Summary
Na+/K+-ATPase, an integral membrane protein, has been studied for over a half century with respect to its transporter function in the plasma membrane, where it expels three Na+ ions from the cell in exchange for two K+ ions. In this study, we demonstrate a functioning Na+/K+-ATPase within HEK293 cell nuclei. This subcellular localization was confirmed by western blotting, ouabain-sensitive ATPase activity of the nuclear membrane fraction, immunocytochemistry and delivery of fluorescently tagged Na+/K+-ATPase α- and β-subunits. In addition, we observed an overlap between nuclear Na+/K+-ATPase and Na/Ca-exchanger (NCX) when nuclei were immunostained with commercially available Na+/K+-ATPase and NCX antibodies, suggesting a concerted physiological coupling between these transporters. In keeping with this, we observed an ATP-dependent, strophanthidin-sensitive Na+ flux into the nuclear envelope (NE) lumen loaded with the Na-sensitive dye, CoroNa-Green. Analogous experiments using Fluo-5N, a low affinity Ca2+ indicator, demonstrated a similar ATP-dependent and strophanthidin-sensitive Ca2+ flux into the NE lumen. Our results reveal an intracellular physiological role for the coordinated efforts of the Na+/K+-ATPase and NCX to actively remove Ca2+ from the nucleoplasm into the NE lumen (i.e. the nucleoplasmic reticulum).
Key words: Na+/K+-ATPase, Nuclear, CoroNa-Green, Fluo5N
Introduction
The Na+/K+-ATPase (Na pump) is an integral membrane protein that plays a central role in ionic homeostasis in animals by mediating the translocation of Na+ and K+ ions against their electrochemical gradients across the membrane (for review see Kaplan, 2002). The Na+/K+-ATPase belongs to a large family of enzymes known as the P-type ATPases (Lutsenko and Kaplan, 1995; Morth et al., 2011). Members of this protein class couple the hydrolysis of ATP to the transmembrane translocation of cations (or lipids in P4 ATPases) and have been identified in all taxonomic phyla. The Na+/K+- and the gastric H+/K+-ATPases are the only members of the P2-type ATPase subfamily, which possess two obligatory subunits a catalytic α-subunit (∼105 kDa) that spans the membrane ten-times and a glycosylated β-subunit (∼55 kDa) with a single transmembrane α-helix, a short cytoplasmic N-terminus, and a large extracellular domain (Kaplan 2002). Four α-subunits and three β-subunits have been identified in vertebrates which associate into several pump isoforms with varying tissue distributions (Blanco and Mercer, 1998; Lingrel, 2010).
Na pump targeting and trafficking has been studied extensively. The quaternary structure plays a crucial role in delivery of the α-subunit to the plasma membrane (PM) as it requires a priori assembly with the β-subunit to escape the endoplasmic reticulum (ER) (Geering, 2001; Gatto et al., 2001). Caplan and co-workers (Dunbar and Caplan, 2000) have shown that basolateral targeting in polarized epithelia is mediated by a signaling region within the 4th transmembrane domain of the Na pump α-subunit. However, other studies have suggested a predominant role for the β-subunit in Na pump distribution in polarized epithelia (Rajasekaran et al., 2001). Moreover, it has been demonstrated that cell-cell adherence was disrupted in MDCK cells by expression of a deglycosylated mutant β-subunit, or by an extracellularly bound β-subunit antibody, suggesting a cell morphological role of the Na pump unrelated to ion transport (Vagin et al., 2005).
It is becoming apparent that membrane targeting of Na pump is more complex than merely α/β assembly in the ER and delivery to the PM. Within polarized tissues, Na pump is heavily localized to the lateral membrane near the apical tight junctions (Rajasekaran et al., 2008). Trafficking of Na+/K+-ATPase has been shown to differ between cell types and can be influenced by the number of different Na pump isoforms that are expressed within a particular cell type. Song et al. (Song et al., 2006) have shown that the α-subunit N-terminus is important for Na+/K+-ATPase trafficking and delivery to the PM in neurons that express both α1 and α2 isoforms, but each isoform is delivered to separate microdomains. This observation provides an explanation for how cardiac glycoside inhibition of Na+ pump can increase the driving force for Na+/Ca2+ exchange without altering bulk cytoplasmic Na+ concentrations. Rather, specifically blocking α2 within PM microdomains adjacent to the sarcoplasmic reticulum (called ‘plasmerosome’), provides a mechanism to increase Ca2+ sequestration into internal stores without a rise in cytosolic Ca2+ (see Blaustein et al., 2009). This example demonstrates that the subcellular location of Na+/K+-ATPase is as critical as its enzymology in contributing to cell biology.
Extending the targeting complexity above, there have been two reports of intracellular Na pump function (Gatto et al., 2001; Garner, 2002). Gatto et al. (Gatto et al., 2001) monitored Na pump assembly, maturation, and delivery to the PM via cell fractionation experiments in insect cells heterologously expressing Na pumps. They observed that immediately upon α-β subunit assembly within the ER, the Na+/K+-ATPase was fully functional. A subsequent study by Garner (Garner, 2002) revealed an endogenous functioning Na pump within the nuclear envelope (NE) of liver cells. However, a compelling physiological role for a nuclear Na+/K+-ATPase remained elusive.
Since these intracellular Na pump reports, a study describing Na/Ca exchanger (NCX) activity within the NE was published (Wu et al., 2009). Ledeen and co-workers suggested that nuclear NCX contributes to nucleoplasmic Ca2+ signaling by actively extruding Ca2+ ions out of the nucleoplasm and into the NE lumen (Wu et al., 2009). However, given that the active transport of Ca2+ via NCX requires exploitation of a Na+ gradient, it seems inescapable that for this function to happen Na+ must accumulate within the NE lumen to a greater concentration than in the nucleoplasm. Taken together with the observations of an ER/Nuc Na+/K+-ATPase (Gatto et al., 2001; Garner, 2002), a hypothetical role for intracellular Na pump arises. Namely, Na pump in either the ER or the NE would actively accumulate Na+ within the contiguous space that makes up both the ER lumen and the NE lumen. Hence, an ER or NE operating Na pump would provide the necessary driving force for nuclear NCX activity and thus contribute to nucleoplasmic Ca2+ homeostasis.
In the present study, we set out to determine whether HEK293 cells have an operational intracellular Na pump and if it plays a functional role in intracellular Ca2+ sequestration. We report the presence of Na+/K+-ATPase in HEK293 cell nuclei confirmed by western blotting, immunocytochemistry, and expression of a fluorescently tagged Na pump α-subunit in live cells. Intracellular Na+/K+-ATPase function was measured as cardiac glycoside-sensitive ATPase in nuclear membrane preparations as well as Na+ transport into the NE lumen of isolated HEK293 nuclei. Changes in Na+ concentration within the NE lumen were determined via the Na+-sensitive fluorescent probe, CoroNa-Green. The observed Na+ transport was both ATP-dependent and strophanthidin-sensitive. Since the main function of Na pump is to generate and maintain Na+ gradients across membranes, we tested the hypothesis that the role of nuclear Na+/K+-ATPase is to provide the driving force for the Ca2+ extrusion into the NE lumen via NCX. We observed an overlap between endogenously expressed Na+/K+-ATPase and NCX within the NE of HEK293 cells. Moreover, we demonstrate a Na pump-dependent NE luminal Ca2+ uptake in isolated nuclei from HEK293 cells. Thus, the Na pump may play an important role in regulating nuclear calcium levels.
Results
Na+/K+-ATPase is present in the nuclear envelope of HEK293 cells
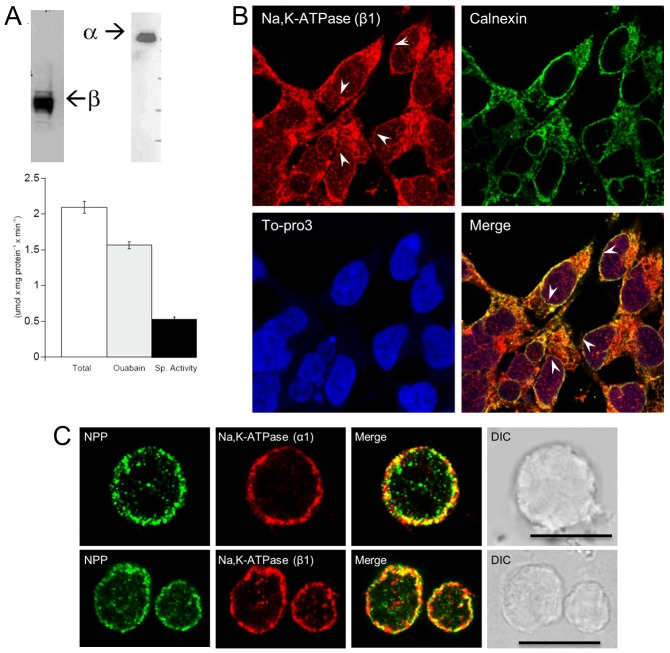
Nuclei from HEK293 cells were isolated and nuclear membrane proteins were separated via SDS-PAGE (Laemmli, 1970) and electrotransferred onto a PVDF membrane (Towbin et al., 1979). The membrane was probed with antibodies against the Na pump α and β subunits, revealing the presence of both subunits within the NE (Fig. 1A). In addition, ouabain-sensitive ATP hydrolysis was measured from the nuclear membrane preparation, demonstrating that NE Na+/K+-ATPase is functional (Fig. 1A).
Fig. 1.
Endogenous Na+/K+-ATPase α- and β-subunits present in HEK293 nuclei. (A) Immunoblot analysis of the isolated nuclear membrane preparation from HEK293 cells revealed the presence of β- and α-subunits of Na+/K+-ATPase. The bar graph represents ATP hydrolysis measurements performed on the nuclear membrane preparation. Ouabain-sensitive ATPase activity (black bar) is the difference between total ATPase (white bar) and ATPase in the presence 0.5 mM ouabain (gray bar). Ouabain-sensitive ATPase activity is consistent with mature functioning Na+/K+-ATPase within HEK293 cell nuclei. Values are means ± s.e.m. of measurements from three different nuclei preparations. (B) Immunostaining of HEK293 cells. Permeabilized HEK293 cells were immunostained with both anti-Na+/K+-ATPase β1-subunit (Alexa Fluor 568 secondary) and anti-calnexin (Alexa Fluor 488 secondary) antibodies, which revealed endogenous Na+/K+-ATPase in NE and ER as apparent from the overlap between Na+/K+-ATPase and calnexin antibodies. To-Pro3 DNA staining was done to visualize the nuclei. Arrowheads indicate Na+/K+-ATPase in the NE. (C) Immunostaining of HEK293 nuclei. Isolated nuclei from HEK293 cells were double-stained with anti-Na+/K+-ATPase α- or β-subunit (Alexa Fluor 568 secondary) and anti-NPP/NE (Alexa Fluor 488 secondary), a marker to delineate the NE. Endogenous Na+/K+-ATPase α- and β-subunits can be clearly seen in the NE of HEK293 cells and confirmed by their overlap with the NPP staining. Scale bars: 11.9 µm.
The Na+/K+-ATPase has long been a marker for PM in cell fractionation (Meier et al., 1984; Holtbäck et al., 1999; Van Dyke, 2004; Pohl et al., 2005). Since nuclear membrane fractionation pools may contain some minor PM contamination, we wanted to use additional methods to determine whether Na pumps were in the NE. Immunocytochemistry was performed on HEK293 cells and their isolated nuclei with antibodies against Na pump α- and β-subunits to confirm the western blotting and ATPase activity results. Confocal imaging of immunostained cells and isolated nuclei revealed the presence of Na+/K+-ATPase in the NE (Fig. 1B,C). HEK293 cells were permeabilized and immunostained with both anti-Na+/K+-ATPase β-subunit and anti-calnexin. Calnexin has been shown to be present in ER and the inner NE (Cala et al., 1993; Gilchrist and Pierce, 1993; Ohsako et al., 1994). We observed a clear overlap between Na+/K+-ATPase and calnexin signals showing that the Na+/K+-ATPase is present in ER as well as NE (Fig. 1B). It has earlier been reported that Na+/K+-ATPase is functional in the ER (Gatto et al., 2001). To show that the Na+/K+-ATPase in NE is indeed the perinuclear region, we stained the nuclei with To-Pro3, a nuclear DNA stain (Fig. 1B). As further confirmation, isolated nuclei from HEK293 cells were incubated with anti-Na+/K+-ATPase α- or β-subunit and also with antibodies against nuclear pore protein (NPP), a NE marker (Fig. 1C). Both proteins were clearly present and merging the images revealed significant signal overlap (Fig. 1C), consistent with Na pump residing within the NE. In the absence of primary antibodies, there was no background labeling via either conjugated secondary antibody (data not shown). We obtained similar results via staining isolated nuclei with another resident nuclear protein, i.e. the retinoblastoma-protein associated protein 46 (RbAp46), a component of the mSin3 co-repressor complex (Guan et al., 1998). Anti-RbAp46, an antibody to a nucleoplasmic histone chaperone (RbAp46), more ubiquitously stained nuclei whereas the Na+/K+-ATPase β-subunit was contained within the NE (data not shown).
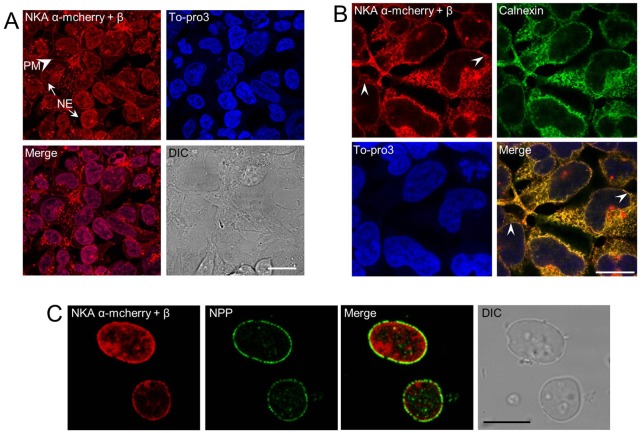
In addition to performing immunocytochemistry on isolated nuclei, we made a stable HEK293 cell line expressing both the fluorescently tagged Na pump α-subunit (i.e. α-mcherry) and the untagged beta-subunit to directly observe its localization in living cells. Fig. 2A–C shows the stable cell line expressing α-mcherry exhibiting both PM and NE localization. Moreover, the cells were stained with To-Pro3 iodide (a DNA stain), which revealed that the intracellular organelles with α-mcherry fluorescence were indeed nuclei (Fig. 2A). Cells were fixed and permeabilized to stain the DNA so as to demarcate the nuclei in cells. Due to permeabilization of fixed cells, Na+/K+-ATPase signal in the PM significantly decreased. We also observed that the Na+/K+-ATPase α-mcherry signal in the background of the nucleus was obscuring a clear distinction between the nucleus and the PM (Fig. 2A). In order to get a closer look at the nuclear α-mcherry distribution, we permeabilized the cells and stained them with anti-calnexin antibody and To-Pro3 (Fig. 2B). Again, consistent with the results above, we observed that the Na+/K+-ATPase α-mcherry localized to ER and NE. A merge between Na+/K+-ATPase α-mcherry, calnexin, and To-Pro3 signals confirms Na+/K+-ATPase localization to both ER and NE. Since ER is extensively spread throughout the cell, the Na+/K+-ATPase α-mcherry in ER looked like a background signal. Note that the PM signal in Fig. 2B is not apparent due to extensive permeabilization of cells. Moreover, we observed that the levels of heterologous and endogenous Na+/K+-ATPase in ER were comparable. We isolated nuclei from this stable cell line and they clearly show α-mcherry in the NE (Fig. 2C). For completeness, nuclei from α-mcherry and β-subunit expressing HEK293 cells were isolated, fixed, and immunostained with anti-NPP antibody. Confocal imaging of these nuclei revealed an overlap/co-localization between mcherry-tagged Na pump α-subunit and the NPP antibody staining (Fig. 2C).
Fig. 2.
Dual expression of Na+/K+-ATPase α-mcherry and β-subunits in HEK293 cells. (A) HEK293 cells stably expressing both Na+/K+-ATPase α-subunit tagged with mcherry and wild-type β-subunit, wherein the Na+/K+-ATPase α-mcherry fusion protein can be seen to localize to both PM (arrowhead) and NE (arrow). The PM signal was reduced due to permeabilization of the PM in order to get efficient DNA staining with To-Pro3. An overlap between Na+/K+-ATPase α-mcherry and To-Pro3 DNA stain in HEK293 nuclei (purple) confirms the nuclear localization of Na+/K+-ATPase. The haziness of the α-mcherry signal in the background of nuclei is due to ER localization of the expressed protein. Scale bar: 24 µm. (B) To confirm the ER localization, we permeabilized the cells expressing mcherry-tagged α-subunit and immunostained them with anti-calnexin antibody. Merging Na+/K+-ATPase α-mcherry, calnexin and To-Pro3 stained images shows a clear overlap between Na+/K+-ATPase α-mcherry signal and calnexin staining, thus confirming that the expressed protein is present in ER along with NE. The extensive area of the ER tends to obscure the distinction between PM- and NE-localized mcherry-tagged Na+/K+-ATPase α (in A). Arrowheads highlight Na+/K+-ATPase α-mcherry in the NE. Scale bar: 11.9 µm. (C) Isolated nuclei from HEK293 stable cell line expressing Na+/K+-ATPase α-mcherry revealing the presence of the fluorescently-tagged Na+/K+-ATPase α-subunit in the NE. The same nuclei were immunostained with anti-NPP/NE (followed by Alexa Fluor 488 secondary) to show the NE staining. Co-localization of Na+/K+-ATPase-mcherry and NPP confirms the presence of Na+/K+-ATPase in the NE. The differential interference contrast (DIC) image of the immunostained HEK293 nuclei is shown. Scale bar: 11.9 µm.
NE consists of two membranes: an outer membrane that is contiguous with the ER membrane and an inner membrane which faces the nucleoplasm. The lumen between the outer and inner NE membranes is continuous with the ER lumen. Careful investigation of the Na+/K+-ATPase amino acid sequence revealed no known nuclear localization signals. The single report of nuclear Na pump suggests that it resides within the inner NE in liver cells (Garner, 2002). Thus, we sought to identify whether Na pump resides within the inner or outer (or both) NE membranes in kidney cells. The NE inner and outer membranes can be biochemically separated via a Na-citrate washing method (Gilchrist and Pierce, 1993), which preferentially removes the outer NE leaving the inner NE intact. We used this method (previously applied by several groups; e.g. Humbert et al., 1996; Xie et al., 2004) to effectively isolate outer from inner NE fractions; the respective membrane proteins were then separated via SDS-PAGE. Fig. 3A shows the immunostaining (anti-Na+/K+-ATPase β-subunit antibody) of the electrotransferred membrane proteins from the two respective membrane pools. Na pump is present almost exclusively in the inner NE. The same blot was reprobed with anti-lamin B (Fig. 3B), a protein that is tightly associated with the nucleoplasmic (inner) side of the NE to demonstrate the quality of membrane separation. This result clearly shows that Na pump is present in the inner NE but not in outer NE.
Fig. 3.
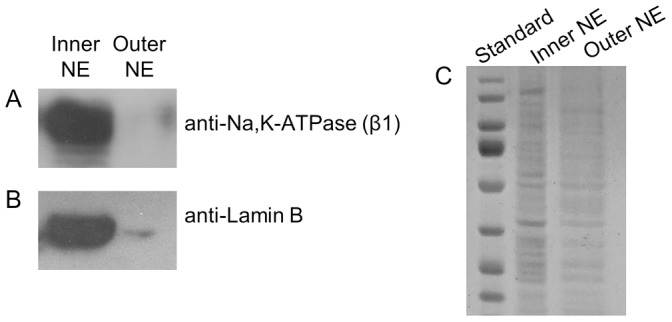
Na+/K+-ATPase resides within the inner membrane of the NE. The inner NE membranes were separated from outer NE membranes using sodium-citrate. (A) Immunoblot probed with anti-Na+/K+-ATPase β-subunit on the separated inner and outer nuclear membrane fractions from isolated HEK293 nuclei. (B) The same immunoblot as in A was stripped and re-probed with anti-Lamin B; a marker for inner NE membrane. (C) Coomassie Brilliant Blue-stained SDS PAGE gel showing that similar levels of inner and outer nuclear membrane protein were loaded on the gel.
Considering the proximity of trans-Golgi network (TGN) to the perinuclear region and that the Na+/K+-ATPase has been shown to be endocytosed in kinase signaling cascades (Liu and Shapiro, 2007), we wanted to determine the localization patterns of TGN and lysosomal markers (TGN46 and Lamp1, respectively) relative to the intracellular Na+/K+-ATPase. We observed that TGN46 localized discontinuously in the perinuclear region, which was distinctly different from that of the intracellular Na+/K+-ATPase. On the other hand, Lamp1 appeared to be exclusively cytosolic in its localization (Fig. 4A). To further confirm that nuclear Na+/K+-ATPase was not associated with the TGN, we examined HEK293 NE proteins via western blotting and found only faint traces of TGN46 but no Lamp1 in whole nuclear extracts (Fig. 4B). Furthermore, separating the outer NE membrane from the inner NE membrane (see Materials and Methods) revealed that TGN46 is present only in the outer membrane of the NE (Fig. 4C). This is in direct contrast to our observations that the nuclear Na+/K+-ATPase is exclusively found in the inner NE (see Fig. 4C). Consequently, it appears that the Na+/K+-ATPase is specifically targeted to the NE, rather than an association with other intracellular organelles such as TGN and lysosomes.
Fig. 4.
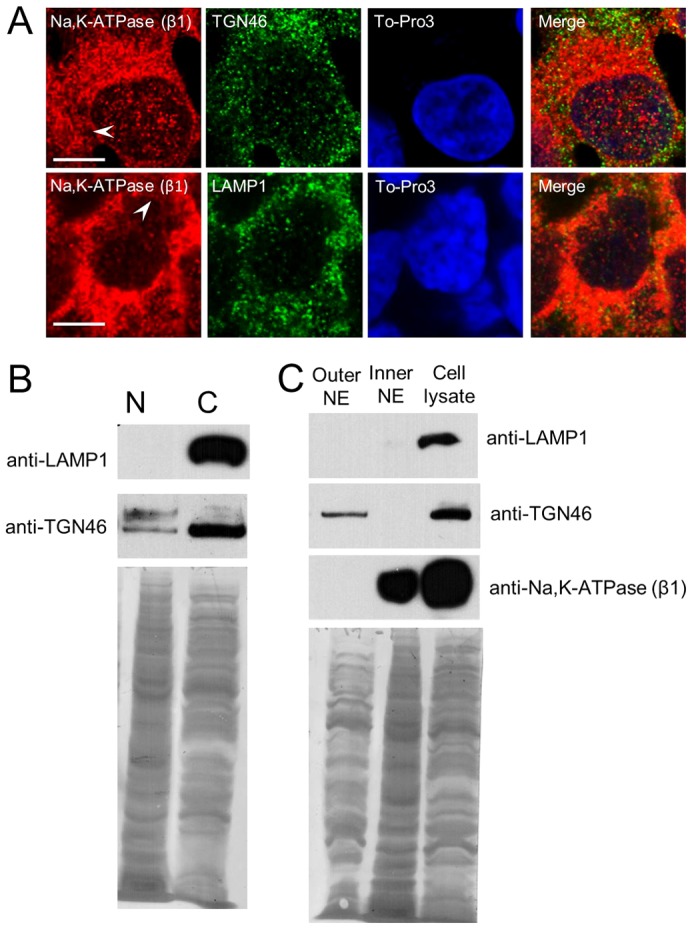
Determining the presence of trans-Golgi network and lysosomal markers relative to Na+/K+-ATPase in HEK293 cells and nuclei. (A) Intracellular localization of Na+/K+-ATPase (β-subunit) in comparison with TGN46 and Lamp1. Whereas Na+/K+-ATPase had distinct perinuclear localization, TGN46 appeared to have discontinuous localization in the perinuclear region; Lamp1 was predominantly cytosolic. Merging Na+/K+-ATPase, TGN46 (or Lamp1) -stained cells and To-Pro-stained nuclei shows co-localization in regions surrounding the nucleus but not in the NE. Arrowheads indicate the presence of Na+/K+-ATPase in the NE. Scale bars: 6 µm. (B) Western blot analysis of HEK293 nuclear fraction for the presence of any TGN and lysosomal contamination. Immunoblot probed with anti-Lamp1 antibody revealed its exclusive presence in the cell lysate fraction (lane C), but not in the nuclear fraction (lane N). The blot probed with anti-TGN46 showed slight traces of TGN46 in the nuclear fraction. The bottom panel is the Amido Black-stained blot, showing similar amounts of proteins loaded on the gel. (C) Determination of the presence of Lamp1 and TGN46 in the outer and inner NE membranes, compared to Na+/K+-ATPase localization. No traces of Lamp1 were detected in either NE fractions, whereas traces of TGN46 were found in only the outer NE membrane. Na+/K+-ATPase was present exclusively in the inner NE membrane.
Na+/K+-ATPase function within the NE
Gatto and co-workers (Gatto et al., 2001) demonstrated that Na pump is active within the ER when heterologously expressed in insect cells and here we have also shown endogenous Na+/K+-ATPase activity in HEK293 nuclei (Fig. 1A). In order to determine if this intracellular Na+/K+-ATPase activity is involved directly in Na+ loading the NE lumen, we used the Na+-sensitive fluorescent probe, CoroNa-Green acetoxy methylester (AM), to monitor changes in NE luminal [Na+]. CoroNa-Green AM was used to load the NE lumen with the dye upon cleavage from resident esterases; the fluorescence intensity of CoroNa increases upon binding Na+. Residual dye within the nucleoplasm was easily removed by washing the nuclei with dye-free buffer as evidenced by the intensity of CoroNa fluorescence being much higher in the nuclear periphery than the central nucleoplasmic portion.
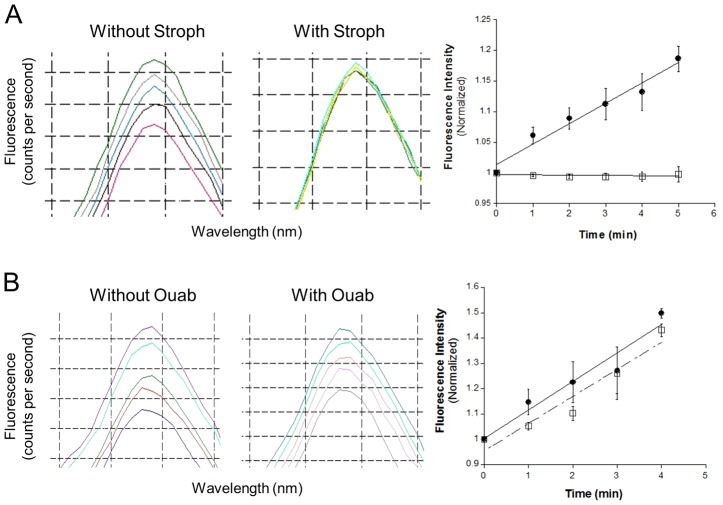
CoroNa has been used to effectively resolve changes in [Na+] in the presence of physiological concentrations of other monovalent cations (Meier et al., 2006). Here we measured changes in [Na+] within the NE of isolated nuclei. CoroNa-loaded nuclei were suspended in Na+-containing assay buffer (see Materials and Methods) and basal fluorescence was measured for 3 minutes (λEx = 494; λEm = 514). Fluorescence intensity was measured at 1 minute intervals for 3–5 minutes after addition of 1 mM Mg-ATP (final concentration). An ATP-dependent rise in fluorescence intensity due to the rise in intraluminal [Na+] was observed in untreated nuclei (Fig. 5A,B, filled circles). In order to determine whether the Na pump contributed to this Na+ transport, nuclei were pretreated with 0.5 mM of the cardiotonic steroids, strophanthidin (Fig. 5A, open squares) or ouabain (Fig. 5B, open squares). Strophanthidin abolished the ATP-dependent Na+ accumulation, whereas ouabain had little or no effect (see Fig. 5A,B, open squares). Given that the binding site for these specific Na pump inhibitors lies on the extracytoplasmic (i.e. luminal) side of the membrane, the greater inhibitory potency of strophanthidin over ouabain is consistent with the increased membrane permeability of the aglycone (Polvani and Blostein, 1988). These experiments in intact ‘closed’ nuclei differ from the ATPase experiments (Fig. 1A), where the membranes were ‘broken’ and afforded ouabain access to the luminal side of the Na pump.
Fig. 5.
Na+ transport into NE lumen is ATP-dependent and strophanthidin-sensitive. The nuclear envelope lumen from isolated HEK293 cell nuclei were loaded with CoroNa-Green AM. CoroNa-loaded nuclei were then divided into two equal fractions out of which one fraction was pretreated with either 0.5 mM strophanthidin (Stroph) or ouabain (Ouab); the untreated fraction was used as a control. (A) Representative raw traces of excitation scans at 1-minute intervals. The untreated fraction of CoroNa-loaded nuclei exhibited an increase in fluorescence (filled circles) after addition of 1 mM ATP (final concentration) but the nuclear fraction pretreated with strophanthidin (open squares) did not. (B) Both the untreated (filled circles) and the ouabain-treated (open squares) nuclear fractions exhibited an increase in CoroNa-fluorescence after the addition of 1 mM ATP, consistent with the inability of the membrane impermeable ouabain to reach the extracytoplasmic (intraluminal) surface of the Na+/K+-ATPase. Fluorescence measurements were taken at excitation and emission wavelengths of CoroNa at 494 nm and 514 nm, respectively. Arbitrary fluorescence intensity values were normalized to the basal fluorescence prior to ATP addition.
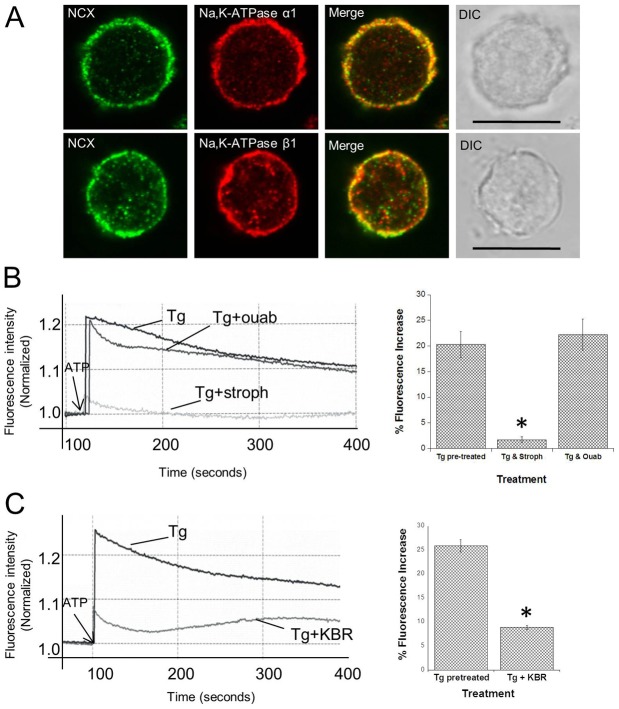
Until recently, the ATP dependent Ca2+ uptake from the cytoplasm into the nuclear calcium store (NE lumen) was thought to be mediated only by SERCA (Gerasimenko et al., 1995). But Wu et al. (Wu et al., 2009) have shown that the calcium uptake in differentiated nerve (NG108-15) and glial (C6) cells also occurs from the nucleoplasmic side through NCX present in the inner NE. Since NCX exploits Na+ gradients for Ca2+ transport, we hypothesized that nuclear Na pump may be responsible for energizing nuclear NCX-mediated Ca2+ transport. After confirming the presence of nuclear NCX within HEK293 NE via immunocytochemistry (Fig. 6A), we measured [Ca+2] changes in the NE lumen with Fluo-5N (a low-affinity Ca2+ indicator). Fluo-5N was successfully utilized in isolated nuclei for measuring [Ca2+] in NE (Zima et al., 2007). Fluo-5N-loaded HEK293 nuclei were pretreated with thapsigargin (2 µM) to specifically inhibit SERCA mediated Ca2+ transport (Takemura et al., 1989). Once the basal fluorescence stabilized (∼2–3 minutes), addition of 1 mM Mg-ATP generated a rapid increase in Fluo-5N fluorescence corresponding to an increase in NE luminal [Ca2+] (Fig. 6B, first bar); an effect significantly reduced in nuclei pretreated with both thapsigargin (2 µM) and strophanthidin (0.5 mM) (Fig. 6B, second bar). In contrast, pretreatment with 0.5 mM ouabain did not inhibit ATP-dependent Ca2+ transport into the NE lumen in the presence of thapsigargin (Fig. 6B, third bar). The latter result was expected given the results observed with CoroNa (Fig. 5B).
Fig. 6.
Na+/K+-ATPase and NCX are physiologically coupled in HEK293 nuclei. (A) Na+/K+-ATPase and NCX co-localize in HEK293 nuclei. Immunocytochemistry was performed on HEK293 nuclei using anti-NCX with Alexa-Fluor-488-conjugated secondary antibody and anti- Na+/K+-ATPase α- or β-subunit and Alexa-Fluor-568-conjugated secondary antibodies. An overlap between Na+/K+-ATPase and NCX can be seen in the merged images, revealing their co-localization. (B) Ca2+ transport into NE lumen is ATP-dependent and strophanthidin-sensitive. Isolated nuclei from HEK293 cells were loaded with Fluo-5N AM. All Fluo-5N-loaded nuclei were then treated with 2 µM thapsigargin (Tg) to block any SERCA-mediated Ca2+ transport and subsequently divided into two equal fractions. One fraction was pretreated with either 0.5 mM strophanthidin or ouabain and the other received only the initial Tg treatment and served as control. The Tg-only-treated fraction of Fluo-5N-loaded nuclei exhibited an increase in the fluorescence after the addition of 1 mM ATP, but the Ca2+ transient was almost completely abolished in the nuclear fraction pretreated with strophanthidin. The ouabain-pretreated nuclei fractions exhibited an increase in the Fluo-5N-fluorescence after the addition of 1 mM ATP, consistent with the inability of the membrane-impermeable ouabain to reach the extracytoplasmic (intraluminal) surface of the Na+/K+-ATPase. The bar graph represents percentage increase in fluorescence after ATP addition. Fluorescence measurements were taken at excitation and emission wavelengths of Fluo-5N at 490 nm and 515 nm, respectively. Arbitrary fluorescence intensity values were normalized to the basal fluorescence prior to ATP addition. (C) NCX-mediated Ca2+ transport into HEK293 nuclei is sensitive to the NCX1-inhibitor, KB-R7943. With ATP addition, there was a Ca2+ transient in Tg-pretreated nuclei, which was significantly reduced upon addition of KB-R7943. The bar graph represents percentage increase in fluorescence upon ATP addition, and shows means ± s.e.m. of five different measurements; *P<0.01.
To reaffirm that the Ca2+ transient observed upon ATP addition was NCX-mediated, we used KB-R7943 (KBR), a potent reverse mode inhibitor of NCX. KBR has also been shown to inhibit the forward mode of NCX1 completely at 30–50 µM (Iwamoto et al., 1996; Kim et al., 2005), which is relevant for the present study. We observed a significant decrease in the Ca2+ transport into the NE lumen upon addition of 50 µM KBR to thapsigargin-pretreated nuclei (Fig. 6C). We also observed that under the same conditions, there was no significant effect of KBR on the Na+ transport. Iwamoto et al. (Iwamoto et al., 1996) have shown that KBR did not significantly influence the activities of Na+/K+-ATPase and other ion transporters. Although KBR is known to affect other ion pathways at these higher concentrations (Iwamoto et al., 1996; Kim et al., 2005), the simplest explanation for observations in this study is NCX inhibition.
Discussion
Our results show that both components of the Na pump heterodimer (i.e. α- and β-subunits) are assembled and trafficked to the NE of HEK293 cells. Specifically, the Na pump appears to exclusively reside within the inner membrane of NE. Similarly, we identified that the NCX also resides within this membrane compartment. We demonstrated that the membrane permeable, Na pump-specific inhibitor, strophanthidin abolished ATP-dependent Na+ transport into the NE lumen, confirming that the Na pump functions intracellularly. Moreover, this Na+/K+-ATPase activity is functionally coupled to NCX mediated Ca2+ extrusion from the nucleoplasm as strophanthidin also inhibits ATP-dependent Ca2+ uptake into the NE lumen. These findings suggest that the coupled actions of Na pump and NCX play an important role in nuclear calcium homeostasis.
Intracellular localized Na+/K+-ATPase
Since its discovery over 50 years ago, the Na/K ATPase has been described almost exclusively as a PM transporter. Indeed, this has been accepted to the point that its presence in high amounts has been used as a staple to confirm PM enrichment in cellular membrane preparations (Ohlendieck et al., 1991; Pohl et al., 2005) as well as PM localization of other membrane proteins (Wallace et al., 2010). Although it is clear that the principal location for Na+/K+-ATPase is within the PM, our results indicate that the Na+/K+-ATPase is specifically targeted to, and has an important function within, intracellular membranes, at least in some cell types.
Using heterologous expression in insect cells, we previously reported that in order for the Na pump to escape the ER, it required assembly of the α-β complex (Gatto et al., 2001). Recently, it has been shown in COS cells that the Na pump α-subunit physically interacts with β-coatomer protein (β-COP) shortly after translation and threading into the ER, and is targeted for degradation (Morton et al., 2010). In order to ‘rescue’ the α-subunit from ER degradation, its interactions with β-COP must be replaced with an association between the Na pump α- and β-subunits (Morton et al., 2010). Interestingly, we observed that not only was α-β assembly required for proper trafficking of the complex, but that assembly immediately produced a significant ouabain-sensitive Na pump in ER microsomes (Gatto et al., 2001). We suggested that the Na pump might be working in the ER in vivo, but were unsure what physiological purpose might be served by this function. Similarly, the gastric H+/K+-ATPase functions while sequestered within intracellular vesicles and the resulting acidic environment in these vesicles activates thiophilic sulfenamides (e.g. omeprazole) to specifically inhibit the H+/K+-ATPase (Swarts et al., 1996; Lambrecht et al., 1998).
Earlier reports regarding organellar localized Na+/K+-ATPase (Gatto et al., 2001; Garner, 2002) did not elaborate on the physiological role of intracellular pumps. Indeed, there have been several reports revealing apparent Na pump localization within the NE, but, in most instances, the observations were either not elaborated upon or dismissed. Below we discuss some published manuscripts that appear to have clear nuclear Na pump.
In order to study the effects of dopamine on the trafficking of Na pump, Bertorello and co-workers (Bertorello et al., 2003) expressed GFP-tagged α-subunit in A549 cells. They observed that dopamine enhanced Na pump mobility towards the PM, except for ‘perinuclear’ Na pump (see figure 3 in Bertorello et al., 2003). Although the observed perinuclear Na+/K+-ATPase may be adjacent, immobile ER Na pump, it may also simply be enzyme targeted to the NE. Similarly, work from the Jorgensen laboratory (Kristensen et al., 2003) studying protein kinase A and C mediated Na pump trafficking in COS-1 cells observed that EGFP-tagged α-subunit produced discernible intracellular labeling. Specifically, they observed an intense fluorescence of Na pump-EGFP near nuclei, which they ‘presumed’ corresponded to ER and Golgi (see figure 3 in Kristensen et al., 2003); we suggest that these data reveal a NE targeted Na pump. In these two examples, the investigators were monitoring the trafficking of GFP-tagged α-subunits, which one might argue could be erroneously trafficked due to the foreign tag. However, Delprat and co-workers (Delprat et al., 2007) also show what appears to be perinuclear Na pump in both the stria vascularis of the inner ear (see figure 2 in Delprat et al., 2007) and in PC-12 cells (see figure 3 in Delprat et al., 2007) via immunocytochemistry with anti-Na pump α-subunit antibody. In the examples given here, as well as others not mentioned, one can see Na pump which appears to be perinuclear. However, given the much larger PM than inner NE membrane, the preponderant localization will always appear to be the PM and the much fainter staining near the nucleus was usually ignored as investigators did not suspect that the enzyme may function there.
Interestingly, two previous reports acknowledge the existence of nuclear Na pump (Garner, 2002; Liu et al., 2004). In isolated hepatic cell nuclei, it was shown that the Na pump resided and functioned within the inner NE (Garner, 2002). Here we observed the same localization and function in kidney cells. One functional difference is that ouabain appeared to inhibit hepatic nuclear Na+/K+-ATPase activity, whereas in our experiments only the membrane permeable cardiotonic steroid, strophanthidin, was able to inhibit nuclear Na+/K+-ATPase (compare figure 4 and table 4 in Garner, 2002 with Fig. 5 here). The difference between tissues may be due to the hepatic membranes being slightly permeable to ouabain, whereas HEK293 nuclei membranes were not. This would allow ouabain access to the NE lumen and therefore to the extracytoplasmic face of the Na+/K+-ATPase in hepatic cells, but not HEK293 cells (Fig. 7). Whether this reflects an actual tissue difference or is introduced during the nuclei isolation procedure remains to be determined.
Fig. 7.
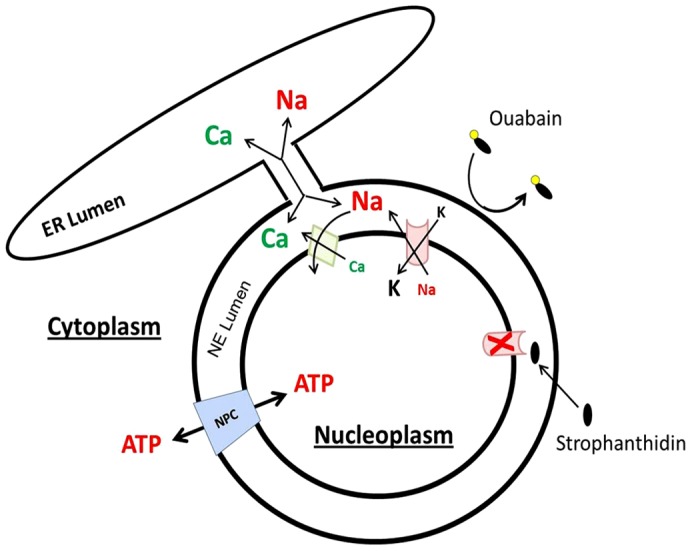
Cartoon showing that the ER lumen is freely diffusible with the space between the inner and outer membranes of the NE (NE lumen). The nuclear pore complexes (NPCs) allow substances to equilibrate between the cytoplasm and nucleoplasm, but the rate of diffusion into and out of the nucleus remains uncertain. In our nuclear flux experiments, the NPCs were used to allow ATP access to the nucleoplasm and the intracellular surface of the Na+/K+-ATPase (red cylinder with concave cardiotonic steroid binding site within the NE lumen). The sugar moiety on ouabain (black oval with yellow circle) significantly decreases its membrane permeability and thus cannot gain access to the intraluminal binding site, whereas the aglycone strophanthidin, diffuses into the NE lumen to block the Na+/K+-ATPase (red ‘X’ signifies a blocked Na+/K+-ATPase). The NCX (green diamond) also resides within the inner NE and uses the high Na+ concentration in the NE lumen (maintained by nuclear Na+/K+-ATPase) to energize Ca2+ uptake from the nucleoplasm to the NE lumen.
In experiments to elucidate the ouabain-receptor cell signaling properties of the Na pump in LLC-PK1 cells, Liu and co-workers observed increased nuclear localization of Na pump after ouabain treatment (Liu et al., 2004). They demonstrated that the enzyme present within the NE was identical to that within the PM and hypothesized that it may function as an ion transporter or transcriptional regulator (or both). Although, we did not investigate the signaling properties of nuclear Na pump, we did observe efficient Na+ transport that contributes to secondary active transport of nuclear Ca2+, which likely plays a role in transcriptional regulation.
Na+/K+-ATPase and nuclear Ca2+ homeostasis
Calcium regulates many facets of cell function and this is especially evident in excitable tissues such as nerves and muscle. Intracellular Ca2+ levels are tightly regulated through several homeostatic mechanisms. These mechanisms apply not only to extracellular Ca2+ whose entry and exit are controlled by a series of channels and pumps, but also to intracellular stores such as mitochondria (Moreau and Parekh, 2008; Szabadkai and Duchen, 2008), ER (Mikoshiba, 2007) and Golgi (Sepúlveda et al., 2008). In addition, reports of Ca2+ signaling systems in the nucleus have substantially increased over the past two decades. Recent studies have clearly implicated that gene transcription and cell cycle progression are directly influenced by nucleoplasmic Ca2+ signals (Hardingham et al., 1997; Nicotera and Rossi, 1994; Thompson et al., 2003). However, whether nuclear Ca2+ signals are generated autonomously within nuclei or follow cytoplasmic Ca2+ signals remains controversial (Bootman et al., 2009; Gerasimenko and Gerasimenko, 2004). Nonetheless, in either case it is becoming clear that nucleoplasmic Ca2+ removal occurs directly across the inner membrane of the NE. In keeping with this, several proteins involved in calcium signaling have been observed within the NE including: SERCA (Humbert et al., 1996), NCX (Xie et al., 2002; Xie et al., 2004), phosphoinositol lipid kinases (Divecha and Irvine, 1995), ryanodine receptor (Xie et al., 2004), IP3 receptor (Santella and Kyozuka, 1997), and now the Na pump. Moreover, the NE has been suggested to function as a Ca2+ storage organelle, i.e. the ‘nucleoplasmic reticulum’, in some cells (Bootman et al., 2009; Fedirko et al., 2009).
Given the size of nuclear pores, why are autonomous Ca2+ transporters required in the NE? It has been argued that the rate of Ca2+ diffusion from the nucleus to the cytoplasm is sufficiently slow, which makes local nuclear [Ca2+] changes physiologically relevant (Al-Mohanna et al., 1994; Badminton et al., 1996) and several studies have reported differentially regulated Ca2+ gradients between cytosol and nucleoplasm (Gerasimenko et al., 2003; Ledeen and Wu, 2006; Marchenko and Thomas, 2006). Localization of a NCX splice variant to the inner NE (Ledeen and Wu, 2007) contributes to selective reduction of nucleoplasmic Ca2+. Given the high Ca2+ concentration within the nucleoplasmic reticulum, Ca2+ removal from the nucleoplasm is ‘uphill’ and thus NCX requires a Na+ gradient to energize this active transport. Here we have demonstrated that both the Na pump and NCX reside within the NE and that the Na pump functions to load the nucleoplasmic reticulum with Na+. More importantly, consistent with a Na+/K+-ATPase driven Na/Ca exchange, we observed that specific inhibition of the Na+/K+-ATPase with the membrane permeable strophanthidin blocked Ca2+ uptake into the nucleoplasmic reticulum.
In conclusion, our study clearly demonstrates that the Na pump plays an important intracellular physiological role by loading the nucleoplasmic reticulum with Na+; this Na+-gradient then drives NCX-mediated Ca2+ extrusion from the nucleoplasm in kidney cells. The work of Garner (Garner, 2002) suggests that the Na pump may play a similar role in liver cells. Moreover, given that several different cell lines appear to have Na pump residing in the NE, this intracellular Na pump function may be a widespread phenomenon of cell physiology. Investigations are underway to determine whether Na pump plays a similar role in other cell types. High intracellular Ca2+ concentrations have been implicated in DNA fragmentation and apoptosis (Ramirez-Ortega et al., 2007), which may suggest a cytoprotective role conferred by Na pump-dependent NCX transport in the nucleus.
Materials and Methods
Plasmid construction, cell culture, and transfection
Sheep Na+/K+-ATPase α and β open reading frames were PCR amplified from pFASTBAC dual-Na+/K+-ATPase plasmid, which was cloned and characterized as described previously (Gatto et al., 2001; Laughery et al., 2003; Laughery et al., 2004). For heterologous expression of both α- and β-subunits concomitantly, Na pump α-mcherry and β-fragments were cloned into pBud v4 (Invitrogen, Carlsbad, CA) into NotI/KpnI and SalI/XbaI sites, respectively. HEK293 cells were grown in Dulbecco's Modified Eagles Medium supplemented with 10% (v/v) fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA), 1% (v/v) pencillin-streptomycin solution (10,000 U/ml penicillin and 10 mg/ml streptomycin), and 4 mM L-glutamine in a humidified CO2 (5%) incubator at 37°C. HEK293 cells were transfected with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to manufacturer's instructions. The expression of our fluorescently-tagged Na+/K+-ATPase constructs was documented 48–72 hours after transfection. Stably transfected cells were selected with 250 µg/ml Zeocin.
Isolation of nuclei from HEK293 cells
Nuclei were isolated from HEK293 cells as described by O'Malley et al. (O'Malley et al., 2003). Briefly, cells were grown to confluence (∼2–3 days), harvested by scraping, and pelleted via centrifugation at 500×g for 5 minutes. The pellet was resuspended in 10 volumes of Buffer A (in mM): 2 MgCl2, 25 KCl, 10 HEPES pH 7.5 and 1× protease inhibitor cocktail (Proteoblock, Fermentas, Glen Burnie, MD). Cells were lysed via dounce homogenization (Wheaton glass homogenizer, 20–30 strokes) after incubation on ice for 15 minutes. The homogenate was overlaid on 1.1 M sucrose in buffer A and centrifuged at 800×g for 10 minutes. The nuclear pellet was resuspended in 0.25 M sucrose in buffer A and overlaid again on 1.1 M sucrose in buffer A and centrifuged at 800×g for 10 minutes for higher purity of the nuclear fraction. The final nuclear pellet was resuspended in Buffer N (0.25 M sucrose in Buffer A).
Immunocytochemistry
Na pump anti-α, Na pump anti-β, mouse anti-NPP, mouse anti-calnexin (Santa Cruz Biotechnology, CA) antibodies were used for staining HEK293 cells and their isolated nuclei. For staining, HEK293 cells or isolated nuclei were allowed to attach to poly-L-lysine coated coverslips overnight. Cells were allowed to grow to 60–80% confluency. Cells were briefly washed with 1× PBS and incubated with 4% paraformaldehyde for 30 minutes in a humidified 37°C chamber. Fixed cells were permeabilized with 0.2% Triton X-100 for 15 minutes followed by blocking with 2% BSA supplemented with 0.05% Triton X-100 for 1 hour. Permeabilized cells or isolated nuclei were incubated with primary antibody overnight at 4°C followed by three washes with 1× PBS for 10 minutes each. Alexa Fluor 488 or 568 conjugated secondary antibody (Invitrogen, Carlsbad, CA) incubation was done at room temperature for 2 hours followed by three washes with 1× PBS for 10 minutes each. Primary and secondary antibodies were diluted in PBS supplemented with 1% BSA (for staining cells and isolated nuclei) and 0.01% Triton X-100 (cells only). The coverslip with cells or nuclei was then mounted in the Prolong Gold mounting medium (Invitrogen, Carlsbad, CA) and imaged under a confocal laser scanning microscope (SP2; Leica) equipped with a 63× oil immersion objective.
Na+/K+-ATPase assay
ATP hydrolysis was measured as reported previously (Gatto et al., 1997). Briefly, the Na+/K+-ATPase assay contained 500 µl of assay medium containing (in mM) 0.6 EGTA, 156 NaCl, 24 KCl, 3.6 MgCl2, 3.6 ATP, 60 imidazole (pH 7.2), 10 sodium azide, and 100 µl of the nuclear membrane preparation containing 10 µg of protein. The assay mixture was incubated at 37°C for 30 minutes, and the phosphate released was determined colorimetrically (absorbance at 850 nm) as a molybdenum complex (Brotherus et al., 1981). Specific Na+/K+-ATPase activity in this ‘broken’ membrane preparation was the difference between the ATP hydrolysis measured in the absence and presence of 0.5 mM ouabain.
Separation of outer NE from inner NE
Outer NE was separated from inner NE in isolated nuclei from HEK293 cells following citric acid extraction as described in Gilchrist et al. (Gilchrist et al., 1993). Briefly, frozen stock of nuclei was diluted in nuclei resuspension buffer containing (in mM): 2 MgCl2, 25 KCl, 10 HEPES (pH 7.5), 250 sucrose, and 1% citric acid to 3 mg/ml and incubated on ice for 15 minutes. The fraction containing inner NE was pelleted at 1000×g for 10 minutes in a microcentrifuge (Eppendorf model 5417R). The nuclear pellet containing inner NE was resuspended in nuclei resuspension buffer to 2 mg/ml. 5–10 µg of protein was solubilized with Laemmli sample buffer (1∶1∶1 v/v 8 M urea, 10% SDS, 125 mM Tris-HCl, pH 6.8 with 5% v/v β-mercaptoethanol) to give a final volume of 50 µl. The supernatant containing the outer NE fraction was precipitated with 10% (v/v) trichloroacetic acid.
SDS PAGE and western blotting
Inner and outer nuclear membrane fractions were loaded on a 10% SDS-PAGE Laemmli gel (Laemmli, 1970). After electrophoresis, proteins were transferred onto PVDF membranes via electroblotting in 10 mM CAPS, 10% (v/v) MeOH, pH 11.0, for 2 hours at a constant current of 180 mAmp (Matsudaira, 1987). The PVDF membrane was blocked with soymilk (Galva et al., 2012) for 15 minutes and then incubated with 1∶5000 mouse anti-Na pump β-subunit antibody (Affinity Bioreagents Inc.) or 1∶1000 goat anti-lamin (Santa Cruz Biotech), for 1 hour at room temperature. After three washes with PBS containing 0.1% Tween-20 (v/v), the membrane was incubated with HRP-conjugated secondary antibody at 1∶5000 (Pierce, Rockford, IL) for 1 hour at room temperature. The membrane was then washed three times with PBS containing 0.1% Tween-20 and the proteins were visualized via chemiluminescent detection of peroxidase activity using the SuperSignal substrate kit (Pierce, Rockford, IL).
Fluorimetric measurements
ATP-dependent Na+ transport
Isolated nuclei from HEK293 cells were incubated with 20 µM membrane permeable fluorescent Na+ indicator CoroNa-Green AM (Invitrogen, Carlsbad, CA). CoroNa-Green AM suspension was prepared according to manufacturer's instructions. Subsequently, nuclei resuspended in the Na+ assay buffer containing (in mM): 145 KCl, 10 NaCl, 3 MgCl2, 20 Tris-HCl (pH 7.2), were allowed to incubate with CoroNa-Green AM for 2 hours at 4°C. Nuclei were then washed in Na+ assay buffer three to four times. Fluorescence measurements of CoroNa-loaded nuclei were taken with a PTI fluorescence spectrometer (Photon Technology International, Princeton, NJ) with constant stirring in a quartz cuvette. Excitation and emission intensities of CoroNa peaked at 494 nm and 516 nm, respectively at room temperature. Background (basal) fluorescence in CoroNa-loaded nuclei was determined for the first 3 minutes, after which 5 µl of 100 mM MgATP (final [ATP] = 1 mM) was added and the fluorescence change was documented every minute for 5 minutes. For pretreatment of nuclei with cardiac glyocosides, 0.5 mM ouabain (membrane impermeable) or strophanthidin (membrane permeable) was added and incubated for at least 1 hour before taking fluorescence measurements. Displayed data shows background subtracted fluorescence levels gathered and analyzed using FelixGx software (PTI, Birmingham, NJ).
ATP-dependent, SERCA-independent, Ca2+ transport
Isolated nuclei from HEK293 cells, resuspended in Ca2+ assay buffer containing (in mM): 140 KCl, 10 NaCl, 0.1 EGTA, 0.075 CaCl2, 10 HEPES (pH 7.2), were incubated with 20 µM Fluo-5N AM (Invitrogen, Carlsbad, CA), a Ca2+ sensitive indicator, for 3–4 hours at 4°C. Subsequently, nuclei were washed in Ca2+ assay buffer three times. Fluorescence measurements were taken as described for CoroNa loaded nuclei. Fluo-5N fluorescence was measured at single excitation and emission wavelengths of 494 nm and 516 nm, respectively at room temperature. In addition to 0.5 mM ouabain or strophanthidin pretreatments, all Fluo-5N loaded nuclei were pretreated with 2 µM thapsigargin to inhibit SERCA-mediated Ca2+ transport.
Acknowledgments
We thank Dr Heinrich Matthies (Vanderbilt University) for technical assistance with the preliminary immunocytochemistry images of isolated nuclei. We also thank Dr Mark Milanick (University of Missouri-Columbia) and Dr Lauren Saunders (Illinois State University) for helpful discussions on the manuscript.
Footnotes
Funding
This work was supported by the National Institutes of Health [grant numbers GM-061583 and DK-83859 to C.G.]. Deposited in PMC for release after 12 months.
References
- Al–Mohanna F. A., Caddy K. W. T., Bolsover S. R. (1994). The nucleus is insulated from large cytosolic calcium ion changes. Nature 367, 745–750 10.1038/367745a0 [DOI] [PubMed] [Google Scholar]
- Badminton M. N., Campbell A. K., Rembold C. M. (1996). Differential regulation of nuclear and cytosolic Ca2+ in HeLa cells. J. Biol. Chem. 271, 31210–31214 10.1074/jbc.271.49.31210 [DOI] [PubMed] [Google Scholar]
- Bertorello A. M., Komarova Y., Smith K., Leibiger I. B., Efendiev R., Pedemonte C. H., Borisy G., Sznajder J. I. (2003). Analysis of Na+,K+-ATPase motion and incorporation into the plasma membrane in response to G protein-coupled receptor signals in living cells. Mol. Biol. Cell 14, 1149–1157 10.1091/mbc.E02-06-0367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco G., Mercer R. W. (1998). Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am. J. Physiol. 275, F633–F650 [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Zhang J., Chen L., Song H., Raina H., Kinsey S. P., Izuka M., Iwamoto T., Kotlikoff M. I., Lingrel J. B.et al. (2009). The pump, the exchanger, and endogenous ouabain: signaling mechanisms that link salt retention to hypertension. Hypertension 53, 291–298 10.1161/HYPERTENSIONAHA.108.119974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman M. D., Fearnley C., Smyrnias I., MacDonald F., Roderick H. L. (2009). An update on nuclear calcium signalling. J. Cell Sci. 122, 2337–2350 10.1242/jcs.028100 [DOI] [PubMed] [Google Scholar]
- Brotherus J. R., Møller J. V., Jørgensen P. L. (1981). Soluble and active renal Na, K-ATPase with maximum protein molecular mass 170,000 +/- 9,000 daltons; formation of larger units by secondary aggregation. Biochem. Biophys. Res. Commun. 100, 146–154 10.1016/S0006-291X(81)80075-7 [DOI] [PubMed] [Google Scholar]
- Cala S. E., Ulbright C., Kelley J. S., Jones L. R. (1993). Purification of a 90-kDa protein (Band VII) from cardiac sarcoplasmic reticulum. Identification as calnexin and localization of casein kinase II phosphorylation sites. J. Biol. Chem. 268, 2969–2975 [PubMed] [Google Scholar]
- Delprat B., Schaer D., Roy S., Wang J., Puel J-L., Geering K.2007). FXYD6 is a novel regulator of Na,K-ATPase expressed in the inner ear. J. Biol. Chem. 2827450–7456 10.1074/jbc.M609872200 [DOI] [PubMed] [Google Scholar]
- Divecha N., Irvine R. F.1995). Phospholipid signaling. Cell 80269–278 10.1016/0092-8674(95)90409-3 [DOI] [PubMed] [Google Scholar]
- Dunbar L. A., Caplan M. J. (2000). The cell biology of ion pumps: sorting and regulation. Eur. J. Cell Biol. 79, 557–563 10.1078/0171-9335-00079 [DOI] [PubMed] [Google Scholar]
- Fedirko N., Gerasimenko J. V., Tepikin A. V., Gerasimenko O. V. (2009). Regulation of early response genes in pancreatic acinar cells: external calcium and nuclear calcium signalling aspects. Acta Physiol. (Oxf.) 195, 51–60 10.1111/j.1748-1716.2008.01935.x [DOI] [PubMed] [Google Scholar]
- Galva C., Gatto C., Milanick M. (2012). Soymilk: an effective and inexpensive blocking agent for immunoblotting. Anal. Biochem. 426, 22–23 10.1016/j.ab.2012.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. H. (2002). Na,K-ATPase in the nuclear envelope regulates Na+: K+ gradients in hepatocyte nuclei. J. Membr. Biol. 187, 97–115 10.1007/s00232-001-0155-5 [DOI] [PubMed] [Google Scholar]
- Gatto C., Lutsenko S., Kaplan J. H. (1997). Chemical modification with Dihydro-4,4′-diisothiocyanostilbene-2,2′-disulfonate reveals the distance between K480and K501in the ATP-Binding domain of the Na,K-ATPase. Arch. Biochem. Biophys. 340, 90–100 10.1006/abbi.1997.9879 [DOI] [PubMed] [Google Scholar]
- Gatto C., McLoud S. M., Kaplan J. H. (2001). Heterologous expression of Na(+)-K(+)-ATPase in insect cells: intracellular distribution of pump subunits. Am. J. Physiol. Cell Physiol. 281, C982–C992 [DOI] [PubMed] [Google Scholar]
- Geering K. (2001). The functional role of beta subunits in oligomeric P-type ATPases. J. Bioenerg. Biomembr. 33, 425–438 10.1023/A:1010623724749 [DOI] [PubMed] [Google Scholar]
- Gerasimenko O., Gerasimenko J. (2004). New aspects of nuclear calcium signalling. J. Cell Sci. 117, 3087–3094 10.1242/jcs.01295 [DOI] [PubMed] [Google Scholar]
- Gerasimenko O. V., Gerasimenko J. V., Tepikin A. V., Petersen O. H. (1995). ATP-dependent accumulation and inositol trisphosphate- or cyclic ADP-ribose-mediated release of Ca2+ from the nuclear envelope. Cell 80, 439–444 10.1016/0092-8674(95)90494-8 [DOI] [PubMed] [Google Scholar]
- Gerasimenko J. V., Maruyama Y., Yano K., Dolman N. J., Tepikin A. V., Petersen O. H., Gerasimenko O. V. (2003). NAADP mobilizes Ca2+ from a thapsigargin-sensitive store in the nuclear envelope by activating ryanodine receptors. J. Cell Biol. 163, 271–282 10.1083/jcb.200306134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist J. S., Pierce G. N. (1993). Identification and purification of a calcium-binding protein in hepatic nuclear membranes. J. Biol. Chem. 268, 4291–4299 [PubMed] [Google Scholar]
- Guan L-S., Rauchman M., Wang Z-Y. (1998). Induction of Rb-associated protein (RbAp46) by Wilms' tumor suppressor WT1 mediates growth inhibition. J. Biol. Chem. 273, 27047–27050 10.1074/jbc.273.42.27047 [DOI] [PubMed] [Google Scholar]
- Hardingham G. E., Chawla S., Johnson C. M., Bading H. (1997). Distinct functions of nuclear and cytoplasmic calcium in the control of gene expression. Nature 385, 260–265 10.1038/385260a0 [DOI] [PubMed] [Google Scholar]
- Holtbäck U., Brismar H., DiBona G. F., Fu M., Greengard P., Aperia A. (1999). Receptor recruitment: a mechanism for interactions between G protein-coupled receptors. Proc. Natl. Acad. Sci. USA 96, 7271–7275 10.1073/pnas.96.13.7271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert J. P., Matter N., Artault J. C., Köppler P., Malviya A. N. (1996). Inositol 1,4,5-trisphosphate receptor is located to the inner nuclear membrane vindicating regulation of nuclear calcium signaling by inositol 1,4,5-trisphosphate. Discrete distribution of inositol phosphate receptors to inner and outer nuclear membranes. J. Biol. Chem. 271, 478–485 [DOI] [PubMed] [Google Scholar]
- Iwamoto T., Watano T., Shigekawa M. (1996). A novel isothiourea derivative selectively inhibits the reverse mode of Na+/Ca2+ exchange in cells expressing NCX1. J. Biol. Chem. 271, 22391–22397 10.1074/jbc.271.37.22391 [DOI] [PubMed] [Google Scholar]
- Kaplan J. H. (2002). Biochemistry of Na,K-ATPase. Annu. Rev. Biochem. 71, 511–535 10.1146/annurev.biochem.71.102201.141218 [DOI] [PubMed] [Google Scholar]
- Kim M. Y., Seol G. H., Liang G. H., Kim J. A., Suh S. H. (2005). Na+-K+ pump activation inhibits endothelium-dependent relaxation by activating the forward mode of Na+/Ca2+ exchanger in mouse aorta. Am. J. Physiol. Heart Circ. Physiol. 289, H2020–H2029 10.1152/ajpheart.00908.2004 [DOI] [PubMed] [Google Scholar]
- Kristensen B., Birkelund S., Jorgensen P. L. (2003). Trafficking of Na,K-ATPase fused to enhanced green fluorescent protein is mediated by protein kinase A or C. J. Membr. Biol. 191, 25–36 10.1007/s00232-002-1043-3 [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 10.1038/227680a0 [DOI] [PubMed] [Google Scholar]
- Lambrecht N., Corbett Z., Bayle D., Karlish S. J. D., Sachs G. (1998). Identification of the site of inhibition by omeprazole of a alpha-beta fusion protein of the H,K-ATPase using site-directed mutagenesis. J. Biol. Chem. 273, 13719–13728 10.1074/jbc.273.22.13719 [DOI] [PubMed] [Google Scholar]
- Laughery M. D., Todd M. L., Kaplan J. H. (2003). Mutational analysis of alpha-beta subunit interactions in the delivery of Na,K-ATPase heterodimers to the plasma membrane. J. Biol. Chem. 278, 34794–34803 10.1074/jbc.M302899200 [DOI] [PubMed] [Google Scholar]
- Laughery M. D., Todd M. L., Kaplan J. H. (2004). Oligomerization of the Na,K-ATPase in cell membranes. J. Biol. Chem. 279, 36339–36348 10.1074/jbc.M402778200 [DOI] [PubMed] [Google Scholar]
- Ledeen R. W., Wu G. (2006). GM1 ganglioside: another nuclear lipid that modulates nuclear calcium. GM1 potentiates the nuclear sodium-calcium exchanger. Can. J. Physiol. Pharmacol. 84, 393–402 10.1139/y05-133 [DOI] [PubMed] [Google Scholar]
- Ledeen R. W., Wu G. (2007). Sodium-calcium exchangers in the nucleus: an unexpected locus and an unusual regulatory mechanism. Ann. N. Y. Acad. Sci. 1099, 494–506 10.1196/annals.1387.057 [DOI] [PubMed] [Google Scholar]
- Lingrel J. B. (2010). The physiological significance of the cardiotonic steroid/ouabain-binding site of the Na,K-ATPase. Annu. Rev. Physiol. 72, 395–412 10.1146/annurev-physiol-021909-135725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Shapiro J. I. (2007). Regulation of sodium pump endocytosis by cardiotonic steroids: Molecular mechanisms and physiological implications. Pathophysiology 14, 171–181 10.1016/j.pathophys.2007.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Kesiry R., Periyasamy S. M., Malhotra D., Xie Z., Shapiro J. I. (2004). Ouabain induces endocytosis of plasmalemmal Na/K-ATPase in LLC-PK1 cells by a clathrin-dependent mechanism. Kidney Int. 66, 227–241 10.1111/j.1523-1755.2004.00723.x [DOI] [PubMed] [Google Scholar]
- Lutsenko S., Kaplan J. H. (1995). Organization of P-type ATPases: significance of structural diversity. Biochemistry 34, 15607–15613 10.1021/bi00048a001 [DOI] [PubMed] [Google Scholar]
- Marchenko S. M., Thomas R. C. (2006). Nuclear Ca2+ signalling in cerebellar Purkinje neurons. Cerebellum 5, 36–42 10.1080/14734220600554438 [DOI] [PubMed] [Google Scholar]
- Matsudaira P. (1987). Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J. Biol. Chem. 262, 10035–10038 [PubMed] [Google Scholar]
- Meier P. J., Sztul E. S., Reuben A., Boyer J. L. (1984). Structural and functional polarity of canalicular and basolateral plasma membrane vesicles isolated in high yield from rat liver. J. Cell Biol. 98, 991–1000 10.1083/jcb.98.3.991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier S. D., Kovalchuk Y., Rose C. R. (2006). Properties of the new fluorescent Na+ indicator CoroNa Green: comparison with SBFI and confocal Na+ imaging. J. Neurosci. Methods 155, 251–259 10.1016/j.jneumeth.2006.01.009 [DOI] [PubMed] [Google Scholar]
- Mikoshiba K. (2007). IP3 receptor/Ca2+ channel: from discovery to new signaling concepts. J. Neurochem. 102, 1426–1446 10.1111/j.1471-4159.2007.04825.x [DOI] [PubMed] [Google Scholar]
- Moreau B., Parekh A. B. (2008). Ca2+-dependent inactivation of the mitochondrial Ca2+ uniporter involves proton flux through the ATP synthase. Curr. Biol. 18, 855–859 10.1016/j.cub.2008.05.026 [DOI] [PubMed] [Google Scholar]
- Morth J. P., Pedersen B. P., Buch–Pedersen M. J., Andersen J. P., Vilsen B., Palmgren M. G., Nissen P. A. (2011). A structural overview of the plasma membrane Na+,K+-ATPase and H+-ATPase ion pumps. Nat. Rev. Mol. Cell Biol. 12, 60–70 10.1038/nrm3031 [DOI] [PubMed] [Google Scholar]
- Morton M. J., Farr G. A., Hull M., Capendeguy O., Horisberger J-D., Caplan M. J. (2010). Association with β-COP regulates the trafficking of the newly synthesized Na,K-ATPase. J. Biol. Chem. 285, 33737–33746 10.1074/jbc.M110.141119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotera P., Rossi A. D. (1994). Nuclear Ca2+: physiological regulation and role in apoptosis. Mol. Cell. Biochem. 135, 89–98 10.1007/BF00925964 [DOI] [PubMed] [Google Scholar]
- O'Malley K. L., Jong Y-J., Gonchar Y., Burkhalter A., Romano C. (2003). Activation of metabotropic glutamate receptor mGlu5 on nuclear membranes mediates intranuclear Ca2+ changes in heterologous cell types and neurons. J. Biol. Chem. 278, 28210–28219 10.1074/jbc.M300792200 [DOI] [PubMed] [Google Scholar]
- Ohlendieck K., Ervasti J. M., Matsumura K., Kahl S. D., Leveille C. J., Campbell K. P. (1991). Dystrophin-related protein is localized to neuromuscular junctions of adult skeletal muscle. Neuron 7, 499–508 10.1016/0896-6273(91)90301-F [DOI] [PubMed] [Google Scholar]
- Ohsako S., Bunick D., Hess R. A., Nishida T., Kurohmaru M., Hayashi Y. (1994). Characterization of a testis specific protein localized to endoplasmic reticulum of spermatogenic cells. Anat. Rec. 238, 335–348 10.1002/ar.1092380308 [DOI] [PubMed] [Google Scholar]
- Pohl J., Ring A., Korkmaz U., Ehehalt R., Stremmel W. (2005). FAT/CD36-mediated long-chain fatty acid uptake in adipocytes requires plasma membrane rafts. Mol. Biol. Cell 16, 24–31 10.1091/mbc.E04-07-0616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polvani C., Blostein R. (1988). Protons as substitutes for sodium and potassium in the sodium pump reaction. J. Biol. Chem. 263, 16757–16763 [PubMed] [Google Scholar]
- Rajasekaran S. A., Palmer L. G., Quan K., Harper J. F., Ball W. J., Jr, Bander N. H., Peralta Soler A., Rajasekaran A. K. (2001). Na,K-ATPase beta-subunit is required for epithelial polarization, suppression of invasion, and cell motility. Mol. Biol. Cell 12, 279–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran S. A., Beyenbach K. W., Rajasekaran A. K. (2008). Interactions of tight junctions with membrane channels and transporters. Biochim. Biophys. Acta 1778, 757–769 10.1016/j.bbamem.2007.11.007 [DOI] [PubMed] [Google Scholar]
- Ramirez–Ortega M., Zarco G., Maldonado V., Carillo J. F., Ramos P., Ceballos G., Melendez Z. J., Garcia N., Zazueta C., Chanona J.et al. (2007). Is digitalis compound-induced cardiotoxicity, mediated through guinea-pig cardiomyocytes apoptosis? Eur. J. Pharmacol. 566, 34–42 10.1016/j.ejphar.2007.03.033 [DOI] [PubMed] [Google Scholar]
- Santella L., Kyozuka K. (1997). Effects of 1-methyladenine on nuclear Ca2+ transients and meiosis resumption in starfish oocytes are mimicked by the nuclear injection of inositol 1,4,5-trisphosphate and cADP-ribose. Cell Calcium 22, 11–20 10.1016/S0143-4160(97)90085-3 [DOI] [PubMed] [Google Scholar]
- Sepúlveda M. R., Marcos D., Berrocal M., Raeymaekers L., Mata A. M., Wuytack F. (2008). Activity and localization of the secretory pathway Ca2+-ATPase isoform 1 (SPCA1) in different areas of the mouse brain during postnatal development. Mol. Cell. Neurosci. 38, 461–473 10.1016/j.mcn.2008.02.012 [DOI] [PubMed] [Google Scholar]
- Song H., Lee M. Y., Kinsey S. P., Weber D. J., Blaustein M. P. (2006). An N-terminal sequence targets and tethers Na+ pump α2 subunits to specialized plasma membrane microdomains. J. Biol. Chem. 281, 12929–12940 10.1074/jbc.M507450200 [DOI] [PubMed] [Google Scholar]
- Swarts H. G. P., Klaassen C. H. W., de Boer M., Fransen J. A. M., De Pont J. J. H. H. M. (1996). Role of negatively charged residues in the fifth and sixth transmembrane domains of the catalytic subunit of gastric H+,K+-ATPase. J. Biol. Chem. 271, 29764–29772 10.1074/jbc.271.47.29764 [DOI] [PubMed] [Google Scholar]
- Szabadkai G., Duchen M. R. (2008). Mitochondria: the hub of cellular Ca2+ signaling. Physiology (Bethesda) 23, 84–94 10.1152/physiol.00046.2007 [DOI] [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr (1989). Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J. Biol. Chem. 264, 12266–12271 [PubMed] [Google Scholar]
- Thompson M., Andrade V. A., Andrade S. J., Pusl T., Ortega J. M., Goes A. M., Leite M. F. (2003). Inhibition of the TEF/TEAD transcription factor activity by nuclear calcium and distinct kinase pathways. Biochem. Biophys. Res. Commun. 301, 267–274 10.1016/S0006-291X(02)03024-3 [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. (1979). Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76, 4350–4354 10.1073/pnas.76.9.4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagin O., Turdikulova S., Yakubov I., Sachs G. (2005). Use of the H,K-ATPase beta subunit to identify multiple sorting pathways for plasma membrane delivery in polarized cells. J. Biol. Chem. 280, 14741–14754 10.1074/jbc.M412657200 [DOI] [PubMed] [Google Scholar]
- Van Dyke R. W. (2004). Heterotrimeric G protein subunits are located on rat liver endosomes. BMC Physiol. 4, 4 http://www.biomedcentral.com/1472-6793/4/1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace D. F., Harris J. M., Subramaniam V. N. (2010). Functional analysis and theoretical modeling of ferroportin reveals clustering of mutations according to phenotype. Am. J. Physiol. Cell Physiol. 298, C75–C84 10.1152/ajpcell.00621.2008 [DOI] [PubMed] [Google Scholar]
- Wu G., Xie X., Lu Z-H., Ledeen R. W. (2009). Sodium-calcium exchanger complexed with GM1 ganglioside in nuclear membrane transfers calcium from nucleoplasm to endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 106, 10829–10834 10.1073/pnas.0903408106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., Wu G., Lu Z-H., Ledeen R. W. (2002). Potentiation of a sodium-calcium exchanger in the nuclear envelope by nuclear GM1 ganglioside. J. Neurochem. 81, 1185–1195 10.1046/j.1471-4159.2002.00917.x [DOI] [PubMed] [Google Scholar]
- Xie X., Wu G., Ledeen R. W. (2004). C6 cells express a sodium-calcium exchanger/GM1 complex in the nuclear envelope but have no exchanger in the plasma membrane: comparison to astrocytes. J. Neurosci. Res. 76, 363–375 10.1002/jnr.20068 [DOI] [PubMed] [Google Scholar]
- Zima A. V., Bare D. J., Mignery G. A., Blatter L. A. (2007). IP3-dependent nuclear Ca2+ signalling in the mammalian heart. J. Physiol. 584, 601–611 10.1113/jphysiol.2007.140731 [DOI] [PMC free article] [PubMed] [Google Scholar]