A novel dominant-negative technology aids analysis of peptide hormones in plants.
Abstract
In recent years, peptide hormones have been recognized as important signal molecules in plants. Genetic characterization of such peptides is challenging since they are usually encoded by small genes. As a proof of concept, we used the well-characterized stem cell-restricting CLAVATA3 (CLV3) to develop an antagonistic peptide technology by transformations of wild-type Arabidopsis (Arabidopsis thaliana) with constructs carrying the full-length CLV3 with every residue in the peptide-coding region replaced, one at a time, by alanine. Analyses of transgenic plants allowed us to identify one line exhibiting a dominant-negative clv3-like phenotype, with enlarged shoot apical meristems and increased numbers of floral organs. We then performed second dimensional amino acid substitutions to replace the glycine residue individually with the other 18 possible proteinaceous amino acids. Examination of transgenic plants showed that a glycine-to-threonine substitution gave the strongest antagonistic effect in the wild type, in which over 70% of transgenic lines showed the clv3-like phenotype. Among these substitutions, a negative correlation was observed between the antagonistic effects in the wild type and the complementation efficiencies in clv3. We also demonstrated that such an antagonistic peptide technology is applicable to other CLV3/EMBRYO SURROUNDING REGION (CLE) genes, CLE8 and CLE22, as well as in vitro treatments. We believe this technology provides a powerful tool for functional dissection of widely occurring CLE genes in plants.
In animals, small peptides are important signal molecules in neural and endocrinal systems (Feld and Hirschberg, 1996; Edlund and Jessell, 1999). In recent years, over a dozen different types of peptide hormones have been identified in plants, regulating both developmental and adaptive responses, usually through interacting with Leu-rich repeat receptor kinases localized in plasma membranes of neighboring cells (Boller and Felix, 2009; De Smet et al., 2009; Katsir et al., 2011). These peptides are often produced from genes with small open reading frames, after posttranslational processing (Matsubayashi, 2011). In addition, peptide hormones, such as CLAVATA3/EMBRYO SURROUNDING REGION (CLV3/ESR [CLE]), systemin, PHYTOSULFOKINE, AtPEP1, and EPIDERMAL PATTERNING FACTOR1 (EPF1), often have paralogs in genomes (Cock and McCormick, 2001; Yang et al., 2001; Pearce and Ryan, 2003; Huffaker et al., 2007; Hara et al., 2007). Bioinformatics analyses revealed that the Arabidopsis (Arabidopsis thaliana) genome contains 33,809 small open reading frames (Lease and Walker, 2006).
CLV3 acts as a secreted 12- or 13-amino acid glycosylated peptide (Kondo et al., 2006; Ohyama et al., 2009) to restrict the number of stem cells in shoot apical meristems (SAMs), through a CLV1-CLV2-SOL2 (for SUPPRESSOR OF LLP1 2, also called CORYNE)-RECEPTOR-LIKE PROTEIN KINASE2 (RPK2) receptor kinase-mediated pathway (Clark et al., 1993; Jeong et al., 1999; Miwa et al., 2008; Müller et al., 2008; Kinoshita et al., 2010; Zhu et al., 2010). All CLE family members, of which there are 83 in Arabidopsis and 89 in rice (Oryza sativa), carry a putative signal peptide and share a conserved 12-amino acid core CLE motif (Oelkers et al., 2008). Overexpression of CLE genes often shows a common dwarf and short-root phenotype (Strabala et al., 2006; Jun et al., 2010), which may not reflect their endogenous functions. Due to redundancies and difficulties in identifying mutants of these small genes, studies of CLE members are challenging. Only a few CLE genes have been genetically characterized, in particular, CLV3, CLE8, CLE40, and CLE41 in Arabidopsis and FLORAL ORGAN NUMBER4 (FON4), FON2-LIKE CLE PROTEIN1 (FCP1), and FON2 SPARE1 in rice (Fletcher et al., 1999; Hobe et al., 2003; Chu et al., 2006; Suzaki et al., 2008, 2009; Etchells and Turner, 2010; Fiume and Fletcher, 2012), while functions of other CLE members remain unknown.
As a proof of concept, we used the well-characterized CLV3 gene to develop an antagonistic peptide technology for functionally dissecting CLE family members in Arabidopsis. A series of constructs carrying Ala substitutions in every amino acid residue in the core CLE motif of CLV3, expressed under the endogenous CLV3 regulatory elements, were made and introduced to wild-type Arabidopsis by transformation. This allowed us to identify the conserved Gly residue in the middle of the CLE motif was vulnerable for generating the dominant-negative clv3-like phenotype. We then performed second dimensional amino acid substitutions to replace the Gly with all other 18 possible proteinaceous amino acids, one at a time, and observed that the substitution of the Gly residue by Thr generated the strongest dominant-negative clv3-like phenotype. Further experiments showed that this technology can potentially be applied to in vitro-synthesized peptides and for functional characterization of other CLE members.
RESULTS AND DISCUSSION
Transgenic Plants Carrying CLV3 with the Gly-to-Ala Substitution in the Core CLE Motif Showed a Dominant-Negative clv3-Like Phenotype
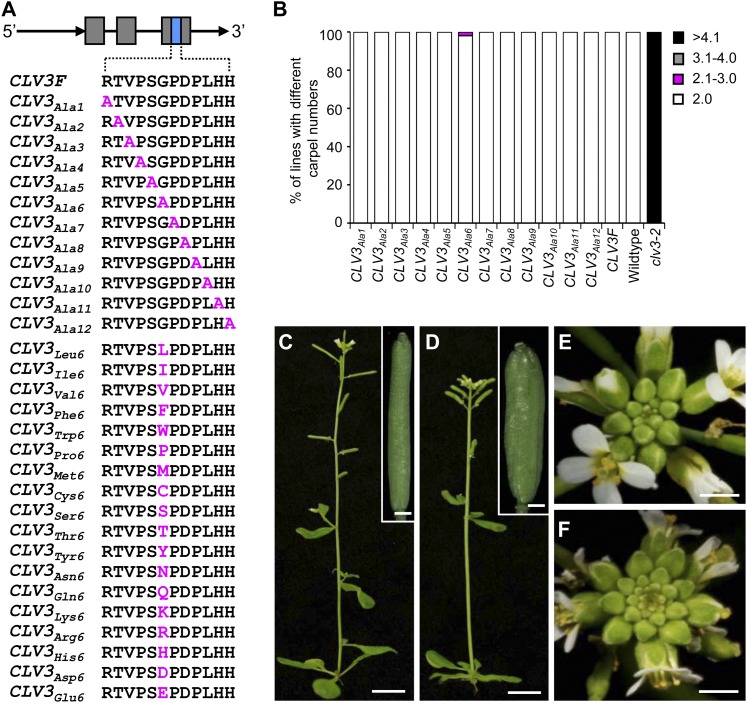
The full-length 3,932-bp CLV3 genomic sequence including a 1,857-bp 5′-upstream sequence, a 559-bp coding region, and a 1,516-bp 3′-downstream sequence was amplified (named CLV3F) and cloned as previously described (Song et al., 2012). Using CLV3F as the template, 12 constructs (named CLV3Ala1–12) were made via PCR-based site-directed mutagenesis to replace each of the 12 residues with Ala, one at a time, in the core CLE motif of CLV3 (Fig. 1A; Song et al., 2012), and transformed into wild-type Arabidopsis (ecotype Landsberg erecta [Ler]) to obtain at least 30 independent transgenic plants for each construct. We screened these transgenic plants for the multicarpel clv3 mutant-like phenotype. As controls, wild-type and transgenic plants carrying CLV3F consistently showed two-carpel siliques (Fig. 1B). Although most plants carrying CLV3Ala1–12 showed no phenotype, we identified that one plant carrying CLV3Ala6, with the Gly residue at the position 6 substituted by Ala, exhibited multiple carpels (three to four carpels) in siliques (Fig. 1, B–D), resembling a weak clv3 phenotype. Sequencing of the endogenous CLV3 locus from the transgenic plant revealed no mutations, suggesting that the observed phenotype is not likely due to contamination from a recessive clv3 mutant.
Figure 1.
Amino acid-substituted CLV3 constructs and transgenic analyses in the wild type. A, Constructs made for two-dimensional substitutions of CLV3. A full-length CLV3 genomic sequence (CLV3F) including endogenous regulatory elements was used as the template for first dimensional Ala substitutions in the 12-amino acid peptide-coding region of CLV3 (named CLV3Ala1–12) and second dimensional substitutions of the Gly residue at position 6 with all other 18 possible proteinaceous amino acids. B, Effects of 12 Ala-substituted CLV3s in generating dominant-negative clv3-like multicarpel phenotypes in the wild type. For each Ala-substituted CLV3, at least 30 transformants were obtained. The wild-type (Ler), clv3-2, and CLV3F transgenic plants were used as controls. C and D, Wild-type plant (C) with two-carpel siliques (inset in C) and the CLV3Ala6 transgenic plant (D) with multicarpel siliques (inset in D). Bars = 10 mm for C and D and 0.7 mm for the insets. E and F, Inflorescences of the wild type (E) and the BC1 plant of the CLV3Ala6 transgenic line (F). Bars = 2 mm.
The CLV3Ala6 transgenic line was backcrossed to the wild type, and BC1 plants still exhibited the clv3-like phenotype (Fig. 1, E and F), suggesting a dominant trait. Phenotypic analyses in the T2 generation showed that the phenotype can be stably transmitted. Transgenic plants carrying the remaining 11 constructs did not give the clv3-like phenotype (Fig. 1B), suggesting that the Gly residue in the CLE motif is vulnerable in creating the antagonistic effect. It is plausible that peptides produced by the CLV3Ala6 transgene are able to compete with the endogenous CLV3 peptides antagonistically to bind the CLV1-CLV2-SOL2-RPK2 receptor kinases and then block downstream signal transduction.
Optimization of the Antagonistic Peptide Technology through Second Dimensional Amino Acid Substitutions
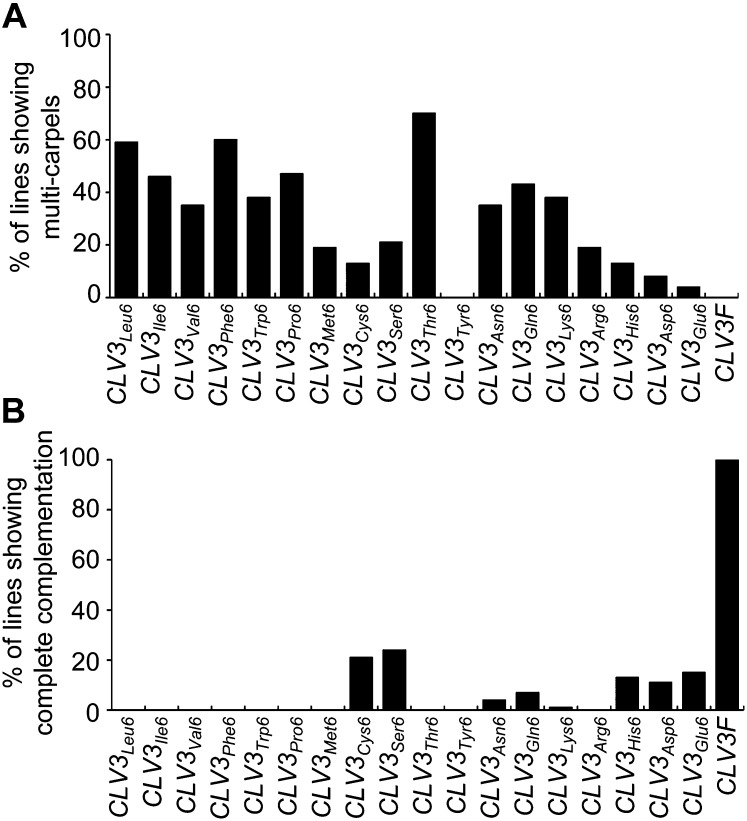
Since the Gly-to-Ala substitution in CLV3 generated only a mild antagonistic effect in the wild type (Fig. 1B), we performed second dimensional amino acid substitutions by replacing the Gly residue, one at a time, with all other 18 possible proteinaceous amino acids through site-directed mutagenesis (Fig. 1A). All constructs, CLV3Leu6, CLV3Ile6, CLV3Val6, CLV3Phe6, CLV3Trp6, CLV3Pro6, CLV3Met6, CLV3Cys6, CLV3Ser6, CLV3Thr6, CLV3Tyr6, CLV3Asn6, CLV3Gln6, CLV3Lys6, CLV3Arg6, CLV3His6, CLV3Asp6, and CLV3Glu6, were transformed into wild-type Arabidopsis (Ler), and at least 30 independent transformants were obtained for each construct. For each T1 transgenic plant, 15 siliques (five from the bottom, five from the middle, and five from the top of the inflorescence) were excised and examined under a dissection microscope for carpel numbers. Efficiencies of these constructs in creating the dominant-negative clv3-like phenotype are summarized in Figure 2A, based on examination of over 700 independent transgenic lines. Strikingly, all these constructs, excluding CLV3Tyr6, were able to produce some plants with the clv3-like phenotype (Fig. 2A). Plants carrying CLV3Thr6 showed the highest antagonistic effect, with approximately 70% of the CLV3Thr6 transgenic lines exhibiting the multicarpel phenotype (Figs. 2A and 3A). Constructs of CLV3Phe6, CLV3Leu6, CLV3Pro6, CLV3Ile6, CLV3Gln6, CLV3Lys6, CLV3Trp6, CLV3Val6, and CLV3Asn6 gave a moderate antagonistic effect, in which 35% to 60% of lines showed the clv3-like phenotype (Fig. 2A). The other seven constructs, including CLV3Ser6, CLV3Met6, CLV3Arg6, CLV3Cys6, CLV3His6, CLV3Asp6, and CLV3Glu6, gave only a weak antagonistic effect (Fig. 2A). Transformation of CLV3Tyr6 led to no antagonistic effect, and all 102 CLV3Tyr6 transgenic lines examined resembled wild-type plants. Results from phenotypic analyses in 43 individual transgenic plants carrying CLV3Thr6 are shown in Figure 3A. The carpel numbers per silique varied from two to seven among different transgenic lines (Fig. 3, A and B). No two-carpel siliques were observed in some lines, such as numbers 8, 11, 21, and 38, suggesting that there was a very strong antagonistic effect (Fig. 3A). Further analyses revealed increased numbers of flower buds in inflorescences (Fig. 3C), and enlarged SAMs in seedlings (Fig. 3D), which resemble the clv3 phenotype (Fig. 3, C and D). By comparison to constructs that gave high percentages of plants with the clv3-like phenotype, we noticed that substitutions of the Gly residue with other nonpolar amino acids carrying a longer side chain, such as Leu, Ile, Val, Phe, Tyr, and Pro, were more likely to generate the antagonistic effect (Fig. 2A). Interaction analyses between these antagonistic peptides and CLV1, CLV2, SOL2, and RPK2 receptor proteins may help to further understand the observed antagonistic effect.
Figure 2.
Antagonistic effects and complementation efficiencies of second dimensional amino acid-substituted constructs. A, Effects of 18 second dimensional amino acid-substituted CLV3 in generating the multicarpel clv3-like phenotype in the wild type. B, Effects of second dimensional amino acid-substituted CLV3 in complementing clv3-2 mutants. For each amino acid-substituted CLV3, at least 30 independent transformants were obtained. CLV3F was used as a control.
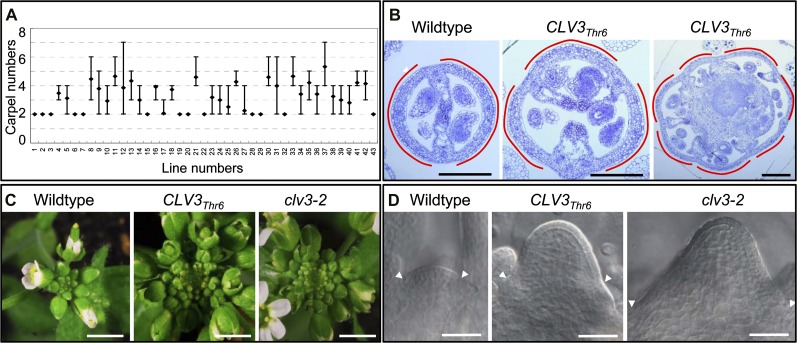
Figure 3.
Effects of CLV3Thr6 in generating the clv3-like phenotype. A, Among 43 individual plants transformed with CLV3Thr6 examined, 30 showed a multicarpel clv3-like phenotype. Note that wild-type Arabidopsis has two carpels in siliques, and the antagonistic effect was observed in transgenic lines with more than two carpels. The diamond indicates the average carpel number, while the top and bottom bars represent the most and least carpel numbers, respectively. B, Transverse sections through siliques from CLV3Thr6 transgenic plants with three (middle) and seven (right) carpels compared with the wild type (left) with two carpels. The red curves indicate the carpels. Bars = 200 µm. C, Inflorescences of wild-type, CLV3Thr6 transgenic, and clv3-2 plants. Compared with the wild type (left), inflorescences in CLV3Thr6 transgenic plants (middle) had supernumerary flowers, as observed in clv3-2 mutants (right). Bars = 3 mm. D, A DIC image showing the enlarged SAM in the CLV3Thr6 transgenic plant (middle) compared with those from the wild type (left) and clv3-2 (right). Arrowheads indicate the margins of SAMs. Bars = 50 µm.
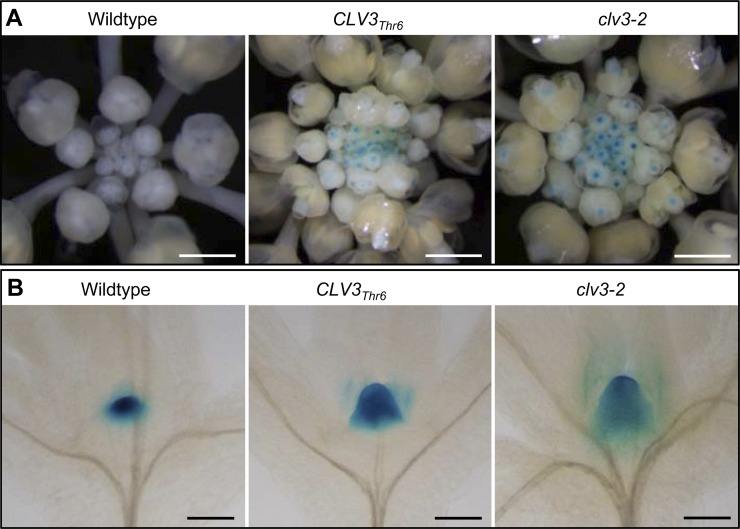
To elucidate if the CLV3Thr6 transgene indeed interferes with stem cell maintenance in SAMs, we crossed the CLV3Thr6 transgenic plant with the stem cell reporter line pCLV3::GUS (Lenhard et al., 2002) and examined the GUS expression in the F2 generation. We observed a prolonged CLV3 expression in floral buds (Fig. 4A) and an enlarged CLV3 expression domain in SAMs (Fig. 4B), as in clv3-2 (Fig. 4), indicating that stem cell maintenance in these CLV3Thr6 transgenic plants was indeed disturbed. This result confirmed that the phenotype produced by the CLV3Thr6 transgene in wild-type background is similar to the phenotype of clv3.
Figure 4.
GUS expression in inflorescences and SAMs of the wild-type, CLV3Thr6 transgenic, and clv3-2 plants carrying pCLV3::GUS. A, GUS expression in inflorescences. Compared with the wild type (left), extended GUS expression were observed in flower buds of CLV3Thr6 transgenic plants (center), as in clv3-2 (right). Pictures were taken under a dissection microscope. Bars = 3 mm. B, GUS expression in SAMs. Compared with the wild type (left), the GUS expression domain was significantly enlarged in the SAM of CLV3Thr6 transgenic plants (center), similar to clv3-2 (right). Pictures were taken under a DIC microscope after clearing. Bars = 150 µm.
To exclude the possibility that the clv3-like phenotype of CLV3Thr6 transgenic plants resulted from cosuppression of CLV3, we examined the expression of endogenous CLV3 in CLV3Thr6 transgenic plants with real-time quantitative PCR. Shoot apices were dissected from seedlings of wild-type (Ler) and CLV3Thr6 transgenic plants in parallel for RNA extraction. Real-time quantitative PCRs were performed using the same forward primer and two reverse primers in which two 3′ terminal nucleotides were unique to discriminate between the endogenous CLV3 and the CLV3Thr6 transgene. No significant reduction of endogenous CLV3 expression was observed in CLV3Thr6 transgenic plants when compared with that in Ler wild type (P = 0.182; Supplemental Fig. S1), suggesting that there is no cosuppression occurring. Notably, the expression level of the CLV3Thr6 transgene was higher than that of endogenous CLV3 in the transgenic line (Supplemental Fig. S1).
Complementation Efficiencies of Second Dimensional Substitution Constructs in clv3-2
To characterize the relationship between antagonistic effects in the wild type and complementation efficiencies in clv3-2, we transformed the second dimensional substitution constructs described above into clv3-2 mutants and analyzed their complementation efficiencies. The results showed that approximately 20% to 30% of T1 transgenic plants carrying CLV3Ser6 orCLV3Cys6 (Fig. 2B), and 10% to 20% of lines carrying CLV3His6, CLV3Asp6, or CLV3Glu6 exhibited complete complementation, producing plants with two-carpel siliques (Fig. 2B). Less than 10% of transgenic lines carrying CLV3Gln6, CLV3Asn6, or CLV3His6 showed complete complementation (Fig. 2B). No complementation was observed in transgenic clv3-2 plants carrying CLV3Leu6, CLV3Ile6, CLV3Val6, CLV3Phe6, CLV3Trp6, CLV3Pro6, CLV3Met6, CLV3Thr6, CLV3Tyr6, or CLV3Arg6 (Fig. 2B), suggesting that these substitutions disrupted the function of CLV3 completely.
A somewhat negative correlation was observed between the efficiencies of the antagonistic effect in the wild-type (Fig. 2A) and the complementation effect in clv3-2 (Fig. 2B) in the second dimensional substitution constructs. In particular, the CLV3Thr6 construct produced the strongest antagonistic effect in the wild type but showed no complementation in clv3-2 (Fig. 2, A and B), whereas CLV3Glu6 showed the weakest antagonistic effect in the wild type but a relatively high complementation efficiency in clv3-2 (Fig. 2, A and B). This negative correlation would be expected if peptides produced by constructs with dominant-negative effect bind more strongly with the CLV3 receptors than the endogenous one but fail to execute signal transduction, thereby producing the observed antagonistic effect. For the same reason, such a construct should have a reduced complementation capacity in clv3-2. The CLV3Tyr6 construct was an exception to this observed trend, as neither an antagonistic nor a complementation effect was observed (Fig. 2, A and B). It is possible that the CLV3Tyr6 peptide produced with the Gly-to-Tyr substitution lost both of the activities of interacting with CLV3 receptors and executing downstream signal transduction.
The reason that the substitutions of the Gly residue with other amino acids gave rise to the antagonistic effect in the wild type remains to be elucidated. Studies in mouse have shown that substitution of the most critical residue in the immunogenic Hb(64-76) peptide resulted in a complete loss of downstream responses in T cells, while substitutions of two secondarily important residues created antagonistic effects (Evavold et al., 1993). Our previous in vivo complementation experiments for Ala-substituted CLV3 (Song et al., 2012) have ranked this Gly residue as the third most important one for complementing clv3-2 among the 12 residues in the core CLE motif. Substitution of the Gly with other nonpolar amino acids with a longer side chain would restrict the rotation of the peptide produced, which may in turn lead to stronger receptor binding. It is plausible that the Gly-to-Thr substitution in CLV3 led to a nonfunctional yet strong receptor-binding peptide, preventing downstream signal transduction, and thereby generating the antagonistic effect.
The Antagonistic Effect in Vitro
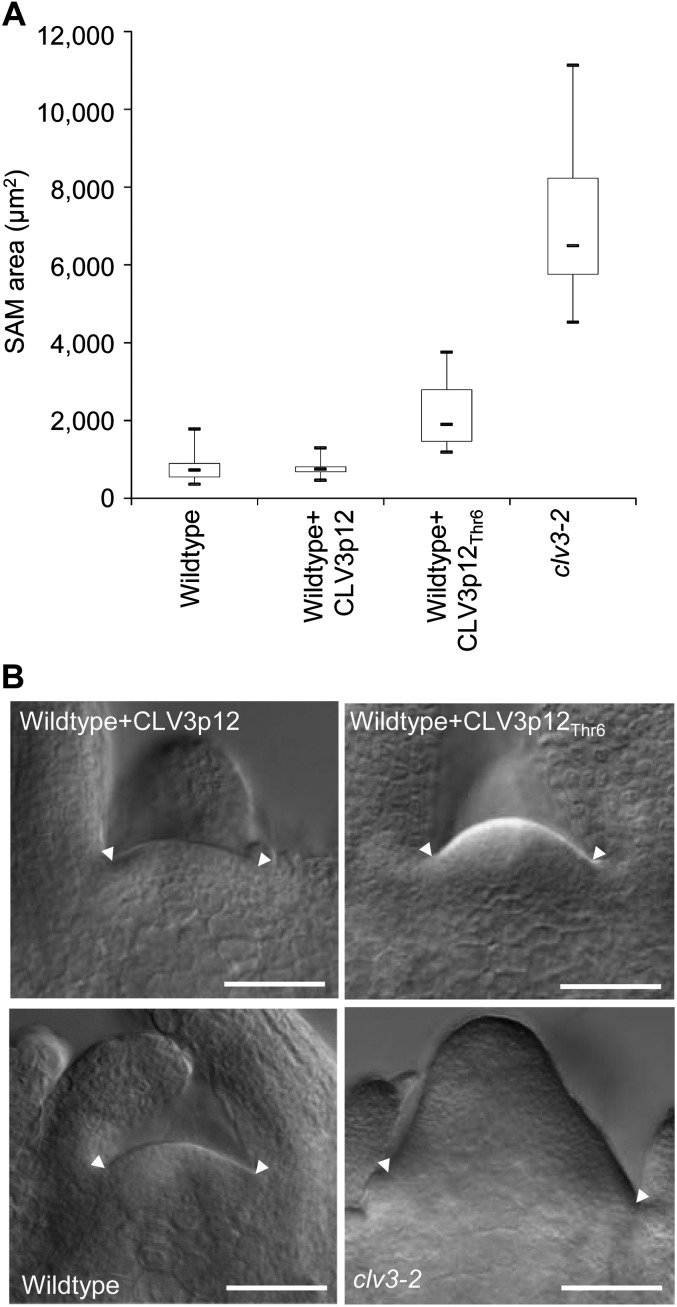
It has been reported previously that synthetic 12- to 14-amino acid peptides corresponding to the CLE motif of CLV3 are functional in vitro in complementing clv3-2 (Fiers et al., 2005; Kondo et al., 2006; Ohyama et al., 2009). To investigate if the antagonistic effect also occurs in vitro, synthetic 12-amino acid CLV3 peptides with the same Gly residue replaced by Thr (named CLV3p12Thr6) were applied to Ler wild-type seedlings in a liquid culture at a concentration of 10 µm, as previously described (Song et al., 2012). To prevent the potential degradation of the applied peptides, media with fresh peptides was replaced every day during the 9-d treatment. Under a differential interference contrast (DIC) microscope, enlarged SAMs were observed in CLV3p12Thr6-treated seedlings, while no enlargements were observed in the control samples incubated in media without peptide (Fig. 5, A and B). A slight reduction of SAM size was observed in seedlings treated with normal CLV3 peptides (named CLV3p12), as reported previously (Kinoshita et al., 2007; Fig. 5, A and B). This result confirmed the presence of the antagonistic effect of the CLV3p12Thr6 peptide in vitro. We noticed that the sizes of SAMs in CLV3p12Thr6-treated seedlings were much smaller than those in transgenic plants carrying CLV3Thr6 (Figs. 3D and 5B), which suggests that the peptides applied in vitro were less effective than the peptides produced in vivo by the transgene.
Figure 5.
Enlarged SAMs observed in wild-type seedlings treated with synthetic CLV3p12Thr6 peptides in vitro. A, Box plots of the areas of SAMs in wild-type seedlings treated with CLV3p12, or CLV3p12Thr6 peptides, compared with those in the wild-type and clv3-2 seedlings. Areas of SAMs were measured with ImageJ software after pictures of median sections were taken under a DIC microscope. B, DIC images of shoot apices from wild-type seedlings treated with CLV3p12 (top left) or CLV3p12Thr6 (top right) for 9 d compared with untreated ones in the wild type (bottom left) and clv3-2 (bottom right). Arrowheads indicate margins of SAMs. Bars = 50 µm.
To clarify if the antagonistic effect resulted from competition between CLV3p12Thr6 and CLV3p12, we applied CLV3p12 in combination with 1, 2, and 10 times the amount of CLV3p12Thr6 to Ler wild-type seedlings. Media with fresh peptides was replaced every day, as described above. After a 9-d treatment, shoot apices were dissected and sizes of SAMs were measured under a DIC microscope. The meristem size of 500 μm2 was used to assign meristems with a significant reduction after peptide treatments. About 17% of meristems treated with CLV3p12 were above 500 µm2 in size. Under the treatments with mixed CLV3p12 and CLV3p12Thr6, significantly increased numbers of SAMs were larger than 500 µm2 (Supplemental Fig. S2). As the concentration of CLV3p12Thr6 increased, the percentage of lines with SAMs larger than 500 µm2 increased accordingly (Supplemental Fig. S2). These results suggested that CLV3p12Thr6 can compete with CLV3p12 in vitro (Supplemental Fig. S2). It is noteworthy that even when 10 times more CLV3p12Thr6 was added to the medium, a complete loss of CLV3p12 activity was still not observed. Since the endogenous mature CLV3 peptide has both hydroxylation and glycosylation modifications that contribute to the CLV3 activity (Ohyama et al., 2009; Shinohara and Matsubayashi, 2012), the competition observed in vitro may not entirely represent the in vivo status.
Application of the Antagonistic Peptide Technology to CLE8
It was reported recently that mutation or down-regulation of CLE8 in Arabidopsis leads to defective embryo and endosperm development (Fiume and Fletcher, 2012). We used CLE8 as an additional target to examine if the antagonistic peptide technology can be used for functional dissection of other CLE genes in Arabidopsis. A CLE8Thr6 construct was made, with the Gly residue at the position 6 replaced by Thr, and expressed under the native CLE8 regulatory elements including both the 5′-upstream (1,881 bp) and 3′-downstream (1,455 bp) genomic regions. We used the same Arabidopsis Columbia-0 (Col-0) ecotype as in the cle8-1 study reported before (Fiume and Fletcher, 2012) to perform this experiment to avoid potential interference from the genetic background.
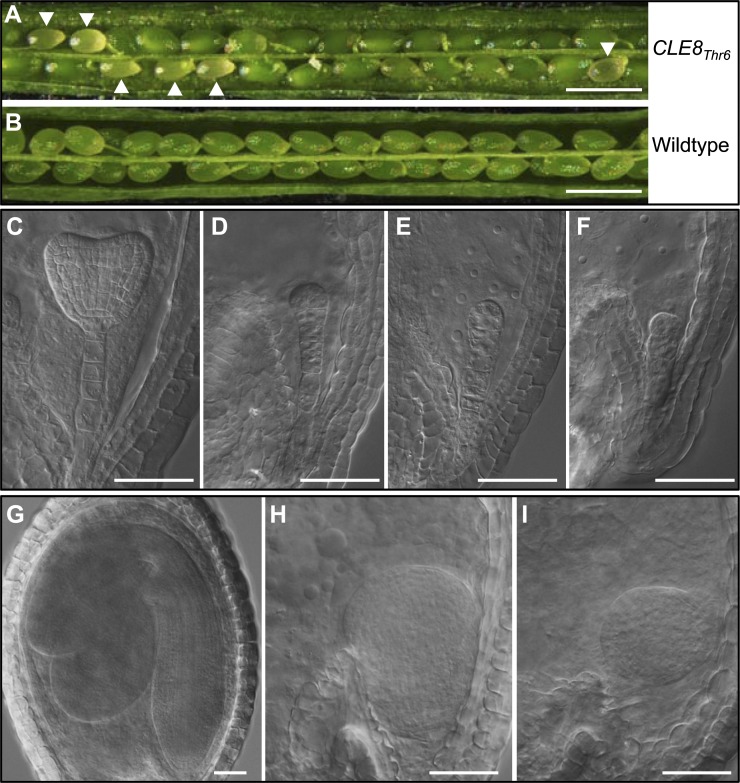
Among 78 independent CLE8Thr6 T1 transgenic lines examined, eight showed embryo-lethal phenotypes (Fig. 6, A and B). The percentages of embryo-lethal seeds in these transgenic lines varied from 6% to 30% (n > 200 each). Phenotypes of defective embryos were similar to those reported in cle8-1 mutants and CLE8 down-regulated plants (Fiume and Fletcher, 2012), with abnormal cell division in the suspensor and the lower portion of the embryo at the proglobular stage (Fig. 6, C–F). When wild-type embryos reached the cotyledonary stage (Fig. 6G), some embryos in CLE8Thr6 transgenic lines were arrested, with altered cell division patterns (Fig. 6, H and I). The embryo defect phenotype was also observed in the BC1 generation when the transgenic plants were pollinated reciprocally with the wild type, suggesting a dominant trait. This result suggested that the Gly-to-Thr substitution in CLE8 is able to mimic the cle8-1 mutant phenotype, indicating that the antagonistic peptide technology can be applied to elucidate additional CLE genes.
Figure 6.
The embryo-lethal phenotypes in CLE8Thr6 transgenic plants. A and B, An opened silique of CLE8Thr6 transgenic plant (A) showing green wild-type ovules and transparent aborted ovules (indicated by arrowheads) compared with the wild-type silique (B) at the same stage. Bars = 1 mm. C to F, In CLE8Thr6 transgenic plants, both wild-type (C) and abnormal embryos (D–F) were observed in siliques 5 d after pollination. Bars = 50 µm. G to I, A wild-type cotyledonary embryo (G) and embryos with abnormal cell division pattern (H and I) in the same silique from a CLE8Thr6 transgenic plant. Bars = 50 µm.
Application of the Antagonistic Peptide Technology to CLE22
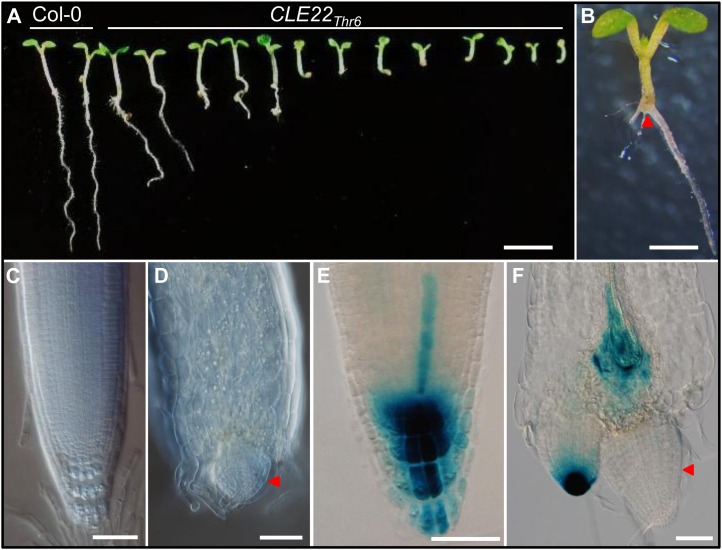
To further verify the applicability of the technology, CLE22 with unknown function was chosen as another target for the Gly-to-Thr substitution. A previous report has shown that CLE22 is expressed in differentiating vascular bundles (Jun et al., 2010). The CLE22Thr6 construct was made and transformed into the Col-0 wild-type background and expressed under the CLE22 native promoter including 1,495-bp 5′-upstream and 1,243-bp 3′-downstream sequences. In the T2 generation, we observed that some transgenic seedlings exhibited a very short root phenotype (Fig. 7, A and B). Detailed examination revealed an almost immediate termination of the root meristem (Fig. 7, C and D). We speculated that the short-root phenotype is the consequence of arrested cell division and differentiation in roots. After crossing the CLE22Thr6 transgenic plant with a DR5::GUS marker line (Ulmasov et al., 1997), we observed no GUS expression in the defective primary root meristem, suggesting the auxin maximum has disappeared. It remains to be elucidated whether the CLE22Thr6 antagonistic effect interferes directly with the auxin flow in roots or whether it interferes with vascular differentiation first and consequently disrupts auxin flow (Fig. 7, E and F).
Figure 7.
CLE22Thr6 transgenic plants exhibited a short-root phenotype. A, Progeny of one CLE22Thr6 transgenic plant, showing different degrees of the short-root phenotype. Bar = 5 mm. B, A seedling of a CLE22Thr6 transgenic plant, showing termination of the primary root (arrowhead). Bar = 1 mm. C and D, Compared with the root meristems in Col-0 wild type (C), CLE22Thr6 transgenic plants (D) showed the aberrant root meristem (arrowhead). Bar = 50 μm. E and F, DR5::GUS expression in root meristems in Col-0 wild-type (E) and CLE22Thr6 transgenic (F) plants, showing the absence of GUS expression in the primary root meristem in the transgenic plant (arrowhead). Bar = 50 μm. Five-day-old seedlings were used for phenotypic observation and expression analyses.
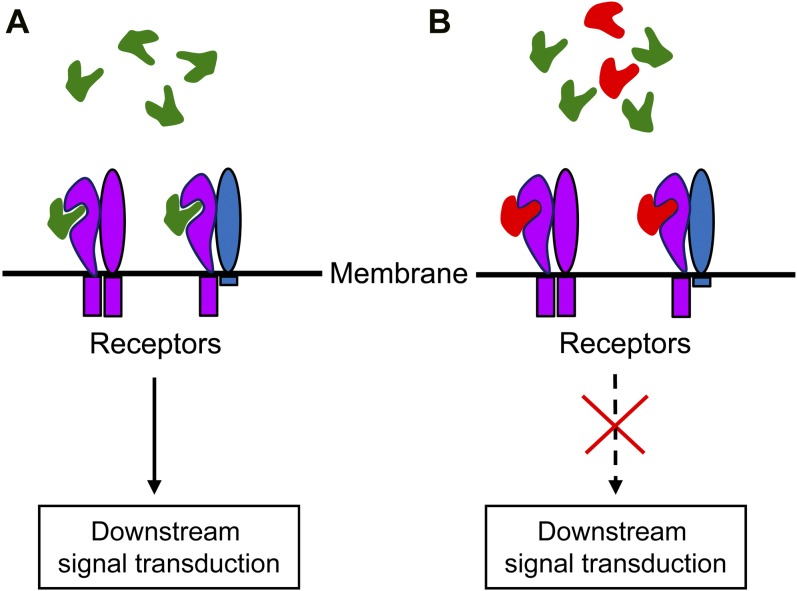
Among all proteinaceous amino acids, Gly is the most flexible one, which may give peptides a free rotation property. The Gly-to-Thr substitutions in the core CLE motif of CLV3, CLE8, and CLE22 may have compromised the flexibility of the peptides produced, leading to stronger interaction with corresponding receptors but disrupted downstream signal transduction, therefore creating the observed antagonistic effects (Fig. 8). Sequence alignment showed that the Gly residue located in the middle of the core CLE motif is highly conserved in the CLE family, with 90.4% identity among the 198 CLE members examined (Supplemental Fig. S3). The conserved Gly residue has also been found in several other types of peptide hormones, such as AtPEPs and EPFs in plants (Supplemental Fig. S4). Targeted expression of these genes with the Gly-to-Thr substitution may potentially help to characterize their functions.
Figure 8.
Schematic model of the antagonistic peptide technology. A, Endogenous CLE peptides (green) bind to its receptors, leading to downstream signal transduction. B, Peptides with the Gly-to-Thr substitution (red) bind more tightly and competitively to the receptors but fail to trigger downstream signal transduction. [See online article for color version of this figure.]
CONCLUSION
Taken together, through two-dimensional amino acid substitutions of CLV3 and expressed under its endogenous promoters in the wild type, we identified the Gly in the peptide-coding region as the vulnerable residue in creating the antagonistic effect. Among all proteinaceous amino acids tested, substitution of the Gly residue by Thr in CLV3 gave the strongest dominant clv3 mutant-like phenotype. We further showed that the antagonistic peptide technology is effective in generating dominant-negative phenotype in CLE8 and CLE22. We believe that the technology provides a powerful tool for the functional dissection of CLEs in plants and may potentially be used in the study of other peptide hormones.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Wild-type Arabidopsis (Arabidopsis thaliana) ecotype Ler was used for all experiments except as otherwise noted. Seeds of Ler and clv3-2 (in Ler background) were surface sterilized for 2 h in a desiccator with chlorine gas, as previously reported (Fiers et al., 2005), and plated on one-half-strength Murashige and Skoog basal salt medium containing 1% Suc, 0.05% MES, and 1% agar (pH 5.8). After a 2-d vernalization at 4°C, plates were cultured in a growth room with 16 h light per day at 21°C ± 1°C. After a 5-d culture, seedlings were transferred to pots filled with 1:1 mixed soil and vermiculite and grown under the same temperature and light regime. Transformation was performed via an Agrobacterium tumefaciens-mediated floral dip method (Clough and Bent, 1998). Transgenic plants were obtained under the selection of 25 mg L−1 glufosinate ammonium (Sigma-Aldrich).
Molecular Cloning
A CLV3 genomic sequence (3,932 bp) containing 5′- and 3′-regulatory elements was cloned into a pDONR221 vector (Life Technologies) and then subcloned into a pBGWFS7 binary vector (Karimi et al., 2002) to produce the CLV3F construct. Ala-substituted CLV3 constructs, CLV3Ala1, CLV3 Ala2, CLV3Ala3, CLV3Ala4, CLV3Ala5, CLV3Ala6, CLV3Ala7, CLV3Ala8, CLV3Ala9, CLV3Ala10, CLV3Ala11, and CLV3Ala12, were made as described previously (Song et al., 2012). For the second dimensional substitutions, the Gly residue at position 6 of the core CLE motif of CLV3 was replaced by all 18 possible proteinaceous amino acids, one at a time, with a PCR-based site-directed mutagenesis kit (Transgen) to produce the constructs CLV3Leu6, CLV3Ile6, CLV3Val6, CLV3Phe6, CLV3Trp6, CLV3Pro6, CLV3Met6, CLV3Cys6, CLV3Ser6, CLV3Thr6, CLV3Tyr6, CLV3Asn6, CLV3Gln6, CLV3Lys6, CLV3Arg6, CLV3His6, CLV3Asp6, and CLV3Glu6. Full-length 3,597-bp CLE8 and a 3,050-bp CLE22 genomic sequences containing 5′ and 3′ regulatory elements were cloned into the pDONR221 vector to produce pDONR221-CLE8F and pDONR221-CLE22F, respectively. A Gly-to-Thr substitution was introduced to the CLE motif of CLE8 and CLE22 via a site-directed mutagenesis to produce the CLE8Thr6 and CLE22Thr6 constructs.
Tissue Clearing
Samples of shoot apices, ovules, and roots were cleared as described (Sabatini et al., 1999) and observed under a DIC microscope (Leica DM4500). SAM areas were measured with ImageJ software as reported previously (Fiers et al., 2005).
In Vitro Peptide Assay
CLV3p12 (RTVPSGPDPLHH) and CLV3p12Thr6 (RTVPSTPDPLHH) peptides (≥90% purity) were synthesized commercially (AuGCT Biotechnology). In vitro treatments of Ler wild-type seedlings with 10 µm CLV3p12 and CLV3p12Thr6 peptides were performed as previously described (Song et al., 2012). For competition assays, Ler wild-type seedlings were treated with different concentrations of CLV3p12Thr6 combined with 10 µm CLV3p in liquid one-half-strength Murashige and Skoog media. Media with peptides were refreshed every day. After a 9-d treatment, shoot apices were excised under a dissection microscope, cleared, and observed as previously described (Sabatini et al., 1999).
GUS Assay
Seedlings and inflorescences of Ler wild-type, clv3-2, and CLV3Thr6 transgenic plants carrying pCLV3::GUS (Lenhard et al., 2002) were examined for GUS expression as previously described (Fiers et al., 2004). Roots of Col-0 wild-type and CLE22Thr6 transgenic plants carrying DR5::GUS were examined for GUS expression as previously described (Ulmasov et al., 1997). After the dehydration with 70%, 85%, 90%, and 100% ethanol, seedlings were cleared and observed under a DIC microscope (Sabatini et al., 1999). Inflorescences were observed under a dissection microscope directly following dehydration.
Histological Analyses
Siliques of CLV3Thr6 transgenic plants were fixed in a modified formaldehyde-acetic acid solution (Liu et al., 1993) for 12 h and embedded in LR White (The London Resin Company) as described in the manufacturer’s manual. Embedded siliques were sectioned at 2-µm thickness using a microtome (Leica EM UC7) and stained with 0.1% toluidine blue.
Real-Time Quantitative PCR
An RNA isolation kit (Tiangen) was used to extract total RNA from shoot apices excised from seedlings of Ler wild-type and 30 to 40 CLV3Thr6 transgenic T2 plants. Reverse transcription was performed using a FastQuant RT kit (Tiangen). Real-time quantitative PCR was performed in a Rotor-Gene 3000 Thermocycler (Corbett Research) with a SYBR Premix ExTaq II kit (Takara). The relative expression levels were normalized against EIF4A through the use of a modified cycle threshold method (Livak and Schmittgen, 2001). The primers used are listed in Supplemental Table S1.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Real-time quantitative PCR analyses of endogenous CLV3 and CLV3Thr6 transgene expression in the wild type and CLV3Thr6 transgenic plants.
Supplemental Figure S2. In vitro competition assay.
Supplemental Figure S3. Alignments of the core CLE motifs in CLE family members.
Supplemental Figure S4. Alignments of the conserved motifs in AtPEP and EPF family members.
Supplemental Table S1. List of primers used.
Acknowledgments
We thank Da-Li Yu and Chen Li for assistance and John Hugh Snyder for critical reading of the article.
Glossary
- SAM
shoot apical meristem
- Ler
Landsberg erecta
- DIC
differential interference contrast
- Col-0
Columbia-0
References
- Boller T, Felix G. (2009) A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol 60: 379–406 [DOI] [PubMed] [Google Scholar]
- Chu H, Qian Q, Liang W, Yin C, Tan H, Yao X, Yuan Z, Yang J, Huang H, Luo D, et al. (2006) The FLORAL ORGAN NUMBER4 gene encoding a putative ortholog of Arabidopsis CLAVATA3 regulates apical meristem size in rice. Plant Physiol 142: 1039–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SE, Running MP, Meyerowitz EM. (1993) CLAVATA1, a regulator of meristem and flower development in Arabidopsis. Development 119: 397–418 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cock JM, McCormick S. (2001) A large family of genes that share homology with CLAVATA3. Plant Physiol 126: 939–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet I, Voss U, Jürgens G, Beeckman T. (2009) Receptor-like kinases shape the plant. Nat Cell Biol 11: 1166–1173 [DOI] [PubMed] [Google Scholar]
- Edlund T, Jessell TM. (1999) Progression from extrinsic to intrinsic signaling in cell fate specification: a view from the nervous system. Cell 96: 211–224 [DOI] [PubMed] [Google Scholar]
- Etchells JP, Turner SR. (2010) The PXY-CLE41 receptor ligand pair defines a multifunctional pathway that controls the rate and orientation of vascular cell division. Development 137: 767–77420147378 [Google Scholar]
- Evavold BD, Sloan-Lancaster J, Allen PM. (1993) Tickling the TCR: selective T-cell functions stimulated by altered peptide ligands. Immunol Today 14: 602–609 [DOI] [PubMed] [Google Scholar]
- Feld S, Hirschberg R. (1996) Growth hormone, the insulin-like growth factor system, and the kidney. Endocr Rev 17: 423–480 [DOI] [PubMed] [Google Scholar]
- Fiers M, Golemiec E, Xu J, van der Geest L, Heidstra R, Stiekema W, Liu CM. (2005) The 14-amino acid CLV3, CLE19, and CLE40 peptides trigger consumption of the root meristem in Arabidopsis through a CLAVATA2-dependent pathway. Plant Cell 17: 2542–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers M, Hause G, Boutilier K, Casamitjana-Martinez E, Weijers D, Offringa R, van der Geest L, van Lookeren Campagne M, Liu CM. (2004) Mis-expression of the CLV3/ESR-like gene CLE19 in Arabidopsis leads to a consumption of root meristem. Gene 327: 37–49 [DOI] [PubMed] [Google Scholar]
- Fiume E, Fletcher JC. (2012) Regulation of Arabidopsis embryo and endosperm development by the polypeptide signaling molecule CLE8. Plant Cell 24: 1000–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JC, Brand U, Running MP, Simon R, Meyerowitz EM. (1999) Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science 283: 1911–1914 [DOI] [PubMed] [Google Scholar]
- Hara K, Kajita R, Torii KU, Bergmann DC, Kakimoto T. (2007) The secretory peptide gene EPF1 enforces the stomatal one-cell-spacing rule. Genes Dev 21: 1720–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobe M, Müller R, Grünewald M, Brand U, Simon R. (2003) Loss of CLE40, a protein functionally equivalent to the stem cell restricting signal CLV3, enhances root waving in Arabidopsis. Dev Genes Evol 213: 371–381 [DOI] [PubMed] [Google Scholar]
- Huffaker A, Ryan CA. (2007) Endogenous peptide defense signals in Arabidopsis differentially amplify signaling for the innate immune response. Proc Natl Acad Sci USA 104: 10732–10736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S, Trotochaud AE, Clark SE. (1999) The Arabidopsis CLAVATA2 gene encodes a receptor-like protein required for the stability of the CLAVATA1 receptor-like kinase. Plant Cell 11: 1925–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JH, Fiume E, Roeder AHK, Meng L, Sharma VK, Osmont KS, Baker C, Ha CM, Meyerowitz EM, Feldman LJ, et al. (2010) Comprehensive analysis of CLE polypeptide signaling gene expression and overexpression activity in Arabidopsis. Plant Physiol 154: 1721–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M, Inzé D, Depicker A. (2002) GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7: 193–195 [DOI] [PubMed] [Google Scholar]
- Katsir L, Davies KA, Bergmann DC, Laux T. (2011) Peptide signaling in plant development. Curr Biol 21: R356–R364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita A, Betsuyaku S, Osakabe Y, Mizuno S, Nagawa S, Stahl Y, Simon R, Yamaguchi-Shinozaki K, Fukuda H, Sawa S. (2010) RPK2 is an essential receptor-like kinase that transmits the CLV3 signal in Arabidopsis. Development 137: 3911–3920 [DOI] [PubMed] [Google Scholar]
- Kinoshita A, Nakamura Y, Sasaki E, Kyozuka J, Fukuda H, Sawa S. (2007) Gain-of-function phenotypes of chemically synthetic CLAVATA3/ESR-related (CLE) peptides in Arabidopsis thaliana and Oryza sativa. Plant Cell Physiol 48: 1821–1825 [DOI] [PubMed] [Google Scholar]
- Kondo T, Sawa S, Kinoshita A, Mizuno S, Kakimoto T, Fukuda H, Sakagami Y. (2006) A plant peptide encoded by CLV3 identified by in situ MALDI-TOF MS analysis. Science 313: 845–848 [DOI] [PubMed] [Google Scholar]
- Lease KA, Walker JC. (2006) The Arabidopsis unannotated secreted peptide database, a resource for plant peptidomics. Plant Physiol 142: 831–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhard M, Jürgens G, Laux T. (2002) The WUSCHEL and SHOOTMERISTEMLESS genes fulfill complementary roles in Arabidopsis shoot meristem regulation. Development 129: 3195–3206 [DOI] [PubMed] [Google Scholar]
- Liu C, Xu Z, Chua NH. (1993) Auxin polar transport is essential for the establishment of bilateral symmetry during early plant embryogenesis. Plant Cell 5: 621–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT Method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Matsubayashi Y. (2011) Post-translational modifications in secreted peptide hormones in plants. Plant Cell Physiol 52: 5–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa H, Betsuyaku S, Iwamoto K, Kinoshita A, Fukuda H, Sawa S. (2008) The receptor-like kinase SOL2 mediates CLE signaling in Arabidopsis. Plant Cell Physiol 49: 1752–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R, Bleckmann A, Simon R. (2008) The receptor kinase CORYNE of Arabidopsis transmits the stem cell-limiting signal CLAVATA3 independently of CLAVATA1. Plant Cell 20: 934–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelkers K, Goffard N, Weiller GF, Gresshoff PM, Mathesius U, Frickey T. (2008) Bioinformatic analysis of the CLE signaling peptide family. BMC Plant Biol 8: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama K, Shinohara H, Ogawa-Ohnishi M, Matsubayashi Y. (2009) A glycopeptide regulating stem cell fate in Arabidopsis thaliana. Nat Chem Biol 5: 578–580 [DOI] [PubMed] [Google Scholar]
- Pearce G, Ryan CA. (2003) Systemic signaling in tomato plants for defense against herbivores. Isolation and characterization of three novel defense-signaling glycopeptide hormones coded in a single precursor gene. J Biol Chem 278: 30044–30050 [DOI] [PubMed] [Google Scholar]
- Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey PN, Leyser O, Bechtold N, Weisbeek P, et al. (1999) An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99: 463–472 [DOI] [PubMed] [Google Scholar]
- Shinohara H, Matsubayashi Y. (2012) Chemical synthesis of Arabidopsis CLV3 glycopeptide reveals the impact of Hyp arabinosylation on peptide conformation and activity. Plant Cell Physiol (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XF, Yu DL, Xu TT, Ren SC, Guo P, Liu CM. (2012) Contributions of individual amino acid residues to the endogenous CLV3 function in shoot apical meristem maintenance in Arabidopsis. Mol Plant 5: 515–523 [DOI] [PubMed] [Google Scholar]
- Strabala TJ, O’donnell PJ, Smit AM, Ampomah-Dwamena C, Martin EJ, Netzler N, Nieuwenhuizen NJ, Quinn BD, Foote HCC, Hudson KR. (2006) Gain-of-function phenotypes of many CLAVATA3/ESR genes, including four new family members, correlate with tandem variations in the conserved CLAVATA3/ESR domain. Plant Physiol 140: 1331–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzaki T, Ohneda M, Toriba T, Yoshida A, Hirano HY. (2009) FON2 SPARE1 redundantly regulates floral meristem maintenance with FLORAL ORGAN NUMBER2 in rice. PLoS Genet 5: e1000693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzaki T, Yoshida A, Hirano HY. (2008) Functional diversification of CLAVATA3-related CLE proteins in meristem maintenance in rice. Plant Cell 20: 2049–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9: 1963–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Matsubayashi Y, Nakamura K, Sakagami Y. (2001) Diversity of Arabidopsis genes encoding precursors for phytosulfokine, a peptide growth factor. Plant Physiol 127: 842–851 [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Wang Y, Li R, Song X, Wang Q, Huang S, Jin JB, Liu CM, Lin J. (2010) Analysis of interactions among the CLAVATA3 receptors reveals a direct interaction between CLAVATA2 and CORYNE in Arabidopsis. Plant J 61: 223–233 [DOI] [PubMed] [Google Scholar]