Yariv phenylglycosides specifically bind to β-1,3-galactan main chains of arabinogalactan proteins.
Abstract
Yariv phenylglycosides [1,3,5-tri(p-glycosyloxyphenylazo)-2,4,6-trihydroxybenzene] are a group of chemical compounds that selectively bind to arabinogalactan proteins (AGPs), a type of plant proteoglycan. Yariv phenylglycosides are widely used as cytochemical reagents to perturb the molecular functions of AGPs as well as for the detection, quantification, purification, and staining of AGPs. However, the target structure in AGPs to which Yariv phenylglycosides bind has not been determined. Here, we identify the structural element of AGPs required for the interaction with Yariv phenylglycosides by stepwise trimming of the arabinogalactan moieties using combinations of specific glycoside hydrolases. Whereas the precipitation with Yariv phenylglycosides (Yariv reactivity) of radish (Raphanus sativus) root AGP was not reduced after enzyme treatment to remove α-l-arabinofuranosyl and β-glucuronosyl residues and β-1,6-galactan side chains, it was completely lost after degradation of the β-1,3-galactan main chains. In addition, Yariv reactivity of gum arabic, a commercial product of acacia (Acacia senegal) AGPs, increased rather than decreased during the repeated degradation of β-1,6-galactan side chains by Smith degradation. Among various oligosaccharides corresponding to partial structures of AGPs, β-1,3-galactooligosaccharides longer than β-1,3-galactoheptaose exhibited significant precipitation with Yariv in a radial diffusion assay on agar. A pull-down assay using oligosaccharides cross linked to hydrazine beads detected an interaction of β-1,3-galactooligosaccharides longer than β-1,3-galactopentaose with Yariv phenylglycoside. To the contrary, no interaction with Yariv was detected for β-1,6-galactooligosaccharides of any length. Therefore, we conclude that Yariv phenylglycosides should be considered specific binding reagents for β-1,3-galactan chains longer than five residues, and seven residues are sufficient for cross linking, leading to precipitation of the Yariv phenylglycosides.
Arabinogalactan proteins (AGPs) are a type of plant proteoglycans consisting of a Hyp-rich core protein and large arabinogalactan (AG) moieties (Fincher et al., 1983; Nothnagel, 1997). Although there are many molecular species of AGP differentiated by their core proteins, the AG moieties commonly comprise β-1,3-galactan main chains and β-1,6-galactan side chains, to which l-Ara and other auxiliary sugars, such as GlcA, 4-O-methyl-GlcA, l-Fuc, l-Rha, and Xyl, are attached (Fincher et al., 1983; Nothnagel, 1997; Seifert and Roberts, 2007). A commercial product of AGPs prepared from the acacia (Acacia senegal) tree is known as gum arabic and utilized as a food stabilizer. In the Japanese herbal remedy Juzen-Taiho-To, AGs from Astragalus membranaceus are the active ingredient (Majewska-Sawka and Nothnagel, 2000; Kiyohara et al., 2002). In intact plants, AGPs are implicated in various physiological events and serve as extracellular constituents and signaling molecules. For instance, an AGP from stylar transmitting tissue attracts pollen tubes and stimulates their elongation in tobacco (Nicotiana tabacum; Cheung et al., 1995).
Yariv phenylglycosides [1,3,5-tri(p-glycosyloxyphenylazo)-2,4,6-trihydroxybenzene] are a group of chemical compounds that were initially developed as carbohydrate antigens for the purification of anti-glycoside antibody and sugar-binding protein (Yariv et al., 1962, 1967a). It then turned out that Yariv phenylglycosides specifically precipitate AGPs (Yariv et al., 1967b; Jermyn and Yeow, 1975). The specific interaction of AGPs with Yariv phenylglycosides forming brown-red precipitate is called Yariv reactivity and has been recognized as an important criterion in the definition of AGPs, even though a number of AGPs do not exhibit Yariv reactivity. Nevertheless, the structure involved in the interaction with Yariv phenylglycoside is presumed to be conserved in many AGPs. The interaction of Yariv phenylglycosides with AGP depends on the glycosyl residues attached to the phenylazotrihydroxybenzene core. In particular, β-glucosyl Yariv phenylglycoside (β-Glc-Yariv) and β-galactosyl Yariv phenylglycoside (β-Gal-Yariv) bind to AGPs, whereas α-glucosyl Yariv and α-galactosyl Yariv (α-Gal-Yariv) do not bind to AGPs (Jermyn and Yeow, 1975; Larkin, 1977, 1978; Nothnagel and Lyon, 1986). Because of the specific interaction with the β-glycosyl Yariv phenylglycosides (β-Yarivs), AGPs were formerly called “β-lectins” (Jermyn and Yeow, 1975; Gleeson and Jermyn, 1979; Nothnagel and Lyon, 1986).
The β-Yarivs are useful tools for staining, detection, and quantification of AGPs. Using β-Glc-Yariv, β-lectins were shown to exist in angiosperm, gymnosperm, fern, moss, and liverwort, illustrating the wide distribution of AGPs in the plant kingdom (Jermyn and Yeow, 1975; Clarke et al., 1978). In addition, β-Yarivs are also used as chemical reagents in the purification of AGPs. A nonclassical AGP, xylogen, which is a signaling molecule inducing the differentiation to tracheary elements, has been purified from the culture medium of zinnia (Zinnia elegans) cells by precipitation with β-Glc-Yariv (Motose et al., 2004). As the treatment with β-Yarivs causes the perturbation of various physiological processes in plants, β-Yarivs are reliable cytochemical reagents to explore AGP functions. Application of β-Yarivs to cultured cells of Arabidopsis (Arabidopsis thaliana) induced programmed cell death, demonstrating the involvement of AGPs in the determination of cell fate (Gao and Showalter, 1999). In tobacco cultured cells, the treatment with β-Yarivs has indicated a possible role of AGPs in the orientation of cortical microtubules and the polymerization of F-actin (Sardar et al., 2006).
Although Yariv phenylglycosides have been extensively utilized in studies of AGPs over 40 years, the identification of the target structures on AGPs required for β-Yariv reactivity remains elusive (Nothnagel, 1997; Seifert and Roberts, 2007). It has been proposed that β-Yarivs bind to the Hyp-rich core protein, based on the observation that deglycosylation treatment with hydrogen fluoride did not abolish the Yariv reactivity of gum arabic and a tobacco AGP (Akiyama et al., 1984). To the contrary, other reports have asserted the importance of the carbohydrate moieties for Yariv reactivity (Komalavilas et al., 1991). However, with regard to the specific carbohydrate structure required for interaction with β-Yarivs, the results were not always consistent: neither α-l-arabinofuranosyl residues nor β-1,6-galactan side chains were found to be involved in Yariv reactivity of AGPs from Gladiolus spp., radish (Raphanus sativus), and grape (Vitis vinifera; Gleeson and Clarke, 1979; Tsumuraya et al., 1987; Saulnier et al., 1992); partial acid hydrolysis to remove α-l-arabinofuranosyl residues diminished Yariv reactivity of a rose (Rosa spp.) AGP (Komalavilas et al., 1991); and mugwort (Artemisia vulgaris) pollen O-glycans consisting of a β-1,6-galactan core and branched α-l-arabinofuranosyl side chains precipitated with β-Glc-Yariv (Léonard et al., 2005). Accordingly, it has also been suggested that Yariv reactivity depends on the overall physical and chemical properties rather than a specific structural feature of AGPs.
In this study, we demonstrate that the peptide component of AGPs is not required for Yariv reactivity. By sequentially trimming the AG moieties of AGPs with sets of specific glycoside hydrolases, we show that β-Gal-Yariv binds to the β-1,3-galactan main chains of radish root AGP. We confirm that β-1,6-galactan side chains are not necessary for Yariv reactivity, we identify β-1,3-galactopentaose (β-1,3-Gal5) as the smallest carbohydrate structure to interact with β-Gal-Yariv, and we show that β-1,3-galactoheptaose (β-1,3-Gal7) or longer β-1,3-galactosyl chains are required for the formation of insoluble precipitate with Yariv phenylglycoside. Based on computational modeling, a possible interaction mechanism between β-Gal-Yariv and β-1,3-galactan is suggested.
RESULTS
Yariv Reactivity of AGP Core Protein
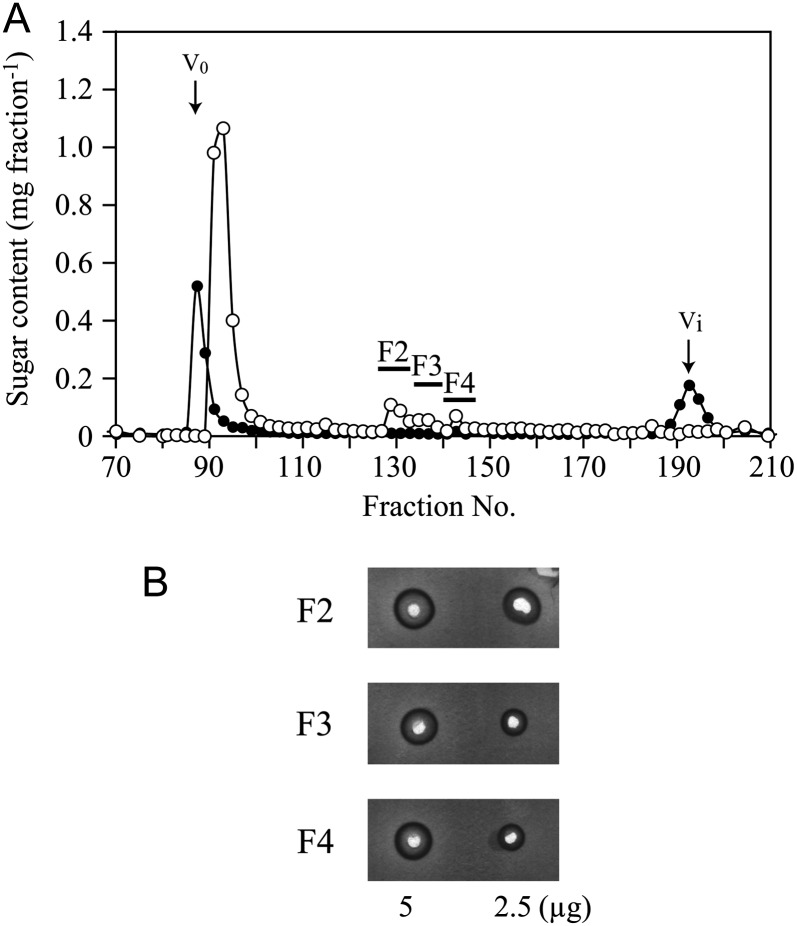
We first investigated whether the Hyp-rich core protein is responsible for the interaction with Yariv phenylglycosides, as proposed by Akiyama et al. (1984). The β-1,3-galactan main chains are attached to Hyp residues of the core protein via O-glycosidic linkages (Tan et al., 2004). The peptide linkages are susceptible to base treatment, but the glycosyl linkages are resistant; therefore, smaller AG molecules linked to Hyp residues (Hyp-AG) can be released by base hydrolysis of AGP. Base treatment of gum arabic and consecutive gel-permeation chromatography yielded three Hyp-AG fractions, designated F2, F3, and F4 (Fig. 1A). Yariv reactivity of the Hyp-AG fractions was assessed by radial gel diffusion assay and was quantified based on the area of the halo formed on an agarose gel containing β-Gal-Yariv (van Holst and Clarke, 1985). These fractions showed clear Yariv reactivity, although the reactivity was relatively weak compared with native gum arabic (fractions F2, F3, and F4 were 28%, 23%, and 23%, respectively, as reactive with β-Gal-Yariv as the intact gum arabic based on sugar weight; Fig. 1B). Since these fractions contain AG linked to Hyp, these results indicate that the core protein may not be required for Yariv reactivity.
Figure 1.
Yariv reactivity of Hyp-AGs. A, Hyp-AGs released from gum arabic by base treatment were purified by gel-permeation chromatography. On the basis of the comparison of the elution profiles for base-treated gum arabic (white circles) and a mixture of gum arabic and Gal (black circles), Hyp-AG fractions F2, F3, and F4 were identified. The sugar content in the fractions was determined by the phenol-sulfuric acid method. V0 and Vi indicate void volume and inner volume, respectively. B, The Yariv reactivity of F2, F3, and F4 was examined by radial diffusion assay.
Effect of Sequential Enzymatic Carbohydrate Trimming on the Yariv Reactivity of Radish AGP
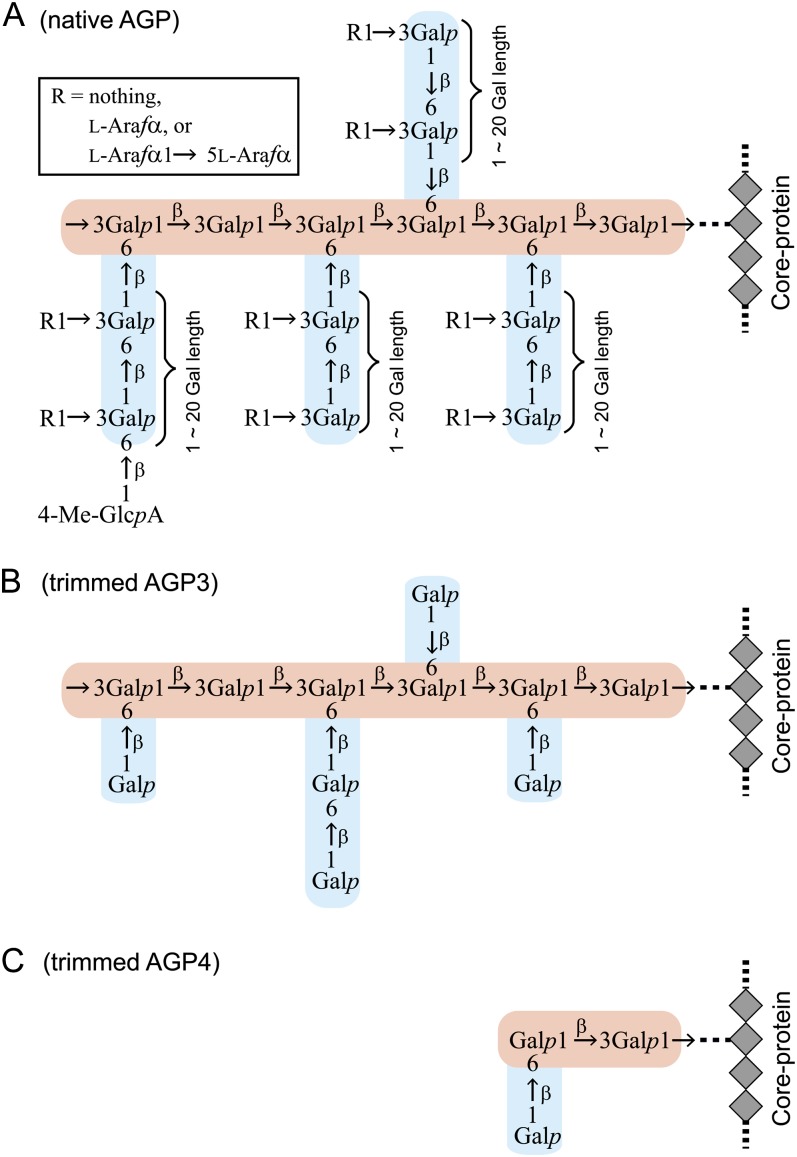
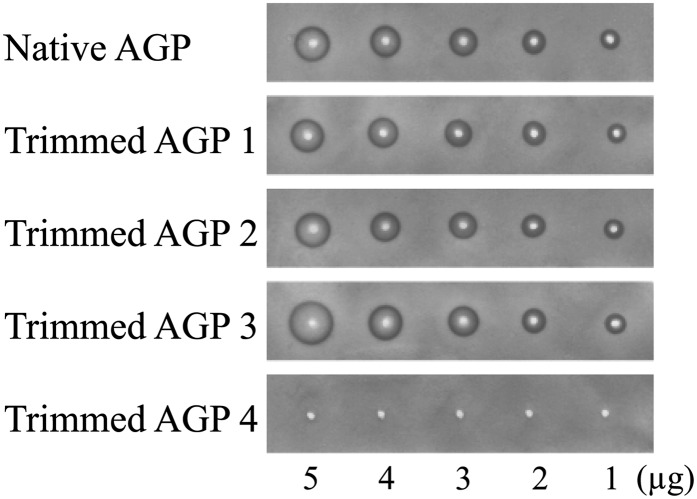
To investigate the carbohydrate component of AGPs that is important for Yariv reactivity, we sequentially trimmed the carbohydrate moieties of radish root AGP. As in many other AGPs (Tryfona et al., 2010, 2012), the carbohydrate moieties of radish root AGP consist of β-1,3-galactan main chains and β-1,6-galactan side chains, to which l-Ara and 4-O-methyl-GlcA are attached (Fig. 2; Tsumuraya et al., 1988). The trimming was accomplished with the aid of four AG-specific glycoside hydrolases: α-l-arabinofuranosidase from Neurospora crassa (NcAraf1), β-glucuronidase from Aspergillus niger (AnGlcAase), endo-β-1,6-galactanase from Trichoderma viride (Tv6GAL), and exo-β-1,3-galactanase from Irpex lacteus (Il1,3GAL; Konishi et al., 2008; Kotake et al., 2009; Takata et al., 2010). All proteins were expressed in Pichia pastoris and purified by chromatography. Trimmed AGP1, AGP2, AGP3, and AGP4 were prepared by combinational digestion of native AGP with these enzymes and subsequent gel-permeation chromatography to remove monosaccharides and oligosaccharides released from the AGP. Structural analysis confirmed that α-l-arabinofuranosyl residues were removed in AGP1 resulting from treatment of native AGP with NcAraf1; all α-l-arabinofuranosyl residues and about one-half of the uronosyl residues were lost in AGP2 produced by treatment with NcAraf1 and AnGlcAase; β-1,6-galactan side chains were reduced to short stubs in AGP3 digested with NcAraf1, AnGlcAase, and Tv6GAL; AGP4, the product of hydrolysis by all four enzymes, including the exo-β-1,3-galactanase, lacked long main chains and thus most of its AG moiety (Fig. 1; Table I). Yariv reactivity of the trimmed AGPs was monitored with the radial gel diffusion assay as described above. Surprisingly, among the trimmed AGPs, only trimmed AGP4 lost Yariv reactivity toward β-Gal-Yariv, whereas trimmed AGP1, AGP2, and AGP3 retained the reactivity (Fig. 3). Compared with native and trimmed AGP1 and AGP2, trimmed AGP3 exhibited relatively high Yariv reactivity based on sugar weight (Table II). None of the trimmed AGPs showed any reactivity toward α-Gal-Yariv (Supplemental Fig. S1). Monosaccharide and oligosaccharide fractions, including l-Ara, 4-O-methyl-GlcA, Gal, and β-1,6-Gal2 released from the AGP during the trimming, did not exhibit any Yariv reactivity at all (Supplemental Fig. S1). These results suggested that β-Gal-Yariv binds to the β-1,3-galactan main chains but not to α-l-arabinofuranosyl and 4-O-methyl-glucuronosyl residues and β-1,6-galactan side chains.
Figure 2.
Structures of AG moieties of native and trimmed AGPs. Radish root AGP was subjected to sequential trimming with AG-specific enzymes. Based on the results of methylation analysis, the structures of AG moieties of native AGP (A) and the trimmed AGP3 (B) and AGP4 (C) were inferred (Table I; Tsumuraya et al., 1988). The β-1,3-galactan main chain is shown in pink, and β-1,6-galactan side chains are shown in blue.
Table I. Structures of native and trimmed AGPs.
| Residue | Native AGP | Trimmed AGPs |
|||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Araf→a | 19.2 | 1.0 | 1.1 | 0.8 | 0.7 |
| →2Araf→ | 0.3 | 0.0 | 0.3 | 0.6 | 0.5 |
| →5Araf→ | 3.0 | 0.0 | 0.0 | 0.0 | 1.8 |
| Fucp→ | 0.4 | 0.0 | 0.0 | 0.0 | 0.0 |
| Galp→ | 7.4 | 16.5 | 18.4 | 17.7 | 2.9 |
| →3Galp→ | 12.4 | 8.7 | 4.0 | 4.1 | 0.4 |
| →6Galp→ | 13.9 | 29.1 | 30.9 | 4.2 | 2.4 |
| →3,6Galp→ | 31.2 | 18.3 | 12.1 | 12.7 | 0.9 |
| Uronic acidsb | 12.2 | 11.3 | 5.2 | 4.0 | 0.4 |
| Totalc | 100.0 | 84.9 | 72.0 | 44.1 | 10.0 |
Proportions of methylated sugars are expressed in mol %. bContent of uronic acids was determined by a modified carbazole-sulfuric acid method using GlcA as the standard. cTotal sugar content was determined by the phenol-sulfuric acid method and is expressed in percentage of that of native AGP.
Figure 3.
Yariv reactivity of trimmed AGPs. The Yariv reactivity of trimmed AGPs was examined by radial diffusion assay. The amount of sugar applied is indicated at the bottom. No reactivity toward α-Gal-Yariv was observed (Supplemental Fig. S1).
Table II. Relative Yariv reactivity of polysaccharides.
| Substrate | Relative Reactivitya |
|---|---|
| Gum arabic from acacia tree | 100 |
| β-Galactan I (gum arabic treated by single Smith degradation) | 94 |
| β-Galactan II (gum arabic treated by double Smith degradation) | 81 |
| β-Galactan III (gum arabic treated by triple Smith degradation) | 248 |
| β-Galactan IV (gum arabic treated by quadruple Smith degradation) | 250 |
| Native AGP from radish roots | 72 |
| Trimmed AGP1 (treated with NcAraf1) | 72 |
| Trimmed AGP2 (treated with NcAraf1 and AnGlcAase) | 73 |
| Trimmed AGP3 (treated with NcAraf1, AnGlcAase, and Tv6GAL) | 102 |
| Trimmed AGP4 (treated with NcAraf1, AnGlcAase, Tv6GAL, and Il1,3GAL) | 0 |
Relative Yariv reactivity is expressed in percentage of that toward gum arabic based on the halo area.
Effect of the Removal of β-1,6-Galactan Side Chains on Yariv Reactivity
We next examined the effect on Yariv reactivity of the degradation of β-1,6-galactan side chains of gum arabic, a commercial mixture of AGP, by Smith degradation, to exclude the possible influence of the large number of short stubs of β-1,6-galactan side chains attached to β-1,3-galactan main chains left even after enzymatic trimming in AGP3 (Fig. 3). Smith degradation can break sugar residues with vicinal hydroxyl (OH) groups; therefore, O-3-linked hexoses including β-1,3-galactosyl residues would survive this treatment, while terminal sugars and hexoses in any other linkage such as β-1,6-galactosyl residues would be degraded. As a result, several cycles of Smith degradation are capable of removing the β-1,6-galactan side chains of gum arabic while preserving the β-1,3-galactan main chains (Tsumuraya et al., 1990). In this study, β-galactan I, II, III, and IV were prepared from gum arabic by single, double, triple, and quadruple Smith degradation, respectively. On the basis of structural analysis, the proportions of β-1,3-galactan main chains in native gum arabic and β-galactan I, II, III, and IV were calculated to be 31%, 49%, 58%, 91%, and 93%, respectively (Supplemental Fig. S2; Akiyama et al., 1984; Defaye and Wong, 1986; Tsumuraya et al., 1990; Kotake et al., 2009). Although most of the short stubs of β-1,6-galactan side chains were removed in β-galactan IV, we could not obtain β-1,3-galactan completely lacking β-1,6-galactosyl branches, as the fourth Smith degradation was not effective in further degrading β-1,6-galactan side chains.
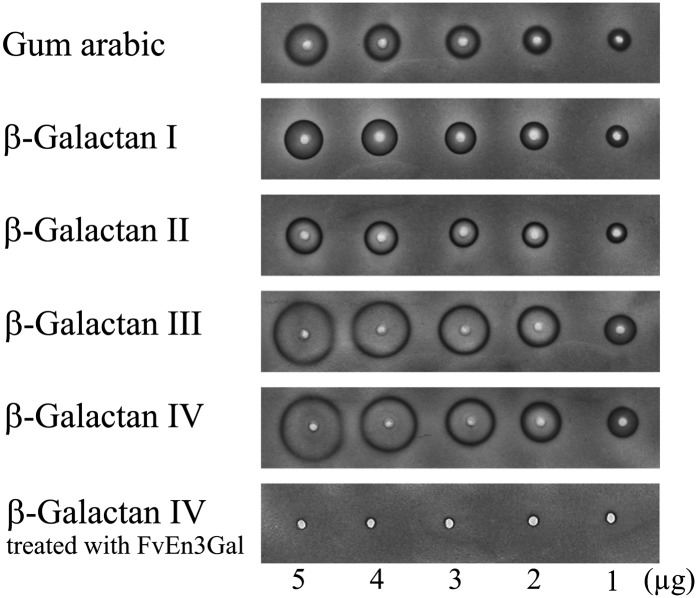
Yariv reactivity of gum arabic was not diminished by the repeated Smith degradation treatments (Fig. 4; Table II). On the contrary, β-galactans III and IV, which have a high proportion of β-1,3-galactan, exhibited significantly higher reactivity than native gum arabic and β-galactan I based on sugar weight. Yariv reactivity of β-galactan IV was lost by treatment with endo-β-1,3-galactanase from winter mushroom (Flammulina velutipes), FvEn3Gal (Kotake et al., 2011). β-Galactan IV also failed to interact with α-Gal-Yariv (Supplemental Fig. S1). Taken together, these data strongly support the hypothesis that β-Gal-Yariv binds to the β-1,3-galactan main chains but not to β-1,6-galactan side chains. Our findings also suggest that neither branching residues of β-1,3-galactan nor remnant β-1,6-galactan side chains are important for Yariv reactivity, since β-galactans III and IV, which have considerably fewer β-1,6-galactosyl branches than intact gum arabic (Table II), exhibited higher Yariv reactivity.
Figure 4.
Yariv reactivity of β-galactans. β-Galactans resulting from single (β-galactan I), double (β-galactan II), triple (β-galactan III), and quadruple (β-galactan IV) Smith degradation of acacia gum were subjected to Yariv radial diffusion assay. The Yariv reactivity of β-galactan IV treated with FvEn3Gal is also shown. The average structures of the β-galactans are shown in Supplemental Figure S2. No reactivity toward α-Gal-Yariv was observed (Supplemental Fig. S1).
Yariv Precipitation with β-1,3-Galactooligosaccharides
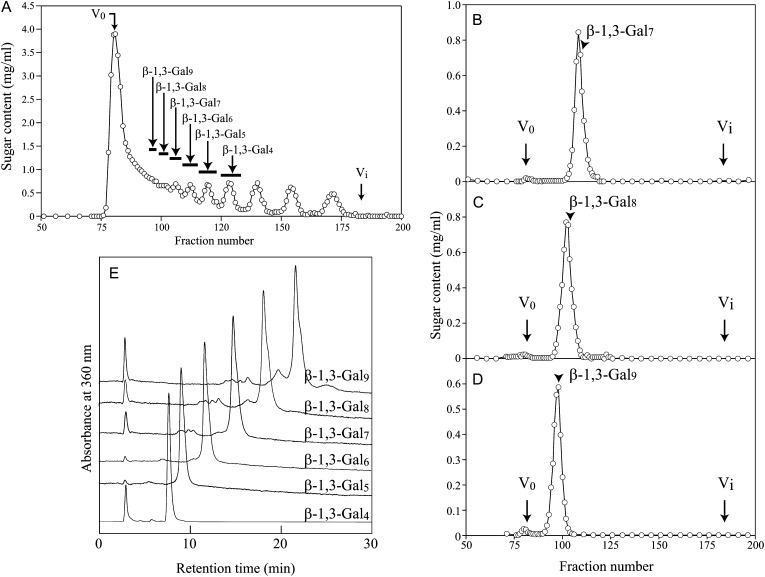
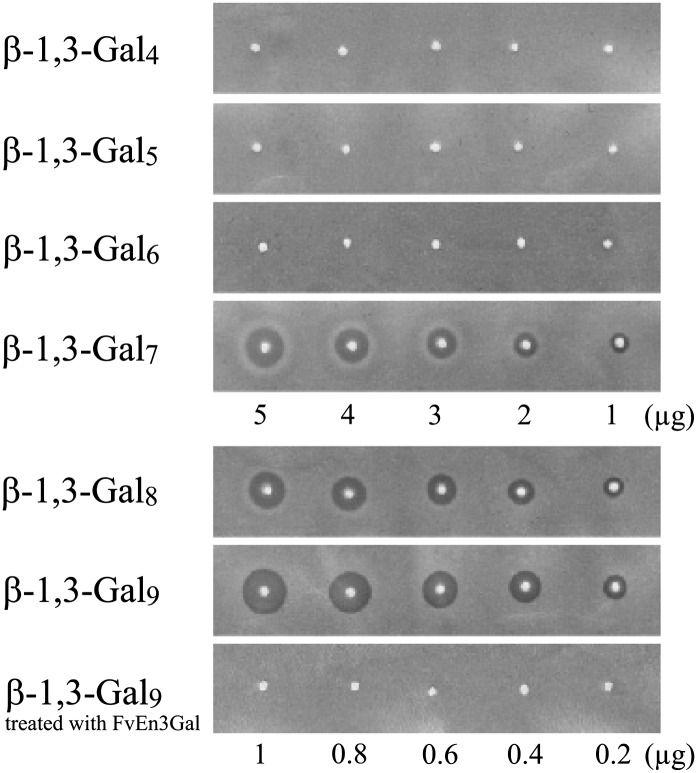
To address the question of how many β-1,3-galactosyl residues are necessary for Yariv precipitation activity, we next examined the Yariv reactivity of a series of β-1,3-galactooligosaccharides (β-1,3-Galn). The oligosaccharides β-1,3-Gal4, β-1,3-Gal5, β-1,3-Gal6, β-1,3-Gal7, β-1,3-Gal8, and β-1,3-Gal9 were prepared from β-galactan IV by partial acid hydrolysis and fractionation by gel-permeation chromatography (Fig. 5). Methylation analysis showed that β-1,3-Gal7, β-1,3-Gal8, and β-1,3-Gal9 still have 0.5, 0.8, and 1.1 β-3,6-galactosyl branches (→3,6Galp→) per molecule on average, which are derived from remnant side chains of β-galactan IV (Supplemental Table S1). Among the oligosaccharides, only β-1,3-Gal7, β-1,3-Gal8, and β-1,3-Gal9 exhibited precipitation activity with β-Gal-Yariv and β-Glc-Yariv, whereas the shorter oligosaccharides, β-1,3-Gal4, β-1,3-Gal5, and β-1,3-Gal6, did not precipitate with either of the two Yariv phenylglycosides tested (Fig. 6; Table III; Supplemental Fig. S3). Compared with β-galactan IV, β-1,3-Gal8 and β-1,3-Gal9 exhibited relatively high Yariv reactivity (Figs. 3 and 6), which probably results from the rapid diffusion of these oligosaccharides on the agarose gel during the assay. Like β-galactan IV, β-1,3-Gal9 lost Yariv reactivity when treated with FvEn3Gal.
Figure 5.
Preparation of β-1,3-galactooligosaccharides (β-1,3-Galn). A, β-1,3-Galn prepared from β-galactan IV by partial acid hydrolysis were separated by gel-permeation chromatography on a Bio-Gel P-2 column. B to D, β-1,3-Gal7 (B), β-1,3-Gal8 (C), and β-1,3-Gal9 (D) were further purified on the same column. In the Yariv assay, the fractions indicated by arrowheads were used. E, To confirm purity, purified β-1,3-Galn were coupled at their reducing terminals with p-aminobenzoic acid ethyl ester and analyzed on the HPLC system. V0 and Vi indicate void volume and inner volume, respectively.
Figure 6.
Yariv reactivity of β-1,3-Galn. The chain length of β-1,3-galactan necessary for β-Gal-Yariv reactivity was investigated. In the assay of β-1,3-Gal8 and β-1,3-Gal9, smaller amounts of samples were used due to their high reactivity. None of them exhibited Yariv reactivity toward α-Gal-Yariv (Supplemental Fig. S3).
Table III. Relative Yariv reactivity of galactooligosaccharides.
| Substrate | Relative Reactivitya |
|---|---|
| β-1,3-Gal4 | 0 |
| β-1,3-Gal5 | 0 |
| β-1,3-Gal6 | 0 |
| β-1,3-Gal7 | 13 |
| β-1,3-Gal8 | 68 |
| β-1,3-Gal9 | 100 |
| β-1,6-Gal6 | 0 |
| β-1,6-Gal7+8b | 0 |
| β-1,6-Gal>8c | 0 |
Relative Yariv reactivity is expressed in percentage of that toward β-1,3-Gal9 based on the halo area. bThis fraction contained both β-1,6-Gal7 and β-1,6-Gal8. cThis fraction was a mixture of β-1,6-Galn higher than β-1,6-Gal8.
Other oligosaccharides, such as β-1,6-Galn, β-GlcA-1,6-β-Gal-1,6-Gal, and α-l-Araf-1,3-β-Gal-1,6-Gal, corresponding to partial structures of β-1,6-galactan side chains of AGPs did not exhibit any Yariv reactivity (Supplemental Fig. S3), demonstrating that β-1,6-galactan side chains and attached α-l-arabinofuranosyl and β-glucuronosyl residues are not necessary for Yariv reactivity.
Detection of the Interaction of β-1,3-Galn with β-Gal-Yariv
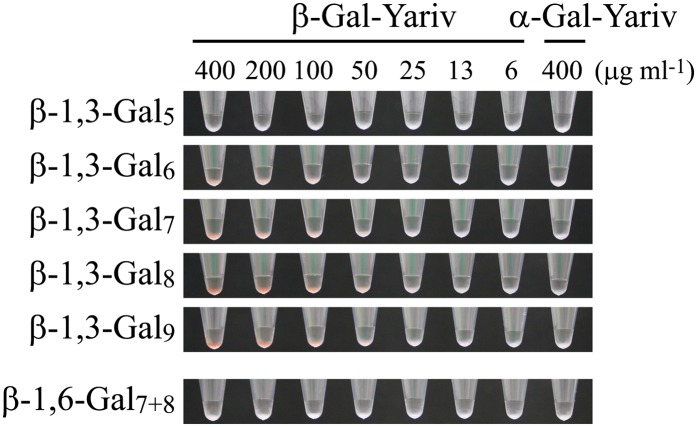
The formation of an insoluble precipitate on the agarose gel likely results from cross linking of the glycan and Yariv and, therefore, may require the binding of at least two Yariv phenylglycosides to each oligosaccharide. It is also conceivable that a weak interaction of a short β-1,3-Galn with Yariv phenylglycoside may not be detected. In order to detect weak interactions with Yariv phenylglycoside, a pull-down assay using β-1,3-Gal5, β-1,3-Gal6, β-1,3-Gal7, β-1,3-Gal8, and β-1,3-Gal9 cross linked to hydrazide beads was performed. The beads were suspended in β-Gal-Yariv solution at concentrations from 6.25 to 400 µg mL−1, and Yariv phenylglycoside coprecipitated with beads was observed. β-1,3-Gal6, β-1,3-Gal7, β-1,3-Gal8, and β-1,3-Gal9 beads formed an apparent red-color precipitate (Fig. 7). Since the β-1,3-Gal6 beads did not precipitate β-Gal-Yariv except at the higher β-Gal-Yariv concentrations (above 100 µg mL−1), the interaction between β-Gal-Yariv and β-1,3-Gal6 beads may be weak compared with longer β-1,3-Galn beads. β-1,3-Gal5 beads did not precipitate β-Gal-Yariv at all. Given that a galactosyl residue at the reducing end of the β-1,3-Gal6 is lost in the cross-linking reaction with the bead hydrazide group, we conclude that β-1,3-Gal5 is able to interact with β-Gal-Yariv. None of β-1,3-Galn beads precipitated α-Gal-Yariv. Consistent with the radial gel-diffusion assay findings, no interaction between β-1,6-Gal7+8 beads (a mixture of β-1,6-Gal7 beads and β-1,6-Gal8 beads) and β-Gal-Yariv was detected. Combining all the data from the base hydrolysis of the peptide backbone, the stepwise trimming of the carbohydrate component of AGPs, and the pull-down assay, we propose that β-Yarivs should be considered specific binding reagents for β-1,3-galactan chains longer than five residues.
Figure 7.
Interaction of β-Gal-Yariv with oligosaccharides. For pull-down assays, β-1,3-Gal5, β-1,3-Gal6, β-1,3-Gal7, β-1,3-Gal8, β-1,3-Gal9, and β-1,6-Gal7+8 cross linked to beads were suspended in β-Gal-Yariv solution ranging from 6.25 to 400 µg mL−1 in concentration. As a control experiment, the beads were also suspended in 400 µg mL−1 α-Gal-Yariv solution.
Computational Modeling of β-1,3-Galactan
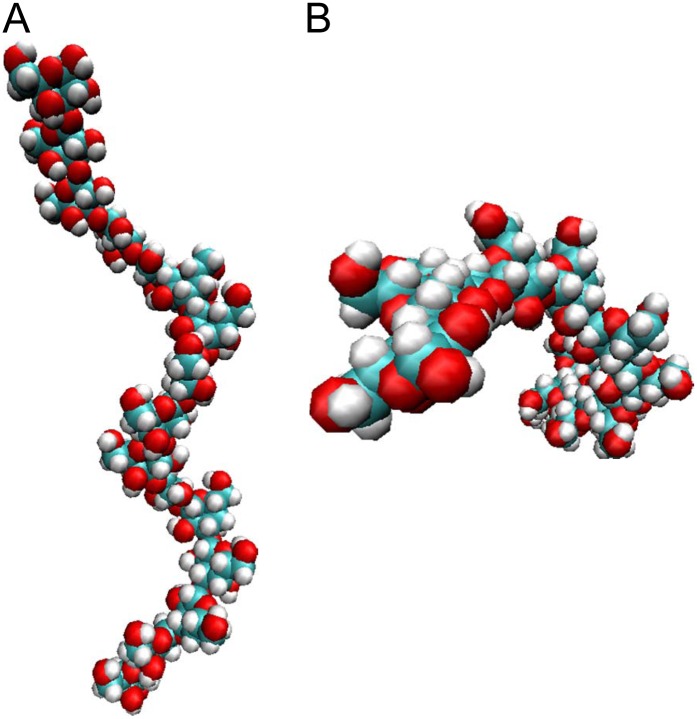
There are only a few reports on the conformation of β-1,3-galactan; it is known, however, that β-1,3-glucan has a right-handed helical structure (Sletmoen and Stokke, 2008). In order to address the question of what mechanism might be responsible for the interaction between β-Yarivs and β-1,3-galactan, molecular dynamics (MD) simulations for β-1,3-galactan were carried out by means of the AMBER11 software package with the all-atom GLYCAM06 force field. In the computer simulations, a straight β-1,3-galactan consisting of 16 galactosyl residues was put into a virtual water box, and the stable conformation was predicted based on the physical movement of the molecule. The simulations suggested that β-1,3-galactan itself forms a right-handed helical structure that has seven or eight galactosyl residues per turn (Fig. 8; Supplemental Fig. S4). The helix is somewhat looser than those reported for β-1,3-glucan and larch (Larix laricina) AG, which both have six galactosyl residues per turn (Chandrasekaran and Janaswamy, 2002). The modeled β-1,3-galactan was flexible in water, undergoing repeated minor conformational changes. Although the modeled structure did not reveal a specific binding site for β-Yarivs, the inside of the helix is relatively hydrophobic, because, as is the case for the β-1,3-glucan helix, OH groups of galactosyl residues other than C-2 OH are facing outward in water (Miyoshi et al., 2004; Sletmoen and Stokke, 2008).
Figure 8.
Computational modeling. The conformation of β-1,3-galactan consisting of 16 galactosyl residues in water was predicted by MD simulations. The simulations for the β-1,3-galactan were performed in a truncated octahedral TIP3P water box with a solvation shell of 12 Å thickness. Side (A) and top (B) views are shown. Note that the modeling may not correctly predict the conformation of the ends of the β-1,3-galactan chain, because of the edge effect.
DISCUSSION
This study indicates, to our knowledge for the first time, that the β-1,3-galactan main chains of AGPs are a target structure for β-Gal-Yariv. This provides an explanation for Yariv binding to most AGPs and AGs, not only the radish root AGP and gum arabic studied here, since the β-1,3-galactan main chain is a conserved carbohydrate structure of AGPs and AGs (Fincher et al., 1983; Nothnagel, 1997; Seifert and Roberts, 2007; Ellis et al., 2010). The core protein is not the target structure, since Hyp-AGs released from gum arabic by degradation of the peptide linkages of the core proteins retained Yariv reactivity. Most importantly, sequential trimming of the carbohydrate component of AGPs with AG-specific glycoside hydrolases, while keeping the core protein intact, abolished Yariv reactivity of AGPs (trimmed AGP4). On the contrary, β-1,3-galactan (β-galactan IV) and β-1,3-Gal9 precipitated with β-Gal-Yariv, but this property was abolished when these molecules were broken down with the FvEn3Gal enzyme. The results presented here are consistent with previous reports suggesting that neither α-l-arabinofuranosyl residues nor β-1,6-galactan side chains are involved in Yariv reactivity (Gleeson and Clarke, 1979; Tsumuraya et al., 1987; Saulnier et al., 1992). However, several other reports have proposed the interaction of β-Yarivs with core proteins or α-l-arabinofuranosyl residues of AGPs. It is unclear why these results are inconsistent with ours, but we suggest that deglycosylation with hydrogen fluoride partially left β-1,3-galactans of the AGPs in the work of Akiyama et al. (1984). Acid hydrolysis of the AG moieties of rose AGP to remove α-l-arabinofuranosyl residues might partially degrade β-1,3-galactans as well as α-l-arabinofuranosyl residues, diminishing the Yariv reactivity (Komalavilas et al., 1991).
Our computational modeling proposed a helical structure for β-1,3-galactan that has seven or eight galactosyl residues per turn. The inside cavity of the helix is relatively hydrophobic and therefore a candidate site for hydrophobic interaction with the phenylazotrihydroxybenzene core of β-Yarivs. A previous equilibrium sedimentation analysis has demonstrated strong self-association of Yariv phenylglycosides in water (Yariv et al., 1962; Woods et al., 1978). Further Yariv hydrophobic interactions, therefore, may cause the formation of large insoluble β-1,3-galactan-Yariv complexes. Our modeling did not explain the specific interaction of β-1,3-galactan with β-glucosyl or β-galactosyl residues of Yariv phenylglycosides, but we propose that these likely bind to Gal residues in the helix. To demonstrate the molecular mechanism for Yariv reactivity, physicochemical analysis of the interaction between β-1,3-galactan and β-Gal-Yariv will now be important.
There are also alternative proposed structures of some AGPs to our model of long-chain β-1,3-galactan. Tan et al. (2010) propose a repetitive main chain of two β-1,3-galactosyl residues and a kink of β-1,6-linked Gal, whereas others postulate a kink (of unknown nature) in the main chain spaced at seven or more Gal residues (Churms et al., 1983; Bacic et al., 1987; Ellis et al., 2010). Although we did not examine the Yariv reactivity of such a kinked main chain, a kink spaced at seven or more residues might not disrupt the helical structure of the main chain, particularly if it is a kink of β-1,6-linked Gal, since the β-1,6-linkage is generally flexible compared with other types of linkages (Rees and Scott, 1971). On the other hand, our results do not appear consistent with structural models for AG moieties that have a short kinked main chain but are nevertheless precipitated with β-Gal-Yariv (Tan et al., 2010; Xu et al., 2010). Additionally, in the case of mugwort pollen, O-glycans were precipitated by β-Glc-Yariv, although they have been reported to lack β-1,3-galactan (Léonard et al., 2005). We cannot exclude the possibility that some AGPs have a target structure for β-Yarivs other than β-1,3-galactan, but our data suggest that reinvestigation for the presence of β-1,3-galactan main chains longer than β-1,3-Gal7 in these AGs is necessary.
Yariv reactivity requires a longer β-1,3-galactosyl chain to make an insoluble precipitate on the agarose gel than it does to bind to the oligosaccharide in the pull-down assay. β-1,3-Gal7 is the smallest oligosaccharide precipitable by Yariv. On the other hand, β-1,3-Gal6 beads are reactive in the Yariv pull-down assay, suggesting that β-1,3-Gal5 is the smallest carbohydrate structure for Yariv interaction, as β-1,3-Gal6 beads have lost a galactosyl residue at the reducing end. Just as the precipitation of proteins with antibodies requires a multivalent interaction, longer oligosaccharides may be required for multiple Yariv glycoside interaction and consequent precipitation.
In view of the fact that β-Yarivs stain tissues of various higher plants, including gymnosperm, fern, moss, and liverwort (Jermyn and Yeow, 1975; Clarke et al., 1978), we propose that β-1,3-galactan, rather than other AG glycan structures or certain features of the core protein, is the widely distributed characteristic distinguishing AGPs in higher plants. The former name β-lectin for AGPs is indicative of their interaction with β-glucosyl and β-galactosyl residues (Pennell et al., 1989). These residues can be found on several cell wall polysaccharides such as cellulose (β-1,4-glucan), β-1,3:1,4-glucan, callose (β-1,3-glucan), xyloglucan, pectic β-1,4-galactan, and AGP. Therefore, it is conceivable that the AGP β-1,3-galactan may interact with any of these polysaccharides. Consistent with this hypothesis, several groups have reported copurification of AGPs with other cell wall polysaccharides (Baldwin et al., 1993; Serpe and Nothnagel, 1995). Since most AGPs are secreted and first localize on the cell surface with their glycosylphosphatidylinositol anchors (Fincher et al., 1983; Sherrier et al., 1999), they may play a role in the modification of cell wall architecture on the cell surface through the interaction of β-1,3-galactan with cell wall polysaccharides. The proposed interaction between β-1,3-galactan and other cell wall components should provide important hints in the elucidation of the many functions of AGPs.
MATERIALS AND METHODS
Preparation of Released Hyp-AGs
Base treatment of gum arabic was performed according to previous studies (Tan et al., 2004, 2010). Peptide linkages of core proteins included in gum arabic were hydrolyzed in 0.44 m NaOH at 105°C for 18 h. The hydrolysate was neutralized with acetic acid to pH 7.0, and then Hyp-AGs were purified by gel-permeation chromatography. The sugar content in the fractions was determined by the phenol-sulfuric acid method (Dubois et al., 1956). The Hyp-AG fractions F2, F3, and F4 were used for radial gel diffusion assay for Yariv reactivity.
Trimming of AGP
NcAraf1, AnGlcAase, Tv6GAL, and Il1,3GAL were prepared as described (Konishi et al., 2008; Kotake et al., 2009; Takata et al., 2010). The purity of the enzymes has been confirmed by SDS-PAGE. Radish (Raphanus sativus) root AGP was digested with NcAraf1, AnGlcAase, Tv6GAL, and Il1,3GAL in 25 mm acetate buffer (pH 4.0) at 37°C for 24 h. The AGP was applied onto a Sephadex G-15 column (2.4 × 23 cm; GE Healthcare) to separate it from released monosaccharides and oligosaccharides. The structures of the trimmed AGPs were determined by methylation analysis with a Shimadzu GC-6A gas chromatograph fitted with a Silar-10C column (0.28 mm × 50 m; Hakomori, 1964; Albersheim et al., 1967). The content of uronic acid was measured by a modified carbazole-sulfuric acid method (Galambos, 1967). In the hydrolysis of β-galactan IV and β-1,3-Gal9, recombinant FvEn3Gal was used (Kotake et al., 2011).
Assay of Yariv Reactivity
Yariv reactivity was determined by radial gel diffusion assay (van Holst and Clarke, 1985). The carbohydrate sample was applied to a gel plate containing 0.004% (w/v) β-Gal-Yariv, 75 mm NaCl, 0.01% (w/v) sodium azide, and 1% (w/v) agarose. The relative reactivity was quantified based on the halo area using gum arabic (Sigma) as the standard and expressed based on equal sugar amount.
Smith Degradation
Smith degradation was performed as described previously (Goldstein et al., 1956; Tsumuraya et al., 1987). Oxidation of gum arabic with 100 mm metaperiodate was carried out in the dark at 7°C for 4 d and terminated by the addition of 1,2-ethanediol. After neutralization with NaOH, the products were reduced with NaBH4 for 12 h and treated with 1 m trifluoroacetic acid for 12 h. The products were washed with 70% (v/v) ethanol, ethanol, acetone, and petroleum ether.
Preparation of Oligosaccharides
We prepared β-1,3-Gal4, β-1,3-Gal5, β-1,3-Gal6, β-1,3-Gal7, β-1,3-Gal8, and β-1,3-Gal9 from β-galactan IV by partial acid hydrolysis with 40 mm trifluoroacetic acid at 100°C for 1 h and purified them by gel-permeation chromatography (Fig. 5). Oligosaccharides β-1,6-Gal4, β-1,6-Gal5, β-1,6-Gal6, β-1,6-Gal7+8, and β-1,6-Gal>8 were prepared from radish root AGP by treatment with NcAraf1, AnGlcAase, and Il1,3GAL and separated on a Bio-Gel P-2 column (Supplemental Fig. S5). To confirm purity, β-1,3-Galn and β-1,6-Galn were coupled at their reducing terminals with p-aminobenzoic acid ethyl ester according to the method of Matsuura and Imaoka (1988) and analyzed on an HPLC system equipped with a TSKgel Amide-80 (4.6 × 250 mm; Tosoh) column (Fig. 5; Supplemental Fig. S5) as described previously (Kotake et al., 2004). Molecular mass of the oligosaccharides was ascertained by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, and their structures were examined by methylation analysis. Other oligosaccharides were prepared as described (Kuroyama et al., 2001; Okemoto et al., 2003).
Pull-Down Assay
β-1,3-Gal5, β-1,3-Gal6, β-1,3-Gal7, β-1,3-Gal8, β-1,3-Gal9, and β-1,6-Gal7+8 cross linked to beads were prepared with a BlotGlyco glycan purification and labeling kit according to the manufacturer’s instructions (Sumitomo Bakelite). In brief, 50 nmol of each oligosaccharide was cross linked to hydrazide groups on the beads, and free oligosaccharides were washed out from the beads. In the pull-down assay, a portion (approximately 5 µL) of the beads was suspended in β-Gal-Yariv solution ranging from 6.25 to 400 µg mL−1 at 25°C for 4 h. After washing the beads with 1 mL of water three times, coprecipitated Yariv phenylglycoside was observed.
Modeling
MD simulations were carried out by using the AMBER11 software package (Case et al., 2010) with the all-atom GLYCAM06 force field (Kirschner et al., 2008) for β-1,3-galactan. The electrostatic interactions were calculated with the particle mesh Ewald method, and the cutoff was 8 Å. Using the LEAP module in AMBER11, the structure was immersed in a truncated octahedral water box with a solvation shell of 12 Å thickness using the TIP3P model for water. The minimization procedure for solvated β-1,3-galactan consisted of two steps. In the first stage, the β-1,3-galactan was kept fixed, and positions of the water were minimized. The solvated structures were then subjected to 500 steps of steepest descent minimization followed by 500 steps of conjugate gradient minimization. During this minimization process, the β-1,3-galactan was kept fixed in its starting conformation using harmonic constraints with a force constant of 500 kcal mol−1 Å−2. In the second stage, the entire system was minimized by 1,000 steps of steepest descent minimization followed by 1,500 steps of conjugate gradient minimization without the restraints. The minimized structure was then subjected to 20 picoseconds of MD, using a 2-femtosecond time step for integration. During the MD simulation, the system was gradually heated from 0 to 300 K using 10 kcal mol−1 Å−2 weak positional restraints on the β-1,3-galactan. The SHAKE algorithm was used, in which all bonds involving hydrogen are constrained. After the system was heated at a constant volume with weak restraints on the complex, MD was performed for 10 nanoseconds with a time step of 2 femtoseconds under constant volume/constant temperature (number of particles, volume, and thermal ensemble) at 300 K. SHAKE was used to constrain bonds involving hydrogen, and the temperature was kept at 300 K with Anderson dynamics.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Yariv reactivity of trimmed AGPs, released monosaccharides and oligosaccharides, and β-galactans.
Supplemental Figure S2. Average structures of native gum arabic and the β-galactans.
Supplemental Figure S3. Yariv reactivity of β-1,3-Galn and other oligosaccharides.
Supplemental Figure S4. Three-dimensional structure of β-1,3-galactan.
Supplemental Figure S5. Preparation of β-1,6-Galn.
Supplemental Table S1. Structures of β-1,3-galactooligosaccharides.
Glossary
- AGP
arabinogalactan protein
- AG
arabinogalactan
- β-Glc-Yariv
β-glucosyl Yariv phenylglycoside
- β-Gal-Yariv
β-galactosyl Yariv phenylglycoside
- β-Yariv
β-glycosyl Yariv phenylglycoside
- α-Gal-Yariv
α-galactosyl Yariv
- MD
molecular dynamics
- OH
hydroxyl
References
- Akiyama Y, Eda S, Kato K. (1984) Gum arabic is a kind of arabinogalactan-protein. Agric Biol Chem 48: 235–237 [Google Scholar]
- Albersheim P, Nevins DJ, English P, Karr A. (1967) A method for the analysis of sugars in plant cell-wall polysaccharides by gas liquid chromatography. Carbohydr Res 5: 340–345 [Google Scholar]
- Bacic A, Churms SC, Stephen AM, Cohen PB, Fincher GB. (1987) Fine structure of the arabinogalactan-protein from Lolium multiflorum. Carbohydr Res 162: 85–93 [Google Scholar]
- Baldwin TC, McCann MC, Roberts K. (1993) A novel hydroxyproline-deficient arabinogalactan protein secreted by suspension-cultured cells of Daucus carota: purification and partial characterization. Plant Physiol 103: 115–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case DA, Darden TA, Cheatham TE, III, Simmerling CL, Wang J, Duke RE, Luo R, Walker RC, Zhang W, Merz KM, et al. (2010) AMBER 11, University of California, San Francisco. http://ambermd.org/doc11/Amber11.pdf [Google Scholar]
- Chandrasekaran R, Janaswamy S. (2002) Morphology of western larch arabinogalactan. Carbohydr Res 337: 2211–2222 [DOI] [PubMed] [Google Scholar]
- Cheung AY, Wang H, Wu H-M. (1995) A floral transmitting tissue-specific glycoprotein attracts pollen tubes and stimulates their growth. Cell 82: 383–393 [DOI] [PubMed] [Google Scholar]
- Churms SC, Stephen AM, Siddiqui IR. (1983) Some new aspects of the molecular structure of Acacia senegal gum (gum arabic). Carbohydr Res 123: 267–279 [Google Scholar]
- Clarke AE, Gleeson PA, Jermyn MA, Knox RB. (1978) Characterization and localization of β-lectins in lower and higher plants. Aust J Plant Physiol 5: 707–722 [Google Scholar]
- Defaye J, Wong E. (1986) Structural studies of gum arabic, the exudate polysaccharide from Acacia senegal. Carbohydr Res 150: 221–231 [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28: 350–356 [Google Scholar]
- Ellis M, Egelund J, Schultz CJ, Bacic A. (2010) Arabinogalactan-proteins: key regulators at the cell surface? Plant Physiol 153: 403–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincher GB, Stone BA, Clarke AE. (1983) Arabinogalactan-proteins: structure, biosynthesis, and function. Annu Rev Plant Physiol 34: 47–70 [Google Scholar]
- Galambos JT. (1967) The reaction of carbazole with carbohydrates. I. Effect of borate and sulfamate on the carbazole color of sugars. Anal Biochem 19: 119–132 [DOI] [PubMed] [Google Scholar]
- Gao M, Showalter AM. (1999) Yariv reagent treatment induces programmed cell death in Arabidopsis cell cultures and implicates arabinogalactan protein involvement. Plant J 19: 321–331 [DOI] [PubMed] [Google Scholar]
- Gleeson PA, Clarke AE. (1979) Structural studies on the major component of Gladiolus style mucilage, an arabinogalactan-protein. Biochem J 181: 607–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson PA, Jermyn MA. (1979) Alteration in the composition of β-lectins caused by chemical and enzymic attack. Aust J Plant Physiol 6: 25–38 [Google Scholar]
- Goldstein IJ, Hay GW, Lewis BA, Smith F. (1956) Controlled degradation of polysaccharides by periodate oxidation, reduction, and hydrolysis. Methods Carbohydr Chem 5: 361–370 [Google Scholar]
- Hakomori S. (1964) A rapid premethylation of glycolipid, and polysaccharide catalyzed by methylsulfinyl carbanion in dimethyl sulfoxide. J Biochem 55: 205–208 [PubMed] [Google Scholar]
- Jermyn MA, Yeow YM. (1975) A class of lectins present in the tissues of seed plants. Aust J Plant Physiol 2: 501–531 [Google Scholar]
- Kirschner KN, Yongye AB, Tschampel SM, González-Outeiriño J, Daniels CR, Foley BL, Woods RJ. (2008) GLYCAM06: a generalizable biomolecular force field. Carbohydrates. J Comput Chem 29: 622–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyohara H, Matsumoto T, Yamada H. (2002) Intestinal immune system modulating polysaccharides in a Japanese herbal (Kampo) medicine, Juzen-Taiho-To. Phytomedicine 9: 614–624 [DOI] [PubMed] [Google Scholar]
- Komalavilas P, Zhu J-K, Nothnagel EA. (1991) Arabinogalactan-proteins from the suspension culture medium and plasma membrane of rose cells. J Biol Chem 266: 15956–15965 [PubMed] [Google Scholar]
- Konishi T, Kotake T, Soraya D, Matsuoka K, Koyama T, Kaneko S, Igarashi K, Samejima M, Tsumuraya Y. (2008) Properties of family 79 β-glucuronidases that hydrolyze β-glucuronosyl and 4-O-methyl-β-glucuronosyl residues of arabinogalactan-protein. Carbohydr Res 343: 1191–1201 [DOI] [PubMed] [Google Scholar]
- Kotake T, Hirata N, Degi Y, Ishiguro M, Kitazawa K, Takata R, Ichinose H, Kaneko S, Igarashi K, Samejima M, et al. (2011) Endo-β-1,3-galactanase from winter mushroom Flammulina velutipes. J Biol Chem 286: 27848–27854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotake T, Kaneko S, Kubomoto A, Haque MA, Kobayashi H, Tsumuraya Y. (2004) Molecular cloning and expression in Escherichia coli of a Trichoderma viride endo-β-(1→6)-galactanase gene. Biochem J 377: 749–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotake T, Kitazawa K, Takata R, Okabe K, Ichinose H, Kaneko S, Tsumuraya Y. (2009) Molecular cloning and expression in Pichia pastoris of a Irpex lacteus exo-β-(1→3)-galactanase gene. Biosci Biotechnol Biochem 73: 2303–2309 [DOI] [PubMed] [Google Scholar]
- Kuroyama H, Tsutsui N, Hashimoto Y, Tsumuraya Y. (2001) Purification and characterization of a β-glucuronidase from Aspergillus niger. Carbohydr Res 333: 27–39 [DOI] [PubMed] [Google Scholar]
- Larkin PJ. (1977) Plant protoplast agglutination and membrane-bound β-lectins. J Cell Sci 26: 31–46 [DOI] [PubMed] [Google Scholar]
- Larkin PJ. (1978) Plant protoplast agglutination by artificial carbohydrate antigens. J Cell Sci 30: 283–292 [DOI] [PubMed] [Google Scholar]
- Léonard R, Petersen BO, Himly M, Kaar W, Wopfner N, Kolarich D, van Ree R, Ebner C, Duus JØ, Ferreira F, et al. (2005) Two novel types of O-glycans on the mugwort pollen allergen Art v 1 and their role in antibody binding. J Biol Chem 280: 7932–7940 [DOI] [PubMed] [Google Scholar]
- Majewska-Sawka A, Nothnagel EA. (2000) The multiple roles of arabinogalactan proteins in plant development. Plant Physiol 122: 3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura F, Imaoka A. (1988) Chromatographic separation of asparagine-linked oligosaccharides labeled with an ultraviolet absorbing compound, p-aminobenzoic acid ethyl ester. Glycoconj J 5: 13–26 [Google Scholar]
- Miyoshi K, Uezu K, Sakurai K, Shinkai S. (2004) Proposal of a new hydrogen-bonding form to maintain curdlan triple helix. Chem Biodivers 1: 916–924 [DOI] [PubMed] [Google Scholar]
- Motose H, Sugiyama M, Fukuda H. (2004) A proteoglycan mediates inductive interaction during plant vascular development. Nature 429: 873–878 [DOI] [PubMed] [Google Scholar]
- Nothnagel EA. (1997) Proteoglycans and related components in plant cells. Int Rev Cytol 174: 195–291 [DOI] [PubMed] [Google Scholar]
- Nothnagel EA, Lyon JL. (1986) Structural requirements for the binding of phenylglycosides to the surface of protoplasts. Plant Physiol 80: 91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okemoto K, Uekita T, Tsumuraya Y, Hashimoto Y, Kasama T. (2003) Purification and characterization of an endo-β-(1→6)-galactanase from Trichoderma viride. Carbohydr Res 338: 219–230 [DOI] [PubMed] [Google Scholar]
- Pennell RI, Knox JP, Scofield GN, Selvendran RR, Roberts K. (1989) A family of abundant plasma membrane-associated glycoproteins related to the arabinogalactan proteins is unique to flowering plants. J Cell Biol 108: 1967–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees DA, Scott WE. (1971) Polysaccharide conformation. Part VI. Computer model-building for linear and branched pyranoglycans. Correlations with biological function. Preliminary assessment of inter-residue forces in aqueous solution. Further interpretation of optical rotation in terms of chain conformation. J Chem Soc B 1971: 469–479 [Google Scholar]
- Sardar HS, Yang J, Showalter AM. (2006) Molecular interactions of arabinogalactan proteins with cortical microtubules and F-actin in Bright Yellow-2 tobacco cultured cells. Plant Physiol 142: 1469–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saulnier L, Brillouet JM, Moutounet M, Hervé du Penhoat C, Michon V. (1992) New investigations of the structure of grape arabinogalactan-protein. Carbohydr Res 224: 219–235 [DOI] [PubMed] [Google Scholar]
- Seifert GJ, Roberts K. (2007) The biology of arabinogalactan proteins. Annu Rev Plant Biol 58: 137–161 [DOI] [PubMed] [Google Scholar]
- Serpe MD, Nothnagel EA. (1995) Fractionation and structural characterization of arabinogalactan-proteins from the cell wall of rose cells. Plant Physiol 109: 1007–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrier DJ, Prime TA, Dupree P. (1999) Glycosylphosphatidylinositol-anchored cell surface proteins from Arabidopsis. Electrophoresis 20: 2027–2035 [DOI] [PubMed] [Google Scholar]
- Sletmoen M, Stokke BT. (2008) Higher order structure of (1,3)-β-D-glucans and its influence on their biological activities and complexation abilities. Biopolymers 89: 310–321 [DOI] [PubMed] [Google Scholar]
- Tan L, Qiu F, Lamport DT, Kieliszewski MJ. (2004) Structure of a hydroxyproline (Hyp)-arabinogalactan polysaccharide from repetitive Ala-Hyp expressed in transgenic Nicotiana tabacum. J Biol Chem 279: 13156–13165 [DOI] [PubMed] [Google Scholar]
- Tan L, Varnai P, Lamport DT, Yuan C, Xu J, Qiu F, Kieliszewski MJ. (2010) Plant O-hydroxyproline arabinogalactans are composed of repeating trigalactosyl subunits with short bifurcated side chains. J Biol Chem 285: 24575–24583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata R, Tokita K, Mori S, Shimoda R, Harada N, Ichinose H, Kaneko S, Igarashi K, Samejima M, Tsumuraya Y, et al. (2010) Degradation of carbohydrate moieties of arabinogalactan-proteins by glycoside hydrolases from Neurospora crassa. Carbohydr Res 345: 2516–2522 [DOI] [PubMed] [Google Scholar]
- Tryfona T, Liang H-C, Kotake T, Kaneko S, Marsh J, Ichinose H, Lovegrove A, Tsumuraya Y, Shewry PR, Stephens E, et al. (2010) Carbohydrate structural analysis of wheat flour arabinogalactan protein. Carbohydr Res 345: 2648–2656 [DOI] [PubMed] [Google Scholar]
- Tryfona T, Liang H-C, Kotake T, Tsumuraya Y, Stephens E, Dupree P. (2012) Structural characterization of Arabidopsis leaf arabinogalactan polysaccharides. Plant Physiol 160: 653–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumuraya Y, Hashimoto Y, Yamamoto S. (1987) An L-arabino-D-galactan and an L-arabino-D-galactan-containing proteoglycanfrom radish (Raphanus sativus) seeds. Carbohydr Res 161: 113–126 [Google Scholar]
- Tsumuraya Y, Mochizuki N, Hashimoto Y, Kovác P. (1990) Purification of an exo-β-(1→3)-D-galactanase of Irpex lacteus (Polyporus tulipiferae) and its action on arabinogalactan-proteins. J Biol Chem 265: 7207–7215 [PubMed] [Google Scholar]
- Tsumuraya Y, Ogura K, Hashimoto Y, Mukoyama H, Yamamoto S. (1988) Arabinogalactan-proteins from primary and mature roots of radish (Raphanus sativus L.). Plant Physiol 86: 155–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Holst G-J, Clarke AE. (1985) Quantification of arabinogalactan-protein in plant extracts by single radial gel diffusion. Anal Biochem 148: 446–450 [DOI] [PubMed] [Google Scholar]
- Woods EF, Lilley GG, Jermyn MA. (1978) The self-association of glycosyl phenyazo dyes (Yariv antigens). Aust J Chem 31: 2225–2238 [Google Scholar]
- Xu J, Okada S, Tan L, Goodrum KJ, Kopchick JJ, Kieliszewski MJ. (2010) Human growth hormone expressed in tobacco cells as an arabinogalactan-protein fusion glycoprotein has a prolonged serum life. Transgenic Res 19: 849–867 [DOI] [PubMed] [Google Scholar]
- Yariv J, Kalb AJ, Katchalski E. (1967a) Isolation of an L-fucose binding protein from Lotus tetragonolobus seed. Nature 215: 890–891 [DOI] [PubMed] [Google Scholar]
- Yariv J, Lis H, Katchalski E. (1967b) Precipitation of arabic acid and some seed polysaccharides by glycosylphenylazo dyes. Biochem J 105: 1C–2C [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yariv J, Rapport MM, Graf L. (1962) The interaction of glycosides and saccharides with antibody to the corresponding phenylazo glycosides. Biochem J 85: 383–388 [DOI] [PMC free article] [PubMed] [Google Scholar]