesterol depletion by cyclodextrins increases ionomycin-induced lysosome secretion (Chen et al., 2010; Hissa et al., 2012). Conversely, cholesterol accumulation in late endosomes and lysosomes paralyzes late endocytic traffic by a variety of mechanisms including inhibition of rabGTPases, which can interfere with the recruitment of molecular motors to lysosomes, and thus reduce the population of pre-docked lysosomes at the plasma membrane, and abnormal sequestration of SNARE proteins, which would interfere with the fusion process (Fraldi et al., 2010; Lebrand et al., 2002).
The AP-1 clathrin adaptor complex regulates the basolateral exocytosis of lysosomes
Lysosomal membrane proteins LAMP1 and LAMP2 are transported from the trans-Golgi network to lysosomes via clathrin-coated transport intermediates. LAMPs contain C-terminal tyrosine-based sorting signals (YXXΦ), which bind the medium (μ) subunits of clathrin-adaptors AP-1, AP-2, AP-3 and AP-4 (Ohno et al., 1998). In polarized MDCK cells, newly synthesized LAMP2 is first transported to the basolateral membrane, endocytosed via clathrin and AP-2 and then sorted to lysosomes (Nabi et al., 1991). We have recently shown that clathrin regulates basolateral polarity (Deborde et al., 2008); this regulation is mediated by AP-1B (Fölsch et al., 1999; Gonzalez and Rodriguez-Boulan, 2009; Gravotta et al., 2007) and AP-1A (Carvajal-Gonzalez et al., 2012; Gravotta et al., 2012). The ubiquitously expressed AP-1A and the epithelial-specific AP-1B have identical γ, β1, σ1 subunits, but differ in their medium (μ) subunits, which are 80% identical: AP-1A has μ1A, whereas AP-1B has μ1B (Ohno et al., 1999). Pertinently, μ1B was shown to participate in membrane targeting and lysosomal transport of LAMP1 in some studies (Höning et al., 1996; Sugimoto et al., 2002) but not in others (Karlsson and Carlsson, 1998; Laulagnier et al., 2011; Tazeh et al., 2009).
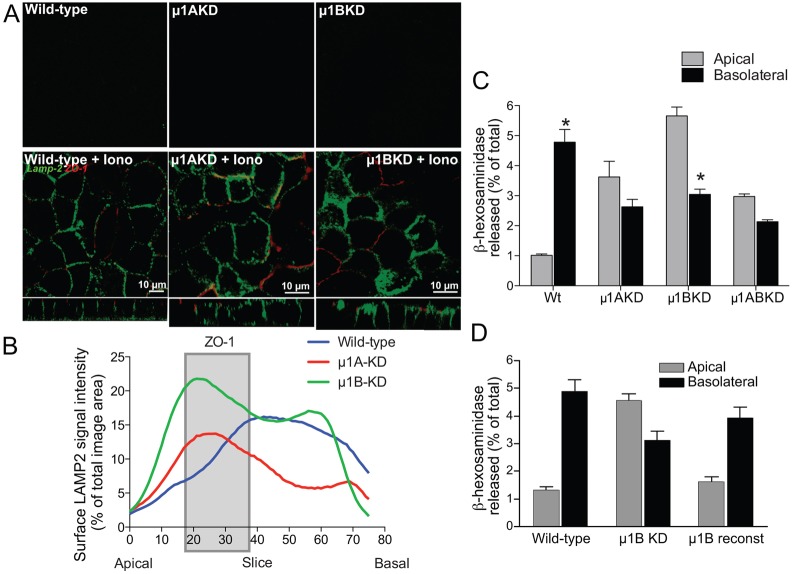
To investigate whether AP-1 participates in LAMP trafficking or lysosome exocytosis, we generated stable lines of MDCK cells lacking μ1A [μ1AKD (Carvajal-Gonzalez et al., 2012)] or μ1B [μ1BKD (Gravotta et al., 2007); supplementary material Fig. S7A]. Although LAMP2 levels in μ1BKD cells were ∼17% higher compared to wild-type and μ1AKD cells (supplementary material Fig. S7B,C), we did not detect LAMP2 on the cell surface (Fig. 3A) or differences in the intracellular distribution of LAMP2 in μ1AKD or μ1BKD cells, compared to wild-type MDCK (supplementary material Fig. S3). Ionomycin induced the appearance of LAMP2 on both the apical and basolateral membranes of μ1AKD and μ1BKD cells, in contrast to its basolateral localization in wild-type cells (Fig. 3A). Quantification of surface LAMP2 signal showed that in wild-type cells, almost all the fluorescence was below ZO-1 (basolateral) whereas μ1AKD and μ1BKD cells showed two bumps in LAMP2 fluorescence, one above ZO-1 (apical) and the other below, indicating that loss of either μ1 subunit resulted in non-polar exocytosis (Fig. 3B). This loss of polarity was confirmed by the non-polar secretion of β-hex in μ1AKD, μ1BKD and μ1ABKD double knockdown cells compared to basolateral secretion in wild-type cells (Fig. 3C). Reconstitution of μ1B activity in μ1BKD cells (Diaz et al., 2009) restored basolateral polarity of lysosome exocytosis (Fig. 3D). Taken together, these data suggest that AP-1 regulates the basolateral fusion of lysosomes. Both AP-1A and AP-1B have been shown to mediate basolateral protein trafficking in polarized epithelia: whereas AP-1A promotes exit of basolateral proteins from the trans-Golgi network (TGN), AP-1B sorts proteins at the common recycling endosome (CRE) (Carvajal-Gonzalez et al., 2012; Gravotta et al., 2012; Gravotta et al., 2007). However, protein trafficking from the TGN and endosomes occurs along a continuum and these organelles cooperate to sort membrane proteins to their correct destinations: for instance, in the absence of AP-1A, AP-1B has been shown to relocate to the TGN and mediate cargo exit (Carvajal-Gonzalez et al., 2012; Gravotta et al., 2012; Gravotta et al., 2007). In the case of lysosome exocytosis, it is likely that AP-1A and AP-1B cooperate in the basolateral targeting of a protein critical for this process. What could be the putative AP-1 cargo that specifies basolateral exocytosis?
Fig. 3.
The AP-1 adaptor is a crucial regulator of polarized lysosome exocytosis. (A) LAMP2 on the surface of wild-type MDCK cells or cells lacking μ1A (μ1AKD) or μ1B (μ1BKD) after ionomycin (Iono) treatment. (B) Quantification of surface LAMP2 fluorescence (expressed as a percentage of total fluorescence) in each confocal slice, numbered starting from the top of the monolayer. The gray bar indicates slices where ZO-1 is found and demarcates the apical and basolateral domains. (C) β-hex release from wild-type (wt), μ1AKD, μ1BKD or μ1ABKD double knockdown cells. *P<0.001, compared with corresponding apical values. (D) β-hex release from wild-type, μ1BKD and μ1BKD cells with reconstituted μ1B.
The t-SNARE syntaxin 4 interacts with the AP-1 adaptor complex and directs lysosome fusion
Exocytosis of lysosomes and secretory granules requires interactions between lysosomal v-SNARE VAMP7 and t-SNAREs syntaxin 4 and SNAP-23 (Rao et al., 2004). In polarized MDCK cells and in the kidney in vivo, syntaxin 4 localizes to the basolateral membrane (Low et al., 1996). Because AP-1 sorts basolateral proteins (Fölsch et al., 1999; Gravotta et al., 2012; Gravotta et al., 2007), we asked whether syntaxin 4 is an AP-1 cargo. In wild-type, μ1AKD and μ1BKD MDCK cells, endogenous syntaxin 4 coimmunoprecipitated with γ-adaptin (Fig. 4A). Our data also show that syntaxin 4 is a specific cargo for AP-1 because syntaxin 2, a t-SNARE implicated in lysosome exocytosis in platelets (Chen et al., 2000), did not co-immunoprecipitate with γ-adaptin (Fig. 4A). Immunofluorescence analysis showed that endogenous syntaxin 4 is expressed at low levels in wild-type MDCK cells and localizes mainly at or below the tight junction (Fig. 4B). In μ1AKD and μ1BKD cells, we observed a few cells with syntaxin 4 at or near the apical membrane (Fig. 4B). To further clarify this, we generated stable cell lines of wild-type, μ1AKD and μ1BKD cells expressing myc-tagged syntaxin 4. Exogenous syntaxin 4 was found exclusively at the basolateral membrane in wild-type MDCK cells as has been previously reported (Low et al., 1996); in μ1AKD and μ1BKD cells, syntaxin 4-myc was found on both the apical and basolateral membranes (supplementary material Fig. S8). However, we were unable to detect a direct interaction between syntaxin 4 and μ1A or μ1B by yeast two-hybrid analysis, likely due to low binding affinity or the participation of a linker protein. While our studies were underway, it was reported that syntaxin 4 is nonpolar in a porcine proximal tubule cell line, LLCPK1, which lacks μ1B (Reales et al., 2011). To further elucidate how AP-1 and syntaxin function in polarized lysosome secretion, we used shRNA-mediated knockdown of endogenous syntaxin 4 (Fig. 4C) (Torres et al., 2011). In cells treated with the scrambled shRNA, ionomycin induced basolateral β-hex release in wild-type MDCK, and non-polar secretion in μ1AKD and μ1BKD cells. However, syntaxin 4 depletion inhibited basolateral lysosome exocytosis in wild-type cells, and both apical and basolateral exocytosis in μ1AKD and μ1BKD cells in response to ionomycin (Fig. 4D).
Fig. 4.
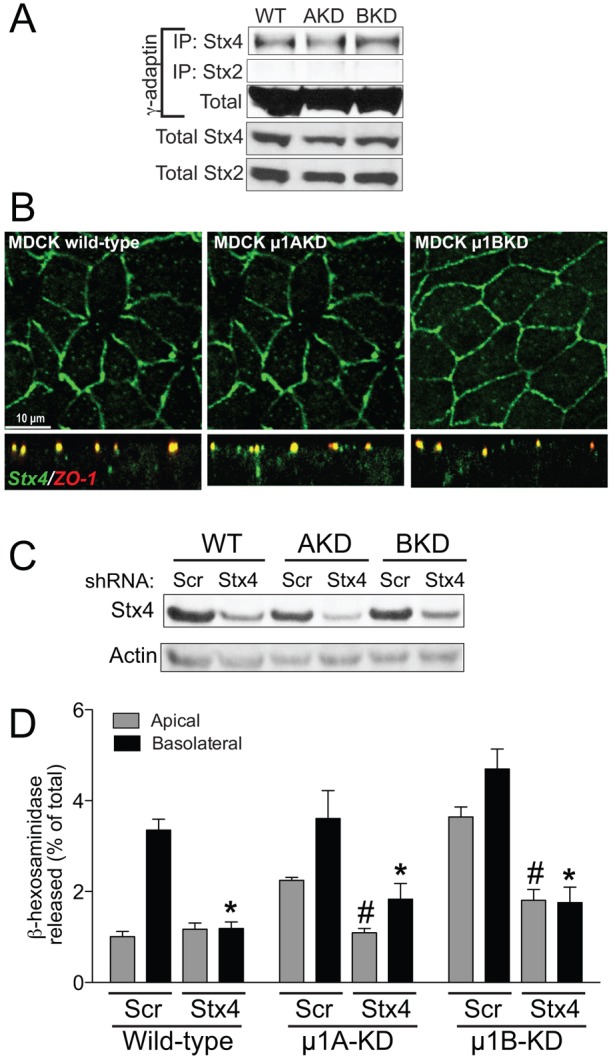
Syntaxin 4 interacts with AP-1 and is essential for lysosome exocytosis. (A) Endogenous syntaxin 4 (Stx4) or syntaxin 2 (Stx2) were immunoprecipitated from wild-type (WT), μ1AKD and μ1BKD MDCK cell lysates (300 µg each) and immunoblotted for γ-adaptin. Inputs: 30 µg each of total lysates probed for γ-adaptin, syntaxin 4 and syntaxin 2. (B) Immunostaining for endogenous syntaxin 4 (green) in wild-type, μ1AKD and μ1BKD cells. ZO-1 is in red. (C) Representative western blot showing shRNA-mediated knockdown of endogenous syntaxin 4 in wild-type, μ1AKD and μ1BKD cells; Scr, scrambled sequence. Actin was the loading control. (D) β-hex release from cells transfected with either scrambled or syntaxin 4 shRNA constructs. *P<0.05, compared with basolateral release from corresponding Scr cells; #P<0.05 compared with apical release from corresponding Scr cells.
Taken together, our data and recently published work (Carvajal-Gonzalez et al., 2012; Gravotta et al., 2012; Gravotta et al., 2007) support a model where, in wild-type MDCK cells, newly synthesized syntaxin 4 is transported out of the TGN by AP-1A and sorted to the basolateral membrane by endosomal AP-1B. As has been demonstrated for other basolateral proteins, μ1B can likely compensate for the absence of μ1A (μ1AKD cells) by mediating the exit of syntaxin 4 from the TGN and promoting increased trafficking to the CRE (Carvajal-Gonzalez et al., 2012; Gravotta et al., 2012; Gravotta et al., 2007). At the CRE, μ1B is required to sort syntaxin 4 to the basolateral membrane and in μ1BKD cells, syntaxin 4 is rerouted to the apical membrane, similar to other basolateral proteins (Carvajal-Gonzalez et al., 2012; Gravotta et al., 2012; Gravotta et al., 2007). In μ1AKD cells, high levels of syntaxin 4 in the CRE could overwhelm the μ1B sorting apparatus, which would also lead to mis-localization of syntaxin 4 to the apical surface. At the membrane, syntaxin 4 clusters are stabilized by association with the actin cytoskeleton and cholesterol-enriched microdomains. Conditions that cause apical redistribution of syntaxin 4 (actin depolymerization, membrane cholesterol extraction, absence of μ1A or μ1B) lead to nonpolar calcium-induced lysosome secretion. Apical exocytosis of lysosomes has been documented in vivo in hepatocytes (Gross et al., 1989) and the proximal tubule of the kidney (Fujita et al., 1998), both of which lack μ1B (Ohno et al., 1999; Schreiner et al., 2010). Indeed, syntaxin 4 is apical in hepatocytes (Fujita et al., 1998) and in LLC-PK1 cells (Reales et al., 2011), providing further support for the role of μ1B in restricting syntaxin 4 to the basolateral membrane and regulating the polarity of lysosome fusion.
How then does apical wound healing occur in the vast majority of epithelia that do express AP-1B? Recent reports have implicated galectin-7 in primary cilia of kidney epithelia and rab11-dependent expulsion of microvilli in intestinal epithelia (Los et al., 2011; Rondanino et al., 2011) in apical membrane repair. Apart from wound healing, lysosome exocytosis can be exploited for treating lysosome storage disorders by promoting cellular clearance of accumulated debris (Chen et al., 2010; Medina et al., 2011). Insight into the molecular mechanisms involved in polarized lysosome exocytosis as detailed in this study will aid our understanding of these diverse processes central to both epithelial physiology and pathology.
Materials and Methods
Cells
MDCK Type II cells were cultured in DMEM (Cellgro) with 5% FBS (Gemini Biosciences) on semi-permeable Transwell® filters (BD Biosciences). Cells were plated at confluence (∼250,000/cm2) for four days after which they are fully polarized. Stable cell lines lacking μ1A or μ1B, μ1AB double knockdown cells and reconstitution of μ1B into μ1BKD cells were generated and maintained as described elsewhere (Carvajal-Gonzalez et al., 2012; Diaz et al., 2009; Gravotta et al., 2012; Gravotta et al., 2007).
Lysosome exocytosis
Cells were rinsed in recording medium (HBSS with 4.5 g/l glucose, 20 mM HEPES) and incubated with ionomycin (Sigma) for 10 minutes at 37°C. Alternatively, streptolysin O (SLO) was used to trigger exocytosis (Idone et al., 2008). SLO [150 ng/ml, (Abcam)] was thiol-activated and bound to cells in Ca2+-free medium for 5 minutes at 4°C and pore formation was induced by replacing the medium with fresh recording medium at 37°C for 10 minutes. Filters were immediately transferred to ice and analyzed for surface LAMP2 or β-hex activity (see below). Other drugs used were (Sigma): 1 U/ml thrombin, cytochalasin D (1 µM, 1 hour), nocodazole (10 µM, 1 hour), U18666A (1 µM, 16 hours), methyl-β-cyclodextrin (1 mM, 1 hour).
Detection of cell-surface LAMP2
Cells on ice were incubated with a monoclonal antibody to the lumenal domain of LAMP2 (Nabi et al., 1991) in recording medium+1% BSA for 30 minutes at 4°C, fixed with 2% paraformaldehyde, permeabilized and stained for ZO-1 and Alexa-Fluor-conjugated secondary antibodies (Invitrogen) for 30 minutes. Cells were imaged by confocal microscopy (Leica SP2 or Andor Revolution XD) using a 63× 1.4 NA or 60×, 1.4 NA oil objective. For each set of experiments, the laser power, voltage and offset were identical for a given fluorophore. Z-stacks were acquired for six different fields, ∼150 cells/field, with fields that showed as little variance in the height of the cells in Z as possible. To quantify surface LAMP2 fluorescence, images from each cell line with corresponding Z-stacks (same number of slices) were analyzed using Andor IQ2 software. The percent area occupied by the LAMP2 signal in each Z slice was calculated using the Analysis feature in IQ2, and plotted as a function of confocal slice. ZO-1 staining was used to demarcate the apical and basolateral domains of the cell.
Measurement of β-hex activity
After drug treatments, apical and basolateral media were collected, centrifuged at 100 g for 5 minutes to pellet dead cells and 10,000×g for 5 minutes to pellet debris. Cells were lysed in 0.5 ml PBS+1% NP-40 for total β-hex activity. To measure enzyme activity, 350 µl of supernatant was incubated for 20 minutes with 50 µl of 6 mM 4-methyl-umbelliferyl-N-acetyl-β-D-glucosaminide (Sigma) in sodium citrate-phosphate buffer, pH 4.5 (Rodríguez et al., 1999). Fluorescence was measured after stopping the reaction with 100 µl 2 M Na2CO3, 1.1 M glycine (365 nm excitation, 450 nm emission, SpectraMax Gemini microplate reader (Molecular Devices)). Cell extracts were diluted 1∶50 before assay for total cellular β-hex activity.
Immunofluorescence staining
To determine the polarity of endogenous syntaxin 4, wild-type, μ1AKD and μ1BKD MDCK cells were fixed, permeabilized and stained with mouse α-syntaxin 4 (BD Biosciences, 1∶400) and rat α-ZO-1, and Alexa-conjugated secondary antibodies and imaged by spinning disk confocal microscopy (Andor Revolution XD) using a 60×1.4 NA oil objective. Drug treated cells were labeled with rhodamine-phalloidin (Cytoskeleton, Inc.) to visualize the actin cytoskeleton or stained with mouse α-beta-tubulin (Sigma) to visualize microtubules.
Immunoprecipitation and immunoblotting
300 µg of total protein from wild-type, μ1AKD and μ1BKD cell lysates were immuprecipitated with 5 µg of α-syntaxin 4 or α-syntaxin 2 (Abcam) using the Dynabead Protein G immunoprecipitation kit (Invitrogen) and probed for γ-adaptin (Sigma) after SDS-PAGE. Total cell lysates (30 µg) were subjected to SDS-PAGE and probed for γ-adaptin, syntaxin 4 and syntaxin 2.
RNA interference
Syntaxin 4 shRNA (5′-CCGGATTGAGAAGAACATC-3′ (Torres et al., 2011) and a scrambled sequence were subcloned into a pGFP-B-RS vector (Origene). Cells were transfected by Amaxa nucleofection (Lonza) and plated on to Transwell filters for experiments. Syntaxin 4 knockdown was confirmed by immunoblotting using a rabbit polyclonal antibody (Sigma).
Statistical analysis
Data were analyzed by a two-tailed t-test or one-way ANOVA with the Bonferroni post-test (GraphPad Prism). Unless otherwise stated, data are presented as mean ± s.e.m. of more than six independent measurements.
Supplementary Material
Acknowledgments
We thank Lalita Lakkaraju for invaluable support and inspiration.
Footnotes
Funding
This work was supported by grants from the National Institutes of Health [grant numbers P30EY016665 core grant (Department of Ophthalmology & Visual Sciences, UW-Madison) and EY08538 to E.R.B.]; the American Health Assistance Foundation (to A.L. and E.R.B.); the Karl Kirchgessner Foundation; Carl and Mildred Reeves Foundation; and the McPherson Eye Research Institute (to A.L.); the Dyson Foundation (to E.R.B.); the European Molecular Biology Organization Fellowship (to J.M.C.-G.); and Research to Prevent Blindness. A.L. is the Retina Research Foundation Rebecca Meyer Brown Professor and the recipient of a career development award from the Research to Prevent Blindness Foundation. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.109421/-/DC1
References
- Andrews N. W. (2002). Lysosomes and the plasma membrane: trypanosomes reveal a secret relationship. J. Cell Biol. 158, 389–394 10.1083/jcb.200205110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal–Gonzalez J. M., Gravotta D., Mattera R., Diaz F., Perez Bay A., Roman A. C., Schreiner R. P., Thuenauer R., Bonifacino J. S., Rodriguez–Boulan E. (2012). Basolateral sorting of the coxsackie and adenovirus receptor through interaction of a canonical YXXPhi motif with the clathrin adaptors AP-1A and AP-1B. Proc. Natl. Acad. Sci. USA 109, 3820–3825 10.1073/pnas.1117949109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviston J. P., Zajac A. L., Tokito M., Holzbaur E. L. (2011). Huntingtin coordinates the dynein-mediated dynamic positioning of endosomes and lysosomes. Mol. Biol. Cell 22, 478–492 10.1091/mbc.E10-03-0233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Lemons P. P., Schraw T., Whiteheart S. W. (2000). Molecular mechanisms of platelet exocytosis: role of SNAP-23 and syntaxin 2 and 4 in lysosome release. Blood 96, 1782–1788 [PubMed] [Google Scholar]
- Chen F. W., Li C., Ioannou Y. A. (2010). Cyclodextrin induces calcium-dependent lysosomal exocytosis. PLoS ONE 5, e15054 10.1371/journal.pone.0015054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deborde S., Perret E., Gravotta D., Deora A., Salvarezza S., Schreiner R., Rodriguez–Boulan E. (2008). Clathrin is a key regulator of basolateral polarity. Nature 452, 719–723 10.1038/nature06828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz F., Gravotta D., Deora A., Schreiner R., Schoggins J., Falck–Pedersen E., Rodriguez–Boulan E. (2009). Clathrin adaptor AP1B controls adenovirus infectivity of epithelial cells. Proc. Natl. Acad. Sci. USA 106, 11143–11148 10.1073/pnas.0811227106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divangahi M., Chen M., Gan H., Desjardins D., Hickman T. T., Lee D. M., Fortune S., Behar S. M., Remold H. G. (2009). Mycobacterium tuberculosis evades macrophage defenses by inhibiting plasma membrane repair. Nat. Immunol. 10, 899–906 10.1038/ni.1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehre C., Rossi A. H., Abdullah L. H., De Pestel K., Hill S., Olsen J. C., Davis C. W. (2005). Barrier role of actin filaments in regulated mucin secretion from airway goblet cells. Am. J. Physiol. Cell. Physiol. 288, C46–C56 [DOI] [PubMed] [Google Scholar]
- Fölsch H., Ohno H., Bonifacino J. S., Mellman I. (1999). A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell 99, 189–198 10.1016/S0092-8674(00)81650-5 [DOI] [PubMed] [Google Scholar]
- Fraldi A., Annunziata F., Lombardi A., Kaiser H. J., Medina D. L., Spampanato C., Fedele A. O., Polishchuk R., Sorrentino N. C., Simons K.et al. (2010). Lysosomal fusion and SNARE function are impaired by cholesterol accumulation in lysosomal storage disorders. EMBO J. 29, 3607–3620 10.1038/emboj.2010.237 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Fujita H., Tuma P. L., Finnegan C. M., Locco L., Hubbard A. L. (1998). Endogenous syntaxins 2, 3 and 4 exhibit distinct but overlapping patterns of expression at the hepatocyte plasma membrane. Biochem. J. 329, 527–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A., Rodriguez–Boulan E. (2009). Clathrin and AP1B: key roles in basolateral trafficking through trans-endosomal routes. FEBS Lett. 583, 3784–3795 10.1016/j.febslet.2009.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravotta D., Deora A., Perret E., Oyanadel C., Soza A., Schreiner R., Gonzalez A., Rodriguez–Boulan E. (2007). AP1B sorts basolateral proteins in recycling and biosynthetic routes of MDCK cells. Proc. Natl. Acad. Sci. USA 104, 1564–1569 10.1073/pnas.0610700104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravotta D., Carvajal–Gonzalez J. M., Mattera R., Deborde S., Banfelder J. R., Bonifacino J. S., Rodriguez–Boulan E. (2012). The clathrin adaptor AP-1A mediates basolateral polarity. Dev. Cell 22, 811–823 10.1016/j.devcel.2012.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J. B., Jr, Myers B. M., Kost L. J., Kuntz S. M., LaRusso N. F. (1989). Biliary copper excretion by hepatocyte lysosomes in the rat. Major excretory pathway in experimental copper overload. J. Clin. Invest. 83, 30–39 10.1172/JCI113873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hissa B., Duarte J. G., Kelles L. F., Santos F. P., del Puerto H. L., Gazzinelli–Guimaraes P. H., de Paula A. M., Agero U., Mesquita O. N., Guatimosim C.et al. (2012). Membrane cholesterol regulates lysosome-plasma membrane fusion events and modulates Trypanosoma cruzi invasion of host cells. PLoS Negl. Trop. Dis. 6, e1583 10.1371/journal.pntd.0001583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höning S., Griffith J., Geuze H. J., Hunziker W. (1996). The tyrosine-based lysosomal targeting signal in lamp-1 mediates sorting into Golgi-derived clathrin-coated vesicles. EMBO J. 15, 5230–5239 [PMC free article] [PubMed] [Google Scholar]
- Huynh K. K., Gershenzon E., Grinstein S. (2008). Cholesterol accumulation by macrophages impairs phagosome maturation. J. Biol. Chem. 283, 35745–35755 10.1074/jbc.M806232200 [DOI] [PubMed] [Google Scholar]
- Idone V., Tam C., Goss J. W., Toomre D., Pypaert M., Andrews N. W. (2008). Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J. Cell Biol. 180, 905–914 10.1083/jcb.200708010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal J. K., Andrews N. W., Simon S. M. (2002). Membrane proximal lysosomes are the major vesicles responsible for calcium-dependent exocytosis in nonsecretory cells. J. Cell Biol. 159, 625–635 10.1083/jcb.200208154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson K., Carlsson S. R. (1998). Sorting of lysosomal membrane glycoproteins lamp-1 and lamp-2 into vesicles distinct from mannose 6-phosphate receptor/gamma-adaptin vesicles at the trans-Golgi network. J. Biol. Chem. 273, 18966–18973 10.1074/jbc.273.30.18966 [DOI] [PubMed] [Google Scholar]
- Laulagnier K., Schieber N. L., Maritzen T., Haucke V., Parton R. G., Gruenberg J. (2011). Role of AP1 and Gadkin in the traffic of secretory endo-lysosomes. Mol. Biol. Cell 22, 2068–2082 10.1091/mbc.E11-03-0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrand C., Corti M., Goodson H., Cosson P., Cavalli V., Mayran N., Fauré J., Gruenberg J. (2002). Late endosome motility depends on lipids via the small GTPase Rab7. EMBO J. 21, 1289–1300 10.1093/emboj/21.6.1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Ropert N., Koulakoff A., Giaume C., Oheim M. (2008). Lysosomes are the major vesicular compartment undergoing Ca2+-regulated exocytosis from cortical astrocytes. J. Neurosci. 28, 7648–7658 10.1523/JNEUROSCI.0744-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Los F. C., Kao C. Y., Smitham J., McDonald K. L., Ha C., Peixoto C. A., Aroian R. V. (2011). RAB-5- and RAB-11-dependent vesicle-trafficking pathways are required for plasma membrane repair after attack by bacterial pore-forming toxin. Cell Host Microbe 9, 147–157 10.1016/j.chom.2011.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low S. H., Chapin S. J., Weimbs T., Kömüves L. G., Bennett M. K., Mostov K. E. (1996). Differential localization of syntaxin isoforms in polarized Madin-Darby canine kidney cells. Mol. Biol. Cell 7, 2007–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low S. H., Vasanji A., Nanduri J., He M., Sharma N., Koo M., Drazba J., Weimbs T. (2006). Syntaxins 3 and 4 are concentrated in separate clusters on the plasma membrane before the establishment of cell polarity. Mol. Biol. Cell 17, 977–989 10.1091/mbc.E05-05-0462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzio J. P., Pryor P. R., Bright N. A. (2007). Lysosomes: fusion and function. Nat. Rev. Mol. Cell Biol. 8, 622–632 10.1038/nrm2217 [DOI] [PubMed] [Google Scholar]
- McNeil P. L., Kirchhausen T. (2005). An emergency response team for membrane repair. Nat. Rev. Mol. Cell Biol. 6, 499–505 10.1038/nrm1665 [DOI] [PubMed] [Google Scholar]
- Medina D. L., Fraldi A., Bouche V., Annunziata F., Mansueto G., Spampanato C., Puri C., Pignata A., Martina J. A., Sardiello M.et al. (2011). Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Dev. Cell 21, 421–430 10.1016/j.devcel.2011.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldolesi J. (2003). Surface wound healing: a new, general function of eukaryotic cells. J. Cell. Mol. Med. 7, 197–203 10.1111/j.1582-4934.2003.tb00220.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muallem S., Kwiatkowska K., Xu X., Yin H. L. (1995). Actin filament disassembly is a sufficient final trigger for exocytosis in nonexcitable cells. J. Cell Biol. 128, 589–598 10.1083/jcb.128.4.589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi I. R., Le Bivic A., Fambrough D., Rodriguez–Boulan E. (1991). An endogenous MDCK lysosomal membrane glycoprotein is targeted basolaterally before delivery to lysosomes. J. Cell Biol. 115, 1573–1584 10.1083/jcb.115.6.1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H., Aguilar R. C., Yeh D., Taura D., Saito T., Bonifacino J. S. (1998). The medium subunits of adaptor complexes recognize distinct but overlapping sets of tyrosine-based sorting signals. J. Biol. Chem. 273, 25915–25921 10.1074/jbc.273.40.25915 [DOI] [PubMed] [Google Scholar]
- Ohno H., Tomemori T., Nakatsu F., Okazaki Y., Aguilar R. C., Foelsch H., Mellman I., Saito T., Shirasawa T., Bonifacino J. S. (1999). Mu1B, a novel adaptor medium chain expressed in polarized epithelial cells. FEBS Lett. 449, 215–220 10.1016/S0014-5793(99)00432-9 [DOI] [PubMed] [Google Scholar]
- Predescu S. A., Predescu D. N., Shimizu K., Klein I. K., Malik A. B. (2005). Cholesterol-dependent syntaxin-4 and SNAP-23 clustering regulates caveolar fusion with the endothelial plasma membrane. J. Biol. Chem. 280, 37130–37138 10.1074/jbc.M505659200 [DOI] [PubMed] [Google Scholar]
- Rao S. K., Huynh C., Proux–Gillardeaux V., Galli T., Andrews N. W. (2004). Identification of SNAREs involved in synaptotagmin VII-regulated lysosomal exocytosis. J. Biol. Chem. 279, 20471–20479 10.1074/jbc.M400798200 [DOI] [PubMed] [Google Scholar]
- Reales E., Sharma N., Low S. H., Fölsch H., Weimbs T. (2011). Basolateral sorting of syntaxin 4 is dependent on its N-terminal domain and the AP1B clathrin adaptor, and required for the epithelial cell polarity. PLoS ONE 6, e21181 10.1371/journal.pone.0021181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez A., Webster P., Ortego J., Andrews N. W. (1997). Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells. J. Cell Biol. 137, 93–104 10.1083/jcb.137.1.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez A., Martinez I., Chung A., Berlot C. H., Andrews N. W. (1999). cAMP regulates Ca2+-dependent exocytosis of lysosomes and lysosome-mediated cell invasion by trypanosomes. J. Biol. Chem. 274, 16754–16759 10.1074/jbc.274.24.16754 [DOI] [PubMed] [Google Scholar]
- Rondanino C., Poland P. A., Kinlough C. L., Li H., Rbaibi Y., Myerburg M. M., Al–bataineh M. M., Kashlan O. B., Pastor–Soler N. M., Hallows K. R.et al. (2011). Galectin-7 modulates the length of the primary cilia and wound repair in polarized kidney epithelial cells. Am. J. Physiol. Renal Physiol. 301, F622–F633 10.1152/ajprenal.00134.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkman S., Andrews N. W., Nussenzweig V., Robbins E. S. (1988). Trypanosoma cruzi invade a mammalian epithelial cell in a polarized manner. Cell 55, 157–165 10.1016/0092-8674(88)90018-9 [DOI] [PubMed] [Google Scholar]
- Schreiner R., Frindt G., Diaz F., Carvajal–Gonzalez J. M., Perez Bay A. E., Palmer L. G., Marshansky V., Brown D., Philp N. J., Rodriguez–Boulan E. (2010). The absence of a clathrin adapter confers unique polarity essential to proximal tubule function. Kidney Int. 78, 382–388 10.1038/ki.2010.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcombe J., Bossi G., Griffiths G. M. (2004). Linking albinism and immunity: the secrets of secretory lysosomes. Science 305, 55–59 10.1126/science.1095291 [DOI] [PubMed] [Google Scholar]
- Sugimoto H., Sugahara M., Fölsch H., Koide Y., Nakatsu F., Tanaka N., Nishimura T., Furukawa M., Mullins C., Nakamura N.et al. (2002). Differential recognition of tyrosine-based basolateral signals by AP-1B subunit mu1B in polarized epithelial cells. Mol. Biol. Cell 13, 2374–2382 10.1091/mbc.E01-10-0096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazeh N. N., Silverman J. S., Schwartz K. J., Sevova E. S., Sutterwala S. S., Bangs J. D. (2009). Role of AP-1 in developmentally regulated lysosomal trafficking in Trypanosoma brucei. Eukaryot. Cell 8, 1352–1361 10.1128/EC.00156-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres J., Funk H. M., Zegers M. M., ter Beest M. B. (2011). The syntaxin 4 N terminus regulates its basolateral targeting by munc18c-dependent and -independent mechanisms. J. Biol. Chem. 286, 10834–10846 10.1074/jbc.M110.186668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Chen G., Zhou W., Song A., Xu T., Luo Q., Wang W., Gu X. S., Duan S. (2007). Regulated ATP release from astrocytes through lysosome exocytosis. Nat. Cell Biol. 9, 945–953 10.1038/ncb1620 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.